Abstract
PDT efficacy depends on the concentration of photosensitizer, oxygen, and light delivery in patient tissues. In this study, we measure the in-vivo distribution of important dosimetric parameters, namely the tissue optical properties (absorption μa (λ) and scattering μs′ (λ) coefficients), photofrin concentration (cphotofrin), blood oxygen saturation (%StO2), and total hemoglobin concentration (THC), before and after PDT. We characterize the inter- and intra-patient heterogeneity of these quantities and explore how these properties change as a result of PDT treatment. The result suggests the need for real-time dosimetry during PDT to optimize the treatment condition depending on the optical and physiological properties.
Keywords: photodynamic therapy, tissue optical properties, blood oxygen saturation, total hemoglobin concentration, diffuse reflectance
INTRODUCTION
Photodynamic therapy (PDT) has been approved by the US Food and Drug Administration for the treatment of various malignant and premalignant conditions [1, 2]. PDT efficacy depends on the availability and dynamic interactions of photosensitizer, light and oxygen. Tissue optical properties influence the delivered light dose and impact PDT outcome. In-vivo measurements of these quantities enable determination of explicit and implicit dose factors affecting PDT and helps to understand the underlying biophysical mechanism of PDT.
At the University of Pennsylvania we are evaluating intrapleural photofrin-mediated photodynamic therapy in a phase II clinical trial as an adjuvant to lung-sparing surgery to treat patients with malignant pleural mesothelioma [3, 4]. In this study, we measure the in-vivo distribution of important dosimetric parameters, namely the tissue optical properties (absorption μa (λ) and reduced scattering μs′ (λ) coefficients), photofrin concentration (cphotofrin), blood oxygen saturation (%StO2), and total hemoglobin concentration (THC), using a diffuse reflectance instrument and algorithms based on the diffusion equation. Measurements were performed at different locations in the pleural cavities of 13 patients, with a broadband diffuse reflectance spectroscopy system over a wide spectral range (500 to 800 nm) before and after PDT treatment. We characterize the heterogeneity of these quantities with respect to location, and patient, and we explore how these properties change as a result of PDT treatment.
METHODS
2.1 Patient treatment
The patients in this study were enrolled in a Phase II randomized clinical trial of Photofrin-mediated PDT for pleural mesothelioma treatment. Patients were administered Photofrin (Pinnacle Biologics, Chicago, IL, USA) at 2 mg/kg of body weight 24 h prior to intra-operative PDT. PDT was performed in the operating room, immediately following radical pleurectomy and debulking of tumor. The PDT treatment was performed with 632 nm light provided by a KTP-pumped dye laser (model 630 XP, Laserscope, Inc., San Jose, CA, USA) to a total fluence of 60 J cm−2. Light was delivered to the pleural cavity via an optical fiber inserted into modified endotracheal tube filled with 0.1% intralipid to produce an extended isotropic light source. Light source was circulated around in the pleural cavity, which was filled with 0.01% intralipid solution, during the PDT to deliver light equally to all sites in the cavity. The instantaneous light fluence rate and the cumulative light dose were monitored continuously using 8 isotropic detectors (Medlight, Switzerland) sutured to 8 strategic locations within the pleural cavity wall. These locations are anterior chest wall (ACW), posterior chest wall (PCW), posterior mediastinum (PM), diaphragm (Diaph), anterior sulcus (AS), posterior sulcus (PS), apex, and pericardium (Peri). Treatment was complete when the cumulative light fluence at each site reached to the prescribed light dose of 60 J cm−2.
2.2 Optical properties measurements
Diffuse reflectance measurements were acquired before and after PDT treatment using a specially designed fiber optic-based contact probe consisting of one source fiber, coupled to an air-cooled quartz-tungsten-halogen (QTH) lamp (Avalight HAL-S, Avantes, Inc., Louisville, CO, USA), and 9 detection fibers spaced at distances from 1.4 to 8.7 mm from the source. Measurements were made with the probe in contact with the interior surface of the pleural cavity, at sites adjacent to the 8 isotropic detectors. Measurements were also made at lung and aorta in some patients and the results were presented in this paper. The reflected light was collected by the detection fibers which are imaged via a spectrograph onto a CCD, to measure radially-resolved diffuse reflectance. Background signal was measured in the same tissue with the white light source turned off, and then subtracted from each measurement. Each tissue spectrum is divided by the spectrum obtained with the same light source and detector in an integrating sphere to account for the wavelength-dependence of the white light source power and CCD response. The diffuse reflectance spectra are fitted with a nonlinear fitting algorithm implemented in the Matlab programming environment to extract the values of tissue optical properties. Details of the probe design and fitting algorithm have been described previously [5].
