Abstract
Interleukin11 was developed to reduce chemotherapy-induced thrombocytopenia, however, its clinical use was limited by severe adverse effects in humans. PEGylated -IL-11 (BBT-059), developed by Bolder Biotechnology, Inc., exhibited a longer half-life in rodents and induced longer-lasting increases in hematopoietic cells than IL-11. A single dose of 1.2 mg kg−1 of BBT-059, administered subcutaneously (SC) to CD2F1 mice (12-14 weeks, male) was found to be safe in a 14-day toxicity study. The drug demonstrated its efficacy both as a prophylactic countermeasure and a mitigator in CD2F1 mice exposed to Co-60 gamma total body irradiation (TBI). A single dose of 0.3 mg kg−1, administered either 24 h pre-, 4 h post-, or 24 h post-irradiation increased the survival of mice to 70-100% from lethal doses of radiation. Pre-administration (−24 h) of the drug conferred a significantly (p <0.05) higher survival compared to 24 h post-TBI. There was significantly accelerated recovery from radiation-induced peripheral blood neutropenia and thrombocytopenia in animals pre-treated with BBT-059. The drug also increased bone marrow cellularity and megakaryocytes and accelerated multi-lineage hematopoietic recovery. In addition, BBT-059 inhibited the induction of radiation-induced hematopoietic biomarkers, thrombopoietin (TPO), erythropoietin (EPO) and Flt-3 ligand (Flt3L). These results indicate that BBT-059 is a promising radiation countermeasure, demonstrating its potential to be used both pre- and post-radiation for hematopoietic acute radiation syndrome with a broad window for medical management in a radiological or nuclear event.
Keywords: Radiation countermeasure, PEG-IL11, Bone marrow recovery, Cytokines
INTRODUCTION
Because of the growing threat of global nuclear or radiological terrorism, development of prophylactic, mitigator and therapeutic measures against radiation injury is a national priority (Singh, Newman et al. 2014). In the case of an unintentional or deliberate acute radiation exposure, medical care would focus primarily on giving prophylactic countermeasures to the first responders and uniformed personal before deployment to the radiation exposed field for rescue missions (Singh, Newman et al. 2014). Radiation prophylactic agents are also useful for civilians expected to be exposed to radiation fallout fields during evacuation procedures (Singh, Newman et al. 2014). Mitigators are needed to protect first responders as well as civilians exposed to acute radiation, and therapeutics are needed for treatment after symptoms develop, mainly due to late-arising injuries (Singh, Newman et al. 2014). Although, many medical countermeasures for acute radiation syndrome (Barshishat-Kupper, Mungunsukh et al.) are in different stages of development (Ghosh, Kulkarni et al. 2009, Ghosh, Perkins et al. 2009, Singh, Newman et al. 2014), only granulocyte colony-stimulating factor (G-CSF) (filgrastim, Neupogen) and PEGylated G-CSF (pegfilgrastim, Neulasta) have been approved for hematopoietic ARS (H-ARS) by the FDA and they are in the strategic national stockpile (SNS); however, filgrastim requires multiple administration until recovery of neutrophils (Administration 2015, Administration 2015) . Therefore, agents that demonstrate a broad window of efficacy are very important for the development of medical management after a radiological/nuclear event.
Interleukin-11 (20 kDa) is a multifunctional cytokine of the interleukin 6 family with anti-inflammatory and hematopoietic proliferative properties, being a key regulator of megakaryocyte maturation (Hauer-Jensen 2014). In addition to hematopoietic protection, a cytoprotective role of IL-11 to protect intestinal crypt cells was also documented and recombinant human IL-11 was used for the treatment of inflammatory bowel disease (Potten 1995, Potten 1996). Systemic administration of IL-11 in mice was found to reduce intestinal mucosal injury and protect crypt cells after exposure to total body radiation (TBI). Although recombinant human IL-11 was developed and marketed to reduce chemotherapy-induced thrombocytopenia, its clinical use is limited by requirement of multiple daily injections and severe adverse effects in humans, including significant fluid retention and multi-organ failure. IL-11 was found to be effective both as a prophylactic countermeasure and as a mitigator after TBI in rodent models (Potten 1995, Burnett, Biju et al. 2013). In order to reduce the toxicity and increase the efficacy, the authors developed PEGylated IL-11 (BBT-059) [Bolder Biotechnology, Inc (BBT)], which demonstrated a longer half-life in plasma in rodents and increased the induction period of hematopoietic stem cells compared to IL-11 (Lee, Park et al. 2012, Plett, Chua et al. 2014). A single dose of BBT-059 stimulated circulating platelets in rats that lasted up to 10 days (Lee, Park et al. 2012).
In an earlier study by Plett et al, a single dose of BBT-059 was found to increase survival and increased white blood cells, platelets, lymphocytes, and neutrophils in C57BL/6 mice exposed to lethal doses of total body irradiation resulting in 50% mortality in 30 days (LD50/30) or 70% mortality in 30 days (LD70/30) (Plett, Chua et al. 2014). Genetic variations in humans may contribute to the considerable difference in the extent and rate of loss of cells after TBI, resulting in radiation-induced lymphocyte apoptosis (Kusunoki, 2008, Schnarr, 2007). Previous studies reported that inherent variation in mouse strains causes changes in radiosensitivity, resulting in differences in survival from TBI (Williams, Brown et al. 2010). This is mainly due to changes in bacterial populations, which stimulate innate immune responses differentially. These results suggested that radioprotectors and mitigators of the immune system should be tested in more than one strain of mouse to ensure its effectiveness in all populations irrespective of genetic variations (Williams, Brown et al. 2010). In this study, we demonstrate, for the first time, that BBT-059 is effective as a radiation countermeasure given 24 h pre-TBI as well as 4 h post-TBI. We also validated the efficacy of BBT-059 in the CD2F1 mouse strain when administered 24 h post-TBI. In addition, we have shown that BBT-059, administered 24 h pre-TBI, accelerated recovery from radiation-induced peripheral blood cytopenia and restoration of sternal bone marrow, protected bone marrow progenitor cells, as well as attenuated protein biomarkers of H-ARS.
MATERIALS AND METHODS
Mice
Twelve to fourteen week old CD2F1 male mice used in these studies were purchased from Envigo Corporation, Indianapolis, IN, USA. The mice were housed in the Armed Forces Radiobiology Research Institute’s (AFRRI) vivarium accredited by the Association for Assessment and Accreditation of Laboratory Animal Care-International. The animals received Harlan Teklad Rodent Diet 8604 and acidified water (pH 2.5 – 3.0) ad libitum and were housed under 12 h light/dark cycle and were acclimatized for 2 weeks before the start of each study. All procedures in these studies were performed under an approved protocol by the Department of Defense Institutional Animal Care Use Committee (IACUC).
Drug preparation
BBT-059 is a long acting PEGylated IL-11 analog created using site-specific PEGylation technology and is modified with a single branched 40 kDa-PEG at a cysteine residue (PEG-*179C) added to the C-terminus of the protein (Lee, Park et al. 2012) . In these studies, BBT-059 was prepared in formulation buffer (10mM sodium phosphate, 4% mannitol, 1% sucrose, pH 6.2) at the specific doses used in studies. Formulation buffer and saline (9%) were used as controls. Drug and controls were injected as a single dose (0.1 mL) subcutaneously (SC) at the nape of the neck.
Irradiation
The experimental animals received a single exposure of 60Co gamma TBI at an estimated dose rate of 0.6 Gy min−1 in the AFRRI radiation facility. The mice were placed in ventilated Lucite boxes arranged in an array using plastic racks during the exposure. The alanine/ESR (electron spin resonance) dosimetry system (American Society for Testing and Material Standard E 1607) was used to measure dose rates to water in cores of acrylic mouse phantoms as described earlier (Ghosh, Kulkarni et al. 2009). The radiation field was uniform within ± 2%.
