Abstract
The widely used influenza subunit vaccine would benefit from increased protection rates in vulnerable populations. Skin immunization by microneedle (MN) patch can increase vaccine immunogenicity, as well as increase vaccination coverage due to simplified administration. To further increase immunogenicity, we used granulocyte-macrophage colony stimulating factor (GM-CSF), an immunomodulatory cytokine already approved for skin cancer therapy and cancer support treatment. GM-CSF has been shown to be upregulated in skin following MN insertion. The GM-CSF-adjuvanted vaccine induced robust and long-lived antibody responses cross-reactive to homosubtypic and heterosubtypic influenza viruses. Addition of GM-CSF resulted in increased memory B cell persistence relative to groups given influenza vaccine alone and led to rapid lung viral clearance following lethal infection with homologous virus in the mouse model. Here we demonstrate that successful incorporation of the thermolabile cytokine GM-CSF into MN resulted in improved vaccine-induced protective immunity holding promise as a novel approach to improved influenza vaccination. To our knowledge, this is the first successful incorporation of a cytokine adjuvant into dissolvable MNs, thus advancing and diversifying the rapidly developing field of MN vaccination technology.
Keywords: GM-CSF, Microneedle, Influenza, Adjuvant, Skin, Co-delivery
1. Introduction
Influenza A virus is a common respiratory pathogen that causes seasonal outbreaks, epidemics and occasional pandemics. Although influenza illness mostly presents with benign symptoms in healthy populations and resolves within 7–10 days, it can become life-threatening in elderly adults, pregnant women, infants and people with chronic conditions [1–3]. Annual infection rates for seasonal influenza are 5–10% for adults and 20–30% for children [4]. Of the many influenza subtypes, H1N1 has the highest prevalence in laboratory-confirmed influenza-like illness (ILI), while H3N2 disproportionately affects individuals ≥65-years of age causing higher morbidity and mortality rates [5].
Influenza vaccination is the most effective public health strategy for reducing influenza mortality rate and economic burden of treatment and hospitalization costs and lost productivity and wages [6, 7]. However, during the 2015–2016 flu season influenza vaccination demonstrated modest protection against ILI, with an overall effectiveness of 47% [8]. In addition to the need for increased immunogenicity, current influenza vaccination approaches face obstacles such as limited duration of immunity and lack of protection in high risk groups, such as young children and the elderly, as well as low coverage and participation in vaccination [9–11].
Skin vaccination by microneedle (MN) patch has the potential to overcome the afore-mentioned hurdles observed in vaccination strategies by generating superior immune responses to vaccine antigen and increasing access to vaccination through improved vaccination logistics and greater patient acceptance [12–15]. MN vaccine patches have specifically been shown to enhance influenza vaccination, including increased immunogenicity [12, 13, 16], improved acceptability [17, 18] and long-lasting stability outside the cold-chain [19]. Additionally, a recent phase I clinical trial showed that influenza vaccination by MN patch administered by study personnel or self-administered by study participants was well tolerated, strongly immunogenic and overwhelmingly preferred compared to intramuscular (IM) vaccination [20].
We previously demonstrated that influenza vaccination by MN patch leads to accelerated viral clearance and increased recall immune responses when compared to IM vaccination [21, 22]. Antigen delivery with this novel platform targets the skin-resident antigen-presenting cells (APCs) and drives robust activation and mobilization of innate and adaptive immune cells [23]. Cytokines shape the immune response by modulating the function of cellular targets, making them potential adjuvants for vaccines. Thus, inclusion of cytokines within vaccine-containing MNs could further boost vaccine effectiveness.
Granulocyte-macrophage colony stimulating factor (GM-CSF) is a monomeric 24 kDa cytokine that promotes the maturation of granulocytes and macrophages from bone marrow progenitor cells [24] and is secreted by a wide array of immune cells, keratinocytes, eosinophils, neutrophils, and endothelial cells [25]. GM-CSF is particularly active in the skin, recruiting epidermal dendritic cells (DC) such as Langerhans cells into draining lymph nodes and enhancing activation and antigen presentation [26, 27]. These properties have made GM-CSF an effective therapy to treat neutropenia in cancer and AIDS patients for the past 20 years [28, 29] and a molecule of interest for adjuvant purposes in therapeutic vaccinations against skin cancers and autoimmune skin disorders [30, 31]. However, despite the potential for successful use as an adjuvant and demonstrated safety in cancer clinical trials [32–34], development of GM-CSF as an adjuvant in vaccines against infectious microorganisms has been limited by variability in effectiveness [35].
Due to the immunomodulatory effects and activation of skin immune cells by GM-CSF, we hypothesized that incorporation of this cytokine in MN patches containing influenza subunit vaccine will increase immunogenicity compared to vaccine alone. This is further motivated by prior findings that GM-CSF was identified as a potential adjuvant from a panel of cytokines and chemokines that were upregulated in the skin following insertion of influenza vaccine-coated MN arrays [36]. We tested our hypothesis in the BALB/c mouse model using both intradermal (ID) injection and MN patch delivery. We elaborate on previous work showing the efficacy of MN patches as an improved vaccination technology and develop a pipeline for testing novel adjuvants to enhance the potency of this emerging technology.
2. Materials and methods
2.1. Cells and virus stocks
Madin-Darby canine kidney (MDCK) cells (ATCC, Manassas, VA) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Thermo, Rockford, IL). Influenza virus stocks A/Brisbane/59/2007 (H1N1), A/California/07/2009 (H1N1), and A/Udorn/307/1972 (H3N2) were propagated in MDCK cells. Egg-grown subunit monovalent influenza vaccines, A/Brisbane/59/2007, A/California/07/2009, A/Christchurch/16/2010 and A/Victoria/210/2009 (A/Perth/16/2009-like) were generously provided by Seqirus (Maidenhead, UK). Vaccine processing for MN patch fabrication (reconstitution of lyophilized preparation and concentration), determination of protein concentration and assay of hemagglutinin (HA) content were carried out as previously described [13]. The following in-house MDCK-grown viruses were used for our studies: H1N1 A/California/07/2009, A/California/10/1978, A/FM/1/1947; and H3N2 A/Texas/50/2012, A/Victoria/210/2009, and A/Aichi/2/1968. Mouse adapted A/California/07/2009 and A/Udorn/307/1972 (H3N2) viruses were serially passaged in lungs of BALB/c mice. The LD50 was determined using the Reed-Muench formula [37] and the viral titers were determined by plaque assay [38]. Viruses were sequenced at Operon-MWG (Huntsville, AL) and Macrogen (Seoul, South Korea) and assembled via ClustalW alignment algorithms in MegAlign (DNASTAR Lasergene v7.0, Madison, WI) and BioEdit (Ibis Biosciences, Carlsbad, CA) software.
2.2. Animals
Eight-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) were bred and housed in a biosafety level 1 facility at Emory University’s Division of Animal Resources and viral infection experiments were performed on animals housed in a biosafety level 2 facility at Emory University’s Division of Animal Resources. All experiments were conducted in accordance with protocols approved by Emory University’s Institutional Animal Care and Use Committee (IACUC) in accordance with guidelines with the United States Federal Animal Welfare Act (PL 89-544) and subsequent amendments.
2.3. Immunizations and infections
Vaccines and adjuvant were prepared in sterile Ca2+ and Mg2+ deficient phosphate-buffered saline (PBS). Mice were intradermally (ID) vaccinated with a tuberculin syringe on the caudal site of dorsum with 1 or 3 μg HA H1N1 A/California/07/2009 subunit vaccine alone or 1 μg HA mixed with 100 ng recombinant murine GM-CSF (PeproTech, Rocky Hill, NJ). Mice were infected intranasally (IN) under isoflurane anesthesia with 25xLD50 (1500 p.f.u.) mouse-adapted A/California/07/2009 virus 2 months post vaccination.
The optimal GM-CSF concentration for microneedle (MN) vaccination was determined by an initial screen of GM-CSF doses (5, 20, 100 ng) administered ID with 1 μg HA of H3N2 (A/Victoria/210/2009 (A/Perth/16/2009-like)) subunit vaccine and was compared to 1 μg HA or 5 μg of the same vaccine without adjuvant. Sera was collected 90 days post vaccination (d.p.v.), and mice were infected IN with mouse adapted A/Udorn/307/1972 (H3N2) at a dose of 4xLD50 (35 plaque forming units (p.f.u.)) 4 months post vaccination.
The best route of adjuvanted vaccine delivery was selected by comparing magnitude and breadth of immune responses induced by MN, ID or IM immunization of mice with A/California/07/2009 subunit vaccine. Skin surfaces for MN patch vaccination were prepared as previously described [39]. Mice were anesthetized using xylazine/ketamine cocktail and MN patches were applied with direct pressure for 1 min and left in place for 20 min. Unused and used patches were analyzed by ELISA for vaccine and adjuvant content to determine delivery efficiency. Sera was collected 28, 90, and 120 d.p.v. Mice were infected with 25xLD50 (1500 p.f.u.) mouse-adapted A/California/07/2009 virus 4 months post vaccination.
Cellular immune responses in MN vaccinated cohorts of mice with blank patches or patches containing monovalent subunit vaccine (A/Christchurch/16/2010, a A/California/07/2009-like strain), at 1 μg HA, 3 μg HA, or 1 μg HA mixed with 100 ng recombinant murine GM-CSF. Mice were challenged with 10xLD50 (600 p.f.u.) homologous mouse-adapted A/California/07/2009 virus one month post vaccination. In survival studies, animals were monitored daily for morbidity (body weight loss, hunched posture, ruffled hair and decreased mobility) and mortality for 2 weeks. Mice that lost 25% of their weight were euthanized according to IACUC guidelines. An independent cohort of mice from each group was euthanized at 4 days post-infection to harvest lungs, spleens and lymph nodes.
2.4. Microneedle patch fabrication
MN patches were fabricated in a two-step process with polydimethylsiloxane (PDMS) molds as previously described [13]. To measure stability of GM-CSF in the presence of excipients a first-cast solution was prepared containing recombinant GM-CSF (PeproTech, Rocky Hill, NJ) in 3% w/v poly-vinyl alcohol (PVA, Millipore, Bellerica, MA) in combination with one or more of the following: 0.1% w/v bovine serum albumin (BSA, Sigma), 10% w/v trehalose (Sigma), 1% w/v carboxymethyl cellulose (CMC, Spectrum, New Brunswick, NJ). A second-cast solution was prepared containing 18% w/v PVA and 18% w/v sucrose (Sigma). MN patches were prepared by sequentially casting the first-cast solution to fill the mold cavities and the second-cast solution to cover the mold surface (corresponding to the MN patch backing). The MN patches were removed from the molds and attached to adhesive paper discs. Patches were inspected via light microscopy for integrity and uniformity. Blank placebo patches were made identically, except no antigen was in the first-cast solution. Influenza HA concentration was measured by a modified SRID assay [13, 40].