2.3 Data analysis
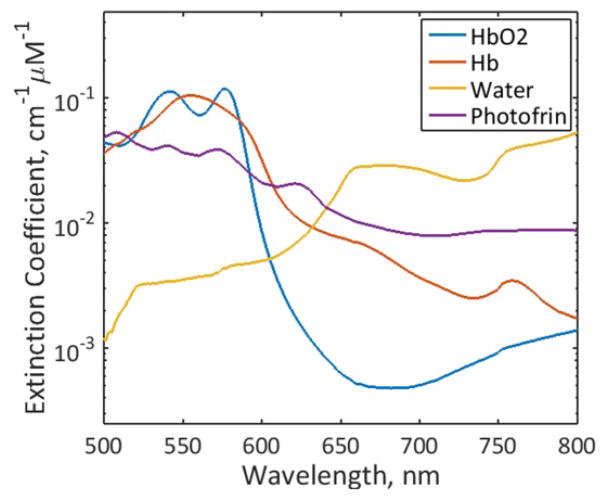
A multi-wavelength algorithm based on diffusion approximation equation was employed to fit all reflectance spectra between 500 nm to 800 nm simultaneously using multiple source-detector separations to extract μa (λ) and μs′ (λ). Then, the concentration of Photofrin and other chromophores were obtained from μa (λ) using μa (λ) = σiciεi (λ), where εi (λ) is the extinction coefficient of i’th chromophore and ci (λ) is the molar concentration of the i’th chromophore. The major chromophores in the spectral region of interest are oxy-(HbO2), deoxy-hemoglobin (Hb), water, and Photofrin and their extinction coefficients are obtained from the literature [4, 6] as shown in Figure 1. The concentrations of all chromophores, cwater, cHb, cHbO2 and cPhotofrin are reconstructed directly using a nonlinearly constrained optimization method, fminsearch, implemented in Matlab.
Figure 1.
Extinction coefficients of HbO2, Hb, water and Photofrin used in the fitting.
RESULTS AND DISCUSSION
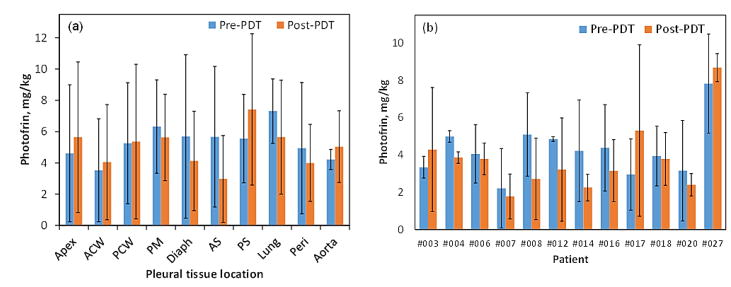
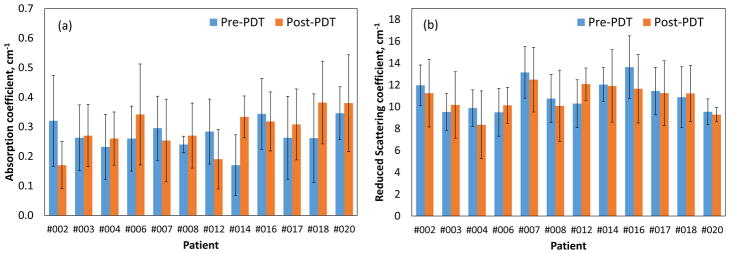
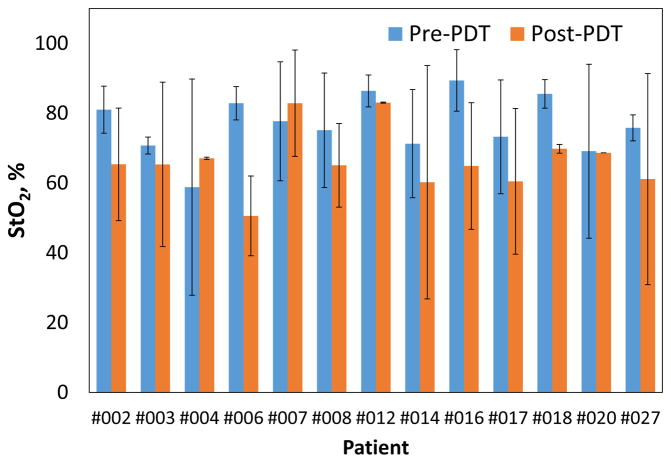
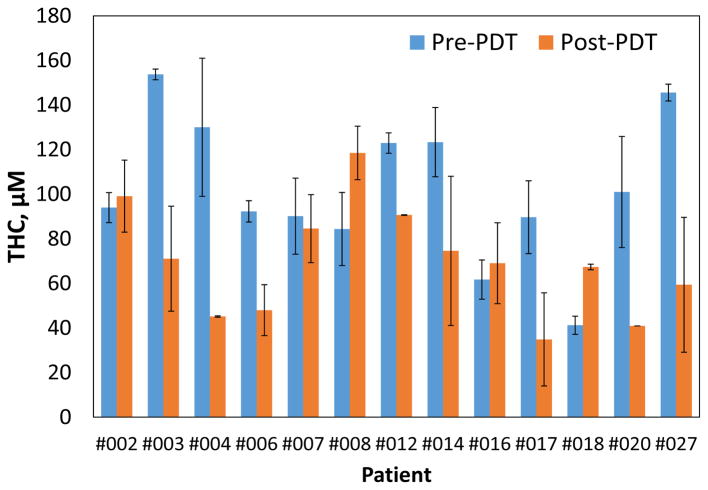
We observed small difference in the mean tissue optical properties; pre-PDT: μa=0.3±0.17cm−1, μs′=10.69±3.66cm−1 and post-PDT: μa=0.26±0.18 cm−1, μs′=10.82±2.47 cm−1. Fig. 3(a) shows no preferential Photofrin uptake by specific pleural tissue. Large heterogeneity in intra- and inter-patient Photofrin uptake is observed as shown in Fig. 3(b). The mean measured Photofrin concentrations in all patients are 4.96 ± 3.41 mg/kg before PDT and 4.33 ± 2.93 mg/kg after PDT. Mean blood oxygen saturation before PDT is 76.7 ± 13.5% and 63.7 ± 20.3% after PDT across all patients. Mean THC before PDT is 115.4 ± 75.5μM and 71.7 ± 44.1μM after PDT. The optical and physiological properties varied from site to site and patient to patient but similar before and after PDT. The detected large heterogeneity of these quantities among and within patients suggests the need for real-time dosimetry during PDT to optimize the treatment condition depending on the optical and physiological properties.
Figure 3.
Comparison of pre- and post-PDT Photofrin concentration (a) by pleural tissue locations, and (b) by patients. Standard deviations are shown as error bars.
CONCLUSION
