Abstract
BACKGROUND
Emergence from general anesthesia is sometimes associated with emergence agitation (EA). Several putative risk factors for EA have been described. Sleep disordered breathing (SDB) is highly prevalent in children and because it is typically associated with abnormal sleep morphology (snoring, vocalization and thrashing around), SDB may be a critical risk factor for EA. To our knowledge, this has not been studied in the pediatric perioperative setting. We hypothesized that there are differences based on SDB diagnosis in the frequency of EA in children following general anesthesia for ambulatory surgery.
METHODS
A prospective, observational, cohort study of 1076 children aged 4–17 years who underwent elective outpatient surgery was conducted. Differences in probability of EA were assessed by comparing baseline clinical and perioperative parameters using bi-variable and multivariable logistic regression analyses.
RESULTS
Of the 1076 children, 66 (6.1%) had EA. Compared with those without EA, children with EA were younger (p<0.001), more likely to have received midazolam premedication (p=0.048) and more likely to have had mask induction (p<0.001). They were also more likely to have a preoperative diagnosis of baseline SDB (p=0.008). On multivariable analysis, SDB, severe obesity, decreasing age in years, increasing first arousal pain score and intraoperative use of sevoflurane were independently predictive of EA (p<0.05 for all variables). SDB did not appear to moderate the effect of severe obesity on the probability of EA (p=0.124).
CONCLUSIONS
In addition to other known risk factors, SDB and severe obesity may be critical independent predictors of EA in children. Mechanisms underlying these observations deserve further elucidation.
Keywords: Children, Post-anesthesia care unit, delirium, agitation, sleep disordered breathing, obstructive sleep apnea, severe obesity
Introduction
Emergence from general anesthesia (GA) is a passive process characterized by gradual return of consciousness following discontinuation of anesthetic drugs at the end of surgery. In the vast majority of children, this is a variable, largely uneventful process whereby patients return to “street readiness” and are discharged from the post-anesthesia care unit (PACU). However, for some children, emergence from GA is complicated by emergence agitation (EA) which covers a broad spectrum of behavioral disturbances including agitation, restlessness, crying, moaning and non-purposeful movements (1,2). Other terminologies used to describe these physical manifestations include emergence delirium (ED) and post-anesthesia excitement (3).
EA in the ambulatory setting can be quite distressing and may blemish the entire perioperative experience. Furthermore, dealing with children manifesting features of EA in the PACU places enormous burden on the PACU nurses, is time consuming and often delays the time parents are reunited with their children after surgery and it may substantially prolong PACU stay (3–5). EA could also lead to disruption of surgical wounds, loss of intravenous line and may predispose PACU nurses to physical injuries especially if they are trying to restrain a combative overweight or obese child (6,7).
Despite its profound and alarming nature, the etiology of pediatric EA remain largely speculative. Several putative risk factors for EA have been described (1–3, 7–9). Many of these have focused on anesthetic agents, especially volatile anesthetic agents like Sevoflurane (7,8). Very few studies have specifically explored patient-level factors as potential predictors of EA (6,9). For example, EA is reported to be more common in boys and in younger children (9). One increasingly prevalent patient-specific factor whose association with EA has hitherto been unexplored is sleep disordered breathing (SDB).
SDB is increasingly prevalent in the general and the pediatric surgical population (10). SDB refers to a spectrum of airway and systemic disorders including habitual snoring, obstructive hypoventilation (OH), and obstructive sleep apnea (OSA) (11,12). Given that abnormal sleep behavior (snoring, vocalization and thrashing around) (12) are features of sleep disordered breathing (SDB), and the increasing prevalence of SDB among the pediatric surgical population, the present report describes the incidence of EA in patients undergoing elective ambulatory surgery and specifically explored the relationship between SDB and EA using data from an on-going prospective observational study. The hypothesis tested was that the proportion of children manifesting features of PACU EA will be higher among those with SDB compared with their peers without SDB.
Methods
Study Design
This report is an excerpt of a larger on-going prospective, cross-sectional study to determine the incidence and risk factors for postoperative pain requiring treatment in the PACU among children aged 4–17 years undergoing elective, ambulatory surgical procedures at the Mott Children’s Hospital (Ann Arbor, MI). The Institutional Review Board of the University of Michigan approved the study. Present analyses included patients recruited from January 24, 2015 to April 04, 2016.
