Abstract
Background and objectives
Proteinuria is used as an indicator of FSGS disease activity, but its use as a clinical trial end point is not universally accepted. The goal of this study was to refine proteinuria definitions associated with long-term kidney survival.
Design, setting, participants, & measurements
Data on 466 patients with primary FSGS with proteinuria (urine protein-to-creatinine ratio >1 g/g) were analyzed from five independent cohorts. Proteinuria by months 1, 4, and 8 after study baseline was categorized by conventional definitions of complete (<0.3 g/g) and partial remission (<3.5 g/g and 50% reduction in proteinuria). Novel remission definitions were explored using receiver operating curves. Kaplan–Meier methods were used to estimate the associations of proteinuria with progression to ESRD or a 50% loss in kidney function. Propensity score–adjusted Cox proportional hazards models were used to adjust for baseline proteinuria, eGFR, and therapy.
Results
In the initial derivation cohort, conventional partial remission was not associated with kidney survival. A novel definition of partial remission (40% proteinuria reduction and proteinuria<1.5 g/g) on the basis of receiver operating curve analyses of 89 patients was identified (Sensitivity=0.70; Specificity=0.77). In the validation cohort analyses, complete remission was associated with better prognosis (6 out of 41 patients progressed to kidney failure; 6.6 per 100 patient-years) as was the novel partial remission (13 out of 71 progressed; 8.5 per 100 patient-years), compared with those with no response (51 out of 116 progressed; 20.1 per 100 patient-years). Conventional partial remission at month 8, but not month 4, was also associated with better response (19 out of 85 patients progressed; risk=10.4 per 100 patient-years). Propensity score–adjusted analyses showed the novel partial remission was associated with less progression at months 4 and 8 (month 4: hazard ratio, 0.50; P=0.01; month 8: hazard ratio, 0.30; P=0.002).
Conclusions
Reaching either a complete or partial remission using a novel or conventional definition was associated with better long-term outcomes in patients with FSGS.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2018_02_20_CJASNPodcast_18_3_T.mp3
Keywords: FSGS; proteinuria; surrogate endpoint; Humans; Glomerulosclerosis, Focal Segmental; glomerular filtration rate; creatinine; Proportional Hazards Models; Propensity Score; Goals; kidney; Renal Insufficiency; Kidney Failure, Chronic; Prognosis; Cohort Studies
Introduction
Primary glomerular diseases, including FSGS, represent an important cause of ESRD (1). There is a paucity of proven therapies for these disorders. As a result, there is a pressing need to identify novel treatments and to test their efficacy in randomized clinical trials (2,3). However, there are several major obstacles to the conduct of these studies. Primary glomerular diseases are rare and traditional study designs are often not feasible. Therefore, the formation of clinical consortia composed of high-quality sites with the capacity and expertise to enroll participants into studies has partially alleviated the problem of patient and practice participation in rare disease trials. Novel trial designs are available and need to be used for rare disease clinical trials (4). Finally, prolonged treatment periods extending for at least 3 years are generally required to document changes in hard clinical outcomes, namely onset of ESRD, for patients with FSGS.
Rather than an end point of ESRD, most FSGS clinical trials rely upon changes in proteinuria as an indicator of efficacy (5). The advantage of this approach is that the effect of a test intervention on proteinuria can be documented in a shorter period of time and with fewer patients, thereby reducing the costs and logistic burden of a clinical trial. However, changes in proteinuria have not been accepted as a valid surrogate marker of hard clinical outcomes for clinical trials conducted for drug development and product registration for FSGS. There are observational studies in pediatric and adult patients with FSGS and membranous nephropathy that document gradations in kidney survival on the basis of reduction in proteinuria (6). There is a willingness of regulatory agencies to consider a prolonged complete remission of proteinuria as a meaningful indicator of drug efficacy in specific disease circumstances, e.g., podocytopathies (6,7). However, to date, partial proteinuria reduction is not recognized as a validated surrogate end point in glomerular disease trials (6). Additionally, a substantial proportion of patients fail to achieve a complete remission, and an acceptable definition of partial remission may improve trial feasibility by increasing the number of patients likely to reach the surrogate end point.
Earlier attempts to validate proteinuria as a meaningful end point for CKD have suffered from the inclusion of a heterogeneous group of diseases, including diabetic nephropathy (7). Changes in proteinuria may be more closely linked to outcomes in disorders in which it represents the major pathophysiologic disturbance in the disease pathway. This is more likely to be the case in podocytopathies such as FSGS. In addition, previous studies defined partial remission loosely as at least a 50% reduction in proteinuria to a level <3.5 (g/g) (8,9). There was no published statistical effort to derive and optimize this definition. Therefore, this study was designed to determine whether a novel, data-derived definition of partial proteinuria remission could be developed that more accurately predicts clinically meaningful outcomes compared with the standard definition of partial remission for children and adults with FSGS.
Materials and Methods
After Institutional Review Board approval, five cohorts of patients with biopsy-confirmed primary FSGS were used in these analyses. All studies included both pediatric and adult patients.
