Abstract
Recent publications have stated that the blood pressure measurement technique used in SPRINT was unattended. However, the SPRINT protocol does not address the issue of attendance.
A survey was conducted immediately after SPRINT closeout visits were completed to inquire whether blood pressure measurements were usually attended or unattended by staff.
There were 4082 participants at 38 sites that measured blood pressure after leaving the participant alone the entire time (Always Alone), 2247 at 25 sites that had personnel in the room the entire time (Never Alone), 1746 at 19 sites that left the participant alone only during the rest period (Alone for Rest), and 570 at 6 sites that left the participant alone only during the blood pressure readings (Alone for Blood Pressure Measurement). Similar systolic and diastolic blood pressures within randomized groups were noted during follow-up at the majority of visits in all four measurement categories. In the Always Alone and Never Alone categories, the Intensive group had a similarly reduced risk for the primary outcome compared with the Standard group (Hazard Ratio = 0.62; 95% Confidence Interval: 0.51 to 0.76 and Hazard Ratio 0.64; 95% Confidence Interval: 0.46 to 0.91 respectively; pairwise interaction p value = 0.88); risk was not significantly reduced for the Intensive group in the smaller Alone for Rest and the Alone for Blood Pressure Measurement categories.
Similar blood pressure levels and cardiovascular disease risk reduction were observed in the Intensive group in SPRINT participants whether the measurement technique used was primarily attended or unattended.
Keywords: Blood pressure measurement/monitoring, cardiovascular disease, hypertension, high blood pressure
INTRODUCTION
Hypertension is a major risk factor for coronary heart disease, stroke and kidney failure that affects over one third of Americans and nearly 1.4 billion adults worldwide.1–3 The health risks attributed to increasing blood pressure (BP) appear to be continuous, with no evidence of a BP threshold above a systolic BP of 115 mmHg, with cardiovascular disease (CVD) mortality increasing progressively throughout the range of BP.4–6 There is conclusive evidence that BP lowering reduces the risk of CVD in hypertensive persons, including evidence from the recently published Systolic Blood Pressure Intervention Trial (SPRINT).7–10
Knowing how BP is measured is important to understanding BP control and guiding clinicians in appropriate management of hypertension.11 The auscultatory method of BP measurement, which requires staff attendance during the reading, was employed in many landmark hypertension trials and has been the gold standard in clinical practice in the past.12, 13 However, concerns have been expressed regarding the safety of mercury sphygmomanometers and the accuracy of auscultatory readings in routine clinical practice. This and convenience of use has led to progressively greater use of automated oscillometric devices in more recent trials.12–19 The SPRINT trial used programmable automated oscillometric devices to measure BP, but there is confusion in recent publications from investigators not involved with SPRINT regarding whether the BP measurements at the 102 SPRINT clinical sites were unattended.20–23 Concern has also been expressed that the BP readings obtained in SPRINT were not comparable with BP readings in other trials where the measurement was attended and that the intensive treatment goal of <120 mm Hg in SPRINT would actually correspond to higher systolic BP (SBP) values in other trials.22
To assess whether BP measurements were attended or unattended at SPRINT clinics, we conducted a survey immediately after study closeout to inquire whether site staff were usually in the room with the participant (attended) or not in the room (unattended) during the rest period and/or during the BP measurement. We examined whether there were differences in measured clinic BP, and whether there were corresponding differences in SPRINT major outcome results or safety events, based on staff attendance.
METHODS
Data Availability
Anonymized SPRINT data used in these analyses will be publicly available at the NHLBI Biologic Specimen and Data Repository (BioLINCC) during late 2018 via controlled access at www.biolincc.nhlbi.nih.gov/home.
Study Design
SPRINT was a randomized, controlled, open-label trial that randomized participants to a target systolic BP goal of either <140 mm Hg (Standard) or <120 mm Hg (Intensive). The SPRINT trial was conducted from November 2010 until August 2015 in 102 clinical sites organized into five Clinical Center Networks. The rationale, design, protocol, and main results for SPRINT are publicly available.7,16,24 The study was approved by responsible Institutional Review Boards at all participating sites and was registered at ClinicalTrials.gov (NCT 01206062). All participants gave written informed consent and the study conformed to the Declaration of Helsinki and to Title 45, United States, Code of Federal Regulations, Part 46, Protection of Human Subjects.
An independent Data and Safety Monitoring Board (DSMB) monitored trial implementation, unblinded trial results, and safety experiences. Following a recommendation by the trial’s DSMB and with the concurrence of the National Heart, Lung, and Blood Institute, the SPRINT BP intervention was halted on August 20, 2015 after a median follow-up of 3.26 years. Follow-up was censored at the date of last assessment for a study event or on August 20, 2015. This publication is based on a database that was frozen on February 8, 2017 and includes outcome events from baseline until the termination of the trial intervention.
Study Population
The eligibility characteristics of the SPRINT participants have been published and can be found in the online supplement. (please see http://hyper.ahajournals.org for Supplemental Material)7
Interventions
After randomization, SPRINT investigators initiated or adjusted antihypertensive medications to achieve the assigned SBP targets according to a step-care treatment algorithm using antihypertensive drugs approved by U.S. Food and Drug Administration.7, 16
Study BP measurement
All SPRINT sites were provided with the Professional Digital Blood Pressure Monitor (Omron Healthcare, Lake Forest, IL) model 907XL for BP measurement in the trial. A central training session was held in September 2010 and again in March 2014 to train clinic investigators and staff in study procedures. The Clinical Center Networks and Coordinating Center also organized training sessions using conference calls, webinars, and on-site training. Training on BP measurement technique emphasized proper positioning of participants, measurement of arm circumference and use of proper cuff size, and the importance of a 5-minute rest period prior to obtaining the three seated BPs. During the rest and BP measurement periods, the participant was neither completing questionnaires, talking nor texting. The Clinical Center Networks tracked performance of clinical sites and conducted standardized site visits which included assessment of BP measurement.
