Abstract
Background and objectives
The safety of intravenous iron dosing in dialysis is uncertain. Higher-dose intravenous iron may be associated with a higher risk of infections, cardiovascular events, hospitalizations, and mortality. This systematic review aimed to determine the safety of higher-dose versus lower-dose intravenous iron, oral iron, or no iron supplementation in adult patients treated with dialysis.
Design, setting, participants, & measurements
We searched Medline, EMBASE, Cochrane library, and CINAHL from inception to January 6, 2017 for randomized, controlled trials and observational studies comparing higher-dose intravenous iron with lower-dose intravenous iron, oral iron, or no iron in patients treated with dialysis that had all-cause mortality, infection, cardiovascular events, or hospitalizations as outcomes.
Results
Of the 2231 eligible studies, seven randomized, controlled trials and 15 observational studies met inclusion criteria. The randomized, controlled trials showed no association between higher-dose intravenous iron (>400 mg/mo for most studies) and mortality (six studies; n=970; pooled relative risk, 0.93; 95% confidence interval, 0.47 to 1.84; follow-up ranging from 35 days to 26 months) or infection (four studies; n=743; relative risk, 1.02; 95% confidence interval, 0.74 to 1.41). The observational studies showed no association between higher-dose intravenous iron (>200 mg/mo for most studies) and mortality (eight studies; n=241,408; hazard ratio, 1.09; 95% confidence interval, 0.98 to 1.21; follow-up ranging from 3 to 24 months), infection (eight studies; n=135,532; pooled hazard ratio, 1.13; 95% confidence interval, 0.99 to 1.28), cardiovascular events (seven studies; n=135,675; hazard ratio, 1.18; 95% confidence interval, 0.90 to 1.56), or hospitalizations (five studies; n=134,324; hazard ratio, 1.08; 95% confidence interval, 0.97 to 1.19).
Conclusions
Higher-dose intravenous iron does not seem to be associated with higher risk of mortality, infection, cardiovascular events, or hospitalizations in adult patients on dialysis. Strength of this finding is limited by small numbers of participants and events in the randomized, controlled trials and statistical heterogeneity in observational studies.
Keywords: anemia; intravenous; chronic kidney disease; dialysis; ferryl iron; Iron; Risk; Follow-Up Studies; Administration, Intravenous; Libraries; Uncertainty; Proportional Hazards Models; hospitalization; renal dialysis; Randomized Controlled Trials as Topic
Introduction
Anemia (1) is common in patients with CKD and kidney failure, and it is associated with morbidity and mortality (2). Intravenous iron and erythropoietin-stimulating agents (ESAs) minimize red blood cell transfusions and their infectious and immunologic complications, and they may marginally improve self-reported physical function in patients with CKD (3). The adverse effects of ESAs are well recognized, with large randomized trials (4–6) showing a strong association of higher hemoglobin targets with increased risks of thrombosis and cardiovascular events.
The safety of intravenous iron dosing in dialysis is less certain. Intravenous iron has negative effects on the immune system in vitro (7) and variable effects on infection risks (8) and outcomes (9). It promotes oxidative stress (10) due to an increase in free iron availability, but the effect on cardiovascular outcomes is unclear. Intravenous iron utilization strategies in dialysis vary widely across countries (11), and guidelines (12–14) suggest intravenous iron use below targets of transferrin saturation 30% and ferritin 500 ng/ml but do not offer any absolute upper limits. This clinical uncertainty reflects a lack of adequately powered and long-term studies balancing benefits, risks, and costs of intravenous iron versus ESAs. In 2011, Medicare instituted a bundled repayment system for dialysis services, including intravenous iron and ESAs, leading to the financial incentive of reducing the utilization of ESAs by increasing the use of intravenous iron. This did occur (15) but may not be persistent due to lower hemoglobin targets (16), and it does not seem to be causing any harm (17). However, there remain ongoing concerns regarding the safety of this practice.
Previous systematic reviews and meta-analyses on iron utilization in anemia have been conducted in the general population (18), and they excluded patients with CKD and patients with kidney failure. Furthermore, these studies compared only routes and did not compare doses of iron supplementation (19); they focused on biochemical markers rather than clinically relevant outcomes (10) and did not report the full spectrum of adverse events. A recent systematic review and meta-analysis found no increased risk of all-cause mortality or adverse events with intravenous iron compared with oral iron, but it was limited in sample size by including only randomized, controlled trials (RCTs) and combining studies of both patients with CKD predialysis and those treated with dialysis (20). Thus, we conducted a systematic review and meta-analysis of RCTs and observational studies evaluating the safety of intravenous iron dosing in the management of anemia in patients treated with dialysis.
Materials and Methods
Data Sources and Searches
We developed and followed a protocol that included population, intervention, comparison, and outcomes criteria for a search strategy (protocol not registered) (protocol is in Supplemental Table 1). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (21) guideline for RCTs and the Meta-Analysis of Observational Studies in Epidemiology guideline (22) for observational studies for meta-analyses and systematic reviews. Our goal was to identify all RCTs and observational studies in which higher-dose intravenous iron use was compared with lower-dose intravenous iron, oral iron, or no iron in an adult dialysis population (hemodialysis or peritoneal dialysis). Outcomes of interest included all-cause mortality, infection, cardiovascular events, or hospitalizations, with a follow-up of at least 30 days. Only articles published in the English language were included. We did not place any restriction on sample size. Databases searched for articles included Medline, PubMed, EMBASE, the Cochrane library, and CINAHL databases. The search strategy was developed in consultation with a medical librarian (L.H.), and it was tailored to each database using a combination of keywords and MeSH terms covering the concepts “intravenous iron” and “renal dialysis” (Supplemental Table 1). The extent of the search was from inception of the databases to January 6, 2017. All abstracts retrieved were downloaded into Refworks, version 2.0.
Study Selection
Two individuals (I.H. and M.B.) independently reviewed titles and abstracts identified by the search strategy and excluded those that did not meet inclusion criteria. For those articles that remained, full texts were retrieved and reviewed for inclusion. A third individual (N.T.) reviewed any conflicts, and final decisions were made by consensus. Reference lists of full texts selected for inclusion were reviewed to identify any additional articles.
