SUMMARY
Therapy of invasive infections due to multidrug-resistant Enterobacteriaceae (MDR-E) is challenging, and some of the few active drugs are not available in many countries. For extended-spectrum β-lactamase and AmpC producers, carbapenems are the drugs of choice, but alternatives are needed because the rate of carbapenem resistance is rising. Potential active drugs include classic and newer β-lactam–β-lactamase inhibitor combinations, cephamycins, temocillin, aminoglycosides, tigecycline, fosfomycin, and, rarely, fluoroquinolones or trimethoprim-sulfamethoxazole. These drugs might be considered in some specific situations. AmpC producers are resistant to cephamycins, but cefepime is an option. In the case of carbapenemase-producing Enterobacteriaceae (CPE), only some “second-line” drugs, such as polymyxins, tigecycline, aminoglycosides, and fosfomycin, may be active; double carbapenems can also be considered in specific situations. Combination therapy is associated with better outcomes for high-risk patients, such as those in septic shock or with pneumonia. Ceftazidime-avibactam was recently approved and is active against KPC and OXA-48 producers; the available experience is scarce but promising, although development of resistance is a concern. New drugs active against some CPE isolates are in different stages of development, including meropenem-vaborbactam, imipenem-relebactam, plazomicin, cefiderocol, eravacycline, and aztreonam-avibactam. Overall, therapy of MDR-E infection must be individualized according to the susceptibility profile, type, and severity of infection and the features of the patient.
KEYWORDS: multidrug resistance, antimicrobial therapy, extended-spectrum β-lactamases, carbapenemases, bloodstream infections, mortality
INTRODUCTION
The emergence and spread of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae have become a public health problem in recent decades (1). Enterobacteriaceae are common pathogens and common causes of different types of community- and hospital-acquired infections, so antimicrobial resistance in these bacteria has significant potential impacts on antibiotic use and patient outcomes. Treatment of infections caused by MDR and XDR Enterobacteriaceae is challenging, with limited antimicrobials available and limited evidence of their efficacy. The previous paradigm, with a specific drug serving as the drug of choice across most clinical situations, no longer holds. Meanwhile, an increasing body of knowledge suggests that therapy can be individualized in accordance with the source and severity of infection and the susceptibility profile of the bacteria, among other factors. In order to help physicians make decisions for treatment of infections caused by MDR and XDR Enterobacteriaceae, a review of the available data is necessary.
The objective of this article is to review the potential therapeutic options for the treatment of infections due to extended-spectrum-β-lactamase (ESBL)-, AmpC-, and carbapenemase-producing Enterobacteriaceae. This review includes mainly clinical studies, prioritizing controlled studies when available, and includes noncomparative studies only when these provide information relevant to specific populations. In vitro and animal studies are also included only if considered necessary in the absence of clinical studies. The target infections are invasive ones, such as hospital-acquired pneumonia (HAP), complicated urinary tract infections (cUTI), complicated intra-abdominal infections (cIAI), and any bacteremic infection. MDR has been defined for epidemiological purposes as acquired nonsusceptibility to at least one agent in three or more antimicrobial categories, and XDR has been defined as nonsusceptibility to at least one agent in all but two or fewer antimicrobial categories (2). Here, however, we consider the most important MDR and XDR Enterobacteriaceae with specific mechanisms of resistance, such as those that produce ESBLs, AmpC β-lactamases, and carbapenemases, which are typically MDR according to the above criteria because of the β-lactamases produced but are also frequently resistant to some non-β-lactam antibiotics and so represent a therapeutic challenge. Also, most studies refer to bacteria that produce these mechanisms of resistance.
Readers should be aware that randomized controlled trials (RCT) are scarce in this field. Most available clinical studies are observational in design (frequently retrospective cohort studies) or are case series and anecdotal reports. RCT data on specific syndromes, based on MDR Enterobacteriaceae analyzed post hoc, are also considered. However, many studies suffer from important limitations, including potential selection and information biases as well as a lack of adequate control for confounding. Lack of statistical power is also a major consideration in studies not finding differences in efficacy between compared drugs.
Information is stratified into empirical and targeted therapy categories wherever possible. Nonetheless, decisions about empirical therapy should be made in accordance with local rates for the pathogens considered, together with individual risk factors and infection severity. Because of important differences in local epidemiology, rules about when empirical therapy against specific resistant bacteria should be started cannot be generalized.
The use of one or another drug may depend on the results of susceptibility testing. While this is beyond the objective of this review, it should be noted that the determination of the MIC for some antimicrobials may not be fully reliable, depending on the methods used; also, a ±1 dilution variability in MIC determination is accepted. Finally, the breakpoints for susceptibility recommended by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee for Antimicrobal Susceptibility Testing (EUCAST) differ for some antimicrobials.
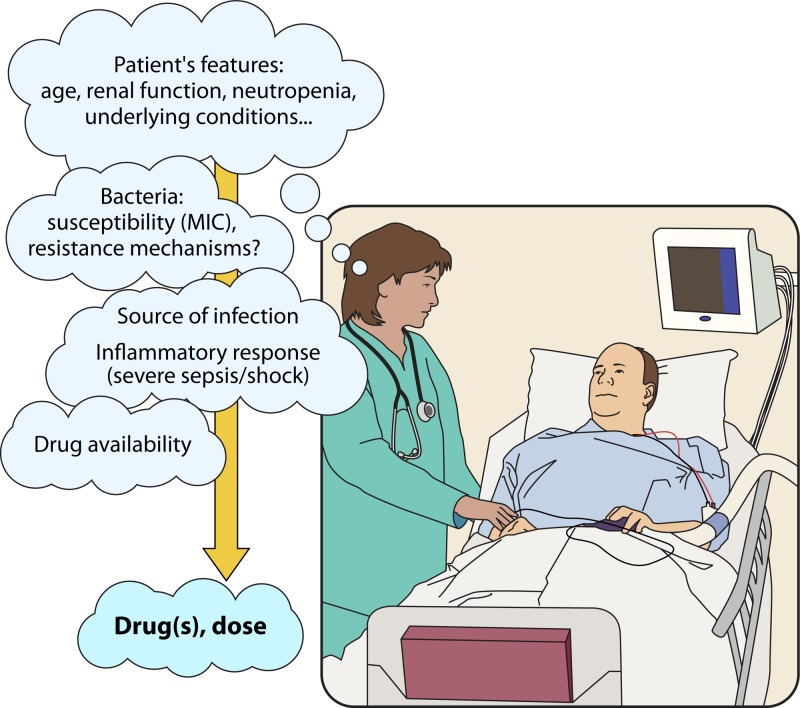
Finally, while it is taken for granted that the general principles for the management of infectious diseases apply, the paramount importance of these principles cannot be stressed enough and include support therapy when needed, rapid and effective source control whenever possible, and consideration of patient characteristics (immunosuppression, renal function, etc.), the severity of systemic inflammatory response syndrome, and the source of infection for the selection of an antimicrobial regimen (Figure 1).
FIG 1.
Aspects to be considered in the decision-making process for antimicrobial therapy of patients with infections due to ESBL-, AmpC-, or carbapenemase-producing Enterobacteriaceae.
THERAPY AGAINST ESBL- AND AmpC-PRODUCING ENTEROBACTERIACEAE
Both ESBL and AmpC producers are typically resistant to some or all cephalosporins, but they exhibit some differences, as follows. ESBLs are inhibited by β-lactam inhibitors and do not hydrolyze cephamycins, while AmpC enzymes are not inhibited by classic β-lactam inhibitors and confer resistance to cephamycins but do not efficiently hydrolyze cefepime (3–5). ESBLs are typically encoded by plasmid-borne genes (3, 4), whereas AmpC can be encoded by plasmid genes or be produced as a result of derepression of chromosomal genes in some Enterobacteriaceae (typically Enterobacter spp., Serratia marcescens, Citrobacter freundii, Providencia spp., and Morganella morganii). The latter will test as susceptible to cephalosporins if AmpC production is not derepressed, but resistance can develop while on treatment with these drugs (5). Finally, chromosomally encoded AmpC can be overproduced in Escherichia coli (5). Since some laboratories do not routinely identify the specific mechanism of resistance to cephalosporins, as this is not recommended for the purpose of treatment decisions by CLSI or EUCAST (but only for epidemiological reasons) and the type of cephalosporinase cannot always be differentiated phenotypically, both ESBL and AmpC producers are reviewed here. Most available information concerns ESBL-producing Enterobacteriaceae (ESBL-E); potentially active drugs against these bacteria are reviewed in Table 1.
TABLE 1.
