Abstract
Background and objectives
Patients undergoing hemodialysis with a frequency other than thrice weekly are not included in current clinical performance metrics for dialysis adequacy. The weekly standard Kt/Vurea incorporates treatment frequency, but there are limited data on its association with clinical outcomes.
Design, setting, participants, & measurements
We used multivariable regression to examine the association of dialysis standard Kt/Vurea with BP and metabolic control (serum potassium, calcium, bicarbonate, and phosphorus) in patients incidental to dialysis treated with home (n=2373) or in-center hemodialysis (n=109,273). We further used Cox survival models to examine the association of dialysis standard Kt/Vurea with mortality, hospitalization, and among patients on home hemodialysis, transfer to in-center hemodialysis.
Results
After adjustment for potential confounders, patients with dialysis standard Kt/Vurea <2.1 had higher BPs compared with patients with standard Kt/Vurea 2.1 to <2.3 (3.4 mm Hg higher [P<0.001] for home hemodialysis and 0.9 mm Hg higher [P<0.001] for in-center hemodialysis). There were no clinically meaningful associations between dialysis standard Kt/Vurea and markers of metabolic control, irrespective of dialysis modality. There was no association between dialysis standard Kt/Vurea and risk for mortality, hospitalization, or transfer to in-center hemodialysis among patients undergoing home hemodialysis. Among patients on in-center hemodialysis, dialysis standard Kt/Vurea <2.1 was associated with higher risk (adjusted hazard ratio, 1.11; 95% confidence interval, 1.07 to 1.14) and standard Kt/Vurea ≥2.3 was associated with lower risk (adjusted hazard ratio, 0.97; 95% confidence interval, 0.94 to 0.99) for death compared with standard Kt/Vurea 2.1 to <2.3. Additional analyses limited to patients with available data on residual kidney function showed similar relationships of dialysis and total (dialysis plus kidney) standard Kt/Vurea with outcomes.
Conclusions
Current targets for standard Kt/Vurea have limited utility in identifying individuals at increased risk for adverse clinical outcomes for those undergoing home hemodialysis but may enhance risk stratification for in-center hemodialysis.
Keywords: hemodialysis adequacy; mortality risk; Epidemiology and outcomes; Hemodialysis, Home; renal dialysis; calcium bicarbonate; blood pressure; Phosphorus; Potassium; Confidence Intervals; Odds Ratio; Bicarbonates; Blood Pressure Determination; Risk; hospitalization
Introduction
In the United States, the prevalent population of individuals with ESRD undergoing maintenance dialysis currently exceeds 460,000 (1). Although the majority of these patients are treated with conventional thrice weekly in-center hemodialysis, there has been recent growth in the adoption of home dialysis modalities, including short daily or frequent home hemodialysis (2). Despite this growth, patients treated with hemodialysis schedules other than thrice weekly, including most patients on home hemodialysis, are currently excluded in the calculation of the dialysis adequacy quality measure that is a part of the Centers for Medicare and Medicaid Services Quality Incentive Program (QIP) for ESRD in the United States (3). This is because identified targets for single-session fractional urea clearance or single-pool Kt/V (spKt/V) have been validated against clinical outcomes only in patients undergoing hemodialysis thrice weekly (4,5).
Current clinical practice guidelines recommend that the dose of dialysis for hemodialysis schedules other than thrice weekly be measured by determination of the weekly standard Kt/V (stdKt/V) defined as the weekly urea generation rate factored by the average predialysis serum urea concentration during a week normalized to the total volume of distribution of urea (6). Current clinical practice guidelines for hemodialysis adequacy suggest a target stdKt/V of 2.3, with a minimum delivered dose of 2.1 (6). The stdKt/V was derived to obtain a measure of dialysis adequacy that is independent of frequency and that would allow comparisons across dialysis modalities (7–9). However, to date, no study has assessed the association of achieved stdKt/V with clinical outcomes in patients undergoing home or in-center hemodialysis.
Using nationally representative data from a large dialysis provider in the United States, we undertook this study to examine the association of achieved stdKt/V with markers of volume and metabolic control in patients undergoing home and in-center hemodialysis. We further sought to determine associations of dialysis stdKt/V with three patient-centered clinical outcomes: all-cause mortality, hospitalization, and for patients undergoing home hemodialysis, transfer to in-center hemodialysis.
Materials and Methods
Data Source
The study population comprised patients ≥18 years of age who started maintenance dialysis from 2007 to 2011, received care at one of the facilities operated by DaVita Inc. for at least 60 days, and were either ever treated with home hemodialysis or exclusively treated with conventional thrice weekly in-center hemodialysis for ≥45 days (Supplemental Figure 1) (10). Follow-up data were available through December 31, 2011. For each patient, the follow-up period was divided into 91-day periods from the date of first dialysis with that modality. Results of laboratory tests, hemodynamic parameters, and parenteral medications were averaged for each 91-day period.
Exposure
The primary exposure in the primary analysis was dialysis stdKt/V calculated in the first 91-day period after initiation of in-center or home hemodialysis (the baseline period). For patients on home hemodialysis, the baseline period may have been preceded by a period of treatment with another dialysis modality. Patients were divided into three groups on the basis of mean stdKt/V during the baseline period: <2.1, 2.1–2.3, and >2.3. To determine stdKt/V, we first calculated for each patient the spKt/V for each dialysis session in the baseline period in which predialysis and postdialysis BUN concentrations were measured, typically once monthly. The spKt/V was estimated using the formula derived by Daugirdas et al. (11) for higher dialysis frequencies. We then calculated the equilibrated Kt/V (eKt/V) as described by Tattersal et al. (12). Next, we calculated the stdKt/V, S, from a fixed volume model as (7)
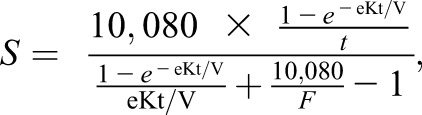 |
where t is the treatment time in minutes and F is the number of dialysis treatments in the week preceding blood sample collection. Finally, we calculated stdKt/V using a variable volume model as (13)
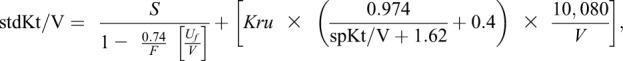 |
where S is the stdKt/V derived from the fixed volume model, F is the number of dialysis treatments per week, Uf is the weekly ultrafiltration volume in milliliters, Kru is the residual native kidney urea clearance in milliliters per minute, and V is the urea distribution volume in milliliters. We used the dialysis stdKt/V (excluding the Kru term) as the primary exposure in the main analysis. In additional analyses restricted to the 15.5% of patients on home hemodialysis and 29.5% of patients on in-center hemodialysis with data on residual kidney function, we also independently examined the dialysis, kidney, and total (dialysis + kidney) stdKt/V.
