Introduction
Volume management is a fundamental challenge of care of patients on hemodialysis (HD). Determining and achieving optimal volume status aim at preventing interdialytic volume overload and minimizing intradialytic hypotension (IDH). Although the negative consequences of volume overload are well acknowledged, the long-term clinical consequences of IDH have only more recently been recognized. Recurring episodes of IDH result in a series of cumulative multisystem ischemic insults, leading to end organ dysfunction, such as congestive heart failure, cardiac arrhythmias, cognitive impairment, and loss of residual kidney function (1). The two clinical scenarios presented make a case for the importance of understanding the underlying pathophysiology and differential diagnosis of IDH.
Patient 1
E.M. is a 63-year-old man with diabetes mellitus type 2, obesity, and ESKD secondary to diabetic nephropathy. He has been on thrice weekly maintenance HD for the past 6 months. He has maintained a urine output of 600 ml/d supported by furosemide 120 mg/d. Other medications included amlodipine and insulin with basal-bolus regimen. Physical examination was unremarkable. Home BP recordings and those during HD showed poorly controlled hypertension. Laboratory tests revealed poor glycemic control (hemoglobin A1c 8.9%; 74 mmol/mol) and malnutrition (serum albumin 2.4 g/dl). Chest radiograph showed increased cardiothoracic ratio; left ventricular ejection fraction was 56%. Standard pool Kt/V was 1.5.
His HD prescription was for thrice weekly 4-hour treatments to achieve a dry weight of 105 kg (body mass index of 36 kg/m2). QB was 350 ml/min, QD was 500 ml/min, HCO3− was 32 mEq/L, Na+D was 138 mEq/L, and dialysate temperature was 36.5°C. His vascular access was a well functioning left radiocephalic fistula.
In the past 3 months, >50% of his HD treatments resulted in frequent episodes of IDH, malaise, and cramping, often leading to ultrafiltration rate reduction, saline boluses, early discontinuation of HD sessions, and failure to reach the target dry weight. He also complained of significant post-HD fatigue. His average interdialytic weight gains were 5% body weight (16 kg/wk), with an average weekly ultrafiltration rate of 11.5 ml/h per kilogram.
Conceptual Framework for Clinical Approach
Dry Weight
This represents something of a theoretical construct in patients on dialysis, and its practical definition still generate ongoing controversy. We need to consider two potential approaches.
“Absolute” dry weight—an ideal function of body weight and extracellular volume that can be measured and normalized to objective assessment of subjects without kidney disease
“Functional” dry weight—the body weight related to the minimal extracellular volume necessary to maintain an optimal end organ perfusion (during HD) influenced by personal tolerance of overhydration and volume removal
Earlier definitions of dry weight promoted aggressive volume removal strategies, risk of cardiovascular stress, and IDH. Clinicians face the challenge of making a correct assessment of the patient’s volume status given the limitations of poor accuracy of the physical examination, BP, and routine medical imaging. Bioimpedance spectroscopy, especially when coupled with an absolute total body water measurement (deuterium dilution technique), is able to guide volume management while also detecting changes in body composition over time (2). However, evidence supporting the effectiveness of bioimpedance devices in improving clinical outcomes is limited (3). Therefore, given the current technological limitations to defining an “absolute” dry weight, we reluctantly recommend allowing some degree of permissive hypervolemia (4), allowing a slight extracellular volume excess to prevent organ hypoperfusion, and achieving a “functional” dry weight.
