Abstract
Central venous catheters are used frequently in patients on hemodialysis as a bridge to a permanent vascular access. They are prone to frequent complications, including catheter-related bloodstream infection, catheter dysfunction, and central vein obstruction. There is a compelling need to develop new drugs or devices to prevent central venous catheter complications. We convened a multidisciplinary panel of experts to propose standardized definitions of catheter end points to guide the design of future clinical trials seeking approval from the Food and Drug Administration. Our workgroup suggests diagnosing catheter-related bloodstream infection in catheter-dependent patients on hemodialysis with a clinical suspicion of infection (fever, rigors, altered mental status, or unexplained hypotension), blood cultures growing the same organism from the catheter hub and a peripheral vein (or the dialysis bloodline), and absence of evidence for an alternative source of infection. Catheter dysfunction is defined as the inability of a central venous catheter to (1) complete a single dialysis session without triggering recurrent pressure alarms or (2) reproducibly deliver a mean dialysis blood flow of >300 ml/min (with arterial and venous pressures being within the hemodialysis unit parameters) on two consecutive dialysis sessions or provide a Kt/V≥1.2 in 4 hours or less. Catheter dysfunction is defined only if it persists, despite attempts to reposition the patient, reverse the arterial and venous lines, or forcefully flush the catheter. Central vein obstruction is suspected in patients with >70% stenosis of a central vein by contrast venography or the equivalent, ipsilateral upper extremity edema, and an existing or prior history of a central venous catheter. There is some uncertainty about the specific criteria for these diagnoses, and the workgroup has also proposed future high-priority studies to resolve these questions.
Keywords: central vein, vascular access, dialysis access, Bacteremia, Blood Culture, Catheter-Related Infections, Central Venous Catheters, Chills, Constriction, Pathologic, Edema, Humans, hypotension, Phlebography, renal dialysis, Uncertainty, United States, United States Food and Drug Administration, Veins, Venous Pressure
Introduction
Tunneled central venous catheters (CVCs) are frequently used to deliver chronic hemodialysis. Although CVCs are far inferior to a permanent vascular access (arteriovenous fistula [AVF] or arteriovenous graft [AVG]), they serve as a critical bridge until permanent access can be placed and cannulated successfully for dialysis (1). Each year, approximately 110,000 patients initiate hemodialysis in the United States, and approximately 80% of them require a CVC either because they have an immature AVF that is not ready to use or because they have not had a permanent access placed before dialysis initiation (2). CVCs are also needed as a bridge access in patients on dialysis whose existing AVF or AVG fails until a new permanent access can be established. Finally, a subset (<10%) of patients on hemodialysis have a permanent requirement for a CVC, because they have exhausted all options for placement of a permanent vascular access.
The major complications of CVCs include catheter-related bloodstream infections (CRBSIs), catheter dysfunction (due to intraluminal thrombosis, fibrin sheath occlusion, malposition, or other mechanical complication), and central vein obstruction (CVO) (3–7). CRBSI may result in substantial morbidity, systemic complications, hospitalizations, and death. Catheter dysfunction impairs the delivery of adequate dialysis and often requires salvage by instillation of a thrombolytic agent into the catheter lumen or catheter exchange. CVO causes physical debility (severe edema of an extremity, head, or neck; pain; or neurologic symptoms) and may impair the function of existing or future ipsilateral permanent vascular access (8,9). Given that the widespread use of CVCs is likely to persist in the foreseeable future, it is imperative to develop drugs or products that minimize the complications of CVCs. This document proposes standardized definitions for each of these CVC end points (CRBSI, catheter dysfunction, and CVO) in future clinical trials along with the rationale for each definition. In addition, we propose future high-priority studies to further refine the proposed definitions of catheter end points. Discussion of specific therapies that may prevent CVC complications is beyond the scope of this paper.