Outcomes
We assessed the association of category of baseline dialysis stdKt/V with markers of volume and metabolic control in the baseline period. These markers included interdialytic weight gain, predialysis BP, and serum potassium, calcium, phosphorus, and bicarbonate. Additionally, we assessed the association of category of dialysis stdKt/V with three clinical outcomes: all-cause mortality, time to first hospitalization, and time to transfer to in-center hemodialysis (among patients treated with home hemodialysis). Clinical outcomes were ascertained via the dialysis facility electronic medical record. For analyses of treatment outcomes, censoring occurred at kidney transplantation, transfer to a dialysis facility operated by another dialysis provider, or end of follow-up. Follow-up was complete for all patients before censoring.
Statistical Analyses
Data were complete for age, sex, diabetes, and cardiovascular comorbidities. Data for race and body mass index (BMI) were missing for <1% of the cohort. Vascular access type was missing for <2% of the cohort. Missing data were handled in adjusted analyses using a listwise deletion approach with the exception of vascular access type, where a missing data category was used. Using multivariable linear regression, we determined the association of category of baseline dialysis stdKt/V with levels of markers of volume and metabolic control in the first 91-day period after initiation of home or in-center hemodialysis. Linear regression models included age, sex, race/ethnicity, BMI, coexisting diabetes, history of congestive heart failure (CHF), history of atherosclerotic heart disease (ASHD), serum albumin, and vascular access type as model covariates.
Using Cox proportional hazards regression models, we performed time to event survival analyses to determine the association of dialysis stdKt/V with all-cause mortality over study follow-up. Three nested hierarchical models were examined: (1) unadjusted; (2) adjusted for age, sex, and race/ethnicity; and (3) additionally adjusted for BMI, diabetes, CHF, ASHD, serum albumin, and vascular access type. We tested for effect modification by sex in survival analyses through assessment of the significance of the first-order interaction term. Additionally, for analyses of home hemodialysis, we assessed for evidence of effect modification by time from initiation of any dialysis to the start of home hemodialysis (dialysis vintage) as well as by dialysis session frequency (less than five versus five or more sessions weekly). For all analyses, the referent group comprised patients with dialysis stdKt/V of 2.1 to <2.3. The predetermined level of significance (α) was set at 0.05. We constructed restricted cubic splines with three degrees of freedom to illustrate the continuous relationship between dialysis stdKt/V and clinical outcomes. We adjusted splines models for age, sex, race/ethnicity, BMI, diabetes, CHF, ASHD, serum albumin, and vascular access type.
For patients with available information on residual kidney function, we repeated survival analyses to assess the independent association of category of dialysis stdKt/V and tertile of kidney stdKt/V with clinical outcomes. In this restricted cohort, we used the same nested models as in the primary analysis, with the addition of dialysis stdKt/V or kidney stdKt/V as covariates where appropriate. We further constructed adjusted restricted cubic splines to examine the continuous relationship of dialysis, renal, and total stdKt/V with clinical outcomes. There were few outcome events among patients on home hemodialysis with data on residual kidney function (Supplemental Tables 1 and 2). Thus, the results of survival and spline analyses in the restricted cohort are reported only for patients on in-center hemodialysis.
Among the home hemodialysis cohort, we conducted a series of sensitivity analyses. First, we repeated Cox regression analyses with additional adjustment for hemodialysis treatment frequency in the fully adjusted model. Second, we repeated survival analyses with the exposure as quintile of baseline stdKt/V rather than clinical categories of <2.1, 2.1–2.3, and >2.3. Third, we analyzed our data using baseline stdKt/V as a binary exposure at two different cutoff points: <2.1 versus ≥2.1 and <2.3 versus ≥2.3. Fourth, for both the in-center hemodialysis cohort and the home hemodialysis cohort, we repeated survival analyses, treating stdKt/V as a time-varying exposure updated at the start of each 91-day period of follow-up.
Institutional review boards at the Los Angeles Biomedical Research Institute and the University of Washington approved the study as exempt from informed consent. Analyses followed Strengthening the Reporting of Observational studies in Epidemiology guidelines (14). Statistical analyses used SAS, version 9.3 (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
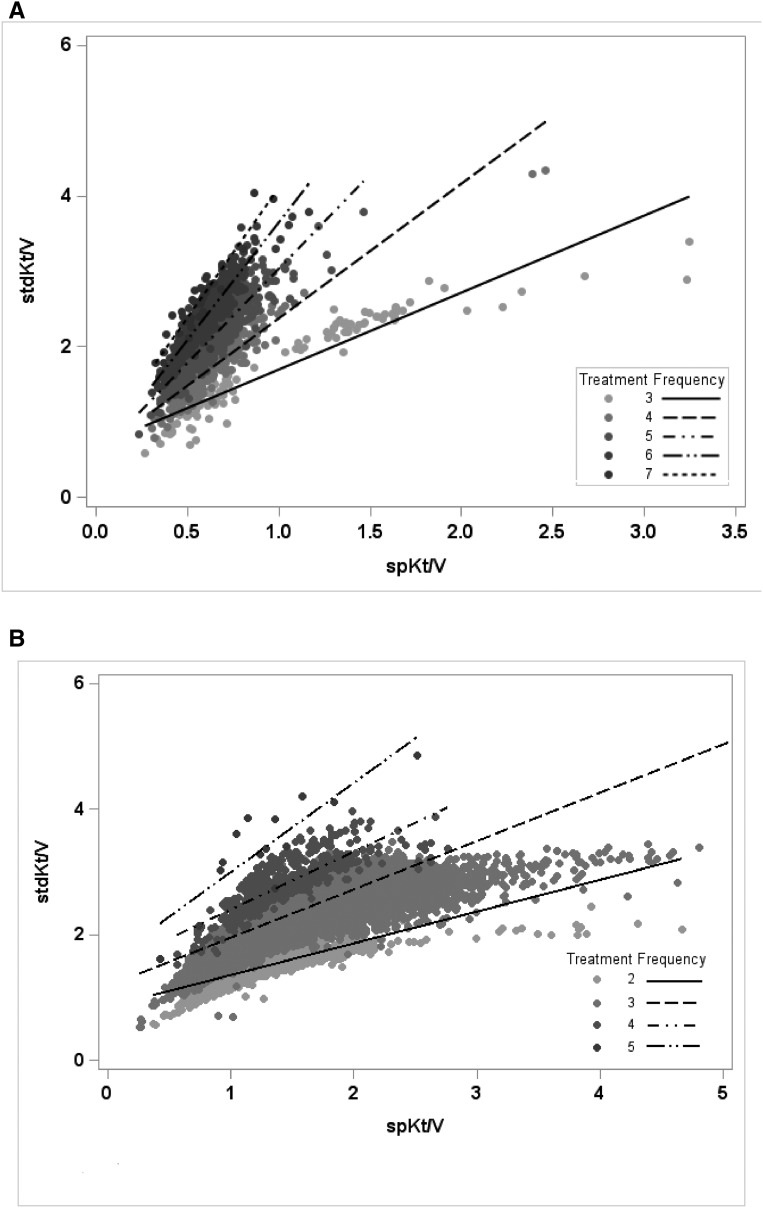
The study cohort included 2373 patients on home hemodialysis from 405 facilities in 42 United States states and 109,273 patients on in-center hemodialysis from 1776 facilities in 45 states. Overall, 182 patients treated with home hemodialysis died, 1138 were hospitalized at least once, and 353 patients transferred to in-center hemodialysis over 2539 patient-years of follow-up (Supplemental Table 1). Of the patients who transferred to in-center hemodialysis, 92 (3.8% of the total home hemodialysis population) eventually returned to home hemodialysis. Irrespective of dialysis modality, patients with dialysis stdKt/V ≥2.3 were less likely to be black and had lower BMI compared with patients with stdKt/V<2.1 (Tables 1 and 2). The relationship between dialysis spKt/V and stdKt/V within the study cohort was curvilinear, consistent with previous model-based nomogram derivations (Figure 1) (7,9).