To measure stability of GM-CSF in the presence of influenza vaccine, the first-cast solution was prepared containing A/Brisbane/59/07 and murine recombinant GM-CSF in 3% w/v poly-vinyl alcohol (PVA; Sigma-Aldrich St Louis, MO) or in combination with 10% w/v trehalose (Sigma) and 3% PVA. Based on pilot delivery efficiency data after microneedle insertion [13], we encapsulated 20 ng GM-CSF per patch for a final working dose of 10 ng and 7 μg HA for a working concentration of 5 μg.
2.5. Bioactivity assays for GM-CSF
Bioactivity of GM-CSF was tested with proliferation assays using 2 cell types: murine bone marrow cells harvested from naïve female mice and TF-1 human erythroleukemia cell line (ATCC® CRL-2003, Manassas, VA). TF-1 cell growth and survival is dependent on GM-CSF or IL-3 [41]. Cells were treated with either solubilized or dried and reconstituted microneedle coating solutions mixed with GM-CSF. Bone marrow was collected from the femurs of two naïve mice, centrifuged at 1000 RPM for 7 min, and incubated in RBC Lysing Buffer Hybri-Max (Sigma-Aldrich, St. Louis, MO) for 10 min. Cells were washed and re-suspended in RPMI with 10% FCS and 1% Penicillin-Streptomycin RPMI (Corning Cellgro, Thermo Fisher Scientific, Waltham, MA). Bioactivity was tested in bone marrow cells using: (i) first-cast solution (with 10% trehalose) without GM-CSF, (ii) GM-CSF suspended in RPMI-10 (200 μl of 200 ng/μl) (iii) first-cast solution mixed with 1:1 with GM-CSF (to reach a final concentration of 100 μg), with GM-CSF and (iv) first-cast solution with GM-CSF dried in microcentrifuge tubes and re-suspended in 200 μl RPMI-10. After 2 days, cells were imaged via Axio Scope software (Carl Zeiss Microscopy, LLC, Thornwood, NY). Cell activation and proliferation was visualized with light microscopy throughout the incubation period. Every 2 days the cells were supplemented with RPMI-10. Following a 4-day incubation, we determined viable cells in proliferation with the colorimetric Promega Cell Titer 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI). An additional liquid vaccine formulation control was included to ensure that the fabrication process did not cause a loss in activity.
Bioactivity of GM-CSF (10 ng delivery concentration) encapsulated in MN patches was tested in TF-1 cells with formulations used in the mold: 1) 0.1% w/v albumin 2) 0.1% albumin and 10% w/v trehalose 3) 1% CMC w/v and 10% trehalose or 4) 1% w/v gelatin for 4 days. Three patches from each group were resuspended in 200 μl of RPMI-10 to overlay the cells. An additional liquid formulation control for each group was included to ensure that the fabrication process did not cause a loss in activity. As a second liquid control, we air-dried the same formulations in microtubes and reconstituted them in RPMI-10. At the end of 3-day incubation period cell proliferation was tested with Promega cell proliferation assay.
Subsequently bioactivity of GM-CSF with vaccine and MN excipients was measured in TF-1 cells by resuspending MN fabricated with GM-CSF (10 ng delivery concentration) and subunit influenza vaccine (A/Brisbane/59/2007) (5 μg HA delivery concentration) in RPMI-10. The following controls were used in TF-1 cell proliferation: 1) GM-CSF dissolved in 200 PBS μl 2) GM-CSF with 0.1% BSA in RPMI-10 3) GM-CSF with 10% trehalose in RPMI-10 4) GM-CSF with vaccine and 10% trehalose in RPMI-10. At the end of 3-day incubation period cell proliferation was tested with Promega cell proliferation assay.
2.6. Characterization and quantitation of humoral immune responses
Serum samples were collected at 14, 28, 56 or 60, 90 and 120 (d.p.v.). Antibody titers were quantified with ELISA as described previously with biotinylated anti-IgG, IgG1, IgG2 antibodies (Southern Biotech, Birmingham, AL) against 100 ng/well monovalent H1N1 A/California/07/2009 subunit vaccine, A/Christchurch/16/2010 subunit vaccine, or recombinant HA A/California/07/2009 from BEI Resources (Manassas, VA) [42]. Hemagglutination inhibition (HAI) assays were performed according to the WHO laboratory diagnostics manual using washed turkey red blood cells [43]. Virus-neutralizing (VN) antibody titers were determined by microneutralization assay for A/Perth/16/2009 and A/California/07/2009, as described previously [22]. IgG titers against chimeric HA proteins containing exotic HA head subtypes and commonly found seasonal HA stalks were measured to determine the antibody targets from H1N1 vaccinated mice; these chimeric proteins (cH6/1 and cH14/3) were created in a baculovirus system as described previously [44]. Influenza-specific antibody avidity was measured by ELISA in the presence of increasing concentrations (0–2.0 M) of the chaotropic agent guanidine thiocyanate (GTC) (Sigma), as previously described [42]. The avidity index was determined using Prism 7.03 Software (GraphPad, La Jolla, CA) by calculating the molar concentration of the chaotropic agent required to reduce the initial optical density by 50%.
2.7. Antigen-specific cellular activity in spleen, lungs, and bone marrow
Lymphocytes were isolated from spleen and lung tissue 7 and 14 d.p.v. and 4 days post infection (d.p.i.). Bone marrow was isolated from mice 60 days d.p.v. Antibody secreting cells (ASCs) were quantified by overlaying 1 × 106 cells/well in ELISPOT plates coated with 200 ng/well H1N1 A/Christchurch/16/2010. ASCs were incubated at 37 °C for 16 h, washed, and then influenza-specific antibodies were detected using isotype-specific, biotinylated murine Ig antibodies. Cytokine secreting cells (CSC) were quantified by overlaying 5 × 105 cells/well in ELISPOT plates (EMD Millipore, Burlington, MA) coated with 100 ng/well of capture antibody (BD Biosciences, San Jose, CA). Cells were incubated with 200 ng/well H1N1 A/Christchurch/16/2010 for 48 h at 37 °C. ASC and CSC plates were washed and incubated with 100 ng/well biotinylated detection antibodies (IL-4 and IFN-γ, BD Biosciences; IgA, IgM, and IgG, Southern Biotech, Birmingham, AL) and developed with streptavidin-HRP and diaminobenzidine. Spots were counted via ImmunoSpot Reader 5.0 (Cellular Technology Limited, Shaker Heights, OH), normalized to 1 × 106 cells/well and plotted with GraphPad Prism software.
2.8. Flow cytometry identification of activated cellular subsets
Skin draining (inguinal) lymph nodes were collected at 7 d.p.v. to evaluate the presence of activated T follicular helper cells (Tfh) and germinal center (GC) B cells by flow cytometry. Single-cell suspensions were incubated with anti-mouse CD16/32 antibody for 10 min on ice. Cells were then incubated with CD3ε (145-2C11), CD4 (GK1.5), PD-1 (29F.1A12), CXCR5 (L138D7), CD19 (6D5), Fas (Jo2) and GL7 (GL7) on ice for 30 min. Antibodies were purchased from BioLegend (San Diego, CA) and BD Biosciences (San Jose, CA). Cells were washed and fixed in 2% paraformaldehyde. Samples were acquired on a LSR II flow cytometer (BD Biosciences) and data were analyzed with FlowJo (FlowJo LLC, BD, Franklin Lakes, NJ) (Suppl Fig. 1).
2.9. Quantification of cytokines following infection
Lungs were weighed and homogenized in 0.5 ml DMEM and 1× Halt Protease Inhibitor. Lysates were diluted in 1× PBS and cytokines (GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-10, TNF-α) were quantified via a BioPlex Pro Mouse Cytokine 8-plex Assay (Bio-Rad, Hercules, California) at the Emory Vaccine Center Virology Core and normalized for dilution factor and original tissue concentration.
2.10. Measurement of lung viral titers
Viral titers in lung homogenates were quantified via plaque assay in MDCK cells [39]. Viral titers were assessed per gram of tissue.
2.11. Statistics
For ELISA, ELISPOT and cell-based assays, the statistical significance of differences between two groups was calculated by two-tailed unpaired Student’s t-test. Log2 converted HAI and VN titers were analyzed with one or two-way ANOVA with Bonferroni post-hoc test. Unless otherwise stated, antibody assays were performed in duplicate. For survival curves, statistics were calculated using a Log-rank (Mantel-Cox) test. Non-linear regression analyses were performed to determine the IC50 (95% confidence interval). A p-value < 0.05 was considered significant.
3. Results
3.1. Intradermal vaccination screen identified GM-CSF as a promising adjuvant in microneedle influenza immunization
The efficacy of GM-CSF as vaccine adjuvant for skin immunization was initially carried out with ID injection studies. The dose of 100 ng for GM-CSF was based on published literature [45]. The immune responses elicited to unadjuvanted A/California/07/2009 subunit vaccine at low or high dose (1 μg HA and 3 μg HA respectively) were compared to those induced by adjuvanted low dose vaccine (Fig. 1). Mice that received the adjuvanted vaccine demonstrated 4-fold higher anti-A/California/07/2009 HAI titers (p = 0.07) when compared to mice vaccinated with low dose unadjuvanted vaccine and similar HAI titers compared to the high-dose vaccinated group (p = 0.025) at 8 weeks post-vaccination (Fig. 1A).
Fig. 1.

Adjuvantation with 100 ng GM-CSF in intradermal H1N1 A/California/07/09 subunit vaccination improves antibody responses and enhances protection to lethal virus challenge. BALB/c mice (n = 4) were vaccinated ID with PBS, 1 μg HA, 3 μg HA, or 1 μg HA/100 ng GM-CSF. (A) Serum influenza-specific HAI titers are plotted at 8 weeks post-vaccination. Statistics for panel (A) were performed with Mann-Whitney test. Mice were challenged with 25xLD50 homologous virus 3 months post-vaccination and (B) survival and (C) body weight changes were recorded for 13 days. Percent body weight values are expressed as mean ± SEM. Statistics for panel (B) were performed with Mantel-Cox test.