Fourteen-day acute toxicity study
To determine the safety of BBT-059 in CD2F1 mice, a 14-day toxicity study was conducted (Swift, Pessu et al. 2014). Two groups of CD2F1 male mice (n=6 per group) were subcutaneously administered either BBT-059 (1.2 mg kg−1) or its vehicle formulation buffer. The animals were monitored for acute (1 to 4 h) signs of toxicity after administration of drug, then daily for 14 days. Signs of acute toxicity included decreased activity, squinting eyes, hunching, labored breathing or mortality. Weights of the animals were recorded at various intervals during the study. All animals were euthanized on day 14 and a gross necropsy was carried out for any abnormal pathology in all major organs.
Survival studies
The initial survival studies consisted of testing one drug dose of BBT-059 (0.3 mg kg−1), one route of administration (SC), three drug regimens (one pre- and two post-TBI) and one radiation dose (LD70/30 [70% mortality over 30 day period] = 9.25 Gy). CD2F1 male mice were weighed, animals outside ±10% of the mean weight excluded, and randomized into groups of four animals per box. There were 24 animals per treatment group (6 boxes) for BBT-059 and its vehicle. The mice received SC administration of either BBT-059 or formulation buffer (the vehicle) at 24 h prior to TBI, 4 h post-TBI, and 24 h post-TBI. Post irradiation, the mice were monitored daily (three times a day when necessary) for 30 days and surviving animals were euthanized at the completion of the observational period. Survival data was plotted as Kaplan-Meier plots and statistical significance of the survival differences was determined by Log-rank test using GraphPad Prism 7 software. For BBT-059 dose response survival studies, each group of mice (n=24) was administered a different dose of BBT-059 ranging from 0.01 to 1.2 mg kg−1 at either 24 h prior or 24 h post-TBI.
Hematology
To study hematological recovery in CD2F1 male mice, BBT-059 (0.3 mg kg−1) or its vehicle (n= 10 per group) were administered SC 24 hour prior to irradiation. All animals were identified by a tail tattoo. The experimental animals received either 0 or 7 Gy radiation (non-lethal dose) at a dose rate 0.6 Gy min−1 in the AFRRI 60Co gamma radiation facility. Blood (20 μl) was collected from submandibular vein from all mice in EDTA tubes at eight time-points, 2 h (represented as day 0 in the graph) and days 1, 3, 7, 10, 14, 21, and 30 post drug administration) and Complete blood counts (CBC) and differential analysis was performed using HESKA Element HT (TM) 5 Analyzer system. Animals were euthanized at the end of the study.
Collection of blood and tissues for various assays
BBT-059 (0.3 mg kg−1) or its vehicle (n= 6 per group) were administered SC 24 hour prior to irradiation. The experimental animals received either 0 or 7 Gy radiation (non-lethal dose) at a dose rate ~0.6 Gy min−1 in the AFRRI 60Co gamma radiation facility. Blood was collected from inferior vena cava under anesthesia on days 0 (2 h post-TBI), 1, 2, 3, 7, 15, and 30 after exposure at 7 Gy or unirradiated mice followed by euthanasia. Serum was separated and used in ELISA as described below. Femurs, sternum and spleen were collected and processed as described below.
Hematopoietic Progenitor Clonogenic Assay
Clonogenicity of mouse bone marrow cells was quantified in standard semisolid cultures using 1 mL of Methocult GF+ system for mouse cells (Stem Cell Technologies Inc., Vancouver, BC) according to the manufacturer’s instructions. Briefly, colony forming units (CFU) were assayed on days 0 (2 h post-TBI), 1, 3, 7, 15, and 30 after exposure at 7 Gy or unirradiated mice. Cells from three femurs from different animals were pooled, washed twice with IMDM and seeded at 1 to 5 × 104 cells per dish in 35-cm cell culture dishes (BD Biosciences). Each sample was plated in duplicate to be scored 14 days after plating. Granulocyte-macrophage colony forming units (CFU-GM), granulocyte-erythrocyte-monocyte-macrophage CFU (CFU-GEMM), colony-forming unit-erythroid (CFU-E) and erythroid burst-forming units (BFU-E) were identified and quantified following the manufacturer’s instructions. Colonies were counted 14 days after plating using a Nikon TS100F microscope. Fifty or more cells were considered one colony. Data are expressed as mean ± standard error of mean (SEM). Statistical significance was determined between irradiated vehicle treated and BBT-059-treated groups.
Sternal histopathology
Following blood collection, animals were euthanized, and the sterna were collected on 0 (2 h post-TBI), 1, 3, 7, 15 and 30 days post-TBI. The sterna were fixed in a 20:1 volume of fixative (10% buffered formalin) to tissue for at least 24 h and up to 7 days. Fixed sterna were decalcified for 3 h in 12–18% sodium EDTA (pH 7.4–7.5) and specimens dehydrated using graded ethanol concentrations and embedded in paraffin. Longitudinal 5 μm sections were stained with regular hematoxylin and eosin (H&E) stain. A board-certified veterinary pathologist conducted blinded histopathological evaluation of these samples. The bone marrow was evaluated in situ within sternebrae and graded for total cellularity and megakaryocyte numbers averaged per 10 high power fields at 40x magnification using a BX41 Olympus microscope (Minneapolis, MN). The grade scale used for cellularity is as follows: Grade 1: < 10%; Grade 2: 11-30%; Grade 3: 31-60%; Grade 4: 61-89%; Grade 5: > 90%. Images were captured with an Olympus DP70 camera and imported into Adobe Photoshop (version CS5) for analysis.
Circulating levels of EPO, TPO, and Flt3L
Mouse Thrombopoietin (TPO) Quantikine ELISA, mouse Erythropoietin (EPO) Quantikine ELISA and mouse/Rat Flt3 ligand (Flt3L) quantikine ELISA kits were purchased from R&D Systems Inc (Minneapolis, MN). The cytokine detection limits were 18 pg mL−1, >20 pg mL−1 and >5 pg mL−1for TPO, EPO and Flt3L ELISAs, respectively. The quantitative levels of EPO, TPO and Flt3L were evaluated from serum samples collected on days 0 (2 h post-TBI), 2, 3, 7 and 15 post-TBI following standard protocols from the vendor. Data represented are mean ± standard error of the mean (Semenza) for n=6 mice.
Western blots analysis
Spleens were isolated and snap-frozen from all groups at different time points of radiation and were stored at −80°C. Spleens were homogenized and lysed in ice cold RIPA buffer (Sc24948A Santa Cruz Biotechnology) in the presence of protease inhibitors. The homogenates were centrifuged at 12000 g for 10 min at 4°C, and the total protein concentrations were determined using a BCA protein assay. After adjusting the concentrations, Laemmli sample buffer was added to 1X final concentration. Samples were denatured by boiling at 100°C for 5 minutes and were run on 4-12% SDS-PAGE gels at 80 V, then transferred to PVDF membranes by semi-dry transfer. Membranes were blocked with 4% bovine serum albumin (BSA) and immunoblots were developed using anti-EPO (Sc7956, Santa Cruz Biotechnology, diluted 1:1000) and HIF-2 alpha (NB100-122 Novus Biologicals, diluted 1:500). Beta-actin (Sc47778, Santa Cruz Biotechnology) was used as an internal control. The intensities of specific bands corresponding to the proteins of interest were measured by using ImageJ software.
