Abstract
Background and objectives
Poor cognition can affect educational attainment, but the extent of neurocognitive impairment in children with CKD is not well understood. This systematic review assessed global and domain-specific cognition and academic skills in children with CKD and whether these outcomes varied with CKD stage.
Design, setting, participants, & measurements
Electronic databases were searched for observational studies of children with CKD ages 21 years old or younger that assessed neurocognitive or educational outcomes. Risk of bias was assessed using a modified Newcastle–Ottawa scale. We used random effects models and expressed the estimates as mean differences with 95% confidence intervals stratified by CKD stage.
Results
Thirty-four studies (25 cross-sectional, n=2095; nine cohort, n=991) were included. The overall risk of bias was high because of selection and measurement biases. The global cognition (full-scale intelligence quotient) of children with CKD was classified as low average. Compared with the general population, the mean differences (95% confidence intervals) in full-scale intelligence quotient were −10.5 (95% confidence interval, −13.2 to −7.72; all CKD stages, n=758), −9.39 (95% confidence interval, −12.6 to −6.18; mild to moderate stage CKD, n=582), −16.2 (95% confidence interval, −33.2 to 0.86; dialysis, n=23), and −11.2 (95% confidence interval, −17.8 to −4.50; transplant, n=153). Direct comparisons showed that children with mild to moderate stage CKD and kidney transplants scored 11.2 (95% confidence interval, 2.98 to 19.4) and 10.1 (95% confidence interval, −1.81 to 22.0) full-scale intelligence quotient points higher than children on dialysis. Children with CKD also had lower scores than the general population in executive function and memory (verbal and visual) domains. Compared with children without CKD, the mean differences in academic skills (n=518) ranged from −15.7 to −1.22 for mathematics, from −9.04 to −0.17 for reading, and from −14.2 to 2.53 for spelling.
Conclusions
Children with CKD may have low-average cognition compared with the general population, with mild deficits observed across academic skills, executive function, and visual and verbal memory. Limited evidence suggests that children on dialysis may be at greatest risk compared with children with mild to moderate stage CKD and transplant recipients.
Keywords: chronic kidney disease; pediatrics; Epidemiology and outcomes; transplant outcomes; dialysis; Neurocognition; Education; Child; Adolescent; Humans; Cross-Sectional Studies; Executive Function; Reading; Transplant Recipients; kidney transplantation; Confidence Intervals; Cohort Studies; Cognition; Renal Insufficiency, Chronic; Memory; Mathematics; Bias; Intelligence
Introduction
CKD has known detrimental effects on children’s physical health and wellbeing, and there is increasing awareness of its potential effect on neurocognitive function, academic outcomes, and mental health (1–3). The pathophysiologic effects of advanced uremia and anemia seen in CKD may alter brain metabolism and impair neurocognitive function through changes to neuronal myelination and synaptic development (4). CKD treatment regimens can also disrupt school attendance and compromise academic achievement, with potential consequences for educational and vocational attainment as children transition to adulthood (4,5).
In children and adolescents with CKD, evidence regarding the effect of reduced kidney function on neurocognitive function varies substantially across studies. A number of studies have identified lower nonverbal intelligence quotient (IQ) and motor performance in children with kidney disease compared with healthy age-matched controls (6–11). Some have found no significant differences in memory (verbal and nonverbal) between children with and without CKD (11). Others have reported specific deficits in complex auditory attention, verbal working memory, and the recognition of emotional states (11,12). The extent and patterns of neurocognitive and academic impairment in children may also vary by CKD stage. The overall IQ score for children treated with hemodialysis seems to be lower than that of children with moderate stage CKD and those with kidney transplants (13–15). Nonetheless, several studies have found improvement in IQ, attention, and mental processing speed after transplantation (16,17).
The objectives of this systematic review were to assess global and domain-specific neurocognitive function and academic skills in children with CKD and whether these outcomes worsen with advancing CKD stage.
Materials and Methods
We conducted a systematic review of observational studies on the basis of standard methods and reporting criteria in the Meta-Analysis of Observational Studies in Epidemiology guidelines (18). The protocol was registered with the International Prospective Register of Systematic Reviews (CRD42014013056).
Studies were included if they assessed neurocognitive or academic outcomes in children and adolescents (ages <21 years old) with CKD. We included studies where comparisons were made between patients with CKD and patients without CKD, either indirectly using normative population data or directly using matched non-CKD cohorts and/or between different stages of CKD (mild to moderate stage CKD, dialysis, and transplant). Prospective cohort and cross-sectional designs were eligible.
MEDLINE, Embase, and PsycINFO were searched from database inception to December 2016, with no language restrictions (Supplemental Table 1). We also hand searched the reference lists of identified studies and review articles. Two authors (K.C. and A.v.Z.) screened the titles and abstracts, and they independently assessed the full-text articles to identify studies that satisfied the inclusion criteria. A third reviewer (G.W.) adjudicated where disagreement arose.
Data extraction was carried out using standard extraction forms. Studies reported in non-English languages were translated before assessment. Where more than one publication of a study existed, only the publication with the most complete data was included for meta-analysis. We sought data on the age, socioeconomic status, duration of CKD of study participants, and raw scores from cognitive testing from study authors and reported these findings where available.
