SUMMARY
Global environmental changes caused by natural and human activities have accelerated in the past 200 years. The increase in greenhouse gases is predicted to continue to raise global temperature and change water availability in the 21st century. Plant diseases are deeply influenced by the environment; a susceptible host will not be infected by a virulent pathogen if the environmental conditions are not conducive for disease. The change in CO2 concentrations, temperature, and water availability can have positive, neutral, or negative effects on disease development, as each disease may respond differently to these variations. However, the concept of disease optima could potentially apply to all pathosystems. Plant resistance pathways, including pattern-triggered immunity and effector-triggered immunity, RNA interference, and defense hormone networks, are all affected by environmental factors. On the pathogen side, virulence mechanisms, such as the production of toxins and virulence proteins, as well as pathogen reproduction and survival are influenced by temperature and humidity. For practical reasons, most laboratory investigations into plant-pathogen interactions at the molecular level focus on well-established pathosystems and use a few static environmental conditions that capture only a fraction of the dynamic plant-pathogen-environment interactions that occur in nature. There is great need for future research to increasingly use dynamic environmental conditions in order to fully understand the multidimensional nature of plant-pathogen interactions and produce disease-resistant crop plants that are resilient to climate change.
Plant diseases can have devastating economic, social and/or ecological consequences on a global scale. Some of the most infamous plant diseases, e.g., potato late blight in Ireland in the 1840s [1] and chestnut blight in the United States in the early 1900s [2], resulted in massive human death and migration and/or drastic changes to the landscape. Not only do many plant diseases persist for centuries, but also new ones continue to emerge worldwide. Estimates of direct production losses for the major agricultural crops by biotic stress have been projected to be around 20% to 40% [3, 4]. Together with associated indirect losses in crop quality and marketability, plant diseases are widely considered to be one of the most formidable obstacles to achieving global food security in the face of the rising human population in the 21st century. For plant scientists, a global challenge is how to speed up the understanding of the molecular, epidemiological and ecological bases of plant diseases and develop truly effective and long-lasting solutions for preventing, reducing, or managing some of the most devastating plant diseases facing modern agriculture today and in the future.
Not every disease is equal. Some plant pathogens have a more devastating socioeconomic effect than others, partly because of the host crop species they infect. We list some of the most important plant diseases in Table 1. Pathogens that commonly infect plants are diverse, ranging from intracellular viruses and bacteria, to those that live extracellularly including other bacteria, fungi, oomycetes, and nematodes. Depending on how pathogens acquire their nutrients, they can be classified as biotrophs or necrotrophs. Biotrophs can only obtain nutrients from living host cells, while necrotrophs usually kill host cells in order to release nutrients. Necrotrophs are also often able to live as saprophytes. In nature, pathogen nutrient acquisition spans a continuum from biotrophy to necrotrophy, with many plant pathogens being hemibiotrophs, showing an initial biotrophic phase before eventually killing the host.
Table 1.
Examples of the most destructive diseases and pathogens for crops with the highest area of production.
| Crop | Most destructive diseases and pathogens |
|---|---|
| Banana and plantain | Banana bunchy top virus (BBTV), black sigatoka by Mycosphaerella fijiensis , and Panama disease by Fusarium oxysporum f. sp. cubense [5, 6, 7] |
| Barley | Fusarium head blight by Fusarium graminearum, powdery mildew by Blumeria graminis f. sp. hordei, and stem rust by Puccinia graminis f. sp. hordei [8] |
| Cassava | African cassava mosaic virus (ACMVD), bacterial blight by Xanthomonas axonopodis pv. manihotis, and cassava brown streak virus (CBSV) [9] |
| Cotton | Bacterial blight by Xanthomonas citri pv. malvacearum, Fusarium wilt by Fusarium oxysporum f. sp. vasinfectum, and Verticillium wilt by Verticillium dahliae [10, 11, 12] |
| Maize | Aspergillus ear rot by Aspergillus flavus, Giberella stalk and ear rot by Fusarium graminearum, and grey leaf spot by Cercospora zeae-maydis [13, 14, 15] |
| Palm fruit | Basal stem rot by Ganoderma boninense, and bud rot by Phytophthora palmivora [16, 17] |
| Peanut | Groundnut rosette disease [a combination of groundnut rosette virus (GNV), its satellite RNA, and groundnut rosette assistor virus (GRAV)] [18] |
| Potato | Brown rot of potato by Ralstonia solanacearum, and late blight by Phytophthora infestans [1, 19] |
| Rapeseed and mustard | Phoma stem canker by Leptosphaeria maculans, and Sclerotinia stem rot by Sclerotinia sclerotiorum [20, 21] |
| Rice | Rice blast by Magnaporthe oryzae, rice bacterial blight by Xanthomonas oryzae pv. oryzae, and sheath blight by Rhizoctonia solani [22, 23, 24] |
| Sorghum and millet | Anthracnose by Colletotrichum sublineolum, and Turcicum leaf blight by Exserohilum turcicum [25] |
| Soybean | Soybean cyst nematode disease by Heterodera glycines, and Asian soybean rust by Phakopsora pachyrhizi [26] |
| Sugar beet | Cercospora leaf spot by Cercospora beticola, and rhizomania by beet necrotic yellow vein virus (BNYVV) [27, 28] |
| Sugarcane | Ratoon stunting by Leifsonia xyli subsp. xyli, and red rot by Colletotrichum falcatum [29, 30] |
| Sweet potato | Sweet potato virus disease [SPVD; a combination of sweet potato feathery mottle virus (SPFMV) and sweet potato chlorotic stunt virus (SPCSV)] [31] |
| Tomato | Late blight by Phytophthora infestans, and tomato yellow leaf curl virus (TYLCV) [1, 32] |
| Wheat | Fusarium head blight by Fusarium graminearum, stem rust by Puccinia graminis, and wheat yellow rust by P. striiformis [33, 34, 35] |
| Yam | Anthracnose by Colletotrichum gloeosporioides, and yam mosaic virus (YMV) [36] |
Pathogens and plants do not interact in isolation. The famous “disease triangle” concept in plant pathology highlights the interaction of both pathogens and plants with the environment. For disease to occur, a susceptible plant host, a virulent pathogen, and the proper environmental conditions are required, as lack of favorable conditions for any of these three factors results in a failure for disease to develop [37]. The effect of environmental variables (e.g., high temperature) on pathogens and plants can have favorable, neutral or negative outcomes on plant disease development. Both pathogens and plants have an optimal environmental condition for their growth and reproduction, with an ideal environmental condition that favors disease. The further the environmental conditions deviate from this “disease optimum”, the fewer disease symptoms will occur on the plant (Figure 1).
Figure 1. Impact of environmental conditions on plant pathogen interactions.
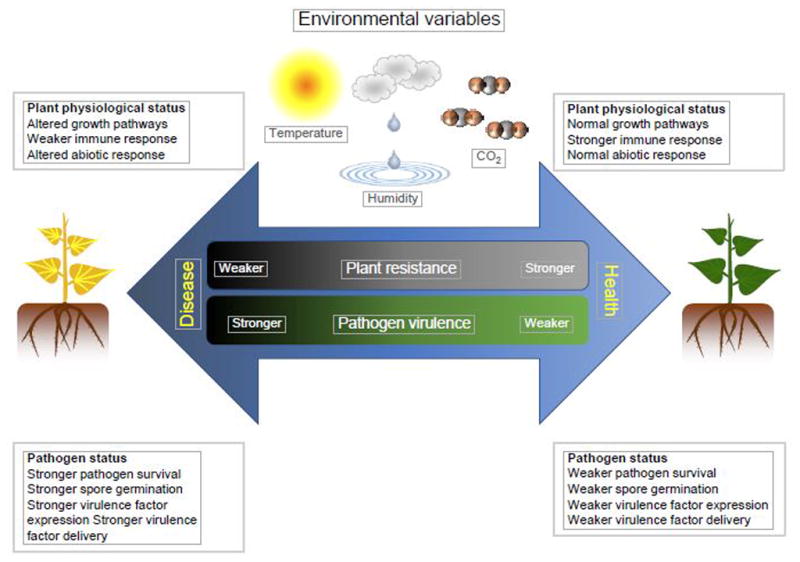
The environment-host-pathogen tripartite interaction operates within a continuum, from interactions fully conducive for disease (disease optima) to those that maintain healthy plants. Environmental conditions can have profound effects on a host plant’s physiological state, including its growth, immune signaling and abiotic stress response, as well as a pathogen’s survival, germination, and expression and delivery of virulence factors. These variable environmental conditions can render the same plant being fully susceptible to being fully resistant, while the pathogen could range from being able to cause severe disease to being only weakly pathogenic. The three most important environmental variables predicted to change in this century are atmospheric CO2 concentration, temperature, and water availability.
Due to genetic, environmental, and cultural reasons, not all cultivated crops have the same importance in human nutrition. Ten crops account for 58% of the total global cultivated area (Table 2). With respect to food security, only four crops contribute to up to 50% of the daily worldwide human calorie uptake (Table 2). In this review, we will highlight the effect of the environment on plant diseases, whenever possible, focusing on the crops with the highest worldwide production, as these crops are fundamental for our global food security. We will also discuss studies that elucidated the molecular mechanisms controlling plant resistance and pathogen virulence, which often use model plant-pathogen systems.
Table 2.
Crops with the highest area harvested worldwide.
| Crop | Area harvested (Million hectares) | Production (Million metric tons) | Calories (C) consumed per day | % of calories of a 2884-caloriea diet |
|---|---|---|---|---|
| Rice | 162.72 | 741.48 | 544 | 18.9 |
| Wheat | 220.42 | 729.01 | 527 | 18.3 |
| Sugarcane | 27.12 | 1884.25 | 213 | 7.4 |
| Sugar beet | 4.47 | 269.71 | ||
| Maize | 184.80 | 1037.79 | 155 | 5.4 |
| Soybean | 117.55 | 306.52 | 96 | 3.3 |
| Potato | 19.10 | 381.68 | 64 | 2.2 |
| Palm fruit (for oil) | 18.70 | 274.62 | 59 | 2.0 |
| Peanut (including its shell) | 26.54 | 43.92 | 38 | 1.3 |
| Cassava | 23.87 | 268.28 | 37 | 1.3 |
| Sunflower seed | 25.20 | 41.42 | 36 | 1.2 |
| Rapeseed and mustard | 36.93 | 74.48 | 36 | 1.2 |
| Banana and plantain | 5.39 | 114.13 | 29 | 1.0 |
| Sorghum | 44.96 | 68.94 | 28 | 1.0 |
| Millet | 31.43 | 28.38 | 27 | 0.9 |
| Sweet potato | 8.35 | 106.60 | 22 | 0.8 |
| Yam | 7.76 | 68.13 | 13 | 0.5 |
| Cotton (seed) | 34.75 | 79.07 | 13 | 0.5 |
| Tomato | 5.02 | 170.75 | 11 | 0.4 |
| Barley | 49.43 | 144.49 | 7 | 0.2 |
| Watermelons | 3.48 | 111.01 | –b | –b |
For 2014, the total worldwide area harvested was 1,384 million hectares, while production was 8,889 million metric tons. Almost 68% of the world calorie consumption is obtained from these 19 crops. Data for calories consumed per day from sugarcane and sugar beet combined. Data from FAOSTAT (Statistics Division, Food and Agriculture Organization of the United Nations), 2013 and 2014.