Data Sources
The Nephrotic Syndrome Study Network (NEPTUNE) is an ongoing, prospective observational cohort study that enrolls patients with evidence of proteinuria at the time of their first kidney biopsy (10). NEPTUNE includes cases with underlying diseases other than FSGS; however, the analyses in this report were limited to patients with FSGS. Patients are followed in 6-month intervals for a maximum of 5 years.
The National Institutes of Health-funded FSGS Clinical Trial (FSGS-CT) study was a multicenter randomized study of children and adults with steroid-resistant primary FSGS (11). Patients who participated in the FSGS-CT were treated at clinical centers in the United States and were randomized between November of 2004 and May of 2008. The efficacy of treatment for 12 months with cyclosporine was compared with a combination of oral pulse dexamethasone and mycophenolate mofetil. Of the 192 patients enrolled, 138 were randomized to cyclosporine (n=72) or to mycophenolate/dexamethasone (n=66). Follow-up labs were collected at weeks 2, 4, 6, 8, 14, 20, 26, 32, 38, 44, 52, 65, and 78; and months 24, 30, 36, 42, 48, and 54.
The NephCure Accelerating Cures Network (NACI) Registry is an ongoing study of patients with primary nephrotic syndrome that began enrollment in November of 2015. Patients enrolled in NACI from seven participating Internal Medicine and Pediatric Nephrology practices in the United States provide consent to share both retrospective and prospective data from their electronic health records, including demographic, diagnosis, biopsy, laboratory, vital signs including BP, and medication data. Patients may consent to join the registry at any point in their disease course, but retrospective data are available from the time of disease onset. The diagnosis of primary FSGS was confirmed by each patient’s nephrologist.
The University of Michigan Nephrotic Syndrome Registry (NS-REG) is an electronic health record extract of 650 patients with confirmed primary nephrotic syndrome, including 373 patients with FSGS. Patients were seen within the University of Michigan Health system at least once between 2009 and 2014. Retrospective laboratory data were available from 2004. Primary nephrotic syndrome diagnosis was confirmed by physician chart-review.
The Clinical Phenotyping Resource and Biobank Core (C-PROBE) cohort study is an ongoing prospective observational cohort study of multiethnic patients with prevalent CKD and functions as one of three complementary cores within the George M. O’Brien Michigan Kidney Translational Core Center (12). Patients in the C-PROBE study are enrolled at sites in the United States, beginning in 2009. Eligible candidates for the C-PROBE study are patients with CKD, stages 1–4, followed within the site nephrology practices or undergoing kidney biopsy. Exclusion criteria include patients receiving chronic dialysis or who have had a kidney transplant. Data collection at annual study visits includes medical, social, and family histories; kidney-specific clinical and demographic information was obtained from chart review. Retrospective laboratory data were available up to 3 years before study entry.
Definitions
In each data source, proteinuria measurements (i.e., urine protein-to-creatinine ratios) were taken from 24-hour urine samples or spot urine samples if 24-hour urine samples were not available. Eligible patients from each cohort include patients with primary FSGS with proteinuria>1 g/g and an eGFR>30 ml/min per 1.73 m2 within the same week. For the prospective NEPTUNE and FSGS-CT studies, patients were required to satisfy these criteria at their baseline visit. For the registry/retrospective NACI, NS-REG, and C-PROBE studies without defined baseline visits, patients were eligible for analyses at the time they first met these entry criteria.
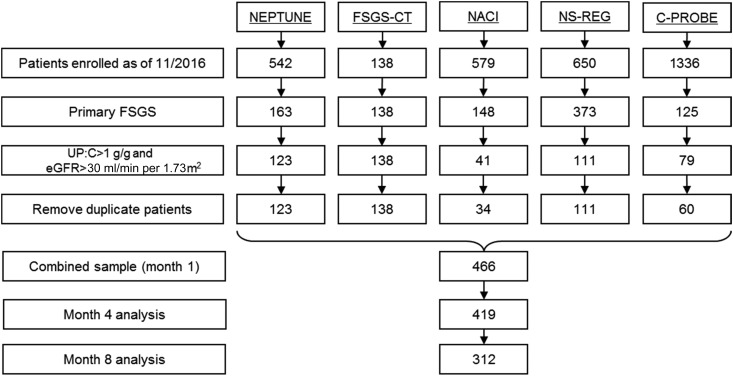
Remission status was assigned by months 1, 4, and 8 after baseline. The baseline proteinuria was used as a reference value when determining relative reduction in proteinuria. The following conventional proteinuria remission definitions were used a priori: (1) complete remission (proteinuria<0.3 g/g), (2) partial remission (≥50% reduction in proteinuria from baseline, and proteinuria between 0.3 and 3.5 g/g), and (3) no response (did not meet either of the above two criteria). Patients without proteinuria and/or serum creatinine data beyond months 4 and 8 were excluded from months 4 and 8 analyses (Figure 1). No imputations were made in cases of missing data.
Figure 1.