Infection of all vaccinated groups, 2 months post-vaccination with 25xLD50 homologous virus, conferred complete protection of the GM-CSF/1 μg HA and the unadjuvanted 3 μg HA vaccine groups (p = 0.04 when comparing survival rates to unadjuvanted 1 μg vaccine group) (Fig. 1B) and minimal morbidity with 5% body weight loss by day 6 (Fig. 1C). In contrast, the group immunized with low-dose unadjuvanted vaccine showed 80% mortality and 20% body weight loss by the survivors. We observed that addition of GM-CSF as an adjuvant can boost protective immune responses comparable to a three-fold higher vaccine dose, thereby conserving the amount of vaccine required for protection.
H3N2 influenza A viruses are known to cause significant disease burden worldwide and the H3N2 strains in inactivated trivalent influenza vaccine have lower efficacy than the H1N1 strains. Hence, we assessed GM-CSF’s ability to boost immune responses to a monovalent H3N2 subunit vaccine using a series of GM-CSF concentrations to optimize the adjuvant dose-response curve. Mice were ID vaccinated with 1 μg HA or 5 μg HA H3N2 A/Victoria/210/2009 (A/Perth/16/2009-like) and a range of GM-CSF concentrations (5, 20, 100 ng) (Fig. 2). Administration of 100 ng GM-CSF with 1 μg HA vaccine doubled the VN titers when compared to unadjuvanted low-dose vaccine at 90 d.p.v. (Fig. 2A). Following infection with 4xLD50 of A/Udorn/307/1972 4 months post-vaccination, the co-delivery of 100 ng GM-CSF increased survival rates (Fig. 2B) and prevented significant body weight loss of infected survivors (Fig. 2C) relative to mice that received a low-dose vaccine alone. Thus, we demonstrated that the adjuvanted formulation can improve protective immunity in a second clinically significant influenza subtype.
Fig. 2.
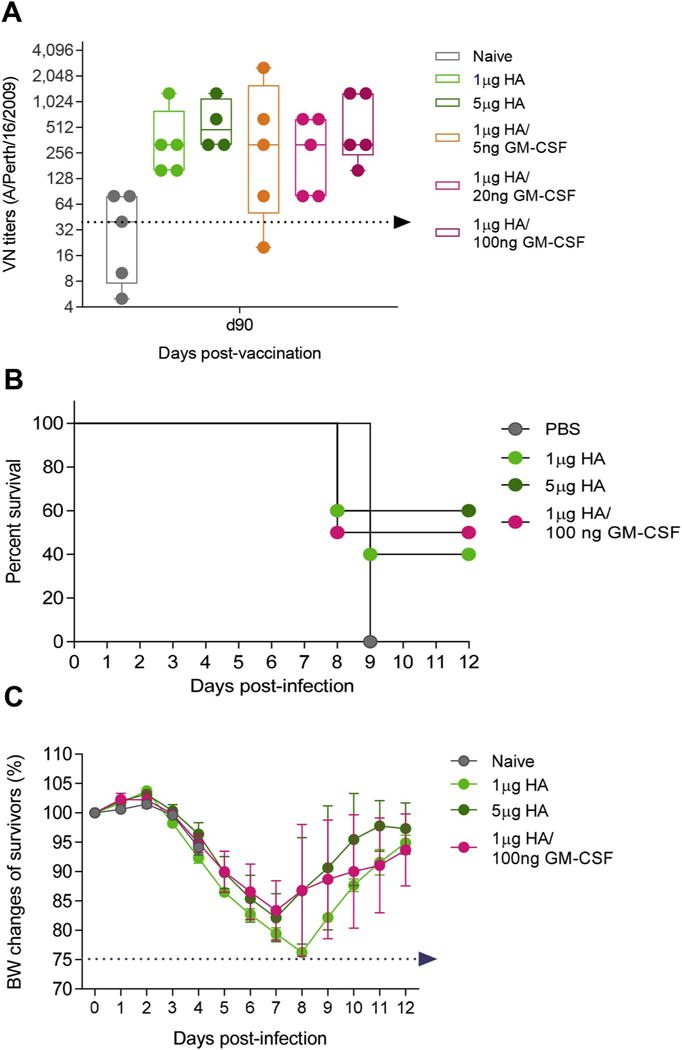
GM-CSF is an effective adjuvant at low doses in H3N2 vaccinations. Mice were vaccinated ID with 1 μg HA H3N2 A/Victoria/210/2009 with a range of GM-CSF concentrations: 5 ng, 20 ng, and 100 ng. A high vaccine dose (5 μg) was used as a control for superlative immune responses. Serum virus-neutralizing (VN) titers were assessed at 90 d.p.v. Vaccinated mice were challenged with 4xLD50 mouse-adapted H3N2 A/Udorn/307/1972 for (C) survival and (D) body weight changes. VN titers were expressed as GMean ± 95% CI. Percent body weight values are expressed as mean ± SEM. Statistics for survival were performed with Mantel-Cox test.
3.2. Effect of MN patch formulation on GM-CSF’s ability to induce proliferation of bone marrow cells
The proliferative capacity of recombinant murine GM-CSF was initially tested in bone marrow cells (Fig. 3A). Cells were stimulated with increasing concentrations of GM-CSF (0.6–400 ng per well) and supernatants were collected at 60 and 120 min of incubation to test for proliferation. We observed a time-dependent and dose-dependent response that was more significant above concentrations of 25 ng of GM-CSF/well. The maximum bone marrow proliferation occurred after 120 min of cellular exposure at 200 ng GM-CSF.
Fig. 3.
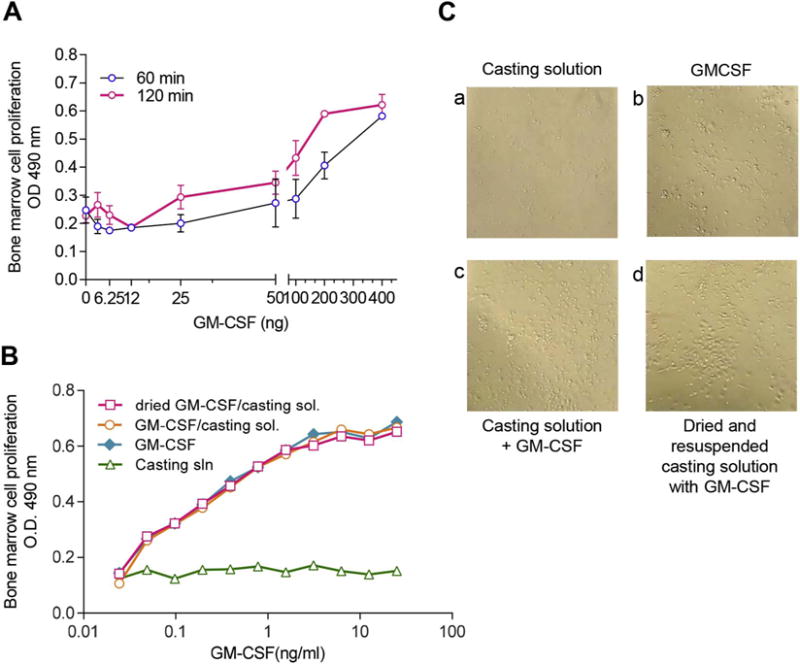
GM-CSF retains proliferative capacity in bone marrow cells following MN patch fabrication and dissolution. (A) Dose-responsive cellular activation of bone marrow cells by murine GM-CSF in PBS at 60 and 120 min. (B) Bone marrow cells (5 × 105 cells/well) from naïve mice were incubated for 48 h with (a) “first-cast” casting solution, (b) GM-CSF dissolved in PBS, (c) GM-CSF mixed 1:1 with casting solution, or (d) GM-CSF in casting solution air-dried in microcentrifuge tubes and reconstituted in PBS. Bioactivity was measured by determining cell proliferation at 48 h post-exposure. (C) Bone marrow cells grown in tissue culture were imaged at 48 h post-exposure to GM-CSF in formulations described in (B).
Since MN patch fabrication may affect the bioactivity of GM-CSF, we measured the ability of GM-CSF to induce activation and proliferation of bone marrow cells before and after incorporation and drying in casting solutions used to fabricate MN patches (Fig. 3B). Bone marrow cells in culture were overlaid with casting solution alone (Fig. 3Ba), murine recombinant GM-CSF dissolved in PBS (Fig. 3Bb), GM-CSF dissolved in first-casting solution (Fig. 3Bc) and GM-CSF in first-casting solution, air dried and resuspended in culture medium (Fig. 3Bd). Visualization of cells with light microscopy showed that inclusion of GM-CSF in the first-casting solution in either liquid or dried formulation caused distinct morphological changes in bone marrow cultures similarly to GM-CSF dissolved in PBS, suggesting that the cytokine retained its biological activity. We also found that the same formulations induced bone marrow cell proliferation and increased the overall cell numbers 4-fold compared to the control (Fig. 3C). Thus, GM-CSF remained stable upon casting processes fundamental to MN production and retained the capacity to proliferate bone marrow cells following reconstitution.
3.3. Stability and bioactivity of GM-CSF following MN patch fabrication with excipients
We next analyzed whether GM-CSF (10 ng delivery concentration) would retain bioactivity following incorporation in MN patches using different formulations. The formulations included trehalose CMC, or gelatin, which have previously been used as structural and stabilizing excipients in MN patch fabrications [13, 46] and BSA alone or mixed with trehalose. Bioactivity of reconstituted patches was compared to lyophilized GM-CSF dissolved in PBS and from reconstituted dried solution. Bioactivity was determined by overlaying solutions on TF-1 cells and measuring proliferative capacity (Fig. 4A). Among the formulations studied, MN patches containing 10 ng GM-CSF and fabricated using casting solutions comprised of 0.1% albumin and 10% trehalose generated the highest cellular proliferation within the standard curve (0.31–20 ng/ml). Formulations that induced the highest cellular proliferation in Fig. 4A were reformulated with vaccine, excipients, and GM-CSF in order to validate bioactivity following co-incorporation of vaccine and adjuvant (Fig. 4B).
Fig. 4.

GM-CSF retains bioactivity following fabrication with a range of MN excipients. (A) The effect of first-cast solution excipients on GM-CSF stability and bioactivity was assessed by the proliferative capacity of GM-CSF using TF-1 cells. Triplicate MN patches containing 10 ng GM-CSF fabricated using various excipients in the first-cast solution were dissolved in culture medium and overlaid on TF-1 cells. They were compared to GM-CSF in fresh solution (Sol A–D) or air-dried and reconstituted in RPMI formulations (Dried A–D). (B) The effect of influenza vaccine (5 μg HA delivery dose) on GM-CSF bioactivity when co-formulated in MN patches was assessed by TF-1 cell proliferation and compared to patches with GM-CSF alone. OD values for panels (A) and (B) are expressed as mean ± SEM. Dotted lines indicate upper and lower detection limits of the assay as determined by a standard curve, and “sol” and “dried” indicate casting solutions that were directly applied to TF-1 cells or dried and reconstituted before application. Each sample was run in duplicates in TF-1 cells. Images of MN patches containing sulforhodamine red dye were taken (Ca) before and (Cb) after application to skin in mice.