Average calories consumed in the world. A calorie (C) is equivalent to 1 kcal.
No data available.
ENVIRONMENTAL FACTORS AFFECTING PLANT DISEASE
Many environmental conditions affect plant disease development, including temperature, light and water availability, soil fertility, wind speeds, and atmospheric ozone, methane and CO2 concentration. Among these, three are predicted to most likely change and affect the climate in this century, namely CO2 concentration, temperature, and water availability. Therefore, this review will focus on these three environmental factors. Readers are referred to excellent reviews that discuss the effects on disease of other environmental factors [38, 39, 40, 41].
Atmospheric carbon dioxide concentration
Data collected across the globe show a dramatic increase in atmospheric CO2 concentrations since the dawn of the industrial revolution. Current atmospheric CO2 concentration has surpassed the 400-ppm threshold (from less than 285 ppm at the start of the 19th century [42]) mainly because of anthropogenic influence [43]. How this will affect future climate conditions is still a matter of debate. It is projected that the increase in greenhouse gases will cause an increase in global temperature between 1.5 °C and 4.8 °C by the end of the century relative to the temperature before the industrial era (year 1800). A 0.85 °C increase [90% uncertaintly interval between 0.65 and 1.06 °C] has been observed so far [43]), with weather extremes (such as heat waves, droughts, floods, and heavy rains) predicted to increase in severity and/or duration [44, 45]. For more information on climate change, please refer to the latest Intergovernmental Panel on Climate Change report [43], the U.S. Global Research Program report [46] and NASA (https://climate.nasa.gov/).
Globally, predicted future increases in temperature and regional changes in water availability [43] will change the areas in which crops will be produced, and the vector and pathogen populations causing disease [47]. Pathogen and pest distribution has even been observed to move towards the poles as global temperature increases [48]. By 2050, crop production is predicted to decrease throughout the world, especially in the poorest and more vulnerable countries (this study did not take into account the effect of increased CO2 on photosynthesis [49, 50]).
Higher CO2 concentrations are expected to increase the photosynthetic rate and crop yield of C3 plants. Depending on the crop studied; there is between a 5 to a 40% increase in yield in free-air CO2 enrichment (FACE) experiments for the predicted CO2 concentrations that will be reached by the end of the century [51]. C4 plants (e.g., corn and sugarcane), however, will not benefit from this increase in atmospheric CO2 due to their already inherent CO2 concentration mechanisms [52]. While increased CO2 concentrations can increase the yields of C3 crop plants, they also increase disease severity in rice and wheat (620 and 780 ppm, respectively), two of our staple crops with the highest worldwide production [53, 54]. For the fungal pathogen Fusarium graminearum, elevated CO2 levels not only increased the susceptibility of wheat varieties (irrespective of the resistant and susceptible genotypes evaluated), but also increased the virulence of the fungal isolate [54], resulting in more severe disease overall. In contrast, in some oomycete-plant interactions such as between soybean and Peronospora manshurica, CO2 concentrations of 550 ppm decreased the severity of the disease by more than 50% [55]. The complicated relationship between CO2 concentrations and disease is further reflected for the potato late blight disease caused by the oomycete Phytophthora infestans. A modeling study assessing the expected risk of late blight infection in potato-growing areas of Scotland for the next 60 years predicted an increase in Phytophthora infection during the first half of the potato growing season, but a decrease in infection for the second half [56]. The model did not separate the effect of increased CO2 concentration from that of increased temperature, and as such, the predicted effect was most likely only indirectly influenced by CO2. For tripartite biotic interactions involving a plant (wheat), a virus (barley yellow dwarf virus, BYDV), and a vector (the aphid Rhopalosiphum padi), non-infected plants had lower aphid populations at elevated CO2, but higher CO2 concentrations showed no effect on overall BDYV infection, highlighting complicated effects of CO2 concentrations on the different biotic interacting components [57].
These disparate reports on the effects of CO2 concentrations on plant-pathogen interactions are not sufficient to draw any unifying principles and, therefore, underscore the great need to expand research in this area. Most importantly, as different plant-pathogen interactions involve pathogens of different lifestyles (e.g., biotrophic vs. necrotrophic) and engagement of different plant defense pathways, increased efforts should be devoted in determining whether the different effects could be attributed to different pathogen lifestyles and/or the CO2 modulation of plant defense pathways.
Temperature
For every plant-pathogen interaction, there is an optimal temperature range at which disease develops. For example, 15 °C is optimal for Globodera pallida nematodes to infect potato plants [58], daytime temperatures of 35 °C and nighttime temperatures of 27 °C are most favorable for Xanthomonas oryzae bacteria to colonize rice [59], whereas temperature between 26 °C to 31 °C are idea l for papaya ringspot virus (PRSV) to infect papaya [60]. However, temperature averages might not always be good predictors of the potential for an infection. The rust pathogen Puccinia striiformis was found to be unable to cause infections in the laboratory if inoculated in wheat seedlings at constant temperatures over 21 °C, while in the field, infections occurred even when temperatures fluctuated between 18 °C to 30 °C [61]. This apparent discrepancy seemed to result from field temperatures being optimal for infection at night, as the fungus was able to survive and cause infection if the lethal temperature did not exceed a certain time threshold [62]. Also, even when the same average temperatures were used to study the interaction between P. infestans and potato, small 5 °C fluctuations in temperature caused plants to b e more susceptible than daily constant temperatures [63]. These findings have important implications, as extremes in temperature (e.g., heat waves) have become more frequent, despite the predicted small increase in global average temperature due to climate change. Consequently, in order to truly understand the effect of temperature on disease development, future research should use conditions that resemble the dynamic conditions observed in nature (e.g., changing temperature conditions for plant growth throughout the day).
It is anticipated that the projected increase in global temperature will most likely change the regional distribution in which a crop is susceptible to a particular pathogen. For areas outside of the tropics, a global trend is the higher prevalence of pathogen inocula overwintering for the next crop-growing season, with potential for more severe and frequent epidemics [64]. This will be particularly relevant for pathogens that already possess cold-, heat- or desiccation-tolerant surviving structures, some of which may last several years, even under adverse conditions [65, 66]. Another outcome of warming temperatures is that new pathogen strains better adapted to these temperatures may become prevalent. For P. infestans, variability in the response to temperature has been observed in populations from different geographic regions [67]. In the interaction between the rust fungus Puccinia striiformis and wheat, new pathogen races that are more aggressive in causing disease at higher temperatures have appeared since the year 2000 and have become more prevalent worldwide in only a few years [68, 69]. It is important to note, however, that some diseases that can potentially cause an epidemic never develop into one simply because of transient temperature shifts. For example, temperatures fluctuating between a low of 12 °C and a high of 25 °C are conducive for soybean rust. However, exposing infected plants to as short as 1 hour at 37 °C abolishes symptom development [70]. Transient temperature shifts, such as those that are predicted to occur more frequently this century [44], might stop or further enhance potential future epidemics. Increased studies on the distribution and variability in the virulence of pathogen strains are required to evaluate the future potential of disease outbreaks and infer the structure of future sweeping pathogen populations.
For tripartite biotic interactions involving a pathogen-transmitting vector,, temperature could profoundly influence disease incidence and/or severity by affecting the vector. This is especially important for viruses, the majority of which are transmitted by insects [71]. Banana bunchy top virus (BBTV) is transmitted by an aphid (Pentalonia nigronervosa) whose fastest development, highest fecundity, and lowest mortality occur at 25 °C [72], which is the same temperature at which transmission of BBTV is most efficient [73]. Temperatures favorable for the aphid could explain epidemics in banana, even if the conditions are suboptimal for BBTV replication. For many diseases, vector populations are an important factor that needs to be thoroughly considered when deciding on strategies for disease prediction and control under changing climate conditions.
Water availability
Most plant diseases are favored by conditions of rain, high air humidity and high soil moisture. In particular, the virulence of pathogens that infect aerial tissues is greatly promoted by rain and high humidity. For example, the virulence of the fungus Sclerotinia sclerotiorum increases as air humidity increases, with highest disease development in lettuce plants when the air relative humidity surpasses 80% [74]. For fruit rot caused by Phytophthora capsici, pathogen growth and disease symptoms were highest at close to 100% relative humidity [75]. For many fungal pathogens, the duration in which the leaf has water on its surface (i.e., leaf wetness) is critical for disease development, with many devastating plant pathogens such as Magnaporthe oryzae (rice blast fungus) or Puccinia striiformis (stripe rust fungus) requiring a minimum of 5 hours of leaf wetness for disease to occur [76]. During clear-night skies with light winds, the duration of leaf wetness is even longer due to dew accumulation in leaves [77]. As air can hold more water vapor at higher temperatures, the possibility for dew accumulation (and consequently, pathogen infection) increases at higher temperatures. On the other hand, soil moisture is more critical than air humidity for soil-inhabiting pathogens, many of which cause plant wilt diseases. Lower soil moisture decreases the incidence of infection of Ralstonia solanacearum in tomato plants [78]. In fact, balancing moisture levels to reduce disease incidence but still favor plant growth is an important cultural practice for disease management [79].
Contrary to the effect observed for Ralstonia infection of tomato plants, drought conditions caused more aggressive infections by Magnaporthe oryzae in rice, resulting in larger pathogen populations and more visible disease symptoms [80]. In the interaction between potato and the bacterial scab pathogen Streptomyces spp., lower soil moisture also favors disease development, and as such, increasing soil moisture can be used as a strategy to control this disease [81]. These two examples are exceptions, as there are few plant-pathogen interactions in which low humidity favors disease development.
Some of the effect of high humidity on pathogens does not directly translate into crop yield reductions but on reduction of product marketability, as exemplified by several pathogenic fungi (Fusarium spp. and Aspergillus spp.) that produce mycotoxins. Presence of mycotoxins even in minute quantities prevents the marketability of the crop. In wheat plants infected with F. graminearum (head blight fungus), higher humidity increased the concentration of the mycotoxin deoxynivalenol [82, 83], which prevented the grain from being sold.
Combined effect of multiple environmental factors
Predicting the combined effect of changing environmental conditions on disease is not straightforward [84, 85]. In Arabidopsis, combined heat, drought and turnip mosaic virus (TuMV) infection cause a more severe reduction in plant growth than each individual factor alone [86]. For many fungal pathogens, the combination of warm temperatures and high humidity provide the optimum conditions for disease development [74]. In the interaction between Botrytis cinerea and grapes, both air relative humidity close to 100% and temperatures between 20 and 25 °C were required for optimal disease [87], and deviations from this optimum resulted in a drastic decrease in disease incidence. In the field, plants and pathogens experience multiple environmental factors. How the combined effects of multiple environmental conditions impact the disease outcome remains one of the most outstanding and challenging questions for the future study of plant-pathogen interactions.
MOLECULAR MECHANISMS UNDERLYING ENVIRONMENTAL MODULATION OF PLANT IMMUNITY
Plants have evolved sophisticated defense mechanisms to fend off pathogen attacks. These mechanisms include PAMP-triggered immunity (PTI), effector-triggered immunity (ETI), RNA interference (RNAi), and intricate defense hormonal regulation (Figure 2). Many excellent reviews have discussed the current understanding of these mechanisms [88, 89, 90, 91, 92, 93]. In this review, we focus on the emerging studies aimed to understand how environmental conditions modulate these essential defense mechanisms.