Flow diagram of included patients from 5 FSGS cohorts. C-PROBE, Clinical Phenotyping Resource and Biobank Core; FSGS-CT, National Institutes of Health-funded National Institute of Diabetes and Digestive and Kidney Diseases FSGS Clinical Trial; NACI, NephCure Accelerating Cures Network; NEPTUNE, Nephrotic Syndrome Study Network; NS-REG, University of Michigan Nephrotic Syndrome Registry; UP:C, urine protein:creatinine ratio.
The main end point of interest was reaching ESRD or a 50% reduction in eGFR from baseline. ESRD was defined by the patient meeting at least one of the following criteria: (1) two consecutive eGFRs<15 ml/min per 1.73 m2 ≥40 days apart, (2) kidney transplant, (3) dialysis duration ≥40 days, and (4) withdrawal from study for reasons of “ESRD.” All end points were identified during follow-up to a maximum of 168 months (median=27; interquartile range, 13–43).
Statistical Analyses
For the NEPTUNE prospective study, three separate parallel Kaplan–Meier analyses with log-rank tests and analogous parallel Cox proportional hazard models were conducted at each study visit, where proteinuria was assessed (months 1, 4, and 8) using remission status at that visit to predict time to ESRD/50% reduction in eGFR. The at-risk time for the time-to-event analyses began at the time of the study visit. For example, in the month 1 analysis, change in proteinuria was assessed between baseline and month 1, and patients were categorized into complete, partial, or no remission. Remission status was used as a predictor in survival analyses beginning from month 1. Receiver operating characteristic curve analyses were conducted using the NEPTUNE data as a derivation sample to determine the optimal threshold of proteinuria in predicting progression to ESRD/50% reduction in eGFR by month 48. These curves were visually inspected for the strongest points of inflection, and the accuracy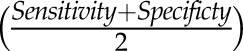 of each threshold was calculated. These parallel results were explored to determine if there was a predictive threshold across visits. The fitted model was then applied to the FSGS-CT data as a confirmation sample to determine if the fitted model is a stronger predictor than the uninformed model in the FSGS-CT confirmation sample. The FSGS-CT sample was also explored as a parallel derivation sample. A separate receiver operating characteristic analysis was conducted to test for an optimal threshold in percentage reduction in proteinuria in predicting progression to ESRD/50% reduction in eGFR.
of each threshold was calculated. These parallel results were explored to determine if there was a predictive threshold across visits. The fitted model was then applied to the FSGS-CT data as a confirmation sample to determine if the fitted model is a stronger predictor than the uninformed model in the FSGS-CT confirmation sample. The FSGS-CT sample was also explored as a parallel derivation sample. A separate receiver operating characteristic analysis was conducted to test for an optimal threshold in percentage reduction in proteinuria in predicting progression to ESRD/50% reduction in eGFR.
Novel FSGS proteinuria remission end points were defined on the basis of the results of the receiver operating characteristic analyses in the NEPTUNE and FSGS-CT derivation-confirmation samples. A third step of pooled analyses was conducted using the FSGS-CT plus the final three datasets to assess confounding factors. Because the NACI and NS-REG studies were retrospective registry studies and did not have defined study visits, we used remission definitions to reflect the best remission status by a given time point instead of the remission status at a given time point (as was done in the NEPTUNE and FSGS-CT analyses). Cumulative remission status was defined at months 1, 4, and 8 after baseline to match the NEPTUNE visit structure. Pooled Kaplan–Meier and Cox proportional hazards models were conducted at each visit. In each of the parallel models, the at-risk time began at the time of the study visit (i.e., when proteinuria was assessed). Propensity score analyses were conducted to adjust for possible confounding factors while conserving statistical power (12). Logistic regression analyses were conducted to calculate propensity scores where the outcome of interest was reaching the novel proteinuria end point, and the predictors included baseline proteinuria, baseline eGFR, exposure to steroids, and exposure to calcineurin inhibitors before the visit of interest. These scores were then used in propensity score–stratified and –adjusted Cox proportional hazards models. In all analyses, patients with incomplete proteinuria data at a given study visit were excluded from that visit’s analysis. All analyses were conducted in SASv9.4.
Results
A total of 466 patients with biopsy-proven FSGS, proteinuria>1 g/g, and an eGFR>30 ml/min per 1.73 m2 at baseline were used in these analyses (Figure 1). The characteristics of each sample are described in Table 1. The pooled sample included 39% pediatric patients, 32% patients of black race, and 52% with nephrotic-range proteinuria (>3.5 g/g) at their baseline visit. The median eGFR at baseline across all samples was 83 ml/min per 1.73 m2 (interquartile range, 53–116 ml/min per 1.73 m2). Patient characteristics were similar across samples with certain key distinctions. The NEPTUNE and FSGS-CT study participants had the highest degrees of proteinuria at baseline (medians of 4.2 and 4.0, respectively) and the FSGS-CT was primarily composed of pediatric patients (67%), whereas all other samples included a majority of adults.
Table 1.