Data Extraction and Quality Assessment
We created a data extraction form to capture relevant information from the included RCTs and observational studies. Extracted data included study characteristics (first author, year of publication, sample size for each group, duration of intervention, treatment regimen and dosing for each group, and follow-up period), participants’ characteristics (age; sex; dialysis modality and duration; inclusion criteria for transferrin saturation, serum ferritin, and hemoglobin; baseline transferrin saturation, serum ferritin, and hemoglobin; and ESA dose) for potential subgroup analyses, and our prespecified safety outcomes (all-cause mortality, infection, cardiovascular events, and hospitalizations). If a study contained more than two intravenous dosing regimens, the highest iron group and the lowest iron group were used for the analysis. For most observational studies and RCTs, the higher iron group doses exceeded 200 and 400 mg/mo, respectively, and conversely, the lower iron arms were consistently <200 mg/mo. Authors were contacted if their study did not meet this criterion to clarify dosing categories. In addition, if studies had analyses for several dosing periods, the longest dosing period was used for our analysis. Two reviewers (I.H. and M.B.) independently extracted data; inconsistencies were corrected and resolved by consensus and consultation with a third reviewer (D.C).
For RCTs, two reviewers (I.H. and M.B.) assessed studies for their quality of reporting and risk of bias using the Cochrane Collaboration tool for assessing risk of bias (23). Bias was assessed in the following domains at the study level: selection bias including random sequence generation and allocation concealment, performance bias, detection bias, attrition bias, reporting bias, and other bias. Three categories were used in summary judgment of the quality of each domain both within individual studies and across all studies: high (bias may alter results seriously), low (bias, if present, is unlikely to alter results seriously), and unclear (a risk of bias raises some doubt about results). Sensitivity analyses were performed by excluding studies designated as high risk of bias in one or more domains.
For observational studies, two reviewers (I.H. and M.B.) assessed studies for their quality of reporting using the Newcastle–Ottawa Scale (24). Bias was assessed in the following domains at the study level: selection of study groups, comparability of groups, and ascertainment of the exposure or outcome of interest. Use of appropriate statistical methods to account for time-dependent confounding was assessed as a potential domain of bias in addition to the Newcastle–Ottawa Scale assessment. Conflict was resolved by a third reviewer (D.C.).
Data Synthesis and Analyses
A meta-analysis was performed on all-cause mortality, infection, cardiovascular events, and hospitalizations using the DerSimonian and Laird method. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated using fixed effects and random effects models. For studies that did not provide HRs, relative risks (RRs) were calculated from events data, and they were used to estimate the HRs. Heterogeneity between studies was analyzed using the Cochran Q and I2 statistics for measures of inconsistency (25) as well as τ2 in the random effects model. The software program Review Manager, Version 5.3 was used for the analysis (26).
Results
A flow diagram outlining the selection strategy is shown in Supplemental Figure 1. Our search strategy identified 2231 articles after removal of duplicates, of which 330 were selected for full text review, with seven RCTs and 15 observational studies deemed eligible for inclusion.
RCTs
Characteristics of Selected Studies.
Study and participant characteristics are summarized in Supplemental Table 2 and Table 1. Of the seven included RCTs, two compared higher- with lower-dose intravenous iron, two compared intravenous with oral iron, two compared intravenous with no iron, and one compared intravenous with either oral or no iron. The intravenous iron formulations used were iron dextran, iron sucrose, ferric gluconate, and ferumoxytol. Six studies reported all-cause mortality, and four reported infection. No study reported cardiovascular events, and only two studies reported hospitalizations; as such, no meta-analysis for these outcomes was performed.
Table 1.
Characteristics of included randomized, controlled trials
| Study, First Author, Year of Publication, Year of Study, Study Name/Data Source | Inclusion criteria: Modality, TSAT, Ferritin, HGB, Iron, ESA, Other | n: All, Active, Comparator | Iron Strategy, Active versus Comparator | Iron Targets | ESA Strategy | HGB Targets | Duration | 1° Outcome | 2° Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Fishbane et al. (28), (1995), N/A, N/A | HD≥3 mo | 75 | ID 100 mg iv 2× weekly during regular HD | N/A | EPO to achieve HCT target with 2000 U increase/decrease if below/above target | HCT 30%–34% | 4 mo | N/A | N/A |
| >15% | 25 | versus | |||||||
| >100 | 50 | Oral iron (FS 325 mg po three times per day or iron polysaccharide 150 mg po two times per day) with rescue 1 g ID over ten HD if ferritin <50 ng/ml, TSAT<12.5% | |||||||
| N/A | |||||||||
| Oral≥3 mo, no iv ID for at least 6 mo | |||||||||
| EPO≥3 mo | |||||||||
| No recent bleeding, transfusions, hematologic | |||||||||
