It is long known that cellular NAD levels are a critical regulator of metabolism and bioenergetics. The intracellular NAD pool consists of both oxidized (NAD+) and reduced (NADH) forms. NAD+ is the main hydride acceptor in intermediary metabolism. Electrons derived from substrate catabolism are carried by NADH and used for oxidative phosphorylation and biosynthetic reactions. These reduction-oxidation (redox) reactions are not only essential for mitochondrial function and cell metabolism but also serve as important modulators of cell signaling1, 2. NAD+ functions as a co-substrate for sirtuin deacylases, ADP-ribose transferases, and cyclic ADP-ribose synthases that govern post-translational modification of proteins, DNA repair and inflammatory responses2.
The cellular NAD+ level is determined by the NAD(H) pool size as well as its redox state. The former is dependent on cellular NAD+ consumption and regeneration, while the latter is regulated by cell metabolism and mitochondrial function (Figure 1). Emerging evidence suggests that derangements in the myocardial NAD pool are causally linked to metabolic remodeling and mitochondrial dysfunction in the failing heart. Stabilizing the intracellular NAD+ level represents a promising therapeutic strategy to improve myocardial bioenergetics and cardiac function1, 3, 4. Reported in this issue of Circulation, Diguet et al report exciting data suggesting that supplementation with a NAD+ precursor, nicotinamide riboside (NR), reduces cardiac dysfunction in preclinical models of heart failure5.
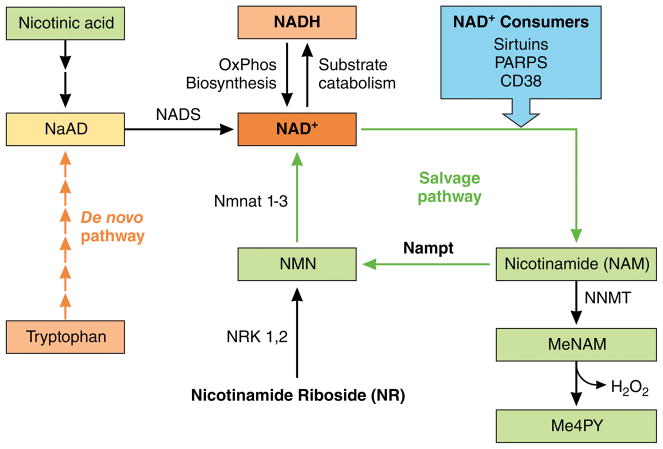
Figure 1.
NAD+ biosynthesis, consumption and salvage pathways. The intracellular NAD+ pool consists of both oxidized (NAD+) and reduced (NADH) forms. Electrons derived from substrate catabolism are carried by NADH and used for oxidative phosphorylation and biosynthetic reactions. NAD+ also functions as a co-substrate for sirtuins, ADP-ribose transferases, and cyclic ADP-ribose synthases (CD38). Eukaryotes synthesize NAD+ from the amino acid tryptophan via the de novo pathway or NAD+ can be salvaged from nicotinamide (NAM) and converted into nicotinamide mononucleotide (NMN) by nicotinamide phosphoribosyltransferase (Nampt). NMN can also be generated by the phosphorylation of NR by nicotinamide riboside kinase (Nrk).
In a genetic mouse model of dilated cardiomyopathy, induced by deletion of serum response factor in the heart (SRF-hKO), Diguet et al. found that supplementation of NR in the diet significantly reduced left ventricular contractile dysfunction and chamber dilation. A similar, albeit moderate, effect was also observed in mice with pressure overload-induced hypertrophy and dysfunction. These observations are consistent with prior work demonstrating the beneficial effects of increasing NAD levels on cardiac hypertrophy and function in models of agonist-induced pathological hypertrophy6, chronic pressure overload7, and mitochondrial cardiomyopathy associated with Friedreich’s ataxia7. Therefore, this study adds support to the emerging concept of increasing NAD levels as a therapeutic strategy for heart failure.