Next, we tested the bioactivity of 10 ng GM-CSF mixed with subunit influenza vaccine (A/Brisbane/59/2007) (5 μg delivery concentration) and encapsulated in MN patches to enhance TF-1 cell proliferation. These patches were dissolved in RPMI-10 and overlaid on TF-1 cells for 3 days. Bioactivity was compared to patches containing GM-CSF alone, GM-CSF formulated with BSA or trehalose and GM-CSF mixed with vaccine and trehalose (Fig. 4B). Cells cultured in the presence of RPMI-alone were the negative control group. Each group of reconstituted patches was compared to the same formulation in solution. Since reconstituted patches induced cell proliferation at similar levels, we concluded that the formulation that stabilized the adjuvants was also suitable for influenza vaccine.
Mechanical stability of MN patches formulated for GM-CSF delivery was also studied. MN patches containing a red dye were fabricated using a casting solution comprising 0.1% albumin and 10% trehalose and applied to skin. Before application, the red dye was localized mostly in the MNs and not in the patch backing (Fig. 4Ca). After application to the skin, the MNs (and red dye) dissolved in the skin (Fig. 4Cb). Altogether, these results demonstrate that MN patches were fabricated with GM-CSF that retained bioactivity and could be used to puncture and dissolve in skin.
3.4. Humoral and protective immune responses following delivery of recombinant murine GM-CSF-adjuvanted H1N1 vaccine
We previously demonstrated that MN patch delivery of antigen in skin can augment immune responses to influenza vaccination. The goal of this study was to determine if incorporation of GM-CSF into MN patches could induce superior and dose-sparing immune responses compared to conventional vaccination (IM or ID) without adjuvant. Mice were vaccinated either cutaneously (MN patches or ID injection) or systemically (IM injection) with the same vaccine (H1N1 A/California/07/2009) whereas the GM-CSF dose was 100 ng per patch and 200 ng for the IM and ID routes as determined in the in vitro bioactivity studies (Fig. 3).
While MN patch vaccination generated superior vaccine-specific IgG responses when compared to IM and ID routes at 28, 90 and 120 d.p.v., we observed no differences in IgG titers among low- or high-dose unadjuvanted vaccine or in the presence of GM-CSF (Fig. 5A). In contrast, the immunopotentiating effects of GM-CSF were demonstrated in antibody cross-reactivity of MN patch vaccinated mice against a heterologous (homosubtypic) seasonal influenza strain H1N1 A/New Caledonia/20/1999 (Fig. 5B) and the heterosubtypic H3N2 A/Brisbane/10/2007 strain (Fig. 5C). The adjuvanted vaccine induced equivalent antigen-specific IgG serum titers to high vaccine doses at 120 d.p.v. Notably, vaccination in the presence of 2-fold higher dose GM-CSF generated comparable responses across ID and IM groups at 75% lower titers than those achieved with MN patch vaccination.
Fig. 5.
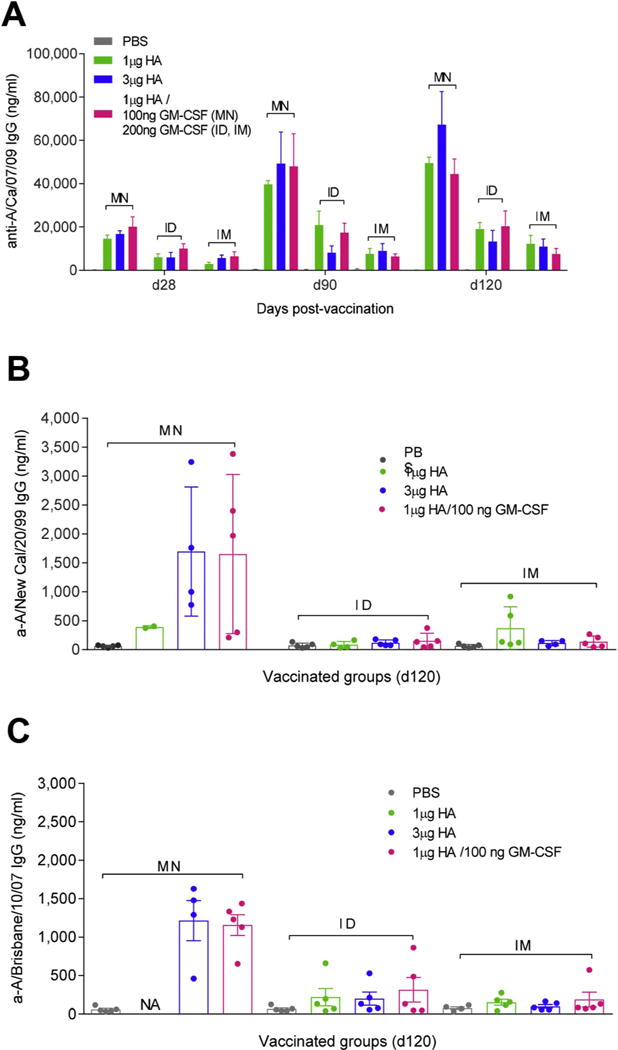
The inclusion of GM-CSF in MN patches in H1N1 influenza vaccination is dose-sparing and generates superior IgG expression against homologous and heterologous HAs compared to ID and IM vaccination. BALB/c mice were vaccinated with PBS, 1 μg HA, 3 μg HA A/California/07/2009 subunit vaccine or 1 μg HA mixed with 100 ng murine GM-CSF in MN patch and 200 ng in solution for IM and ID injection. Serum IgG titers against (A) H1N1 A/California/07/2009 are plotted for days 28, 90, and 120 d.p.v. (B) H1N1 A/New Caledonia/20/1999-specific and (C) H3N2 A/Brisbane/10/2007-specific IgG titers were determined at 120 days post-vaccination. Values are expressed as mean ± SEM.
These data indicate that MN patch vaccination induced robust long-lived antibody responses, and that co-administration with GM-CSF further potentiated heterologous immune responses after MN patch vaccination, but not after ID or IM vaccination. Thus, the combination of MN patch vaccination and GM-CSF adjuvantation may play an important role on increasing breadth of immunity.
While all vaccinated groups showed protection (higher than 80%) (Fig. 6A, C, E) following infection with homologous virus 4 months post-vaccination, only the MN group demonstrated least body weight losses (Fig. 6B) compared to the ID group (Fig. 6D) or IM group (Fig. 6F). Contrary to the initial ID vaccination data (Fig. 1) where administration of GM-CSF with low dose of vaccine improved protection compared to vaccine alone, in this experiment all vaccinated groups irrespectively of route of administration survived lethal infection. These findings are likely due to antibody maturation, considering that the infection study in this experiment took place 4 months post vaccination compared to 2 months in the initial ID vaccination study.
Fig. 6.
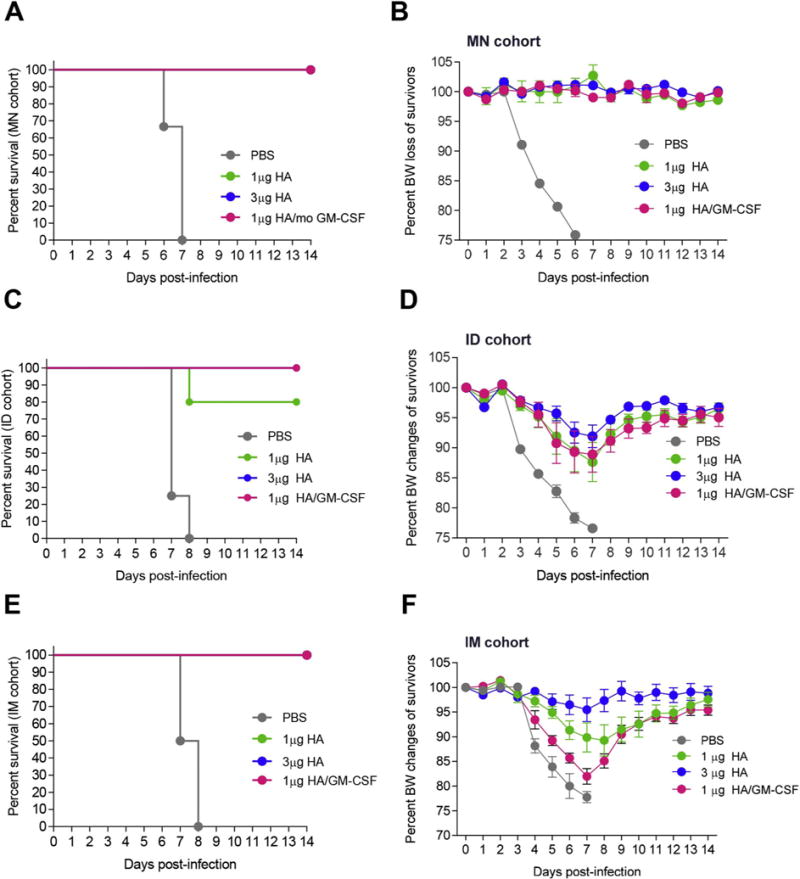
H1N1 subunit vaccination with GM-CSF improved protection of mice from lethal H1N1 influenza challenge. Mice vaccinated with a single dose of A/California/07/2009 subunit vaccine via MN (A, B), ID (C, D), or IM (E, F) routes were challenged 17 weeks post-vaccination with 25xLD50 homologous H1N1 A/California/07/2009 virus. Survival rates (A, C, E) and body weight changes (B, D, F) were recorded for 14 days post-challenge. Percent body weight values are expressed as mean ± SEM.
3.5. Longevity of antibody responses to MN vaccination with subunit influenza vaccine and GM-CSF
To assess the potential of GM-CSF to enhance long-term humoral and cellular immune memory, we fabricated MN patches containing low dose, high dose or low dose H1N1 vaccine (A/Christchurch/16/2010, A/California/07/2009-like) with GM-CSF in an experimental design similar to the initial screening experiments in which we demonstrated superior immune responses after MN patch vaccination compared to the needle and syringe approach (Fig. 7A).
Fig. 7.