Additional information about BP measurement technique was also provided to the sites in the SPRINT protocol and SPRINT Manual of Procedures (MOP).7, 24, 25 The SPRINT protocol stated that “Seated BP and pulse are measured at each clinic visit after a rest period using an automated device, or manual devices if necessary. The preferred method is the automated device as it offers reduced potential for observer biases and decreased demand on staff in terms of training and effort in data collection.” The SPRINT protocol did not address the issue of staff attendance during the BP measurement. The SPRINT MOP recommended that the staff leave the room during the rest period, but return to take the BPs at the end of the 5-minute rest, but did not require staff attendance or absence during the BP measurement. The Omron Digital Blood Pressure Monitor that was used in SPRINT could be programmed to incorporate the 5-minute rest and then initiate the three BP measurements automatically after the 5 minutes had elapsed. Coordinators were instructed how to program the Omron device during training. The coordinators could have been in or out of the room during the 5-minute rest period and/or during the time the Omron was automatically taking the BP. (please see http://hyper.ahajournals.org for Supplemental Material)
Study outcomes
Definitions of study primary outcomes and serious adverse events (SAEs) have been published and can be found in the online supplement. (please see http://hyper.ahajournals.org for Supplemental Material)7, 16, 24
Which BP measurement technique used (attended or unattended) in individual SPRINT clinics was not recorded during the trial, but was assessed by a survey immediately after the close-out period (please see http://hyper.ahajournals.org for Supplemental Material). In the survey, clinic sites were asked to indicate how often BP measurements were taken at their site when: 1) participants were alone during the 5 minute rest period and also during the 3 BP measurements (Always Alone - AA); 2) study personnel were in the room during the entire time (Never Alone - NA); 3) participants were alone during the rest period but study staff were present during the 3 BP measurements (Alone for Rest - AR); 4) study personnel were in the room during rest but the participant was alone during the 3 BP measurements (Alone for BP Measurement - ABM) or 5) other. Each clinic was asked to provide a single, consensus response. Based on these responses, 88 sites and participants at each site were placed into one of the four mutually exclusive categories of BP measurement technique (AA, NA, AR, ABM) if they used that technique at least 50% of the time and more than any other. Most sites predominantly used one technique, and after weighting the survey responses by the total number of SPRINT BP examinations performed at each clinic, we calculate that 92% of examinations in AA clinics were performed with staff out of the room for both rest and BP measurements, 82% of examinations in NA clinics were performed with staff in the room for both rest and BP measurements, 85% of examinations in AR clinics were performed with staff out of the room for rest but present for BP measurements, and 89% of examinations in ABM clinics were performed with staff present for rest but out of the room when BP measurements were taken. Fourteen sites (716 participants) that had substantial turnover during the close-out period or that indicated that they did not use any one of the BP measurement techniques 1–4 ≥50% of the time were excluded from these analyses (please see http://hyper.ahajournals.org for Supplemental Material). Sites were also asked to rate how confident they were in their response to the BP measurement technique(s) used in their clinic. Of the 88 sites included in these analyses, 83 (94%) indicated they were extremely or very confident in their responses.
Statistical Analysis
BP measurement techniques 1–4 were treated as post-hoc categories for use in participant-level analyses. Standard descriptive statistics (means and standard deviations [SD] for continuous variables; frequencies and percentages for categorical variables) were used unless otherwise noted.
Baseline characteristics were compared by treatment group and BP measurement technique using row-by-column frequency tables for categorical variables and analysis of variance models for continuous variables. We report the p-value for the marginal effect of BP measurement technique after controlling for randomized group, using the Cochran-Mantel-Haenszel statistic for categorical variables and the marginal type III sum of squares for continuous variables. For baseline BPs, we also used general linear models to examine differences between treatment groups and BP measurement techniques after adjustment for age, gender, black race, Hispanic ethnicity, chronic kidney disease (CKD), clinical and subclinical CVD, number of chronic diseases, current and former smoking status, statin use, aspirin use, Framingham risk score, number of BP medications at enrollment, body mass index (BMI), weight, and heart rate.
The mean of the 3 BP measurements (usually obtained from the Omron display) was recorded at visits by the site staff and used for these analyses. We examined average SBP, average diastolic BP (DBP), and average number of medications prescribed during follow-up using mixed linear models. We used contrasts and pairwise comparisons with no adjustment for multiple comparisons to examine differences between techniques.
For CVD and safety outcomes, Cox proportional hazards analyses were used to calculate hazard ratios (HR) with 95% confidence intervals (CI) between treatment groups in each of the 4 BP measurement technique categories. Differences between randomized groups by BP measurement techniques were also examined in adjusted proportional hazard analyses after controlling for the baseline characteristics age, gender, black race, Hispanic ethnicity, CKD, clinical and subclinical CVD, number of chronic diseases, current and former smoking status, statin use, aspirin use, Framingham risk score, SBP and DBP, number of BP medications at enrollment, BMI, weight, and heart rate. In both adjusted and unadjusted models, contrasts were used to compare hazard ratios between the AA and NA categories.
We also performed several sensitivity analyses, including analyses repeated after collapsing the BP measurement techniques into two categories, attended (NA + AR) vs. unattended (AA + ABM) blood pressure measurements, analyses repeated after restricting classification of BP techniques to sites using that technique for at least 85% of BP exams, and models for blood pressures and prescribed medications fit separately for each visit. Results of these analyses are presented in the online supplement (please see http://hyper.ahajournals.org).
RESULTS
SPRINT randomized 9,361 participants to either the Intensive or Standard arm. A total of 38 sites (4082 participants) were classified to the AA category, 25 sites (2247 participants) were classified to the NA category, 19 sites (1746 participants) were classified to the AR category, and 6 sites (570 participants) were classified to the ABM category (please see http://hyper.ahajournals.org for Supplemental Material). Thus, a total of 44 sites with 3,993 participants had BP measured while the staff were in the room (attended, NA + AR), and 44 sites with 4652 participants had BP measured while the staff were out of the room (unattended, AA + ABM). (Figure 1 Consort Diagram).
Figure 1.