| Fudin et al. (29), (1998), N/A, N/A | Incident HD | 48 | iv ferric gluconate 62.5 mg qHD | N/A | N/A | N/A | 12, 26 mo | RBC transfusions | ferritin |
| TSAT<21% | 24 | versus | |||||||
| N/A | 12 | Oral ferrous sulfate 160 mg po daily | |||||||
| <78 | 12 | versus | |||||||
| N/A | None | ||||||||
| N/A | |||||||||
| Depleted sternal bone marrow iron stores | |||||||||
| Besarab et al. (30), (2000), N/A, N/A | HD | 42 | ID 4–6 100 mg doses during consecutive HD to increase TSAT>30% and 25–150 mg/wk to maintain TSAT 30%–50% | TSAT 20%–30% during 16–20 wk run-in phase, then | EPO to achieve HGB target | Steady state if maximal HGB variation <1.2 g/dl and EPO<500 U per dose during last 6 wk of run-in phase, 9.5–12 g/dl targeting baseline value, algorithm | 6 mo | ESA dose by power calculations | N/A |
| TSAT 19%–30%, ferritin 150–600 HGB≥9.5 g/L with MCV>80 fl | 23 | versus | 30%–50% active versus | ||||||
| N/A | 19 | ID 25–150 mg/wk to maintain TSAT 20%–30% | 20%–30% comparator | ||||||
| Stable EPO dose over previous 3 mo (±25%) >700 U 3× per 1 wk | |||||||||
| N/A | |||||||||
| Singh et al. (31), (2006), N/A, N/A | Incident or prevalent PD | 126 | IS 1000 mg iv over 28-d period as 300 mg day 1 and day 15, 400 mg day 28 | N/A | ESA at same dose before randomization, unchanged throughout study period | N/A | 8 wk with withdrawal if modality change, intervention for anemia management | Change from baseline to highest HGB | HGB response; iron stores; BP; adverse events before, during, after infusion; SAE indicates fatal or life-threatening disability or incapacity; hazard; anemia intervention management (RBC transfusion, increase in ESA, iron administration not in study protocol) |
| TSAT≤25% | 80 | versus | |||||||
| Ferritin≤500 | 46 | No iron | |||||||
| HGB≥9.5 and ≤11.5 | |||||||||
| No iv or po iron for 4 wk | |||||||||
| ESA (DPO or EPO) without dose change for 8 wk | |||||||||
| N/A | |||||||||
| Coyne et al. (32), (2007), 2004–2006, DRIVE, N/A | HD≥90 d | 134 | FG 125 mg iv ×8=1 g | N/A | EPO dose was raised by 25% and maintained for the entire study | N/A | Baseline ≤1 wk, 6 wk | Change from baseline to week 6 in HGB | Percentage of responders (HGB increase of ≥2 g/dl during the study) and time to response, change in baseline of CHr, TSAT, ferritin, CRP |
| TSAT≤25%, | 68 | versus | |||||||
| Ferritin 500–1200 | 66 | None | |||||||
| HGB≤11.0 g/dl | |||||||||
| ≤125 mg/wk iv iron previous 4 wk | |||||||||
| Stable dosage of EPO≥225 IU/kg per week or ≥22500 IU/wk for ≥2 wk | |||||||||
| N/A | |||||||||
| Provenzano et al. (33), (2009), N/A, N/A | HD≥90 d, TSAT≤30% ferritin≤600 | 230 | FX 510 mg iv ×2 during sequential HD within 5±3 d | N/A | ESA dose was required to remain constant throughout the study | N/A | 35 d but nonrandomized readmission phase | Change in HGB from baseline to 35 d | Proportion of patients achieving a ≥1-g/dl increase in HGB at day 35; change in HGB at day 21; change in TSAT, ferritin, SI, TIBC, CHr at days 21 and 35; safety analysis |
| HGB≤11.5 | 114 | versus | |||||||
| N/A | 116 | Ferrous fumarate 50 mg for 200 mg elemental iron daily ×21 d | |||||||
| Stable ±25% ESA dose for 10 d | |||||||||
| N/A | |||||||||
| Lewis et al. (34), (2015), 2010–2012, N/A | 3× Weekly HD or PD for ≥3 mo | active control trial | FC 1 g (210 mg ferric iron) tablets with protocol titration schedule (have iv iron dose 55 mg/mo) | At the discretion of the site | At the discretion of the treating physician | N/A | 2 wk Washout, 52 wk active control, 4 wk placebo control | Change in phosphate at 4 wk placebo control period | Changes over 52 wk in ferritin, TSAT, cumulative doses of iv iron, ESA |
| TSAT<50% | 441 | versus | |||||||
| Ferritin<1000 | 292 | Calcium acetate 667 mg capsules, sevalamer 800 mg tablets titrated by FDA-approved package inserts | |||||||
| N/A | 149 | and iv iron prohibited if ferritin >1000 ng/ml or TSAT>30% (have iron dose 115 mg/mo) | |||||||
| N/A | Placebo control trial | ||||||||
| N/A | 192 | ||||||||
| 3–18 Doses phosphate binders, phosphate≥2.5 and ≤8.0 mg/dl | 96 | ||||||||
| 96 |
TSAT, transferring saturation; HGB, hemoglobin; ESA, erythropoietin-stimulating agent; N/A, not reported; HD, hemodialysis; iv, intravenous; ID, iron dextran; EPO, erythropoietin; FS, ferrous sulfate; po, per ora; HCT, hematocrit; qHD, with each hemodialysis; RBC, red blood cell; MCV, mean corpuscular volume; DPO, darbopoetin; IS, iron sucrose; SAE, serious adverse event; DRIVE, dialysis patients' response to IV iron with elevated ferritin study; FG, ferric gluconate; CHr, reticulocyte hemoglobin content; CRP, C-reactive protein; FX, ferumoxytol; SI, serum iron; TIBC, total iron binding capacity; PD, peritoneal dialysis; FC, ferric citrate; FDA, Food and Drug Agency.
Mortality.
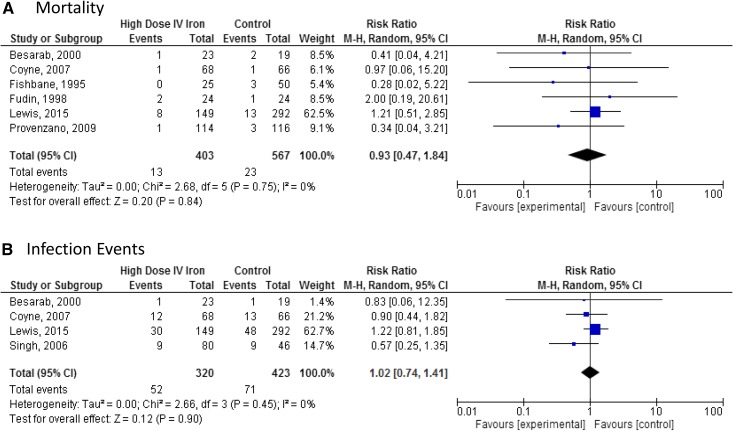
Meta-analysis of six studies (n=970) showed no difference in all-cause mortality with higher-dose intravenous iron treatment: RR, 0.93; 95% CI, 0.47 to 1.84 in the random effects model (Figure 1A). There was no heterogeneity between studies (τ2=0.00; χ2=2.68; df=5; P=0.75; I2=0%).
Figure 1.
No statistically significant difference in either mortality or infection events in meta-analyses of randomized controlled trials. Meta-analysis comparing the safety of high-dose intravenous (IV) iron versus control in randomized, controlled trials. (A) Mortality. (B) Infection events. 95% CI, 95% confidence interval; M-H, Mantel-Haenszel.