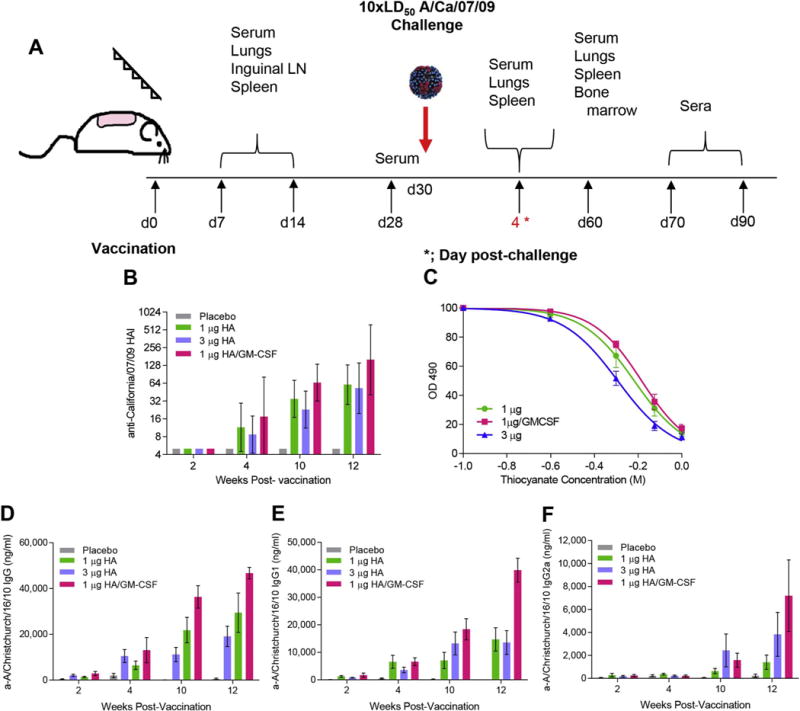
GM-CSF improved antibody responses and avidity when included in MN patch vaccination. (A) Cohorts of BALB/c mice were vaccinated with MN patches containing vaccine formulation solution (placebo), 1 μg HA, 3 μg HA, or 1 μg HA of A/Christchurch/16/2010 mixed with recombinant murine GM-CSF. A total of 100 mice (n = 25 per group) were used for this study. Serum samples were collected and analyzed at the time points depicted. (B) HAI titers against A/California/07/2009 virus (GMean ± 95% CI), and (C) antibody avidity against A/Christchurch/16/2010 subunit vaccine (n = 5). Serum binding A/Christchurch/16/2010-specific antibody titers were assessed with ELISA; (D) IgG, (E) IgG1, and (F) IgG2a (n = 5). Statistics were performed with Mann-Whitney test. Antibody values are expressed as mean ± SEM.
We found that GM-CSF-adjuvanted patches increased by 2-fold A/California/07/2009-specific HAI titers up to 12 weeks post-vaccination (Fig. 7B) and enhanced IgG binding avidity to subunit vaccine (Fig. 7C), showing improved antibody specificity for the influenza antigen compared to non-adjuvanted patch vaccination. Addition of GM-CSF to the low-dose vaccine formulation augmented more than two-fold the levels of anti-A/Christchurch/16/2010 IgG antibodies when compared to the same dose of vaccine alone (p = 0.004 at 10 weeks and p = 0.001 for 12 weeks post-vaccination) (Fig. 7D). When comparing the IgG1 titers between the same groups, we found a 2-fold difference by week 10 post-vaccination (p = 0.05), which was further increased by week 12 (p = 0.003). Notably, the adjuvanted formulation also induced 4-fold higher IgG1 responses than the unadjuvanted high-dose vaccine by week 12 (p = 0.003) (Fig. 7E). Although we did not see similar statistically significant differences in IgG2a titers among vaccinated groups due to a higher spread of responses, the antibody levels followed the same trend as in IgG1 subclass (Fig. 7F). Thus, the inclusion of GM-CSF in MN patches increased the production, specificity and duration of antibodies in response to influenza vaccination.
Given the increase in long-term antibody expression for GM-CSF-adjuvanted MN patches, we also investigated whether mice vaccinated against influenza virus with GM-CSF had increased systemic B cell activation and memory responses. Indeed, co-administration of vaccine with GM-CSF doubled the number of influenza-specific IgM-secreting cells (Fig. 8A) and tripled the number of influenza-specific IgG-secreting cells (Fig. 8B) relative to non-adjuvanted low dose vaccine at 14 d.p.v. Most importantly, GM-CSF increased the number of vaccine-specific IgG-secreting cells in the bone marrow 60 d.p.v by 50% compared to all non-adjuvanted groups (Fig. 8C–D). These results, combined with serological analysis, demonstrate that GM-CSF improved the development of humoral immunity through increased antibody avidity and IgG subtype expression, systemic IgM and IgG cell activation, and maintenance of vaccine-specific IgG-secreting cells in the bone marrow.
Fig. 8.
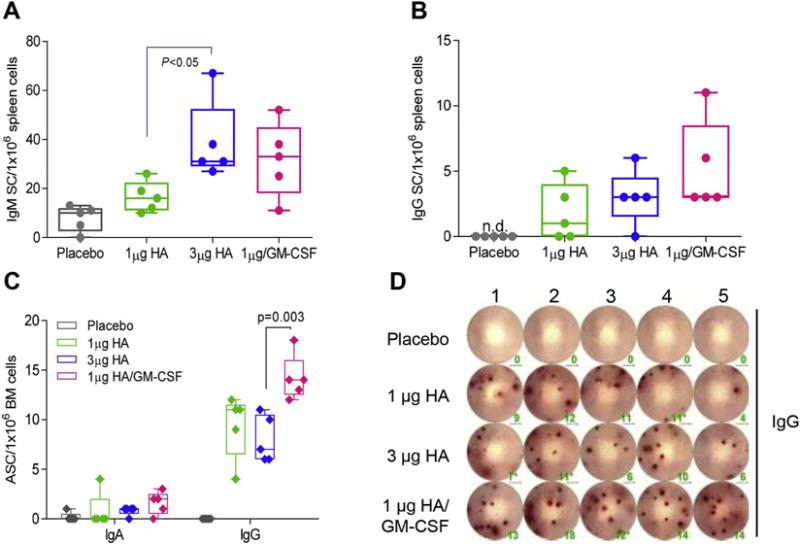
Inclusion of GM-CSF enhanced activation of B cells in spleen and bone marrow. Lymphocytes were isolated from the spleen 14 days post-MN vaccination to enumerate vaccine-specific ASCs. (A) IgM-secreting cells and (B) IgG-secreting cells were measured in ELISpot plates coated with 200 ng vaccine/well and incubated for 16 h post isolation. (C, D) Bone marrow (BM) was harvested from mice 60 days post-MN vaccination to quantify vaccine-specific IgG SCs. All ASCs were enumerated in ELISPOT plates coated with A/California/07/2009 recombinant protein and normalized per 1 × 106 lymphocytes or BM cells respectively.
3.6. T cell activation in the inguinal lymph nodes (ILN) and spleen
Protective immune responses to influenza virus infection require both antibody responses to neutralize free virus and facilitate its opsonization and virus-specific cellular responses to kill infected cells and coordinate immune responses [47]. Healthy adults expressing higher numbers of vaccine-specific CD8+ IFN-γ-secreting cells were less likely to have enhanced viral pathogenesis during the 2009 H1N1 pandemic [48]. Thus, we investigated whether GM-CSF-adjuvanted vaccination could boost the number of activated T cells in draining inguinal lymph nodes (ILN) and the numbers of systemic vaccine-specific cytokine secreting cells. GM-CSF adjuvant-containing patches tripled the frequency of activated follicular CD8+ T cells (CD8+CXCR5+PD-1+) as early as day 7 (Fig. 9A–E). In contrast, GM-CSF did not increase the frequency of CD4+ T follicular helper cells (CD4+, CXCR5+, PD-1+) (Suppl. Fig. 2A). These findings suggest that GM-CSF adjuvant improves protective immunity following vaccination by enhancing activation of virus-specific CD8+ cytotoxic lymphocytes (CTLs).
Fig. 9.
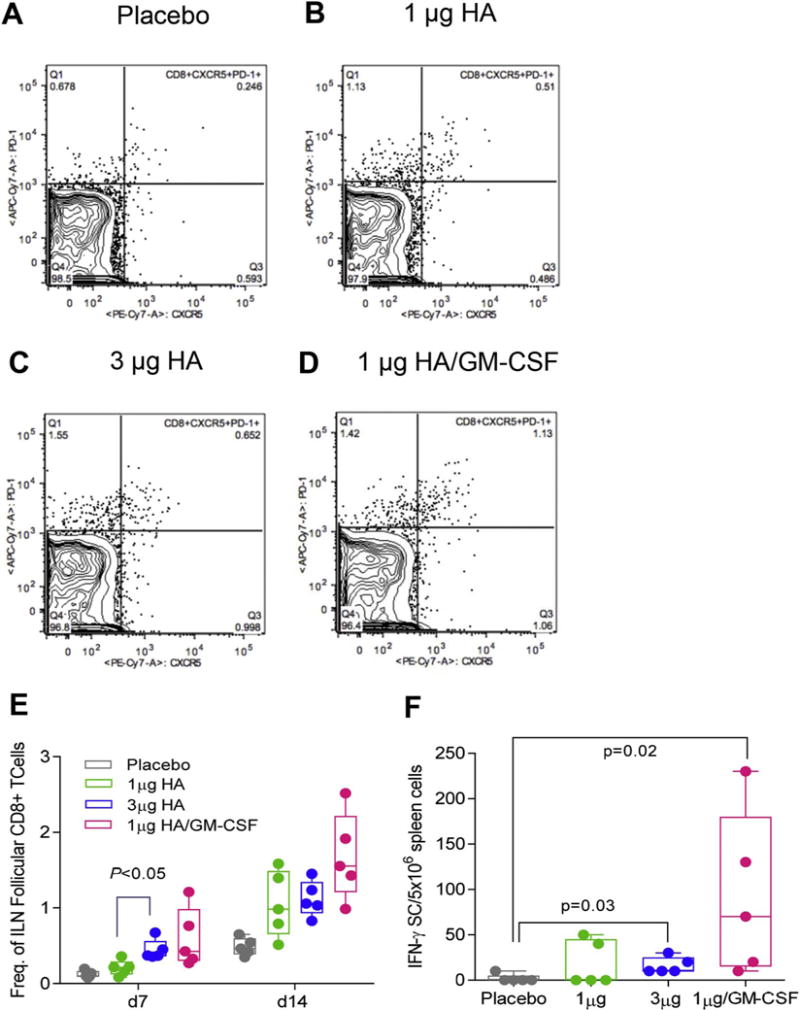
MN patch vaccination with GM-CSF increased CD8+ T cell responses in inguinal lymph nodes (ILN) and vaccine-specific IFN-γ responses in the spleen. Inguinal lymph nodes and spleens were harvested from mice 7 and 14 d.p.v. and measured for activation and vaccine-specific cytokine secretion. CD8+ follicular helper cells (CD8+CDXCR5+PD-1+) were determined for each sample, and representative images for (A) placebo, (B) 1 μg HA, (C) 1 μg HA, and (D) 1 μg HA are shown. (E) Frequency of CD8+ follicular helper cells in ILN were quantified in FlowJo and plotted in GraphPad Prism 7. (E) Vaccine-specific IFN-γ were isolated from the spleen at day 7 and enumerated in ELISPOT plates following stimulation with A/Christchurch/16/2010 subunit vaccine. Values are expressed as mean ± SEM and statistics were performed with Mann-Whitney test.