Risk of Bias Assessment
Two authors (K.C. and M.D.) undertook independent quality assessment of the included studies. We used a modified Newcastle–Ottawa scale to assess risk of bias. Differences were resolved by discussion until consensus was achieved.
Data Synthesis and Statistical Analyses
We summarized the overall and domain-specific neurocognitive and academic estimates between those with and without CKD using random effects models stratified by CKD stage (mild to moderate stage CKD, dialysis, and transplant), with the summary estimates expressed as mean differences with 95% confidence intervals (95% CIs) and effect sizes reported as Cohen d values. We compared estimates against the population norm for standardized measures of intelligence (Wechsler scales: mean =100, SD=15; IQ classification: 80–89 low average, 90–109 average, and 110–119 high average [19]), academic achievement (Wechsler Individual Achievement Test [WIAT] and Wide Range Achievement Test [WRAT]), attention (Conners Continuous Performance Test [CPT]), executive function (Behavior Rating Inventory of Executive Function [BRIEF]), and memory (Wide Range Assessment of Memory and Learning [WRAML]). Where possible, comparisons were also made between children with CKD and age-matched controls. Unless specified, a lower score was indicative of poorer outcomes. We also compared the overall and domain-specific cognitive and academic estimates between CKD stages if data were available.
Heterogeneity was assessed using the Cochran Q test and the I2 statistic. Possible sources of heterogeneity were investigated using subgroup analysis and metaregression (random effects model) on publication year, country of publication, study design, study size, and risk of bias. Meta-regression was conducted where ten or more studies for the specified outcomes were available. Funnel plots were generated to assess publication bias. All analyses were conducted using Review Manager Version 5 and Comprehensive Meta-Analysis Version 3.
Results
Characteristics of Included Studies
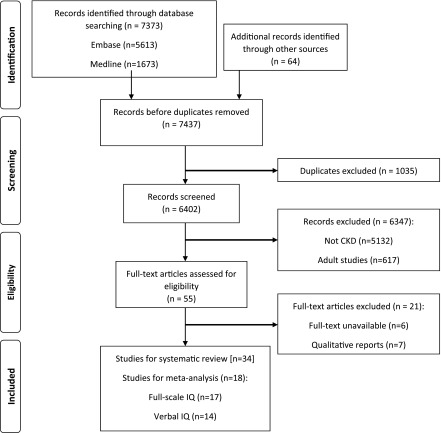
Of 7437 records identified, 34 studies (25 cross-sectional and nine cohort) with 3086 children and adolescents were included, and findings from 18 studies were used in the meta-analyses (Figure 1). The studies were conducted in nine countries, including the United States (59%), The Netherlands (9%), Canada (6%), Egypt (6%), and Finland (6%). Of those with CKD (n=2446), 2092 (85.5%) had mild to moderate stage CKD, 115 (4.7%) were on dialysis, and 239 (9.8%) had kidney transplants. Neurocognitive function was measured by 51 different tests, with full-scale IQ being the most frequently reported outcome followed by verbal and performance IQ, memory, attention, and executive function (Figure 2 A–C, Supplemental Tables 2 and 3, Table 1).
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses flowchart. Thirty-four studies were included for systematic review (25 cross-sectional, 9 cohort). IQ, intelligence quotient.
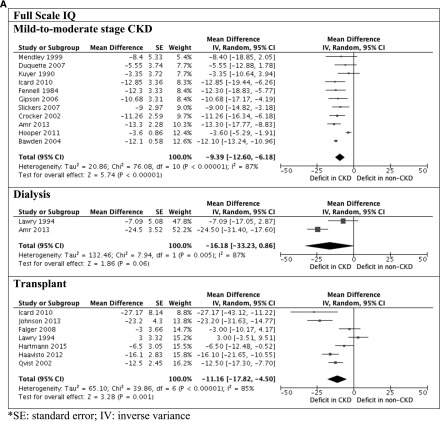
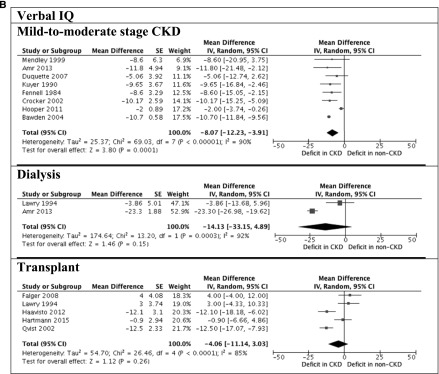
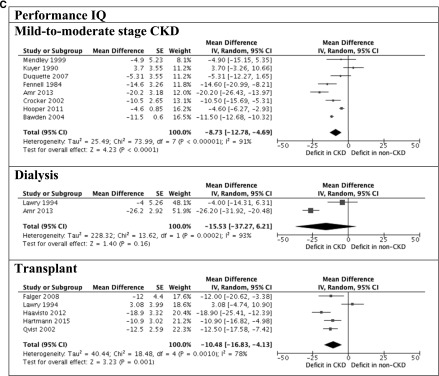
Figure 2.
Significant deficits observed in children with CKD. A shows full-scale intelligence quotient (IQ) between children with and without CKD. B shows verbal and performance IQ between children with and without CKD. 95% CI, 95% confidence interval; df, degree of freedom; IV, inverse variance; SE, SEM.
Table 1.