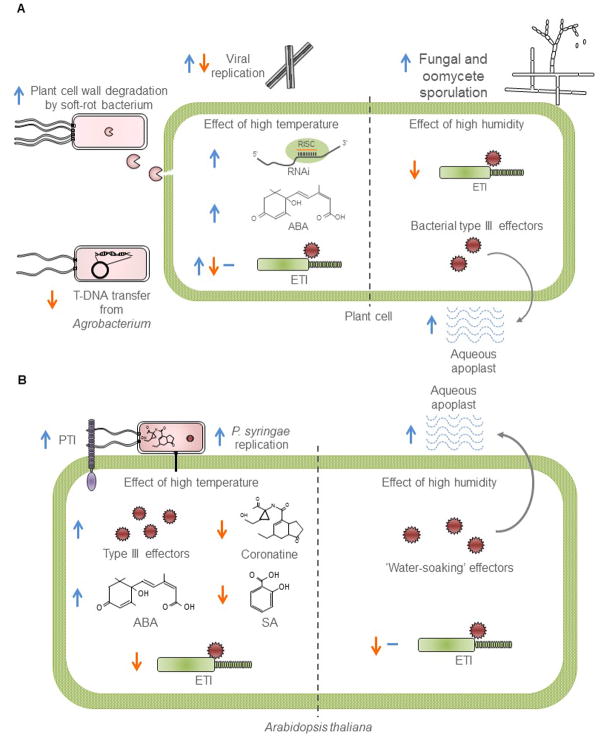
Figure 2. Environmental impact on the molecular mechanisms influencing disease.
(A) A diagram depicting examples of plant-pathogen-environment triangular interactions. High temperature (left half of plant cell) increases the production of bacterial plant cell wall-degrading enzymes by soft-rotting bacteria, while it decreases transfer DNA (T-DNA) delivery by Agrobacterium spp. Viral accumulation can be positively or negatively influenced by elevated temperature, however, the plant antiviral RNA interference (RNAi) mechanisms are generally increased by warm temperatures. Abscisic acid (ABA) accumulation is increased, whereas effector-triggered immunity (ETI) can be unaffected, heightened, or rendered ineffective at high temperatures. High humidity (left half of cell) increases sporulation by fungal and oomycete pathogens, and allows bacterial effectors to establish an aqueous apoplast in infected leaves. In tomato, ETI is negatively affected by high humidity. Abbreviation: RISC, RNA-induced silencing complex. (B) A diagram depicting the current knowledge of the Arabidopsis-Pseudomonas-environment triangular interaction. Elevated temperature increases effector translocation into plant cells and decreases production of the toxin coronatine from P. syringae. On the other hand, elevated temperature increases ABA and decreases salicylic acid (SA) hormone concentrations in Arabidopsis. A decrease in ETI and an increase in PAMP-triggered immunity (PTI) at elevated temperature have been reported. Higher air humidity conditions favor P. syringae, which produce effectors that establish an aqueous apoplast in the infected leaves to favor disease. Also, higher humidity affects some aspects of the ETI response.
Environmental impact on PAMP-triggered immunity (PTI)
PTI is a mechanism in which conserved molecules of microbes (pathogen- or microbe-associated molecular patterns, PAMP or MAMP, respectively) are recognized by plant plasma-membrane-localized pattern recognition receptors (PRR). MAMP recognition causes a signaling cascade that includes protein phosphorylation, reactive oxygen species (ROS) production, Ca2+ concentration increases, and gene activation that lead, through a yet unknown mechanism, to halt microbial growth [88]. PTI is thought to be part of a mechanism that prevents multiplication of the vast number of nonpathogenic microbes that plants encounter in nature.
There is emerging evidence that humidity and water availability could influence the effectiveness of PTI. Virulent bacterial pathogens often cause the leaf apoplast (intercellular spaces) to be water-soaked as part of its infection cycle [94, 95]. Keeping leaves water-soaked in the apoplast under high humidity allows PTI-inducing non-pathogenic Pseudomonas bacteria to grow to a significant level in Arabidopsis, bean, and tobacco plants [96].
Stomata are microscopic openings in leaves and stems that are involved in CO2 and O2 exchange with the atmosphere, and in water loss through transpiration. Under drought stress, the balance between the biosynthesis and catabolism of the hormone abscisic acid (ABA) favors increased concentration of this hormone in stomatal guard cells, which leads to stomatal closure [97]. In contrast, under high humidity, stomata open while ABA accumulation decreases [98]. Stomata are also commonly used as entry points for leaf pathogens. PTI activation triggered the closure of stomata after the recognition of MAMPs by stomatal guard cells [99, 100], as part of the plant defense against bacterial entry into the leaf apoplast. It was recently shown that high humidity conditions blocked PTI-induced stomatal closure in both Arabidopsis and bean plants [101], suggesting that stomatal defense is another point of environmental regulation of PTI.
Likewise, PTI signaling can be altered by temperature changes. In Arabidopsis, short warm-temperature treatments (28 °C for 15 minutes) induced higher PTI-associated MAPK (mitogen-activated protein kinase) phosphorylation and PTI marker gene expression after PAMP exposure, suggesting that PTI may be enhanced at warm temperatures [102].
Environmental impact on effector-triggered immunity (ETI)
A widely used and arguably the most effective form of genetically controlled resistance against plant pathogens is ETI. During ETI, a virulence-promoting pathogen effector or its activity in plant cells is sensed by a plant resistance (R) protein, most of which are NLR (nucleotide-binding domain and leucine-rich repeat) proteins [92]. This recognition triggers a signaling cascade that usually culminates in a hypersensitive response (HR), a type of programmed cell death thought to contain the pathogen at the site of infection [93]. While effective against biotrophic pathogens, ETI is ineffective against (and sometimes can be taken advantage by) necrotrophs, which, as already mentioned, obtain their nutrients from dead host tissues [103].
In many pathosystems, high temperatures compromise ETI. For example, the tobacco N protein for the resistance against tobacco mosaic virus (TMV, [104]), several tomato Cf proteins for resistance against the leaf mold pathogen Cladosporium fulvum [105], and several R proteins that confer resistance to the bacterial pathogen Pseudomonas syringae (RPM1, RPS2, and RPS4 from Arabidopsis) or potato virus X (PVX, recognized by Rx in potato) [106] do not mount an effective ETI at temperatures higher than 30 °C. Contrary to most R proteins, however, X a7, a rice disease resistance protein against Xanthomonas oryzae, is more effective at higher temperatures than at lower temperatures [107]. The suppressive effect of high temperature on ETI is also evident in certain “autoimmune” mutant plants in which ETI and spontaneous cell death are constitutively activated. A prominent example is some Arabidopsis snc1 mutant alleles. SNC1 is an Arabidopsis NLR gene in which several mutations cause an autoimmune phenotype and increase resistance to P. syringae at 22 °C but not at 28 °C [108]. Transcription of the SNC1 gene and the autoimmune phenotype of a snc1 allele are suppressed by a gain-of-function allele of the pseudokinase ZED1 at 22 °C [109]. ZED1 is an Arabidopsis protein required for the recognition of the bacterial effector HopZ1a by the R protein ZAR1 [110]. It seems that SNC1 and ZED1 proteins are part of the same high temperature-sensitive ETI pathway [109].
The effect of temperature on ETI appears to depend on the duration of the temperature regime. Recognition of P. syringae effector AvrRpt2 by Arabidopsis RPS2 and subsequent inhibition of bacterial growth is lost if plants are grown for 3 weeks at 28 °C prior to infection [106]. However, if plants had been acclimated for only 1 day at 28 °C, ETI containment of growth is still present, even though the ETI-associated HR cell death is absent [111]. Therefore, it seems that acclimation at high temperatures for short periods of time might only disarm part of the ETI-signaling pathways. The variable results from using different durations of temperature treatment suggests a need to conduct future temperature experiments with diurnally fluctuating temperature cycles and heat waves that resemble those experienced by crops in the field.
How high temperature suppresses ETI is not fully understood. Several studies suggest that one effect may be at the level of nuclear localization of some R proteins. For example, autoimmunity-associated mutations in SNC1 cause increased SNC1 nuclear localization at 22°C. A reduction of nuclear localization at 28 °C is associated with suppression of autoimmune phenotypes. Similarly, nuclear localization of the R protein N was observed after recognition of the TMV coat protein at 22 °C but not at 28 °C [108]. A genetic suppressor screen identified an Arabidopsis mutant, aba2-21 (an allele of a gene involved in ABA biosynthesis), that restores SNC1 autoimmunity at high temperatures [112]. The aba2-21 increases the nuclear localization of SNC1 and RPS4 (which recognizes the Pseudomonas effector AvrRps4) at 28 °C [112], therefore, the lack of R protein nuclear accumulation might cause ETI failure at high temperatures. It is important to note that not all R proteins show nuclear localization [113, 114], so this phenomenon likely applies to only a subset of R proteins.
As with high temperatures, high humidity can also interfere with ETI-associated HR. For example, the response to C. fulvum Avr4 and Avr9 effectors by tomato Cf R proteins is dramatically reduced at air humidity levels above 95% [115]. Similarly, the ETI-associated HR is compromised under high humidity conditions for the recognition of the bacterial effector AvrRpt2 by RPS2 in Arabidopsis [95]. However, under conditions of high humidity, ETI-associated pathogen population restriction is still evident in certain Arabidopsis accessions [116].
The suppressive effect of high humidity on ETI extends to autoimmune mutants. The Arabidopsis ssi4 mutant, which carries a mutation in an NLR-type R protein, shows spontaneous ETI-like cell death associated with increased levels of pathogen resistance (and the level of the defense hormone salicylic acid [SA]). These phenotypes are abolished under high humidity [117], suggesting the possibility that some R genes could be rendered less effective under high humidity conditions during agricultural production.
Environmental impact on quantitative resistance traits
Partial quantitative resistance to pathogens, which is less characterized at the molecular level than canonical PTI or ETI, reduces but does not completely abolish disease symptoms. It is usually controlled by quantitative trait loci (QTL), which collectively confer resistance [118]. In wheat, a kinase containing a START domain, Yr36, conferred wide-spectrum partial resistance to several races of Puccinia striiformis at temperatures as high as 35 °C, but not at temperatures below 20 °C [119]. Sr13, an NLR gene, is another wheat gene that confers partial resistance to P. striiformis. Plants carrying Sr13 induced the transcription of resistance markers and resistance to P. striiformis only under high temperatures conditions [120]. As partial quantitative resistance is less prone to go through cycles of boom and bust compared to R genes, a moderate level of protection would be provided in plants carrying this type of resistance even under the higher temperature conditions that are predicted to occur in the future.
Environmental impact on RNA interference (RNAi)
RNAi is a widespread defense mechanism against viruses [121]. During viral replication, double stranded viral RNA intermediates are recognized and cleaved by the RNAi cellular machinery, generating small interfering RNAs (siRNA). These siRNAs are then used to “silence” the production of new viral particles by targeting virus RNAs [121]. The importance of RNAi in viral defense can be inferred by the plethora of RNAi suppressors present in plant viruses [89]. Temperature affects the efficacy of RNAi-based plant defense. At low temperatures (15 °C compared to temperatures between 21 °C to 27 °C), RNAi viral defenses in Nicotiana benthamiana are compromised against Cymbidium ringspot virus (CymRSV, an orchid-infecting virus) [122]. However, these results may not be generalized, as grape plants are able to mount efficient RNAi against transgenes even at temperatures as low as 4 °C, whereas Arabidopsis silencing mechanisms are not functional below 10 °C [123]. As with low temperatures, high temperatures also modulate RNAi defenses. In cassava, DNA geminiviruses, including the causal agents of cassava mosaic disease, cause more symptoms and have higher viral titres at 25 °C, compared to those observed a t 30 °C. The virus-specific siRNAs concentration is lower at 25 °C, indicating that the antiviral RNAi pathways may be enhanced at higher temperatures (or that RNAi suppressors are less active) [124].