Table 1.
Characteristics of patients ever treated with home hemodialysis in the first 91-day period after initiation of home hemodialysis by category of dialysis standard Kt/V
| Characteristic | Total | Category of Dialysis stdKt/V | ||
|---|---|---|---|---|
| <2.1 | 2.1 to <2.3 | ≥2.3 | ||
| N | 2373 | 1221 | 496 | 656 |
| Age, yr | 53±15 | 52±14 | 53±15 | 53±15 |
| Sex, % women | 33 | 26 | 34 | 48 |
| Body mass index, kg/m2 | 29.7±7.7 | 30.2±7.9 | 30.0±7.4 | 28.7±7.7 |
| Weekly treatment frequency | 5 (4–5) | 5 (4–5) | 5 (5–5) | 5 (5–6) |
| Mean treatment time, mina | 166±32 | 165±29 | 165±32 | 166±36 |
| Time from initiation of dialysis to start of home hemodialysis, d | 277 (99–541) | 244 (78–498) | 307 (112–551) | 306 (132–605) |
| Weekly interdialytic weight gain, kg | 5.9±3.7 | 5.2±3.5 | 6.4±3.5 | 6.8±3.7 |
| Race, % | ||||
| Whitea | 69 | 68 | 72 | 71 |
| Black | 20 | 23 | 17 | 19 |
| Asiana | 2 | 2 | 2 | 3 |
| Hispanica | 6 | 5 | 7 | 6 |
| Othera | 2 | 2 | 2 | 2 |
| Primary insurance, % | ||||
| Medicarea | 39 | 39 | 39 | 42 |
| Medicaida | 3 | 3 | 4 | 4 |
| Other insurancea | 57 | 58 | 57 | 55 |
| Access type, % | ||||
| CVC | 20 | 16 | 22 | 27 |
| AV fistula | 70 | 73 | 68 | 64 |
| AV grafta | 10 | 10 | 10 | 9 |
| Facility region, % | ||||
| Northeasta | 16 | 16 | 15 | 17 |
| Midwesta | 26 | 25 | 27 | 26 |
| Southa | 38 | 37 | 36 | 39 |
| Westa | 20 | 21 | 23 | 18 |
| Coexisting illnesses, % | ||||
| Diabetesa | 60 | 60 | 61 | 58 |
| Hypertensiona | 73 | 73 | 70 | 73 |
| Atherosclerotic heart diseasea | 27 | 26 | 27 | 28 |
| Congestive heart failure | 49 | 47 | 51 | 51 |
| Other cardiovascular disease | 22 | 21 | 20 | 26 |
| Cerebrovascular diseasea | 1 | 1 | <1 | 2 |
| Chronic obstructive pulmonary diseasea | 5 | 6 | 4 | 7 |
| History of malignancya | 4 | 4 | 4 | 4 |
| Medications iv | ||||
| Iron, median monthly dosea | 200 (50–367) | 200 (67–367) | 200 (46–367) | 200 (33–367) |
| ESA, median weekly dosea | 5574 (0–12,310) | 5555 (0–12,258) | 5401 (0–11,967) | 5687 (0–12,571) |
| Laboratory data | ||||
| Albumin, g/dla | 4.0±0.4 | 4.0±0.4 | 4.0±0.4 | 4.0±0.5 |
| Creatinine, mg/dla | 7.6±3.1 | 7.3±3.2 | 7.7±3.0 | 7.4±2.9 |
| Potassium, mEq/L | 4.4±0.6 | 4.4±0.6 | 4.3±0.5 | 4.3±0.6 |
| Sodium, mEq/L | 137±3 | 138±3 | 138±3 | 137±3 |
| Bicarbonate, mg/dl | 24±2 | 24±2 | 24±2 | 24±25 |
| Hemoglobin, g/dla | 11.2±1.3 | 11.2±1.3 | 11.2±1.3 | 11.3±1.3 |
| Ferritin, ng/ml | 367 (196–619) | 331 (182–560) | 394 (227–658) | 407 (210–703) |
| Total iron binding capacity, mg/dla | 250±48 | 251±46 | 250±48 | 248±50 |
| Iron saturation, % | 28±10 | 27±10 | 28±10 | 29±10 |
| Calcium, mg/dl | 8.9±0.6 | 8.9±0.6 | 9.0±0.6 | 9.0±0.6 |
| Phosphorus, mg/dla | 5.1±1.2 | 5.1±1.2 | 5.1±1.1 | 5.0±1.2 |
| Intact parathyroid hormone, pg/ml | 339 (206–535) | 350 (222–555) | 329 (193–485) | 332 (191–538) |
| Alkaline phosphatase, U/L | 80 (62–104) | 77 (60–100) | 81 (64–105) | 84 (65–115) |
| WBC, ×103/μl | 7.1±2.8 | 7.0±2.2 | 7.3±3.6 | 7.3±3.1 |
| Lymphocytes, % of total WBCsa | 24±8 | 24±8 | 23±8 | 23±9 |
Data are presented as mean±SD, median (interquartile range), or percentage. Values presented are from the first 91-day period after initiation of home hemodialysis. stdKt/V, standard Kt/V; CVC, central venous catheter; AV, arteriovenous; iv, intravenous; ESA, erythropoiesis stimulating agent; WBC, white blood cell.
Indicates P>0.05 for the difference among stdKt/V categories using ANOVA, a Kruskal–Wallis test, or a chi-squared test. P<0.05 for all other comparisons.
Table 2.