Characteristics of included studies
| Characteristic | N (%) |
|---|---|
| Study related | |
| Year of publication | |
| 2010 to present | 14 (41) |
| 2000–2009 | 10 (29) |
| Pre-2000 | 10 (29) |
| Country of publication | |
| United States | 20 (59) |
| The Netherlands | 3 (9) |
| Canada | 2 (6) |
| Egypt | 2 (6) |
| Finland | 2 (6) |
| Other | 5 (15) |
| No. of patients per study (n) | |
| 0–99 | 27 (79) |
| 100–199 | 5 (15) |
| 200+ | 2 (6) |
| Study design | |
| Cross-sectional | 25 (74) |
| Cohort | 9 (26) |
| Patient related | |
| Mean age, yra | |
| 0–6 | 3 (9) |
| 7–12 | 16 (47) |
| 13+ | 13 (38) |
| Stages of CKDb | |
| Mild to moderate stage CKD | 25 (74) |
| Dialysis | 6 (18) |
| Transplant | 15 (44) |
| Use of controlsb | |
| Direct non-CKD controls | 19 (56) |
| Normative population data | 28 (82) |
| Test related | |
| Outcome domains | |
| Intelligence | 25 (74) |
| Academic achievement | 10 (29) |
| Attention | 11 (32) |
| Executive function | 9 (26) |
| Memory | 12 (35) |
| Intelligence | |
| Wechsler scales (IQ) | 22 (65) |
| Stanford Binet Intelligence Test | 2 (6) |
| Test of nonverbal intelligence | 1 (3) |
| Other | 9 (26) |
| Attention | |
| Conners Continuous Performance Task | 5 (15) |
| Continuous performance taskc | 3 (9) |
| Gordon Diagnostic System | 2 (6) |
| Other | 8 (24) |
| Executive function | |
| Behavior Rating Inventory of Executive Function | 5 (15) |
| Delis–Kaplan Executive Function System | 2 (6) |
| Halstead–Reitan Category Test | 2 (6) |
| Other | 8 (24) |
| Memory | |
| Wide range assessment of memory and learning | 5 (15) |
| Nonverbal selective reminding test | 2 (6) |
| Other | 9 (26) |
| Academic achievement | |
| Wechsler Individual Achievement Test | 6 (18) |
| Wide Range Achievement Test | 3 (9) |
| Peabody Individual Achievement Test | 2 (6) |
| Other | 2 (6) |
| Subtestsc | |
| Full-scale IQ | 21 (62) |
| Verbal IQ | 18 (53) |
| Performance IQ | 16 (47) |
| Reading | 8 (24) |
| Arithmetic | 8 (24) |
| Spelling | 4 (12) |
IQ, intelligence quotient.
Two studies did not specify.
There is overlap in the number of studies.
Not all studies reporting outcomes contained data suitable for meta-analysis.
Risk of Bias and Publication Bias Assessment
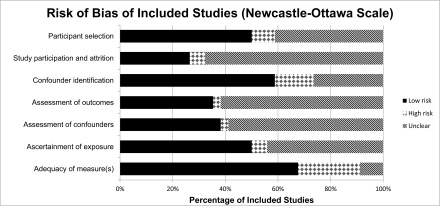
The overall risk of bias was high (Figure 3). Seventeen (50%) studies described the recruitment strategies, and only nine (26%) had participation rates of >70%. Twenty-three (68%) studies did not report an objective assessment of the exposure (CKD) with blinding to outcome. Conduct of statistical methods was adequate in 21 (62%) studies. Adjustment for confounders was conducted in 20 (59%) studies. Asymmetry of funnel plots also indicated potential publication bias for studies conducted with different CKD groups (Supplemental Table 4).
Figure 3.
Risk of bias assessment. The overall risk of bias was high.
Association between CKD and Neurocognitive Functioning
Intelligence (25 Studies, 1446 Patients).
The Wechsler scales were the most frequently used instruments (n=22 studies) for assessing intellectual functioning (20). The mean global intelligence (full-scale IQ) of all children with CKD was 92.7 (SD=16.2; n=758). Children with mild to moderate stage CKD scored −9.39 (95% CI, −12.6 to −6.18), −8.07 (95% CI, −12.2 to −3.90), and −8.73 (95% CI, −12.8 to −4.69) points on full-scale IQ, verbal IQ, and performance IQ, respectively, compared with the general population. The full-scale IQ, verbal IQ, and performance IQ scores were −16.2 (95% CI, −33.2 to 0.86), −14.1 (95% CI, −33.2 to 4.89), and −15.5 (95% CI, −37.3 to 6.21) points for children on dialysis compared with the general population, respectively. Transplant recipients scored −11.2 (95% CI, −17.8 to −4.50), −4.06 (95% CI, −11.1 to 3.03), and −10.5 (95% CI, −16.8 to −4.13) points on full-scale IQ, verbal IQ, and performance IQ, respectively, compared with the population norm.