Data Source and Subject Profiles
We enrolled patients on randomly selected weekdays during the preoperative interview. All patients scheduled for outpatient surgery on selected days were approached for possible enrollment. Perioperative caregivers (anesthesiologists and nurses) did not know subject recruitment days in advance nor were they aware of the study’s hypotheses. In keeping with routine clinical care, all perioperative interventions were at the discretion of the anesthesia care givers. Trained research assistants (RAs) collected baseline clinical and anthropometric data on study enrollees. Patients with cardiac disease, severe respiratory disorders, or those with severe neuro-cognitive impairment were excluded from the study.
Outcome measures
Our primary outcome measure was the occurrence of PACU emergence agitation, defined as presence of non-purposeful movements, restlessness, or thrashing; incoherence; inconsolability; and unresponsiveness (2,4). EA was recorded as a categorical (yes/no) variable by experienced PACU nurses. Our secondary objective was the association of EA with PACU length of stay, defined as the time in minutes from PACU admission to PACU discharge.
Covariates
Primary exposure variable was SDB defined as the presence of one or more of the following: history of OSA diagnosis, habitual snoring or witnessed cessation of breathing during sleep. OSA diagnosis was sought by the specific question, “has your child ever been diagnosed with OSA?” Habitual snoring was defined as parental or care giver report of loud snoring in the child for at least 3 or more nights per week (11).
Anesthetics and perioperative medications (including preoperative midazolam), mode of induction of general anesthesia (mask vs. intravenous), and intraoperative airway device used (face mask, laryngeal mask airway or endotracheal tube), were documented for all patients. Other recorded variables include, age (yr.), sex, surgical specialty, height (cm), weight (Kg), body mass index (BMI), as well as duration of surgery and anesthesia. Body Mass Index (BMI) was calculated as weight in kilograms divided by the square of the height in meters (BMI = kg/m2). BMI was then transformed into a categorical variable for the grouping of children into high and normal BMI classes. Weight status among children and adolescents aged 2 to 19 years is most frequently defined based on BMI percentiles (13). Here, normal BMI indicates sex-specific BMI between the 5th-84th percentile, while high BMI (overweight and obese) denotes sex-specific BMI ≥ 85th percentile according to reference growth charts from the National Center for Health Statistics (NCHS)/Centers for Disease Control and Prevention (CDC) (14). Using BMI percentile identifies children with excess adiposity with acceptable accuracy particularly the upper end of the sex-specific BMI distribution (15). Additionally, the 85th percentile for BMI is a reasonable cutoff because previous studies have established that it provided a sensitivity of 67% in males and 75% in females for total body fat screening (14). Relatedly, we also defined severe obesity according to the recommendations of the American Heart Association as age and sex-specific BMI percentile greater than 120% of the 95th percentile or a BMI greater than 35kg/m2 whichever is lower (16).
Intraoperative analgesia use was recorded as a categorical (yes/no) variable as well as by type (opioid or non-opioid). Perioperative management including choice and timing of analgesic as well as the type volatile anesthetic was at the discretion of the anesthesia care giver. Study subjects were observed throughout their PACU stay by experienced PACU nurses and trained RAs. Pain was scored by each patient’s bedside nurse on arousal or within 15minutes of PACU admission and at scheduled intervals throughout the PACU stay in accordance with routine clinical practice using the Faces Legs Activity Cry Consolability (FLACC) behavioral observation instrument (17) or the 0–10 Numerical Rating Scale (NRS) which are widely utilized valid and reliable scales for the age range included in our study (18).
Statistical Analysis
Data analyses were carried out using PASW Statistics v.22.0 program for Windows (SPSS Inc. Chicago, IL). The primary hypothesis tested was that the rates of PACU EA would differ based on preoperative SDB category. Basic descriptive statistics, including means, standard deviations and percentages were calculated for demographic and clinical data. Crude incidence of SDB, EA, BMI categories were described as simple proportions. Univariate factors associated with PACU EA were assessed with Pearson’s Chi-squared or t-test as necessary. Numeric PACU pain scores (0–10 scale) were transformed thus: <3 = mild pain, ≥4 = moderately severe pain and ≥7 = severe pain).