Infection.
Meta-analysis of four studies (n=743) showed no difference in infection with higher-dose intravenous iron treatment: RR, 1.02; 95% CI, 0.74 to 1.41 in the random effects model (Figure 1B). There was no heterogeneity between studies (τ2=0.00; χ2=2.66; df=3; P=0.45; I2=0%).
Quality of Reporting and Risk of Bias.
No study was found to be at low risk of bias (Supplemental Figure 2). Performance bias was consistently an issue, with all studies being labeled as either high risk due their open-label methodology or unclear, because attempts at blinding were not reported. Attrition bias was also frequently observed. No study adequately described allocation concealment or blinding of outcome assessment (Supplemental Figure 3).
Sensitivity Analyses.
Sensitivity analysis was performed by removing studies designated as high risk of bias in one or more domains, with the exception of performance bias. Results remained unchanged and were not statistically significant (Supplemental Figures 4 and 5).
Observational Studies
Characteristics of Selected Studies.
Study and participant characteristics are summarized in Supplemental Table 3 and Table 2. Eight studies compared higher-dose intravenous iron with lower-dose intravenous iron, one study compared intravenous iron with oral iron, and six studies compared intravenous iron with no iron. Eight studies reported all-cause mortality, eight studies reported infection, seven studies reported cardiovascular events, and five studies reported hospitalizations.
Table 2.
Characteristics of observational studies
| Study: First Author, Year Of Publication, Year Of Study, Study Name/Data Source | Inclusion Criteria: Modality, TSAT, Ferritin, HGB, Iron, ESA, Other | n | Iron Strategy: Active Versus Comparator | Iron Targets | ESA Strategy | HGB Targets | Duration | 1° Outcome | 2° Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Canziani et al. (35), (2001), N/A, N/A | Chronic HD >3 mo, 3× per week for 4 h | 111 | IS 100 mg ×10=28 treatment d | TSAT<20% | EPO doses were not changed during the study | N/A | 150 d | Infectious episodes | N/A |
| =1 g/mo | TSAT<20% | ||||||||
| IS 100 mg ×20=70 treatment d | TSAT 20%–50% | ||||||||
| =2 g/150 d =400 mg/mo | |||||||||
| IS 100 mg ×10=70 treatment d | |||||||||
| =1 g/150 d =200 mg/mo | |||||||||
| Hoen et al. (36), (2002), 1994–1995, EPIBACDIAL | Chronic HD 2–3× per week | 513 | iv iron (polymaltose ferric hydroxide) | N/A | N/A | N/A | 1 yr | Bacteremia | N/A |
| Hospital or self-care units | Oral iron (FS, ferrous fumarate, ferrous asorbate) | ||||||||
| iv or oral iron for at least 6 mo | |||||||||
| Feldman et al. (37, 38), (2002 and 2004), 1996–1998, Fresenius Medical Corporation | HD | 27,280 | None | N/A | N/A | N/A | 6-mo baseline, follow-up 6 mo rolling intervals, 6, 12 mo lagged time-dependent analyses | Censored death by time-dependent models 0–6, 6–12, and 12–18 mo | N/A |
| >0–700 mg | |||||||||
| >700–1000 mg | |||||||||
| >1000–1800 mg | |||||||||
| >1800 mg | |||||||||
| Kalantar-Zadeh et al. (39), (2005), 2001–2003, DaVita | Maintenance HD >3 mo | 58,058 | IG, IS, ID<5% but no strategies | N/A | N/A | N/A | Eight quarters (3 mo per quarter) | Mortality | N/A |
| Kopelman et al. (40), (2007), N/A, N/A | HD with | 39 | iv FG if ferritin>800 ng/ml and HGB<11 dl and TSAT<25% versus standard of care practice protocol, which held iv iron if ferritin >800 ng/ml | Nurse anemia manager | Nurse anemia manager | Nurse anemia manager | 3 mo | N/A | N/A |
| TSAT<25%, ferritin >800 | |||||||||
| Group 1 =“FID” HGB <11, TSAT<25%, ferritin >800, ≥250 mg FG in 3 mo | |||||||||
| Group 2 =“no FID” ferritin >800, TSAT<25%, <250 mg FG in 3 mo | |||||||||
| Kapoian et al. (41), (2008), N/A, DRIVE-2 | DRIVE participant, chronic HD ≥90 d, TSAT≤25%, ferritin 500–1200 | 112 | Discretion of physicians (ID, FG, IS) | Discretion of physicians | Discretion of physicians | Discretion of physicians | 6 wk | EPO dose at 6 wk | Mean change from end of DRIVE HGB, TSAT, ferritin to end of DRIVE-2, safety adverse events |
| HGB≤11.0 g/dl, EPO≥225 U/kg or ≥22,500 U/wk with no change ≥14 d, ≤125 mg of iv iron in last 4 wk | EPO | ||||||||
| Pollak et al. (42), (2009), 1998–2006, N/A | ICHD | 1774 | As per attending nephrologist, anemia management team | N/A | As per attending nephrologist, anemia management team, iv | N/A | Until death or June 30, 2007 | Censored survival | KMC for survival no/low groups dramatically different, so pooling is inappropriate |
| 1998–2006 | ID, IS, FG | “Low versus medium versus high” ≤12.1, 12.1–27.7, >27.7 103 U/wk | |||||||
| “No/low versus medium versus high” = no, 1–202, 203–455, >455 | |||||||||