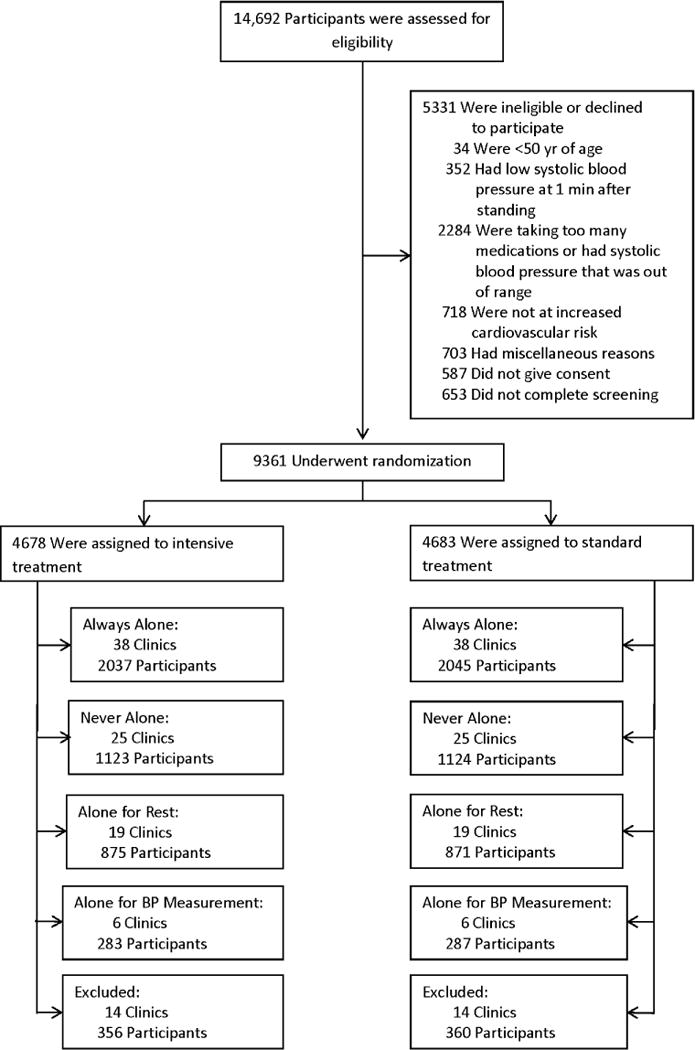
CONSORT diagram
Analyses of the baseline participant characteristics for the four BP measurement technique categories by treatment assignment revealed significant differences (Table 1 and please see http://hyper.ahajournals.org for Supplemental Material Tables S1 and S2). The participants in the NA and ABM categories were younger; the participants in ABM category were more likely to be Hispanic, female, and a non-smoker and less likely to use statin or aspirin therapy; the participants in the AA category were more likely to have CKD; the participants in the AA and NA categories were more likely to have CVD and have a higher Framingham risk score; and the participants in the NA category were most likely to be a current smoker. Both the Intensive and Standard treatment groups in the ABM category had higher average SBP readings (p value =0.001) and lower DBP readings (p value <0.001) at baseline compared with the other BP measurement technique categories (Table 1). After adjustment for other variables listed in Table 1, the DBP readings at baseline remained lowest in the ABM category (p value <0.001). The baseline SBP differences were attenuated but remained significantly different, with the NA group having lower average SBP than the other groups (mean adjusted SBPs were 139.1 mm Hg in the NA group vs. 139.9, 140.2, and 139.9 mm Hg in the AA, AR and ABM groups, respectively, p = 0.049)
Table 1.
Baseline Characteristics of SPRINT Participants Stratified by Randomized Group and Blood Pressure Technique
| Characteristics | Always Alone (n =4082) |
Never Alone (n =2247) |
Alone for Rest (n =1746) |
Alone for BP Measurement (n=570) |
P value* | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intensive | Standard | Intensive | Standard | Intensive | Standard | Intensive | Standard | ||
| No. Clinics | 38 | 25 | 19 | 6 | |||||
| No. Randomized | 2037 (100) | 2045 (100) | 1123 (100) | 1124 (100) | 875 (100) | 871 (100) | 283 (100) | 287 (100) | |
| Age 50–64 | 730 (35.8) | 767 (37.5) | 565 (50.3) | 552 (49.1) | 303 (34.6) | 296 (34.0) | 132 (46.6) | 130 (45.3) | <0.001 |
| 65–74 | 674 (33.1) | 656 (32.1) | 298 (26.5) | 290 (25.8) | 286 (32.7) | 287 (33.0) | 93 (32.9) | 104 (36.2) | |
| ≥75 | 633 (31.1) | 622 (30.4) | 260 (23.2) | 282 (25.1) | 286 (32.7) | 288 (33.1) | 58 (20.4) | 53 (18.5) | |
| Female Gender | 668 (32.8) | 620 (30.3) | 340 (30.3) | 349 (31.0) | 351 (40.1) | 376 (43.2) | 138 (48.8) | 139 (48.4) | <0.001 |
| Black Race | 595 (29.2) | 625 (30.6) | 409 (36.4) | 419 (37.3) | 244 (27.9) | 228 (26.2) | 81 (28.6) | 78 (27.2) | <0.001 |
| Hispanic Ethnicity | 64 (3.1) | 45 (2.2) | 193 (17.2) | 182 (16.2) | 26 (3.0) | 26 (3.0) | 183 (64.7) | 187 (65.2) | <0.001 |
| Chronic kidney disease‡ | 633 (31.2) | 633 (31.0) | 282 (25.1) | 270 (24.0) | 254 (29.0) | 254 (29.2) | 57 (20.1) | 67 (23.3) | <0.001 |
| Cardiovascular disease | 438 (21.5) | 448 (21.9) | 238 (21.2) | 237 (21.1) | 145 (16.5) | 144 (16.5) | 54 (19.1) | 51 (17.8) | <0.001 |
| Clinical | 276 (13.6) | 279 (13.6) | 154 (13.7) | 151 (13.4) | 84 (9.6) | 81 (9.9) | 27 (9.5) | 23 (8.0) | <0.001 |
| Subclinical | 174 (8.5) | 178 (8.7) | 87 (7.8) | 100 (8.9) | 51 (5.8) | 58 (6.7) | 38 (13.4) | 35 (12.2) | <0.001 |
| Framingham 10-yr CVD risk score-% | 25.8±12.9 | 26.0±12.5 | 25.1±13.1 | 24.8±12.8 | 24.0±11.5 | 23.8±11.9 | 23.6±12.8 | 22.5±11.5 | <0.001 |