For studies that included age-matched controls, children with mild to moderate stage CKD scored −16.2 (95% CI, −22.2 to −10.1), −7.84 (95% CI, −13.5 to −2.20), and −11.7 (95% CI, −13.2 to −10.2) points on full-scale IQ, verbal IQ, and performance IQ, respectively, compared children without kidney disease. Children on dialysis scored −29.5 (95% CI, −38.5 to −20.5), −18.2 (95% CI, −23.2 to −13.2), and −21.1 (95% CI, −29.2 to −13.0) points on full-scale IQ, verbal IQ, and performance IQ, respectively, compared with age-matched controls. The full-scale IQ for transplant recipients was −29.4 (95% CI, −53.9 to −4.77) points compared with age-matched controls. Five studies (n=168) compared intellectual functioning across CKD stage. Compared with children on dialysis, the average full-scale IQ score was 11.2 points higher among children with mild to moderate stage CKD (95% CI, 2.98 to 19.4). There may be improvement after transplantation, because transplant recipients scored 10.1 (95% CI, −1.81 to 22.0) points higher in full-scale IQ than children on dialysis.
Attention (13 Studies, 1366 Patients).
The Conners' CPT (five studies; n=875) was the most frequently used instrument to assess attention, with higher T scores (mean =50; SD=10) reflecting greater impairment (21,22). Children with CKD had equivalent scores to the population norms for variability (0.10; 95% CI, −1.02 to 1.22), but scores were 1.70 (95% CI, 0.32 to 3.08) and 1.70 (95% CI, 0.58 to 2.82) points higher in the omission and commission errors subtests, respectively, and −1.80 (95% CI, −3.39 to −0.21) points in reaction time.
Executive Function (11 Studies, 1390 Patients)
Five studies used the BRIEF, which assesses executive function using T scores (mean =50; SD=10), where higher T scores indicate greater impairment (23). Compared with the general population, children with mild to moderate stage CKD scored 3.50 (95% CI, 1.97 to 5.03), 5.90 (95% CI, 4.34 to 7.46), and 5.20 (95% CI, 3.64 to 6.76) points higher in the behavior regulation, metacognition, and global executive indices of the BRIEF, respectively. This pattern of deficit was less consistent among transplant recipients, who scored −1.70 (95% CI, −5.88 to 2.48) points compared with the general population in behavior regulation but 11.4 (95% CI, 0.75 to 22.1) and 5.80 (95% CI, −2.11 to 13.7) points higher in the metacognition and global executive indices, respectively.
Memory (12 Studies, 615 Patients)
The WRAML was used to assess three domains of memory (verbal memory, visual memory, and acquisition of new learning) in five studies (mean =10; SD=3). Compared with children without CKD, those with mild to moderate stage CKD scored lower in the subtests of verbal memory (story memory, sentence memory, and number/letter) and visual memory (picture memory, design memory, and finger windows). Transplant recipients scored −11.2 (95% CI, −18.4 to −4.01), −11.3 (95% CI, −17.1 to −5.55), and −13.4 (95% CI, −19.5 to −7.26) points in verbal, visual and screening memory, respectively, compared with the general population.
Association between CKD and Educational Outcomes (Ten Studies, 808 Patients)
The WIAT and the WRAT were used to measure academic skills (mean =100; SD=15), with outcomes commonly reported as reading, mathematics, and spelling (Supplemental Table 2) (24–26). Overall, the scores for children with mild to moderate stage CKD were −5.19 (95% CI, −8.14 to −2.25), −1.50 (95% CI, −2.56 to −0.43), −9.58 (95% CI, −16.0 to 3.12), and −5.18 (95% CI, −8.28 to −2.09) points in reading accuracy, reading comprehension, mathematics, and spelling, respectively, compared with the general population (Supplemental Figure 1). Transplant recipients scored −9.31 (95% CI, −16.7 to −1.90), −12.0 (95% CI, −13.0 to −11.1), and −9.20 (95% CI, −11.2 to −7.25) points on reading accuracy, mathematics, and spelling compared with the general population. The greatest differences were seen in children on dialysis, who scored −10.0 (95% CI, −11.5 to −8.46), −11.0 (95% CI, −12.5 to −9.46), and −15.0 (95% CI, −16.5 to −13.5) points on reading, arithmetic, and spelling, respectively, compared with the population norm.
In studies with healthy age-matched controls, children with mild to moderate stage CKD scored −0.60 (95% CI, −8.44 to 7.24) and −7.25 (95% CI, −8.89 to −5.62) points in reading accuracy and mathematics, respectively, but 0.40 (95% CI, −1.42 to 2.22) and 4.76 (95% CI, −0.52 to 10.0) points higher in reading comprehension and spelling, respectively. For children on dialysis, reading, arithmetic, and spelling scores were lower by −10.0 (95% CI, −11.9 to −8.08), −3.00 (95% CI, −4.92 to −1.08), and −11.0 (95% CI, −12.5 to −9.46) points, respectively, compared with the age-matched controls. Transplant recipients scored −7.00 (95% CI, −8.39 to −5.61), −10.0 (95% CI, −11.4 to −8.61), and −7.00 (95% CI, −8.39 to −5.61) points on reading, arithmetic, and spelling, respectively, compared with age-matched controls. Compared with patients on dialysis, transplant recipients generally had higher scores on reading, arithmetic, and spelling.
Effect of Disease Severity, Duration of Disease, and Age of Onset
Four studies considered the effect of variables, such disease severity, duration of CKD, and age of disease onset, on intellectual functioning and academic achievement in children across different CKD stages. Compared with children with mild to moderate stage CKD, those with advanced stage CKD had lower full-scale IQ and memory function. Children with prolonged duration of reduced kidney function seemed to have lower memory function and academic skills compared with children who experienced shorter duration of CKD. However, the age of disease onset did not seem to have an effect on the neurocognitive function in children with CKD.