RESULTS
Survival of lethally irradiated mice from a single dose of BBT-059
BBT-059 was found to be safe in CD2F1 male mice when administered once at 1.2 mg kg−1 body weight. The animals showed no symptoms of toxicity and gained weight during the study in a similar manner as the naïve group (data not shown). No abnormal pathology was found in any of the major organs during gross necropsy.
Survival of CD2F1 mice following TBI with 9.35 Gy at the estimated rate of 0.6 Gy min−1 and therapy with a single dose of BBT-059 at 0.3 mg kg−1 or formulation buffer was tested. When BBT-059 was administered 24 h prior to TBI (Fig 1A), the drug treated group had 96% survival as compared to 29% in vehicle treated group. When administered 4 h post-TBI (Fig 1B), BBT-059 treated mice had similar % survival as seen in pre-TBI. In the case of 24 h post-TBI administration of BBT-059 (Fig 1C), the drug treated group showed 75% survival whereas vehicle treated group had 25% survival. Log-rank test p values ranged from <0.0001 – 0.0005 for the survival curves when compared to the respective vehicle-treated groups.
Fig 1.
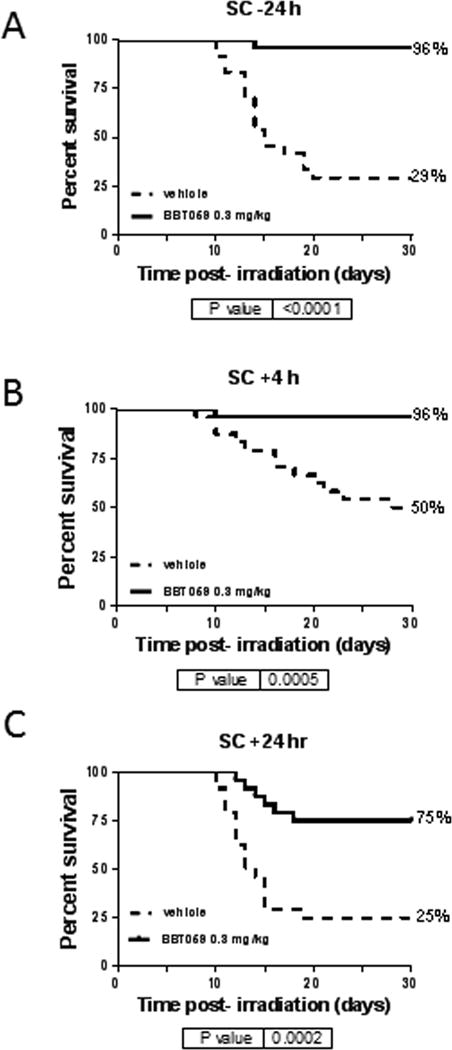
Survival of CD2F1 mice following total body irradiation with 9.35 Gy at the estimated rate of 0.6 Gy min−1and administered a single dose of BBT-059 at 0.3 mg kg−1 (solid line) or formulation buffer (10 mM sodium phosphate, 4% mannitol, 1% sucrose, pH 6.2) as vehicle (dashed line). BBT-059 was administered either 24 h prior to TBI (A), 4 h post-TBI (B) or 24 h post-TBI (C). Kaplan-Meier survival curves were plotted using GraphPad software; n=24 mice per group and trend in survival is compared between vehicle and drug-treated groups (Log-rank test p <0.0001 – 0.0005).
Drug dose response studies
In order to determine the optimum dose of BBT-059 administered 24 h pre-TBI, a range of drug doses (0.01 - 1.2 mg kg−1) were tested in mice (n=24) at 9.75 Gy (LD90/30) (Fig 2A). Percent survival at the lower doses (0.01 and 0.05 mg kg−1) did not show dose response, however, it was observed at higher doses (0.3 to 1.2 mg kg−1). Hence, in the succeeding study the radiation dose was increased to 10.5 Gy (LD100/30) (Fig 2B). At BBT-059 doses of 0.3 and 0.6 mg kg−1, more than 90% survival was observed, whereas 46% survival was observed at 0.1 mg kg−1, exhibiting a good dose response. A dose of 1.2 mg kg−1 was not as effective, resulting in 79% survival (Fig 2B). All animals from the saline group died by day 18 and 4% survival was seen in the vehicle (formulation buffer) group, as expected for an LD100 dose of radiation.
Fig 2.
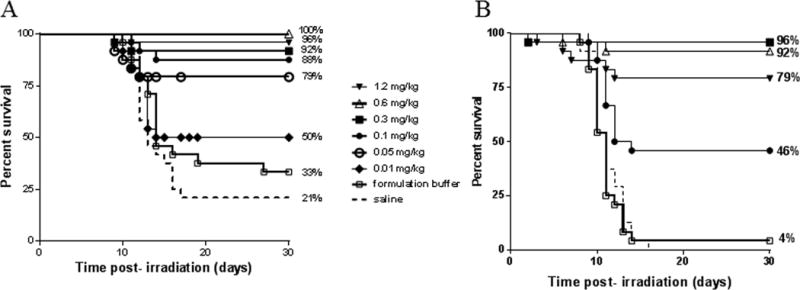
Optimization of BBT-059 dose to improve survival of lethally irradiated mice. A) Effect of increasing BBT-059 dose administered SC 24 h prior to 9.75 Gy TBI (n=24/group). B) Effect of increasing BBT-059 dose administered SC 24 h prior to 10.5 Gy TBI (n=24/group). Survival curves shown here are saline (dashed line), formulation buffer (□), and BBT-059 doses 0.01 mg kg−1 (◆), 0.05 mg kg−1 (○), 0.1 mg kg−1 (●), 0.3 mg kg−1 (■), 0.6 mg kg−1 (Δ) and 1.2 mg kg−1 (▼). Percent survival on day 30 post-TBI is shown at the end of each curve.
To determine the optimum dose of BBT-059 administered 24 h post-TBI, efficacy of four doses ranging from 0.1 to 0.8 mg kg−1 were tested. A clear dose response to % survival was achieved with 0.1 and 0.3 mg kg−1 resulting in 67% and 71% survival respectively (Fig 3). Similar to data shown in Fig 2B, higher doses of BBT-059 did not result in better survival.
Fig 3.
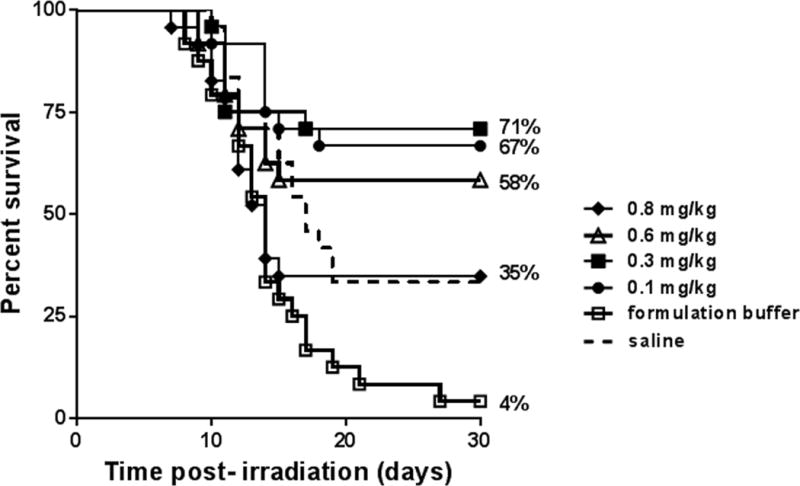
Optimization of BBT-059 dose to improve survival of lethally irradiated mice when administered SC 24 h post-TBI 9.35 Gy (n=24/group). Survival curves shown here are saline (dashed line), formulation buffer (□), and BBT-059 doses 0.1 mg kg-1 (●), 0.3 mg kg-1 (■), 0.6 mg kg-1 (Δ) and 0.8 mg kg-1 (◆). Percent survival on day 30 post-TBI is shown at the end of each curve.