Ultrafiltration Rate and Interdialytic Weight Gain
Ultrafiltration rate is the ratio between ultrafiltration volume and HD treatment volume indexed by post-HD body weight. Large interdialytic weight gains—the amount of volume that the patient introduces between two HD sessions—require higher ultrafiltration rates to achieve dry weight within fixed HD treatment times. When ultrafiltration rate exceeds capillary refilling rate, a rapid reduction in intravascular volume results in IDH, even if extracellular volume is normal or increased. Additionally, high ultrafiltration rates unwittingly may contribute to chronic volume overload: hemodynamic instability may lead to the early termination of HD sessions, ultrafiltration rate reduction, and upward dry weight adjustments. High interdialytic weight gains and ultrafiltration rates as well as HD treatment time below 4 hours per session have all consistently been associated with increased risk of IDH, morbidity and mortality (5). Therefore, reducing interdialytic weight gains and increasing HD treatment time provide a strategy to reduce ultrafiltration rates while still achieving dry weight.
Reducing interdialytic weight gain is achievable by increasing urine output with diuretics, when possible, and controlling the main factors driving thirst: restricting dietary sodium, reducing dialysate sodium concentration, and in diabetic patients, preventing hyperglycemia. Xerostomia, a subjective feeling of dry mouth, needs also to be considered as a driver to water ingestion. Reducing dialysate sodium improves interdialytic weight gains and hypertension; however, its benefits are controversial on IDH frequency and in IDH-prone patients (6). Therefore, we recommend reducing dialysate sodium as a personalized intervention in stable patients with clinical suspicion of sodium overload, potentially in conjunction with other interventions to improve hemodynamic stability.
Extending HD treatment time by increasing HD session frequency and/or length is also effective in abrogating hemodynamic instability and negative consequences, such as myocardial stunning (7).
The awareness of the relationship between ultrafiltration rate and mortality has recently led the technical expert panel of the US Centers for Medicare and Medicaid Services for the ESKD Quality Incentive Program (a mandatory federal pay-for-performance program) to introduce a ultrafiltration rate threshold (13 ml/h per kilogram) as a quality metric to improve outcomes in patients on HD. United States HD facilities will face financial penalties whenever the prescribed ultrafiltration rate goal is not met. Meeting the ultrafiltration rate goal—while also maintaining volume balance—requires strict control of interdialytic weight gain and flexibility for HD centers to increase HD treatment time as needed. As reported by the US Dialysis Outcomes and Practice Patterns Study database (8), current average treatment times delivered in United States HD centers are below the recommended thrice weekly 4-hour sessions; we are concerned that the implementation of an ultrafiltration rate threshold might paradoxically cause greater harm than benefit, turning a clinically desirable “permissive” hypervolemia into pathologic hypervolemia.
Dialysate Cooling to Improve Hemodynamic Tolerability of HD
Standard HD treatments using “normal” body temperature dialysate commonly result in heat accumulation, peripheral vascular resistance reduction, heart rate and skin blood flow increase—impairing the vasomotor response of HD patients to hemodynamic stress. Our research program has shown that reducing dialysate temperature (individualized to the patients core temperature; potentially as low as 35°C) is effective in preventing IDH and end organ damage (9,10). Dialysate cooling improves baroreflex sensitivity, potentially helping to restore vasoregulatory reserve in IDH-prone patients with autonomic dysfunction (10).
Patient Management and Clinical Course
Hemodynamic instability during HD in this patient is most likely a combination of high interdialytic weight gain, inappropriately short treatment times and autonomic dysfunction. His management, therefore, required a mixed approach to his multifactorial challenges. To address his high interdialytic weight gain, we focused on both dietary salt restriction (especially avoiding processed and convenience foods) and glycemic control. A dietary salt restriction to 5 g/d was prescribed and reinforced. Basal insulin dose was increased by 20%, and furosemide was increased to 250 mg/d. Na+D was reduced to 136 mEq/L to achieve more complete clearance of the interdialytic sodium load.
A shift to a frequent HD regimen (5 d/wk for 4 hours per treatment) was agreed on to achieve a gradual dry weight reduction (0.3 kg per session whenever possible). Dialysate temperature was reduced to 35.5°C (0.5°C below core temperature) to improve hemodynamic tolerability during volume removal. No symptoms were reported.