Multivariable Regression Analysis
To fit a multivariable logistic regression model, we estimated the Pr(Y=1|X), where Y is the binary dependent variable (PACU EA yes/no) and X is the vector of the covariates. The probability of PACU EA was thus modeled with age, gender, SDB history, use of midazolam premedication, mask induction, intra-operative opioid use and first recorded PACU pain score included as predictors. Given the known relationship between SDB and severe obesity () and in order to determine whether this relationship moderated the occurrence of EA, we included the interaction between SDB and severe obesity as covariates in the logistic regression model. The results from the logistic regression analyses are presented as adjusted odds ratios (ORs) with 95% confidence intervals (CI). Goodness of fit of the logistic regression models was assessed using the Hosmer-Lemeshow test (19). Furthermore, the model’s prediction strength was assessed using Receiver operating characteristic (ROC) analyses and area under the curve (AUC). All reported P values were two-sided and a P value of <0.05 was considered to be statistically significant.
Results
Of the 1076 children, more than half (55.8 %) were boys. The mean age of the subjects was 9.64±4.04 years and about one third (31.6%) had a preoperative history consistent with SDB. EA was documented in 66 (6.1%) patients. The characteristics of the 1076 study participants are summarized in Table 1. A wide variety of surgical procedures were performed (otolaryngological, orthopedic, ophthalmologic, urologic, general surgical, dental and plastic surgery). Expectedly, patients with EA were significantly younger than the control group (Table 1). Other demographic and clinical factors with statistically significant association with EA include history of severe obesity, preoperative premedication with midazolam, mask induction, and use of inhalational anesthetic agents. On the other hand, IV induction (p=0.01) and intraoperative administration of Propofol (p=0.001) were associated with decreased rates of EA (Table 1).
Table 1.
Patient perioperative characteristics according to EA categories
| Variables | EA (Cases) (N=66) |
No EA (Controls) (N=1010) |
p-value |
|---|---|---|---|
| Continuous variables (Mean ±SD) | |||