Subgroup Analyses
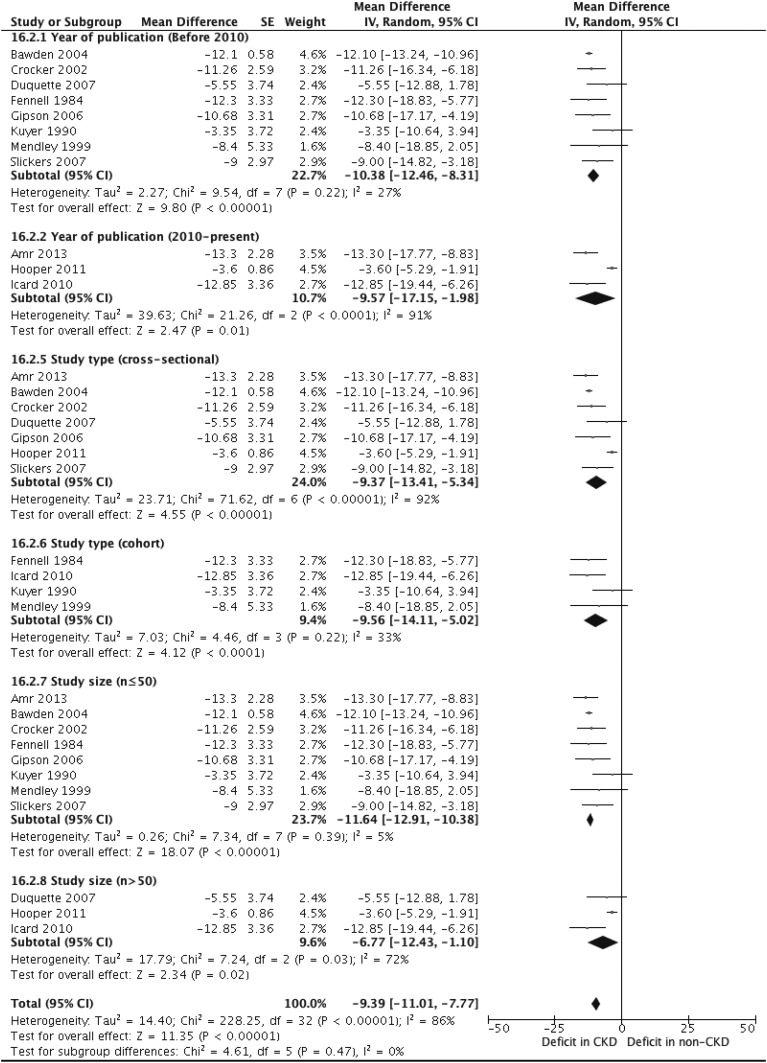
With the exception of study sample size, the full-scale IQ scores did not significantly vary with our hypothesized study-level sources of variability (Figure 4). The mean differences in full-scale IQ scores between children with CKD and the general population in studies published before and after 2010 were −10.4 (95% CI, −12.5 to −8.31) and −9.57 (95% CI, −17.2 to −1.98), respectively. The estimates for cross-sectional and cohort studies were −9.37 (95% CI, −13.4 to −5.34) and −9.56 (95% CI, −14.1 to −5.02), respectively. For studies with a smaller sample size (n≤50), the mean difference was −11.7 (95% CI, −12.9 to −10.4) compared with −6.77 (95% CI, −12.4 to −1.10) for studies with larger sample sizes (n>50).
Figure 4.
Subgroup analyses of full-scale intelligence quotient in children with and without CKD (mild to moderate stage CKD). 95% CI, 95% confidence interval; df, degree of freedom; IV, inverse variance; SE, SEM.
Metaregression
A metaregression (n=11 studies) of full-scale IQ scores between children with and without CKD showed that study sample size was the only variable that explained the high degree of heterogeneity between studies (β=5.31; SEM=2.01; P<0.01). The coefficients in full-scale IQ scores between children with CKD and the general population were −0.51 (SEM=3.01; P=0.86), 0.05 (SEM=3.67; P=0.99), 2.58 (SEM=2.51; P=0.30), and −0.72 (SEM=3.41; P=0.83) for year of publication (before and after 2010), study designs (cross-sectional versus cohort studies), country (the United States versus not the United States), and study quality (low versus high risk of bias), respectively.
Discussion
Our study findings suggest that children with CKD may have lower intellectual functioning compared with children without CKD. Deficits were most apparent in global intelligence, with children achieving average to low-average full-scale IQ scores compared with the population norm. Compared with children with mild to moderate stage CKD and those with kidney transplants, children on dialysis had the lowest full-scale IQ scores. The specific patterns of deficit were evident for attention, memory, and executive function domains. For attention, children with CKD had difficulty in holding information and shifting from one stimulus to another but displayed intact function in other areas. Children with CKD had reduced visual and verbal memory compared with children without CKD. For executive function, children with CKD had reduced metacognitive skills but preserved behavioral regulation compared with children without CKD. Similar to scores of intellectual functioning, academic achievement also ranged from average to low average among children with CKD compared with the general population, with the greatest deficit observed in mathematics.