The optimum single dose for BBT-059 as a prophylactic as well as a mitigator was determined to be 0.3 mg kg−1.
Hematological recovery stimulated by BBT-059 administered 24 h pre-TBI
Hematological recovery was measured by comparing peripheral blood cell counts (white blood cells (WBC), red blood cells (RBC), % Hematocrit (%HCT), neutrophils (NEU), platelets (PLT), monocytes (MON) and lymphocytes (LYM)) of irradiated mice treated with BBT-059 or vehicle 24 h pre-TBI. The effects of BBT-059 on cytopenia resulting from radiation injury of NEU, PLT, RBC and %HCT (Fig 4), WBC, MON and LYM (supplementary Fig 1) were significant. There was no significant effect of BBT-059 on non-irradiated mice when compared with vehicle treated group except in the case of PLT. On days 3, 5, 14 and 21, in non-irradiated BBT-059 treated group, a significant increase (p < 0.05) in PLT counts were observed compared to non-irradiated vehicle control.
Fig 4.
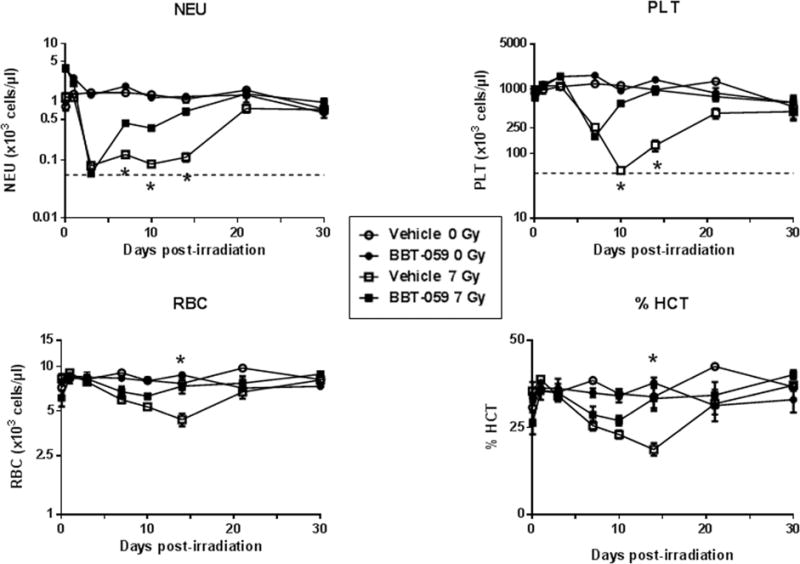
Recovery of peripheral blood cells (neutrophils (NEU), platelets (PLT) and RBCs) of non-irradiated mice treated with vehicle formulation buffer (○) and BBT-059 (●) and irradiated (7 Gy) mice treated with formulation buffer (□) and BBT-059 (■). Either Formulation buffer or BBT-059 at 0.3 mg kg-1 was administered 24 h prior to irradiation. Day 0 represents day of irradiation. Dashed line represents nadirs for the cell type. Data represented are mean ± standard error of the mean (SEM) for n=10 mice. Significant difference (p < 0.005 – 0.0125) between BBT-059-treated and vehicle-treated irradiated groups by ANOVA is indicated with an asterisk (*). Some data points in the figure do not have error bars that are visible because they are smaller than symbols.
NEU: On day 3 post-TBI, the NEU counts decreased sharply reaching a neutropenia nadir (~58 ± 5 cells μL1) in the irradiated groups compared to those in the non-irradiated control groups (~1437 ± 150 cells μL−1, p < 0.001) (Fig 4). On day 7 post-TBI, the BBT-059 treated group showed significant recovery from neutropenia (430 ± 54 cells μL−1) as compared to the vehicle-treated group (124 ± 15 cells μL−1). NEU cell numbers in the irradiated vehicle-treated group stayed low until day 14 post-TBI, whereas the BBT-059 treated group almost reached the NEU cell numbers in the non-irradiated control groups. By day 30 all four groups had similar NEU cell counts, as the irradiation dose was non-lethal.
PLT: Platelet nadir was reached for the irradiated vehicle-treated group on day 10 but the BBT-059 treated group had a significantly higher (p < 0.0001) cell count (602 × 103 ± 45 × 103 cells μL−1) (Fig 4), protecting the mice from thrombocytopenia. By day 14, there was no significant difference between the non-irradiated controls (989 × 103 ± 125 × 103 cells μL−1) and the irradiated BBT-059 treated group (971 × 103 ± 141 × 103 cells μL−1). By day 30, the cell numbers in both irradiated groups were similar to the non-irradiated controls. This data suggested that BBT-059 protected and aided in faster recovery from radiation induced thrombocytopenia.
WBC, MON and LYM: The irradiated group receiving BBT-059 showed markedly higher WBC and MON counts than the irradiated vehicle-treated group on days 7, 10 and 14 post-TBI, and the differences were statistically significant (p < 0.001). These results indicated that the administration of BBT-059 improved peripheral blood WBC and monocyte counts in irradiated mice. There were significant (p < 0.05) increases in LYM as well when mice were treated with BBT-059 compared to the vehicle-treated group on days 10 and 14 post-TBI.
RBC and %HCT: Changes in the RBC count and % hematocrit (%HCT) in the different groups are shown in Fig 4. On day 14 the %HCT of the irradiated vehicle-treated group (19 ± 2%) was significantly lower than that of the control group (38 ± 2%) or irradiated BBT-059 treated group (33 ± 4%) (p < 0.001). The same effect was also seen in RBC counts, suggesting recovery of peripheral hematopoietic cells with BBT-059 treatment in irradiated mice.
BBT-059 protects hematopoietic progenitor cells from radiation injury when administered 24 h pre-TBI
In addition to detrimental effects on peripheral blood cells, irradiation also negatively affects the hematopoietic progenitor cells. Clonogenic assays were carried out to evaluate the extent of damage caused by irradiation and possible recovery by BBT-059 treatment administered 24 h pre-TBI. Colony forming unit (CFU) assays measured CFU-GM, CFU-GEMM, CFU-E and BFU-E to evaluate the function of hematopoietic cells. As shown in Figure 5, until day 7 post-TBI at 7 Gy, no colonies were observed. On day 7, the total number of colonies found in the vehicle-treated group (5 ± 2 CFU) was significantly lower compared to the BBT-059 treated group (44 ± 2 CFU). Even on day 15 the difference between the vehicle-treated and BBT-059 treated groups with respect to GM, GEMM, BFU-E and CFU-E was significant (p <0.0001). Based on the CFU counts, BBT-059 treated group has recovered from radiation damage. This result suggests that the cellular functions affected by irradiation in hematopoietic progenitor cells can be restored by BBT-059 treatment.
Fig 5.