CRBSIs
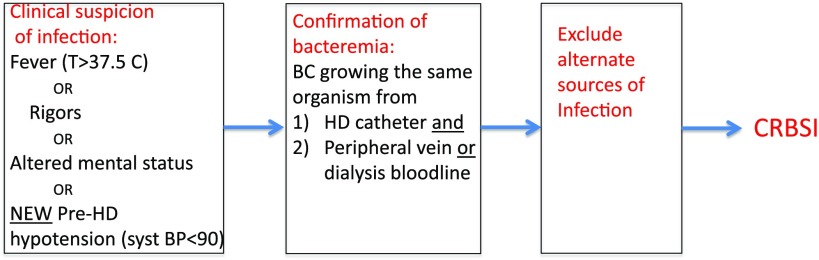
Our workgroup suggests defining a CRBSI as growth of the same organism from the hemodialysis catheter and peripheral venipuncture (or a suitable surrogate, such as the dialysis bloodline) in a patient with clinical signs of an infection and no alternative source of bloodstream infection (Figure 1). CRBSI can be quantified as time to event or the incidence of events per population and time at risk (e.g., CRBSIs per 1000 catheter-days or CRBSIs per 100 patient-months). A definition of CRBSI that is both feasible and reliable is needed for investigators to conduct future studies for regulatory approval of biologics, drugs, or devices to prevent CRBSI in this patient population. The standard definition of CRBSI proposed by the Infectious Diseases Society of America (IDSA) requires (10) (1) clinical signs of infection (fever, rigors, altered mental status, or unexplained hypotension); (2) absence of evidence of an alternate source of bloodstream infection; and (3) positive blood culture obtained from a peripheral vein and one of the following: significant growth culture of a catheter segment matching the organism grown from the blood culture, simultaneously obtained quantitative blood cultures with at least threefold higher number of organisms grown from the catheter-drawn blood culture compared with growth of the same organism from a percutaneously drawn blood culture, or simultaneously obtained routine blood cultures with growth from the catheter-drawn blood culture detected at least 2 hours before growth of the same organism from a percutaneously drawn blood culture. This definition of CRBSI has not been validated specifically in patients on hemodialysis with catheters.
Figure 1.
Proposed criteria for diagnosis of dialysis catheter–related bloodstream infection (CRBSI). BC, blood culture; HD, hemodialysis; syst BP, systolic BP.
Clinical signs of infection may be obscured in patients on dialysis, because they have a lower basal temperature than patients with normal kidney function (11). As a consequence, a 1°C increase in temperature above baseline in patients on dialysis may be below the absolute temperature cutoff (38°C) used by many institutions to signal the need for blood cultures. Fever should be defined as a lower threshold temperature (e.g., 37.5°C) in patients on hemodialysis so as to minimize false negatives (patients who actually have CRBSI but are not diagnosed, because blood cultures are not obtained due to subtle symptomatology). Equally important, patients on hemodialysis with CRBSI frequently present with symptoms or signs other than fever, such as rigors, altered mental status, or unexplained hypotension. For example, in one large single-center study of catheter-dependent patients on hemodialysis, fever was a presenting symptom in only 47% of confirmed cases of CRBSI (12).
Obtaining peripheral vein blood cultures is challenging in patients treated in freestanding dialysis units for three reasons. First, in many patients on dialysis, peripheral veins have been exhausted due to prior venipuncture or vascular access (AVF or AVG). Second, even when peripheral veins are available, many experts recommend that they be spared to avoid potential destruction of veins needed for creation of future vascular access. This restriction limits potential phlebotomy sites to distal veins. Third, the dialysis staff rarely performs venipuncture and may lack the proficiency to reliably access peripheral veins. The challenge in obtaining blood cultures from peripheral veins was highlighted in a recent study performed at a hospital-based hemodialysis unit, in which a peripheral vein culture was not feasible 25% of the time, even when attempted by experienced phlebotomists (13). The rate of unsuccessful phlebotomy would be substantially higher when attempted by hemodialysis staff who are much less skilled in phlebotomy. This study also provided convincing evidence that, in lieu of peripheral vein cultures, hemodialysis circuit–derived blood cultures may be acceptable surrogates for defining CRBSI in catheter-dependent patients on hemodialysis (13).