| Age (years) | 7.09±3.35 | 9.81±4.03 | <0.001 |
| Weight (kg) | 30.28±19.65 | 39.14±21.28 | 0.002 |
| BMI (kg/m2) | 18.11±5.95 | 19.02±5.14 | 0.367 |
| Surgery duration | 36.88±29.65 | 38.72±40.53 | 0.743 |
| PACU time (min) | 120.74±50.05 | 103.11±53.06 | 0.017 |
| OR MS equivalents/kg | 0.09±0.06 | 0.08±0.07 | 0.460 |
| PACU MS equivalents/kg | 0.03±0.03 | 0.01±0.02 | <0.001 |
| Categorical variables (%) | |||
| Sex (male) | 62.9 | 55.3 | 0.244 |
| Past surgical history | 58.1 | 66.0 | 0.203 |
| SDB history | 46.8 | 30.7 | 0.008 |
| Mask induction | 91.9 | 66.7 | <0.001 |
| Midazolam premedication | 46.8 | 34.4 | 0.048 |
| Tylenol premedication | 45.2 | 31.7 | 0.011 |
| Overweight/Obese | 35.5 | 28.8 | 0.261 |
| OR opioid | 93.5 | 85.8 | 0.085 |
| OR Non-opioid | 66.1 | 54.0 | 0.063 |
| OR Dexamethasone | 82.3 | 66.8 | 0.011 |
| OR Sevoflurane | 91.9 | 78.8 | 0.012 |
| OR Isoflurane | 79.0 | 66.8 | 0.045 |
| OR Nitrous Oxide | 93.5 | 76.0 | 0.001 |
| OR Propofol | 37.1 | 67.2 | <0.001 |
Abbreviations: EA = Emergence Agitation; BMI = body mass index; OR = Operating room; PACU = post-anesthesia care unit; MS =morphine sulfate; SDB = Sleep Disordered Breathing;
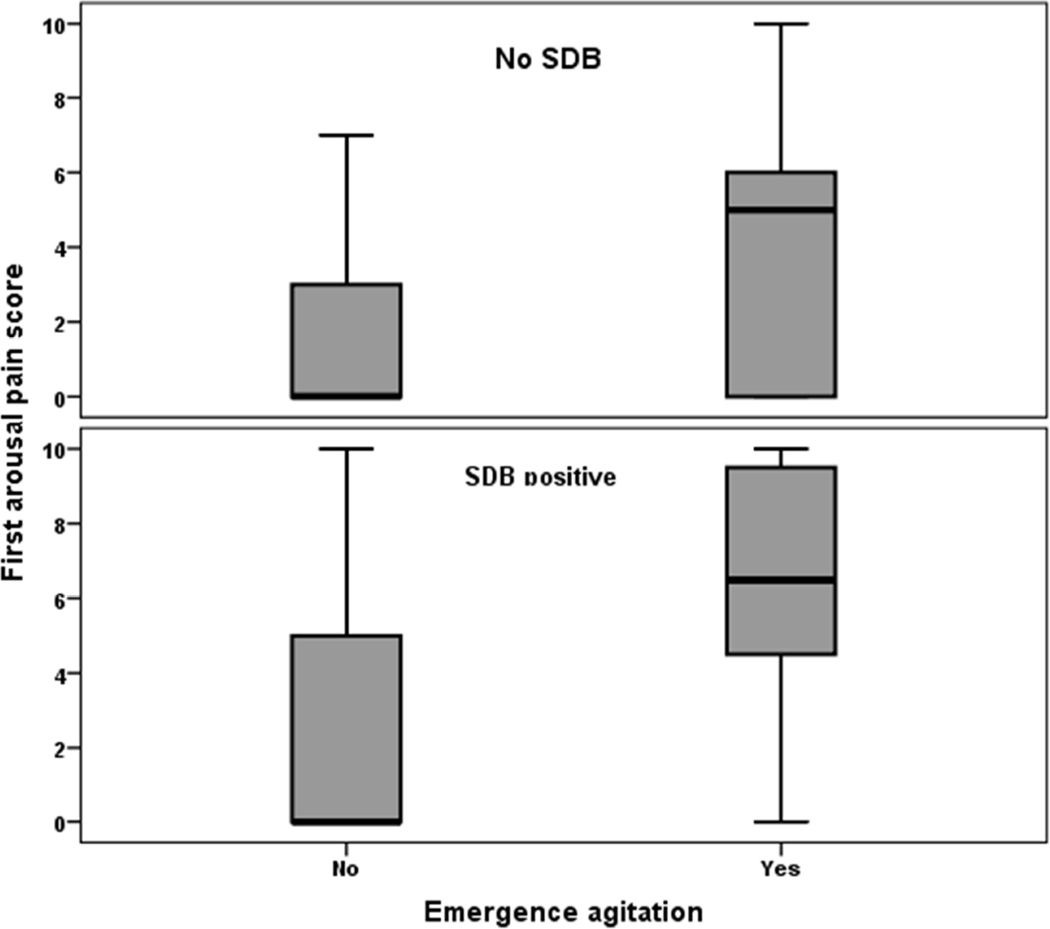
Overall, children with SDB had significantly higher unadjusted odds of EA compared with children without SDB (8.5% vs. 4.5%; OR = 1.98, 95%CI = 1.18–3.33; p=0.008). Similarly, children with severe obesity demonstrated significantly higher rates of EA compared to their peers in lower BMI category (14.8% vs. 5.0%; OR = 3.29, 95%CI = 1.67–6.46; p<0.001). On average, children with EA were assigned significantly higher first arousal pain score in the presence (p<0.001) or absence (p<0.001) of preoperative SDB diagnosis (Fig. 1). PACU IV opioid administration was significantly associated with EA with or without documented pain. Among patients with documented mild pain (pain score <3) more patients with EA received IV opioid compared to those without EA (42.4% vs. 23.3%; p=0.012). A similar pattern was observed among those with pain score >3 (55.2% vs. 33.4%; p=0.019).
Fig. 1.
Boxplot showing the distribution of first arousal pain scores across EA and SDB groups. Children in the EA group had significantly higher first arousal pain scores regardless of SDB grouping.