Summary of positive and negative aspects and dosing of potentially useful drugs in the treatment of infections with ESBL- and AmpC-producing Enterobacteriaceaeb
| Drug | Positive aspects | Negative aspects | Dosing (for adults with normal renal function) and comments |
|---|---|---|---|
| Meropenem, imipenem, doripenem | Reference drugs, usually active | Ecological impact; less experience with doripenem | Standard dosing is recommended |
| Ertapenem | Not active against P. aeruginosaa; usually active; convenient for outpatient therapy and deescalation from other carbapenems | Ecological impact if CPE endemicity/outbreak; doubts in cases of septic shock (insufficient dosing?); anecdotal failures described with development of resistance (porin loss) | 1 g/day in most situations; for septic shock or high-inoculum infections with borderline MIC isolates, use other alternatives or increase dose to 2 g/day |
| Piperacillin-tazobactam | Probably noninferior to carbapenems in UTI and biliary tract infections | False susceptibility with some automated systems; inoculum effect (unrelated to ESBLs); heterogeneous resistance rates (5 to 30% among ESBL producers, higher among AmpC producers); doubts in cases of septic shock, pneumonia (CLSI susceptibility breakpoint too high)? | 4.5 g every 8 h (extended infusion) or every 6 h |
| Amoxicillin-clavulanic acid | No inoculum effect; probably noninferior to carbapenems in UTI and biliary tract infections; not active against P. aeruginosaa; convenient for oral switch | Not available for i.v. use in many countries; heterogeneous resistance rates, usually >40% among ESBL producers; AmpC producers are resistant | Intravenous, 2.2 g/8 h; oral, at least 1.250 g/8 h for UTI |
| Ceftolozane-tazobactam | Areas with large proportions of susceptible isolates | Reserve drug for MDR P. aeruginosa infection; scarce experience so far; 10–30% resistance rates among ESBL producers, lower rates in AmpC producers | 1.5 g/8h; approved for cUTI and cIAI (with metronidazole); consider 3 g/8 h for pneumonia |
| Ceftazidime-avibactam | Large proportion of susceptible isolates | Reserve drug for KPC- or OXA-48-producing Enterobacteriaceae | 2.5 g/8 h; approved for cUTI and cIAI (with metronidazole); in Europe, also approved for HAP in case of limited options |
| Cefotaxime, ceftriaxone, ceftazidime, cefepime | Some ESBL-E may be susceptible; cefepime is usually active against AmpC producers | Most isolates are resistant (except to cefepime in the case of AmpC producers); inoculum effect; ecological impact; clinical data are scarce and contradictory | If used, high doses are recommended (cefotaxime, 1 g/6 h to 2 g/8 h; ceftazidime or cefepime, 2 g/8 h) |
| Cefoxitin, cefotetan, cefmetazole, moxalactam, flomoxef | Not active against P. aeruginosaa; areas with large proportions of susceptible isolates (ESBL producers); probably useful against UTI for stable patients | AmpC producers are resistant; inoculum effect; observational studies with contradictory results; anecdotally described development of resistance during therapy | High doses; close follow-up needed |
| Temocillin | Active against ESBL and AmpC producers; not active against P. aeruginosaa | Not available in many countries; comparative studies are lacking | Probably 2 g every 8 h |
| Gentamicin, tobramycin, amikacin | Active against many ESBL and AmpC producers; useful for UTI | Nephrotoxicity; less efficacious in non-UTI infections; heterogeneous resistance rates | Standard dosing (see Table 2); may be considered empirically as carbapenem-sparing agents (in monotherapy or in combination with a lower-spectrum β-lactam) until microbiological data are available |
| Tigecycline | Active against most ESBL and AmpC producers; not active against P. aeruginosaa | FDA and EMA warnings for use only if other options are unavailable/unsuitable; probably not a good option for UTI or HAP | 100-mg loading dose, 50 mg/12 h; may be an alternative in cIAI |
| Fosfomycin (i.v.) | Noninferior to piperacillin-tazobactam in cUTI (pending publication of data) | Not available in many countries; scant experience; risk of emergence of resistant subpopulations with monotherapy | 4 g/6 h to 6–8 g/8 h |
| Ciprofloxacin, levofloxacin | Potentially useful for fully susceptible isolates; convenient for oral switch | Ecological impact; most isolates are resistant; failures for isolates with MICs of 0.5–1 mg/liter have been described | For i.v. ciprofloxacin, 400 mg/8–12 h; for oral ciprofloxacin, 500–700 mg/12 h; for levofloxacin (i.v., oral), 750 mg/24 h |
| Trimethoprim-sulfamethoxazole | Convenient for oral switch | Most isolates are resistant; scant published experience | i.v. or oral, 160/800 mg/8–12 h |
Considered a positive aspect in terms of antimicrobial stewardship purposes (avoiding selective pressure on P. aeruginosa).
CPE, carbapenemase-producing Enterobacteriaceae; i.v., intravenous.
Carbapenems
Carbapenems have traditionally been considered the drugs of choice for infections caused by enterobacteria producing ESBL and AmpC enzymes (3–5) because they are not affected by these resistance mechanisms. Furthermore, in the case of ESBL-E, they have been associated with lower failure rates than those for other drugs, mostly cephalosporins and fluoroquinolones. A meta-analysis that included 21 observational studies of bacteremic infections caused by ESBL-E up to January 2012 showed that mortality rates for patients who had received empirical or definitive treatment with carbapenems were lower than those for patients treated with cephalosporins, fluoroquinolones, or aminoglycosides; the differences were not significant for β-lactam–β-lactamase inhibitor (BLBLI) combinations (6). Note that many of the studies included in the meta-analysis had significant limitations, including a lack of control for confounding, and it was not always clear whether bacteria were susceptible to the noncarbapenem drugs used. There is very little published experience involving children. A small retrospective study in South Korea included children with ESBL-E UTI treated with carbapenems (4 patients) or “other drugs” (23 patients) and those who switched from a carbapenem to another drug (15 patients); the “other drugs” were cefotaxime, piperacillin-tazobactam, and amikacin (7). All patients were cured, and times to defervescence were similar. Studies comparing carbapenems to specific drugs are reviewed in specific subsections.
Regarding Enterobacteriaceae harboring chromosomal blaAmpC, a recent meta-analysis that included studies with limitations did not find that carbapenems were clearly superior to fluoroquinolones, cefepime, or BLBLIs. In most studies reviewed, 20 to 35% of isolates included showed the derepressed AmpC phenotype (8). The data for plasmid-mediated AmpC producers are scarce.
In summary, the available data still suggest that carbapenems are the reference drugs for treatment of these infections. Nonetheless, the same assumption probably contributed to the significant worldwide increase in the consumption of carbapenems (9), which may be partly linked to the subsequent spread of carbapenem resistance. It is therefore important to take a closer look at potential alternative drugs.
Among carbapenems, most published articles have tended to focus on imipenem and meropenem (3, 6, 10). With respect to other group 2 carbapenems, a post hoc analysis of patients with infections due to ESBL-E included in an RCT comparing doripenem and other drugs against cUTI, cIAI, and HAP analyzed the outcomes of those receiving doripenem (25 patients) or comparators (levofloxacin, imipenem, and piperacillin-tazobactam) (29 patients); the efficacies were similar, but the numbers involved were clearly very limited (11).
Ertapenem is the only group 1 carbapenem, does not have clinically relevant activity against Pseudomonas aeruginosa or Acinetobacter baumannii, and may exert lower selection pressure for resistance on these bacteria than that with other carbapenems (12); such a potential ecological advantage would be lost in environments with high rates of carbapenem-resistant Enterobacteriaceae (CRE) (13), for which the selection pressure would be similar or even higher. Five observational studies were found comparing ertapenem with other carbapenems in bloodstream infections (BSI) due to ESBL producers. There were no significant differences in terms of prognosis for either empirical or targeted therapy (14–18). In one study, however, subgroup analyses of patients who presented with severe sepsis or septic shock showed a trend toward increased mortality with ertapenem (18). A potential explanation for this would be the lower probability of attaining the pharmacokinetic-pharmacodynamic (PK-PD) target in these patients by using the standard dose of 1 g daily. The most common source of infection in all these studies was UTI, and patients with HAP were underrepresented. This is relevant because the probability of PK-PD target attainment with ertapenem has been shown to be low for patients with early-onset ventilator-associated pneumonia (VAP) and hypoalbuminemia (19). A noncomparative study analyzed 20 patients with VAP caused by ESBL-E (mostly Klebsiella pneumoniae), and clinical and microbiological success rates were 80% and 75%, respectively (20). An open, single-center RCT compared deescalation to ertapenem versus continuation with a group 2 carbapenem, including imipenem, meropenem, doripenem, or biapenem, in patients with infections due to ESBL-E (32 and 34 patients, respectively) (21); 40% had a UTI and 16% had HAP. Overall, 50% of patients were bacteremic, and the ESBL-E was Klebsiella pneumoniae in 32% of cases. There were no significant differences in clinical cure (94% with ertapenem and 79% with other carbapenems), microbiological eradication (100% and 96%, respectively), or mortality (9% and 29%, respectively). With respect to children, data from two noncomparative studies of UTI due to ESBL-E gave promising results (22, 23). Ertapenem is also suitable for outpatient parenteral antimicrobial therapy (OPAT); experience so far comes from uncontrolled studies showing good results (24–27) and one study comparing it with oral fosfomycin (discussed below) (28).
Results from in vitro models suggest that regrowth occurs in isolates with a MIC of 1 mg/liter (intermediate susceptibility) exposed to ertapenem (29) and that resistant subpopulations of ESBL-producing E. coli may emerge during therapy at 1 g/day, while a dose of 1.5 to 2 g/day shows better bacterial killing (30). Contrary to expectations, extended infusions or fractionated dosing showed no benefits. Development of resistance to ertapenem (31–33) and other carbapenems (34) during or after treatment with ertapenem has been described anecdotally, mostly as a consequence of porin loss in complex infections. In any case, caution may be needed in using ertapenem for high-inoculum infections with inadequate source control or that are impossible to control/remove. In such circumstances, the use of a higher dose or an alternative drug would seem reasonable.
Classic BLBLIs
ESBLs are inhibited by β-lactam inhibitors (3, 4), and classic BLBLIs, such as amoxicillin-clavulanic acid, ampicillin-sulbactam, piperacillin-tazobactam, ticarcillin-sulbactam, and cefoperazone-sulbactam, are active against ESBL producers in the absence of other mechanisms of resistance. Nonetheless, β-lactamase hyperproduction and coproduction of plasmid-mediated AmpC enzymes, among other factors, can affect inhibitor activity. BLBLI resistance rates in ESBL producers show important geographical differences and are high in some areas (35–37). Furthermore, some automated systems may fail to detect resistance to piperacillin-tazobactam, as described for isolates coproducing CTX-M-15 and OXA-1 (38).