| SBP, mmHg | 139.6±15.9 | 139.9±15.2 | 139.2±15.8 | 138.8±15.3 | 140.0±15.2 | 140.0±15.9 | 142.5±15.0 | 141.1±14.4 | 0.001 |
| DBP, mmHg | 77.9±11.6 | 78.0±12.0 | 79.3±12.1 | 78.7±11.6 | 77.8±12.1 | 77.1±12.8 | 76.9±11.0 | 76.2±10.5 | <0.001 |
| Pulse, bpm | 65.9±11.7 | 66.1±12.2 | 66.6±11.2 | 66.7±11.7 | 66.4±12.0 | 65.5±10.9 | 65.8±10.1 | 67.0±10.2 | 0.11 |
| # of BP Medications | 1.9±1.0 | 1.9±1.0 | 1.9±1.0 | 1.9±1.0 | 1.7±1.1 | 1.7±1.1 | 1.7±1.0 | 1.6±0.9 | <0.001 |
| Weight, lbs. | 192.2±41.5 | 193.8±41.8 | 194.0±40.4 | 191.0±40.7 | 186.3±41.4 | 186.2±42.1 | 183.4±38.2 | 181.5±38.2 | <0.001 |
| BMI, Kg/m2 | 30.0±5.8 | 29.9±5.7 | 30.2±5.7 | 29.9±5.7 | 29.3±5.6 | 29.2±5.7 | 29.6±5.2 | 29.4±5.0 | <0.001 |
| # of Chronic Diseases | 2.8±1.7 | 1.8±1.7 | 2.6±1.7 | 2.6±1.7 | 2.7±1.7 | 2.7±1.7 | 2.2±1.5. | 2.2±1.5 | <0.001 |
| Smoking Status | |||||||||
| Current | 271 (13.3) | 256 (12.6) | 189 (16.9) | 165 (14.7) | 85 (9.7) | 96 (11.1) | 37 (13.1) | 33 (11.5) | <0.001 |
| Past | 919 (45.2) | 911 (44.7) | 469 (41.9) | 472 (42.1) | 356 (40.8) | 389 (44.8) | 97 (34.3) | 79 (27.6) | <0.001 |
| Statin use | 935 (46.3) | 938 (46.3) | 458 (41.0) | 497 (44.5) | 358 (41.1) | 382 (44.2) | 97 (34.4) | 113 (39.8) | <0.001 |
| Aspirin use | 1106 (54.6) | 1116 (54.8) | 574 (51.2) | 553 (49.2) | 442 (50.5) | 415 (47.8) | 103 (36.4) | 107 (37.4) | <0.001 |
Test for differences among BP measurement techniques after controlling for randomized group assignment. Cardiovascular disease (CVD), blood pressure (BP), body mass index (BMI)
Average BPs over time are presented by treatment group and BP measurement technique category in Figure 2, Table 2, and Supplemental Tables S3 and S4 (please see http://hyper.ahajournals.org). The post-randomization differences in average SBP and DBP among the 4 BP measurement technique categories in the Intervention arm were relatively small (SBP range 120.6 – 122.2 mm Hg and DBP range 67.4 – 68.3 mm Hg respectively). A similar finding was noted for the 4 BP measurement technique categories in the Standard group (SBP range 134.4 – 135.4 and DBP range 73.0 – 75.0 respectively) (Table 2). At the majority of follow-up visits, similar SBPs within randomized groups were noted among participants in all four BP measurement technique categories. Heterogeneity in treatment effect by BP measurement technique was detected for SBP at only the 27-month visit (interaction p value = 0.020). Similarly, no heterogeneity of treatment effect was detected for DBP by BP measurement technique category through month 24 of follow-up. In a sensitivity analysis, average SBP and DBP by treatment group over time for attended (NA + AR) blood pressure measurement was also compared with unattended (AA + ABM) measurement and revealed essentially no difference whether the staff were in the room or not (attended Intensive SBP mean = 121.5 mm Hg compared with unattended Intensive SBP mean = 121.5 mm Hg and attended Standard SBP mean = 134.5mm Hg compared with unattended Standard SBP mean = 134.7 mm Hg, all p values > 0.05) Additional information on BP is available in Supplemental Tables S5 and S6) (please see http://hyper.ahajournals.org)
Figure 2.
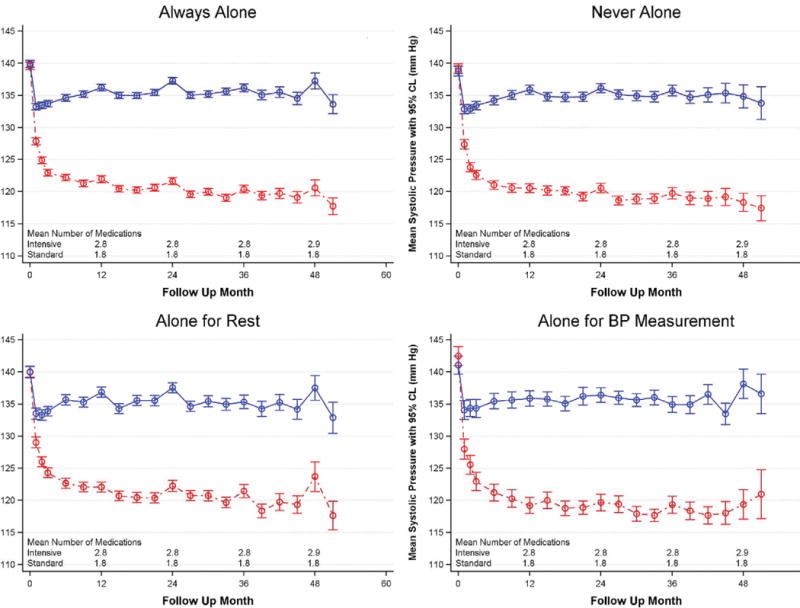
Systolic Blood Pressure by BP Measurement Technique Reported by SPRINT Staff over Time. Means and confidence intervals for standard group participants are shown in blue, while those for intensive group participants are shown in red.
Table 2.
