Most in the nephrology community would agree on the necessity of intravenous iron to treat anemia for patients receiving dialysis. The cumulative average yearly blood loss is high among individuals receiving hemodialysis and leads to an estimated loss of over 2 g of iron per annum (1). Functional iron deficiency results from enhanced iron utilization from erythropoiesis stimulating agents (ESAs). Administration of oral iron cannot reliably replete stores to match ongoing demands because of impaired intestinal absorption mediated by regulatory proteins, such as hepcidin and ferroportin (2).
There is less agreement, however, about the safest strategy of intravenous iron administration, namely dosing (3). Contemporary practice patterns reveal two general approaches for iron repletion—low dose and high dose. Low dose involves 25–50 mg weekly, whereas high dose includes 100 mg administered for five to ten consecutive hemodialysis sessions, leading to a cumulative monthly dose of approximately 200 mg with the former approach and 500–1000 mg with the latter approach. In any given year, a singular patient may be prescribed a combination of low- and high-dose therapies on the basis of monthly laboratory parameters—hemoglobin, transferrin saturation, and ferritin. Dosing approaches in peritoneal dialysis are less well characterized than those in hemodialysis.
The amount of iron delivered in a single session and cumulatively over a month to a patient receiving high-dose therapy contrasts starkly with the 20–25 mg that are handled daily by homeostatic pathways involving the bone marrow, macrophages, senescent red blood cells, enterocytes, hepatocytes, the spleen, and iron binding proteins (2). Thus, there have been concerns that high doses of any intravenous iron formulation—with its oxyhydroxide core and a dynamic ratio of labile ferric and ferrous iron—could overwhelm these pathways and lead to clinical disease. These safety concerns fall into potentially overlapping categories of hypersensitivity, cardiovascular disease, infections, iron overload, and mortality.
Against this backdrop of uncertainty regarding dosing, Hougen et al. (4) have conducted a comprehensive meta-analysis and systematic review of the clinical safety of intravenous iron in the dialysis population in this issue of the Clinical Journal of the American Society of Nephrology (CJASN). Separately analyzing seven randomized, controlled trials and 15 observational studies, the authors find that high-dose intravenous iron (defined as >400 mg/mo for the clinical trials and >200 mg/mo in the observational studies) does not show an adverse safety signal compared with low-dose intravenous iron with respect to all-cause mortality, infection, cardiovascular disease, or hospitalizations. The study duly notes the common limitations of meta-analysis and takes the appropriate steps to assess statistical heterogeneity, quality of reporting, and risk of publication bias. The authors also acknowledge the potential implications of their findings—notably, a greater confidence in the option of high-dose intravenous iron for patients and perhaps, a validation of the recent trends of increased intravenous iron use nationally.
Can the nephrology community now rest easy with intravenous iron on the basis of the findings of this comprehensive study? Before responding in the affirmative, we must consider the findings of the study in the broader context of two sets of issues. The first set relates to a common concern of meta-analyses, heterogeneity, and the extent to which we can believe that observed variability across studies supports a summary estimate. The second set revolves around the potential benefits of high-dose intravenous iron so that we can better weigh the observed safety in the current meta-analysis.
Although the clinical trials did not show significant statistical heterogeneity, there seems to be important clinical differences among the studies. First and foremost, the studies span 20 years of clinical practice and are potentially subject to the changing consensus on ESAs and target hemoglobin levels. In fact, the listed ESA strategies for individual studies vary widely in table 1 of the meta-analysis in ref. 4, with some being highly prescriptive and others having great latitude. Second, several different intravenous iron formulations were used. Currently, we do not know whether the different intravenous iron formulations have different safety profiles (5) other than hypersensitivity/anaphylaxis (6). Third, one study’s primary goal was to determine the efficacy of an iron-based binder rather than the efficacy of intravenous iron dosing on hemoglobin level. These differences affect the appropriateness of use of one overall estimate to summarize all of the included studies (7). The existence of such clinical variability, also termed clinical heterogeneity, would be expected to lead to some degree of statistical heterogeneity in the results; however, tests for heterogeneity have low power and may fail to reach statistical significance, even when a moderate degree of genuine heterogeneity exists (7).