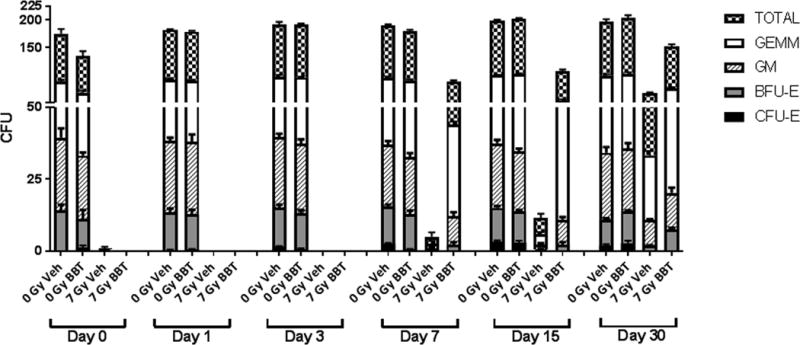
BBT-059 accelerates hematopoietic progenitor cell recovery after a non-lethal dose of radiation (7 Gy) in CD2F1 mice (n=6 per group) when administered 24 h prior to irradiation. Clonogenic potential of bone marrow cells was assessed by a CFU assay. Colony forming units (CFU) were assayed on days 0 (2 h post-TBI), 1, 3, 7, 15, and 30 after exposure. Cells from three femurs were pooled, counted, and each sample plated in duplicate to be scored 14 days after plating. Data are expressed as mean ± Standard error of mean (SEM). Statistical significance was determined between irradiated vehicle treated and BBT-059-treated groups.
BBT-059 restores bone-marrow cellularity when administered 24 h pre-TBI
Bone marrow architecture and cellularity of mice treated 24 h pre-TBI with vehicle or BBT-059 was evaluated by the AFRRI pathologist (Fig 6). Megakaryocytes were evaluated by averaging the number of cells per 10 (40x) high power fields (HPFs). Bone marrow cellularity was determined by evaluating the amount of adipose (fat) tissue versus hematopoietic cells (minus, mature red blood cells) on one (10x) high power field (HPF). Cellularity was assigned a “grade”, which correlated with a “percentage range” of cellularity; an average was obtained for each group. The grading scheme was: Grade 1: < 10%; Grade 2: 11-30%; Grade 3: 31-60%; Grade 4: 61-89%; Grade 5: > 90% cellularity (Fig 6 graph). Seven Gy Irradiated specimens (vehicle-treated – RV, BBT-059 treated – RD) were compared to respective non-irradiated controls (vehicle-treated NRV, BBT-059 treated NRD). Samples were collected on different days post-TBI. The extent of recovery from radiation damage was estimated from the H&E stained slides and quantitated as number of megakaryocytes and % cellularity (Fig 6). When compared to non-irradiated controls (NRV or NRD) irradiated samples show significant damage in the vehicle treated group (Paunesku, Vogt et al.) and much less in the BBT-059 treated group on day 1. On day 3 in the irradiated specimens hardly any megakaryocytes could be seen, but by day 7, the BBT-059 treated group showed recovery. By day 15 there were significant differences in the irradiated vehicle-treated and BBT-059 treated groups with respect to number of megakaryocytes as well as % cellularity. By day 30, even though the vehicle treated groups recovered, they still remained lower than the BBT-059 treated group.
Fig 6.
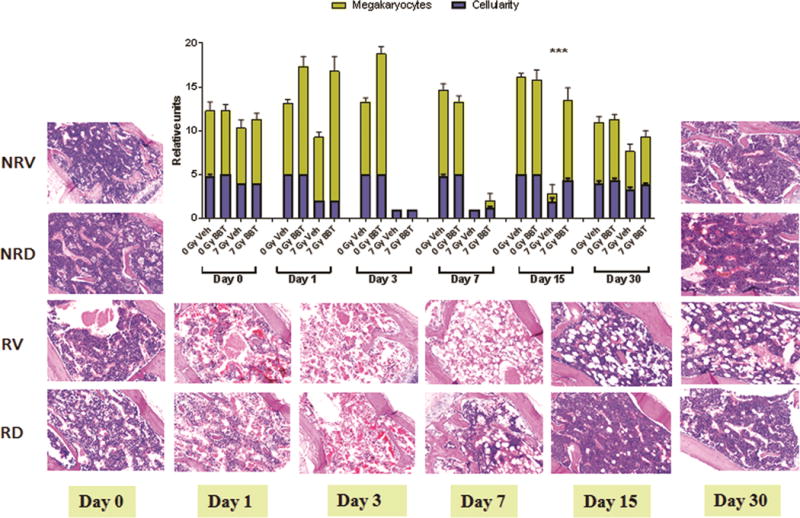
BBT-059 treatment promoted sternal bone marrow hematopoietic cell recovery after non-lethal dose of TBI (7 Gy) when administered 24 h prior to TBI. Representative sternal bone marrow sections are shown for non-irradiated vehicle (NRV) and BBT-059 (NRD) treated mice from days 0 and 30, and from vehicle (RV) and BBT-059 (RD) treated irradiated mice from days 0 (2 hr post-TBI), 1, 3, 7, 15 and 30 post-TBI. Bone marrow cellularity and megakaryocyte numbers were quantitated from histological sections from days 0, 1, 3, 7, 15 and 30. Significant increase in bone marrow cellularity and megakaryocytes were observed after 15 days post-TBI in the BBT-059 treatment group.. Data represented are mean ± standard error of the mean (SEM) for n=6 mice. Percent (%) range of Cellularity: Grade 1: < 10%; Grade 2: 11-30%; Grade 3: 31-60%; Grade 4: 61-89%; Grade 5: > 90%).
BBT-059 attenuates radiation-induced induction of EPO, TPO, and Flt3L when administered 24 h pre-TBI
The levels of EPO, TPO and Flt3L were evaluated by ELISA from sera from blood samples collected on days 0 (2 h post-TBI), 1, 2, 3, 7 and 15 post-TBI (Fig 7). Mice were treated with vehicle or BBT-059 24 h prior to TBI. Serum samples from four groups of mice were compared. Two groups of mice (n=6 per group), administered either vehicle formulation buffer (NRV) or BBT-059 (NRD) but not irradiated were used as controls. The levels of cytokines or biomarker in these controls were then compared to the levels observed in 7 Gy irradiated vehicle-treated (RV) and BBT-059 treated (RD) serum samples.
Fig 7.
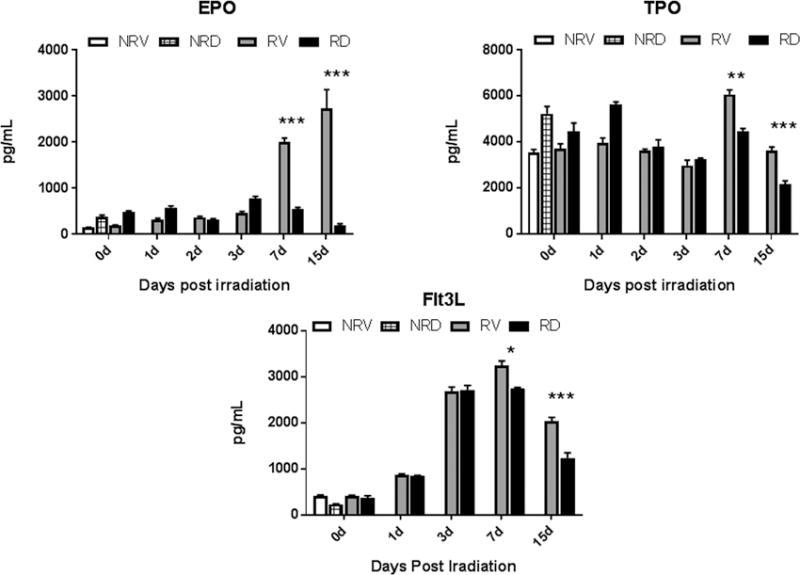
BBT-059 administration 24 h prior to TBI inhibited radiation-induced elevated synthesis of EPO, TPO and Flt3L in mouse serum when compared to vehicle treated animals. The levels of EPO, TPO and Flt3L were evaluated from serum from samples collected on days 0 (2 h post-TBI), 1, 2, 3, 7 and 15 post-TBI by ELISA. Data represented are mean ± standard error of the mean (SEM) for n=6 mice per group.