Lastly, quantitative blood cultures or differential time to blood culture positivity have not been validated in patients on hemodialysis and are not available at referral laboratories used by the large dialysis organizations. It is unknown whether the use of differential time to culture sensitivity is valid for the diagnosis of CRBSI in patients with dialysis catheters. In fact, a recent single-center study reported that the diagnostic criteria for differential time to positivity were fulfilled in only one third of patients on hemodialysis with CRBSI determined on the basis of blood culture and clinical data (13).
Applying the standard definition of CRBSI developed for patients not on dialysis to patients on hemodialysis with catheters has major implications in terms of the likelihood of diagnosing CRBSI. For example, one randomized clinical trial (RCT) evaluated the efficacy of methylene blue–containing catheter locks in prevention of CRBSI in patients with hemodialysis catheters (14). A definite diagnosis of CRBSI required a temperature ≥38°C, positive blood cultures for the same organism obtained from the catheter lumen and a peripheral vein, and no identifiable source of bacteremia other than the catheter (the IDSA criteria). Patients were classified as having a concordant CRBSI if the temperature was ≤38°C but they met the other two criteria, and they were classified as having a probable CRBSI if a peripheral blood culture could not be obtained but the other two criteria were met. A total of 26 CRBSIs were documented in this study, of which only nine (or 35%) met the criteria for a definite CRBSI. When all three categories of CRBSI were included, methylene blue–containing catheter locks reduced the frequency of CRBSI by 71% in comparison with conventional heparin locks (P<0.01). If, however, only definite CRBSIs were included, the study lock solution reduced the frequency of CRBSI by approximately 50%, but this difference was no longer statistically significant. In other words, requiring that patients with hemodialysis catheters meet the definition of CRBSI developed for nondialysis catheters would substantially increase the size (and cost) of an RCT designed to evaluate whether a new therapy prevents CRBSI in patients on hemodialysis with catheters.
An alternative approach taken at most United States dialysis units is to obtain a blood culture from the catheter hub before initiation of the dialysis session and a second blood culture from the dialysis bloodline after the dialysis session has been initiated (as a surrogate for the peripheral vein culture). Using this approach, CRBSI is diagnosed in a patient with symptoms suggestive of infection who has growth of the same organism in blood cultures obtained from the catheter hub and the dialysis bloodline and no evidence of an alternate source of infection (15,16). There are limited published data evaluating whether cultures obtained from the dialysis bloodline represent a valid surrogate for direct peripheral vein cultures. A recent single-center study reported that use of bloodline cultures in lieu of peripheral vein cultures actually improved the sensitivity and specificity for the diagnosis of CRBSI in patients with hemodialysis catheters (13). This finding was attributed primarily to a higher risk of contaminants when blood cultures were obtained from peripheral veins.
Our workgroup suggests defining a CRBSI as growth of the same organism from the hemodialysis catheter and peripheral venipuncture (or a suitable surrogate, such as the dialysis bloodline) in a patient with clinical signs of an infection and no alternative source of bloodstream infection (Figure 1). Because clinicians are frequently not present at the dialysis unit when the patient develops a clinical suspicion of CRBSI, the process of ruling out noncatheter sources of bloodstream infection needs to be reliably performed by the dialysis nurse. We propose that the study protocols include a simple validated checklist to evaluate other likely potential sources of infection. Demonstration of a differential time to positivity as part of the definition of CRBSI in patients with hemodialysis catheters would not be required. However, such information should be collected in CRBSI prevention research trials to evaluate its utility.
A separate area of uncertainty is how to diagnose CRBSI if the symptoms suggestive of infection arise during the hemodialysis session rather than being present at the onset. In this scenario, the opportunity to obtain blood cultures through the catheter hub before initiation of the dialysis session has been missed. What is typically done at the dialysis units in this scenario is to obtain two separate sets of cultures from the dialysis bloodline.
The workgroup also recommends several high-priority clinical research studies to validate whether such an alternative definition of CRBSI is both valid and feasible in patients with hemodialysis catheters (Table 1).
Table 1.