Characteristics of patients with FSGS from five independent cohort studies
| Characteristic | NEPTUNE (n=123) | FSGS-CT (n=138) | NACI (n=34) | NS-REG (n=111) | C-PROBE (n=60) |
|---|---|---|---|---|---|
| Age (Median, IQR) | 30 (14–47) | 15 (11–23) | 19 (14–29) | 34 (20–53) | 41 (21–54) |
| Pediatric (<18) | 44 (36) | 93 (67) | 12 (35) | 18 (16) | 13 (22) |
| Adult (18+) | 79 (64) | 45 (33) | 22 (65) | 93 (84) | 47 (78) |
| Sex, n (%) | |||||
| Female | 51 (41) | 65 (47) | 17 (50) | 58 (52) | 25 (42) |
| Male | 72 (59) | 73 (53) | 17 (50) | 53 (48) | 35 (58) |
| Race, n (%) | |||||
| Asian/Asian American | 9 (7) | 3 (2) | 8 (24) | 4 (4) | 0 (0) |
| Black | 34 (23) | 53 (38) | 8 (24) | 26 (23) | 26 (43) |
| White | 66 (54) | 78 (57) | 17 (50) | 74 (67) | 31 (52) |
| Other | 14 (11) | 4 (3) | 1 (3) | 7 (6) | 3 (5) |
| Hypertension at baseline, n (%) | 75 (61) | 80 (58) | 18 (56) | 30 (27) | 51 (85) |
| Urine protein-to-creatinine ratio at entry, median (IQR) | 4.2 (2.1–8.1) | 4.0 (2.2–8.3) | 3.6 (1.9–7.5) | 3.4 (1.7–7.2) | 2.4 (1.6–7.4) |
| Nephrotic range proteinuria at entry (>3.5) | 71 (58) | 77 (56) | 17 (50) | 53 (48) | 23 (38) |
| eGFR at entry, median (IQR) | 77 (49–101) | 112 (76–180) | 71 (43–122) | 66 (41–96) | 72 (50–109) |
| ESRD/50% reduction in eGFR during follow-up, n (%) | 31 (25) | 40 (29) | 7 (21) | 55 (50) | 10 (17) |
Categoric variables are displayed as frequency (percent) and continuous variables as median (25th, 75th percentile). NEPTUNE, Nephrotic Syndrome Study Network; FSGS-CT, The National Institutes of Health-funded FSGS Clinical Trial; NACI, The NephCure Accelerating Cures Institute; NS-REG, University of Michigan Nephrotic Syndrome Registry; C-PROBE, The Clinical Phenotyping Resource and Biobank Core; IQR, interquartile range.
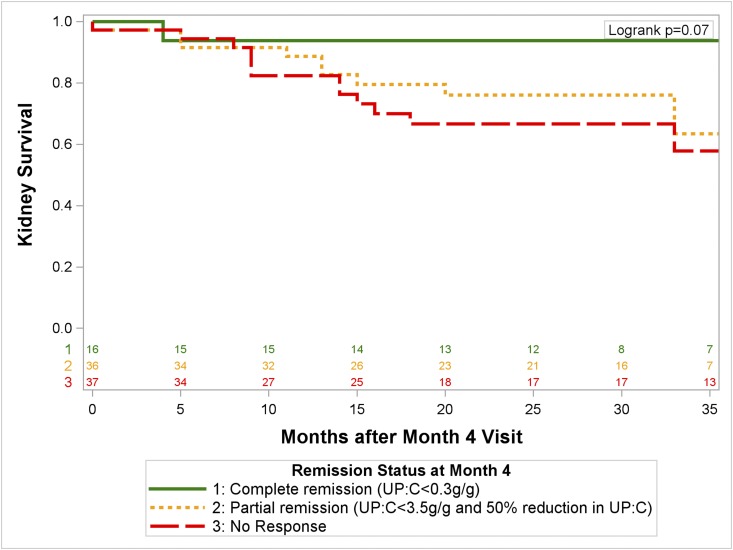
The NEPTUNE sample was used as the initial derivation cohort. At study month 4, 16 (18%) patients were in a complete remission, 36 (40%) were in a partial remission, according to conventional definitions, and 37 (41%) had no response. Figure 2 shows that patients in complete remission at the month 4 visit did not generally progress to kidney failure. However, those in a partial remission in this analysis did not differ from those with no response. The same trend was identified at months 1 and 8.
Figure 2.
No improvement in kidney survival in children and adults with primary FSGS in the NEPTUNE study by conventional definitions of partial proteinuria remission at 4 months after biopsy. Complete remission, urine protein-to-creatinine ratio (UP:C) <0.3 g/g; partial remission, UP:C 0.3 to <3.5 g/g; no remission includes all other patients.