There have been concerns about the efficacy of BLBLIs against infections due to susceptible ESBL producers (3), even though similar concerns do not exist for Enterobacteriaceae producing other β-lactamases, such as TEM-1 or SHV-1. The arguments for such concerns include the inoculum effect with piperacillin-tazobactam. This effect, however, also occurs with non-ESBL-E organisms and is therefore not related to ESBL production (39). Animal model studies have suggested that the activity of piperacillin-tazobactam against ESBL producers depends, as expected, on the level of exposure and that use of low doses (3.375 g every 6 h) is insufficient (40–42), but they have also confirmed in vivo that a higher inoculum is associated with lower efficacy (43, 44). It should be noted that amoxicillin-clavulanic acid is not affected by the inoculum effect in vitro or in vivo (39, 43). Finally, some anecdotal failures with piperacillin-tazobactam have been described (45).
In regard to comparative clinical studies, a post hoc analysis of several prospective Spanish cohorts of patients with bacteremia caused by ESBL-producing E. coli did not find that in vitro-active BLBLIs (piperacillin-tazobactam and amoxicillin-clavulanic acid) had a deleterious impact on mortality or length of stay compared to that with carbapenems for either empirical or targeted therapy (46). The study included specific definitions for exposure, and control for confounding variables was performed by multivariate analysis with use of a propensity score for receiving BLBLI. For interpretation purposes, the following important aspects of this study should be considered: only E. coli cases were included, the source of the BSI was the biliary or urinary tract in more than half of patients, high doses of piperacillin-tazobactam were used (mainly 4.5 g every 6 h), and the MIC of piperacillin-tazobactam was ≤4 mg/liter for 65% of patients treated with this antibiotic. Two meta-analyses published in 2015, one including all pathogens (47) and the other restricted to ESBL producers (6), did not find superiority of carbapenems over BLBLIs. However, a later study in the United States that included patients with BSI due to ESBL producers, mostly K. pneumoniae, found higher mortality with empirical piperacillin-tazobactam than with carbapenems after controlling for confounders (48). In that study, only patients receiving a carbapenem as definitive treatment were included; those who continued with piperacillin-tazobactam as definitive treatment (who were probably doing well) were excluded, which may have caused a selection bias. The most frequent dosage of piperacillin-tazobactam was 3.375 g every 6 h, and the MIC of piperacillin-tazobactam was ≤4 mg/liter for only 40% of isolates. A small study including only patients with Proteus mirabilis BSI found higher mortality for patients treated with piperacillin-tazobactam, but there was no control for confounders (49). Several other studies in which carbapenems did not show superiority over BLBLIs in patients with BSI were performed later (50–53). Two of these deserve further comment. One was an analysis of the international retrospective cohort INCREMENT, which compared 170 and 195 patients treated empirically with BLBLIs and carbapenems, respectively, and 92 and 509 patients treated with the respective definitive therapies (51). In the overall and subgroup analyses, BLBLIs did not show higher rates of mortality or clinical failure than those with carbapenems. The other was also a retrospective international cohort study (BICAR), performed with neutropenic patients and including 48 and 126 patients treated empirically with a BLBLI (mostly piperacillin-tazobactam) and a carbapenem, respectively; the patient numbers for targeted therapy were 17 and 234, respectively. Thirty-day mortality rates were 20.8% and 13.4% for empirical BLBLIs and carbapenems, respectively, and 5.8% and 15.8% for the respective targeted therapies (53). Treatment with a BLBLI was not shown to be associated with worse outcomes than those with carbapenems in multivariate analysis or after propensity score matching of patients. Other studies that included only UTI showed similar results (54, 55). An open randomized controlled trial performed in 3 hospitals compared the efficacies of piperacillin-tazobactam (4.5 g every 6 h) and ertapenem (1 g per day) in patients with UTI due to ESBL-E (56). Patients with obstruction of the urinary tract or prostatitis were excluded. Thirty-three patients were included in each arm; 27% and 21%, respectively, were bacteremic and 24 and 33%, respectively, had septic shock. The rates of clinical success, microbiological success, and mortality were 94%, 97%, and 6%, respectively, with piperacillin-tazobactam and 97%, 97%, and 6%, respectively, with ertapenem.
It is possible that not all BLBLIs are equally effective, with differences due to the inhibitory capacity of the β-lactamase inhibitor or to the activity of the β-lactam. There are nevertheless very few comparative data for different BLBLIs. As shown above, piperacillin-tazobactam, but not amoxicillin-clavulanic acid, shows reduced activity at high inoculum concentrations both in vivo and in vitro (39, 43). In the Spanish post hoc analysis of prospective cohorts of patients with BSI due to ESBL-producing E. coli, the 30-day mortality rate was 11.4% with piperacillin-tazobactam (4/35 patients) and 8.1% with amoxicillin-clavulanic acid (3/37 patients) (46). For susceptible isolates, the MIC distributions with piperacillin-tazobactam were extremely wide, with 10, 8, 4, 6, and 7 isolates showing MICs of ≤1, 2, 4, 8, and 16 mg/liter, respectively, while all isolates showed a MIC of 4 or 8 mg/liter with amoxicillin-clavulanic acid. A subsequent analysis showed differences in mortality according to the MIC of piperacillin-tazobactam (0/18 patients for isolates with MICs of ≤2 mg/liter and 36.8% for isolates with MICs of >2 mg/liter; relative risk [RR] = 0.13; 95% confidence interval [CI] for RR, 0.01 to 0.98) (57); note that all mortality was in patients with sources other than the urinary tract. A randomized controlled study (MERINO trial) comparing piperacillin-tazobactam with meropenem for the treatment of cephalosporin-resistant Enterobacteriaceae is recruiting at the time of this writing (58). Data for other BLBLIs, such as ampicillin-sulbactam, are lacking.
The above data strongly suggest that, in many situations, BLBLIs are suitable alternatives to carbapenems for the treatment of many invasive infections caused by ESBL producers if the intended BLBLI is active in vitro. The data are more solid for cUTI and biliary tract infections, including bacteremia. We still recommend a carbapenem for patients with high-inoculum infections (for example, undrained abscesses or pneumonia) or for patients with septic shock, for whom there are few available data. The recommended dosage for piperacillin-tazobactam is 4.5 g every 6 h, or possibly 4.5 g every 8 h if administered by extended infusion (59). Also, amoxicillin-clavulanate seems to be a good option for susceptible isolates in countries where this drug is available for intravenous administration. There are too few data on other BLBLIs to provide recommendations.
In regard to organisms harboring chromosomally carried blaAmpC genes, a meta-analysis of BSI caused by Enterobacter, Citrobacter, and Serratia species showed that treatment with piperacillin-tazobactam was not associated with increased mortality compared to that with carbapenems (8). A retrospective cohort study studied 165 patients with BSI due to these microorganisms, 85% of which were in fact AmpC producers. Eighty-eight patients received targeted therapy with piperacillin-tazobactam and 77 with meropenem or cefepime (60). Mortality rates were 10% and 12%, respectively, while in 41 propensity-matched pairs, mortality rates were 15% and 7%, respectively (odds ratio [OR] = 0.50; 95% CI = 0.13 to 2.0). We found no comparative studies of plasmid-mediated AmpC producers. Despite the major limitations of the studies included, the results suggest that an in vitro-active BLBLI would be effective against these organisms.
Newer BLBLIs (Ceftolozane-Tazobactam and Ceftazidime-Avibactam)
Ceftolozane-tazobactam combines a new cephalosporin (ceftolozane) with enhanced antipseudomonal activity with a classic β-lactamase inhibitor (tazobactam). The drug was approved by the FDA and the European Medicines Agency (EMA) for treatment of cIAI (in combination with metronidazole) and cUTI, including pyelonephritis. This compound has been shown to be active in vitro against >90% and 42 to 98% of ESBL-producing E. coli and K. pneumoniae isolates, respectively (61). One study analyzed the outcomes for 150 patients with infection due to ESBL-E in pivotal trials of ceftolozane-tazobactam against cUTI (the comparator was levofloxacin) and cIAI (the comparator was meropenem) (62). Rates of clinical cure and microbiological eradication were higher with ceftolozane-tazobactam (98.1% and 72.2%, respectively) than with levofloxacin (82.6% and 47.8%, respectively) against cUTI; 82% of isolates were susceptible to ceftolozane-tazobactam, whereas only 25% were susceptible to levofloxacin. Against cIAI, ceftolozane-tazobactam and meropenem outcomes were similar (clinical cure rates were 95.8% and 88.5%, respectively; the same percentages were found for microbiological eradication).
Ceftazidime-avibactam combines a well-known third-generation cephalosporin with a new (non-β-lactam) β-lactamase inhibitor. It was recently approved by the FDA and the EMA for treating cUTI and cIAI (the latter in combination with metronidazole); the EMA also includes an indication for HAP and other infections due to Gram-negative bacteria with limited treatment options. Avibactam inhibits class A enzymes, including ESBLs and Klebsiella pneumoniae carbapenemases (KPC), as well as class C and some OXA β-lactamases, but is not active against metallo-β-lactamases (MBLs) (61). In the pivotal trial against cUTI, ceftazidime-avibactam and doripenem were compared. Clinical cure among patients with ceftazidime-resistant isolates (mostly due to ESBL production) was 89.3% (67/75 patients) with ceftazidime-avibactam and 89.3% (75/84 patients) with doripenem (63). In the pivotal trial for treatment of cIAI, ceftazidime-avibactam plus metronidazole showed a rate of clinical response against ceftazidime-nonsusceptible Enterobacteriaceae (around 80% were ESBL producers) similar to that with meropenem (81.8% [36/44 patients] versus 85.5% [53/62 patients]) (64), and it showed an efficacy similar to that of the best available therapy (mostly carbapenems) in a pathogen-directed trial of patients with cUTI and cIAI caused by ceftazidime-resistant Enterobacteriaceae (65).