Average post-randomization blood pressures and medications, controlling for subject, visit and clinical site
| Variable | Always Alone | Never Alone | Alone for Rest | Alone for BP Measurement |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Systolic BP | ||||
| Intensive Participants | 121.4 (120.7, 122.0)a | 121.0 (120.2, 121.8)a | 122.2 (121.3, 123.1)a | 120.6 (119.1, 122.2)a |
| Standard Participants | 134.4 (133.8, 135.1)a | 134.4 (133.6, 135.1)a | 134.7 (133.8, 135.6)a | 135.4 (133.8, 136.9)a |
| Delta | 13.1 (12.6, 13.5)a | 13.3 (12.7, 13.9)a | 12.5 (11.9,13.2)a | 14.7 (13.5,15.9) |
| Diastolic BP | ||||
| Intensive Participants | 67.9 (66.9, 69.0)a | 68.3, (67.0, 69.6)a | 67.4 (65.9, 68.9)a | 68.0 (65.4, 70.6)a |
| Standard Participants | 74.6 (73.6, 75.7)a | 75.0 (73.7, 76.3)a | 74.0 (72.5, 75.5)a | 73.0 (70.4, 75.7)a |
| Delta | 6.7 (6.2, 7.2)a | 6.7 (6.0, 7.4)ab | 6.6 (5.8, 7.3)ab | 5.0 (3.7, 6.4)b |
| Number of Medications | ||||
| Intensive Participants | 2.7 (2.7, 2.8)ab | 2.8 (2.7, 2.9)b | 2.7 (2.6, 2.8)a | 2.5 (2.4, 2.7)a |
| Standard Participants | 1.8 (1.8, 1.9)a | 1.9 (1.8, 2.0)a | 1.8 (1.7, 1.9)a | 1.8 (1.6, 1.9)a |
| Delta | 0.9 (0.8, 1.0)a | 0.9 (0.9, 1.0)a | 0.9 (0.8, 1.0)a | 0.8 (0.6, 0.9)a |
Within rows, cells with the superscript “a” are not different (p>0.05) from other cells with the superscript “a” or “ab”, but are different (p<0.05) from cells with the superscript “b” or with no superscript at all. Similarly, within rows cells with the superscript “b” are not different (p>0.05) from other cells with the superscript “b” or “ab” but are different (p≤0.05) from cells with the superscript “a” or with no superscript at all.
Average medication use over time is presented by treatment group and BP measurement technique categories in Table 2, and Supplemental Table S7 (please see http://hyper.ahajournals.org). At the majority of follow-up visits, similar average number of medication use within randomized groups was noted among participants in all four BP measurement technique categories.
When the larger AA and NA groups were compared with one another, there was no evidence of heterogeneity of treatment effect by BP measurement technique for the primary outcome (pairwise interaction p-value = 0.88). However, when all four measurement technique categories were compared, heterogeneity of treatment effect was detected for the primary outcome (interaction p-value = 0.005) (Table 3). In both the AA and NA categories the Intensive treatment group had a reduced risk of the primary outcome compared to the Standard treatment group (HR = 0.62; 95% CI: 0.51 to 0.76 and 0.64; 95% CI: 0.46 to 0.91 respectively); however, no significant difference between the treatment groups among participants in the smaller AR and the ABM categories was detected.
Table 3.
Primary and all-cause mortality outcomes stratified by treatment group and blood pressure technique
| Outcome | Intensive Arm | Standard Arm | Intensive vs. Standard Hazard Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BP Technique | N | Events | % Per year | N | Events | % Per year | HR | 95% CI | Interaction P-value | |
| Primary | Always Alone | 2037 | 101 | 1.5 | 2045 | 159 | 2.5 | 0.62 | 0.51,0.76 | 0.005 |
| Never Alone | 1123 | 68 | 1.9 | 1124 | 103 | 3.0 | 0.64 | 0.46,0.91 | ||
| Alone for Rest | 875 | 50 | 1.8 | 871 | 51 | 1.9 | 0.98 | 0.76,1.25 | ||
| Alone for BP Measurement | 283 | 20 | 2.1 | 287 | 15 | 1.5 | 1.39 | 0.78,2.49 | ||
| All death | Always Alone | 2037 | 64 | 1.0 | 2045 | 98 | 1.5 | 0.65 | 0.47,0.88 | 0.28 |
| Never Alone | 1123 | 46 | 1.3 | 1124 | 60 | 1.7 | 0.76 | 0.53,1.11 | ||
| Alone for Rest | 875 | 19 | 0.7 | 871 | 32 | 1.1 | 0.59 | 0.37,0.94 | ||
| Alone for BP Measurement | 283 | 10 | 1.0 | 287 | 7 | 0.7 | 1.48 | 0.63,3.05 | ||
No heterogeneity of treatment effect was detected for total mortality by BP measurement technique (interaction p-value = 0.28) (Table 3). In the AA and AR categories, the Intensive group compared to the Standard group had a reduced risk of total mortality (HR= 0.65; 95% CI: 0.47 to 0.88 and HR= 0.59; 95% CI: 0.37 to 0.94 respectively). A similar point estimate of reduced risk for total mortality in the Intensive group compared to the Standard group was seen in the NA category. No difference between the Intensive and Standard groups in total mortality was detected in the much smaller ABM category (Table 3). When only the larger AA and NA groups are compared to one another, no evidence of heterogeneity of treatment effect by BP measurement technique was detected (interaction p-value = 0.51). In both categories the Intensive treatment group had a reduced risk of total mortality compared to the Standard treatment group.
Since there were substantial differences in baseline characteristics by BP measurement technique, Cox proportional hazards analyses for the primary outcome and total mortality adjusted for the baseline variables listed in Table 1 were conducted. After adjustment, the heterogeneity of treatment effect by BP measurement technique for the primary outcome remained significant (interaction p-value = 0.008). Similar to the unadjusted analyses, the adjusted analyses showed no significant heterogeneity of treatment effect by BP measurement technique for total mortality (interaction p-value = 0.42) (Supplemental Table S8; please see http://hyper.ahajournals.org).
Additional sensitivity analyses were conducted in clinics that used the BP measurement technique ≥ 85% of the time and no heterogeneity of treatment effect was detected for the primary outcome or for total mortality (Supplemental Tables S9 and S10; please see http://hyper.ahajournals.org).