To determine whether inclusion of GM-CSF in MN patches leads to the increased generation of vaccine-specific T cells, we enumerated vaccine-specific IFN-γ and IL-4 producing lymphocytes in ILN at 7 and 14 d.p.v. By day 14, the GM-CSF/low-dose vaccine group induced a 3-fold increase in the number of IFN-γ secreting lymphocytes when compared to placebo (p = 0.02), low dose (p > 0.05) and high dose vaccine groups (p = 0.03) (Fig. 9F). Mice receiving adjuvanted vaccine showed a 50% enhancement in the number of IL-4 secreting lymphocytes (Suppl. Fig. 2B). Thus, in addition to previous results demonstrating the increase of vaccine-specific antibodies, the use of GM-CSF as an adjuvant in MN patches also exerted a positive effect on CTL activation and vaccine-specific IFN-γ and IL-4 secreting cell numbers, providing a range of cellular and humoral immune responses for an effective response to influenza viral infection.
3.7. Antibody response to H1N1 and H3N2 viruses
Effective influenza vaccination must demonstrate long-lasting and broadly neutralizing immune responses against commonly circulating influenza virus strains in order to reduce disease burden [49]. Breadth of immunity adds value to current seasonal influenza vaccines and it is the goal of successful universal vaccine candidates [50]. In order to measure the range of influenza-specific antibody responses elicited in animals that received the vaccine alone or in combination with GM-CSF, serum collected 3 months post-vaccination was tested for binding to various HAs within the same subtype (H1N1, group 1) or different subtype (H3N2, group 2).
With the exception of significantly higher antibody IgG titers to a homologous-like virus (anti-A/California/07/09) (Fig. 10A), the GM-CSF adjuvanted vaccine generated comparable IgG titers to un-adjuvanted vaccine against homosubtypic strains (Suppl. Fig. 3A, B). Interestingly, the adjuvanted vaccine induced 3-fold increase of IgG antibodies against 3 circulating H3N2 strains; A/Texas/50/2012 (Fig. 10B), A/Victoria/210/2009 (Suppl. Fig. 3C), A/Aichi/2/1969 (Suppl. Fig. 3D) relative to non-adjuvanted vaccine. Using recombinant baculovirus proteins containing rare HA heads (H6 and H14) and common seasonal stalks (H1 and H3), it was possible to determine whether the inclusion of GM-CSF as an adjuvant increased the number of antibodies able to bind to H1 and H3 stocks. While the combination of GM-CSF with low vaccine dose did not increase the antibody response to H1 stalks compared to vaccine alone (Fig. 10C), it significantly increased the titer of antibodies binding to H3 stalks (Fig. 10D). These data suggest that the mechanism by which GM-CSF adjuvants increase antibody binding to H3N2 viruses is likely related to enhanced reactivity with H3 stalk rather than the H3 head or receptor binding domain. Thus, co-administration of GM-CSF with a licensed influenza vaccine encapsulated in MN patches generated antibodies with significant cross-reactivity, demonstrating the potential of this adjuvant to expand immune protection across various influenza subtypes.
Fig. 10.
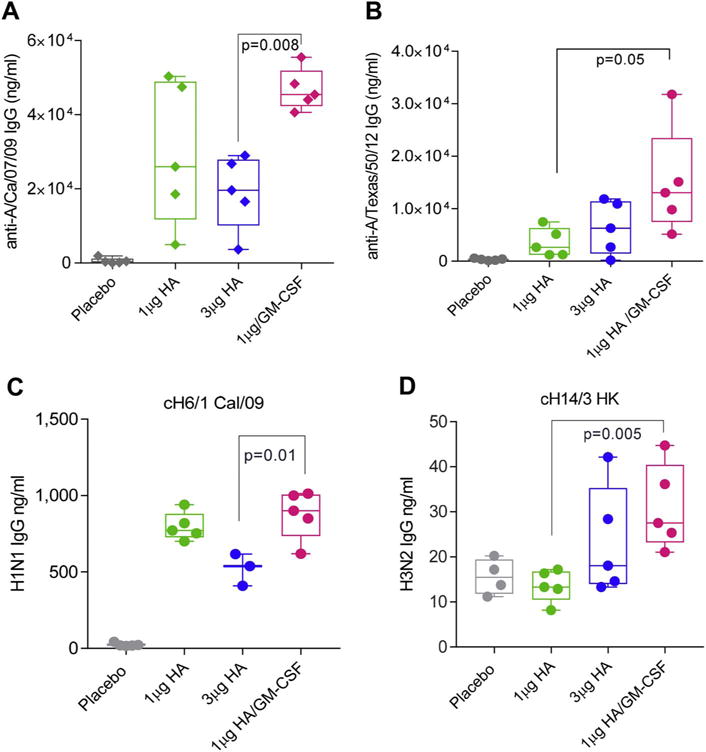
GM-CSF adjuvanted MN patch immunization improved cross-reactive neutralization responses between homologous and heterologous viral strains. Serum was collected from mice 90 days post-A/Christchurch/16/2010 vaccination with dissolving MN. IgG reactivity with monovalent subunit vaccines for (A) H1N1 A/California/07/2009 and (B) H3N2 A/Texas/50/2012 was measured with ELISA. IgG reactivity with chimeric HA proteins was measured with ELISA against (C) H1 stalks (cH6/1 Cal/09) and (D) H3 stalks (cH14/3 HK). Statistics performed in Mann-Whitney test for comparison of 1 μg HA/GM-CSF vs. 1 μg HA groups.
3.8. Viral growth and immune responses to lethal challenge following MN patch vaccination with GM-CSF adjuvant
To determine the effect of skin vaccination with GM-CSF on protective immunity and identify the implicated mechanisms, mice were infected 30 days post-vaccination with 10xLD50 A/California/07/2009. Survival and weights changes were monitored for 14 days post-infection. A second infected cohort of mice was sacrificed at 4 days post-infection to determine viral titers and cytokine profiles in the lungs and assess vaccine effectiveness in accelerating viral clearance and reducing virus-induced inflammation. Although all vaccinated groups maintained their weight and survived lethal infection (data not shown), immunization with adjuvanted vaccine induced a 2.7-log reduction in viral titers when compared to mice vaccinated with placebo MN patches (p = 0.02), whereas immunization with high-dose vaccine was 42% less effective (2.5-log reduction in viral titers) (p = 0.02). Mice vaccinated with low-dose vaccine in the absence of GM-CSF had one-log decrease in titers compared to placebo infected mice (p = 0.03) (Fig. 11A).
Fig. 11.
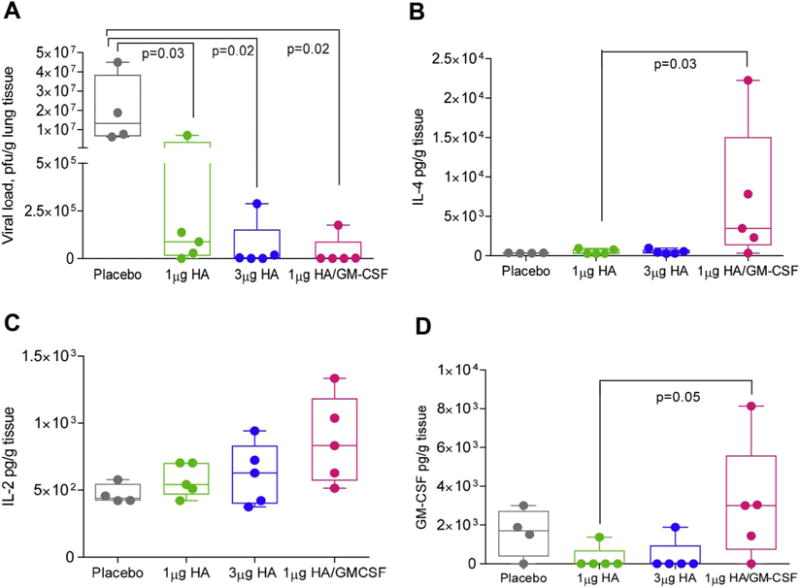
Th1 cytokine responses to viral infection were increased in GM-CSF-adjuvanted MN vaccinated mice. Mice vaccinated with A/Christchurch/16/2010 MN were infected with 10xLD50 mouse-adapted A/California/07/2009 virus 30 d.p.v. (A) Viral load (p.f.u.) was measured in lung lysates 4 days post-infection. Cytokine expression in lung lysates was quantified in a Bio-plex Pro Mouse Cytokine 8-plex assay. (B) IL-4 (C) IL-2 and (D) GM-CSF levels. Values are expressed as mean ± SEM. Analysis of Mann-Whitney test between 1 μg HA and 1 μg HA/100 ng GM-CSF reached statistical significance of p < 0.5 in ELISPOT assays for IL-4 and GM-CSF secretion.
Lung lysates taken from vaccinated mice 4 days after lethal infection and tested for eight major Th1, Th2 and inflammatory cytokines showed significant expression of IL-4, IL-2 and GM-CSF. These cytokines were significantly elevated following lethal infection of mice that received adjuvanted vaccine when compared to the other vaccinated groups (Fig. 11B–D). Early expression of GM-CSF in the lungs has been shown to promote alveolar macrophage activation and recruitment in order to reduce viral replication [51, 52]. These findings may explain efficient viral clearance seen shortly after infection (Fig. 11A). In contrast, IL-2 and IL-4 play important roles in reducing CTL-mediated immunopathogenesis [53] and promoting tissue repair through activation of innate lymphoid cells, respectively [54]. These studies point to a potential role of GM-CSF in influenza vaccination by MN patch to elicit a unique signature of cytokines that promote elimination of virus from the respiratory compartment while reducing byproduct inflammation and tissue damage due to an overactive immune system.