| Kuo et al. (43), (2012), 2004–2005, N/A | Chronic 3× weekly HD | 1239 | Ferric chloride hexahydrate | N/A | N/A | N/A | 12 mo | CV events (MI, stroke, CHF, complicated PVD, sudden death), all-cause mortality | Endothelial dysfunction markers in subgroup in 10-wk randomized trial of 40 versus 80 mg versus placebo weekly |
| >6 mo dialysis vintage, life expectancy>6 mo | |||||||||
| Brookhart et al. (44), (2013), 2004–2008, USRDS | HD≥9 mo | 117,050 | Low dose: 1–200 mg/mo versus high dose: >200 mg/mo | N/A | N/A | N/A | 6-mo baseline, 1-mo exposure, 3-mo follow-up | Hospitalization for infection, death attributed to infection, composite outcome with sensitivity analysis for definition of infection | N/A |
| DaVita and USRDS | Bolus versus maintenance | ||||||||
| Kshirsagar et al. (45), (2013), 2004–2008, USRDS | ICHD≥9 mo, at least one TSAT measurement, without FG, IS≥9 dialysis sessions | 117,050 | None versus low dose (1–200 mg/mo) versus high dose (>200 mg/mo) | 6-mo baseline, 1-mo exposure, 3-mo follow-up | Death attributed to CV, hospitalization for MI, hospitalization for stroke, composite | N/A | |||
| Davita and USRDS | Bolus (iron on consecutive dialysis sessions of at least 100 mg) versus maintenance with potential to excess 600 mg within 30 d) | ||||||||
| Freburger et al. (46), (2014), 2006–2010, USRDS | HD≥9 mo with at least one TSAT measurement without FG, IS≥9 dialysis sessions | 6605 | None versus bolus (two consecutive iron doses of at least 100 mg with potential to exceed 600 mg within 30 d) versus maintenance | N/A | N/A | N/A | 6-mo baseline, 1-mo exposure, 3-mo follow-up periods | Death from any cause, hospitalized for pneumonia, infection, MI, stroke, iv antibiotics, CV-related death | Infection-related composite outcomes, CV composite outcome |
| Renal Research Institute and USRDS | |||||||||
| Kuragano et al. (47), (2014), 2007–2009, TRAP | HD≥1 yr | 1095 | Oral iron versus iv iron versus oral and iv iron | N/A | N/A | 10–11 g/dl | 2 yr | Dose of ESA and iron to maintain HGB 10–11 g/dl, CCVD, infection, hospitalization, death | N/A |
| Low versus high dose | |||||||||
| Miskulin et al. (48), (2014), 2003–2008, DEcIDE | Incident ICHD, <60 d in between incident date and first ICHD treatment | 21,233 with 14,078, 12,646, and 10,899 for 1, 3, and 6 mo analyses, respectively | No versus low >0–150 mg versus moderate >150–350 mg versus high >350 mg/mo | N/A | N/A | N/A | 90-d baseline; 30-d exposure; 1-, 3-, and 6-mo rolling window follow-up but <4 yr | Censored all-cause mortality | Infection-related mortality, CV mortality |
| Bailie et al. (49), (2015), N/A, DOPPS, 2 (2002–2004), 3 (2005–2008), 4 (2009–2011) | HD, ≥5 mo | 32,435 | 60% IS, 24% FG, 11% iron polymaltose,2% ID in Europe, Australia, New Zealand | N/A | N/A | N/A | 1 mo after 4-mo iv iron dose ascertainment period until death or transfer, modality change, loss to follow-up, study end | All-cause mortality | CV-related mortality, infection-related mortality, non-CV/noninfection–related mortality, first hospitalization |
| 71% IS, 20% FG, 8% ID in North America | |||||||||
| Tangri et al. (50), (2015), 2003–2008, DEcIDE | Incident ICHD, <60 d in between incident date and first ICHD treatment | 9544, 8580, 7416 for 1, 3, and 6 mo analyses, respectively | No versus low >0–150 mg versus moderate >150–350 mg versus high >350 mg/mo | N/A | N/A | N/A | 90-d baseline; 30-d exposure; 1-, 3-, and 6-mo rolling window follow-up | Censored all-cause hospitalization 30 d after 1-, 3-, and 6-mo windows | Censored hospitalization attributable to infectious and CV causes, composite of hospitalizations, and death |
TSAT, transferring saturation; HGB, hemoglobin; ESA, erythropoietin-stimulating agent; N/A, not applicable; HD, hemodialysis; IS, iron sucrose; EPO, erythropoietin; iv, intravenous; EPIBACDIAL, a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients; FS, ferrous sulfate; IG, iron glucose; ID, iron dextran; FID, functional iron deficiency; FG, ferric gluconate; DRIVE-2, dialysis patients' response to IV iron with elevated ferritin study II; DRIVE, dialysis patients' response to IV iron with elevated ferritin study; ICHD, in-center hemodialysis; KMC, Kaplan–Meier curve; CV, cardiovascular; MI, myocardial infarction; CHF, congestive heart failure; PVD, peripheral vascular disease; USRDS, United States Renal Data System; TRAP, prospective study of treatment for renal anemia on prognosis in hemodialysis patients; CCVD, chronic cerebrovascular disorders; DECIDE, developing evidence to inform decisions about effectiveness; DOPPS, dialysis outcomes and practice patterns study.
Mortality.
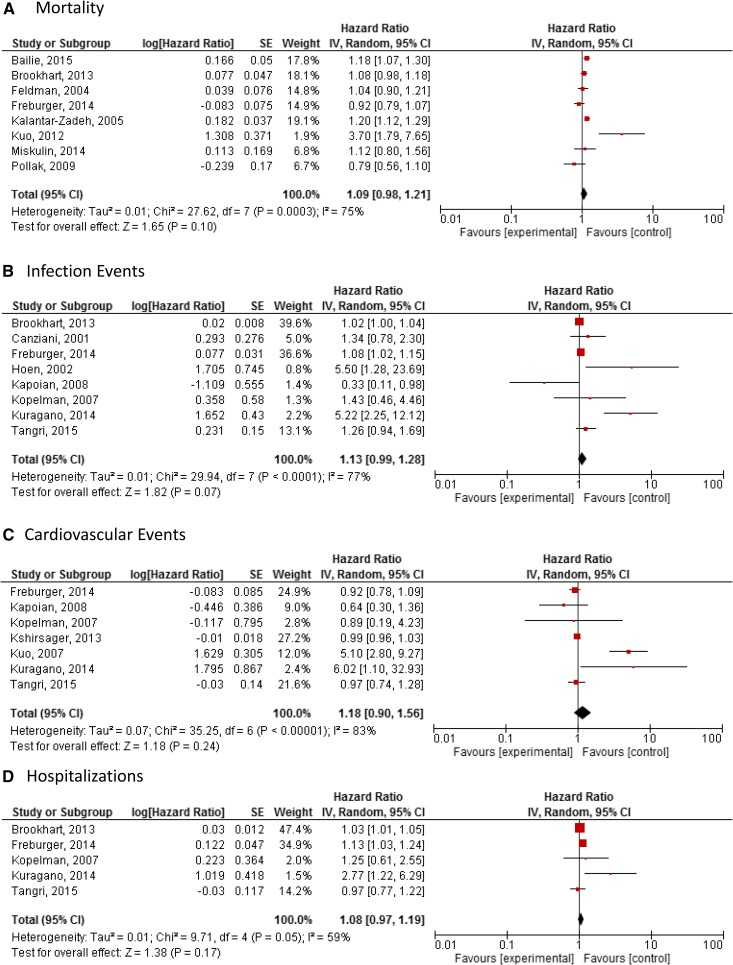
Meta-analysis of eight studies (n=241,408) showed no difference in mortality with higher-dose intravenous iron treatment: HR, 1.09; 95% CI, 0.98 to 1.21 random effects model (Figure 2A). There was considerable heterogeneity between studies (P=0.003; I2=75%).