The available data therefore support the efficacy of both new BLBLIs against susceptible ESBL producers in patients with cUTI, and also of ceftazidime-avibactam against cIAI, although it should be noted that the resistance rate among ESBL producers is higher for ceftolozane-tazobactam than for ceftazidime-avibactam (61). However, because of their potential added value against XDR organisms (XDR P. aeruginosa in the case of ceftolozane-tazobactam and KPC- or OXA-48-producing Enterobacteriaceae in the case of ceftazidime-avibactam), it seems prudent to reserve these drugs for these particular organisms. We found no studies providing clinical data on infections caused by AmpC producers.
Oxyiminocephalosporins (Cefotaxime, Ceftriaxone, Ceftazidime, and Cefepime)
According to present breakpoints recommended by EUCAST (66) and CLSI (67), some ESBL-E are susceptible to cephalosporins (68, 69). Producers of TEM and SHV types of ESBLs are susceptible to cefotaxime more frequently than CTX-M producers are, and the opposite is the case for ceftazidime and cefepime. This is because different ESBL types vary in the ability to hydrolyze specific cephalosporins (3, 4). The proportion of AmpC producers (by either plasmid-borne genes or derepressed or hyperexpressed chromosomal genes) that are susceptible to cephalosporins (except cefepime) is lower (70).
Before 2010, Enterobacteriaceae with cephalosporin MICs of ≤8 mg/liter were considered susceptible. Patients with BSI due to ESBL-E treated with cephalosporins had worse outcomes than expected, even when isolates showed MICs within the range of susceptibility (71), which prompted the recommendation to report all ESBL-E as resistant. However, PK-PD stochastic models suggested that the breakpoints for cephalosporins were too high and that outcome was dependent only on the probability of attaining the PK-PD target, regardless of ESBL production (72, 73). As a result, EUCAST and CLSI lowered the susceptibility breakpoints of cephalosporins for Enterobacteriaceae (as of 2017, isolates with MICs of ≤1 mg/liter are susceptible according to EUCAST breakpoints [66]; breakpoints according to CLSI are ≤1 mg/liter for cefotaxime, ≤2 mg/liter for cefepime, and ≤4 mg/liter for ceftazidime [67]), and it is recommended to report the susceptibility as found, irrespective of ESBL production.
Clinical data on outcomes for patients with infections caused by ESBL-E who were treated with active cephalosporins versus other options are limited and sometimes contradictory (68, 74–79). Goethaert et al. found similar mortality rates for 21 and 23 patients with BSI due to TEM-23-producing Enterobacter aerogenes who were treated empirically with cefepime (2 g every 8 h) and carbapenems, respectively (74). Most patients received combination therapy, and there was no adjustment for confounders. Chopra et al. found an adjusted OR for mortality of 1.66 (95% CI = 0.71 to 3.87) for patients treated with cefepime (dose not specified) compared to that for carbapenems in patients with ESBL-E BSI (76). Lee et al. found higher mortality with cefepime (1 to 2 g every 8 h) than with carbapenems, using multivariate analysis and propensity score matching (77). The outcomes were somewhat worse for isolates with cefepime MICs of 2 to 8 mg/liter than for those with MICs of ≤1 mg/liter. Finally, Wang et al. found a trend toward higher mortality with cefepime (2 g every 8 h) than that with carbapenems in a propensity score-matched analysis (hazard ratio [HR] = 2.87; 95% CI = 0.88 to 9.41) (78). In a study of Enterobacter cloacae bacteremia, ESBL production was independently associated with increased mortality in patients treated with cefepime, even after controlling for the MIC (79). Another study evaluated the impact of the cefotaxime or ceftriaxone MIC on the outcomes for 409 patients with community-onset bacteremia due to community-onset BSI due to Enterobacteriaceae (mostly E. coli) who were treated empirically with these drugs (80); 94% of isolates were susceptible (MICs of ≤1 mg/liter). Patients with susceptible isolates had a lower risk of mortality in adjusted analysis, but no comparisons with different drugs were given. The arguments against the use of cephalosporins include the inoculum effect shown in in vitro and in vivo models (44, 81–83) and the possibility of hyperexpression of blaESBL genes (84).
In view of the data available so far, we would not recommend using a cephalosporin with in vitro susceptibility as targeted therapy for patients with invasive infections due to ESBL producers. For patients who received an active cephalosporin empirically, we recommend switching to an alternative drug as targeted therapy, except for stable patients with nonobstructive UTI or if the source of infection has been removed. If a cephalosporin is to be used, a high dose is recommended.
AmpC producers are usually susceptible to cefepime unless other mechanisms of resistance also exist. An observational study of BSI due to Enterobacter cloacae found a higher mortality for patients treated with cefepime than for those treated with carbapenems when the isolates had MICs of 4 to 8 mg/liter (79). The meta-analysis mentioned found no significant differences in outcomes for patients with BSI caused by Enterobacteriaceae harboring chromosomally encoded AmpC who were treated with cefepime or carbapenems, although only a minority of patients included had isolates with derepressed AmpC (8). Tamma et al. compared mortality rates for hospitalized patients with blood, bronchoalveolar lavage, or intra-abdominal fluid cultures growing AmpC-producing Enterobacter spp., Serratia spp., or Citrobacter spp. with derepressed AmpC and treated with cefepime (1 to 2 g every 8 h) or meropenem; after comparing 32 propensity score-matched pairs, no effect on mortality was demonstrated (31% and 34%, respectively) (85). This contrasts with the fact that cefepime is also less active in vitro and in vivo with high inocula of AmpC producers (86–88). More clinical comparative studies of cefepime against derepressed AmpC mutants and plasmid-mediated AmpC producers are needed.
In summary, at high doses, cefepime seems to be a reasonable alternative to carbapenems for the treatment of invasive infections caused by susceptible Enterobacteriaceae with chromosomally encoded AmpC. There is very little experience regarding the efficacy of cefepime against plasmid-mediated AmpC producers.
Cephamycins
The inability of ESBLs to efficiently hydrolyze cephamycins, which include cefoxitin, cefotetan, cefmetazole, moxalactam, and flomoxef, means that cephamycins are active against ESBL producers in the absence of other resistance mechanisms (2). Cephamycins are not active against AmpC producers. The use of these drugs was discouraged after early anecdotal reports of development of resistance in ESBL producers during treatment due to porin loss (89, 90). Later, several observational studies comparing the efficacies of cephamycins (mainly flomoxef and cefmetazole) and carbapenems in infections due to ESBL producers were published (91–97). The studies included patients with BSI, predominantly UTI, and one included only patients with pyelonephritis (93). In all but two studies (95, 96), there were small numbers of patients treated with cephamycins, ranging from 7 to 29. Only one study showed worse outcomes with these drugs (92), but most had limited or inadequate control for confounders and low statistical power. In most of the studies, the patients who received carbapenems seemed to be more seriously ill. Matsumura et al. found similar mortality rates among patients receiving targeted therapy for 59 patients treated with flomoxef or cefmetazole and 54 treated with carbapenems, after propensity score adjustment (95). Lee et al. found similar mortality rates with flomoxef and carbapenems when the MIC of flomoxef was ≤1 mg/liter but not when it was 4 to 8 mg/liter (96).
The available data suggest that cephamycins may be an alternative to carbapenems for some nonsevere infections, particularly UTI, where they can serve as carbapenem-sparing options. More data are needed for other types of infection and more seriously ill patients. In any case, high doses and close follow-up are recommended.
Temocillin
Temocillin is active against Enterobacteriaceae and is stable against hydrolysis by ESBLs and AmpC β-lactamases; it has little useful activity against Pseudomonas spp. (98, 99). Unfortunately, it is currently available for intravenous use in only a few countries (such as the United Kingdom and Belgium), and there is very little published experience regarding its use against these pathogens. In a murine model of UTI, the efficacy of temocillin was similar to that of imipenem against CTX-M-15-producing E. coli (100). Balakrishan et al. (101) reported 92 patients with infections due to Enterobacteriaceae (41 had UTI and 42 BSI from diverse sources) who were treated with temocillin; 53 of the isolates were ESBL or derepressed AmpC producers. Clinical and microbiologial cure rates were 86% and 84%, respectively. In a crude analysis, ESBL or AmpC production had no impact on outcome. No clinical studies have been found comparing temocillin with carbapenems or other antibiotics in infections caused by ESBL- or AmpC-producing Enterobacteriaceae. Its efficacy seems to correlate with higher doses (2 g every 12 h), although recent pharmacokinetic-pharmacodynamic data suggest 2 g every 8 h (or in continuous infusion) as the optimal dose for a susceptibility breakpoint of ≤16 mg/liter (102). More clinical studies, and particularly RCT, are needed to establish the role of temocillin in the treatment of ESBL and AmpC producers.