The optical properties, drug concentration, total hemoglobin concentration and blood oxygen saturation of 13 patients with malignant pleural mesothelioma are measured before and after Photofrin-mediated PDT, using a broadband diffuse reflectance spectroscopy system, and compared with respect to tissue location and patient. We observed substantial heterogeneity in optical and physiological property measurements among and within patients, which suggests the need for real-time dosimetry during PDT to optimize the treatment condition based on the optical and physiological properties.
Figure 2.
Inter-patient comparison of average pre- and post PDT pleural tissues (a) absorption coefficients and, (b) reduced scattering coefficients at the treatment wavelength (632 nm). Standard deviations of various tissue locations within individual patient are shown.
Figure 4.
Inter-patient comparison of blood oxygen saturation, %StO2 pre- and post-PDT. Standard deviations of various tissue locations within individual patient are shown as error bar.
Figure 5.
Inter-patient comparison of pre- and post-PDT total hemoglobin concentration.
Acknowledgments
The authors would like to thank all the PDT group members at the hospital of the University of Pennsylvania, Carmen Rodriguez, Theresa M. Busch, Sunil Singhal, Charles B. Simone II, Sally McNulty, Joann Miller and Min Yuan. This work was supported by NIH grants R01 CA 154562, P01 CA087971 and by the Department of Radiation Oncology, University of Pennsylvania.
References
- 1.Zhu TC, Finlay JC. The role of photodynamic therapy (PDT) physics. Med Phys. 2008;35(7):3127–36. doi: 10.1118/1.2937440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008;53(9):R61. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 3.Ong YH, Kim MM, Finlay JC, Dimofte A, Cengel KA, Zhu TC. Four-Channel PDT Dose Dosimetry for Pleural Photodynamic Therapy. Proc SPIE. 2017;10047:1004717–1. [Google Scholar]
- 4.Ong YH, Kim MM, Finlay JC, Dimofte A, Singhal S, Glatstein E, Cengel KA, Zhu TC. PDT dose dosimetry for Photofrin-mediated pleural photodynamic therapy (pPDT) Phys Med Biol. 2017;63(1):015031. doi: 10.1088/1361-6560/aa9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlay JC, Zhu TC, Dimofte A, Friedberg JS, Hahn SM. Diffuse reflectance spectra measured in vivo in human tissues during Photofrin-mediated pleural photodynamic therapy. Proc SPIE. 2006;6139:61390. doi: 10.1117/12.647016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang HW, Zhu TC, Putt ME, Solonenko M, Metz J, Dimofte A, Miles J, Fraker DL, Glatstein E, Hahn SM, Yodh AG. Broadband reflectance measurements of light penetration, blood oxygenation, hemoglobin concentration, and drug concentration in human intraperitoneal tissues before and after photodynamic therapy. J Biomed Opt. 2005;10(1):14004. doi: 10.1117/1.1854679. [DOI] [PubMed] [Google Scholar]