Figure 2.
No statistically significant difference in either mortality, infection events, cardiovascular events, or hospitalizations in meta-analyses of observational studies. Meta-analysis comparing the safety of high-dose intravenous iron versus control in observational studies. (A) Mortality. (B) Infection events. (C) Cardiovascular events. (D) Hospitalizations. 95% CI, 95% confidence interval; SE, SEM.
Infection.
Meta-analysis of eight studies (n=135,532) showed no difference in infection with higher-dose intravenous iron treatment: HR, 1.13; 95% CI, 0.99 to 1.28 random effects model (Figure 2B). There was considerable heterogeneity between studies (P<0.001; I2=77%).
Cardiovascular Events.
Meta-analysis of seven studies (n=135,675) showed no difference in cardiovascular events with higher-dose intravenous iron treatment: HR, 1.18; 95% CI, 0.90 to 1.56 random effects model (Figure 2C). There was considerable heterogeneity between studies (P<0.001; I2=83%).
Hospitalizations.
Meta-analysis of five studies (n=134,324) showed no difference in hospitalization with higher-dose intravenous iron treatment: HR, 1.08; 95% CI, 0.97 to 1.19) random effects model (Figure 2D). There was substantial heterogeneity between studies (P=0.05; I2=59%).
Quality of Reporting and Risk of Bias.
Seven studies were found to have a low risk of bias in all categories. One study was found to have a high risk of bias in the category of assessment of outcomes, and four studies were high risk of bias due to use of inappropriate statistical methods (Supplemental Figures 6 and 7).
Sensitivity Analyses.
Sensitivity analysis was performed by removing studies designated as high risk of bias in one or more domains. Results remained unchanged and were not statistically significant (Supplemental Figures 8–11).
Discussion
In this systematic review and meta-analysis of seven RCTs and 15 observational studies of >140,000 participants, we did not find evidence of increased risk of infection, cardiovascular events, hospitalizations, or mortality with the use of higher-dose intravenous iron compared with lower doses of iron in patients treated with dialysis. Of note, a possibility of harm was observed with a nonstatistically significant trend toward harm in observational studies, which was partially mitigated by the sensitivity analysis. Taken together, these findings suggest that use of higher doses of intravenous iron and lower doses of ESA may not be harmful, although additional randomized trials are needed to strengthen these findings.
Litton et al. (18) performed a systematic review and meta-analysis of RCTs addressing the efficacy and safety of intravenous iron compared with either oral iron or no iron supplementation in the general population and found that intravenous iron increased hemoglobin and reduced the need for red blood cell transfusion. There was no difference in mortality or serious adverse events (n=19 studies; RR, 1.1; 95% CI, 0.8 to 1.1), but intravenous iron was associated with a borderline increase in risk of infection. Their findings in the nondialysis population confirm our results for all-cause mortality and cardiovascular morbidity but contradict our finding and the findings of a previous meta-analysis of patients treated with dialysis, which show no infection risk in randomized trials (10). We hypothesize that this is likely to due to a reporting bias favoring positive infectious events in the nondialysis literature, because infections were rarely a predefined outcome and considered exploratory. In the dialysis literature, where infection has been reported to be of significant concern, our meta-analysis of randomized trials suggests no increase in risk.
A recent meta-analysis in patients treated with dialysis and those with nondialysis CKD also compared intravenous iron with oral iron (20). These investigators found an excess risk of short-term adverse effects with intravenous iron compared with oral formulations but no long-term effects on patient safety. Their findings are concordant with our findings but omit comparisons of higher versus lower doses of intravenous iron treated with dialysis and the accumulating evidence from well conducted observational studies (20). To our knowledge, this is the first systematic review of observational studies of intravenous iron in patients treated with dialysis. Although there is increased potential for time-dependent confounding with intravenous iron, iron indices, ESA dose, and hemoglobin in patients treated with dialysis, several large recent studies have chosen robust statistical methods, such as marginal structure models, in efforts to appropriately account for these potential confounders. Given the large number of patients in these studies and similarities in the dosing comparisons and statistical methods, we felt that it was appropriate to summarize their findings using meta-analysis despite the statistical heterogeneity. In our subgroup analysis restricted to studies using rigorous statistical methods or those with a lower risk of bias, our primary findings of no risk with intravenous iron were again confirmed.
Our study has important clinical, research, and health policy implications. From a clinical perspective, our findings on the basis of the currently available literature suggest that use of higher doses of intravenous iron in patients treated with dialysis may not increase risk of infections, hospitalizations, cardiovascular events, or mortality, although further study is warranted. Several national and international descriptive studies indicate that ESA minimization and higher iron dosing may already be happening since the publication of the Trial to Reduce Cardiovascular Events with Aranesp Therapy (6) and the bundling of dialysis payments in United States, although perhaps not persistently due to lower hemoglobin targets. Our findings cautiously suggest that the change in clinical practice might not increase the risk of adverse events. In fact, surveillance systems have not detected any increase in mortality rates for patients treated with dialysis in the higher-intravenous iron dosing era. From a research perspective, simple, large randomized trials with updated meta-analyses comparing intravenous iron dosing and strategies in the context of anemia management with ESAs or novel therapies with long-term follow-up to assess safety, efficacy, and cost are needed. One such trial is already under way and will add to the RCT evidence for intravenous iron (27). From a policy perspective, with further confirmatory research, agencies and programs could incorporate the option of higher-dose intravenous iron in dialysis framed by this evidence in the context of patient values, preferences, and economic implications.