Clinically, scores at least one SD lower in overall intelligence and domain-specific cognitive domains place children at increased risk of poor academic performance at school, reduced quality of life, and poor mental health, with potential implications for vocational attainment and financial independence as they transition into adulthood (3,27,28). We found at least one-SD lower intellectual functioning (full-scale IQ and performance IQ), academic achievement (total achievement subdomain), and executive function (initiation, sustaining, and BRIEF metacognition index subtests) among children with CKD compared with those without CKD. Because the SEM of measurement is approximately five standard points for Wechsler scales and our findings show differences of more than five standard points, such cognitive deficits would be considered clinically meaningful (29).
There are also data suggesting that children with more advanced stage CKD, increased duration of disease, and younger age of disease onset are associated with an increased risk of neurocognitive deficit and poor academic outcomes (15,28). By comparison, studies of children with epilepsy also show deficits in intellectual functioning: full-scale IQ mean =85.0 (SD=20.7; n=69), verbal IQ mean =86.9 (SD=22.6; n=69), and performance IQ mean =84.5 (SD=19.4; n=69) (30). Prior studies have reported that children with chronic illnesses, such as chronic liver disease (CLD), experienced comparable deficit in intellectual functioning, attention, and executive function, but a significant improvement in IQ scores was observed in children with CLD after transplantation (full-scale IQ mean =94.7; SD=13.5; effect size =−0.35; n=134) (31,32). Such deficit in cognitive function may be associated with poorer academic achievement, health literacy, and psychologic wellbeing in children with CLD (33–35).
Unique metabolic, biochemical, and neurodegenerative mechanisms may partially explain why the overall intellectual function of children with CKD is reduced compared with the general population. Increased plasma levels of uremic solutes may cause neurotoxic demyelination and impair synaptic development (4). CKD-related anemia and poor nutrition may also reduce the delivery of oxygen to the brain and alter brain metabolism, whereas secondary hyperparathyroidism can potentially interfere with neurotransmission by increasing calcium levels in the brain (36). It has been proposed that dialysis itself may also lead to cognitive impairment through rapid changes in BP causing brain hypoperfusion as well as the presence of gaseous microemboli, edema, and ongoing deposition of hemosiderin in cerebral parenchyma (37). These intradialytic BP changes in addition to the increased severity and chronicity of pathophysiologic effects related to ESKD, such as hypertension and poor nutrition, may result in reduced cognitive function among children on dialysis compared with those with other CKD stages.
Alongside inherent pathophysiologic factors, the therapeutic regimens for CKD may also compromise academic achievement (2,3). First, the frequency of sleep disturbances in children with CKD may result in poor concentration, excessive daytime sleepiness, and lower academic performance (38). Second, the interactions of complex medication routines and strict dialysis cycles may decrease attentional control, working memory, and executive function, cognitive domains that are important to children’s ability to acquire, understand, and retain information in social and educational environments (37,39,40). In particular, lower mathematic scores may be attributed to the reduced overall neurocognitive function of children with CKD, with fluid reasoning and processing speed having direct and indirect effects on mathematic ability (41,42). Studies examining the relationship between intelligence (full-scale IQ) and academic performance reported a moderate and statistically significant correlation moderated by factors, such as attendance, motivation, home and school environments, and cultural demographics (3,43). Across all CKD stages, we have shown that intelligence is largely consistent with academic skills. Third, ongoing dialysis sessions and recovery from transplant surgeries may reduce the amount and regularity of time spent in the classroom, with chronic absenteeism potentially preceding loss of interest, withdrawal, and poor school progression (2,44,45). Importantly, low-average cognition and academic skills suggest that children receiving dialysis may be at greatest risk compared with those in other CKD groups. Therefore, educational support programs should aim to minimize deficits in attention, memory, and executive function and navigate the logistic restrictions of rigorous management regimens for CKD through a multidisciplinary approach. Identification of the biopsychosocial factors associated with improved neurocognitive and educational outcomes and the involvement of relevant expert teams may inform the development of a comprehensive post-transplant rehabilitation service for children and adolescents with CKD.
This systematic review synthesizes all published evidence on global and domain-specific cognitive and academic outcomes in children and adolescents with CKD, including variation across CKD stage. Our findings provide relevant prognostic information on how advancing CKD stage may affect neurocognitive and academic outcomes, while exploring the effect of publication year, study size, and study design on these outcomes. Nonetheless, there are some potential limitations. We combined all studies of intelligence (IQ) that used the Wechsler scales. However, the composition of IQ has evolved over time, with newer versions of Wechsler Intelligence Scale for Children focusing more on fluid reasoning than crystallized knowledge (46–48). Because of the variability in exposure groups, outcome measures, and reporting, meta-analyses of estimates for outcomes including attention, executive function, and memory were not possible. Although heterogeneity was partially explained by study size, not all of the sources of variability could be identified. Small sample sizes may have insufficient power and thus, reduce the likelihood that a statistically significant result reflects a true effect. Nonetheless, additional factors to consider for the unexplained heterogeneity include participant demographics, such as age, socioeconomic status, severity of CKD, duration of disease, and age of onset; study-level factors, such as use of direct controls (versus normative population data); and the breadth of outcome measures used. Data on CKD-related clinical factors, such as low birth weight, hypertension, and nutritional status, were also not available from included studies. As such, we could not assess the potential confounding effects of these factors on the association between reduced kidney function and cognition in our analyses. Lastly, because the majority of studies have cross-sectional designs, we could not assess the longitudinal change in cognitive function with advancing CKD stage.