EPO: EPO levels in NRV and NRD were found to be 144 ± 6 pg mL1 and 376 ± 36 pg mL−1, respectively (Fig 7). The difference in the EPO levels between NRV and NRD is not significant (p = 0.25). A gradual increase in EPO levels was seen in the irradiated groups (RV and RD) by day 3. On day 7, in vehicle-treated RV group EPO levels were significantly higher (1998 ± 86 pg mL−1) than in the BBT-059 treated RD group (552 ± 28 pg mL−1). On day 15, EPO levels in vehicle-treated specimens (RV) were around 14 fold higher (2734 ± 406 pg mL−1) than in the BBT-059 treated RD group (192 ± 36 pg mL−1) indicating protection and recovery from radiation damage as a result of BBT-059 treatment.
TPO: Basal levels of TPO in controls NRV and NRD (Fig 7) were 3532 ± 144 pg mL−1 and 5238 ± 305 pg mL−1, respectively. There was not much change in TPO levels in the early time points (days 0 (2 h post-TBI), 1-3) in irradiated groups (RV and RD) compared to non-irradiated controls (NRV and NRD). On day 7, TPO levels went up in the irradiated vehicle-treated group (6069 ± 191 pg mL−1) whereas in the BBT-059 treated RD group the TPO levels were maintained at similar levels as in the non-irradiated controls (4469 ± 118 pg mL−1). By day 15, there was a significant protection (p <0.0001) seen in BBT-059 treated group as TPO levels were still much higher in vehicle-treated group.
Flt3L: A radiation-induced biomarker of hematopoiesis, Flt3 ligand levels were also tested in serum (Fig 7). Similar to the cytokines tested above, non-irradiated vehicle-treated (NRV) and BBT-059 treated (NRD) mice were used as controls with Flt3L levels of 415 ± 22 pg mL−1 and 238 ± 7 pg mL−1, respectively. Though there was an increase in Flt3L levels on days 1 and 3, there was no difference between RV and RD. On day 7, the Flt3L level in BBT-059 treated group (2739 ± 29 pg mL−1) dropped significantly (p <0.05) as compared to vehicle-treated group (3247 ± 96 pg mL−1). These results suggested that the administration of BBT-059 either protected or assisted in the recovery from damage caused by irradiation.
BBT-059 inhibits radiation-induced expression of EPO and HIF-2 alpha in spleen when administered 24 h pre-TBI
EPO is the hormonal regulator for the production of red cells and provides the model for oxygen-regulated gene expression like hypoxia-inducible factor (HIF) (Eckardt and Kurtz 2005). Hypoxia inducible factor-2 alpha (HIF-2 alpha) has appeared as the transcription factor which regulates EPO synthesis in the spleen. After irradiation, the amount of HIF-2 alpha increased significantly in vehicle-treated mice after 15 days, however there was no change in the expression in vehicle and BBT-059 treated mice as compared to non-irradiated controls on days 0 (2 h post-TBI) and 3. This increase in HIF-2 alpha expression was also accompanied by a significant increase in EPO after 15 days (Fig 8). The irradiated mice that were treated with BBT-059 had significantly lower (p <0.05) levels of HIF-2 alpha and EPO expression as compared to the vehicle-treated group. Based on these findings, we postulate that HIF-2 alpha and EPO expression are at high levels on day 15 post-irradiation. This data shows that BBT-059 inhibited the expression of hypoxic suppression by reducing the HIF-2 alpha and EPO expression, which suggested its role in protection against radiation injury.
Fig 8.
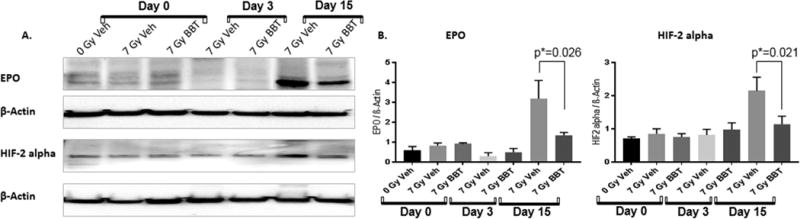
A) Western blot analysis of EPO and HIF-2 alpha in spleens of non-irradiated vehicle on day 0 (0 Gy veh), 7 Gy irradiated vehicle (7 Gy Veh) and 7 Gy BBT-059 treated (7 Gy BBT) on days 0 (2 h post-TBI), 3, and 15. B) Quantification of samples was done after analyzing three independent experiments from different samples and the bar graph shows the quantification of the ratio of protein of interest and its respective beta-Actin control.
Discussion
Development of medical countermeasures against radiation (prophylactic as well as mitigators) continues to be a significant capability shortfall and an unmet need for military personnel as well as exposed civilians. Here, we present a novel countermeasure, BBT-059, which demonstrate radioprotective as well as radiomitigative efficacy in CD2F1 mice exposed to TBI. This data also indicates that the drug has shown significantly better efficacy as a prophylactic countermeasure than it does as a mitigator. Although G-CSF is approved as a radiation countermeasure for ARS by the FDA, it is effective only when given after exposure as a multiple dose regimen, which may not be feasible for a mass casualty situation. In addition, modest effects in animal survival were observed with other cytokines, interleukin-1 (IL-1), interleukin-3 (IL-3), interleukin-6 (IL-6), interleukin-11 (IL-11), interleukin-12 (IL-12), EPO, TPO and granulocyte macrophage colony stimulating factors (GM-CSF) from sublethal doses of TBI (Weiss, 2009, Herodin, 2003, MacVittie, 2002, Patchen, 1992, Tanikawa, 1990, Van der Meeren, 2002, and Whitnall, 2000). Plett et al. published efficacy of various PEGylated growth factors or cytokines such as, G-CSF, GM-CSF, stem cell factor (SCF), tumor necrosis factor-alpha (TNF-alpha) or interferon-γ (INF-γ) (Plett, Chua et al. 2014). IL-11 in combination with SCF protected mice from TBI when administered prophylactically. Orally administered IL-11 was found to be effective in mice exposed to lethal dose of TBI (Hauer-Jensen 2014). IL-11 was given at various daily doses starting 24 h post-TBI, which continued up to 5 days (Hauer-Jensen 2014). Survival efficacy of a single (24 h post-TBI) and a 3-dose regimen (given on days 1, 3, and 5 post-TBI) of BBT-059 was compared in C57BL/6 mice (males and females) by Plett et al (Plett, Chua et al. 2014). The authors showed that 3-dose regimen of BBT-059 demonstrated better efficacy than single dose administration (given on days 1, 3, and 5 post-TBI). Plett et al (2014) also demonstrated efficacy of BBT-059 in both male and female C57BL/6 mice when administered 24 h post-TBI. In this study, we have shown that the drug is effective in the CD2F1 male mouse strain, which indicates that BBT-059 is effective in multiple strains and sexes. We also have shown that BBT-059 is effective starting from 24 h pre-radiation to 24 h post-radiation exposure, which is a fairly long window for protection of military personnel (before sending them to fields with probable radiation exposure for clean-up operations) as well as civilian population in a radiation fall-out field.