There was no difference in total SAEs between the Intensive and Standard groups in any of the four BP measurement technique categories (Table 4). Further, we could detect no significant heterogeneity of treatment effect for total SAEs (interaction p-value = 0.57) or for any of the other monitored conditions of interest (syncope, hypotension, injurious falls, bradycardia, acute kidney injury, or electrolyte abnormality) by BP measurement technique (all other interaction p-values > 0.05). In a model adjusting for the baseline characteristic differences by BP measurement technique, the findings did not substantially change in SAEs or other monitored conditions. (Supplemental Table S11; please see http://hyper.ahajournals.org).
Table 4.
SAE and other monitored adverse event outcomes stratified by treatment group and blood pressure technique
| Outcome | Intensive Arm | Standard Arm | Intensive vs. Standard Hazard Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BP Technique | N | Events | % Per year | N | Events | % Per year | HR | 95% CI | Interaction P-value | |
| Any SAE | Always Alone | 2037 | 814 | 15.4 | 2045 | 793 | 15.0 | 1.03 | 0.95,1.12 | 0.57 |
| Never Alone | 1123 | 428 | 14.7 | 1124 | 419 | 14.3 | 1.02 | 0.85,1.23 | ||
| Alone for Rest | 875 | 330 | 14.6 | 871 | 333 | 14.8 | 0.99 | 0.88,1.10 | ||
| Alone for BP Measurement | 283 | 73 | 8.9 | 287 | 67 | 7.7 | 1.16 | 0.96,1.41 | ||
| Syncope | Always Alone | 2037 | 52 | 0.8 | 2045 | 29 | 0.4 | 1.79 | 1.20,2.68 | 0.14 |
| Never Alone | 1123 | 18 | 0.5 | 1124 | 15 | 0.4 | 1.19 | 0.61,2.34 | ||
| Alone for Rest | 875 | 14 | 0.5 | 871 | 17 | 0.6 | 0.82 | 0.46,1.46 | ||
| Alone for BP Measurement | 283 | 4 | 0.4 | 287 | 6 | 0.6 | 0.68 | 0.11,4.42 | ||
| Hypotension | Always Alone | 2037 | 45 | 0.7 | 2045 | 26 | 0.4 | 1.73 | 1.03,2.92 | 0.48 |
| Never Alone | 1123 | 25 | 0.7 | 1124 | 10 | 0.3 | 2.50 | 1.30,4.78 | ||
| Alone for Rest | 875 | 16 | 0.6 | 871 | 13 | 0.5 | 1.22 | 0.55,2.71 | ||
| Alone for BP Measurement | 283 | 3 | 0.3 | 287 | 5 | 0.5 | 0.61 | 0.04,9.99 | ||
| Injurious Fall | Never Alone | 1123 | 27 | 0.8 | 1124 | 28 | 0.8 | 0.95 | 0.57,1.59 | 0.64 |
| Always Alone | 2037 | 42 | 0.6 | 2045 | 38 | 0.6 | 1.10 | 0.66,1.84 | ||
| Alone for Rest | 875 | 19 | 0.7 | 871 | 23 | 0.8 | 0.82 | 0.49,1.38 | ||
| Alone for BP Measurement | 283 | 5 | 0.5 | 287 | 2 | 0.2 | 2.59 | 0.40,16.76 | ||
| Bradycardia | Always Alone | 2037 | 36 | 0.5 | 2045 | 31 | 0.5 | 1.15 | 0.74,1.81 | – |
| Never Alone | 1123 | 18 | 0.5 | 1124 | 17 | 0.5 | 1.05 | 0.60,1.84 | ||
| Alone for Rest | 875 | 12 | 0.4 | 871 | 17 | 0.6 | 0.70 | 0.31,1.58 | ||
| Alone for BP Measurement | 283 | 4 | 0.4 | 287 | 0 | 0 | – | – | ||
| Acute Kidney Injury | Always Alone | 2037 | 91 | 1.4 | 2045 | 61 | 0.9 | 1.50 | 1.11,2.03 | 0.41 |
| Never Alone | 1123 | 46 | 1.3 | 1124 | 22 | 0.6 | 2.08 | 1.16,3.75 | ||
| Alone for Rest | 875 | 30 | 1.1 | 871 | 14 | 0.5 | 2.15 | 1.22,3.77 | ||
| Alone for BP Measurement | 283 | 8 | 0.8 | 287 | 7 | 0.7 | 1.18 | 0.62,2.26 | ||
| Electrolyte Abnormal | Always Alone | 2037 | 66 | 1 | 2045 | 48 | 0.7 | 1.38 | 1.04,1.83 | 0.38 |
| Never Alone | 1123 | 22 | 0.6 | 1124 | 24 | 0.7 | 0.91 | 0.50,1.67 | ||
| Alone for Rest | 875 | 28 | 1 | 871 | 19 | 0.8 | 1.47 | 0.80,2.72 | ||
| Alone for BP Measurement | 283 | 6 | 0.6 | 287 | 2 | 0.2 | 3.10 | 0.78,12.38 | ||
DISCUSSION
Blood pressure in SPRINT was measured in a standardized method in both attended and unattended conditions. Similar SBP differences (12–14 mm Hg) between the Intensive and Standard groups were seen in all four BP measurement technique categories regardless of staff attendance and these differences were well maintained over the course of the trial. Overall, there was no compelling evidence in SPRINT that unattended BP measurements, as in the AA (always alone) category, led to lower SBP at baseline or during follow-up, compared to the NA (never alone) category. In addition to similar SBP differences between randomized groups, the AA and NA categories had very similar mean SBP values (with only 1–2 mm Hg difference) at baseline and throughout follow-up, but this does not rule out a true BP difference between the groups.