Characteristics of patients treated exclusively with thrice weekly in-center hemodialysis in the first 91-day period after initiation of in-center hemodialysis by category of dialysis standard Kt/V
| Characteristic | Total | Category of Dialysis stdKt/V | ||
|---|---|---|---|---|
| <2.1 | 2.1 to <2.3 | ≥2.3 | ||
| N | 109,273 | 29,440 | 29,845 | 49,988 |
| Age, yr | 63±15 | 60±15 | 63±15 | 65±15 |
| Sex, % women | 43 | 27 | 36 | 57 |
| Body mass index, kg/m2 | 28±7 | 31±8 | 29±7 | 26±6 |
| Weekly treatment frequency | 3 (3–3) | 3 (3–3) | 3 (3–3) | 3 (3–3) |
| Mean treatment time, min | 212±23 | 205±25 | 211±23 | 215±22 |
| Weekly interdialytic weight gain, kg | 5.7±2.9 | 5.3±2.9 | 5.6±2.8 | 5.9±2.9 |
| Race, % | ||||
| White | 47 | 48 | 48 | 46 |
| Black | 31 | 35 | 32 | 29 |
| Asian | 3 | 2 | 3 | 4 |
| Hispanic | 15 | 12 | 14 | 17 |
| Other | 4 | 3 | 3 | 4 |
| Primary insurance, % | ||||
| Medicare | 54 | 49 | 53 | 56 |
| Medicaid | 6 | 8 | 7 | 7 |
| Other insurance | 40 | 43 | 40 | 37 |
| Access type, % | ||||
| CVC | 78 | 74 | 77 | 81 |
| AV fistula | 15 | 20 | 16 | 12 |
| AV graft | 4 | 3 | 4 | 5 |
| Unknown | 3 | 3 | 2 | 3 |
| Facility region, % | ||||
| Northeast | 13 | 11 | 13 | 13 |
| Midwesta | 18 | 18 | 19 | 18 |
| South | 43 | 41 | 43 | 44 |
| West | 25 | 28 | 25 | 24 |
| Coexisting illnesses, % | ||||
| Diabetes | 58 | 61 | 59 | 57 |
| Hypertension | 51 | 49 | 51 | 53 |
| Atherosclerotic heart diseasea | 14 | 14 | 15 | 15 |
| Congestive heart failure | 37 | 39 | 37 | 36 |
| Other cardiovascular disease | 15 | 14 | 15 | 16 |
| Cerebrovascular disease | 2 | 2 | 2 | 2 |
| Chronic obstructive pulmonary disease | 5 | 5 | 5 | 5 |
| History of malignancya | 2 | 2 | 2 | 2 |
| Medications | ||||
| Iron, median monthly dose | 333 (150–483) | 333 (150–500) | 333 (167–500) | 333 (133–467) |
| ESA, median weekly dose | 26,400 (16,500–36,300) | 26,400 (16,500–39,600) | 26,400 (16,500–37,500) | 24,750 (14,850–33,000) |
| Laboratory data | ||||
| Albumin, g/dla | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 |
| Creatinine, mg/dl | 5.9±2.4 | 6.1±2.6 | 6.0±2.4 | 5.7±2.2 |
| Potassium, mEq/L | 4.4±0.5 | 4.4±0.5 | 4.4±0.5 | 4.4±0.5 |
| Sodium, mEq/L | 138±3 | 138±3 | 138±3 | 138±3 |
| Bicarbonate, mg/dl | 24±3 | 23±3 | 24±3 | 24±3 |
| Hemoglobin, g/dla | 11.1±1.2 | 11.1±1.2 | 11.2±1.2 | 11.1±1.2 |
| Ferritin, ng/ml | 282 (164–481) | 250 (147–425) | 272 (161–461) | 308 (179–528) |
| Total iron binding capacity, mg/dl | 225±49 | 229±51 | 227±48 | 22 3±48 |
| Iron saturation, % | 23±9 | 22±9 | 23±9 | 24±9 |
| Calcium, mg/dl | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 |
| Phosphorus, mg/dl | 4.9±1.1 | 5.0±1.2 | 5.0±1.1 | 4.9±1.1 |
| Intact parathyroid hormone, pg/ml | 314 (198–486) | 327 (204–508) | 318 (204–487) | 305 (191–473) |
| Alkaline phosphatase, U/L | 87 (69–115) | 86 (68–113) | 86 (68–113) | 88 (70–116) |
| WBC, ×103/μla | 7.8±2.7 | 7.8±2.7 | 7.8±2.8 | 7.8±2.6 |
| Lymphocytes, % of total WBCsa | 21±8 | 21±7 | 21±7 | 21±8 |
Data are presented as mean±SD, median (interquartile range), or percentage. Values presented are from the first 91-day period after initiation of in-center hemodialysis. stdKt/V, standard Kt/V; CVC, central venous catheter; AV, arteriovenous; iv, intravenous; ESA, erythropoiesis stimulating agent; WBC, white blood cell.
Indicates P>0.001 for the difference among stdKt/V categories using ANOVA, a Kruskal–Wallis test, or a chi-squared test. P<0.001 for all other comparisons.
Figure 1.
Single-pool Kt/V (spKt/V) and standard Kt/V (stdKt/V) in patients undergoing home and in-center hemodialysis are closely correlated after accounting for dialysis treatment frequency. (A) Home hemodialysis linear correlation coefficients: 0.93 for weekly treatment frequency =3, 0.92 for frequency =4, 0.91 for frequency =5, 0.92 for frequency =6, and 0.90 for frequency =7. (B) In-center hemodialysis linear correlation coefficients: 0.79 for weekly treatment frequency =2, 0.87 for frequency =3, 0.83 for frequency =4, and 0.86 for frequency =5.
Association of Dialysis stdKt/V with Markers of Volume and Metabolic Control
Among patients on home hemodialysis, dialysis stdKt/V <2.1 was associated with lower weekly interdialytic weight gain (1.4 kg/wk lower; P<0.001), higher predialysis systolic BP (3.4 mm Hg higher; P<0.001), and lower serum bicarbonate (0.4 mEq/L lower; P<0.01) compared with stdKt/V 2.1 to <2.3 (Table 3). Patients with dialysis stdKt/V ≥2.3 had modestly greater weekly interdialytic weight gain (0.9 kg/wk higher; P<0.001) but similar predialysis systolic BP (P=0.10). There was no association between dialysis stdKt/V and serum phosphorus among patients on home hemodialysis.
Table 3.
Adjusted associations of dialysis standard Kt/V with markers of volume status and metabolic control in patients treated with home hemodialysis and patients treated with in-center hemodialysis
| stdKt/V | Home HD | In-Center HD | ||
|---|---|---|---|---|
| Estimate | P Value | Estimate | P Value | |
| Serum potassium, mEq/L | ||||
| <2.1 | +0.06 | 0.04 | +0.02 | <0.001 |
| 2.1 to <2.3 | 4.0 | Reference | 4.3 | Reference |
| ≥2.3 | +0.0 | 0.90 | +0.00 | 0.20 |
| Serum calcium, mg/dl | ||||
| <2.1 | −0.06 | 0.03 | −0.02 | <0.001 |
| 2.1 to <2.3 | 6.3 | Reference | 9.2 | Reference |
| ≥2.3 | −0.02 | 0.50 | +0.01 | 0.20 |
| Serum phosphorus, mg/dl | ||||
| <2.1 | +0.05 | 0.40 | +0.02 | 0.08 |
| 2.1 to <2.3 | 5.8 | Reference | 5.8 | Reference |
| ≥2.3 | +0.0 | 0.90 | −0.04 | <0.001 |
| Serum bicarbonate, mEq/L | ||||
| <2.1 | −0.4 | <0.001 | −0.3 | <0.001 |
| 2.1 to <2.3 | 23.6 | Reference | 22.8 | Reference |
| ≥2.3 | 0.04 | 0.80 | +0.2 | <0.001 |
| Weekly interdialytic weight gain, kg | ||||
| <2.1 | −1.4 | <0.001 | −0.7 | <0.001 |
| 2.1 to <2.3 | 4.4 | Reference | 6.1 | Reference |
| ≥2.3 | +0.9 | <0.001 | +0.9 | <0.001 |
| Predialysis systolic BP, mm Hg | ||||
| <2.1 | +3.4 | <0.001 | +0.9 | <0.001 |
| 2.1 to <2.3 | 154 | Reference | 148 | Reference |
| ≥2.3 | −1.4 | 0.20 | −0.2 | 0.20 |
For each variable, the reference category comprised patients with stdKt/V 2.1 to <2.3. Plus/minus values indicate change from the reference category. Estimates from multivariable regression models adjusted for age, sex, race/ethnicity, body mass index, coexisting diabetes, history of congestive heart failure, history of atherosclerotic heart disease, serum albumin, and vascular access type. stdKt/V, standard Kt/V; HD, hemodialysis.