Radiation-induced peripheral blood neutropenia and thrombocytopenia can result in infection and hemorrhagic complications that could be lethal (Singh, Newman et al. 2015). In such cases the primary cause of lethality is sepsis which results from opportunistic infection due to declines in white blood cells, eventually developing into multiple-organ failure (Singh, Newman et al. 2015). BBT-059, administered 24 h pre-TBI, accelerated the regeneration and repletion of peripheral blood cells, particularly WBC, NEU, MON, RBC, LYM, and PLT. In earlier studies reported from our laboratory, we have shown that pretreatment of gamma-tocotrienol, enhances recovery of peripheral blood cells in non-lethally irradiated mice (Ghosh, Kulkarni et al. 2009). In this study, we show that BBT-059 appears to be a promising candidate to prevent radiation-induced infections and hemorrhagic complications by significantly accelerating the recovery from neutropenia and thrombocytopenia. It is interesting to note that WBC, RBC, MON, NEU and PLT counts were significantly higher in drug treated animals compared to irradiated control starting from day 4 post-radiation, which indicates that BBT-059 stimulated bone marrow (BM) to protect the hematopoietic system. Radiation-induced BM aplasia is one of the major consequences of apoptosis in hematopoietic stem and progenitor cells. Histopathology of sternal BM was used to evaluate the effect of radiation on overall cellularity and megakaryocyte cells over 30 days. Increased restoration of sternal BM was observed in pre-TBI BBT-059 treated animals and this recovery profile correlates with the peripheral blood recovery data. There was a significant increase in the total cellularity and megakaryocyte counts on day 15 in the drug treated animals. Similarly, recovery of BM stem and progenitor cells was accelerated when BBT-059 was given 24 h before radiation and this is in concordance with our observation with peripheral blood cell counts as well as histopathological analysis of sternal BM. The surviving progenitor cells from drug-treated animals were capable of self-renewal from day 7 onward and there were significantly higher number of colonies of megakaryocyte and erythroid lineages (GEM-CFU, BFU-E, CFU-E, and GM-CFU) on both days 7 and 15 in the drug treated group. In contrast, the progenitor population in vehicle-treated, irradiated animals exhibited poor self-renewal capacity and a resultant lack of colony formation. These findings clearly demonstrate the protective role of BBT-059 towards hematopoietic progenitor cells (HPCs). Therefore, restoring the damaged innate immune system may be one of the mechanisms of radioprotection provided by BBT-059.
The production of hematopoietic cytokines, EPO, TPO, and Flt3L are increased after radiation (Dainiak 2002, Ossetrova, Condliffe et al. 2014). TPO, a hematopoietic growth factor mainly produced by the cells in liver and kidney, regulates megakaryocyte and platelet production (Bartley, Bogenberger et al. 1994, Kaushansky, Lok et al. 1994, Lok, Kaushansky et al. 1994). Administration of recombinant TPO to rodents, nonhuman primates, and humans increased circulating platelets significantly (Lok, Kaushansky et al. 1994). As seen in Fig 7, BBT-059 treated non-irradiated and irradiated mice show small increase in EPO and TPO levels on days 0 and 1 (but no increase in Flt3L). The days 0 and 1 increase in TPO in non-irradiated animals correlates with the increase in megakaryocytes and platelets in non-irradiated BBT-059 treated mice (Fig 6 bar graph). Our data clearly shows a gradual increase in serum TPO levels in irradiated animals, with a maximum on day 7; the increase is significantly attenuated by BBT-059 on both days 7 and 15 post-TBI. Flt3L was found to be a radiation specific biomarker for bone marrow aplasia (Ossetrova, Condliffe et al. 2014, Ossetrova, Sandgren et al. 2014). In this study, we also have shown that serum levels of Flt3L are increased post-TBI with time and attenuated by BBT-059. EPO, a glycoprotein also produced by the cells in liver and kidney, regulates erythropoiesis and promotes survival by proliferation and differentiation of erythroid progenitor cells (Elliott, Pham et al. 2008, Paschos, Lykissas et al. 2008, Ossetrova, Condliffe et al. 2014). EPO is also induced by hypoxia and its receptor is mainly regulated under conditions of reduced oxygen saturation, which can be triggered by a reduction of the hematocrit (Rosenberger, Mandriota et al. 2002, Barshishat-Kupper, Mungunsukh et al. 2011). HIF-2 alpha, facilitates cellular adaptation to hypoxia by stimulating a wide spectrum of biological processes (Semenza 2001). Irradiation can cause hypoxic suppression of certain genes which might be activating HIF-2 alpha. Under hypoxia, HIF signaling is activated (Kaelin and Ratcliffe 2008). HIF-2 appears as the main transcriptional regulator of EPO. In this study, we have shown that circulating EPO levels are increased with time, reaching a peak on day 15 post-TBI. BBT-059 administration significantly reduced the EPO levels on both days 7 and 15 post-TBI. We also have shown that hematocrit levels decline with radiation, however, accelerated recovery from day 10 was observed in the BBT-059 treated group. Since hypoxia can be triggered by a decline in hematocrit, we determined the protein levels of HIF-2 alpha and EPO in spleens from irradiated animals by Western blot. Significantly higher levels of HIF-2 alpha and EPO protein were observed in the irradiated spleens on day 15 post-TBI. Inhibition of the HIF-2 alpha and EPO increases in the BBT-059 treated group suggested the drug’s ability to protect the animals from radiation-induced stress.
As seen in Fig 4, BBT-059 is able to increase RBC and PLT levels post-TBI without increasing serum levels or spleen levels of EPO and TPO (Fig 8). Thus, the mechanism involved here could be of rescuing / accelerating recovery of stem and progenitor cells early on, which are then able to differentiate to form RBCs and PLTs.
Conclusion
In summary, this study reports a promising radiation countermeasure which is unique in its application in preventing as well as mitigating radiation injury with a broad window for medical management. The drug protects the hematopoietic system as well as inhibits the upregulation of hematopoietic cytokines, at least in part, preventing lethal radiation injury. Finally, these data support the notion that BBT-059 should be developed further for potential use for our military personnel in harm’s way as well as for civilians exposed in a mass casualty scenario. Our future plan is to develop BBT-059 further to determine the optimum schedule of administration pre-TBI and a dose reduction factor (DRF).
Supplementary Material
Supl Fig 1. Recovery of peripheral blood cells (white blood cells (WBC), monocytes (MON) and lymphocytes (LYM)) of non-irradiated mice treated with vehicle formulation buffer (○) and BBT-059 (●) and irradiated (7 Gy) mice treated with formulation buffer (□) and BBT-059 (■). Either Formulation buffer or BBT-059 at 0.3 mg kg-1 was administered 24 h prior to irradiation. Day 0 represents day of irradiation. Data represented are mean ± standard error of the mean (SEM) for n=10 mice. Significant difference (p < 0.005 – 0.0125) between BBT-059-treated and vehicle-treated irradiated groups by ANOVA is indicated with an asterisk (*). Some data points in the figure do not have error bars that are visible because they are smaller than symbols.
Acknowledgments
The authors gratefully acknowledge to HM1 Paul O’Brien, Fialka G. Ward, Kefale Wuddie and Zemenu Aschenake for technical assistance and MAJ Leonora Dickson for histopathological evaluation. We also would like to thank Dr. Carmen Rios for her contribution in the scientific discussion and intellectual input. Support for the work was provided by grants from the National Institute of Allergy and Infectious Diseases (AA112044-001-04000 to SG) and the AFRRI Intramural funding RAB23338. The opinions contained herein are the private views of the authors, and are not necessarily those of Armed Forces Radiobiology Research Institute, the Uniformed Services of the University of the Health Sciences, or the Department of Defense.
Footnotes
Conflict of Interest: The following authors VPK, SB, NKS, SS and SPG declare no conflict of interest. CMF and GNC are employees of Bolder Biotechnology and have a financial interest in the company.