Aminoglycosides
Data on the effectiveness and limitations of aminoglycosides in treating Enterobacteriaceae infections can be extrapolated to infections caused by ESBL or AmpC producers. A systematic review and meta-analysis showed that aminoglycosides had efficacies similar to those of comparators against urinary infections but lower efficacies against other types of infection (103). From a general perspective, the aminoglycoside–β-lactam combination for the treatment of sepsis is disappointing, as it does not seem to provide any extra benefit but increases the risk of toxicity (104). The results of a recent observational study also showed that even short-course (median, 2 days) adjunctive empirical gentamicin increased the risk of renal toxicity but did not protect against mortality in patients with severe sepsis or shock in an area with low resistance rates (and, in fact, the addition of gentamicin did not increase the probability of appropriate coverage) (105). Importantly, the proportions of patients treated with vancomycin among those receiving and not receiving gentamicin in that study were 41% and 18%, respectively, although its effect was controlled for in multivariate analysis. It is not known whether the results would be different in areas with high rates of ESBL-E or for patients without shock or not receiving vancomycin. Using INCREMENT cohort data, Palacios-Baena et al. compared the empirical use of drugs other than carbapenems or BLBLIs (86 patients; 43 received an aminoglycoside) and carbapenems (249 patients) for BSI due to ESBL producers for mortality, clinical cure, and length of hospital stay. No significant differences (or trends) in any outcome were shown (106). Toxicity was not formally evaluated, but significant toxicity would be expected to have some effect on length of stay. Smaller studies of cancer patients with BSI (107) and children with UTI (108) also showed a reasonable effectiveness of aminoglycosides against ESBL-producing organisms in these populations. Finally, the variability in serum concentrations achieved may be important for isolates presenting MICs near the breakpoint in critically ill patients, since therapeutic failure against susceptible strains may be expected in these patients if the pharmacodynamic target is not reached (109).
In view of the above-mentioned findings, it seems that using aminoglycosides adds toxicity rather than benefits, and therefore they cannot be recommended as empirical drugs in areas with low rates of resistance to β-lactams or other first-line drugs. Nonetheless, they may still be considered an empirical option in carbapenem-sparing regimens (as monotherapy or combined with a narrower-spectrum β-lactam) in areas where ESBLs and/or AmpC are prevalent, particularly in UTI and sepsis. In any case, the aminoglycoside should immediately be changed to a better-tolerated drug once the susceptibility results are available.
Among the aminoglycosides, amikacin usually provides better coverage against ESBL and AmpC producers (110). Plazomicin is a new aminoglycoside with good activity against ESBL and AmpC producers (111, 112) and is reviewed in the section on carbapenemase producers.
Tigecycline
Tigecycline is a glycylcycline and, as such, is not affected by ESBLs or AmpC β-lactamases. Tigecycline exhibits predominantly bacteriostatic activity. Its spectrum of activity includes Gram-positive bacteria, Enterobacteriaceae (except for members of the Proteae family), A. baumannii, and anaerobes. It is not active against P. aeruginosa (113). The drug is approved in Europe and the United States for the treatment of complicated skin and skin structure infections and cIAI; in the United States, it is also approved for community-acquired bacterial pneumonia. Importantly, both the FDA and the EMA issued warnings because the drug was associated with an increased risk of mortality and clinical failure in meta-analyses of randomized trials (114–117). Hence, tigecycline was recommended only when other options were not available or were unsuitable. Although there is scant clinical experience with infections caused by ESBL producers (118–120), the results would be expected to be similar to those with non-ESBL producers. Because tigecycline is more frequently needed for the treatment of carbapenem-resistant Enterobacteriaceae (CRE), more information is provided in the relevant section.
Fosfomycin
Fosfomycin is an old antibiotic which remains active against most ESBL- and AmpC-producing E. coli and K. pneumoniae (and other MDR Enterobacteriaceae) isolates (121, 122). An oral formulation of fosfomycin trometamol is available in some countries and has been used extensively for the treatment of uncomplicated UTI; it also shows good efficacy against cystitis caused by ESBL-producing strains (123–126). An observational study compared fosfomycin trometamol (89 patients) administered at 3 g every 48 or 72 h with ertapenem (89 patients) as a step-down regimen in patients with invasive infections due to ESBL producers (28); readmission rates were similar (14.6% and 13.5%, respectively).
The intravenous formulation is available in Spain, France, Germany, and Austria, among other countries. In a recent meta-analysis, the efficacy of fosfomycin in randomized trials (most of which were performed more than 15 years ago) was similar to those of comparators for treatment of diverse infectious syndromes, and the drug was well tolerated (127). One of the main problems with this drug is the potential emergence of resistance during therapy, which seems to be less frequent in E. coli than in other bacteria (128). Recent studies suggest that what actually happens is selection of resistant mutants already present when therapy is started (129). Because of this, for severe infections, fosfomycin has traditionally been recommended for use in combination with other drugs (126, 128). The most appropriate dosing schedules range from 4 g every 6 to 8 h to up to 8 g every 8 h (129, 130). For monotherapy, the drug has been tested as empirical therapy (6 g every 8 h) in an RCT of cUTI, including pyelonephritis; a preliminary report of the trial showed that fosfomycin met the noninferiority criteria against piperacillin-tazobactam for overall success (131). It is also being tested compared to ceftriaxone or meropenem as targeted therapy in an RCT of bacteremic UTI due to multidrug-resistant E. coli (132). Until the results of these studies are fully available, no recommendation can be made about the use of this drug for monotherapy against ESBL or AmpC producers.
Fluoroquinolones and Trimethoprim-Sulfamethoxazole
Fluoroquinolone resistance is very frequent among ESBL producers (3, 4) but is not universal. In most cases, resistance is due to chromosomal mutations. Some isolates may also show low-level resistance due to the presence of plasmid-mediated quinolone resistance (PMQR) mechanisms (133).
Tumbarello et al. found that 8 of 16 patients with BSI due to ESBL-E who were treated with ciprofloxacin died. The MICs of ciprofloxacin for all these patients were 0.5 to 1 mg/liter (134). Endimiani et al. described worse results with ciprofloxacin than with imipenem for a small cohort of patients with BSI due to TEM-52-producing K. pneumoniae, which was associated with the fact that the MICs of ciprofloxacin were frequently higher than 0.25 mg/liter (135). According to these data, the current EUCAST susceptibility breakpoint (≤0.25 mg/liter) seems to be more appropriate than the breakpoint of ≤1 mg/liter recommended by CLSI, at least for ESBL-E. The study by Palacios-Baena et al. mentioned above, which examined the outcomes for patients with BSI due to ESBL-E who were treated empirically with active drugs other than BLBLIs or carbapenems, included 19 patients treated with a fluoroquinolone as the only active drug according to CLSI breakpoints, and the mortality rate was 10.5%, similar to that for patients treated with carbapenems (106).
With respect to the impact of PMQR mechanisms, data from an animal model suggest a reduced efficacy of ciprofloxacin or levofloxacin. The presence of qnr genes also increased the mutant prevention concentration (MPC) (136–141). The clinical impact of PMQR mechanisms has been studied in only a few observational studies, with discrepant results. The available data are difficult to interpret, as many isolates also had other mechanisms of resistance and a small number of patients were treated with quinolones (142–144). However, because PMQR (particularly qnr genes) is common in ESBL-E (133), caution is needed in treating patients with quinolones, particularly using CLSI breakpoints.
A small proportion of ESBL-E isolates are susceptible to trimethoprim-sulfamethoxazole. Although no clinical studies specifically investigating the efficacy of this drug were found, the results are expected to be similar to those for non-ESBL producers, and it may therefore be an option mainly for cUTI.
THERAPY AGAINST CARBAPENEM-RESISTANT ENTEROBACTERIACEAE
CRE may arise due to carbapenemase production (currently the most frequent mechanism) or to the combination of permeability problems with production of other β-lactamases, such as ESBLs or AmpC (145–152). Carbapenemases are rapidly spreading worldwide and fall into 3 main groups: KPC enzymes, belonging to Ambler class A; MBLs, belonging to molecular class B and including NDM, VIM, and IMP enzymes, among many others; and OXA enzymes, belonging to class D (in Enterobacteriaceae, OXA-48 is the most prevalent one). Their epidemiology is heterogeneous, and their capacity to hydrolyze carbapenems and other β-lactams is similarly variable (145–147). The most frequent carbapenemase-producing Enterobacteriaceae (CPE) organism so far has been K. pneumoniae, which causes infections predominantly identified as health care-associated infections. The treatment options against these infections are very limited. The most frequently used active antimicrobials so far have been “second-line” agents, including polymyxins, tigecycline, fosfomycin, and (occasionally) aminoglycosides (145–152). Some isolates are susceptible to minocycline, doxycycline, chloramphenicol, trimethoprim-sulfamethoxazole, and temocillin (152–156). The new β-lactamase inhibitors, avibactam and vaborbactam, inhibit KPC (avibactam also inhibits OXA-48) but not MBLs (61, 152).
Because the options are so limited, all potentially active drugs should be tested in vitro. For many patients, it is necessary to create individualized antibiotic therapy regimens in line with the source and severity of infection, susceptibility testing data, and information available from in vitro, in vivo, and clinical studies (see below) (149, 157). Dose modification may also be necessary (Table 2). As with all pathogens, careful evaluation of the clinical significance of a CRE isolate is assumed in order to prevent unnecessary treatment (154). A summary of recommendations for regimens to be considered in the treatment of CRE according to the data presented in the following subsections is found in Table 3. It should be noted that many carbapenemase producers also coproduce ESBLs, and the impact of the production of both enzymes on treatment is not well established.
TABLE 2.