Environmental impact on defense hormone pathways
Stress hormones, particularly ABA, SA, jasmonic acid (JA), and ethylene (ET), are well known regulators of abiotic and/or biotic responses in plants. In particular, ABA is involved in regulating multiple abiotic environmental stresses, including drought, heat, cold, and salinity responses [125], whereas SA [126], JA [127] and ET [128] are involved in regulating responses against pathogens.
Part of the environmental impact on plant disease development may be mediated through regulation of defense hormones and/or the hormone crosstalk between ABA signaling (abiotic responses) and SA/JA/ET signaling (biotic responses) [91]. Indeed, during infection with the hemibiotrophic pathogen P. syringae, both ABA and SA concentrations increase [129]. Furthermore, when ABA biosynthesis and signaling are impaired, plants become more resistant to P. syringae and the necrotroph Botrytis cinerea [129, 130]. Hormonal crosstalk could cause this observed enhanced resistance for P. syringae, as SA accumulation is enhanced in ABA biosynthesis mutants [131]. However, other necrotrophs show the opposite effect. For example, infection of ABA biosynthesis- or signaling-deficient Arabidopsis mutant plants with the necrotrophs Pythium irregulare and Alternaria brassicicola cause disease to develop more aggressively [132]. The conflicting effect of ABA on resistance to fungal necrotrophs (B. cinerea vs. A. brassicicola) requires future in-depth studies.
A recent study examined the effect of warm temperatures on SA-mediated defense in the Arabidopsis-P. syringae interaction. It was found that at 30 °C, Arabidops is plants became more susceptible to P. syringae [133]. In contrast to a high level of SA accumulation normally induced by P. syringae at 23 °C, Arabidopsis failed to accumulate SA in response to P. syringae infection at 30 °C [133]. The biosynthetic pathway for pathogen-inducible SA starts from conversion of chorismate to isochorismate by ICS1 (ISOCHORISMATE SYNTHASE 1), from which SA is synthesized [134]. The lack of SA accumulation correlated with a lack of expression of a major group of SA-responsive genes, including the ICS1 gene. Nuclear entry of NPR1, the master regulator of SA responses, was unaffected at 30 °C [134], and as such, the temperature-sensitive component(s) of SA signaling, which awaits identification, likely lies downstream of NPR1 nuclear localization and/or upstream of ICS1 gene expression.
In contrast to the negative effect of warm temperature on SA defense, exposing Arabidopsis plants to cold temperatures (4 °C) for more than one week increased SA concentration, expression of SA-regulated defense genes, and plant resistance to Pseudomonas infection [135, 136]. CAMTA (calmodulin-binding transcription activator) transcription factors are involved in cold-temperature-mediated expression of SA-responsive genes, including genes involved in SA biosynthesis (e.g., ICS1) [135, 136]. Interestingly, CAMTA transcriptional factors in tomato were also important for negatively regulating the transcription of drought- and defense-associated genes, and resistance against pathogens and drought [137], further implicating a role of CAMTA transcription factors in connecting temperature, drought, and SA-regulated plant responses.
Compared to the increasing mechanistic understanding of the molecular basis of temperature effect on SA-mediated defense, the effect of environmental conditions on JA- and ET-mediated defense pathways is much less understood. One study showed that higher relative air humidity increases the expression of genes required for the biosynthesis and signaling of JA [102]. Further research is needed to investigate JA- and ET-mediated defenses, as they affect plant resistance against many pathogenic necrotrophs.
ENVIRONMENTAL MODULATION OF PATHOGEN VIRULENCE MECHANISMS
Pathogens have evolved many mechanisms to subvert plant defenses and other cellular processes in order to exploit the photosynthate-rich plants. These virulence mechanisms include specialized secretion systems for delivering a variety of virulence factors (collectively called effectors) into the plant cell, sporulation in fungal and oomycete pathogens, and intimate association of viruses (and some other pathogens) with their insect vectors (Figure 2). The expression and/or manifestation of many of these virulence mechanisms can be affected by environmental conditions. The environment could also have profound effects on the epiphytic (colonization of the surface of plants) and/or saprophytic (nutrient acquisition from dead organic matter) phase of certain pathogens.
Effect of temperature on virulence mechanisms of bacterial pathogens
The interaction between environmental conditions and pathogen virulence mechanisms has been better studied in bacterial pathogens compared to other plant pathogens. Agrobacterium tumefaciens is famous for producing tumor-like growth in its hosts, a hallmark of crown gall disease. Agrobacterium pathogenesis is dependent on the formation of an extracellular pilus (T-pilus) involved in the translocation of virulence-promoting DNA and proteins, a function that requires the expression of a large number of virulence (vir) genes [138]. The expression of vir genes is controlled by a two-component system, VirA is a transmembrane sensory protein that detects plant phenolic compounds produced during wounding and phosphorylates VirG, a protein that binds the vir DNA box in vir genes to increase their expression [139]. Temperatures at or above 32 °C reduced vir gene expression and rendered Agrobacterium strains non-pathogenic on Kalanchoe plants, probably due to loss of VirA phosphorylation activity [140]. In addition, temperatures higher than 28 °C inhibited pilus formation and decreased Vir protein stability in some but not all Agrobacterium strains [141], which correlated with the inability of these strains to cause disease at 28 °C.
Soft rotting bacteria are necrotrophs that macerate plant tissues using a multitude of bacterial plant cell wall-degrading enzymes (PCWDE). For Pectobacterium atrosepticum, a soft rotting bacterium, there is a clear correlation between increased virulence at temperatures as high as 35 °C and the production of PCWDE and quorum-sensing signals by certain strains [142]. Quorum-sensing signals are required for the production of macerating enzymes when a threshold bacterial population density has been reached [143], and their temperature-dependence limits Pectobacterium pathogenesis.
In Pseudomonas syringae, temperature has been shown to affect two well-studied virulence mechanisms: the production of phytotoxins and the type III secretion system for the delivery of virulence effector proteins into the plant cells. In P. syringae pv. glycinea, the causal agent of bacterial blight in soybean, the coronatine toxin was preferentially produced in planta under low temperature conditions [144]. A modified two-component system in which the extracellular sensor CorS is involved in thermoregulation is required for coronatine production [145]. Cold-induced coronatine production is not observed in all coronatine-producing strains, even though epidemics for all those coronatine-producing strains are favored by cool and wet weather conditions [144]. In vitro expression of genes for the production of another Pseudomonas toxin, phaseolotoxin, [146] and, more importantly, for the expression of genes that encode the type III protein secretion system in culture are favored by low temperatures [147]. Cold temperature-induced expression of type III secretion genes in vitro, however, is in contrast with the increased translocation of effectors into the plant cell at high temperatures in P. syringae-infected Arabidopsis plants [133], highlighting a need for future investigation to untangle the dichotomy of in vitro vs. in vivo studies with respect to the temperature effects on the virulence of P. syringae and other pathogens.
Effect of humidity on virulence-promoting mechanisms in bacteria
Recent studies suggest that bacterial virulence mechanisms involve the establishment of a disease-promoting liquid environment in the apoplast of infected leaves [94, 95]. In P. syringae, two highly conserved effectors of P. syringae, AvrE and HopM1, are redundantly involved in the establishment of the aqueous apoplast, but only at high humidity [95]. The environment-dependent virulence function of bacterial effectors can be observed not only for AvrE1 and HopM1, but also for P. syringae effector HopAM1, which contributes to virulence only in Arabidopsis plants grown under drought conditions [148]. As such, it is possible that the effect on pathogen virulence of some effectors may become apparent only under certain environmental conditions to which plants are exposed in nature, but not in laboratory conditions.
In another bacterial plant pathogen, Xanthomonas gardneri (which causes bacterial spot disease on tomato) a transcription activator-like (TAL) effector, AvrHah1, causes water soaking in tomato leaves and seems to be involved in promoting the intake of water from the environment into the apoplast [94]. AvrHah1 might affect cell wall permeability in order to make the apoplast more favorable for bacterial survival and/or dispersal [94].
Environmental effect on sporulation by fungal and oomycete pathogens
It is well known that inoculum production and dispersal are critical for disease epidemics in crop fields. In some instances, wind can disperse fungal spores over thousands of kilometers, as has been observed for the wheat stem rust pathogen Puccinia graminis as a critical part of its disease cycle [149]. In maize, Cercospora zeae-maydis required humidity higher than 95% for profuse sporulation, and only under this high humidity condition can the effect of temperature on disease be observed (25–30 °C being the optimum temperature [150]). This requirement for high humidity for sporulation is observed for the majority of fungal and oomycete pathogens [1, 74, 151]. Interestingly, sexual and asexual spore production in F. graminearum required the protein-refolding chaperone HSP90 [152]. This requirement suggests a possible mechanistic connection between temperature response and sporulation in this fungus, as HSP90 is required for heat shock responses in vitro and colonization in planta [152].
Asexual zoospore production in the oomycete Phytophthora infestans requires low temperatures. Temperature regulation of zoospore production involves the expression of a protein phosphatase (PinifC3), which controls the phosphorylation status of RNA polymerase and other transcriptional regulators in this oomycete. PinifC3 transcription is induced at 10 °C, as the gene has a cold-induced pr omoter element [153]. The optimal temperatures by Phytophthora and Fusarium to cause disease epidemics in the field correlate with those required for pathogen sporulation, which is essential for disease dispersal [1, 154].
Thus, environmental conditions appear to have pervasive effects on many steps of pathogen infection, ranging from pathogen sporulation, pathogen growth and virulence gene expression in plants, and the overwintering of inocula, which is critical for initiating infections in subsequent plant-growing seasons. It should be pointed out that climate conditions near the optimum for the multiplication of pathogens could potentially increase the probability of their evolution due to the higher population numbers. This could effectively change the pathogen population structures and create a condition for the emergence of new virulence strains, a cause of significant concern for future disease epidemics (Table 3).
Table 3.
Major causes for concern due to plant diseases in the context of climate change.
Increased temperatures:
|
Increased rainfall/humidity:
|
CONCLUDING REMARKS AND FUTURE OUTLOOK
Clearly, the ongoing changes in climate patterns have potential to threaten the already vulnerable global food security in multiple ways, including exacerbating major plant diseases and creating weather conditions for devastating new diseases to emerge in critical food-producing regions. Based on current climatic trends and plant and pathogen responses to major climatic factors in this century, we predict certain diseases to be of major concern for worldwide food security (Table 3). There is no more urgent time than now to call for intensifying global research efforts to understand how individual and combined environmental conditions influence plant immunity, pathogen virulence and disease development. From limited (and often disparate) literature in this important area of plant research, it is already obvious that the effects of CO2, temperature and humidity and other environmental conditions on disease development could vary depending on plant and pathogen species. Therefore, predicting a general outcome for all diseases may be difficult. An overarching theme, however, is that there are environmental optima for plant immune and pathogen virulence systems to function properly. Environmental deviations from these optima inevitably reduce the strength of plant immunity or pathogen virulence. The final impact on disease reflects the combined environmental effects on plant immunity and pathogen virulence (Figure 1). However, with increased data, it may be possible to develop conceptual theories to unify the differential influences of environmental conditions on different plant diseases. For example, one could apply the mathematical relations used in the metabolic theory of ecology (MTE) to plant-pathogen interactions, as has been done in assessing the effect of climatic factors on human diseases [155]. MTE integrates the metabolic rate, mass and temperature of organisms [156]; the information may be used to potentially predict how host plants and plant pathogens would respond to dynamically changing environments.