4. Discussion
Skin immunization is an innovative route of vaccination which takes advantage of a potent network of antigen-presenting cells (APCs) and other innate immune cells in skin that can interact with naive T and B cells proximal to the site of vaccination in draining lymph nodes [55–58]. We have previously reported the importance of langerin+ dendritic cells (DCs) in modulating adaptive immune responses following cutaneous MN vaccination [59]. GM-CSF can recruit and activate APCs [60] and is a potent inducer of NF-κB expression and nitric acid synthase in epithelial DCs [61]. CD103+ langerin+ DCs have shown GM-CSF dependent activation of follicular CD4+ T helper cells that subsequently led to the expression of IFN-γ and IL-17 [62]. Thus, the presence of GM-CSF as an adjuvant is critical for enhancing the potency of vaccines with limited immunogenicity, such as the licensed subunit influenza vaccine, by promoting antigen capture and DC and T cell activation and regulation [63, 64].
In this study, we investigated the role of GM-CSF to increase the efficiency of skin dendritic cells APCs and the recruitment and priming of antibody-secreting B cells and CD8+ follicular T cells in primary and secondary lymphoid tissues. Here we demonstrate stable co-incorporation of GM-CSF with influenza vaccine in MN patches that can be used to enhance the vaccine-specific humoral and cellular immune responses. We identified that ID administration of GM-CSF with H1N1 or H3N2 vaccines, improved antibody responses and protection against viral challenge [65, 66]. We then identified the formulations for efficient vaccine and adjuvant co-encapsulation within MN patches and delivery in the skin. We also identified adjuvant concentrations required for cellular activation, cytokine stabilization and bioreactivity.
Importantly, we demonstrated the superiority of MN patch vaccination over ID and IM vaccination in enhancement of vaccine-specific antibody responses. Mice cutaneously immunized with H1N1 influenza vaccine and GM-CSF as adjuvant via ID and MN routes showed increased protection with lower morbidity and mortality rates following lethal infection, a phenomenon not seen in IM vaccination. These findings suggest that GM-CSF has unique properties as an adjuvant within the skin that can be exploited when developing MN patch delivery technologies.
Examination of the immune response to GM-CSF as an adjuvant showed increased activation of antigen-specific IFN-γ secreting cells following vaccination, which may explain the persistence of IgG2a antibodies 4 months post-vaccination [67]. Following lethal infection, mice vaccinated against H1N1 virus in the presence of GM-CSF effectively controlled viral growth. Importantly, GM-CSF-adjuvanted H1N1 vaccination improved serum cross-reactivity with a homosubtypic (H1N1) and heterosubtypic (H3N2) viral strains. The broadly cross-reactive antibodies elicited in the presence of adjuvants were directed against the hemagglutinin stalk region. Ultimately, influenza immunization with a trivalent or quadrivalent vaccine co-encapsulated with appropriate adjuvants such as GM-CSF in a MN patch could significantly reduce disease burden due to strain mismatch or drift within an influenza season.
The role of GM-CSF in driving more potent humoral or cellular immune responses has been debated. Previous DNA vaccination studies in rhesus macaques showed that influenza HA responses were improved by the inclusion of recombinant rhGM-CSF [68]. Intramuscular vaccination with GM-CSF encoded in a viral vector increased the frequency of APCs but decreased the antigen-specific CD8+ T cell responses in the draining lymph node [69]. In our studies, MN patch vaccination with GM-CSF increased the number of CD8+ follicular T cells in ILN, highlighting the usefulness of administering this cytokine as an adjuvant during vaccination. Our findings are in alignment with data reported by Chou et al. on co-delivery of GM-CSF with hepatitis B subunit vaccine to skin in a biodegradable hydrogel [70]. The authors demonstrated that presence of GM-CSF increased antibody response to the vaccine and vaccine-specific T cell proliferation in addition to improved activation of APCs in the draining lymph node.
GIFT fusokines, engineered to have peptides from multiple cytokines, have been generated to combine the APC stimulation capabilities of GM-CSF with T cell differentiation signaler IL-4 [71]. These fusokines have shown promise in prophylactic HIV vaccination studies in guinea pigs when anchored via glycolipids to virus-like particles (VLPs) [72]. These reports and our present findings suggest that the potency of GM-CSF as an adjuvant is dependent on administration route and formulation. To our knowledge, this is the first example of the use of MN patches to deliver a bioreactive cytokine as an adjuvant that can augment effective systemic and mucosal immune responses elicited to influenza vaccine.
5. Conclusions
In this study, we repurposed a cytokine already approved for clinical use for stimulating immune cell repopulation following chemotherapy for use as an adjuvant for skin immunization [73, 74]. Our preclinical studies in mice showed enhanced immunogenicity of influenza vaccine with recombinant murine GM-CSF administered with a MN patch, proposing a novel use for this cytokine as an active molecular adjuvant. Additionally, our process provides a pipeline to examine other active recombinant molecules as adjuvants and to optimize combined adjuvant doses for maximized vaccine efficacy and mucosal immunity. Lastly, these data demonstrate the usefulness of GM-CSF as an adjuvant in a single context; however, an effective adjuvant strategy may likely employ a combination of cytokines, TLR ligands or STING agonists [75, 76].
Supplementary Material
Acknowledgments
We thank Nadia Lelutiu for her scientific feedback and editing of this manuscript, Dahnide Taylor-Williams for technical support, and Kiran Gill and David Lee for Emory Vaccine Center core facility expertise with flow cytometry and Luminex assays, respectively.
Research funding: This work was supported by NIH grant 5R01AI111557.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jconrel.2018.02.033.
Footnotes
Competing financial interests
M.R.P. is an inventor of patents licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products (e.g., Micron Biomedical). This potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University.
E.Q.L, L.K.M., E.S.E., E.V.V, J.T.B., S.W., J.P.P., N.B., F.K. R.W.C, I.S. declare that they have no conflicts of interest.
References
- 1.Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008 Sep 12;26(Suppl. 4):D59–66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC, editor. CDC. Seasonal Influenza Vaccine Effectiveness, 2005–2015. Atlanta, GA: CDC; 2015. http://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. [Google Scholar]
- 4.WHO. Influenza (Seasonal) 2011 http://www.who.int/mediacentre/factsheets/fs211/en/
- 5.Gilca R, Skowronski DM, Douville-Fradet M, et al. Mid-season estimates of influenza vaccine effectiveness against influenza A(H3N2) hospitalization in the elderly in Quebec, Canada, January 2015. PLoS One. 2015;10(7):e0132195. doi: 10.1371/journal.pone.0132195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements KM, Meier G, McGarry LJ, Pruttivarasin N, Misurski DA. Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Human Vaccines Immunother. 2014;10(5):1171–1180. doi: 10.4161/hv.28221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007 Jun 28;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Flannery B, Chung J. US Flu VE Network, 2015–16. Centers for Disease Control and Presentation; 2016. Influenza Vaccine Effectiveness, Including LAIV vs IIV in Children and Adolescents. [Google Scholar]
- 9.Kim YC, Jarrahian C, Zehrung D, Mitragotri S, Prausnitz MR. Delivery systems for intradermal vaccination. Curr Top Microbiol Immunol. 2012;351:77–112. doi: 10.1007/82_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012 Jan;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 11.Ciancio BC, Rezza G. Costs and benefits of influenza vaccination: more evidence, same challenges. BMC Public Health. 2014 Aug 08;14:818. doi: 10.1186/1471-2458-14-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Fernando GJ, Crichton ML, et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release. 2011 Jun 30;152(3):349–355. doi: 10.1016/j.jconrel.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Vassilieva EV, Kalluri H, McAllister D, et al. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving micro-needle patch stable at room temperature. Drug Deliv Trans Res. 2015 Aug;5(4):360–371. doi: 10.1007/s13346-015-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caffarel-Salvador E, Donnelly RF. Transdermal drug delivery mediated by microneedle arrays: innovations and barriers to success. Curr Pharm Des. 2016;22(9):1105–1117. doi: 10.2174/1381612822666151216145645. [DOI] [PubMed] [Google Scholar]
- 15.Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Ann Rev Chem Biomol Eng. 2017 Jun 07;8:177–200. doi: 10.1146/annurev-chembioeng-060816-101514. [DOI] [PubMed] [Google Scholar]
- 16.Koutsonanos DG, Compans RW, Skountzou I. Targeting the skin for microneedle delivery of influenza vaccine. Adv Exp Med Biol. 2013;785:121–132. doi: 10.1007/978-1-4614-6217-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014 Apr 1;32(16):1856–1862. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall S, Sahm LJ, Moore AC. Microneedle technology for immunisation: perception, acceptability and suitability for paediatric use. Vaccine. 2016 Feb 03;34(6):723–734. doi: 10.1016/j.vaccine.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Mistilis MJ, Joyce JC, Esser ES, et al. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv Trans Res. 2017 Apr;7(2):195–205. doi: 10.1007/s13346-016-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouphael NG. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017 Aug 12;390(10095):649–658. doi: 10.1016/S0140-6736(17)30575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensley LE, Mulangu S, Asiedu C, et al. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus Species. PLoS Pathog. 2010 May;6(5):e1000904. doi: 10.1371/journal.ppat.1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koutsonanos DG, Vassilieva EV, Stavropoulou A, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skountzou I. Skin immunization with influenza vaccines. Sci Rep. 2015;386:343–369. doi: 10.1007/82_2014_407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Na YR, Jung D, Gu GJ, Seok SH. GM-CSF grown bone marrow derived cells are composed of phenotypically different dendritic cells and macrophages. Mol Cells. 2016 Oct;39(10):734–741. doi: 10.14348/molcells.2016.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conti L, Gessani S. GM-CSF in the generation of dendritic cells from human blood monocyte precursors: recent advances. Immunobiology. 2008;213(9–10):859–870. doi: 10.1016/j.imbio.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Bowne WB, Wolchok JD, Hawkins WG, et al. Injection of DNA encoding granulocyte-macrophage colony-stimulating factor recruits dendritic cells for immune adjuvant effects. Cytokines Cell Mol Ther. 1999 Dec;5(4):217–225. [PubMed] [Google Scholar]