Among patients treated with in-center hemodialysis, higher dialysis stdKt/V was associated with greater interdialytic weight gain, lower serum phosphorus, and higher serum bicarbonate (Table 3). The magnitude of the differences in predialysis BP among categories of dialysis stdKt/V was lower for in-center compared with home hemodialysis. In contrast, the magnitudes of the modest differences in mean concentrations of laboratory parameters among categories of dialysis stdKt/V were similar to those seen among patients on home hemodialysis.
Association of Dialysis stdKt/V with Mortality, Hospitalization, and Transfer to In-Center Hemodialysis
In survival analyses using Cox proportional hazards regression, there was no association of dialysis stdKt/V with all-cause mortality, time to first hospitalization, or transfer to in-center hemodialysis for patients treated with home hemodialysis, irrespective of the level of adjustment for potential confounders (Table 4). There was no evidence of significant effect modification by sex, time since initiation of dialysis (dialysis vintage), or dialysis session frequency on any of the survival analyses among patients on home hemodialysis. Among individuals undergoing in-center hemodialysis, dialysis stdKt/V <2.1 was associated with greater risk for death (adjusted hazard ratio [HR], 1.11; 95% confidence interval [95% CI], 1.07 to 1.14) and greater risk for first hospitalization (adjusted HR, 1.10; 95% CI, 1.08 to 1.12) compared with stdKt/V 2.1 to <2.3 (Table 4).
Table 4.
Associations of dialysis standard Kt/V with clinical outcomes in patients treated with home hemodialysis and in-center hemodialysis
| stdKt/V | Hazard Ratio (95% Confidence Interval) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mortality | Hospitalization | Transfer to In-Center HD | |||||||
| Unadjusted | Minimally Adjusteda | Fully Adjustedb | Unadjusted | Minimally Adjusteda | Fully Adjustedb | Unadjusted | Minimally Adjusteda | Fully Adjustedb | |
| Home HD | |||||||||
| <2.1 | 0.93 (0.66 to 1.33) | 0.95 (0.66 to 1.37) | 1.03 (0.71 to 1.48) | 1.05 (0.91 to 1.23) | 1.07 (0.92 to 1.25) | 1.08 (0.93 to 1.26) | 1.23 (0.94 to 1.61) | 1.23 (0.94 to 1.62) | 1.24 (0.94 to 1.64) |
| mid | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| ≥2.3 | 1.04 (0.71 to 1.52) | 1.22 (0.82 to 1.83) | 1.08 (0.73 to 1.60) | 1.04 (0.88 to 1.23) | 1.05 (0.88 to 1.24) | 1.03 (0.87 to 1.22) | 1.10 (0.81 to 1.49) | 1.11 (0.82 to 1.50) | 1.15 (0.85 to 1.56) |
| In-center HD | |||||||||
| <2.1 | 0.99 (0.96 to 1.02) | 1.08 (1.05 to 1.12) | 1.11 (1.07 to 1.14) | 1.06 (1.04 to 1.08) | 1.09 (1.07 to 1.11) | 1.10 (1.08 to 1.12) | — | — | — |
| mid | Reference | Reference | Reference | Reference | Reference | Reference | — | — | — |
| ≥2.3 | 1.06 (1.03 to 1.09) | 1.02 (0.99 to 1.05) | 0.97 (0.94 to 0.99) | 1.08 (1.06 to 1.09) | 1.03 (1.02 to 1.05) | 1.01 (0.99 to 1.02) | — | — | — |
stdKt/V, standard Kt/V; HD, hemodialysis; —, not applicable.
Data adjusted for age, sex, and race/ethnicity.
Data adjusted for demographic characteristics (age, sex, and race/ethnicity) plus body mass index, diabetes status, history of congestive heart failure, history of atherosclerotic heart disease, serum albumin, and vascular access type.