Recommended dosing for the most frequently used drugs against carbapenem-resistant Enterobacteriaceae (CRE) for patients with normal renal functiona
| Drug | Usual/standard dose(s) | Dosing for CRE and comments |
|---|---|---|
| Meropenem | 1 g/8 h | 2 g/8 h by EI (isolates with MICs of 2–8 mg/liter; for isolates with higher MICs, it is probably not efficacious) |
| Ertapenem | 1 g/24 h | Consider 2 g/day for double-carbapenem regimens |
| Colistinb | From the EMA, loading dose, 6–9 MU, and then 9 MU/day in 2–3 doses; from the FDA, 2.5–5 mg of colistin base activity/kg/day | EMA dose is recommended for severe CRE infections; the need for a loading dose and high continuation dose in patients without severe infection/shock is controversial |
| Polymyxin Bc | From the FDA, 1.5–2.5 mg/kg/day in 2 doses | For mild infections and isolates with MICs of ≤1 mg/liter, the FDA dose is probably appropriate; for severe infections and isolates with MICs of up to 4 mg/liter, a loading dose of 2–2.5 mg/kg followed by 3 mg/kg/day in 2 doses is recommended (controversially) |
| Tigecycline | 100-mg loading dose and then 50 mg/12 h | For HAP, cUTI, BSI, or shock, consider a 200-mg loading dose and then 100 mg/12 h |
| Gentamicin, tobramycin | 5–7 mg/kg/day | For HAP or shock without other options, higher doses (10–15 mg/kg) might be considered, but the risk of toxicity is high; TDM is recommended |
| Amikacin | 15–20 mg/kg/day | For HAP or shock without other options, higher doses (25–30 mg/kg) might be considered, but the risk of toxicity is high; TDM is recommended |
| Fosfomycin | 4 g/6 h to 8 g/8 h | Use in combination; high sodium concn |
| Temocillin | 2 g/8–12 h | KPC producers are occasionally susceptible; continuous infusion improves PK-PD target attainment |
| Aztreonam | 1–2 g/8 h | MBL producers are susceptible if they are not ESBL or AmpC producers |
| Ceftazidime | 1–2 g/8 h | OXA-48 producers are susceptible if they are not ESBL or AmpC producers |
| Ceftazidime-avibactam | 2.5 g/8 h | KPC and OXA-48 producers are frequently susceptible |
| Meropenem-vaborbactam | 2/2 g/8 h | KPC producers are frequently susceptible |
Please refer to the text for explanations and references. EI, extended infusion; EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; HAP, hospital-acquired pneumonia; cUTI, complicated urinary tract infection; BSI, bloodstream infection; MU, million units; TDM, therapeutic drug monitoring; MBL, metallo-β-lactamase.
One million units of colistimethate sodium = 80 mg colistimethate sodium = 34 mg of colistin base activity.
One million units of polymyxin B = 100 mg of colistin base activity.
TABLE 3.
Summary of recommended regimens for treatment of infections caused by carbapenem-resistant Enterobacteriaceaea
| Risk level, therapy type, and isolate susceptibility | Drugs |
|---|---|
| High risk,b combination therapy | |
| Susceptible to a β-lactam (use according to susceptibility) | Backbone: ceftazidime-avibactam (preferred) or meropenem-vaborbactam; alternatively, meropenem (if MIC is ≤8 mg/liter) or ceftazidime or aztreonam |
| Accompanying drug (no data available about the need for combination therapy if ceftazidime-avibactam or meropenem-vaborbactam is used as the backbone): colistin, tigecycline, aminoglycoside, or fosfomycin (if isolate is intermediate to the backbone drug, consider using 2 of these) | |
| Resistant to all β-lactams (including isolates with meropenem MICs of >8 mg/liter), susceptible to at least 2 drugs, including colistin | Backbone: colistin |
| Accompanying drug: tigecycline, aminoglycoside (high risk of nephrotoxicity), or fosfomycin | |
| Resistant to all β-lactams and colistin, susceptible to at least 2 drugs | Backbone: tigecycline or aminoglycoside |
| Accompanying drug: tigecycline or aminoglycoside, fosfomycin | |
| Pandrug-resistant or susceptible to only one drug | Meropenem plus ertapenem or ceftazidime-avibactam plus aztreonam; add any active drug; consider active investigational drugs if available; consider in vitro testing of combinations for synergy |
| Low risk,c monotherapy | |
| According to susceptibility | Ceftazidime-avibactam, meropenem-vaborbactam, meropenem, ceftazidime, aztreonam, colistin, tigecycline, aminoglycoside (if intermediate susceptibility, choose another option or use combination) |
Close clinical and microbiological follow-up is needed. If any of the following is needed, consider the source: colistin, preferred over other accompanying drugs in cases of HAP/VAP; tigecycline, to be considered mostly for cIAI (if used for HAP, BSI, or cUTI, consider double dosing); aminoglycoside, to be considered mostly for cUTI (if needed for HAP, consider a high dose), and TDM is recommended; fosfomycin, to be considered mostly for cUTI but, if needed, also as a third drug for any source. For cIAI, consider adding metronidazole except for with meropenem and tigecycline. It may be wise to reserve the newer drugs (ceftazidime-avibactam and meropenem-vaborbactam) for high-risk patients whenever possible. HAP, hospital-acquired pneumonia; cIAI, complicated intra-abdominal infection; cUTI, complicated urinary tract infection; TDM, therapeutic drug monitoring.
High risk is defined as having septic shock or, for bloodstream infections, an INCREMENT mortality score of ≥8 points (severe sepsis or shock at presentation, 5 points; Pitt score of ≥6, 4 points; Charlson index of ≥2, 3 points; and source of infection other than urinary or biliary tract, 3 points).
Low risk is defined as having an INCREMENT mortality score of <8 points.
Monotherapy versus Combination Therapy
As the efficacy of some frequently in vitro-active drugs against CPE in monotherapy, such as the polymyxins, tigecycline, or fosfomycin, is doubtful (see below), the use of combination therapy for the management of infections caused by these organisms has been explored with the objective of investigating the potential synergistic or additive effects of certain combinations of antimicrobials. Many in vitro studies and some in vivo studies have investigated the effects of double and triple combinations of drugs with different mechanisms of action (158–210). A systematic review of studies of in vitro synergy of polymyxins and carbapenems showed synergy against 50% of carbapenem-resistant isolates (95% CI = 30 to 69%) in time-kill studies (less when the checkerboard or Etest method was used). Combinations were also associated with less development of resistance to colistin in vitro, but data about carbapenems were not provided (211). Antagonism was infrequent. Overall, KPC-producing K. pneumoniae was studied most often. Some conclusions that can be drawn from the data in these studies are as follows: (i) it is difficult to extrapolate findings due to heterogeneity in methodologies, overrepresentation of KPC producers, concurrent mechanisms of resistance, bacterial species, clones, susceptibilities of isolates, and concentrations of antimicrobial agents tested; (ii) the effects of the most frequently tested combinations varied widely; (iii) triple combinations (colistin with carbapenem and rifampin or tigecycline, colistin with double carbapenems) seemed to provide synergistic effects more frequently, although these were less frequently studied, with diverse effects in different strains; (iv) the synergistic effects of combinations including meropenem were more frequent when the MIC was ≤16 mg/liter; and (v) combinations including colistin and rifampin (with or without carbapenems) were frequently synergistic against colistin-resistant isolates. Individual testing to guide therapy in cases with very limited options is desirable, but delays in providing results, the intrinsic difficulties of such studies, and a lack of evidence of clinical correlation should be taken into account.
No RCT were found that compared combination therapy with monotherapy for patients with CPE infections. Designing such a trial would be complex because of the heterogeneous susceptibilities. Observational studies comparing the outcomes for patients treated with monotherapy or combination therapy were reviewed, and control for confounders was taken into account. Most studies focused on or supplied data for BSI (212–229) (Table 4), while others included other types of infection (213, 215, 227, 230–236) (Table 5). Systematic reviews published in 2014 found major limitations in the studies analyzed and therefore could not draw strong conclusions (47, 237). Another systematic review and meta-analysis of infections due to carbapenem-resistant bacteria (not just Enterobacteriaceae) found lower mortality with colistin combinations than with colistin monotherapy, although again, the authors drew attention to the limitations of the studies (238).
TABLE 4.