Our findings suggest that children with CKD may have low-average neurocognitive and academic outcomes. Although cognition seems to worsen with advancing CKD, with the poorest performance observed in children on dialysis, the magnitude and domain-specific patterns of effect are not clearly defined in the existing literature. Well conducted longitudinal studies assessing multiple cognitive domains as children progress through different CKD stages are needed to better elucidate the association between reduced kidney function and neurocognitive function.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to specially thank Dr. Tonya Kara who, although no longer with us, continues to inspire with her courageous character and contributions to things larger than ourselves. We also thank Philip Masson for his assistance in the development of search strategies.
A.v.Z. is supported by a National Health and Medical Research Council (NHMRC) postgraduate scholarship APP1115259. N.N. is partially supported by NHMRC Career Development fellowship 1067066. A.T.-P. is partially supported by NHMRC Program grant BeatCKD APP1092957.
Footnotes
Deceased.
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice, “Responsive Designed Interventions Are Needed to Support Positive Outcomes of Children and Adolescents with CKD,” on pages 357–358.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09650917/-/DCSupplemental.
References
- 1.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ: Epidemiology of chronic kidney disease in children. Pediatr Nephrol 27: 363–373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell LE, Ferris ME, Fenton N, Hooper SR: Health care transition for adolescents with CKD-the journey from pediatric to adult care. Adv Chronic Kidney Dis 18: 384–390, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Kaufman M, Shemesh E, Benton T: The adolescent transplant recipient. Pediatr Clin North Am 57: 575–592, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Icard P, Hooper SR, Gipson DS, Ferris ME: Cognitive improvement in children with CKD after transplant. Pediatr Transplant 14: 887–890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groothoff JW, Grootenhuis M, Dommerholt A, Gruppen MP, Offringa M, Heymans HSA: Impaired cognition and schooling in adults with end stage renal disease since childhood. Arch Dis Child 87: 380–385, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falger J, Latal B, Landolt MA, Lehmann P, Neuhaus TJ, Laube GF: Outcome after renal transplantation. Part I: Intellectual and motor performance. Pediatr Nephrol 23: 1339–1345, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Bawden HN, Acott P, Carter J, Lirenman D, MacDonald GW, McAllister M, McDonnell MC, Shea S, Crocker J: Neuropsychological functioning in end-stage renal disease. Arch Dis Child 89: 644–647, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duquette PJ, Hooper SR, Wetherington CE, Icard PF, Gipson DS: Brief report: Intellectual and academic functioning in pediatric chronic kidney disease. J Pediatr Psychol 32: 1011–1017, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fennell RS, Fennell EB, Carter RL, Mings EL, Klausner AB, Hurst JR: A longitudinal study of the cognitive function of children with renal failure. Pediatr Nephrol 4: 11–15, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Gipson DS, Hooper SR, Duquette PJ, Wetherington CE, Stellwagen KK, Jenkins TL, Ferris ME: Memory and executive functions in pediatric chronic kidney disease. Child Neuropsychol 12: 391–405, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Haavisto A, Korkman M, Holmberg C, Jalanko H, Qvist E: Neuropsychological profile of children with kidney transplants. Nephrol Dial Transplant 27: 2594–2601, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Hartung EA, Kim JY, Laney N, Hooper SR, Radcliffe J, Port AM, Gur RC, Furth SL: Evaluation of neurocognition in youth with CKD using a novel computerized neurocognitive battery. Clin J Am Soc Nephrol 11: 39–46, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawry KW, Brouhard BH, Cunningham RJ: Cognitive functioning and school performance in children with renal failure. Pediatr Nephrol 8: 326–329, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Hooper SR, Gerson AC, Butler RW, Gipson DS, Mendley SR, Lande MB, Shinnar S, Wentz A, Matheson M, Cox C, Furth SL, Warady BA: Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin J Am Soc Nephrol 6: 1824–1830, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouhard BH, Donaldson LA, Lawry KW, McGowan KR, Drotar D, Davis I, Rose S, Cohn RA, Tejani A: Cognitive functioning in children on dialysis and post-transplantation. Pediatr Transplant 4: 261–267, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Fennell RS 3rd, Rasbury WC, Fennell EB, Morris MK: Effects of kidney transplantation on cognitive performance in a pediatric population. Pediatrics 74: 273–278, 1984 [PubMed] [Google Scholar]
- 17.Mendley SR, Zelko FA: Improvement in specific aspects of neurocognitive performance in children after renal transplantation. Kidney Int 56: 318–323, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Saklofske DH, Prifitera A, Weiss LG: WISC-IV Clinical Use and Interpretation: Scientist-Practitioner Perspectives, 1st Ed., Amsterdam, Elsevier Academic Press, 2005 [Google Scholar]
- 20.Na SD, Burns TG: Wechsler intelligence scale for children-V: Test review. Appl Neuropsychol Child 5: 156–160, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Conners CK: Conners Continuous Performance Test 3rd Edition (Conners CPT 3). Pearson Clinical, 2014. Available at: https://www.pearsonclinical.com.au/products/view/548. Accessed August 14, 2017