Basic research into how temperature, humidity and other abiotic stresses reduce plant immune signaling will likely yield environment-vulnerable points in the plant immune system. Such knowledge will hopefully provide a foundation for developing a new generation of plant varieties in which the plant immune system is more resilient to environmental fluctuations. A well-known phenomenon associated with increasing stress tolerance is reduction in plant growth and yield. Recent efforts have been successful to reduce the detrimental effects from the expression of immunity-related genes [157]. It would be interesting to investigate whether such improved balance between plant growth/yield and disease resistance can be maintained under different climatic conditions.
Future breeding programs for new improved varieties should try to incorporate the abiotic tolerance, growth, and biotic resistance variability that favor plant immunity and disfavor pathogen virulence. These traits may be found in close wild relatives of cultivated crops, as they could have evolved combined abiotic and disease resistance strategies over millennia that might not be present in the narrow germplasm from which a modern crop variety is derived. Traits could be introduced into cultivated plant varieties through gene pyramiding, marker-assisted selection, whole genome-wide association mapping (GWAS) analysis and other approaches. Some attempts to incorporate wild germplasm of both abiotic and biotic resistance into cultivated plant species have been carried out. Good examples are the introgression of chromosomal segments of the entire genome of a highly abiotic and biotic stress tolerant wild grass species (Festuca pratensis) into forage grasses [158], and the extraordinary development of an advanced rice breeding line with six genes of resistance to bacterial and fungal diseases, two genes for resistance to an insect pest, and two major QTLs conferring salt and flood tolerance [159]. More effort of this type is needed to incorporate both pathogen resistance and environmental tolerance into commercial varieties. Another approach could be the use of planting mixtures of crop varieties that cover a range of climate and pathogen conditions (an idea that has been effectively employed for years when using multilines, near-isogenic lines that carry different resistance genes). This approach almost doubled the yields in rice fields affected by M. oryzae infection (compared to fields sown with monocultures) [160]. Similarly, planting mixed native landraces of potato in the Andes protects the farmer from the unpredictability of weather and pathogen infection [161].
A major concern of genetic manipulation of plant traits is the reluctance of the public to accept genetically modified crops. For example, many countries have significant restrictions on the use of transgenic organisms (only 28 countries have been planting transgenic organisms as of 2016 [162]). With the advent of the CRISPR (Clustered regularly interspersed short palindromic repeats) technology such concern may be reduced. New technologies will also enable us to more accurately forecast future pathogen epidemics by using nanosensors to detect pathogen populations in the field [163]. In addition, the identification of plants resistant to both disease and environmental stresses could benefit from the use of high-throughput phenotyping technology [164].
Another promising area of research aimed at making crop plants resilient to both abiotic and biotic stresses is that involving the emerging field of plant-microbiome interactions [165]. Disease suppressive soils with enrichment in the bacterial clades Proteobacteria and Firmicutes protect against damping-off disease caused by R. solani [166], and numerous individual “biocontrol” microbes have an ability to fight against pathogens [167, 168]. Specific microbes are also known to enhance tolerance to drought and other abiotic stresses [169]. One can envision harnessing the potential of a community-defined microbiome in both suppressing disease and increasing environmental tolerance in the future to enhance crop resilience and productivity. Readers are referred to recent reviews on this topic [165, 170].
Finally, we would like to point out that, although there is a widespread observation of the environmental effect on diverse diseases caused by a wide range of pathogens with different lifestyles, a major concern is that in-depth studies have been done mostly using only a few model plant species (e.g., Arabidopsis) and with biotrophic pathogens. For many crops with the highest world production, there are only descriptive studies on the environmental effects on major diseases. Knowledge on the molecular phenomena behind plant resistance and pathogen virulence is almost completely absent. This dearth of studies hampers the development of appropriate resistance strategies against the most devastating world plant diseases (Table 1). Also, future studies addressing the effect of environmental factors on plant disease will need to take into account the dynamic nature of conditions in the field. We need controlled growth chambers that more closely resemble the dynamic conditions to which the plants and pathogens are subjected in nature, so that field trials can be reproduced, and any laboratory results can be validated. Current static laboratory experiments, although simple to perform, will not provide complete answers to solving the molecular mysteries of plant diseases. The classically accepted paradigms used to approach plant-pathogen interactions must now change. Future studies of plant-pathogen interactions should increasingly consider the multi-dimensional nature of “plant-pathogen-environment” interactions that are more reflective of what occur in crop fields and natural ecosystems.
Acknowledgments
We would like to thank colleagues who devoted time to discuss the challenges that agricultural production faces today: Dr. Linda Hanson, Dr. Mary Hausbeck, Dr. James Kelly and Dr. Eric Olson from Michigan State University, and Dr. Lina Quesada from North Carolina State University. We would also like to thank Dr. Bethany Huot for her invaluable help and suggestions during the initial planning of this manuscript and for critically reviewing the manuscript. We apologize to any colleagues whose work was not cited due to space limitations.
Footnotes
AUTHOR CONTRIBUTIONS
ACV, CDMC, and SYH wrote the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fry WE, Birch PR, Judelson HS, Grünwald NJ, Danies G, Everts KL, Gevens AJ, Gugino BK, Johnson DA, Johnson SB, et al. Five reasons to consider Phytophthora infestans a reemerging pathogen. Phytopathology. 2015;105:966–981. doi: 10.1094/PHYTO-01-15-0005-FI. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostakis SL. Chestnut blight: The classical problem of an introduced pathogen. Mycologia. 1987;79:23–37. [Google Scholar]
- 3.Oerke EC. Crop losses to pests. J Agric Sci. 2006;144:31–43. [Google Scholar]
- 4.Savary S, Ficke A, Aubertot JN, Hollier C. Crop losses due to diseases and their implications for global food production losses and food security. Food Sec. 2012;4:519–537. [Google Scholar]
- 5.Rybicki EP. A Top Ten list for economically important plant viruses. Arch Virol. 2015;160:17–20. doi: 10.1007/s00705-014-2295-9. [DOI] [PubMed] [Google Scholar]
- 6.Churchill AC. Mycosphaerella fijiensis, the black leaf streak pathogen of banana: progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Mol Plant Pathol. 2011;12:307–328. doi: 10.1111/j.1364-3703.2010.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ordonez N, Seidl MF, Waalwijk C, Drenth A, Kilian A, Thomma BPHJ, Ploetz RC, Kema GHJ. Worse comes to worst: Bananas and Panama disease - When plant and pathogen clones meet. PLoS Pathog. 2015;11:e1005197. doi: 10.1371/journal.ppat.1005197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean R, van Kan JA, Pretorius ZA, Hammond-Kosack KE, di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, et al. The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bart RS, Taylor NJ. New opportunities and challenges to engineer disease resistance in cassava, a staple food of African small-holder farmers. PLoS Pathog. 2017;13:e1006287. doi: 10.1371/journal.ppat.1006287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delannoy E, Lyon BR, Marmey P, Jalloul A, Daniel JF, Montillet JL, Essenberg M, Nicole M. Resistance of cotton towards Xanthomonas campestris pv. malvacearum. Annu Rev Phytopathol. 2005;43:63–82. doi: 10.1146/annurev.phyto.43.040204.140251. [DOI] [PubMed] [Google Scholar]
- 11.Cianchetta AN, Allen TW, Hutmacher RB, Kemerait RC, Kirkpatrick TL, Lawrence GW, Lawrence KS, Mueller JD, Nichols RL, Olsen MW, et al. Survey of Fusarium oxysporum f. sp vasinfectum in the United States. J Cotton Sci. 2015;19:328–336. [Google Scholar]
- 12.Wei F, Fan R, Dong H, Shang W, Xu X, Zhu H, Yang J, Hu X. Threshold microsclerotial inoculum for cotton verticillium wilt determined through wet-sieving and real-time quantitative PCR. Phytopathology. 2015;105:220–229. doi: 10.1094/PHYTO-05-14-0139-R. [DOI] [PubMed] [Google Scholar]
- 13.Fountain JC, Scully BT, Ni X, Kemerait RC, Lee RD, Chen ZY, Guo B. Environmental influences on maize-Aspergillus flavus interactions and aflatoxin production. Front Microbiol. 2014;5:40. doi: 10.3389/fmicb.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munkvold GP. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur J Plant Pathol. 2003;109:705–713. [Google Scholar]
- 15.Ward JMJ, Stromberg EL, Nowell DC, Nutter FW., Jr Gray leaf spot. A disease of global importance in maize production. Plant Dis. 1999;83:884–895. doi: 10.1094/PDIS.1999.83.10.884. [DOI] [PubMed] [Google Scholar]
- 16.Chong KP, Dayou KP, Arnnyitte A. Detection and control of Ganoderma boninense in oil palm crop. Cham: Springer International Publishing; 2017. [Google Scholar]
- 17.Torres GA, Sarria GA, Martinez G, Varon F, Drenth A, Guest DI. Bud rot caused by Phytophthora palmivora: A destructive emerging disease of oil palm. Phytopathology. 2016;106:320–329. doi: 10.1094/PHYTO-09-15-0243-RVW. [DOI] [PubMed] [Google Scholar]
- 18.Naidu RA, Kimmins FM, Deom CM, Subrahmanyam P, Chiyembekeza AJ, van der Merwe PJA. Groundnut rosette. A virus disease affecting groundnut production in sub-Saharan Africa. Plant Dis. 1999;83:700–709. doi: 10.1094/PDIS.1999.83.8.700. [DOI] [PubMed] [Google Scholar]
- 19.Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol. 2012;13:614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitt BDL, Brun H, Barbetti MJ, Rimmer SR. World-wide importance of Phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus) Eur J Plant Pathol. 2006;114:3–15. [Google Scholar]
- 21.Hu Q, Hua W, Yin Y, Zhang X, Liu L, Shi J, Zhao Y, Qin L, Chen C, Wang H. Rapeseed research and production in China. Crop J. 2017;5:127–135. [Google Scholar]