Proposed high-priority studies regarding dialysis catheter end points
| CRBSI |
| Studies to evaluate whether dialysis bloodline cultures are suitable alternatives to direct peripheral venipuncture samples; to facilitate such studies, we propose that future FDA approval studies of suspected catheter infection in patients receiving HD obtain peripheral blood cultures in a subset of patients enrolled to assess their agreement with bloodline cultures, with enough power to adequately address the issues of sensitivity, specificity, and positive and negative predictive value of such cultures |
| Studies to evaluate the use of differential time to positivity or proportional quantitative growth of bloodline and catheter hub blood cultures in HD catheters both before and after initiation of an HD session |
| Studies to assess the predictive value for CRBSI of different temperature thresholds in patients on HD |
| Studies to assess whether CVC intraluminal colonization in asymptomatic patients on dialysis precedes most CRBSI cases and whether it may be a reliable surrogate outcome for future studies (20) |
| Catheter dysfunction |
| Studies to evaluate the variability of dialysis blood flows between consecutive HD sessions; catheters should be matched for design variables, such as luminal radius and catheter tip designs |
| Studies to evaluate the optimal method to document whether the catheter tip is in the right atrium (19); also, tip orientation is important for step tip catheters, because they do not function optimally if the arterial port (short end) is against the venous wall |
CRBSI, catheter-related bloodstream infection; FDA, Food and Drug Administration; HD, hemodialysis; CVC, central venous catheter.
We envision several types of future interventional trials addressing CRBSI. In traditional RCTs, individual study participants are randomly assigned to different treatment arms. Alternatively, pragmatic trials, in which randomization occurs at the dialysis clinic level, may provide comparable information but at a fraction of the cost of a traditional RCT. Likewise, use of collaborative registry networks to support trials can improve data quality, the reliability of metadata analysis, and integration of other relevant large data sources. Future studies may evaluate catheter lock solutions, exit site antimicrobial ointment, impregnated catheter dressings, or catheter devices to prevent CRBSI.
Because there is substantial variation in the frequency of CRBSI between hemodialysis units, a single-arm study comparing outcomes with the published literature is inadequate. The ideal RCT would compare an antimicrobial lock solution, antimicrobial exit site ointment, or antimicrobial venous access device with the current standard, which includes use of chlorhexidine with alcohol for catheter exit site care and routine disinfection of catheter hubs (i.e., scrubbing the hub with antiseptic during catheter connections and disconnections; http://www.cdc.gov/dialysis/prevention-tools/core-nterventions.html) (17). To minimize the potential for bias in the diagnosis of CRBSI, the optimal study will be double blinded (so that neither the patient nor the dialysis nurse know whether the patient is receiving the investigational product or the current standard of care). Some device studies cannot be blinded. To minimize the potential for bias in such studies, there should be an independent data monitoring and safety board (DMSB) that reviews the objective data to assess whether a CRBSI has occurred. In addition to evaluating the primary end point of the study, such RCTs should include several secondary outcomes (Table 2).
Table 2.
Proposed secondary outcomes in dialysis catheter trials
| Catheter dysfunction |
| Use of thrombolytic therapy |
| Antimicrobial resistance—not just of clinical isolates but also of surveillance cultures |
| Catheter removal |
| All-cause hospitalization or hospitalization due to infection |
| Systemic antimicrobial use/exposure |
| Death |
| Any adverse events directly related to the drug on the patient or catheter integrity |
| Cost of care |
| Quality of life |
Catheter Dysfunction
Our workgroup suggests defining catheter dysfunction as the inability of a CVC to (1) complete a single dialysis session without triggering recurrent pressure alarms or (2) reproducibly deliver a mean dialysis blood flow ≥300 ml/min (with arterial and venous pressures being within the hemodialysis unit parameters) or provide a Kt/V>1.2 in 4 hours or less. The workgroup made a distinction between early catheter dysfunction (one that is present during the first few catheter uses for dialysis) and late dysfunction (when the catheter function was initially acceptable, but dysfunction occurred at a later time point). Causes of primary dysfunction include improper positioning, tip malposition, wrong vessel (azygos vein), tip malorientation, kinked catheter, and constrictive suture. Some causes are operator dependent and should be excluded in any early dysfunction catheter study. Secondary dysfunction may be caused by intrinsic thrombosis within the catheter lumen or formation of a fibrin sheath or extrinsic thrombosis encasing the catheter (17).