Despite the compelling evidence that expanding the intracellular NAD pool benefits the failing heart, the question of how it works remains not fully answered. Prior studies found that decreased NAD+ availability was associated with increased protein acetylation in failing myocardium7–9. Those studies suggested protein hyperacetylation was attributable to impaired NAD+-dependent protein deacetylation by sirtuins, especially Sirt3, the mitochondria-localized sirtuin7, 8. The benefit of increasing NAD+ levels was accompanied by a reduction in protein acetylation. However, changes in protein acetylation in the failing heart involve numerous acetylation sites on a large number of proteins. It is challenging to determine the functional significance of specific acetylation sites. Increased lysine acetylation on proteins involved in mitochondrial energy transduction pathways, including fatty acid oxidation, tricarboxylic acid (TCA) cycle, and electron transport chain has been identified in failing hearts7,9. One study suggested that increased protein acetylation increased the sensitivity of mitochondrial permeability transition pore which could be normalized by elevating NAD+ levels7. In contrast, the current study by Diguet et al. did not find evidence of altered protein acetylation in the failing hearts of SRF-hKO mice. In their hands, NR supplementation slightly increased, rather than decreased, global protein acetylation in the heart. The change was similar in control and SRF-hKO hearts suggesting an effect independent of heart failure. Thus, these results argue against a role for protein deacetylation in mediating the benefits of NR supplementation.
Diguet et al. propose that the benefit observed in their study is likely due to improved energy metabolism. This is logical as NAD+ levels and associated redox state are powerful regulators of energy metabolism1, 10. However, the study provided limited experimental evidence and they were largely indirect. The authors found that activities of citrate synthase (CS) and ATP-citrate lyase (ACL) were increased by NR supplementation in SRF-hKO hearts. Moreover, acetylation of several nuclear proteins, e.g. FoxO1 and p53, was elevated. These observations led to speculation that NR supplementation enhanced acetyl-CoA generation which resulted in increased protein acetylation in the nucleus and cytoplasm. This is intriguing and somewhat counterintuitive, as protein hyperacetylation has been found in the failing heart of animal models and patients. It is not clear why in this case promoting protein acetylation and/or cytosolic acetyl-CoA levels would benefit the failing heart. The authors also found that incubation of neonatal rat cardiomyocytes with NR did not increase oxygen consumption or ATP production but increased glycolysis. This is again confusing and difficult to reconcile with the notion of improved energy metabolism triggered by NR. Multiple possibilities can be considered for these observations. The effects of NR on energy metabolism need further investigation by assessing cardiac energetics and mitochondrial function. Additional studies comparing models and stages of heart failure, and ultimately trials in human patients will likely shed light on common underlying mechanisms.
This study also contributes valuable and much needed information on NAD+ biosynthesis and metabolism in heart failure. Eukaryotes synthesize NAD+ from the amino acid tryptophan via the de novo pathway and NAD+ can be salvaged from nicotinamide (NAM) after it is consumed by NAD+-dependent enzymes10,11. In mammalian cells the salvage pathway is considered the primary mechanism to maintain a continuous supply of NAD+. In this pathway, NAM is converted into nicotinamide mononucleotide (NMN) by nicotinamide phosphoribosyltransferase (Nampt). NMN can also be generated by the phosphorylation of NR by nicotinamide riboside kinase 1 or 2 (Nrk). Adenylylation of NMN by nicotinamide mononucleotide adenylyl transferases (NMNAT) forms NAD+ (Figure 1). The basis for decreased NAD+ levels in the failing heart likely involves multiple mechanisms. Impaired biosynthesis or salvage of NAD+, over-activation of NAD+-consuming enzymes, and altered intermediary and mitochondrial metabolism have all been proposed6,7. The Diguet study revealed that the Nampt was uniformly downregulated in the failing hearts of humans and mice, while Nrk2 was upregulated. They suggest that decreased NAD+ salvage via the Nampt reaction in failing heart is an important mechanism for the decreased NAD+ pool, for which the upregulation of Nrk2 failed to compensate in the absence of exogenous NR. Therefore, they advocated for NR supplementation as the strategy of choice for restoring NAD+ levels. While the hypothesis is plausible, it is not mutually exclusive of the alternative strategies such as stimulating Nampt activity or directly providing NMN, both of which have shown efficacy in protection against a variety of disorders associated with depletion of intracellular NAD+, including heart failure, neurodegeneration, and aging 7, 12, 13. Diguet et al. found substantial increases of MeNAM and Me4PY after NR supplementation even though NAM levels were unchanged. The finding seems to indicate that feeding the NAD pool with NR without salvage NAM may lead to overflow of downstream metabolites of NAM (Figure 1). Notably, the conversion of MeNAM to Me4PY is accompanied by ROS generation. The biological significance of these reactions has not been fully investigated but is important for therapeutic application and should be addressed in the future studies.