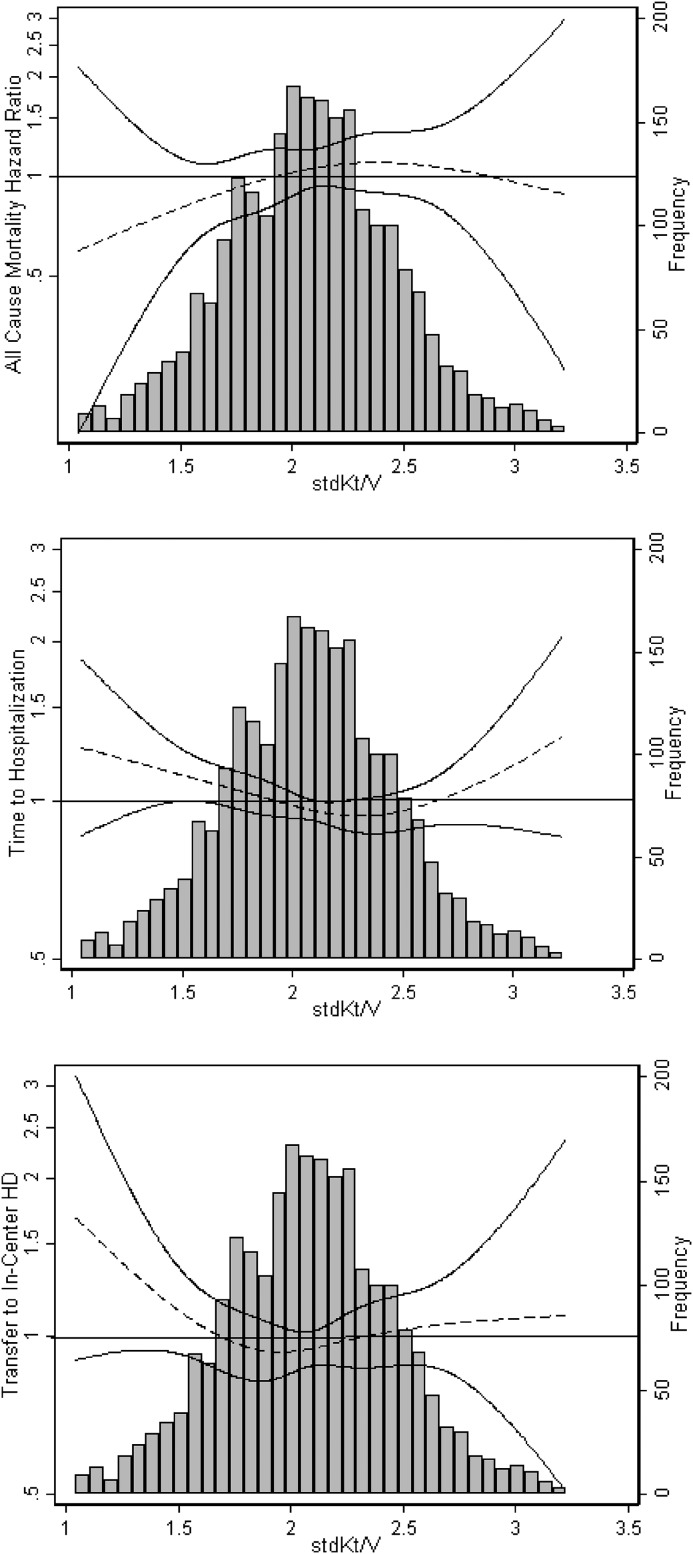
Among patients on in-center hemodialysis, dialysis stdKt/V ≥2.3 was associated with a modestly lower risk for all-cause mortality compared with stdKt/V 2.1 to <2.3 (adjusted HR, 0.97; 95% CI, 0.94 to 0.99). Risk for hospitalization for patients on in-center hemodialysis with dialysis stdKt/V ≥2.3 was not significantly different from that for the reference group. Because there was evidence of a significant statistical interaction by sex for the association of stdKt/V with mortality and hospitalization (P<0.001 for both), analyses were repeated among men and women separately. Stratum-specific HR estimates were not meaningfully different from those observed for the overall cohort (data not shown). For both home and in-center hemodialysis, cubic splines illustrating adjusted continuous relationships generally showed a flat relationship between stdKt/V and risk for clinical outcomes, particularly for stdKt/V values ranging from 2.0 to 3.0 (Figures 2 and 3).
Figure 2.
Dialysis standard Kt/V (stdKt/V) was not associated with all-cause mortality, hospitalization, or transfer to in-center hemodialysis (HD) in patients undergoing home HD. Cubic splines shown are from models adjusted for age, sex, race/ethnicity, body mass index, diabetes status, history of congestive heart failure, history of atherosclerotic heart disease, vascular access type, and serum albumin. Solid lines represent 95% confidence intervals.
Figure 3.
Spline analyses suggested that dialysis stdKt/V less than that typically achieved in clinical practice was associated with higher mortality and hospitalization among patients undergoing in-center hemodialysis. Cubic splines shown are from models adjusted for age, sex, race/ethnicity, body mass index, diabetes status, history of congestive heart failure, history of atherosclerotic heart disease, vascular access type, and serum albumin. Solid lines represent 95% confidence intervals.
Restricted Cohort: Association of Dialysis, Kidney, and Total stdkt/V with Clinical Outcomes among Patients with Residual Native Kidney Function
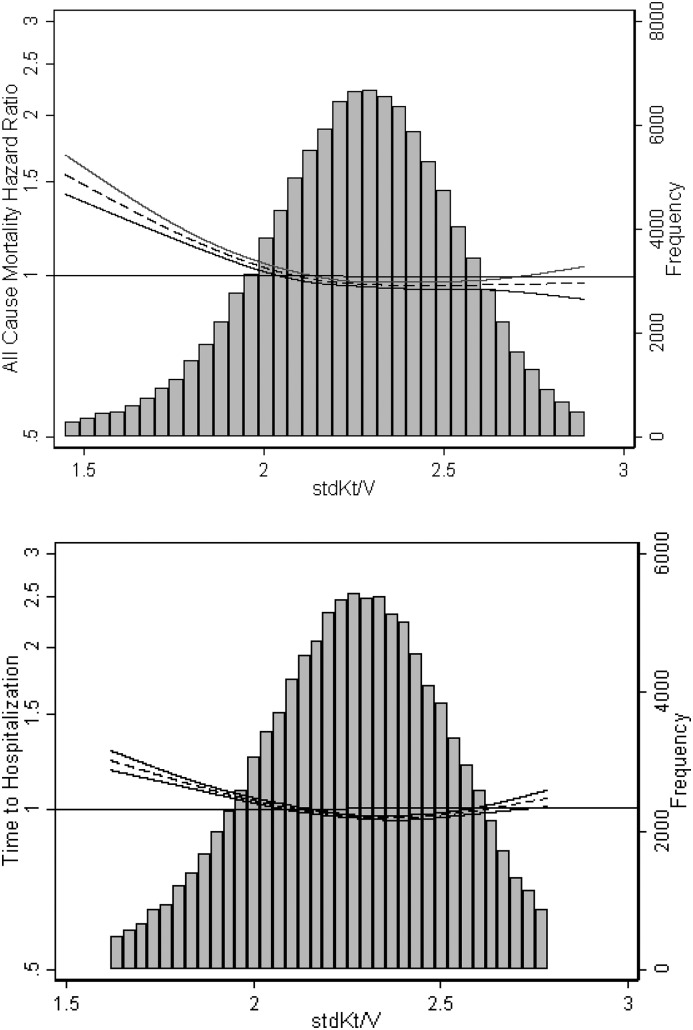
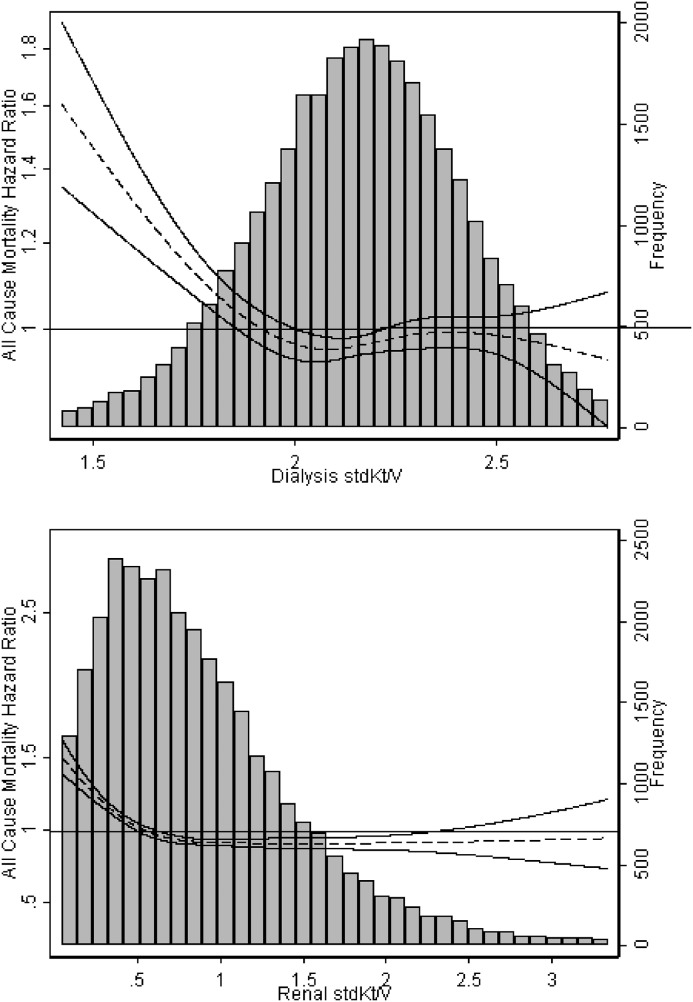
Compared with individuals without data on residual kidney function, individuals with available data were more likely to be white, less likely to be women, and in those undergoing home hemodialysis, less likely to dialyze using an arteriovenous access (Supplemental Table 3). After adjustment for potential confounders and kidney stdKt/V, patients on in-center hemodialysis with dialysis stdKt/V <2.1 had higher risks for death (adjusted HR, 1.07; 95% CI, 1.01 to 1.14) and hospitalization (adjusted HR, 1.08; 95% CI, 1.04 to 1.11) compared with patients with stdKt/V 2.1 to <2.3 (Supplemental Table 4). In models adjusting for kidney stdKt/V, dialysis stdKt/V ≥2.3 was not associated with decreased risk of mortality or hospitalization compared with the referent category. Results of analyses using restricted cubic splines also suggested a threshold effect, with increased risk for adverse outcomes only in patients with stdKt/V<2.1 (Figure 4). In contrast, we observed a graded relationship between tertile of kidney stdKt/V and risk for mortality and hospitalization, with patients in the highest tertile at lowest risk for adverse outcomes (Supplemental Table 4).