Early dysfunction may be an important end point if a manufacturer claims that a given catheter design will be associated with a lower incidence of primary dysfunction. Luminal radius, tip design, catheter length, tip orientation, and catheter location are important when comparing catheters. Luminal radius has the greatest effect on flow. Late dysfunction will be a relevant end point if a company claims that a certain biologic, drug, or device prevents late catheter dysfunction by eliminating catheter thrombosis and fibrin sheath formation. A fibrin sheath is suspected when there is a ball valve inflow dysfunction when the catheter is assessed with a saline flush before initiating hemodialysis. It can be diagnosed with a pullback venogram. Contrast is injected through the catheter after it is partially withdrawn, with its tip near the clavicle showing the fibrin sheath as a wind sock.
The workgroup initially considered selecting an absolute dialysis blood flow threshold (e.g., flow <300 ml/min) to define catheter dysfunction at an arterial dialysis pressure ≥−250 mmHg and a venous dialysis pressure <250 mmHg. However, the minimal acceptable blood flow required to deliver an adequate dose of dialysis (Kt/V) varies by patient characteristics (especially weight and sex) (18). For example, a 50-kg woman may achieve an adequate Kt/V at a dialysis blood flow of 250 ml/min, whereas a 100-kg man may require a minimal blood flow of 400 ml/min. On the basis of this consideration, we propose that catheter dysfunction be defined on the basis of an inadequate dialysis blood flow or an inadequate Kt/V.
Early catheter dysfunction is present when the patient’s catheter is never able to deliver a mean dialysis blood flow ≥300 ml/min (with arterial and venous pressures remaining within the dialysis unit parameters) or deliver a Kt/V>1.2 in ≤4 hours if the mean dialysis blood flow is <300 ml/min. Late catheter dysfunction is defined in patients without early catheter dysfunction who subsequently are (1) unable to complete a single hemodialysis session due to recurrent pressure alarms or (2) unable to deliver a mean dialysis blood flow >300 ml/min (with arterial and venous dialysis pressures remaining within the unit parameters) or a Kt/V≥1.2 in 4 hours or less if the mean dialysis blood flow is <300 ml/min (Figure 2). Catheter dysfunction is declared only if the reduction in dialysis blood flow is not resolved by repositioning the patient, reversing the arterial and venous lines, or forceful flushing of the catheter.
Figure 2.
Proposed criteria for diagnosis of catheter dysfunction. CVC, central venous catheter; HD, hemodialysis.
The workgroup also recommends two high-priority studies for future research regarding catheter dysfunction end points (Table 1).
Future Food and Drug Administration (FDA)–approved studies will likely evaluate either catheter lock solutions or catheter devices to prevent catheter dysfunction. Because there is substantial variation in the frequency of catheter dysfunction between hemodialysis units, a single-arm study comparing outcomes with the published literature is inadequate. The ideal RCT will compare an investigational lock solution with the current standard. To minimize the potential for bias in the diagnosis of catheter dysfunction, the optimal catheter lock study will be double blinded (so that neither the patient nor the dialysis nurse know whether the patient is receiving the investigational lock or standard heparin). Catheter device trials cannot be easily blinded. To minimize the potential for bias in such studies, there should be an independent DMSB that reviews the objective data to assess whether catheter dysfunction has occurred.
CVO
Our workgroup suggests defining CVO in patients with clinical manifestations of central vein stenosis in conjunction with imaging studies showing high-grade stenosis. CVO comprises either stenosis or complete occlusion of the central vein(s). It should be suspected in patients who develop ipsilateral arm swelling after placement of a permanent vascular access (AVF or AVG) in the presence of a tunneled hemodialysis catheter or previous catheters. Depending on the location of the central vein stenosis, swelling of the face, breast (brachiocephalic), or both arms (superior vena cava) may occur (6,8). However, it is quite common to see no arm swelling, even with stenosis of 70% in patients who develop substantial collateral veins (19).
CVO is documented with contrast venography supplemented by Duplex ultrasound. Magnetic resonance venography, computerized tomography venography, or standard contrast venography through an existing catheter at the time of removal may contribute to the diagnosis, but they are insufficient on their own for accurate diagnosis. The correlation between degree of obstruction and clinical symptoms is poor.