Despite the discrepancies in the mechanistic insight, results from the present study corroborate prior reports that targeting NAD+ levels is a promising therapeutic strategy for heart failure. The translational potential is further enhanced by the fact that multiple compounds are available as NAD+ precursors or Nampt activators. Although no information is currently available on the pharmacokinetics or tolerability of these compounds in patients, similar information from healthy volunteers has been reported recently. The co-authors of the Diguet study published dynamic changes of the NAD+ metabolome in human subjects after a single dose of NR 14. Another recent study has described the pharmacokinetics of NR and its effect on blood NAD+ levels in healthy volunteers after 9 days of treatment15. These studies are extremely valuable for moving forward but much more is needed. For example, it is unknown whether similar changes in NAD+ and its metabolites occur in the heart as that detected in the blood. Moreover, pharmacokinetics and safety information in heart failure patients are required before efficacy issues can be addressed. It is also worth mentioning that all the preclinical studies reported so far used a “prevention” approach, i.e. the NAD+ pool was expanded at the same time as heart failure induction. For clinical application, it would be more appropriate to treat the subject when cardiac dysfunction has already developed. This type of study will advance our understanding for both mechanisms and efficacy in a scenario relevant for therapy, thus, critical for translation.
Acknowledgments
Funding sources
This work is supported in part by grants from the National Institutes of Health (R01 HL110349 and HL126209 to RT, and T32 HL007828 to MW).
Footnotes
Disclosures
None.
References
- 1.Ussher JR, Jaswal JS, Lopaschuk GD. Pyridine nucleotide regulation of cardiac intermediary metabolism. Circ Res. 2012;111:628–641. doi: 10.1161/CIRCRESAHA.111.246371. [DOI] [PubMed] [Google Scholar]
- 2.Canto C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CF, Tian R. Mitochondrion as a Target for Heart Failure Therapy- Role of Protein Lysine Acetylation. Circ J. 2015;79:1863–1870. doi: 10.1253/circj.CJ-15-0742. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Karamanlidis G, Tian R. Novel targets for mitochondrial medicine. Sci Transl Med. 2016;8:326rv3. doi: 10.1126/scitranslmed.aac7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diguet Nicolas, Trammell Samuel AJ, Tannous Cynthia, Deloux Robin, Piquereau Jérôme, Mougenot Nathalie, Gouge Anne, Gressette Mélanie, Manoury Boris, Blanc Jocelyne, Breton Marie, Decaux Jean-François, Lavery Gareth, Baczkó István, Zoll Joffrey, Garnier Anne, Li Zhenlin, Brenner Charles, Mericskay Mathias. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.026099. CIRCULATIONAHA.116.026099. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. 2010;285:3133–3144. doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CF, Chavez JD, Garcia-Menendez L, Choi YS, Roe N, Chiao YA, Edgar J, Goo YA, Goodlett DR, Bruce JE, Tian R. Normalization of NAD+ Redox Balance as a Therapy for Heart Failure. Circulation. 2016;134:883–894. doi: 10.1161/CIRCULATIONAHA.116.022495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin AS, Abraham DM, Hershberger KA, Bhatt DP, Mao L, Cui H, Liu J, Liu X, Muehlbauer MJ, Grimsrud PA, Locasale JW, Payne RM, Hirschey MD. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93885. pii: 93885. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi KC, Jr, Margulies KB, Coon JJ, Kelly DP. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;1:e84897. doi: 10.1172/jci.insight.84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, McKnight SL. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell. 2014;158:1324–1334. doi: 10.1016/j.cell.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Airhart SE, Shireman LM, Risler LJ, Anderson GD, Nagana Gowda GA, Raftery D, Tian R, Shen DD, O’Brien KD. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One. 2017;12:e0186459. doi: 10.1371/journal.pone.0186459. [DOI] [PMC free article] [PubMed] [Google Scholar]