Figure 4.
Restricting analyses to patients on in-center hemodialysis with available data on residual native kidney function (n=31,748) did not change the observed association of dialysis stdKt/V with all-cause mortality. Data shown are from models adjusted for age, sex, race/ethnicity, body mass index, diabetes status, history of congestive heart failure, history of atherosclerotic heart disease, vascular access type, and serum albumin. Dialysis stdKt/V models are additionally adjusted for kidney stdKt/V; kidney stdKt/V models are additionally adjusted for dialysis stdKt/V. Solid lines represent 95% confidence intervals.
Sensitivity Analyses
Results of sensitivity survival analyses among the home hemodialysis cohort in which the primary exposure was modeled as quintile of stdKt/V showed no association between quintile of baseline stdKt/V and risk of mortality, hospitalization, or transfer to in-center hemodialysis (Supplemental Table 5). Furthermore, results of additional analyses in which we modeled stdKt/V as a binary exposure with a cutoff of either 2.1 or 2.3 were similar (Supplemental Table 6). The results of sensitivity analyses in which we modeled stdKt/V as a time-varying exposure showed no association of category of stdKt/V with clinical outcomes among the home hemodialysis cohort (Supplemental Table 7). In contrast, in patients undergoing in-center hemodialysis, stdKt/V<2.1 was associated with higher mortality (HR, 1.36; 95% CI, 1.31 to 1.41) and stdKt/V≥2.3 was associated with lower mortality (HR, 0.89; 95% CI, 0.87 to 0.92) compared with patients with stdKt/V 2.1 to <2.3. These associations were similar to those observed in the primary analysis, although modestly stronger in magnitude.
Discussion
In this nationally representative cohort study of patients undergoing home and in-center hemodialysis in the United States, we found that differences in achieved stdKt/V within the range commonly seen in clinical practice were not associated with clinically meaningful differences in laboratory markers of metabolic control, irrespective of dialysis modality. Additionally, in survival analyses accounting for important potential confounding variables, there was no significant association between dialysis stdKt/V and adjusted risk for mortality, hospitalization, or transfer to in-center hemodialysis among patients undergoing home hemodialysis. For patients treated with in-center hemodialysis, our results suggest a higher risk for all-cause death and shorter time to first hospitalization for those in the lowest category of achieved dialysis stdKt/V (i.e., <2.1). These results were not meaningfully different among a restricted cohort of patients on in-center hemodialysis with available information on residual kidney function. Sensitivity analyses categorizing stdKt/V as quintiles, a binary exposure, or a time-varying exposure showed similar results.
Measurement of small solute clearance using the Kt/V and its derivatives remains a widely used index for assessing the adequacy of dialysis (15). In the first year of implementation of the Medicare ESRD QIP in 2012, achievement of a urea reduction ratio of at least 65% was one of three core clinical measures (16). Since that time, although the number of clinical measures required of dialysis facilities to report has increased dramatically, assessment of dialysis adequacy targets remains a cornerstone of the QIP, because it is legislatively mandated and hence, likely to remain a part of the measurement of quality of care (17). The exclusion of patients undergoing dialysis more than three times weekly from the calculation of dialysis facilities’ QIP scores has been noted as a limitation by key national stakeholder groups.
We found a significant association between lower achieved stdKt/V and greater risk for death and hospitalization among patients undergoing in-center hemodialysis, findings that we did not observe for home hemodialysis. A variety of factors may have contributed to these findings. There are important ways in which home hemodialysis differs from in-center hemodialysis that may result in a true difference in the association of dialysis dose with outcomes. For example, many patients on home hemodialysis use machines using a low-dialysate flow approach in contrast to the high dialysis flow used for in-center hemodialysis. Patients undergoing home hemodialysis tend also to have lower per treatment interdialytic weight gain due to shorter interdialytic intervals, with resultant lower ultrafiltration rates and lower incidence of intradialytic hypotension (18). Additionally, although the variable volume equations that we used to calculate stdKt/V accurately incorporate weekly ultrafiltration volume, improved volume control may obscure any potential clinical benefit from higher diffuse or convective small solute clearance. Another possible contributor to our observed disparate findings in home and in-center hemodialysis may be differences in mechanisms contributing to a “dose-targeting bias” for in-center but not home hemodialysis (19). Dose-targeting bias refers to the presence of confounding related to unmeasured patient-level factors, which for example, may prevent patients from achieving a higher Kt/V and are also be related to poor survival (4,20,21). These factors, which include patient motivation, adherence to therapies, or unmeasured coexisting illnesses, may be influential for patients on in-center hemodialysis and less so for patients on home hemodialysis. As another possibility, the absence of an association between stdKt/V and outcomes for home hemodialysis in which patients dialysis more frequently may represent indirect evidence for the importance of the higher peak urea concentrations seen with thrice weekly in-center hemodialysis with respect to adverse clinical outcomes (22). Additionally, given that few patients in our study had measured stdKt/V substantially lower than currently recommended targets, we were unable to determine whether such substandard achieved stdKt/V may indeed be independently associated with worse outcomes. Similarly, few patients in our study achieved stdKt/V values that were substantially higher than currently recommended targets, and thus, we were unable to evaluate for an association of very high stdKt/V with improved clinical outcomes. Of note, data from the Frequent Hemodialysis Network Daily Study and other recent well conducted, single-center cohort studies suggest that stdKt/V substantially higher that what was achieved by patients in our study is associated with better patient and technique survival as well as improvements in other patient-centered outcomes (18,23,24). In clinical practice, such high stdKt/V values are most often encountered with extended hours and/or frequent hemodialysis at home. Finally, the absence of an observed association between stdKt/V and clinical outcomes among patients on home hemodialysis may have been due to limited statistical power to detect a true effect in the smaller home hemodialysis cohort compared with the in-center hemodialysis cohort. This limitation inherent to our study is reflected in wide 95% CIs around the HR estimates from the Cox proportional hazards survival models for home hemodialysis, particular where all-cause mortality was the primary outcome. Given such wide 95% CIs, we are unable to definitively rule out true modest to moderate effect sizes that are within these 95% CIs.