- 27.Yu TWW, Chueh HYY, Tsai CCC, Lin CTT, Qiu JT. Novel GM-CSF-based vaccines: one small step in GM-CSF gene optimization, one giant leap for human vaccines. Human Vaccines Immunother. 2016:1–9. doi: 10.1080/21645515.2016.1221551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manfredi R, Mastroianni A, Coronado O, Chiodo F. Recombinant human granulocyte-macrophage colony-stimulating factor (rHuGM-CSF) in leukopenic patients with advanced HIV disease. J Chemother. 1996 Jun;8(3):214–220. doi: 10.1179/joc.1996.8.3.214. [DOI] [PubMed] [Google Scholar]
- 29.Burdach SE, Muschenich M, Josephs W, et al. Granulocyte-macrophage-colony stimulating factor for prevention of neutropenia and infections in children and adolescents with solid tumors. Results of a prospective randomized study. Cancer. 1995 Aug 01;76(3):510–516. doi: 10.1002/1097-0142(19950801)76:3<510::aid-cncr2820760323>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Tarhini AA, Leng S, Moschos SJ, et al. Safety and immunogenicity of vaccination with MART-1 (26-35, 27L), gp100 (209-217, 210M), and tyrosinase (368-376, 370D) in adjuvant with PF-3512676 and GM-CSF in metastatic melanoma. J Immunother. 2012 May;35(4):359–366. doi: 10.1097/CJI.0b013e31825481fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis. 2004 Dec;57(6):236–247. [PubMed] [Google Scholar]
- 32.Simmons SJ, Tjoa BA, Rogers M, et al. GM-CSF as a systemic adjuvant in a phase II prostate cancer vaccine trial. Prostate. 1999 Jun 01;39(4):291–297. doi: 10.1002/(sici)1097-0045(19990601)39:4<291::aid-pros10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Mittendorf EA, Clifton GT, Holmes JP, et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014 Sep;25(9):1735–1742. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeller C, Michielin O, Ascierto PA, Szabo Z, Blank CU. Systematic review of the use of granulocyte-macrophage colony-stimulating factor in patients with advanced melanoma. Cancer Immunol Immunother. 2016 Sep;65(9):1015–1034. doi: 10.1007/s00262-016-1860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Gao N, Wu J, et al. Variable effects of the co-administration of a GM-CSF-expressing plasmid on the immune response to flavivirus DNA vaccines in mice. Immunol Lett. 2014 Nov;162(1 Pt A):140–148. doi: 10.1016/j.imlet.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 36.del Pilar Martin M, Weldon WC, Zarnitsyn VG, et al. Local response to micro-needle-based influenza immunization in the skin. MBio. 2012;3(2):e00012–00012. doi: 10.1128/mBio.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muench H, Reed LJ. A simple method of estimating fifty percent endpoints. J Hygiene. 1938;29(493) [Google Scholar]
- 38.Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74(11):4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006 Aug 28;24(35–36):6110–6119. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Williams MS. Single-radial-immunodiffusion as an in vitro potency assay for human inactivated viral vaccines. Vet Microbiol. 1993 Nov;37(3–4):253–262. doi: 10.1016/0378-1135(93)90027-5. [DOI] [PubMed] [Google Scholar]
- 41.Klampfer L, Zhang J, Nimer SD. GM-CSF rescues TF-1 cells from growth factor withdrawal-induced, but not differentiation-induced apoptosis: the role of BCL-2 and MCL-1. Cytokine. 1999 Nov;11(11):849–855. doi: 10.1006/cyto.1999.0514. [DOI] [PubMed] [Google Scholar]
- 42.Esser ES, Romanyuk A, Vassilieva EV, et al. Tetanus vaccination with a dissolving microneedle patch confers protective immune responses in pregnancy. J Control Release. 2016 Aug 28;236:47–56. doi: 10.1016/j.jconrel.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 43.WHO/CDS/CSR/NCS. WHO Manual of Animal Influenza Diagnosis and Surveillance. Department of Communicable Disease Surveillance and Response; 2002. [Google Scholar]
- 44.Krammer F, Pica N, Hai R, Margine I, Palese P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol. 2013 Jun;87(12):6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnem Eric M, Stupak Elliot, Schering Corporation, assignee US5679356 A. Use of gm-csf as a vaccine adjuvant US patent. 1997
- 46.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010 Aug;16(8):915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreijtz JH, Fouchier RA, Rimmelzwaan GF. Immune responses to influenza virus infection. Virus Res. 2011 Dec;162(1–2):19–30. doi: 10.1016/j.virusres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 48.Sridhar S, Begom S, Bermingham A, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013 Oct;19(10):1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 49.Jang YH, Seong BL. Options and obstacles for designing a universal influenza vaccine. Viruses. 2014 Aug 18;6(8):3159–3180. doi: 10.3390/v6083159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krammer F, Palese P, Steel J. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol. 2015;386:301–321. doi: 10.1007/82_2014_408. [DOI] [PubMed] [Google Scholar]
- 51.Huang FF, Barnes PF, Feng Y, et al. GM-CSF in the lung protects against lethal influenza infection. Am J Respir Crit Care Med. 2011 Jul 15;184(2):259–268. doi: 10.1164/rccm.201012-2036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sever-Chroneos Z, Murthy A, Davis J, et al. GM-CSF modulates pulmonary resistance to influenza A infection. Antiviral Res. 2011 Nov;92(2):319–328. doi: 10.1016/j.antiviral.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bot A, Holz A, Christen U, et al. Local IL-4 expression in the lung reduces pulmonary influenza-virus-specific secondary cytotoxic T cell responses. Virology. 2000 Mar 30;269(1):66–77. doi: 10.1006/viro.2000.0187. [DOI] [PubMed] [Google Scholar]
- 54.Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011 Nov;12(11):1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawai K, Shimura H, Minagawa M, Ito A, Tomiyama K, Ito M. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J Dermatol Sci. 2002 Dec;30(3):185–194. doi: 10.1016/s0923-1811(02)00105-6. [DOI] [PubMed] [Google Scholar]
- 56.Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol. 2004 Jul 1;173(1):531–541. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- 57.Mitsui H, Inozume T, Kitamura R, Shibagaki N, Shimada S. Polyarginine-mediated protein delivery to dendritic cells presents antigen more efficiently onto MHC class I and class II and elicits superior antitumor immunity. J Invest Dermatol. 2006 Aug;126(8):1804–1812. doi: 10.1038/sj.jid.5700335. [DOI] [PubMed] [Google Scholar]
- 58.Miller LS. Toll-like receptors in skin. Adv Dermatol. 2008;24:71–87. doi: 10.1016/j.yadr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pulit-Penaloza JA, Esser ES, Vassilieva EV, et al. A protective role of murine langerin(+) cells in immune responses to cutaneous vaccination with microneedle patches. Sci Rep. 2014;4:6094. doi: 10.1038/srep06094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krakowski M, Abdelmalik R, Mocnik L, Krahl T, Sarvetnick N. Granulocyte macrophage-colony stimulating factor (GM-CSF) recruits immune cells to the pancreas and delays STZ-induced diabetes. J Pathol. 2002 Jan;196(1):103–112. doi: 10.1002/path.1013. [DOI] [PubMed] [Google Scholar]
- 61.Cruz MT, Duarte CB, Goncalo M, Figueiredo A, Carvalho AP, Lopes MC. Granulocyte-macrophage colony-stimulating factor activates the transcription of nuclear factor kappa B and induces the expression of nitric oxide synthase in a skin dendritic cell line, Immunol. Cell Biol. 2001 Dec;79(6):590–596. doi: 10.1046/j.1440-1711.2001.01041.x. [DOI] [PubMed] [Google Scholar]
- 62.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010 Mar;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005 Apr;11(4 Suppl):S63–68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 64.Pulendran B. Division of labor and cooperation between dendritic cells. Nat Immunol. 2006 Jul;7(7):699–700. doi: 10.1038/ni0706-699. [DOI] [PubMed] [Google Scholar]
- 65.Blanton L, Alabi N, Mustaquim D, et al. Update: influenza activity in the United States during the 2016–17 season and composition of the 2017–18 influenza vaccine. Morbid Mortal Weekly Rep. 2017 Jun 30;66(25):668–676. doi: 10.15585/mmwr.mm6625a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen D, Endres RL, Erickson CA, et al. Epidermal immunization by a needle-free powder delivery technology: immunogenicity of influenza vaccine and protection in mice. Nat Med. 2000 Oct;6(10):1187–1190. doi: 10.1038/80538. [DOI] [PubMed] [Google Scholar]
- 67.Kawano Y, Noma T, Yata J. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J Immunol. 1994 Dec 01;153(11):4948–4958. [PubMed] [Google Scholar]
- 68.Loudon PT, Yager EJ, Lynch DT, et al. GM-CSF increases mucosal and systemic immunogenicity of an H1N1 influenza DNA vaccine administered into the epidermis of non-human primates. PLoS One. 2010;5(6):e11021. doi: 10.1371/journal.pone.0011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wanjalla CN, Goldstein EF, Wirblich C, Schnell MJ. A role for granulocyte-macrophage colony-stimulating factor in the regulation of CD8(+) T cell responses to rabies virus. Virology. 2012 May 10;426(2):120–133. doi: 10.1016/j.virol.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chou HY, Lin XZ, Pan WY, et al. Hydrogel-delivered GM-CSF overcomes non-responsiveness to hepatitis B vaccine through the recruitment and activation of dendritic cells. J Immunol. 2010 Nov 01;185(9):5468–5475. doi: 10.4049/jimmunol.1001875. [DOI] [PubMed] [Google Scholar]
- 71.Deng J, Pennati A, Cohen JB, et al. GIFT4 fusokine converts leukemic B cells into immune helper cells. J Transl Med. 2016 Apr 27;14(1):106. doi: 10.1186/s12967-016-0865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng H, Zhang H, Deng J, et al. Incorporation of a GPI-anchored engineered cytokine as a molecular adjuvant enhances the immunogenicity of HIV VLPs. Sci Rep. 2015 Jul 07;5:11856. doi: 10.1038/srep11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992 Aug;90(2):482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams IR, Ort RJ, Daley D, et al. Constitutive expression of B7-1 (CD80) on mouse keratinocytes does not prevent development of chemically induced skin papillomas and carcinomas. J Immunol. 1996 May 1;156(9):3382–3388. [PubMed] [Google Scholar]
- 75.Williams P, Galipeau J. GM-CSF-based fusion cytokines as ligands for immune modulation. J Immunol. 2011 May 15;186(10):5527–5532. doi: 10.4049/jimmunol.1003699. [DOI] [PubMed] [Google Scholar]
- 76.Zhang C, Wang B, Wang M. GM-CSF and IL-2 as adjuvant enhance the immune effect of protein vaccine against foot-and-mouth disease. Virol J. 2011 Jan 09;8:7. doi: 10.1186/1743-422X-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