SPRINT BP measurements were conducted using methods that were commonly recommended by professional societies and BP guidelines committees at the time the trial started.14, 26–28 These recommendations emphasized the importance of the BP measurement methods, but did not state whether the patient should be attended or unattended.14 Other trials using automatic BP monitors used standardized techniques such as those used in SPRINT, but SPRINT is the first clinical outcome trial where the issue of staff presence or absence during measurement and the effect on obtained BP values has been questioned.17, 22, 29
SPRINT data presented in this report contradict assumptions that all BP measurements taken in SPRINT were unattended and thus cannot be directly compared with other trials where BP measurement was attended.22 A corollary is that these SPRINT data also do not support the suggestion that the intensive treatment goal of <120 mm Hg in SPRINT would actually correspond to substantially higher SBP values in other trials where the BP measurement was assumed to be attended.22 Data from the SPRINT Ambulatory Blood Pressure Ancillary Study (n=897) also support the contention that the BP values obtained at the SPRINT study clinic visit whether attended or unattended are similar to values obtained during 24-hour ambulatory blood pressure monitoring.30
The present SPRINT data are inconsistent with several published reports suggesting that BP values were lower when taken unattended compared to attended BP measurements.31–33 However, many of these previous reports were comparisons of non-standardized office BP or home BP measured with the patient’s device compared with standardized automated unattended BP measurements, where many differences such as patient positioning and rest periods in addition to staff attendance are likely to have played a role in the reported BP differences. Additional research, with more controlled environments, is needed to determine whether and to what extent attendance during the BP measurement affects level of BP.
CVD and total mortality risk were reduced similarly in participants treated to the Intensive compared with Standard goals in clinics using either the NA and AA BP measurement techniques, with no evident increased risk of SAEs related to whether BP measurements were attended or unattended. Furthermore, if attendance during the BP measurement led to higher BP readings compared to unattended measurement, then participants in the Intensive arm in the NA category would have been expected to have been prescribed more medication, possibly resulting in increased SAEs such as syncope or hypotension compared to participants in the intensive arm of the AA category. However, the average number of medications used was not different between these two categories, and there was no difference in overall rates of SAEs, syncope, or hypotension. Thus, it does not appear that attendance at BP measurement sessions in SPRINT led to over-treatment using the SPRINT BP treatment algorithm.
These analyses suggest that using the SPRINT Intensive treatment algorithm and a SBP goal of <120 mm Hg, along with the BP measurement techniques recommended by hypertension guideline committees including use of a validated automated BP device in clinical practice in persons at high risk for CVD, will reduce the risks of CVD and mortality to a similar degree whether the BP measurement is attended or unattended. Furthermore, these findings suggest that staff training to allow for a quiet rest period, proper positioning of the arm and body, use of proper cuff size, and multiple measurements using a validated automated BP device may be more important than whether the BP measurement is attended or unattended.
Not surprisingly, there were baseline differences in the characteristics of participants in the four BP measurement categories as SPRINT did not systematically assign individuals to these categories and made substantial efforts to recruit a diverse group of qualified participants. Of note, the ABM category was much smaller with fewer events compared to the other three BP measurement categories, which could have resulted in less stable risk estimates than in the much larger AA and NA categories.
The strengths of SPRINT include its large sample size, diverse participant population, success in both implementing the protocol and achieving the SBP targets and sustained mean difference in SBP between the two treatment groups throughout the trial, and the robust CVD and mortality outcome differences. Another strength of SPRINT was central staff training, quality assurance monitoring, and use of a validated automated electronic sphygmomanometer, which may have improved standardization and reduced bias.
Limitations of these analyses include use of a post-hoc survey to assess the BP measurement technique, and classifications based on staff recall rather than real-time individual evaluation of staff attendance before and during the BP readings. In addition, staff turnover during the trial may have contributed to incomplete data collection or inaccurate recall of the BP measurement technique used. Thus, there is a potential for misclassification and responses by staff that were socially desirable, which may have influenced the results towards null findings. However, we believe that misclassification was minimal because the majority of study coordinators at each of the clinics reported performing the same BP measurement technique on the majority of occasions with a high level of confidence in their report.
Another limitation of these data includes the fact that both unattended and attended BP measurements were not made in the same individual participant at the same time. Thus, a direct comparison of attended and unattended BP measurement technique in the same individual cannot be made from the SPRINT data, and this question will require future study.
Supplementary Material
PERSPECTIVES
Similar BP levels and CVD risk reduction were observed in SPRINT participants whether the BP measurement technique used was primarily attended (NA) or unattended (AA). To arrive at firmer conclusions, additional research with better methods, is needed to determine whether attendance or other factors during the BP measurement affect level of BP reading.
NOVELTY AND SIGNIFICANCE.
What is new?
Similar BP levels and CVD risk reduction were observed in the Intensive group in SPRINT participants whether the BP measurement technique used was primarily attended or unattended.
What is relevant?
In order to fully realize the benefits and minimize risks associated with following the SPRINT Intensive treatment algorithm, use of a validated automated BP device, staff training to allow for a quiet rest period, proper positioning of the arm and body, use of proper cuff size, and averaging multiple measurements may be more important than whether the BP measurement is attended or unattended.
Summary.
These SPRINT data appear to contradict assumptions that BP taken in SPRINT cannot be directly compared with BP in other trials.
Acknowledgments
SOURCES OF FUNDING
The Systolic Blood Pressure Intervention Trial was funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. and Arbor Pharmaceuticals, LLC. The investigators would also like to acknowledge the SPRINT participants without whom this trial would not have been possible. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, NIH, the U.S. Department of Veterans Affairs, or the United States Government. The ClinicalTrials.gov Identifier: NCT01206062.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS.
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES
Karen C. Johnson, MD, MPH - None
Paul K. Whelton, MB, MD, MSc - None
William C. Cushman, MD - Supported by Eli Lilly institutional grant, uncompensated consulting
for Takeda and Novartis
Jeffrey A. Cutler, MD, MPH - None
Gregory W. Evans, MA - Supported on an institutional grant from AstraZeneca AB
Joni K. Snyder, BSN, MA - None
Walter T. Ambrosius, PhD - None
Srinivasan Beddhu, MD - None
Alfred K. Cheung, MD - None
Lawrence J. Fine, MD, DrPH – None
Cora E. Lewis, MD, MSPH - None
Mahboob Rahman, MD - None
David M. Reboussin, PhD – None
Michael V. Rocco, MD, MSCE - None
Suzanne Oparil, MD - Research grant support from Actelion Pharmaceuticals/George Clinical;
AstraZeneca AB/Duke; Bayer; NIH/NHLBI, NHLBI; Novartis; Rox Medical Inc. Consulting for Scientific Advisory Board/Consultancy: Actelion Clinical Research Inc.; George Clinical Pty Ltd/Actelion; Lundbeck (Neurogenic Orthostatic Hypotension Advisory Board; Novo Nordisk; ROX Medical, Inc.