- 22.Talbot NJ. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu Rev Microbiol. 2003;57:177–202. doi: 10.1146/annurev.micro.57.030502.090957. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Wang S. Rice versus Xanthomonas oryzae pv. oryzae: a unique pathosystem. Curr Opin Plant Biol. 2013;16:188–195. doi: 10.1016/j.pbi.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Yellareddygari SKR, Reddy MS, Kloepper JW, Lawrence KS, Fadamiro H. Rice sheath blight: A review of disease and pathogen management approaches. J Plant Pathol Microb. 2014;5:241. [Google Scholar]
- 25.Sharma I, Kumari N, Sharma V. Sorghum fungal diseases. In: Lichtfouse E, Goyal A, editors. Sustainable agriculture reviews 16. Cham: Springer; 2015. pp. 141–172. [Google Scholar]
- 26.Wrather A, Shannon G, Balardin R, Carregal L, Escobar R, Gupta GK, Ma Z, Morel W, Ploper D, Tenuta A. Effect of diseases on soybean yield in the top eight producing countries in 2006. Plant Health Prog. 2010 doi: 10.1094/PHP-2010-0125-01-RS. [DOI] [Google Scholar]
- 27.Weiland J, Koch G. Sugarbeet leaf spot disease (Cercospora beticola Sacc) Mol Plant Pathol. 2004;5:157–166. doi: 10.1111/j.1364-3703.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 28.McGrann GR, Grimmer MK, Mutasa-Göttgens ES, Stevens M. Progress towards the understanding and control of sugar beet rhizomania disease. Mol Plant Pathol. 2009;10:129–141. doi: 10.1111/j.1364-3703.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comstock JC. Ratoon stunting disease. Sugar Tech. 2002;4:1–6. [Google Scholar]
- 30.Sharma R, Tamta S. A review on red rot: The “cancer” of sugarcane. J Plant Pathol Microbiol. 2015:S1003. [Google Scholar]
- 31.Kokkinos CD, Clark CA, McGregor CE, LaBonte DR. The effect of sweet potato virus disease and its viral components on gene expression levels in sweetpotato. J Amer Soc Hort Sci. 2006;131:657–666. [Google Scholar]
- 32.Moriones E, Navas-Castillo J. Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 2000;71:123–134. doi: 10.1016/s0168-1702(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 33.McMullen M, Jones R, Gallenberg D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997;81:1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- 34.Chen XM. Epidemiology and control of stripe rust [Puccinia striiformis f. sp tritici] on wheat. Can J Plant Pathol. 2005;27:314–337. [Google Scholar]
- 35.Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Bhavani S, Njau P, Herrera-Foessel S, Singh PK, Singh S, Govindan V. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu Rev Phytopathol. 2011;49:465–481. doi: 10.1146/annurev-phyto-072910-095423. [DOI] [PubMed] [Google Scholar]
- 36.Amusa NA, Adegbite AA, Muhammed S, Baiyewu RA. Yam diseases and its management in Nigeria. Afr J Biotechnol. 2003;2:497–502. [Google Scholar]
- 37.Stevens RB. Cultural practices in disease control. In: Horsfall JG, editor. Plant Pathology: An advanced treatise, volume III: The diseases population epidemics and control. London: Academic Press Inc; 1960. pp. 357–429. [Google Scholar]
- 38.Hua J. Modulation of plant immunity by light, circadian rhythm, and temperature. Curr Opin Plant Biol. 2013;16:406–413. doi: 10.1016/j.pbi.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Dordas C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron Sustain Dev. 2008;28:33–46. [Google Scholar]
- 40.Aylor DE. The role of intermittent wind in the dispersal of fungal pathogens. Annu Rev Phytopathol. 1990;28:73–92. [Google Scholar]
- 41.Krupa S, McGrath MT, Andersen CP, Booker FL, Burkey KO, Chappelka AH, Chevone BI, Pell EJ, Zilinskas BA. Ambient ozone and plant health. Plant Dis. 2001;85:4–12. doi: 10.1094/PDIS.2001.85.1.4. [DOI] [PubMed] [Google Scholar]
- 42.Etheridge DM, Steele LP, Langenfelds RL, Francey RJ, Barnola JM, Morgan VI. Natural and anthropogenic changes in atmospheric CO2 over the last 1000 years from air in Antarctic ice and firn. J Geophys Res. 1996;101:4115–4128. [Google Scholar]
- 43.Intergovernmental Panel on Climate Change. Climate change 2014: Synthesis report. In: Pachauri RK, Meyer LA, editors. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva: IPCC; 2014. [Google Scholar]
- 44.Fischer EM, Knutti R. Anthropogenic contribution to global occurrence of heavy-precipitation and high-temperature extremes. Nat Clim Change. 2015;5:560–564. [Google Scholar]
- 45.Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 2004;305:994–997. doi: 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- 46.Wuebbles DJ, Fahey DW, Hibbard KA, Dokken DJ, Stewart BC, Maycock TK, editors. U.S. Global Change Research Program. Climate science special report: Fourth national climate assessment. I. Washington: USGCRP; 2017. [Google Scholar]
- 47.Chakraborty S. Migrate or evolve: options for plant pathogens under climate change. Glob Chang Biol. 2013;19:1985–2000. doi: 10.1111/gcb.12205. [DOI] [PubMed] [Google Scholar]
- 48.Bebber DP, Ramotowski MAT, Gurr SJ. Crop pests and pathogens move polewards in a warming world. Nat Clim Change. 2013;3:985–988. [Google Scholar]
- 49.Wheeler T, von Braun J. Climate change impacts on global food security. Science. 2013;341:508–513. doi: 10.1126/science.1239402. [DOI] [PubMed] [Google Scholar]
- 50.Bierbaum RM, Fay M, Ross-Larson B, editors. World Bank Group. World development report 2010: Development and climate change. Washington: World Bank Group; 2009. [Google Scholar]
- 51.Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005;165:351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 52.Long SP, Ainsworth EA, Leakey AD, Nösberger J, Ort DR. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi T, Ishiguro K, Nakajima T, Kim HY, Okada M, Kobayashi K. Effects of elevated atmospheric CO2 concentration on the infection of rice blast and sheath blight. Phytopathology. 2006;96:425–431. doi: 10.1094/PHYTO-96-0425. [DOI] [PubMed] [Google Scholar]
- 54.Váry Z, Mullins E, McElwain JC, Doohan FM. The severity of wheat diseases increases when plants and pathogens are acclimatized to elevated carbon dioxide. Glob Chang Biol. 2015;21:2661–2669. doi: 10.1111/gcb.12899. [DOI] [PubMed] [Google Scholar]
- 55.Eastburn DM, Degennaro MM, Delucia EH, Dermody O, McElrone AJ. Elevated atmospheric carbon dioxide and ozone alter soybean diseases at SoyFACE. Glob Chang Biol. 2010;16:320–330. [Google Scholar]
- 56.Skelsey P, Cooke DEL, Lynott JS, Lees AK. Crop connectivity under climate change: future environmental and geographic risks of potato late blight in Scotland. Glob Chang Biol. 2016;22:3724–3738. doi: 10.1111/gcb.13368. [DOI] [PubMed] [Google Scholar]
- 57.Trębicki P, Vandegeer RK, Bosque-Pérez NA, Powell KS, Dader B, Freeman AJ, Yen AL, Fitzgerald GJ, Luck JE. Virus infection mediates the effects of elevated CO2 on plants and vectors. Sci Rep. 2016;6:22785. doi: 10.1038/srep22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones LM, Koehler AK, Trnka M, Balek J, Challinor AJ, Atkinson HJ, Urwin PE. Climate change is predicted to alter the current pest status of Globodera pallida and G. rostochiensis in the United Kingdom. Glob Chang Biol. 2017;23:4497–4507. doi: 10.1111/gcb.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horino O, Mew TW, Yamada T. The effect of temperature on the development of bacterial leaf blight on rice. Ann Phytopath Soc Japan. 1982;48:72–75. [Google Scholar]
- 60.Mangrauthia SK, Singh Shakya VP, Jain RK, Praveen S. Ambient temperature perception in papaya for papaya ringspot virus interaction. Virus Genes. 2009;38:429–434. doi: 10.1007/s11262-009-0336-3. [DOI] [PubMed] [Google Scholar]
- 61.Park RF. The role of temperature and rainfall in the epidemiology of Puccinia striiformis f. sp tritici in the summer rainfall area of eastern. Australia Plant Pathol. 1990;39:416–423. [Google Scholar]
- 62.Denis JI. Effect of high temperatures on survival and development of Puccinia striiformis on wheat. Trans Br mycol Soc. 1987;88:91–96. [Google Scholar]
- 63.Shakya SK, Goss EM, Dufault NS, van Bruggen AHC. Potential effects of diurnal temperature oscillations on potato late blight with special reference to climate change. Phytopathology. 2015;105:230–238. doi: 10.1094/PHYTO-05-14-0132-R. [DOI] [PubMed] [Google Scholar]
- 64.Ma L, Qiao J, Kong X, Zou Y, Xu X, Chen X, Hu X. Effect of low temperature and wheat winter-hardiness on survival of Puccinia striiformis f. sp tritici under controlled conditions. PLOS One. 2015;10:e0130691. doi: 10.1371/journal.pone.0130691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ritchie F, Bain R, Mcquilken M. Survival of sclerotia of Rhizoctonia solani AG3PT and effect of soil-borne inoculum density on disease development on potato. J Phytopathol. 2013;161:180–189. [Google Scholar]
- 66.Turkensteen LJ, Flier WG, Wanningen R, Mulder A. Production, survival and infectivity of oospores of Phytophthora infestans. Plant Pathol. 2000;49:688–696. [Google Scholar]
- 67.Mariette N, Androdias A, Mabon R, Corbière R, Marquer B, Montarry J, Andrivon D. Local adaptation to temperature in populations and clonal lineages of the Irish potato famine pathogen Phytophthora infestans. Ecol Evol. 2016;6:6320–6331. doi: 10.1002/ece3.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hovmøller MS, Yahyaoui AH, Milus EA, Justesen AF. Rapid global spread of two aggressive strains of a wheat rust fungus. Mol Ecol. 2008;17:3818–3826. doi: 10.1111/j.1365-294X.2008.03886.x. [DOI] [PubMed] [Google Scholar]
- 69.Milus EA, Kristensen K, Hovmøller MS. Evidence for increased aggressiveness in a recent widespread strain of Puccinia striiformis f. sp tritici causing stripe rust of wheat. Phytopathology. 2009;99:89–94. doi: 10.1094/PHYTO-99-1-0089. [DOI] [PubMed] [Google Scholar]
- 70.Bonde MR, Nester SE, Berner DK. Effects of daily temperature highs on development of Phakopsora pachyrhizi on soybean. Phytopathology. 2012;102:761–768. doi: 10.1094/PHYTO-01-12-0011-R. [DOI] [PubMed] [Google Scholar]
- 71.Whitfield AE, Falk BW, Rotenberg D. Insect vector-mediated transmission of plant viruses. Virology. 2015;479–480:278–289. doi: 10.1016/j.virol.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 72.Robson JD, Wright MG, Almeida RPP. Biology of Pentalonia nigronervosa (Hemiptera, Aphididae) on banana using different rearing methods. Environ Entomol. 2007;36:46–52. doi: 10.1603/0046-225x(2007)36[46:bopnha]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 73.Anhalt MD, Almeida RPP. Effect of temperature, vector life stage, and plant access period on transmission of Banana bunchy top virus to banana. Phytopathology. 2008;98:743–748. doi: 10.1094/PHYTO-98-6-0743. [DOI] [PubMed] [Google Scholar]
- 74.Clarkson JP, Fawcett L, Anthony SG, Young C. A model for Sclerotinia sclerotiorum infection and disease development in lettuce, based on the effects of temperature, relative humidity and ascospore density. PLoS ONE. 2014;9:e94049. doi: 10.1371/journal.pone.0094049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Granke LL, Hausbeck MK. Effects of temperature, humidity, and wounding on development of Phytophthora rot of cucumber fruit. Plant Dis. 2010;94:1417–1424. doi: 10.1094/PDIS-04-10-0258. [DOI] [PubMed] [Google Scholar]