We then conducted the planned receiver operating characteristic curve analysis of continuous values of proteinuria in order to determine the strongest inflection point in the relationship between proteinuria and kidney failure. Continuous proteinuria was a significant predictor of subsequent kidney failure in the NEPTUNE month 4 analysis (area under curve=0.77). This model indicated proteinuria<1.5 g/g to have the strongest sensitivity and specificity in predicting progression to kidney failure (Sensitivity=0.70; Specificity=0.77). This threshold was more sensitive to identifying patients progressing to kidney failure compared with the conventional threshold of 3.5 g/g (Sensitivity=0.41; Specificity=0.89). Using the FSGS-CT study data as a confirmation sample, the fitted model from the NEPTUNE was a stronger predictor than the uninformed model (area under curve=0.78; P<0.001). In a parallel derivation analysis using the FSGS-CT study data, we also identified proteinuria of 1.5 g/g to be the strongest inflection point (Supplemental Figure 1) (Sensitivity=0.71; Specificity=0.81). However, receiver operating characteristic models of percentage reduction in proteinuria from baseline showed no significant relationship with progression to kidney failure.
We used this information to modify the definitions of FSGS proteinuria remission used in Kaplan–Meier analyses. The top two panels of Supplemental Figure 2 use the FSGS proteinuria reduction end point of reaching a proteinuria 0.3 to <1.5 g/g. In both the NEPTUNE and FSGS-CT analyses, this modified partial remission end point was associated with a decreased likelihood of disease progression compared with no remission. We considered the possibility that using an absolute threshold of <1.5 g/g as our only criteria would enable small absolute changes in proteinuria from just above to just below 1.5 g/g to be considered clinically significant. As stated above, receiver operating characteristic analyses did not identify an informative percentage reduction threshold that predicted progression to kidney failure. Consequently, to achieve the goal of added face validity, a threshold of a 40% reduction was chosen as a reasonable value. This was selected on the basis of clinical judgment. The conventional partial remission definition requires a 50% reduction; however, we modified this to a 40% requirement to counterbalance the added stringency from using a threshold proteinuria of 1.5 instead of 3.5 g/g. Thus, we tested the end point of reaching a proteinuria<1.5 g/g and a 40% reduction in proteinuria. This end point was also shown to be associated with better prognosis in both the NEPTUNE and FSGS-CT samples in Supplemental Figure 2.
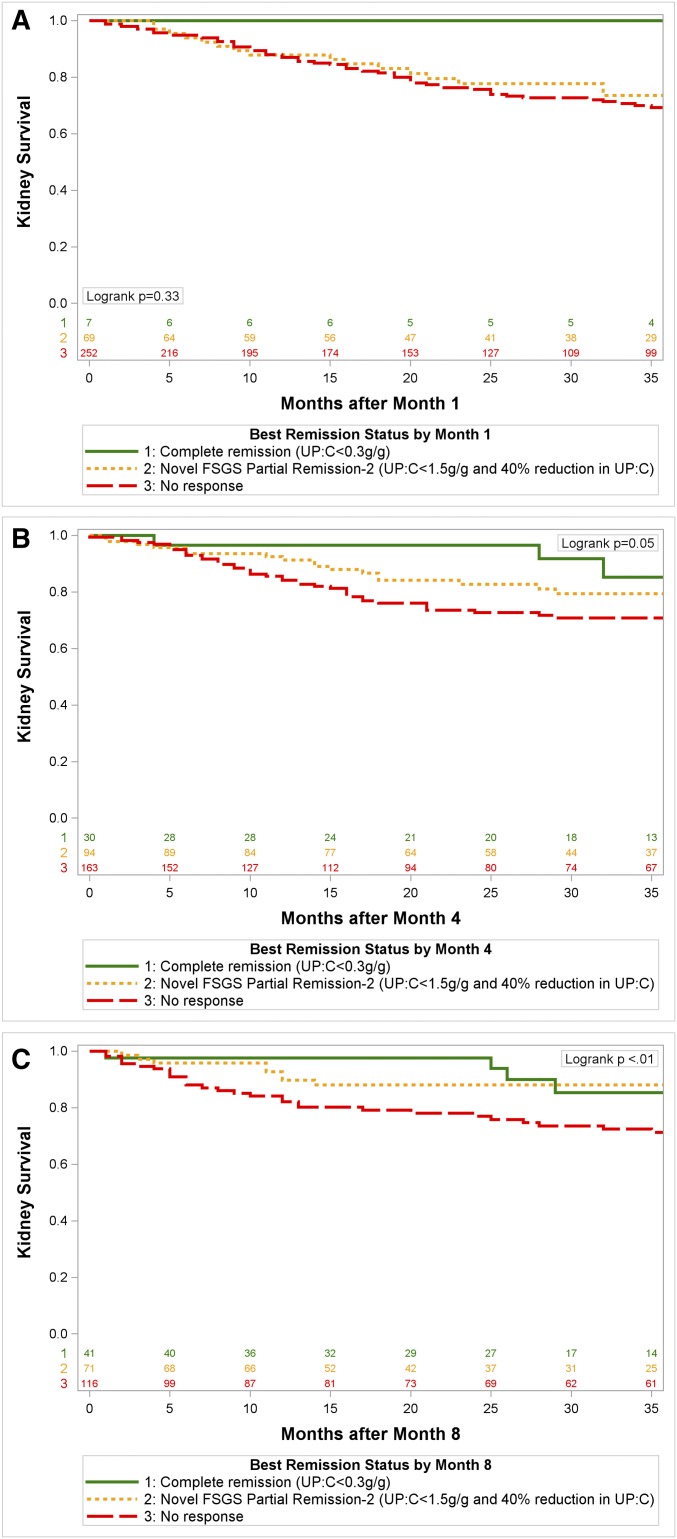
We then tested the robustness of this novel end point using a pooled data analysis from the FSGS-CT, NACI, NS-REG, and C-PROBE study data sources. Each patient was classified as reaching the two-term FSGS proteinuria reduction end point (proteinuria 0.3 to <1.5 g/g and at least a 40% reduction in proteinuria) by months 1, 4, and 8 after baseline. Figure 3 shows a significant association between reaching the novel end point by months 4 and 8 and kidney survival.