The workgroup recommends developing and validating a grading system that relates the anatomic location and distribution of obstructive lesions with the functional implications for current and future hemodialysis access. A successful system will reliably predict outcomes and indicate the expected level of intensity of interventions necessary to achieve successful access and preserve venous capital.
Future FDA-approved studies will likely evaluate catheter composition or design and devices to prevent CVO. Because there is substantial variation in the frequency of catheter-induced CVO in different hemodialysis units and literature reports, a single-arm study comparing outcomes with the published literature is inadequate. The ideal RCT would compare a catheter or device with the current standard. Catheter device trials cannot be easily blinded. To minimize the potential for bias in the diagnosis of catheter-induced CVO, there should be an independent DMSB to review objective data and assess whether CVO has occurred.
Special Pediatric Considerations
In addition to the major end points encountered in adults dialyzing with a CVC (CRBSI, catheter dysfunction, and CVO), there are several end points unique to children using CVCs. These arise from the emphasis on allowing children to live a normal life and engage in activities, such as swimming, sports, and bathing. As a consequence, additional catheter end points should be considered in children.
Because of age as well as possible cognitive delay, children are often not able to refrain from grasping and tugging on the CVC. There is a significant concern for CVC dislodgement, even when the CVC exit site is securely covered and sutured in place. Even if the CVC does not fall out completely, it may be malpositioned into the superior vena cava, potentially diminishing its capacity to deliver adequate hemodialysis and leading to frequent CVC replacement. Products to prevent CVC dislodgement (special dressing, pouch, or more invasive device) would be welcome. Although Kt/V is a commonly used measure of hemodialysis adequacy in adults, other markers of inadequate dialysis in children include poor volume control, hypertension, and growth retardation. Inadequate dialysis is particularly relevant in children, because their nutritional protein intake is not restricted.
Risks associated with CVC replacement include repeated trauma to central vessels (with inherent consequent risks of central venous stenosis and thrombosis) in addition to those associated with general anesthesia. If the procedure is performed in the interventional suite, radiation may be necessary, adding to the lifetime radiation exposure. Hence, we recommend additional catheter end points in children, including the number of CVC exchanges due to malposition and quantification of procedural radiation exposure.
Lifestyle implications in children should be considered in devising study end points. Various barrier mechanisms for the catheter exit site are available, and additional secondary infection end points can include number of exit site infections and the frequency and severity of skin irritation, with possible grading of irritation (skin breakdown, cellulitis, etc.). Activity restrictions implemented in children using CVCs may affect their quality of life due to exclusion from peer activities, such as sports and swimming. Monitoring for implications of these restrictions can yield potential targets of intervention. For example, data collection regarding infection after swimming or showering if allowed could also yield infectious as well as quality of life outcomes. Accordingly, pediatric quality of life surveys should be administered during clinical trials.
Finally, the numerous challenges associated with CVCs have led to consideration of permanent arteriovenous access (AVF and AVG), even in smaller children (15–20 kg).
We cannot anticipate every type of future intervention to address catheter problems. Theoretically, an intervention to prevent one catheter complication could potentially exacerbate another catheter complication. To address the possibility, future catheter studies should record data on multiple catheter end points.