At 3 months, an overall dry weight reduction of 2.4 kg was achieved. Home monitoring showed better glycemic and BP control. Amlodipine was discontinued (further enhancing hemodynamic tolerability during dialysis). Weekly total interdialytic weight gain was reduced to 12.1 kg, and IDH episodes and symptoms were reduced to <10% of the treatments, despite the increased HD treatment frequency.
Patient 2
H.R. is a 28-year-old woman with spina bifida and a history of myelomeningocele closure, a ventriculoperitoneal shunt for hydrocephalus, paraplegia and neurogenic bladder. She had recently started maintenance HD due to reflux nephropathy. Six weeks earlier, she was admitted for a Pseudomonas aeruginosa urosepsis episode treated with piperacillin/tazobactam and ciprofloxacin. Her urinary output was 1000 ml/d, and medications consisted of furosemide, carvedilol, sodium valproate, calcitriol, and darbepoetin.
Physical examination noted a generally reduced muscle mass but no signs of volume overload. Average BP values measured at home and during HD were high normal (133/86 versus 138/89 mm Hg). Her usual interdialytic weight gains were modest (2% body weight). Predialysis bloodwork was Na+ 138 mEq/L, urea 189 mg/dl, and glucose 92 mg/dl, with a plasma osmolality of 313 mOsm/kg.
Her initial HD prescription was thrice weekly 3.5-hour HD sessions using a high-flux 2.1-m2 polysulphone dialyzer. Dry weight was 46.2 kg (body mass index of 20.1 kg/m2), QB was 300 ml/min, QD was 500 ml/min, HCO3− was 32.0 mEq/L, Na+D was 140 mEq/L, K+D was 3.0 mEq/L, and Ca++D was 3.0 mEq/L. Her vascular access was a left internal jugular vein permanent catheter.
After discharge, the patient started developing recurrent episodes of syncope during HD treatment. The episodes were preceded by headache, nausea and vomiting, and they usually occurred 1 hour into HD, even with minimal ultrafiltration rate, and resolved spontaneously 5–10 minutes after ultrafiltration had been suspended. BP fell during most of these episodes by 20–30 mm Hg.
Conceptual Framework for Clinical Approach
Differential Diagnosis of IDH
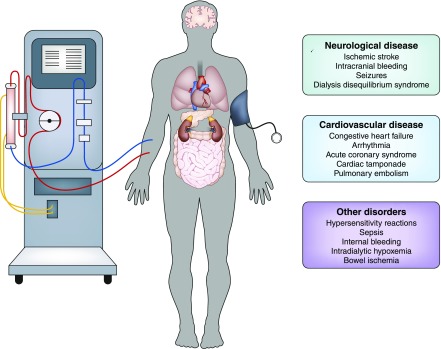
Many acute complications can manifest themselves with hypotension, symptoms associated with organ hypoperfusion (loss of consciousness, chest pain, and abdominal pain), and autonomic nervous system activation. It is important to recognize that not all apparent circulatory collapses during HD represent a classic response to inappropriately high ultrafiltration rate. A detailed discussion of the myriad factors to consider is beyond the scope of this article, but Figure 1 summarizes the most important differential diagnoses of IDH. Given her history of structural neurologic abnormalities and recent sepsis, two clinical entities are particularly important to take into consideration.
Figure 1.
Differential diagnosis of intradialytic hypotension: the clinical presentation of intradialytic hypotension has to be distinguished from several conditions affecting the hemodialysis patient's level of consciousness, hemodynamic stability and tissue oxygenation.
Coning
Uncal and cerebellar tonsillar herniation can present with severe dialysis–induced headache and reduced level of consciousness, and it can result in death. More commonly, this presentation occurs in the setting of either inherited abnormalities predisposing to coning (such as the Chiari malformation with partial hindbrain herniation through the foramen magnum, which is sometimes seen in spina bifida) or after neurosurgery. Cerebrospinal fluid diversion and shunt malfunction also can increase the risk of dialysis-induced brainstem herniation. Limitation of ultrafiltration rate and careful matching of dialysate tonicity are the essentials of management.