Figure 3.
Pooled analysis of 4 FSGS cohorts demonstrate kidney survival is better for patients achieving the novel FSGS partial proteinuria remission or complete remission compared to no remission by month 4, and month 8, but not month 1. Kidney survival is better for patients achieving the novel FSGS partial proteinuria remission or complete remission compared with no remission by month 4 and month 8, but not month 1. UP:C, urine protein:creatinine ratio.
To assess the effect of potential confounding variables using variables suggested by prior literature to be related to the risk for kidney failure in FSGS, propensity score–stratified and –adjusted analyses are shown in Table 2. Propensity scores of reaching the two-term FSGS proteinuria reduction end point were calculated at each visit on the basis of baseline proteinuria, baseline eGFR, and treatment with steroids or calcineurin inhibitors. Propensity score–stratified analyses indicated a significant inverse relationship between reaching the novel end point by months 4 and 8 and disease progression. There was no significant relationship between reaching the FSGS proteinuria reduction end point by month 1 and progression after accounting for propensity scores. Propensity score–adjusted analyses showed the same relationship.
Table 2.
Novel FSGS partial proteinuria remission remained significantly associated with decreased likelihood of disease progression compared with those with no response after propensity score–stratified and propensity score–adjusted Cox proportional hazards analyses
| Proteinuria Assessment | Crude Data | Regression Results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Complete Remission | Partial Remission | No Response | Unadjusted Model | Propensity Score Stratified (Quintiles) | Propensity Score Adjusted | ||||
| # of Events/# of Patients/(Risk per 100 Patient-Years) | # of Events/# of Patients (Risk per 100 Patient-Years) | # of Events/# of Patients (Risk per 100 Patient-Years) | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Novel | |||||||||
| Month 1 | 0/7 (0.0) | 17/69 (10.9) | 62/252 (12.0) | 0.8 (0.5 to 1.4) | 0.43 | 0.8 (0.4 to 1.4) | 0.36 | 0.7 (0.4 to 1.3) | 0.28 |
| Month 4 | 3/30 (4.2) | 17/94 (8.0) | 44/163 (13.0) | 0.5 (0.3 to 0.9) | 0.02 | 0.5 (0.3 to 0.9) | 0.01 | 0.5 (0.3 to 0.8) | 0.003 |
| Month 8 | 6/41 (6.6) | 13/71 (8.5) | 51/116 (20.1) | 0.4 (0.2 to 0.7) | <0.001 | 0.3 (0.1 to 0.5) | 0.002 | 0.3 (0.2 to 0.6) | 0.001 |
| Conventional | |||||||||
| Month 1 | 0/7 (0.0) | 22/88 (11.0) | 57/233 (12.2) | 0.6 (0.3 to 1.1) | 0.06 | 0.4 (0.1 to 1.7) | 0.21 | 0.5 (0.1 to 1.9) | 0.29 |
| Month 4 | 3/30 (4.2) | 24/114 (9.6) | 37/143 (12.4) | 0.8 (0.5 to 1.1) | 0.22 | 0.7 (0.4 to 1.3) | 0.23 | 0.7 (0.4 to 1.2) | 0.19 |
| Month 8 | 6/41 (6.6) | 19/85 (10.4) | 45/104 (20.0) | 0.4 (0.2 to 0.7) | 0.003 | 0.4 (0.2 to 0.8) | 0.01 | 0.4 (0.2 to 0.8) | 0.01 |
Propensity scores were calculated from the following covariates: baseline urine protein-to-creatinine ratio, baseline eGFR, exposure to steroid therapy, and exposure to calcineurin inhibitor therapy. Stratified results are from stratified Cox proportional hazards models where the baseline hazard is stratified by quintiles of propensity scores; adjusted results are from Cox proportional hazards models where the propensity score is included as a covariate. Adjusted analyses were also adjusted for study (National Institutes of Health-funded FSGS Clinical Trial, NephCure Accelerating Cures Institute, University of Michigan Nephrotic Syndrome Registry, or Clinical Phenotyping Resource and Biobank Core). 95% CI, 95% confidence interval; HR, hazard ratio.