Abbreviations: EA = Emergence Agitation; SDB = Sleep Disordered Breathing;
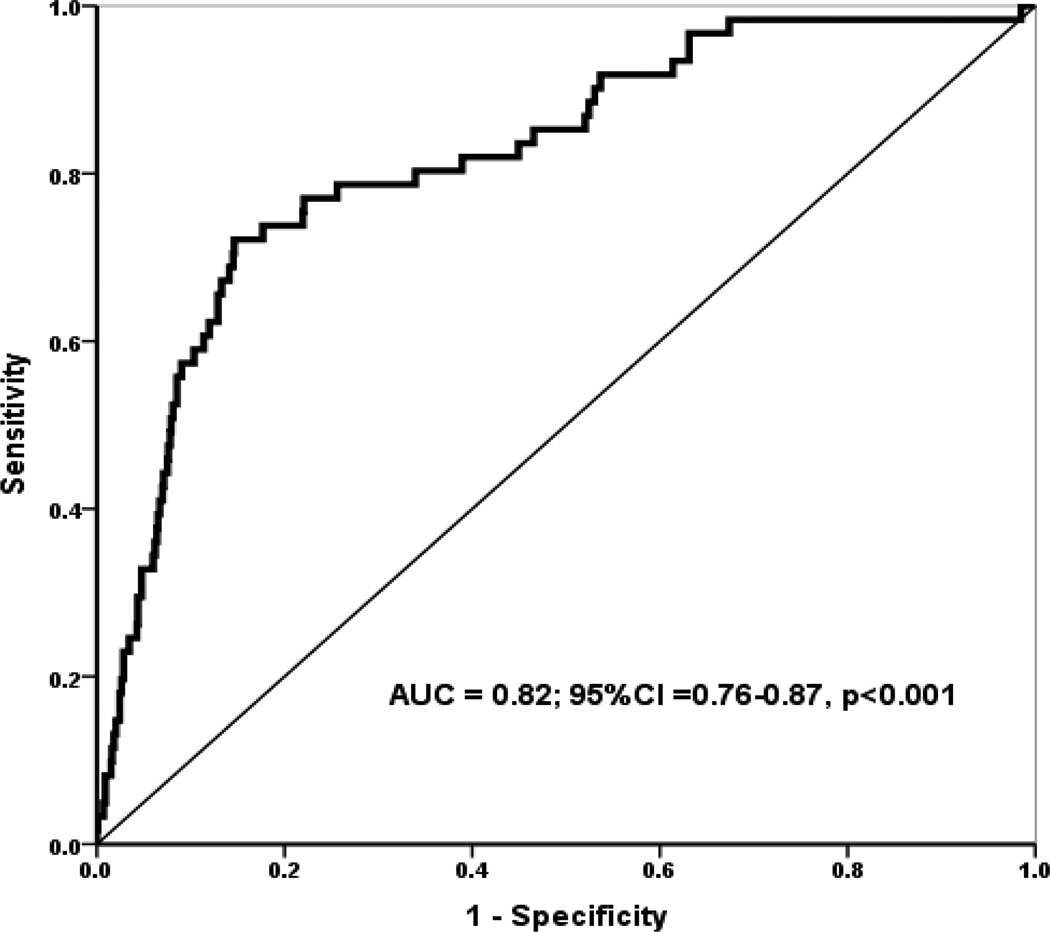
Multivariate logistic regression indicated that the factors detailed in Table 2 were independently associated with the occurrence of EA. Model diagnostic parameters are included in the table. The ROC curve analysis of this model showed good predictive ability - AUC of 0.82; 95% CI = 0.76–0.87; p=0.001 (Fig. 2). Clinically severe obesity was associated with the highest odds of EA. Specifically, when controlling for the other covariates in the model, severe obesity was associated with three-fold higher odds of EA (OR = 3.38; 95% confidence interval = 1.60 – 7.15, p < 0.001). Furthermore, preoperative SDB diagnosis was associated with a 16% increased odds of EA. Interestingly, the interaction term for severe obesity and SD was not statistically significant (OR = 1.19; 95% confidence interval = 0.64 – 1.39, p = 0.124). As shown in Table 2, the predicted probability of EA was associated with increasing first arousal pain score. Specifically, every unit increase in first arousal pain score was associated with a 26% higher odds of EA. Other factors significantly associated with EA in the adjusted model are displayed in Table 2. Of interest, midazolam premedication and ASA status were not significant predictors of EA in our regression model.
Table 2.
Result of binary logistic regression model to estimate the adjusted odds ratio for the factors associated with emergence agitation.