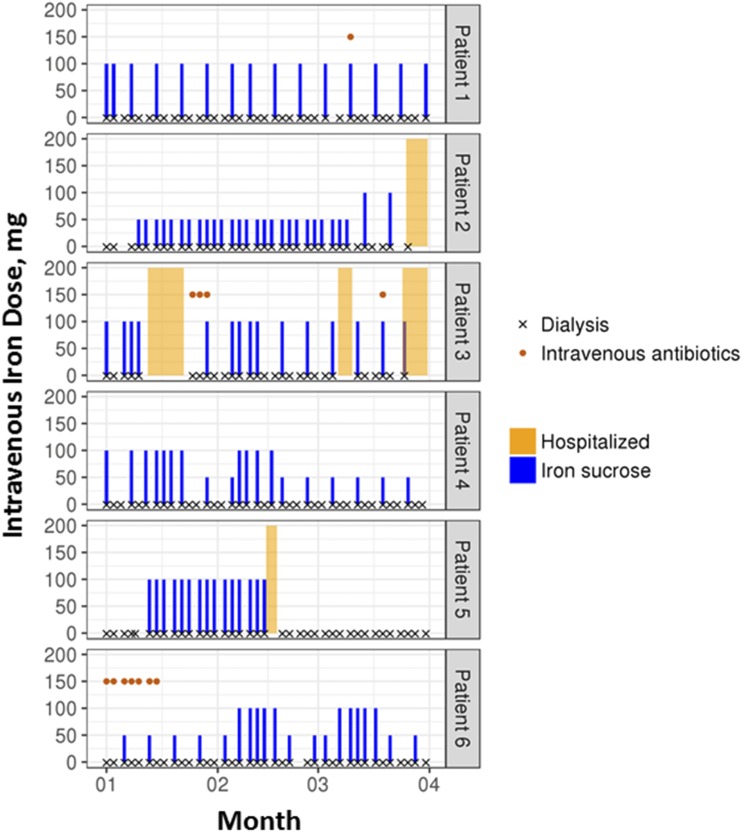
In addition to the substantial statistical heterogeneity across the 15 observational studies included in the meta-analysis, several related issues add further complexity in the interpretation of the results. Cumulative dosing of iron is a common approach to defining exposure in the observational studies included in the meta-analysis; the authors define high dose as >200 mg/mo. This quantity of intravenous iron is modest and may lead to misclassification of exposure given potential overlap with low-dose intravenous iron therapy (50 mg weekly over 4 weeks). Therefore, the safety profiles of high- and low-dose intravenous iron may seem similar. Furthermore, there can be important heterogeneity in the patients’ clinical experiences in the observational studies that is masked by inclusion into common categories of intravenous iron dosing. As an example, we present the clinical trajectory of six different patients who would be categorized in an observational study as having received 1500 mg of intravenous iron over 3 months (Figure 1). Despite the same amount of iron received over a period of 3 months, the patients have different patterns of iron use, clinical characteristics (e.g., infection as indicated by the use of intravenous antibiotics), and encounters with the health care system, including admission to hospital. These factors are also potential independent risk factors for the outcomes of interest, making it difficult to tease apart their effects from those of the cumulative exposure to intravenous iron. Moreover, a reliance on cumulative exposure does not align well with the treatment decisions that clinicians make. In contemporary clinical practice, clinicians rely on protocols to determine intravenous iron dose and frequency; these protocols, in turn, are guided by iron studies, hemoglobin, and other clinical parameters, such as infections. A dynamic clinical approach to intravenous iron administration, therefore, differs markedly from a cumulative average definition of 200 or 400 mg/mo for the next 3 months. As a result, the effect of cumulative intravenous iron dosing on safety outcomes may be challenging to determine or interpret.
Figure 1.
Treatment experience varies among patients all receiving 1500 mg iron sucrose over 3 months. Each panel represents a single patient. Dialysis sessions received are indicated by an × across the bottom of each panel, where a cluster of three ×s indicates a week. Intravenous iron received is indicated by a blue bar, with the height of the bar indicating the dose administered during the session. Despite having received the same amount of total iron over a 3-month period, these six patients differed in (1) treatment experiences (i.e., different dose and frequency of iron treatment), (2) clinical characteristics (e.g., infection episodes indicated by the use of intravenous antibiotics), and (3) health care system encounters (e.g., hospitalizations). For example, patient 4 received a mix of high-dose therapy (100 mg per session) and low-dose therapy (50 mg/wk) throughout the entire period, whereas patient 5 received only high-dose therapy (100 mg per session) within a shorter period of time.