The strengths of this study include its size and inclusion of RCTs and observational studies encompassing representative dialysis populations with a substantial amount of patients and adverse events. Although follow-up duration and number of participants were limited in the RCTs, the observational studies contained much larger sample sizes and periods of observation. In addition, the sensitivity analysis for only studies at low risk of bias strengthens our results. We are confident in our description of the current literature but advise caution in applying our results. In particular, for patients treated with peritoneal dialysis, external validity is limited given that only three studies included patients on peritoneal dialysis, in which intravenous iron is less frequently used given the efficacy of oral iron in this population. Furthermore, the majority of studies were performed in the United States, and thus, applicability to other settings may be limited given differences in patient mix across nations.
We did not specifically evaluate intravenous iron dosing in the context of intravenous iron strategies (bolus versus maintenance), but there is presumably an overlap that could not be addressed due to a lack of individual patient data. We chose to not include hypersensitivity reactions as outcomes of interest given their rarity and unlikely relationship to dosing regimen. Finally, our cutoffs for defining intravenous iron doses as a categorical variable were arbitrarily dichotomized into “higher” and “lower” and did not take into account individualization of therapy on the basis of iron requirements identified by iron studies, clinical needs, physician and system processes, and costs. Ideally, intravenous iron dosing could be analyzed as a continuous variable using individual patient data metaregression, which may identify an absolute threshold or “very high” intravenous iron dosing that is harmful, but this was not possible.
We found no evidence of excess infections, cardiovascular events, hospitalizations, or death associated with intravenous iron in our systematic review and meta-analysis. The small number of participants and events in the RCTs as well as the statistical heterogeneity noted in observational studies limit our certainty regarding safety effects of higher-dose intravenous iron in this population. Simple, large, high-quality RCTs of significant size and freedom from bias will help strengthen these findings.
Disclosures
N.T. has received grant support from Astra Zeneca Inc. Astra Zeneca Inc. manufactures Roxadustat, which is a novel treatment for anemia of CKD.
Supplementary Material
Acknowledgments
The primary funding sources were the Canadian Institutes of Health Research New Investigator Award and the University of Manitoba’s Bachelor of Science in Medicine Program.
The funders had no role in the study’s design, conduct, or reporting or the decision to submit the manuscript for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Rest Easy with Intravenous Iron for Dialysis Patients?: High Dose IV Iron Safety,” on pages 363–365.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05390517/-/DCSupplemental.
References
- 1.Hsu CY, McCulloch CE, Curhan GC: Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: Results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 13: 504–510, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Pisoni RL, Bragg-Gresham JL, Young EW, Akizawa T, Asano Y, Locatelli F, Bommer J, Cruz JM, Kerr PG, Mendelssohn DC, Held PJ, Port FK: Anemia management and outcomes from 12 countries in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 44: 94–111, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Palmer SC, Saglimbene V, Mavridis D, Salanti G, Craig JC, Tonelli M, Wiebe N, Strippoli GF: Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: A network meta-analysis. Cochrane Database Syst Rev 12: CD010590, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A; CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Sonnweber T, Theurl I, Seifert M, Schroll A, Eder S, Mayer G, Weiss G: Impact of iron treatment on immune effector function and cellular iron status of circulating monocytes in dialysis patients. Nephrol Dial Transplant 26: 977–987, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Boelaert JR, Daneels RF, Schurgers ML, Matthys EG, Gordts BZ, Van Landuyt HW: Iron overload in haemodialysis patients increases the risk of bacteraemia: A prospective study. Nephrol Dial Transplant 5: 130–134, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Ishida JH, Marafino BJ, McCulloch CE, Dalrymple LS, Dudley RA, Grimes BA, Johansen KL: Receipt of intravenous iron and clinical outcomes among hemodialysis patients hospitalized for infection. Clin J Am Soc Nephrol 10: 1799–1805, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Susantitaphong P, Alqahtani F, Jaber BL: Efficacy and safety of intravenous iron therapy for functional iron deficiency anemia in hemodialysis patients: A meta-analysis. Am J Nephrol 39: 130–141, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Bailie GR, Larkina M, Goodkin DA, Li Y, Pisoni RL, Bieber B, Mason N, Tong L, Locatelli F, Marshall MR, Inaba M, Robinson BM: Variation in intravenous iron use internationally and over time: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 28: 2570–2579, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Kliger AS, Foley RN, Goldfarb DS, Goldstein SL, Johansen K, Singh A, Szczech L: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis 62: 849–859, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Moist LM, Troyanov S, White CT, Wazny LD, Wilson JA, McFarlane P, Harwood L, Sood MM, Soroka SD, Bass A, Manns BJ: Canadian society of nephrology commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis 62: 860–873, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Locatelli F, Bárány P, Covic A, De Francisco A, Del Vecchio L, Goldsmith D, Hörl W, London G, Vanholder R, Van Biesen W; ERA-EDTA ERBP Advisory Board : Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: A European Renal Best Practice position statement. Nephrol Dial Transplant 28: 1346–1359, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Hirth RA, Turenne MN, Wheeler JR, Nahra TA, Sleeman KK, Zhang W, Messana JA: The initial impact of Medicare’s new prospective payment system for kidney dialysis. Am J Kidney Dis 62: 662–669, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Karaboyas A, Zee J, Morgenstern H, Nolen JG, Hakim R, Kalantar-Zadeh K, Zager P, Pisoni RL, Port FK, Robinson BM: Understanding the recent increase in ferritin levels in united states dialysis patients: Potential impact of changes in intravenous iron and erythropoiesis-stimulating agent dosing. Clin J Am Soc Nephrol 10: 1814–1821, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chertow GM, Liu J, Monda KL, Gilbertson DT, Brookhart MA, Beaubrun AC, Winkelmayer WC, Pollock A, Herzog CA, Ashfaq A, Sturmer T, Rothman KJ, Bradbury BD, Collins AJ: Epoetin alfa and outcomes in dialysis amid regulatory and payment reform. J Am Soc Nephrol 27: 3129–3138, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litton E, Xiao J, Ho KM: Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: Systematic review and meta-analysis of randomised clinical trials. BMJ 347: f4822, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albaramki J, Hodson EM, Craig JC, Webster AC: Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev 1: CD007857, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Shepshelovich D, Rozen-Zvi B, Avni T, Gafter U, Gafter-Gvili A: Intravenous versus oral iron supplementation for the treatment of anemia in CKD: An updated systematic review and meta-analysis. Am J Kidney Dis 68: 677–690, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group : Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 151: 264–269, W64, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group : The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343: d5928, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P: The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 1, 2016