Observational studies providing comparative data on monotherapy and combination therapy for bloodstream infections due to carbapenem-resistant Enterobacteriaceaea
| Reference | Design, no. of sites | Included infections | Carbapenemase(s) | Mortality definition | No. of deaths/no. of patients treated with MT (%) | No. of deaths/no. of patients treated with CT (%) | CT protective or not; adjusted OR (95% CI) for mortality with CTb |
|---|---|---|---|---|---|---|---|
| 212 | Retrospective, 1 site (Turkey) | BSI due to CRE | Mostly K. pneumoniae OXA-48 | 28-day | 2/5 (40) | 16/31 (51.5) | MV analysis not performed |
| 213 | Prospective, 9 sites (Italy) | BSI due to ERTr K. pneumoniae (BSI subanalysis) | Mostly KPC | In-hospital | 4/9 (44.4) | 11/25 (44) | CT not protective (OR not provided) |
| 214 | Retrospective, 2 sites (Greece) | BSI due to CR K. pneumoniae | Mostly KPC, some VIM | 28-day | 32/72 (44.4) | 28/103 (27.2) | CT protective; 0.48 (0.28–0.81) |
| 215 | Retrospective, 3 sites (Brazil) | Infections due to KPC-producing K. pneumoniae (BSI subanalysis) | KPC | 30-day | 15/34 (44.1) | 24/44 (54.4) | CT not protective (OR not provided) |
| 216 | Retrospective, 2 sites (USA) | BSI due to CR K. pneumoniae | Most (probably) KPC | 30-day | 18/68 (26.4) | 28/73 (38.3) | CT not protective; with BL, 1.8 (0.6–5.6); without BL, 1.1 (0.3–3.6) |
| 217 | Retrospective, 16 sites (worldwide) | BSI due to CPE | 74% KPC | 30-day | 85/208 (40.9) | 47/135 (34.8) | CT protective only in high-risk patients; 0.54 (0.32–0.89) |
| 218 | Prospective, 1 site (Spain) | BSI due to KPC-producing K. pneumoniae, COLr | KPC | 30-day | 14/32 (43.8) | 18/72 (25) | CT protective in septic shock |
| 219 | Retrospective, 1 site (India) | Children, BSI due to CRE; includes inactive drugs | 66% K. pneumoniae, 72% NDM | 30-day | Not specified | Not specified | Crude OR = 0.23 (0.05–1.0); MV analysis not performed |
| 220 | Retrospective, 1 site (Spain) | BSI due to OXA-48 producers | OXA-48 | 30-day | 2/7 (28.5) | 13/27 (48.1) | MV analysis not performed |
| 221 | Retrospective, 1 site (Greece)c | BSI due to CS and CR K. pneumoniae in ICU | Mostly KPC | 30-day | 18/57 (31.5) | 7/38 (18.4) | CT protective; 0.23 (0.07–0.75); also with shock |
| 222 | Retrospective, 2 sites (USA) | BSI due to KPC-producing K. pneumoniae; includes inactive drugs | KPC | 28-day | 11/19 (57.8) | 2/15 (13.3) | CT protective; 0.07 (0.009–0.71) |
| 223 | Retrospective, 8 sites (USA) | BSI due to CRE | Mostly KPC | 30-day | 21/55 (38.1) | 22/43 (51.1) | CT not protective (OR not provided) |
| 224 | Retrospective, 4 sites (Greece) | BSI due to CR K. pneumoniae, neutropenic patients | Mostly KPC | 14-day | 5/10 (50) | 11/30 (36.6) | CT protective; 0.25 (0.07–0.81) |
| 225 | Prospective, 13 sites (Italy) | BSI due to CR K. pneumoniae, hematological patients | Not identified | 21-day | 69/77 (89.6) | 40/72 (55.5) | CT protective; 0.52 (0.35–0.77) |
| 226 | Retrospective, 3 sites (Italy) | BSI due to KPC-producing K. pneumoniae | KPC | 30-day | 25/46 (54.3) | 27/71 (34.1) | CT with COL plus TIG-MER protective; 0.11 (0.02–0.60) |
| 227 | Retrospective, 5 sites (Italy)d | Infections due to KPC-producing K. pneumoniae (BSI subanalysis) | KPC | 30-day | 80/156 (51.3) | 93/291 (32) | MV analysis not performed for BSI |
| 228 | Retrospective, 11 sites (South America) | BSI due to CRE | Mostly KPC | 28-day | 5/8 (62.5) | 17/29 (58.6) | MV analysis not performed |
| 229 | Retrospective, 1 site (Greece) | BSI due to KPC-producing K. pneumoniae | KPC | Infection related | 7/15 (46) | 0/20 (0) | CT not included in MV |
Superiority of specific combinations or drugs in monotherapy was not evaluated and cannot be discarded. MT, monotherapy; CT, combination therapy; OR, odds ratio; CI, confidence interval; BSI, bloodstream infection; CRE, carbapenem-resistant Enterobacteriaceae; MV, multivariable; ERTr, ertapenem resistant; CR, carbapenem resistant; BL, β-lactam; CPE, carbapenemase-producing Enterobacteriaceae; COLr, colistin resistant; CS, carbapenem susceptible; TIG-MER, tigecycline-meropenem.
When the adjusted OR was provided for MT (with CT as a reference), the inverse was calculated.
Patients with carbapenem-susceptible and -resistant K. pneumoniae isolates are compared in this study; here we extracted the data for carbapenem-resistant isolates only.
Includes patients from reference 225.
TABLE 5.
Observational studies providing comparative data for monotherapy and combination therapy for different types of infection due to carbapenem-resistant Enterobacteriaceaea
| Reference | Design, no. of sites | Included infections | Carbapenemase(s) | Mortality definition | No. of deaths/no. of patients treated with MT (%) | No. of deaths/no. of patients treated with CT (%) | CT protective or not; adjusted OR (95% CI) for mortality with CTb |
|---|---|---|---|---|---|---|---|
| 213 | Prospective, 9 sites (Italy) | Infections due to ERTr K. pneumoniae | Mostly KPC | In-hospital | 8/37 (21.6) | 17/54 (31.4) | CT not protective (OR not provided) |
| 215 | Retrospective, 3 sites (Brazil) | Infections due to KPC-producing K. pneumoniae | KPC | 30-day | 21/57 (36.8) | 32/61 (52.4) | CT not protective (OR not provided) |
| 227 | Retrospective, 5 sites (Italy)c | Infections due to KPC-producing K. pneumoniae | KPC | 30-day | 118/307 (38.4) | 107/354 (30.2) | CT protective; 0.52 (0.35–0.77) |
| 230 | Retrospective, 17 sites (Taiwan) | ICU infections due to CR K. pneumoniae/E. coli | Mostly AmpC/ESBL plus porin loss | 30-day | 7/23 (30.4) | 5/10 (50) | CT not protective (OR not provided) |
| 231 | Prospective, 1 site (Brazil) | Infections due to CRE | Mostly KPC | Infection related | 6/29 (20.6); for UTI, 6/28 (21.4) | 38/78 (38.7); for UTI, 6/23 (26) | CT not protective (OR not provided) |
| 232 | Retrospective, 1 site (Colombia) | Children, CR K. pneumoniae infections | Not studied | Not specified | 2/19 (10.5) | 9/22 (40.9) | MV analysis not performed |
| 233 | Retrospective, 1 site (Brazil) | HAI due to KPC-producing K. pneumoniae, cancer patients | KPC | 30-day | 8/22 (36.6) | 21/38 (55.2) | CT not protective (OR not provided) |
| 234 | Prospective, 1 site (Greece) | CR K. pneumoniae infections, ICU | KPC | Infection related | Not specified | Not specified | CT not protective (OR not provided) |
| 235 | Retrospective, 1 site (South Africa) | Infections due to OXA-48 producers | OXA-48 | In-hospital | 2/6 (33.3) | 5/13 (38.4) | MV analysis not performed |
| 236 | 2 sites (Brazil) | VAP due to CRE | Not specified | 30-day | 40/66 (60.6) | 6/17 (35.2) | CT not protective (OR not provided) |
Superiority of specific combinations or drugs in monotherapy cannot be discarded. MT, monotherapy; CT, combination therapy; OR, odds ratio; CI, confidence interval; ERTr, ertapenem resistant; CR, carbapenem resistant; ESBL, extended-spectrum β-lactamase; CRE, carbapenem-resistant Enterobacteriaceae; UTI, urinary tract infection; MV, multivariable; ICU, intensive care unit; VAP, ventilator-associated pneumonia; HAI, health care-associated infections.
When the adjusted OR was provided for MT, the inverse was calculated.
Includes patients from reference 225.
Some important methodological issues should be taken into account in analyzing these data. First, the impact of combination therapy is evaluated mostly as targeted therapy and therefore has a risk of survivor bias and confounding by indication. Second, the definitions of exposure to different therapy regimens are heterogeneous, including diverse criteria for number of days from onset of infection to initiation of treatment, duration of treatment, and inclusion of inactive drugs in combination regimens in some studies, as well as different criteria for considering antibiotics to be active (for example, EUCAST versus CLSI breakpoints and susceptible versus nonresistant status). Third, the drugs used are diverse, and therefore it is frequently impossible to evaluate whether specific combinations or drugs in monotherapy are better than others. Fourth, in many studies, the sample size is very limited. Finally, control for confounders is also frequently insufficient.
The most frequent type of bacteria included were KPC-producing K. pneumoniae, although some studies included mainly OXA-48 producers (212, 220, 235), NDM producers (219), or noncarbapenemase producers (230). Some studies focused on specific populations, such as intensive care unit (ICU) patients (221, 230), hematological or cancer patients (224, 225, 233), or children (219, 232). As Tables 4 and 5 show, some studies found combination therapy to be associated with lower mortality rates, while others did not. An analysis of the largest study of BSI, to date (217), showed that using more than one active drug had a protective effect on mortality only in the subset of patients with a high probability of dying (but not in the others) according to the validated INCREMENT CPE mortality score, which includes presentation with severe sepsis or shock, ≥6 points on the Pitt score, ≥2 points on the Charlson index, and a source of BSI other than the urinary or biliary tract (239). The results were corroborated by propensity score matching. Two previous studies found that combination therapy was protective, in a stratified analysis of patients with rapidly fatal underlying diseases or with septic shock (214) and in patients with BSI with a non-UTI source (227). Another study found that combination therapy was associated with lower mortality in patients with septic shock related to BSI due to colistin-resistant, highly carbapenem-resistant, KPC-producing K. pneumoniae (218).
These data suggest that combination therapy may be beneficial for high-risk patients, depending on the underlying situation, source of infection, and presence of septic shock, and also suggest that monotherapy may be enough for lower-risk patients. Note that ceftazidime-avibactam or meropenem-vaborbactam was not used in these studies, and therefore whether combination therapy is needed with these compounds is unknown. More studies are needed for isolates producing MBLs or OXA-48 enzymes and for CRE infections not caused by carbapenemases. The subsections below comment on the use of specific drugs. Curiously, rifampin was not included in the combinations studied despite the fact that several in vitro studies suggested a potential synergy with colistin, as mentioned above. In an RCT comparing colistin and rifampin with colistin monotherapy for serious infections caused by XDR A. baumannii, the combination was not found to provide any obvious benefit (240). The colistin dose in that study was lower than the one presently recommended, and the results cannot be extrapolated to Enterobacteriaceae.