- 76.Magarey RD, Sutton TB, Thayer CL. A simple generic infection model for foliar fungal plant pathogens. Phytopathology. 2005;95:92–100. doi: 10.1094/PHYTO-95-0092. [DOI] [PubMed] [Google Scholar]
- 77.Rowlandson T, Gleason M, Sentelhas P, Gillespie T, Thomas C, Hornbuckle B. Reconsidering leaf wetness duration determination for plant disease management. Plant Dis. 2015;99:310– 319. doi: 10.1094/PDIS-05-14-0529-FE. [DOI] [PubMed] [Google Scholar]
- 78.Islam TMD, Toyota K. Effect of moisture conditions and pre-incubation at low temperature on bacterial wilt of tomato caused by Ralstonia solanacearum. Microbes Environ. 2004;19:244–247. [Google Scholar]
- 79.Juroszek P, von Tiedemann A. Potential strategies and future requirements for plant disease management under a changing climate. Plant Pathol. 2011;60:100–112. [Google Scholar]
- 80.Bidzinski P, Ballini E, Ducasse A, Michel C, Zuluaga P, Genga A, Chiozzotto R, Morel JB. Transcriptional basis of drought-induced susceptibility to the rice blast fungus Magnaporthe oryzae. Front Plant Sci. 2106;7:1558. doi: 10.3389/fpls.2016.01558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johansen TJ, Dees MW, Hermansen A. High soil moisture reduces common scab caused by Streptomyces turgidiscabies and Streptomyces europaeiscabiei in potato. Acta Agric Scand B. 2015;65:193–198. [Google Scholar]
- 82.Beyer M, Verreet JA, Ragab WS. Effect of relative humidity on germination of ascospores and macroconidia of Gibberella zeae and deoxynivalenol production. Int J Food Microbiol. 2005;98:233–240. doi: 10.1016/j.ijfoodmicro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 83.Cowger C, Patton-Ozkurt J, Brown-Guedira G, Perugini L. Post-anthesis moisture increased Fusarium head blight and deoxynivalenol levels in North Carolina winter wheat. Phytopathology. 2009;99:320–327. doi: 10.1094/PHYTO-99-4-0320. [DOI] [PubMed] [Google Scholar]
- 84.Atkinson NJ, Urwin PE. The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot. 2012;63:3523–3543. doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- 85.Rejeb IB, Pastor V, Mauch-Mani B. Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants. 2014;3:458–475. doi: 10.3390/plants3040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prasch CM, Sonnewald U. Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol. 2013;162:1849–1866. doi: 10.1104/pp.113.221044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ciliberti N, Fermaud M, Roudet J, Rossi V. Environmental conditions affect Botrytis cinerea infection of mature grape berries more than the strain or transposon genotype. Phytopathology. 2015;105:1090–1096. doi: 10.1094/PHYTO-10-14-0264-R. [DOI] [PubMed] [Google Scholar]
- 88.Couto D, Zipfel C. Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol. 2016;16:537–552. doi: 10.1038/nri.2016.77. [DOI] [PubMed] [Google Scholar]
- 89.Csorba T, Kontra L, Burgyán J. Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology. 2015;479–480:85–103. doi: 10.1016/j.virol.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 90.Dou D, Zhou JM. Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host Microbe. 2012;12:484–495. doi: 10.1016/j.chom.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 91.Huot B, Yao J, Montgomery BL, He SY. Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant. 2014;7:1267–1287. doi: 10.1093/mp/ssu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones JD, Vance RE, Dangl JL. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354:aaf6395. doi: 10.1126/science.aaf6395. [DOI] [PubMed] [Google Scholar]
- 93.Mukhtar MS, McCormack ME, Argueso CT, Pajerowska-Mukhtar KM. Pathogen tactics to manipulate plant cell death. Curr Biol. 2016;26:R608–R619. doi: 10.1016/j.cub.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 94.Schwartz AR, Morbitzer R, Lahaye T, Staskawicz BJ. TALE-induced bHLH transcription factors that activate a pectate lyase contribute to water soaking in bacterial spot of tomato. Proc Natl Acad Sci USA. 2017;114:E897– E903. doi: 10.1073/pnas.1620407114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xin XF, Nomura K, Aung K, Velásquez AC, Yao J, Boutrot F, Chang JH, Zipfel C, He SY. Bacteria establish an aqueous living space as a crucial virulence mechanism. Nature. 2016;539:524–529. doi: 10.1038/nature20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramos ME. PhD dissertation. Berkeley: University of California; 2010. Bacterial growth in the plant apoplast is limited by nutrient availability. [Google Scholar]
- 97.Daszkowska-Golec A, Szarejko I. Open or close the gate - stomata action under the control of phytohormones in drought stress conditions. Front Plant Sci. 2013;4:138. doi: 10.3389/fpls.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okamoto M, Tanaka Y, Abrams SR, Kamiya Y, Seki M, Nambara E. High humidity induces abscisic acid 8'-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant Physiol. 2009;149:825–834. doi: 10.1104/pp.108.130823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Melotto M, Underwood W, Koczan J, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 100.Panchal S, Melotto M. Stomate-based defense and environmental cues. Plant Signal Behav. 2017;12:e1362517. doi: 10.1080/15592324.2017.1362517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Panchal S, Chitrakar R, Thompson BK, Obulareddy N, Roy D, Hambright WS, Melotto M. Regulation of stomatal defense by air relative humidity. Plant Physiol. 2016;172:2021–2032. doi: 10.1104/pp.16.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng C, Gao X, Feng B, Sheen J, Shan L, He P. Plant immune response to pathogens differs with changing temperatures. Nat Commun. 2013;4:2530. doi: 10.1038/ncomms3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lorang J, Kidarsa T, Bradford CS, Gilbert B, Curtis M, Tzeng SC, Maier CS, Wolpert TJ. Tricking the guard: exploiting plant defense for disease susceptibility. Science. 2012;338:659–662. doi: 10.1126/science.1226743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whitham S, McCormick S, Baker B. The N gene of tobacco confers resistance to tobacco mosaic virus in transgenic tomato. Proc Natl Acad Sci USA. 1996;93:8776–8781. doi: 10.1073/pnas.93.16.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Jong CF, Takken FL, Cai X, de Wit PJ, Joosten MH. Attenuation of Cf-mediated defense responses at elevated temperatures correlates with a decrease in elicitor-binding sites. Mol Plant Microbe Interact. 2002;15:1040–1049. doi: 10.1094/MPMI.2002.15.10.1040. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, Bao Z, Zhu Y, Hua J. Analysis of temperature modulation of plant defense against biotrophic microbes. Mol Plant Microbe Interact. 2009;22:498–506. doi: 10.1094/MPMI-22-5-0498. [DOI] [PubMed] [Google Scholar]
- 107.Webb KM, Oña I, Bai J, Garrett KA, Mew T, Vera Cruz CM, Leach JE. A benefit of high temperature: increased effectiveness of a rice bacterial blight disease resistance gene. New Phytol. 2010;185:568–576. doi: 10.1111/j.1469-8137.2009.03076.x. [DOI] [PubMed] [Google Scholar]
- 108.Zhu Y, Qian W, Hua J. Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathog. 2010;6:e1000844. doi: 10.1371/journal.ppat.1000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Z, Cui D, Liu J, Zhao J, Liu C, Xin W, Li Y, Liu N, Ren D, Tang D, et al. Arabidopsis ZED1-related kinases mediate the temperature-sensitive intersection of immune response and growth homeostasis. New Phytol. 2017;215:711–724. doi: 10.1111/nph.14585. [DOI] [PubMed] [Google Scholar]
- 110.Lewis JD, Lee AH, Hassan JA, Wan J, Hurley B, Jhingree JR, Wang PW, Lo T, Youn JY, Guttman DS, et al. The Arabidopsis ZED1 pseudokinase is required for ZAR1-mediated immunity induced by the Pseudomonas syringae type III effector HopZ1a. Proc Natl Acad Sci USA. 2013;110:18722–18727. doi: 10.1073/pnas.1315520110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Menna A, Nguyen D, Guttman DS, Desveaux D. Elevated temperature differentially influences effector-triggered immunity outputs in Arabidopsis. Front Plant Sci. 2015;6:995. doi: 10.3389/fpls.2015.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mang HG, Qian W, Zhu Y, Qian J, Kang HG, Klessig DF, Hua J. Abscisic acid deficiency antagonizes high-temperature inhibition of disease resistance through enhancing nuclear accumulation of resistance proteins SNC1 and RPS4 in Arabidopsis. Plant Cell. 2012;24:1271–1284. doi: 10.1105/tpc.112.096198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Vries JS, Andriotis VM, Wu AJ, Rathjen JP. Tomato Pto encodes a functional N-myristoylation motif that is required for signal transduction in Nicotiana benthamiana. Plant J. 2006;45:31–45. doi: 10.1111/j.1365-313X.2005.02590.x. [DOI] [PubMed] [Google Scholar]
- 114.Gao Z, Chung EH, Eitas TK, Dangl JL. Plant intracellular innate immune receptor Resistance to Pseudomonas syringae pv. maculicola 1 (RPM1) is activated at, and functions on, the plasma membrane. Proc Natl Acad Sci USA. 2011;108:7619–7624. doi: 10.1073/pnas.1104410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang C, Cai X, Zheng Z. High humidity represses Cf-4/Avr4- and Cf-9/Avr9-dependent hypersensitive cell death and defense gene expression. Planta. 2005;222:947–956. doi: 10.1007/s00425-005-0036-8. [DOI] [PubMed] [Google Scholar]
- 116.Velásquez AC, Oney M, Huot B, Xu S, He SY. Diverse mechanisms of resistance to Pseudomonas syringae in a thousand natural accessions of Arabidopsis thaliana. New Phytol. 2017;214:1673–1687. doi: 10.1111/nph.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou F, Menke FL, Yoshioka K, Moder W, Shirano Y, Klessig DF. High humidity suppresses ssi4-mediated cell death and disease resistance upstream of MAP kinase activation, H2O2 production and defense gene expression. Plant J. 2004;39:920–932. doi: 10.1111/j.1365-313X.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 118.St Clair DA. Quantitative disease resistance and quantitative resistance loci in breeding. Annu Rev Phytopathol. 2010;48:247–268. doi: 10.1146/annurev-phyto-080508-081904. [DOI] [PubMed] [Google Scholar]
- 119.Fu D, Uauy C, Distelfeld A, Blechl A, Epstein L, Chen X, Sela H, Fahima T, Dubcovsky J. A novel kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science. 2009;323:1357–1360. doi: 10.1126/science.1166289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang W, Chen S, Abate Z, Nirmala J, Rouse MN, Dubcovsky J. Identification and characterization of Sr13, a tetraploid wheat gene that confers resistance to the Ug99 stem rust race group. Proc Natl Acad Sci USA. 2017;114:E9483– E9492. doi: 10.1073/pnas.1706277114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ding SW. RNA-based antiviral immunity. Nat Rev Immunol. 2010;10:632–644. doi: 10.1038/nri2824. [DOI] [PubMed] [Google Scholar]