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Cochrane Collaboration : Manager R: (RevMan), Version 5.3, Copenhagen, Denmark, The Nordic Cochrane Centre, 2014 [Google Scholar]
- 27.EU Clinical Trials Register: UK Multicentre Open-Label Randomised Controlled Trial of IV Iron Therapy in Incident Haemodialysis Patients: European Medicines Agency c1995, 2013. Available at: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2013-002267-25. Accessed December 1, 2016
- 28.Fishbane S, Frei GL, Maesaka J: Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis 26: 41–46, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Fudin R, Jaichenko J, Shostak A, Bennett M, Gotloib L: Correction of uremic iron deficiency anemia in hemodialyzed patients: A prospective study. Nephron 79: 299–305, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Besarab A, Amin N, Ahsan M, Vogel SE, Zazuwa G, Frinak S, Zazra JJ, Anandan JV, Gupta A: Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol 11: 530–538, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Singh H, Reed J, Noble S, Cangiano JL, Van Wyck DB; United States Iron Sucrose (Venofer) Clinical Trials Group : Effect of intravenous iron sucrose in peritoneal dialysis patients who receive erythropoiesis-stimulating agents for anemia: A randomized, controlled trial. Clin J Am Soc Nephrol 1: 475–482, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala AR; DRIVE Study Group : Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: Results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) study. J Am Soc Nephrol 18: 975–984, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Provenzano R, Schiller B, Rao M, Coyne D, Brenner L, Pereira BJ: Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol 4: 386–393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis JB, Sika M, Koury MJ, Chuang P, Schulman G, Smith MT, Whittier FC, Linfert DR, Galphin CM, Athreya BP, Nossuli AK, Chang IJ, Blumenthal SS, Manley J, Zeig S, Kant KS, Olivero JJ, Greene T, Dwyer JP; Collaborative Study Group : Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 26: 493–503, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canziani ME, Yumiya ST, Rangel EB, Manfredi SR, Neto MC, Draibe SA: Risk of bacterial infection in patients under intravenous iron therapy: Dose versus length of treatment. Artif Organs 25: 866–869, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Hoen B, Paul-Dauphin A, Kessler M: Intravenous iron administration does not significantly increase the risk of bacteremia in chronic hemodialysis patients. Clin Nephrol 57: 457–461, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Feldman HI, Santanna J, Guo W, Furst H, Franklin E, Joffe M, Marcus S, Faich G: Iron administration and clinical outcomes in hemodialysis patients. J Am Soc Nephrol 13: 734–744, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Feldman HI, Joffe M, Robinson B, Knauss J, Cizman B, Guo W, Franklin-Becker E, Faich G: Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol 15: 1623–1632, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Kalantar-Zadeh K, Regidor DL, McAllister CJ, Michael B, Warnock DG: Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol 16: 3070–3080, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Kopelman RC, Smith L, Peoples L, Biesecker R, Rizkala AR: Functional iron deficiency in hemodialysis patients with high ferritin. Hemodial Int 11: 238–246, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Kapoian T, O’Mara NB, Singh AK, Moran J, Rizkala AR, Geronemus R, Kopelman RC, Dahl NV, Coyne DW: Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol 19: 372–379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollak VE, Lorch JA, Shukla R, Satwah S: The importance of iron in long-term survival of maintenance hemodialysis patients treated with epoetin-alfa and intravenous iron: Analysis of 9.5 years of prospectively collected data. BMC Nephrol 10: 6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo KL, Hung SC, Lin YP, Tang CF, Lee TS, Lin CP, Tarng DC: Intravenous ferric chloride hexahydrate supplementation induced endothelial dysfunction and increased cardiovascular risk among hemodialysis patients. PLoS One 7: e50295, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brookhart MA, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Kshirsagar AV: Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol 24: 1151–1158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kshirsagar AV, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Brookhart MA: Intravenous iron supplementation practices and short-term risk of cardiovascular events in hemodialysis patients. PLoS One 8: e78930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freburger JK, Ellis AR, Kshirsagar AV, Wang L, Brookhart MA: Comparative short-term safety of bolus versus maintenance iron dosing in hemodialysis patients: A replication study. BMC Nephrol 15: 154, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuragano T, Matsumura O, Matsuda A, Hara T, Kiyomoto H, Murata T, Kitamura K, Fujimoto S, Hase H, Joki N, Fukatsu A, Inoue T, Itakura I, Nakanishi T: Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int 86: 845–854, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Miskulin DC, Tangri N, Bandeen-Roche K, Zhou J, McDermott A, Meyer KB, Ephraim PL, Michels WM, Jaar BG, Crews DC, Scialla JJ, Sozio SM, Shafi T, Wu AW, Cook C, Boulware LE; Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) Network Patient Outcomes in End Stage Renal Disease Study Investigators : Intravenous iron exposure and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 9: 1930–1939, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailie GR, Larkina M, Goodkin DA, Li Y, Pisoni RL, Bieber B, Mason N, Tong L, Locatelli F, Marshall MR, Inaba M, Robinson BM: Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int 87: 162–168, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Tangri N, Miskulin DC, Zhou J, Bandeen-Roche K, Michels WM, Ephraim PL, McDermott A, Crews DC, Scialla JJ, Sozio SM, Shafi T, Jaar BG, Meyer K, Boulware LE; DEcIDE Network Patient Outcomes in End-Stage Renal Disease Study Investigators : Effect of intravenous iron use on hospitalizations in patients undergoing hemodialysis: A comparative effectiveness analysis from the DEcIDE-ESRD study. Nephrol Dial Transplant 30: 667–675, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.