| Variables | Odds ratios | 95% CI | p value |
|---|---|---|---|
| First Arousal pain score | 1.26 | 1.17 – 1.36 | <0.001 |
| Severe Obesity | 3.38 | 1.60– 7.15 | 0.001 |
| OR Dexmedetomidine | 0.19 | 0.07 – 0.54 | 0.002 |
| Sex (Male) | 1.32 | 1.05 – 2.30 | 0.036 |
| Age (per year) | 0.89 | 0.81– 0.98 | 0.029 |
| OR Propofol | 0.51 | 0.26 – 0.97 | 0.039 |
| SDB history | 1.16 | 1.05 – 2.04 | 0.040 |
| OR Sevoflurane | 1.19 | 1.02 – 3.45 | 0.037 |
| ASA 1&2 vs. 3 | 0.59 | 0.17 – 2.05 | 0.412 |
| Midazolam premedication | 1.43 | 0.79 – 2.57 | 0.233 |
| SDB*Severe Obesity | 1.19 | 0.64 – 1.39 | 0.124 |
| Pseudo R2= 0.22 | |||
| Hosmer-Lemeshow test = Chi sq = 3.05, df=8; p=0.115 | |||
| N = 1076 | |||
Abbreviations: SDB = Sleep Disordered Breathing; OR = Operating room; ASA = American Society of Anesthesiologists; CI = confidence interval
Fig. 2.
Receiver operator characteristic (ROC) curve evaluating the sensitivity and specificity of the model predicting EA using a panel of clinical and demographic variables. Area under the curve (AUC) for the predictors was 0.82; 95% CI = 0.76–0.87; p <0.001. AUC for the ROC indicates the usefulness of a test (our model) in predicting a binomial outcome (EA yes/no). A value of 0.82 indicates ‘good’ model predictive ability
Abbreviations: EA = Emergence Agitation; CI =confidence interval.
Discussion
The findings of the current study provide new insight into the factors associated with pediatric EA. The central question we asked was whether baseline SDB diagnosis was associated with increased incidence of EA. Using a prospective, observational cross-sectional study design, we studied children and adolescents who underwent elective ambulatory operations, and found an incidence of EA consistent with published data (1–3). We report, for the first time in a large sample of ambulatory pediatric surgical cohort, that baseline SDB was associated with a higher probability of EA. We further found that clinically severe obesity was a strong independent predictor of PACU EA. Importantly, SDB did not appear to moderate the effect of severe obesity on EA in our study cohort. Additionally, our results also confirmed a number of patient level and anesthetic factors previously shown to be predictors of pediatric EA (1–7).
EA is a well described psychologic and physical phenomena displayed by some children recovering from general anesthesia. EA is often described as both a disorder of receptivity (abnormal reception of auditory or visual cues – child is inconsolable even by familiar voices or toys) and of perceptivity (heightened perception of stimuli and hyperactive motor behavior) (20). Although often short-lived, it may be prolonged and can be quite distressing and may blemish the entire perioperative experience (3,4). Managing a child with EA in the PACU can be extremely stressful for caregivers as well as other patients and may delay the time parents are reunited with their children (4). EA could also lead to disruption of surgical wounds, loss of intravenous line and may predispose PACU nurses to physical injuries especially if they are trying to restrain a combative overweight or obese child (2,3).
Reported incidence of EA varies widely from 5–50% depending on the definition used, age range of study subjects and type of anesthesia and surgical procedures (1). We found an incidence of EA of 6.1% in our subjects which could be an indication of our use of a categorical definition and on the wide age range of patients in our study cohort.
SDB is an increasingly prevalent condition in children (21) which increases the likelihood that children presenting for anesthesia and surgery would either have a formal diagnosis of OSA or have many features consistent with SDB, often referred to as clinical or probable OSA (22). Previous investigators have shown that children with preoperative SDB diagnosis demonstrated postoperative negative behaviors that lasted for several days to weeks (23). These reports have focused on negative behaviors occurring after PACU discharge. Our results indicate that EA (a form of postoperative negative behavior) occurs more frequently in children with SDB and this develops in the PACU. To our knowledge, this is the first time this association is being reported.
Mechanisms underlying childhood EA remain largely elusive although several risk factors have been described (1–5). The similarities between general anesthesia and natural sleep (24) and the sleep fragmentation as well as abnormal sleep behavior (thrashing around, vocalization, gross motor movements) described in SDB may help explain some of the observed findings in our subjects with this diagnosis.
Another possibility is the association of hypoxia with SDB. Postoperative respiratory complications are more frequent in children with SDB (25) and some of the observed PACU restlessness may be indicative of hypoxia in our study subjects. Unfortunately, we did not record the PACU oxygen saturation values at the time these patients were classified as having EA by their PACU nurses. There is however, no reason for us to believe that hypoxic children with SDB are more likely to be given a label of EA compared to their peers without SDB.