Nonconvulsive Status Epilepticus
Status epilepticus may present with confusion or more severe reductions in the level of consciousness without obvious convulsive activity. It may mimic collapse from either a catastrophic intracerebral event or acute cardiovascular insufficiency. Events that can precipitate nonconvulsive status include antibiotics (particularly penicillins, cephalosporins, and quinolones), alcohol, drug withdrawal, infection, hypoxia, cerebrovascular accident, and malignancy.
Patient Management and Clinical Course
Chest radiograph, Holter electrocardiogram, and transthoracic echocardiography were all unremarkable. Brain magnetic resonance scanning confirmed a Chiari type 2 malformation with no signs of cerebral edema. Electroencephalography documented generalized, nonspecific abnormalities with no seizure activity. Considering the recent start of maintenance HD and her neurologic history, the focus of the management was on the minimization of potential brain stem compression during exposure to volume and osmolality shifts during HD—significant drops in plasma osmolality due to intradialytic urea removal may also facilitate IDH because of a fall in intravascular volume. She was transferred to a short daily HD regimen (five 2-hour sessions per week), dialysate electrolytes were closely matched to her predialysis plasma electrolytes, and the dialyzer surface was reduced to 0.6 m2. The workup of potential living donors for kidney transplantation was accelerated. One week later, the patient’s symptoms fully resolved, and her prescription was maintained.
Conclusion
The pathophysiology of IDH can differ significantly from patient to patient: understanding its complexities is essential to plan effective prevention strategies and individualize HD prescription. However, the nephrologist cannot ignore that the optimization of HD treatment also requires the patient’s understanding and acceptance, although ultimately, the difficult process of decision making is the patient’s responsibility and should be respected.
Disclosures
Dr. Christopher W. McIntyre received honoraria from Baxter. Dr. Fabio R. Salerno has no disclosures to report.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.McIntyre CW: Recurrent circulatory stress: The dark side of dialysis. Semin Dial 23: 449–451, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Chan C, McIntyre C, Smith D, Spanel P, Davies SJ: Combining near-subject absolute and relative measures of longitudinal hydration in hemodialysis. Clin J Am Soc Nephrol 4: 1791–1798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bioimpedance Devices for the Assessment of Body Fluid Volume for Patients Undergoing Dialysis: A Review of the Clinical Effectiveness, Cost-Effectiveness, and Guidelines 2014. Available at: https://www.cadth.ca/bioimpedance-devices-assessment-body-fluid-volume-patients-undergoing-dialysis-review-clinical. Accessed January 24, 2018
- 4.Huang SHS, Filler G, Lindsay R, McIntyre CW: Euvolemia in hemodialysis patients: A potentially dangerous goal? Semin Dial 28: 1–5, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Flythe JE, Curhan GC, Brunelli SM: Disentangling the ultrafiltration rate-mortality association: The respective roles of session length and weight gain. Clin J Am Soc Nephrol 8: 1151–1161, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh AT, Mc Causland FR: Osmolality and blood pressure stability during hemodialysis. Semin Dial 30: 509–517, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW: Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning). Clin J Am Soc Nephrol 6: 1326–1332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DOPPS practice monitor. 2010–2017. Available at: https://www.dopps.org/dpm/DPMSlideBrowser.aspx?type5Topic&id54. Accessed January 24, 2018.
- 9.Selby NM, Burton JO, Chesterton LJ, McIntyre CW: Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol 1: 1216–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chesterton LJ, Selby NM, Burton JO, McIntyre CW: Cool dialysate reduces asymptomatic intradialytic hypotension and increases baroreflex variability. Hemodial Int 13: 189–196, 2009 [DOI] [PubMed] [Google Scholar]