- 22.Edwards MC, Gardner ES, Chelonis JJ, Schulz EG, Flake RA, Diaz PF: Estimates of the validity and utility of the Conners’ continuous performance test in the assessment of inattentive and/or hyperactive-impulsive behaviors in children. J Abnorm Child Psychol 35: 393–404, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Baron IS: Behavior rating inventory of executive function. Child Neuropsychol 6: 235–238, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D: Wechsler Individual Achievement Test – Australian and New Zealand Standardised, Third Edition (WIAT-III A&NZ). Pearson Clinical, 2016. Available at: https://www.pearsonclinical.com.au/products/view/588. Accessed August 14, 2017
- 25.Wilkinson GS, Robertson GJ: Wide Range Achievement Test 4 (WRAT4). Pearson Clinical, 2006. Available at: https://www.pearsonclinical.com.au/products/view/567. Accessed August 14, 2017
- 26.Anonymous: Peabody Individual Achievement Test (PIAT-R-NU). Available at: http://achievement-test.com/testing-options/peabody. Accessed August 14, 2017
- 27.Murray AM, Knopman DS: Cognitive impairment in CKD: No longer an occult burden. Am J Kidney Dis 56: 615–618, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slickers J, Duquette P, Hooper S, Gipson D: Clinical predictors of neurocognitive deficits in children with chronic kidney disease. Pediatr Nephrol 22: 565–572, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busch RM, Lineweaver TT, Ferguson L, Haut JS: Reliable change indices and standardized regression-based change score norms for evaluating neuropsychological change in children with epilepsy. Epilepsy Behav 47: 45–54, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reilly C, Atkinson P, Das KB, Chin RFM, Aylett SE, Burch V, Gillberg C, Scott RC, Neville BGR: Cognition in school-aged children with “active” epilepsy: A population-based study. J Clin Exp Neuropsychol 37: 429–438, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Sorensen LG, Neighbors K, Martz K, Zelko F, Bucuvalas JC, Alonso EM; Studies of Pediatric Liver Transplantation (SPLIT) and Functional Outcomes Group (FOG) : Cognitive and academic outcomes after pediatric liver transplantation: Functional Outcomes Group (FOG) results. Am J Transplant 11: 303–311, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moser JJ, Veale PM, McAllister DL, Archer DP: A systematic review and quantitative analysis of neurocognitive outcomes in children with four chronic illnesses. Paediatr Anaesth 23: 1084–1096, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Adebäck P, Nemeth A, Fischler B: Cognitive and emotional outcome after pediatric liver transplantation. Pediatr Transplant 7: 385–389, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Kaller T, Schulz KH, Sander K, Boeck A, Rogiers X, Burdelski M: Cognitive abilities in children after liver transplantation. Transplantation 79: 1252–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Kennard BD, Stewart SM, Phelan-McAuliffe D, Waller DA, Bannister M, Fioravani V, Andrews WS: Academic outcome in long-term survivors of pediatric liver transplantation. J Dev Behav Pediatr 20: 17–23, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Arnold R, Issar T, Krishnan AV, Pussell BA: Neurological complications in chronic kidney disease. JRSM Cardiovasc Dis 5: 2048004016677687, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madero M, Sarnak MJ: Does hemodialysis hurt the brain? Semin Dial 24: 266–268, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Etgen T: Kidney disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther 7: 29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duquette PJ: Attention functions in children with pediatric chronic kidney disease. PhD Dissertation. Chapel Hill, NC, University of North Carolina at Chapel Hill, 2007 [Google Scholar]
- 40.Puerta L: Relationship between cognitive processes and academic performance in high school students. Psychol Av Discip 9: 85–100, 2015 [Google Scholar]
- 41.Taub GE, Floyd RG, Keith TZ, McGrew KS: Effects of general and broad cognitive abilities on mathematics achievement. Sch Psychol Q 23: 187–198, 2008 [Google Scholar]
- 42.Reeve RA, Waldecker C: Evidence-based assessment and intervention for dyscalculia and maths disabilities in school psychology. In: Handbook of Australian School Psychology, edited by Thielking M, Terjesen MD, Cham, Switzerland, Springer, 2017, pp 197–213 [Google Scholar]
- 43.Kaufman SB, Reynolds MR, Liu X, Kaufman AS, McGrew KS: Are cognitive g and academic achievement g one and the same? An exploration on the Woodcock-Johnson and Kaufman tests. Intelligence 40: 123–138, 2012 [Google Scholar]
- 44.Quarles D: An Analysis of Tardiness, Absenteeism, and Academic Achievement of 9th Grade Students in a Selected School District in Southeastern Georgia, Orangeburg, South Carolina, ProQuest Dissertations Publishing, 2011 [Google Scholar]
- 45.Baxter SD, Royer JA, Hardin JW, Guinn CH, Devlin CM: The relationship of school absenteeism with body mass index, academic achievement, and socioeconomic status among fourth-grade children. J Sch Health 81: 417–423, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanaya T, Ceci SJ, Scullin MH: The rise and fall of IQ in special ed: Historical trends and their implications. J Sch Psychol 2003; 41: 453–465 [Google Scholar]
- 47.Flynn JR: Problems with IQ gains: The huge vocabulary gap. J Psychoeduc Assess 28: 412–433, 2010 [Google Scholar]
- 48.Wicherts JM, Dolan CV, Hessen DJ, Oosterveld P, van Baal GCM, Boomsma DI, Spange MM: Are intelligence tests measurement invariant over time? Investigating the nature of the Flynn effect. Intelligence 32: 509–537, 2004 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.