Postoperative pain is another potential confounder in our subjects. The association between EA and PACU pain is well documented (7,26) and separating the two is often very difficult. Our study, like those of previous investigators (4, 27) found that children with EA were often assigned higher PACU pain scores. We also found that these children were more likely to receive IV pain medications in the PACU. This agrees with previous studies which showed that PACU nurses will generally treat a child for pain before labelling the child as having EA (4). To this end, EA is a diagnosis of exclusion.
Our observation of a strong association between severe obesity and EA is concerning. Childhood obesity is increasingly prevalent and despite massive health promotion efforts, it remains a major threat to the health of American children (13,14). Current estimates indicates that about 2–7% of children are classifiable as clinically severely obese (BMI > 120% of the 95th age and sex-specific BMI percentile) (16).
In this study, we report for the first time, that severe childhood obesity is associated with a three-fold higher odds of EA. This observation is important for several reasons. First, it may provide another mechanistic explanation for the pathophysiologic basis of EA. Future studies should explore possible role of systemic inflammation in EA in obese children. Second, and on a more practical level, the association of severe obesity with EA creates unique challenges for PACU nurses. Tying to contain or restrain a restless agitated severely obese child could lead to injury to the recovery room staff and/or the patient.
Taken together, the increasing prevalence of severe pediatric obesity coupled with its association with EA suggests that this is a group that demands targeted preventive management. Such “personalized perioperative care” could include avoidance of anesthetic agents known to be strongly associated with EA (5–7) in severely obese children. Additionally, prophylactic use of agents that decrease EA such as Dexmedetomidine and Propofol (26) may be warranted in severely obese children. Clearly, mechanisms underlying the association of severe obesity with EA deserve further elucidation.
Limitations
The strengths of this study include the prospective, consecutive subject enrollment as well as the standardized method of data collection by trained RAs. Despite these, some limitations should be acknowledged. This was a secondary analysis of prospectively collected data hence detailed characterization of phenotype specific for EA were unavailable. Also, EA was recorded as a categorical variable so it was impossible to compare grades or severity of EA. Additionally, diagnosis of SDB was entirely clinical (no polysomnography data) and because symptoms were combined, possibility of misclassification bias exists. However, cost implications of polysomnography makes it impractical to insist that children undergo this test preoperatively. To this end, our data actually mirrors “real-life” clinical practice as most institutions do not insist on preoperative polysomnography in children. Finally, the observational nature of the present study introduces heterogeneity into the data, which increases the possibility of unmeasured covariates that may be influential to the primary outcome variable.
Conclusions
Among a large cohort of children undergoing elective outpatient operations we identified two new risk factors for EA: SDB and clinically severe obesity. Prospective identification of children or specific surgical procedures associated with PACU EA should enable adequate planning and management of these patients in an outpatient setting. Unraveling the separate or joint mechanistic roles of SDB and severe childhood obesity in the context of PACU EA are key next steps in our understanding of postoperative negative behaviors in children.
Acknowledgments
National Institute of General Medical Sciences (NIGMS) grant number K23 GM104354 supported Dr. Nafiu’s work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cole JW, Murray DJ, McAllister JD, et al. Emergence behavior in children: defining the incidence of excitement and agitation following anesthesia. Paediatr Anaesth. 2002;12:442–447. doi: 10.1046/j.1460-9592.2002.00868.x. [DOI] [PubMed] [Google Scholar]
- 2.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 3.Aouad MT, Nasr VG. Emergence agitation in children: an update. Current Opinion in Anesthesiology. 2005;18(6):614–619. doi: 10.1097/01.aco.0000188420.84763.35. [DOI] [PubMed] [Google Scholar]