Finally, we repeated the Cox proportional hazards regressions to test the effect of the conventional definitions in the validation sample (Table 2). These analyses identified the conventional partial proteinuria remission definitions to predict disease progression at month 8 but not by months 1 or 4.
Discussion
This study supports the use of proteinuria as an early end point and marker for progression to kidney failure in patients with FSGS. Complete remission of proteinuria using the <0.3 g/g definition is associated with the best kidney survival. The novel FSGS proteinuria reduction end point of proteinuria 0.3 to <1.5 g/g accompanied with at least a 40% reduction in proteinuria was a robust correlate of kidney survival in patients with primary FSGS. The novel proteinuria metric was associated with better prognosis after accounting for the propensity, or likelihood, of seeing a change in proteinuria. There was also some evidence supporting the use of the conventional partial remission definition in predicting kidney survival as well.
This is one of the largest analyses of well characterized children and adults with primary FSGS, and the only study to use data-derived, empiric proteinuria end points. It supports the application of proteinuria end points to patients across the lifespan, even though the genetic mutations or secondary causes that lead to FSGS differ in children and adults (13). It provides a model for the development of novel proteinuria end points in other primary glomerular diseases. For example, this analysis complements findings supporting the use of early reduction in proteinuria as a surrogate end point in IgA nephropathy (14).
One limitation of this study is the lack of a biologic model describing the link between a proteinuria threshold of 1.5 g/g and a change in underlying pathophysiology. We suggest that the primary cellular disturbance in the podocyte leading to the disease is responsible for causing proteinuria and the progressive decline in GFR. This would apply equally in cases of FSGS caused by genetic abnormalities, circulating factor(s), or adaptive changes in podocyte structure and function. There is ample evidence that proteinuria per se is a progression factor for glomerular disease and reducing proteinuria can slow progression. Interestingly, proteinuria is considered the mechanistic link between glomerular and tubular damage in glomerular disease (15). It would also support the mechanistic link seen between glomerular proteinuria, tubular damage, interstitial damage, and progressive loss of kidney function documented in the NEPTUNE study (16). Additionally, the proteinuria threshold of 1.5 g/g is consistent with a modified definition of partial remission used in severe lupus nephritis in predicting long-term kidney survival (17). We support the inclusion of the 40% reduction in proteinuria as part of the definition to identify “real” changes in proteinuria, and to avoid labeling minor changes (e.g., from 1.55 to 1.45 g/g) as clinically meaningful. However, our analyses of relative change in proteinuria, by themselves, were not statistically significant.
Still, it is worth emphasizing that although the conventional partial remission definition did not predict kidney survival in the NEPTUNE derivation cohort, it was still ultimately associated with better kidney survival in the final validation cohort by month 8. This suggests that although the novel definition may be a strong definition, there are possibly other relevant, but not as discriminative, definitions for proteinuria as well, used to predict better kidney survival in cohorts of patients starting with either nephrotic or subnephrotic proteinuria.
Another limitation of these analyses is the potential lead-time bias introduced by repeating parallel analyses starting from months 1, 4, and 8 instead of a time-varying covariates model. We decided to not use a time-varying covariate approach given the different follow-up windows with proteinuria across studies and the inability to assess proteinuria between study visits and measurements. Multiple testing and missing data across each of the three study visits are limitations as well.
This analysis has several notable strengths. It represents the largest analysis of clinical outcome assessment of proteinuria in a single primary glomerular disease entity. The study cohort is geographically diverse and is consistent with the current characteristics of patients with FSGS in the United States. However, we acknowledge several limitations. Except for the FSGS-CT, treatment was not defined and the effect of therapeutic interventions could not be specifically assessed. The measurements of proteinuria were not centralized in all five data sources and we did not determine albuminuria.
In conclusion, we describe the development of a novel FSGS proteinuria reduction metric derived from a large, clinically representative, pooled cohort of patients with primary FSGS that correlated with kidney survival. Adoption of this metric for use in clinical practice may enable nephrologists to assess the response to treatment and prognosis of individual patients more accurately. If approved by regulatory agencies upon further validation, then incorporation of the end point into the study design and statistical plan may enable more efficient completion of randomized clinical trials designed to test novel therapies for patients with FSGS.
Disclosures
This investigator-initiated work was supported by Retrophin, Inc. and was conducted as an ancillary study to the Nephrotic Syndrome Study Network, Clinical Phenotyping Resource and Biobank Core study, and the NephCure Accelerating Cures Network. The study investigators report the following disclosures: R.K. and S.T. are employees of Retrophin; D.S.G. as part of her employment at University of Michigan is the principal investigator of the NephCure Accelerating Cures Institute Coordinating Center and has research funding from NephCure Kidney International, the National Institutes of Health, the Patient Centered Outcomes Research Institute, Retrophin, Bristol-Myers Squibb, Complexa, and Pfizer.
Supplementary Material
Acknowledgments
We are indebted to the patients and families who graciously participated in these research studies. We would like to thank Lynn Holvenski for her help with the data extractions and dataset preparation.