- 122.Szittya G, Silhavy D, Molnár A, Havelda Z, Lovas A, Lakatos L, Bánfalvi Z, Burgyán J. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 2003;22:633–640. doi: 10.1093/emboj/cdg74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Romon M, Soustre-Gacougnolle I, Schmitt C, Perrin M, Burdloff Y, Chevalier E, Mutterer J, Himber C, Zervudacki J, Montavon T, et al. RNA silencing is resistant to low-temperature in grapevine. PLoS One. 2013;8:e82652. doi: 10.1371/journal.pone.0082652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chellappan P, Vanitharani R, Ogbe F, Fauquet CM. Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol. 2005;138:1828– 1841. doi: 10.1104/pp.105.066563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front Plant Sci. 2017;8:161. doi: 10.3389/fpls.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yan S, Dong X. Perception of the plant immune signal salicylic acid. Curr Opin Plant Biol. 2014;20:64–68. doi: 10.1016/j.pbi.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang L, Zhang F, Melotto M, Yao J, He SY. Jasmonate signaling and manipulation by pathogens and insects. J Exp Bot. 2017;68:1371–1385. doi: 10.1093/jxb/erw478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 129.de Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Rodriguez Egea P, Bögre L, Grant M. Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J. 2007;26:1434–1443. doi: 10.1038/sj.emboj.7601575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Audenaert K, de Meyer GB, Höfte MM. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol. 2002;128:491–501. doi: 10.1104/pp.010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Torres Zabala M, Bennett MH, Truman WH, Grant MR. Antagonism between salicylic and abscisic acid reflects early host-pathogen conflict and moulds plant defence responses. Plant J. 2009;59:375–386. doi: 10.1111/j.1365-313X.2009.03875.x. [DOI] [PubMed] [Google Scholar]
- 132.Adie BA, Pérez-Pérez J, Pérez-Pérez MM, Godoy M, Sánchez-Serrano JJ, Schmelz EA, Solano R. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell. 2007;19:1665–1681. doi: 10.1105/tpc.106.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huot B, Castroverde CDM, Velásquez AC, Hubbard E, Pulman JA, Yoshida Y, Yao J, Howe GA, Childs KL, Tsuda K, et al. Dual impact of elevated temperature on plant defence and bacterial virulence in Arabidopsis. Nat Commun. 2017;8:1808. doi: 10.1038/s41467-017-01674-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 135.Kim Y, Park S, Gilmour SJ, Thomashow MF. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013;75:364–376. doi: 10.1111/tpj.12205. [DOI] [PubMed] [Google Scholar]
- 136.Kim YS, An C, Park S, Gilmour SJ, Wang L, Renna L, Brandizzi F, Grumet R, Thomashow M. CAMTA-mediated regulation of salicylic acid immunity pathway genes in Arabidopsis exposed to low temperature and pathogen infection. Plant Cell. 2017;29:2465–2477. doi: 10.1105/tpc.16.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li X, Huang L, Zhang Y, Ouyang Z, Hong Y, Zhang H, Li D, Song F. Tomato SR/CAMTA transcription factors SlSR1 and SlSR3L negatively regulate disease resistance response and SlSR1L positively modulates drought stress tolerance. BMC Plant Biol. 2014;14:286. doi: 10.1186/s12870-014-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCullen CA, Binns AN. Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu Rev Cell Dev Biol. 2006;22:101–127. doi: 10.1146/annurev.cellbio.22.011105.102022. [DOI] [PubMed] [Google Scholar]
- 139.Jin SG, Roitsch T, Christie PJ, Nester EW. The regulatory VirG protein specifically binds to a cis-acting regulatory sequence involved in transcriptional activation of Agrobacterium tumefaciens virulence genes. J Bacteriol. 1990;172:531–537. doi: 10.1128/jb.172.2.531-537.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jin S, Song YN, Deng WY, Gordon MP, Nester EW. The regulatory VirA protein of Agrobacterium tumefaciens does not function at elevated temperatures. J Bacteriol. 1993;175:6830–6835. doi: 10.1128/jb.175.21.6830-6835.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baron C, Domke N, Beinhofer M, Hapfelmeier S. Elevated temperature differentially affects virulence, VirB protein accumulation, and T-pilus formation in different Agrobacterium tumefaciens and Agrobacterium vitis strains. J Bacteriol. 2001;183:6852–6861. doi: 10.1128/JB.183.23.6852-6861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hasegawa H, Chatterjee A, Cui Y, Chatterjee AK. Elevated temperature enhances virulence of Erwinia carotovora subsp. carotovora strain EC153 to plants and stimulates production of the quorum sensing signal, N-acyl homoserine lactone, and extracellular proteins. Appl Environ Microbiol. 2005;71:4655–4663. doi: 10.1128/AEM.71.8.4655-4663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pirhonen M, Flego D, Heikinheimo R, Palva ET. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993;12:2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weingart H, Stubner S, Schenk A, Ullrich MS. Impact of temperature on in planta expression of genes involved in synthesis of the Pseudomonas syringae phytotoxin coronatine. Mol Plant Microbe Interact. 2004;17:1095–1102. doi: 10.1094/MPMI.2004.17.10.1095. [DOI] [PubMed] [Google Scholar]
- 145.Ullrich M, Peñaloza-Vázquez A, Bailey AM, Bender CL. A modified two-component regulatory system is involved in temperature-dependent biosynthesis of the Pseudomonas syringae phytotoxin coronatine. J Bacteriol. 1995;177:6160–6169. doi: 10.1128/jb.177.21.6160-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aguilera S, López-López K, Nieto Y, Garcidueñas-Piña R, Hernández-Guzmán G, Hernández-Flores JL, Murillo J, Alvarez-Morales A. Functional characterization of the gene cluster from Pseudomonas syringae pv. phaseolicola NPS3121 involved in synthesis of phaseolotoxin. J Bacteriol. 2007;189:2834–2843. doi: 10.1128/JB.01845-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van Dijk K, Fouts DE, Rehm AH, Hill AR, Collmer A, Alfano JR. The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (type III) protein secretion system in a temperature- and pH-sensitive manner. J Bacteriol. 1999;181:4790–4797. doi: 10.1128/jb.181.16.4790-4797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goel AK, Lundberg D, Torres MA, Matthews R, Akimoto-Tomiyama C, Farmer L, Dangl JL, Grant SR. The Pseudomonas syringae type III effector HopAM1 enhances virulence on water-stressed plants. Mol Plant Microbe Interact. 2008;21:361–370. doi: 10.1094/MPMI-21-3-0361. [DOI] [PubMed] [Google Scholar]
- 149.Meyer M, Cox JA, Hitchings MDT, Burgin L, Hort MC, Hodson DP, Gilligan CA. Quantifying airborne dispersal routes of pathogens over continents to safeguard global wheat supply. Nat Plants. 2017;3:780–786. doi: 10.1038/s41477-017-0017-5. [DOI] [PubMed] [Google Scholar]
- 150.Paul PA, Munkvold GP. Influence of temperature and relative humidity on sporulation of Cercospora zeae-maydis and expansion of gray leaf spot lesions on maize leaves. Plant Dis. 2005;89:624–630. doi: 10.1094/PD-89-0624. [DOI] [PubMed] [Google Scholar]
- 151.Manstretta V, Rossi V. Effects of temperature and moisture on development of Fusarium graminearum perithecia in maize stalk residues. Appl Environ Microbiol. 2015;82:184–191. doi: 10.1128/AEM.02436-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bui DC, Lee Y, Lim JY, Fu M, Kim JC, Choi GJ, Son H, Lee YW. Heat shock protein 90 is required for sexual and asexual development, virulence, and heat shock response in Fusarium graminearum. Sci Rep. 2016;6:28154. doi: 10.1038/srep28154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tani S, Judelson H. Activation of zoosporogenesis-specific genes in Phytophthora infestans involves a 7-nucleotide promoter motif and cold-induced membrane rigidity. Eukaryot Cell. 2006;5:745–752. doi: 10.1128/EC.5.4.745-752.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rossi V, Ravanetti A, Pattori E, Giosuè S. Influence of temperature and humidity on the infection of wheat spikes by some fungi causing Fusarium head blight. J Plant Pathol. 2001;83:189–198. [Google Scholar]
- 155.Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- 156.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 157.Xu G, Yuan M, Ai C, Liu L, Zhuang E, Karapetyan S, Wang S, Dong X. uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nature. 2017;545:491–494. doi: 10.1038/nature22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.King J, Armstead I, Harper J, Ramsey L, Snape J, Waugh R, James C, Thomas A, Gasior D, Kelly R, et al. Exploitation of interspecific diversity for monocot crop improvement. Heredity. 2013;110:475–483. doi: 10.1038/hdy.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Das G, Rao GJ. Molecular marker assisted gene stacking for biotic and abiotic stress resistance genes in an elite rice cultivar. Front Plant Sci. 2015;6:698. doi: 10.3389/fpls.2015.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhu Y, Chen H, Fan J, Wang Y, Li Y, Chen J, Fan J, Yang S, Hu L, Leung H, et al. Genetic diversity and disease control in rice. Nature. 2000;406:718–722. doi: 10.1038/35021046. [DOI] [PubMed] [Google Scholar]
- 161.Zimmerer KS. The ecogeography of Andean potatoes. Versatility in farm regions and fields can aid sustainable development. BioScience. 1998;48:445–454. [Google Scholar]
- 162.ISAAA. ISAAA annual report. International Service for the Acquisition of Agri-biotech Applications; 2016. [Google Scholar]
- 163.Kwak SY, Wong MH, Lew TTS, Bisker G, Lee MA, Kaplan A, Dong J, Liu AT, Koman VB, Sinclair R, et al. Nanosensor technology applied to living plant systems. Annu Rev Anal Chem. 2017;10:113–140. doi: 10.1146/annurev-anchem-061516-045310. [DOI] [PubMed] [Google Scholar]
- 164.Fahlgren N, Gehan MA, Baxter I. Lights, camera, action: high-throughput plant phenotyping is ready for a close-up. Curr Opin Plant Biol. 2015;24:93–99. doi: 10.1016/j.pbi.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 165.Hacquard S, Spaepen S, Garrido-Oter R, Schulze-Lefert P. Interplay between innate immunity and the plant microbiota. Annu Rev Phytopathol. 2017;55:565–589. doi: 10.1146/annurev-phyto-080516-035623. [DOI] [PubMed] [Google Scholar]
- 166.Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JH, Piceno YM, DeSantis TZ, Andersen GL, Bakker PA, et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 2011;332:1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- 167.Bardin M, Ajouz S, Comby M, Lopez-Ferber M, Graillot B, Siegwart M, Nicot PC. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front Plant Sci. 2015;6:566. doi: 10.3389/fpls.2015.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Santhanam R, Luu VT, Weinhold A, Goldberg J, Oh Y, Baldwin IT. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc Natl Acad Sci USA. 2015;112:E5013–E5020. doi: 10.1073/pnas.1505765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rolli E, Marasco R, Vigani G, Ettoumi B, Mapelli F, Deangelis ML, Gandolfi C, Casati E, Previtali F, Gerbino R, et al. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ Microbiol. 2015;17:316–331. doi: 10.1111/1462-2920.12439. [DOI] [PubMed] [Google Scholar]
- 170.Busby PE, Soman C, Wagner MR, Friesen ML, Kremer J, Bennett A, Morsy M, Eisen JA, Leach JE, Dangl JL. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017;15:e2001793. doi: 10.1371/journal.pbio.2001793. [DOI] [PMC free article] [PubMed] [Google Scholar]