In conclusion, in a large cohort of patients undergoing home or in-center hemodialysis, differences in dialytic stdKt/V within the range of what is generally achieved in contemporary practice are associated with few clinically meaningful differences in BP or metabolic control. Additionally, current targets for stdKt/V have limited utility in identifying individuals at increased risk for death, hospitalization, or transfer to in-center hemodialysis among patients undergoing home hemodialysis. Given these results, great caution should be exercised in the reliance on stdKt/V as the primary measure of dialysis adequacy among individuals undergoing home hemodialysis. There remains an imperative for the nephrology community to continue to search for further meaningful interventions that will improve outcomes for patients on maintenance dialysis.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants T32DK007467 (to M.B.R.) and R01DK095668 (to K.K.-Z. and R.M.) from the National Institute of Diabetes and Digestive and Kidney Diseases, grant KL2 TR000421 (to M.B.R.) from the National Center for Advancing Translational Sciences, and an unrestricted gift from the Northwest Kidney Centers (to the Kidney Research Institute).
Because Rajnish Mehrotra is the Editor-in-Chief (EIC) of the Clinical Journal of the American Society of Nephrology, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript. Ian de Boer, a Deputy Editor of CJASN, is at the same institution as some of the authors, including the EIC and therefore was also not involved in the peer review process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05680517/-/DCSupplemental.
References
- 1.United States Renal Data System : USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016 [Google Scholar]
- 2.Rivara MB, Mehrotra R: The changing landscape of home dialysis in the United States. Curr Opin Nephrol Hypertens 23: 586–591, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Medicare & Medicaid Services (CMS) ; HHS: Medicare program; end-stage renal disease prospective payment system, and quality incentive program. Final rule. Fed Regist 80: 68968–69077, 2015 [PubMed] [Google Scholar]
- 4.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R; Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, Van Wyck D, Greenland S, Kalantar-Zadeh K: Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis 55: 100–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daugirdas JT, Depner TA, Inrig J, Mehrotra R, Rocco MV, Suri RS, Weiner DE, Greer N, Ishani A, MacDonald R, Olson C, Rutks I, Slinin Y, Wilt TJ, Rocco M, Kramer H, Choi MJ, Samaniego-Picota M, Scheel PJ, Willis K, Joseph J, Brereton L; National Kidney Foundation : KDOQI clinical practice guideline for hemodialysis adequacy: 2015 Update. Am J Kidney Dis 66: 884–930, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Leypoldt JK, Jaber BL, Zimmerman DL: Predicting treatment dose for novel therapies using urea standard Kt/V. Semin Dial 17: 142–145, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Leypoldt JK: Urea standard Kt/V(urea) for assessing dialysis treatment adequacy. Hemodial Int 8: 193–197, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Daugirdas JT: Dialysis dosing for chronic hemodialysis: Beyond Kt/V. Semin Dial 27: 98–107, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, Cheung AK, Brunelli S, Heagerty PJ, Katz R, Molnar MZ, Nissenson A, Ravel V, Streja E, Himmelfarb J, Mehrotra R: Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant 30: 1208–1217, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daugirdas JT, Leypoldt JK, Akonur A, Greene T, Depner TA; FHN Trial Group : Improved equation for estimating single-pool Kt/V at higher dialysis frequencies. Nephrol Dial Transplant 28: 2156–2160, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tattersall JE, DeTakats D, Chamney P, Greenwood RN, Farrington K: The post-hemodialysis rebound: Predicting and quantifying its effect on Kt/V. Kidney Int 50: 2094–2102, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Daugirdas JT, Depner TA, Greene T, Levin NW, Chertow GM, Rocco MV; Frequent Hemodialysis Network Trial Group : Standard Kt/Vurea: A method of calculation that includes effects of fluid removal and residual kidney clearance. Kidney Int 77: 637–644, 2010 [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370: 1453–1457, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Daugirdas JT: Kt/V (and especially its modifications) remains a useful measure of hemodialysis dose. Kidney Int 88: 466–473, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services (CMS), HHS : Medicare program; end-stage renal disease prospective payment system and quality incentive program; ambulance fee schedule; durable medical equipment; and competitive acquisition of certain durable medical equipment prosthetics, orthotics and supplies. Final rule. Fed Regist 76: 70228–70316, 2011 [PubMed] [Google Scholar]
- 17.Centers for Medicare & Medicaid Services (CMS): End-Stage Renal Disease: (ESRD) Quality Incentive Program (QIP) Payment Year 2020 Proposed Measure Technical Specifications. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/PY-2020-NPRM-tech-specs-6-28-16.pdf. Accessed September 22, 2016
- 18.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS; FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daugirdas JT: Dialysis time, survival, and dose-targeting bias. Kidney Int 83: 9–13, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Greene T, Daugirdas J, Depner T, Allon M, Beck G, Chumlea C, Delmez J, Gotch F, Kusek JW, Levin N, Owen W, Schulman G, Star R, Toto R, Eknoyan G; Hemodialysis Study Group : Association of achieved dialysis dose with mortality in the hemodialysis study: An example of “dose-targeting bias”. J Am Soc Nephrol 16: 3371–3380, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Port FK, Ashby VB, Dhingra RK, Roys EC, Wolfe RA: Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol 13: 1061–1066, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Keshaviah P, Collins AJ, Ma JZ, Churchill DN, Thorpe KE: Survival comparison between hemodialysis and peritoneal dialysis based on matched doses of delivered therapy. J Am Soc Nephrol 13[Suppl 1]: S48–S52, 2002 [PubMed] [Google Scholar]
- 23.Lockridge R, Ting G, Kjellstrand CM: Superior patient and technique survival with very high standard Kt/V in quotidian home hemodialysis. Hemodial Int 16: 351–362, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Lockridge RS, Kjellstrand CM: Nightly home hemodialysis: Outcome and factors associated with survival. Hemodial Int 15: 211–218, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.