The Nephrotic Syndrome Study Network (NEPTUNE) is sponsored by the National Institutes of Health (NIH)/National Center for Advancing Translational Sciences and NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (U54DK083912), NephCure Kidney International, and the University of Michigan. Most NEPTUNE participating sites are supported by the NIH-sponsored Clinical and Translational Science Awards and their clinical research–supported facilities. The FSGS Clinical Trial was supported by NIDDK, NIH. The NephCure Accelerating Cures Institute is supported by NephCure Kidney International and the University of Michigan Department of Pediatrics and Communicable Diseases. The University of Michigan Nephrotic Syndrome Registry was supported in part by the NIH/NIDDK O’Brien Kidney Center (2P30-DK-081943) summer research internship (Grace Tsai), the University of Michigan Department of Pediatrics and Communicable Diseases and Honest Broker Office, and the NephCure Accelerating Cures Institute. The Clinical Phenotyping Resource and Biobank Core study is supported, in part, by the O’Brien Renal Center NIH Grant P30-DK081943-01, and the University of Michigan School of Medicine, Department of Internal Medicine, and Department of Pediatrics and Communicable Diseases.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04780517/-/DCSupplemental.
References
- 1.United States Renal Data System : 2015 USRDS Annual Data Report: Epidemiology of Kidney isease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 2.KDIGO : Kidney Disease: Improving Global Outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 139–274, 2012 [Google Scholar]
- 3.Han KH, Kim SH: Recent advances in treatments of primary focal segmental glomerulosclerosis in children. BioMed Res Int 2016. Available at https://www.hindawi.com/journals/bmri/2016/3053706/. Accessed April 17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spino C, Jahnke JS, Selewski DT, Massengill S, Troost J, Gipson DS: Changing the paradigm for the treatment and development of new therapies for FSGS. Front Pediatr 4: 25, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trachtman H: Efficacy and safety of RE-021, a dual endothelin receptor and angiotensin receptor blocker, in patients with focal segmental glomerulosclerosis (FSGS): A randomized, double-blind, active-control, dose-escalation study (2012). Available at https://clinicaltrials.gov/ct2/show/NCT01613118. Accessed April 17, 2017
- 6.Thompson A, Cattran DC, Blank M, Nachman PH: Complete and partial remission as surrogate end points in membranous nephropathy. J Am Soc Nephrol 26: 2930–2937, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, Kasiske B, Hostetter T: Proteinuria as a surrogate outcome in CKD: Report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 54: 205–226, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Gipson DS, Chin H, Presler TP, Jennette C, Ferris ME, Massengill S, Gibson K, Thomas DB: Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol 21: 344–349, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry Group : Focal and segmental glomerulosclerosis: Definition and relevance of a partial remission. J Am Soc Nephrol 16: 1061–1068, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, Sampson MG, Kopp JB, Lemley KV, Nelson PJ, Lienczewski CC, Adler SG, Appel GB, Cattran DC, Choi MJ, Contreras G, Dell KM, Fervenza FC, Gibson KL, Greenbaum LA, Hernandez JD, Hewitt SM, Hingorani SR, Hladunewich M, Hogan MC, Hogan SL, Kaskel FJ, Lieske JC, Meyers KE, Nachman PH, Nast CC, Neu AM, Reich HN, Sedor JR, Sethna CB, Trachtman H, Tuttle KR, Zhdanova O, Zilleruelo GE, Kretzler M: Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83: 749–756, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Hogan SL, Middleton JP, Vehaskari VM, Flynn PA, Powell LM, Vento SM, McMahan JL, Siegel N, D’Agati VD, Friedman AL: Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int 80: 868–878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.C-PROBE : The clinical pehnotyping resource and biobank core (2017). Available at https://kidneycenter.med.umich.edu/c-probe-core. Accessed April 17, 2017
- 13.Santín S, Bullich G, Tazón-Vega B, García-Maset R, Giménez I, Silva I, Ruíz P, Ballarín J, Torra R, Ars E: Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 6: 1139–1148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Mondal H, Greene T, Masaschi T, Locatelli F, Schena FP, Katafuchi R, Appel GB, Maes BD, Li PK, Praga M, Del Vecchio L, Andrulli S, Manno C, Gutierrez E, Mercer A, Carroll KJ, Schmid CH, Levey AS: Early change in urine protein as a surrogate end point in studies of IgA nephropathy: An individual-patient meta-analysis. Am J Kidney Dis 68: 392–401, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Remuzzi G, Benigni A, Remuzzi A: Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116: 288–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariani LH, Martini S, Barisoni L, Canetta PA, Troost JP, Hodgin JB, Palmer M, Rosenberg AZ, Lemley KV, Chien HP, Zee J, Smith A, Appel GB, Trachtman H, Hewitt SM, Kretzler M, Bagnasco SM: Interstitial fibrosis scored on whole-slide digital imaging of kidney biopsies is a predictor of outcome in proteinuric glomerulopathies [published online ahead of print February 27, 2017]. Nephrol Dial Transplant 10.1093/ndt/gfw443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ; Collaborative Study Group : Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol 3: 46–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.