References
- Food and Drug Administration. FDA approves radiation countermeasure. Silver Spring; MD: 2015. Available at: https://www.fda.gov/emergencypreparedness/counterterrorism/medicalcountermeasures/aboutmcmi/ucm443245.htm. Accessed: 30 October 2017. [Google Scholar]
- Food and Drug Administration. Highlights of prescribing information for Neulasta. Silver Spring; MD: 2015. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5186lbl.pdf. Accessed: 30 October 2017. [Google Scholar]
- Barshishat-Kupper M, Mungunsukh O, Tipton AJ, McCart EA, Panganiban RA, Davis TA, Landauer MR, Day RM. Captopril modulates hypoxia-inducible factors and erythropoietin responses in a murine model of total body irradiation. Exp Hematol. 2011;39(3):293–304. doi: 10.1016/j.exphem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Bartley TD, Bogenberger J, Hunt P, Li YS, Lu HS, Martin F, Chang MS, Samal B, Nichol JL, Swift S, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994;77(7):1117–1124. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- Bruechner K, Bergmann R, Santiago A, Mosch B, Yaromina A, Hessel F, Hofheinz F, van den Hoff J, Baumann M, Beuthien-Baumann B. Comparison of [18F]FDG uptake and distribution with hypoxia and proliferation in FaDu human squamous cell carcinoma (hSCC) xenografts after single dose irradiation. Int J Radiat Biol. 2009;85(9):772–780. doi: 10.1080/09553000903043067. [DOI] [PubMed] [Google Scholar]
- Burnett AF, Biju PG, Lui H, Hauer-Jensen M. Oral interleukin 11 as a countermeasure to lethal total-body irradiation in a murine model. Radiat Res. 2013;180(6):595–602. doi: 10.1667/RR13330.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30(6):513–528. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- Eckardt KU, Kurtz A. Regulation of erythropoietin production. Eur J Clin Invest. 2005;35(Suppl 3):13–19. doi: 10.1111/j.1365-2362.2005.01525.x. [DOI] [PubMed] [Google Scholar]
- Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. 2008;36(12):1573–1584. doi: 10.1016/j.exphem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Ghosh SP, Kulkarni S, Hieber K, Toles R, Romanyukha L, Kao TC, Hauer-Jensen M, Kumar KS. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol. 2009;85(7):598–606. doi: 10.1080/09553000902985128. [DOI] [PubMed] [Google Scholar]
- Ghosh SP, Perkins MW, Hieber K, Kulkarni S, Kao TC, Reddy EP, Reddy MV, Maniar M, Seed T, Kumar KS. Radiation protection by a new chemical entity, Ex-Rad: efficacy and mechanisms. Radiat Res. 2009;171(2):173–179. doi: 10.1667/RR1367.1. [DOI] [PubMed] [Google Scholar]
- Hauer-Jensen M. Toward development of interleukin-11 as a medical countermeasure for use in radiological/nuclear emergencies. Dig Dis Sci. 2014;59(7):1349–1351. doi: 10.1007/s10620-014-3074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, Forstrom JW, Buddle MM, Oort PJ, Hagen FS, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369(6481):568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- Lee HT, Park SW, Kim M, Ham A, Anderson LJ, Brown KM, D'Agati VD, Cox GN. Interleukin-11 protects against renal ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2012;303(8):F1216–1224. doi: 10.1152/ajprenal.00220.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S, Kaushansky K, Holly RD, Kuijper JL, Lofton-Day CE, Oort PJ, Grant FJ, Heipel MD, Burkhead SK, Kramer JM, et al. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369(6481):565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- Ossetrova NI, Condliffe DP, Ney PH, Krasnopolsky K, Hieber KP, Rahman A, Sandgren DJ. Early-response biomarkers for assessment of radiation exposure in a mouse total-body irradiation model. Health Phys. 2014;106(6):772–786. doi: 10.1097/HP.0000000000000094. [DOI] [PubMed] [Google Scholar]
- Ossetrova NI, Sandgren DJ, Blakely WF. Protein biomarkers for enhancement of radiation dose and injury assessment in nonhuman primate total-body irradiation model. Radiat Prot Dosimetry. 2014;159(1–4):61–76. doi: 10.1093/rpd/ncu165. [DOI] [PubMed] [Google Scholar]
- Paschos N, Lykissas MG, Beris AE. The role of erythropoietin as an inhibitor of tissue ischemia. Int J Biol Sci. 2008;4(3):161–168. doi: 10.7150/ijbs.4.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunesku T, Vogt S, Irving TC, Lai B, Barrea RA, Maser J, Woloschak GE. Biological applications of X-ray microprobes. Int J Radiat Biol. 2009;85(8):710–713. doi: 10.1080/09553000903009514. [DOI] [PubMed] [Google Scholar]
- Plett PA, Chua HL, Sampson CH, Katz BP, Fam CM, Anderson LJ, Cox GN, Orschell CM. PEGylated G-CSF (BBT-015), GM-CSF (BBT-007), and IL-11 (BBT-059) analogs enhance survival and hematopoietic cell recovery in a mouse model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2014;106(1):7–20. doi: 10.1097/HP.0b013e3182a4dd4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Interleukin-11 protects the clonogenic stem cells in murine small-intestinal crypts from impairment of their reproductive capacity by radiation. Int J Cancer. 1995;62(3):356–361. doi: 10.1002/ijc.2910620321. [DOI] [PubMed] [Google Scholar]
- Potten CS. Protection of the small intestinal clonogenic stem cells from radiation-induced damage by pretreatment with interleukin 11 also increases murine survival time. Stem Cells. 1996;14(4):452–459. doi: 10.1002/stem.140452. [DOI] [PubMed] [Google Scholar]
- Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002;13(7):1721–1732. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13(2):167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- Singh VK, Newman VL, Berg AN, MacVittie TJ. Animal models for acute radiation syndrome drug discovery. Expert Opin Drug Discov. 2015;10(5):497–517. doi: 10.1517/17460441.2015.1023290. [DOI] [PubMed] [Google Scholar]
- Singh VK, V, Newman L, Romaine PL, Wise SY, Seed TM. Radiation countermeasure agents: an update (2011-2014) Expert Opin Ther Pat. 2014;24(11):1229–1255. doi: 10.1517/13543776.2014.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift SN, Pessu RL, Chakraborty K, Villa V, Lombardini E, Ghosh SP. Acute toxicity of subcutaneously administered vitamin E isomers delta- and gamma-tocotrienol in mice. Int J Toxicol. 2014;33(6):450–458. doi: 10.1177/1091581814554929. [DOI] [PubMed] [Google Scholar]
- Vigneulle RM, Rao S, Fasano A, MacVittie TJ. Structural and functional alterations of the gastrointestinal tract following radiation-induced injury in the rhesus monkey. Dig Dis Sci. 2002;47(7):1480–1491. doi: 10.1023/a:1015846514471. [DOI] [PubMed] [Google Scholar]
- Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, Macvittie TJ, Mason KA, Medhora MM, Moulder JE, Okunieff P, Otterson MF, Robbins ME, Smathers JB, McBride WH. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173(4):557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supl Fig 1. Recovery of peripheral blood cells (white blood cells (WBC), monocytes (MON) and lymphocytes (LYM)) of non-irradiated mice treated with vehicle formulation buffer (○) and BBT-059 (●) and irradiated (7 Gy) mice treated with formulation buffer (□) and BBT-059 (■). Either Formulation buffer or BBT-059 at 0.3 mg kg-1 was administered 24 h prior to irradiation. Day 0 represents day of irradiation. Data represented are mean ± standard error of the mean (SEM) for n=10 mice. Significant difference (p < 0.005 – 0.0125) between BBT-059-treated and vehicle-treated irradiated groups by ANOVA is indicated with an asterisk (*). Some data points in the figure do not have error bars that are visible because they are smaller than symbols.