- 4.Voepel-Lewis T, Burke C, Hadden SM, et al. Nurses’ diagnoses and treatment decisions regarding care of the agitated child. J Perianesth Nurs. 2005;20:239–348. doi: 10.1016/j.jopan.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Przybylo HJ, Martini DR, Mazurek AJ, et al. Assessing behaviour in children emerging from anaesthesia: Can we apply psychiatric diagnostic techniques? Paediatr Anaesth. 2003;13:609–616. doi: 10.1046/j.1460-9592.2003.01099.x. [DOI] [PubMed] [Google Scholar]
- 6.Chidambaran V, Sadhasivam S, Diepstraten J, Esslinger H, Cox S, Schnell BM, Samuels P, Inge T, Vinks AA, Knibbe CA. Evaluation of propofol anesthesia in morbidly obese children and adolescents. BMC anesthesiology. 2013;13(1):1. doi: 10.1186/1471-2253-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis PJ, Greenberg JA, Gendelman M, et al. Recovery characteristics of sevoflurane and halothane in preschoolaged children undergoing bilateral myringotomy and pressure equalization tube insertion. Anesth Analg. 1999;88:34–38. doi: 10.1097/00000539-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kain ZN, Caldwell-Andrews AA, Maranets I, McClain B, Gaal D, Mayes LC, Feng R, Zhang H. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99(6):1648–1654. doi: 10.1213/01.ANE.0000136471.36680.97. [DOI] [PubMed] [Google Scholar]
- 9.Uezono S, Goto T, Terui K, et al. Emergence agitation after sevoflurane versus propofol in pediatric patients. Anesth Analg. 2000;91:563–566. doi: 10.1097/00000539-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Tan HL, Kheirandish-Gozal L, Gozal D. Obstructive sleep apnea in children: update on the recognition, treatment and management of persistent disease. Expert Rev Respir Med. 2016 Mar;21:1–9. doi: 10.1586/17476348.2016.1163224. [DOI] [PubMed] [Google Scholar]
- 11.Perez IA, Ward SL. The snoring child. Pediatr Ann. 2008;37(7):465–470. doi: 10.3928/00904481-20080701-11. [DOI] [PubMed] [Google Scholar]
- 12.Dehlink E, Tan HL. Update on paediatric obstructive sleep apnoea. J Thorac Dis. 2016;8(2):224–235. doi: 10.3978/j.issn.2072-1439.2015.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120:S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 15.Lazarus R, Baur L, Webb K, Blyth F. Body mass index in screening for adiposity in children and adolescents: Systematic evaluation using receiver operating characteristic curves. Am J Clin Nutr. 1996;63(4):500–506. doi: 10.1093/ajcn/63.4.500. [DOI] [PubMed] [Google Scholar]
- 16.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90(5):1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 17.Voepel-Lewis T, Merkel S, Tait AR, et al. The reliability and validity of the Face, Legs, Activity, Cry, Consolability observational tool as a measure of pain in children with cognitive impairment. Anesth Analg. 2002;95:1224–1229. doi: 10.1097/00000539-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Voepel-Lewis T, Burke C, Jeffreys N, et al. Do 0–10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth Analg. 2011;112:415–421. doi: 10.1213/ANE.0b013e318203f495. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;15;16(9):965–980. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100:1138–1145. doi: 10.1097/00000542-200405000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, Fedok F, Vlasic V, Graff G. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishman SL, Tawfik KO, Smith DF, Cheung K, Pringle LM, Stephen MJ, Everett TL, Stierer TL. Screening for Pediatric Obstructive Sleep Apnea before Ambulatory Surgery. J Clin Sleep Med. 2015;15;11(7):751–755. doi: 10.5664/jcsm.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tait AR, Voepel-Lewis T, O'Brien LM. Postsurgical behaviors in children with and without symptoms of sleep-disordered breathing. Perioper Med (Lond) 2014;3:8. doi: 10.1186/2047-0525-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giménez S, Romero S, Alonso JF, Mañanas MÁ, Pujol A, Baxarias P, Antonijoan RM. Monitoring sleep depth: analysis of bispectral index (BIS) based on polysomnographic recordings and sleep deprivation. J Clin Monit Comput. 2015 Nov 14; doi: 10.1007/s10877-015-9805-5. [DOI] [PubMed] [Google Scholar]
- 25.Nafiu OO, Prasad Y, Chimbira WT. Association of childhood high body mass index and sleep disordered breathing with perioperative laryngospasm. Int J Pediatr Otorhinolaryngol. 2013;77(12):2044–2048. doi: 10.1016/j.ijporl.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Kanaya A. Emergence agitation in children: risk factors, prevention, and treatment. J Anesth. 2016;30(2):261–267. doi: 10.1007/s00540-015-2098-5. [DOI] [PubMed] [Google Scholar]
- 27.Somaini M, Sahillioğlu E, Marzorati C, Lovisari F, Engelhardt T, Ingelmo PM. Emergence delirium, pain or both? A challenge for clinicians. Paediatr Anaesth. 2015;25(5):524–529. doi: 10.1111/pan.12580. [DOI] [PubMed] [Google Scholar]