Jackson T. Wright, Jr., MD, PhD - None
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics C and Stroke Statistics S. Executive Summary: Heart Disease and Stroke Statistics–2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447–54. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- 4.Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, Greenland P. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Archives of internal medicine. 2001;161:1501–8. doi: 10.1001/archinte.161.12.1501. [DOI] [PubMed] [Google Scholar]
- 5.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 6.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprint Research Group. Wright JT, Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. The New England journal of medicine. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613–22. doi: 10.1097/HJH.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 9.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blood Pressure Lowering Treatment Trialists C Collaboration. Turnbull F, Neal B, Ninomiya T, Algert C, Arima H, Barzi F, Bulpitt C, Chalmers J, Fagard R, Gleason A, Heritier S, Li N, Perkovic V, Woodward M, MacMahon S. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008;336:1121–3. doi: 10.1136/bmj.39548.738368.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Subcommittee of P and Public Education of the American Heart Association Council on High Blood Pressure R. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 12.Borhani NO, Applegate WB, Cutler JA, Davis BR, Furberg CD, Lakatos E, Page L, Perry HM, Smith WM, Probstfield JL. Systolic Hypertension in the Elderly Program (SHEP). Part 1: Rationale and design. Hypertension. 1991;17:II2–15. doi: 10.1161/01.hyp.17.3_suppl.ii2. [DOI] [PubMed] [Google Scholar]
- 13.Probstfield JL, Applegate WB, Borhani NO, Curb JD, Cutler JA, Davis BR, Furberg CD, Hawkins CM, Lakatos E, Page LB, et al. The Systolic Hypertension in the Elderly Program (SHEP): an intervention trial on isolated systolic hypertension. SHEP Cooperative Research Group. Clin Exp Hypertens A. 1989;11:973–89. doi: 10.3109/10641968909035386. [DOI] [PubMed] [Google Scholar]
- 14.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 15.ACCORD Study Group. Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC, Grimm RH, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–46. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cushman WC, Grimm RH, Cutler JA, Evans GW, Capes S, Corson MA, Sadler LS, Alderman MH, Peterson K, Bertoni A, Basile JN, ACCORD Study Group Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:44i–55i. doi: 10.1016/j.amjcard.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. New Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 19.Kjeldsen SE, Hedner T, Jamerson K, Julius S, Haley WE, Zabalgoitia M, Butt AR, Rahman SN, Hansson L. Hypertension optimal treatment (HOT) study: home blood pressure in treated hypertensive subjects. Hypertension. 1998;31:1014–20. doi: 10.1161/01.hyp.31.4.1014. [DOI] [PubMed] [Google Scholar]
- 20.Messerli FH, Kjeldsen SE. Letter by Messerli et al Regarding Article The Implications of Blood Pressure Measurement Methods on Treatment Targets for Blood Pressure. Circulation. 2017;135:e4. doi: 10.1161/CIRCULATIONAHA.116.025301. [DOI] [PubMed] [Google Scholar]
- 21.Bakris G. Response by Bakris to Letter Regarding Article The Implications of Blood Pressure Measurement Methods on Treatment Targets for Blood Pressure. Circulation. 2017;135:e47. doi: 10.1161/CIRCULATIONAHA.116.026534. [DOI] [PubMed] [Google Scholar]
- 22.Kjeldsen SE, Lund-Johansen P, Nilsson PM, Mancia G. Unattended Blood Pressure Measurements in the Systolic Blood Pressure Intervention Trial: Implications for Entry and Achieved Blood Pressure Values Compared With Other Trials. Hypertension. 2016;67:808–12. doi: 10.1161/HYPERTENSIONAHA.116.07257. [DOI] [PubMed] [Google Scholar]
- 23.Schiffrin EL, Calhoun DA, Flack JM. SPRINT Proves that Lower Is Better for Nondiabetic High-Risk Patients, but at a Price. Am J Hypertens. 2016;29:2–4. doi: 10.1093/ajh/hpv190. [DOI] [PubMed] [Google Scholar]
- 24.SPRINT Protocol. https://www.sprinttrial.org/public/dspScience.cfm. Updated November 1. Accessed 8-24-17.
- 25.SPRINT Manual of Operations - Blood Pressure Measurement. https://www.sprinttrial.org/public/dspHome.cfm Updated Novermber 1, 2010. Accessed 8-24-2017.
- 26.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 28.Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can J Cardiol. 2016;32:569–88. doi: 10.1016/j.cjca.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 29.ACCORD Manual of Procedures. https://biolincc.nhlbi.nih.gov/studies/accord/ Updated August 13, 2013. Accessed 10-12-17.
- 30.Drawz PE, Pajewski NM, Bates JT, et al. Effect of Intensive Versus Standard Clinic-Based Hypertension Management on Ambulatory Blood Pressure: Results From the SPRINT (Systolic Blood Pressure Intervention Trial) Ambulatory Blood Pressure Study. Hypertension. 2017;69:42–50. doi: 10.1161/HYPERTENSIONAHA.116.08076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers MG. A Short History of Automated Office Blood Pressure - 15 Years to SPRINT. J Clin Hypertens (Greenwich) 2016;18:721–4. doi: 10.1111/jch.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graves JW, Nash C, Burger K, Bailey K, Sheps SG. Clinical decision-making in hypertension using an automated (BpTRU) measurement device. J Hum Hypertens. 2003;17:823–7. doi: 10.1038/sj.jhh.1001626. [DOI] [PubMed] [Google Scholar]
- 33.Filipovsky J, Seidlerova J, Kratochvil Z, Karnosova P, Hronova M, Mayer O. Automated compared to manual office blood pressure and to home blood pressure in hypertensive patients. Blood Press. 2016;25:228–34. doi: 10.3109/08037051.2015.1134086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized SPRINT data used in these analyses will be publicly available at the NHLBI Biologic Specimen and Data Repository (BioLINCC) during late 2018 via controlled access at www.biolincc.nhlbi.nih.gov/home.