We move to the second set of issues, examining the advantage of high-dose intravenous iron over low-dose intravenous iron. The known benefits of any intravenous iron relate to their effects on erythropoiesis, dosing of ESAs, and possibly, the need for blood transfusions. However, very little is known about the benefit of high-dose intravenous iron versus low-dose intravenous iron on these outcomes. Our previous work suggests a modest increase in hemoglobin of 0.2 mg/dl at 6 weeks of follow-up for patients given high-dose intravenous iron compared with low-dose intravenous iron (8); the effect on ESA dose is clinically insignificant. The cost-savings of high-dose intravenous iron from a reduction in ESA dose is not known. Although highly relevant in the era of value-based care, we may never know the true costs for ESAs and intravenous iron given that large dialysis organizations negotiate preferential rates with pharmaceutical companies and payers. Moreover, does high-dose intravenous iron make patients feel better? Again, there are few data. To date, there is only one study that has shown a modest effect of iron on mental and physical health component scores in the Kidney Disease Quality of Life survey (9).
In conclusion, we may be able to rest easier with high-dose intravenous iron on the basis of the comprehensive meta-analysis of currently available data in this issue of the CJASN (4). However, in our opinion, the findings also highlight the need for further study of the safety and effectiveness of intravenous iron among patients on dialysis, specifically with a focus on optimal dosing pattern and frequency. At least one ongoing clinical trial is addressing these issues (10), and we agree with the study authors that, ultimately, a large, pragmatic clinical trial may be needed. Finally, the paradigm of anemia management in CKD will likely change with the development of oral agents that stimulate erythropoiesis and coordinate oral iron absorption—perhaps obviating the necessity of any intravenous iron at all.
Disclosures
A.V.K. was on an Akebia advisory board in 2017.
Acknowledgments
We thank Dr. Jennifer Flythe for critical review of the editorial.
X.L. is supported by a Thomas O. Pyle Fellowship from Harvard Medical School and Harvard Pilgrim Health Care Institute.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Safety of Intravenous Iron in Dialysis: A Systematic Review and Meta-Analysis,” on pages 457–462.
References
- 1.Sargent JA, Acchiardo SR: Iron requirements in hemodialysis. Blood Purif 22: 112–123, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Andrews NC: Forging a field: The golden age of iron biology. Blood 112: 219–230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macdougall IC, Bircher AJ, Eckardt KU, Obrador GT, Pollock CA, Stenvinkel P, Swinkels DW, Wanner C, Weiss G, Chertow GM; Conference Participants : Iron management in chronic kidney disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int 89: 28–39, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Hougen I, Collister D, Bourrier M, Ferguson T, Hochheim L, Komenda P, Rigatto C, Tangri N: Safety of intravenous iron in dialysis: A systematic review and meta-analysis. Clin J Am Soc Nephrol 13: 457–467, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookhart MA, Freburger JK, Ellis AR, Winkelmayer WC, Wang L, Kshirsagar AV: Comparative short-term safety of sodium ferric gluconate versus iron sucrose in hemodialysis patients. Am J Kidney Dis 67: 119–127, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Graham DJ, Kane RC, Xie D, Wernecke M, Levenson M, MaCurdy TE, Houstoun M, Ryan Q, Wong S, Mott K, Sheu TC, Limb S, Worrall C, Kelman JA, Reichman ME: Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA 314: 2062–2068, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Thompson SG: Why sources of heterogeneity in meta-analysis should be investigated. BMJ 309: 1351–1355, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kshirsagar AV, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Brookhart MA: The comparative short-term effectiveness of iron dosing and formulations in US hemodialysis patients. Am J Med 126: 541.e1–541.e14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freburger JK, Ellis AR, Wang L, Butler AM, Kshirsagar AV, Winkelmayer WC, Brookhart MA: Comparative effectiveness of iron and erythropoiesis-stimulating agent dosing on health-related quality of life in patients receiving hemodialysis. Am J Kidney Dis 67: 271–282, 2016 [DOI] [PubMed] [Google Scholar]
- 10.EU Clinical Trials Register: Proactive IV irOn therapy for HaemodiALysis patients (PIVOTAL): UK Multicentre Open-Label Randomised Controlled Trial of IV Iron Therapy in Incident Haemodialysis Patients. EudraCT 2013-002267-25. Available at: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2013-002267-25. Accessed January 18, 2018