Disclosures
M.A. is a consultant for CorMedix. D.J.B.-M. is an employee of Transonic Systems Inc. *K.A. is a consultant for Otsuka Pharmaceuticals, Alexion Pharmaceuticals, Bard, and Lutonix. K.M.B. is a former employee of NxStage Inc. *D.H.C. is an employee of Abbvie. A.M.E. received a research grant from Baxter. L.M. is a consultant for Bard, Marveo Medical, PurCath, and HRET and received research funding from CareFusion, Bard, and Astrellas. P.R.-C. is a consultant for WL Gore, Medtronic, Bard Peripheral Vascular, Cook, CorMedix, Fibrogen, and TVA; is the chief scientific officer and founder of Inovasc; received research funding from Cook Medical and Bard Peripheral Vascular; and received honoraria from WL Gore, Medtronic, Bard Peripheral Vascular, and Cook. S.S. is a consultant for TVA Medical, Lutonix, and Vascular Therapies and received honoraria from WL Gore. H.W. is a consultant for Proteon Therapeutics and Merit and received honoraria from Proteon Therapeutics. *These individuals are representing themselves and are not representing their employers.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of any Kidney Health Initiative member organization, the Centers for Disease Control and Prevention, or the US Department of Health and Human Services, and mention of trade names, commercial practices, or organizations does not imply endorsement by the US Government. The authors did not receive financial support for preparing this document.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related articles, “Clinical Trial End Points for Hemodialysis Vascular Access: Background, Rationale, and Definitions,” “Definitions and End Points for Interventional Studies for Arteriovenous Dialysis Access,” and “FDA Regulatory Perspectives for Studies on Hemodialysis Vascular Access,” on pages 490–494, 501–512 and 513–518, respectively.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12011116/-/DCSupplemental.
References
- 1.Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Gilbertson DT, Chen SC: The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 4[Suppl 1]: S5–S11, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Allon M: Dialysis catheter-related bacteremia: Treatment and prophylaxis. Am J Kidney Dis 44: 779–791, 2004 [PubMed] [Google Scholar]
- 4.Lok CE, Mokrzycki MH: Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int 79: 587–598, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Mokrzycki MH, Lok CE: Traditional and non-traditional strategies to optimize catheter function: Go with more flow. Kidney Int 78: 1218–1231, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Agarwal AK, Patel BM, Haddad NJ: Central vein stenosis: A nephrologist’s perspective. Semin Dial 20: 53–62, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Vats HS: Complications of catheters: Tunneled and nontunneled. Adv Chronic Kidney Dis 19: 188–194, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Krishna VN, Eason JB, Allon M: Central venous occlusion in the hemodialysis patient. Am J Kidney Dis 68: 803–807, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Shingarev R, Barker-Finkel J, Allon M: Association of hemodialysis central venous catheter use with ipsilateral arteriovenous vascular access survival. Am J Kidney Dis 60: 983–989, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK: Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin Infect Dis 49: 1–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérgola PE, Habiba NM, Johnson JM: Body temperature regulation during hemodialysis in long-term patients: Is it time to change dialysate temperature prescription? Am J Kidney Dis 44: 155–165, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Al-Solaiman Y, Estrada E, Allon M: The spectrum of infections in catheter-dependent hemodialysis patients. Clin J Am Soc Nephrol 6: 2247–2252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quittnat Pelletier F, Joarder M, Poutanen SM, Lok CE: Evaluating approaches for the diagnosis of hemodialysis catheter-related bloodstream infection. Clin J Am Soc Nephrol 11: 847–854, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maki DG, Ash SR, Winger RK, Lavin P; AZEPTIC Trial Investigators: A novel antimicrobial and antithrombotic lock solution for hemodialysis catheters: A multi-center, controlled, randomized trial. Crit Care Med 39: 613–620, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Allon M: Treatment guidelines for dialysis catheter-related bacteremia: An update. Am J Kidney Dis 54: 13–17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johns TS, Mokrzycki MH: Optimal approach for the diagnosis of hemodialysis catheter-related bacteremia. Clin J Am Soc Nephrol 11: 756–758, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenblum A, Wang W, Ball LK, Latham C, Maddux FW, Lacson E Jr.: Hemodialysis catheter care strategies: A cluster-randomized quality improvement initiative. Am J Kidney Dis 63: 259–267, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Moist LM, Hemmelgarn BR, Lok CE: Relationship between blood flow in central venous catheters and hemodialysis adequacy. Clin J Am Soc Nephrol 1: 965–971, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Levit RD, Cohen RM, Kwak A, Shlansky-Goldberg RD, Clark TW, Patel AA, Stavropoulos SW, Mondschein JI, Solomon JA, Tuite CM, Trerotola SO: Asymptomatic central venous stenosis in hemodialysis patients. Radiology 238: 1051–1056, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Mermel LA: What is the evidence for intraluminal colonization of hemodialysis catheters? Kidney Int 86: 28–33, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.