Carbapenems for Treatment of CPE Infections
Carbapenemase activity against carbapenems varies according to the enzyme, and probably the expression levels of carbapenemase genes (145, 149, 241). Some CPE are in fact susceptible to carbapenems according to the susceptibility breakpoints currently recommended by CLSI (≤1 mg/liter for meropenem, imipenem, and doripenem and ≤0.5 mg/liter for ertapenem) (67) and EUCAST (≤2 mg/liter for imipenem and meropenem, ≤1 mg/liter for doripenem, and ≤0.5 mg/liter for ertapenem) (66). This is particularly frequent in OXA-48 producers, as noted in several outbreaks (220, 242). Stochastic modeling data suggest that the probability of reaching the target pharmacodynamic parameter is around 80% for isolates with a MIC of 8 mg/liter if meropenem is administered at 2 g every 8 h by extended infusion (243, 244).
This led to the consideration of carbapenems for treatment of infections with CPE isolates showing susceptibility or low-level resistance to these drugs. There are limited data available for carbapenems as monotherapy. Data from 22 articles analyzing the efficacy of imipenem or meropenem in relation to the MIC found that the clinical cure rate was 69% for isolates with a MIC of 4 mg/liter (32 patients) and 29% for isolates with MICs of >8 mg/liter (7 patients) (244). Efficacy for isolates with a MIC of 4 mg/liter was similar to that for patients with infections due to non-carbapenemase-producing strains. The available information is too limited to recommend carbapenems as monotherapy against carbapenem-susceptible CPE, but carbapenems may be an option for infections that are easy to treat (such as UTI). For isolates with higher MICs or other types of infections, we suggest an alternative drug or a combination therapy (see below).
The use of carbapenems in combination with other drugs has been evaluated in retrospective cohort studies. Some found that adding meropenem at high doses (2 g every 8 h by extended infusion) to another active drug was associated with lower mortality among patients with BSI (214, 226) or diverse types of infections (227) caused by CPE when the MIC was ≤8 mg/liter. Other studies found that the addition of a carbapenem conferred no advantage for patients with BSI (216, 217), and a recent study found that treatment with meropenem at a high dose was independently associated with lower mortality in patients with carbapenem-resistant K. pneumoniae even in the case of isolates with MICs of ≥16 mg/liter (245). In all these studies, the predominant CPE was KPC-producing K. pneumoniae. The reasons for the discrepancies between studies are not clear. Inherent variability in determining MIC may have some influence. With the available information, if ceftazidime-avibactam or meropenem-vaborbactam cannot be used, it would be prudent to consider adding meropenem (using optimized dosing) to another active drug for patients with severe sepsis or shock if the MIC is ≤8 mg/liter, particularly if other in vitro-active drugs are not appropriate for the source of infection (for example, tigecycline for cUTI and tigecycline or aminoglycosides for ventilator-associated pneumonia) or if other combinations carry a high risk of toxicity (for example, colistin and aminoglycosides). It is not clear if carbapenems would also be beneficial in cases of CPE caused by MBLs, OXA-48, or other causes of carbapenem resistance. Some animal model studies did not find that carbapenems had the same efficacy against isolates with similar MICs but different mechanisms of resistance to carbapenems (246, 247), which argues against directly extrapolating the results obtained with KPC producers to other mechanisms of resistance. It should also be pointed out that use of carbapenems may theoretically facilitate the emergence of higher levels of carbapenem resistance due to permeability problems or increased expression of carbapenemases. Hence, it is worth studying carbapenem-sparing regimens.
Double Carbapenems
KPC exhibits a greater affinity for ertapenem than for other carbapenems (248), which led to the hypothesis that use of ertapenem might allow the other carbapenem to act. This seems to work in vitro only if the meropenem MIC is ≤128 mg/liter (249–253), and not for all strains (203). Some small, noncomparative case series have shown promising results (250–253). Ertapenem and meropenem have been found to be synergistic in vitro against other types of carbapenemase-producing Enterobacteriaceae (209). A comparison of 28-day mortality was carried out recently between ICU patients with carbapenem-resistant K. pneumoniae infections (90% were KPC producers) who received double carbapenems, with ertapenem as targeted therapy (48 patients; 35 of these received a third drug), and 96 patients who received other treatment regimens (52 received a combination of drugs) (254). Patients in both treatment arms were matched by SAPS-II score at admission and SOFA score at diagnosis of infection. Half the patients had pneumonia. In a multivariate analysis, double-carbapenem therapy was associated with lower mortality (adjusted OR = 0.33; 95% CI = 0.13 to 0.87), and among the patients treated with double carbapenems, 66% had XDR isolates. Because of significant potential negative ecological effects, and until more data are available, this combination should be considered only when there are no other reasonable options.
Polymyxins
Polymyxins are cationic polypeptide antibiotics, and only polymyxin B and polymyxin E (colistin) are used in clinical practice (255–257). Polymyxins are active against Enterobacteriaceae, except for Proteus spp., Serratia spp., Morganella spp., and Providencia spp. They have been a cornerstone in the management of infections due to CRE in the past, mostly because of being the last resort against these bacteria on many occasions. There is more clinical information available on colistin.
Whether colistin as monotherapy is as efficacious as the so-called first-line drugs against susceptible Enterobacteriaceae (β-lactams and fluoroquinolones) is a matter of controversy. Direct comparisons in observational studies are challenged because patients treated with colistin usually have carbapenem-resistant Enterobacteriaceae and are frequently more seriously ill. A systematic review including mostly patients with MDR P. aeruginosa and A. baumannii found higher mortality and toxicity for patients treated with colistin than for those treated with other drugs, mostly β-lactams (258), although similar data for Enterobacteriaceae are scarce. A randomized trial comparing colistin with meropenem (both combined with levofloxacin) in patients with ventilator-associated pneumonia is under way (259).
With regard to the question of whether colistin is more effective in combination with other drugs, apart from the general information provided above, Hirsch and Tam reviewed 15 articles including 55 patients with KPC-producing K. pneumoniae infections treated with colistin and found that colistin was less effective as monotherapy than in combination (260). In a meta-analysis of infections due to carbapenem-resistant bacteria, Zusman et al. found that polymyxin monotherapy was associated with higher mortality than that with colistin combinations, although the authors drew attention to significant limitations of the studies (238). In the INCREMENT cohort, colistin monotherapy was associated with increased mortality compared to that with combinations including tigecycline, colistin, and carbapenems (217). Overall, the drugs most frequently combined with colistin have been carbapenems, tigecycline, aminoglycosides, and fosfomycin. The potential additive nephrotoxicity of colistin and aminoglycosides is a concern. Two randomized controlled trials comparing colistin versus colistin plus meropenem are being performed; one of them includes patients with severe infections due to carbapenem-resistant Gram-negative bacteria (261), and the other includes patients with BSI or pneumonia due to XDR Gram-negative bacilli (https://clinicaltrials.gov/ct2/show/NCT01597973).
The most appropriate dose of colistin is also controversial. Colistin is administered as a prodrug (colistimethate sodium) that needs to be converted to the active drug. The previous standard dosage regimen recommended for colistin is now considered insufficient by most authors (Table 2) (262–265), and administration of a loading dose followed by a high maintenance dose has been suggested based on pharmacokinetic-pharmacodynamic models (263–266). The European Medicines Agency recommends a 9-million-unit (MU) loading dose for critically ill patients, followed by 9 MU/day in 2 or 3 doses (267). The FDA, however, makes no recommendation about loading dose and recommends 2.5 to 5 mg/kg of body weight/day of colistin base activity for patients with normal renal function (34 mg of colistin base activity = 1 MU) (268). Whether use of a loading dose and higher daily doses is associated with improved efficacy is again controversial. No comparative randomized trials have been found, although several observational or quasi-experimental studies with discrepant results have been published (269–276). In most studies, renal toxicity was more frequent with higher doses. It should also be pointed out that most studies included not only CRE but also P. aeruginosa and A. baumannii. A small randomized trial compared the rates of nephrotoxicity of colistin administered as a 9-MU loading dose followed by 4.5 MU every 12 h or as 2 MU every 8 h (20 patients in each arm); the rates of acute kidney injury based on RIFLE criteria were 60% and 15%, respectively (P = 0.003) (277).
Dosing regimens are not well established for polymyxin B either. Polymyxin B is administered as an active drug and therefore does not need in vivo conversion to be active. In a retrospective cohort study of 151 patients with BSI due to carbapenem-resistant Gram-negative bacteria (102 isolates were Enterobacteriaceae), a dosing regimen of <1.3 mg/kg/day was independently associated with higher mortality (278), thus supporting the standard recommendation of administering 1.5 to 2.5 mg/kg/day in 2 doses. In a multivariate analysis in that study, a daily dose of ≥250 mg was associated with acute kidney injury. However, a population pharmacokinetic study of critically ill patients suggested the use of 3 mg/kg/day in patients with severe infections and isolates with MICs of ≤2 mg/liter (279). Stochastic modeling also suggests the importance of administering a loading dose of polymyxin B (278, 279).
Regarding comparative data on colistin and polymyxin B, the available data suggest that colistin is associated with a higher risk of nephrotoxicity than polymyxin B (280, 281). However, no clear differences in clinical benefits (including cure rates or mortality) have been demonstrated for one over the other so far (281, 282). Dose adjustment is recommended for both drugs in patients with renal insufficiency, according to the FDA label; however, since exposures to polymyxin B are similar in patients with and without renal insufficiency and clearance of the drug is not affected by renal function, the dosing of this drug should probably not be adjusted according to renal function (278, 283, 284).
Resistance to polymyxins is increasing, with outbreaks of colistin-resistant CRE reported in different parts of the world (285–289). Colistin resistance has been associated with an increased risk of mortality (213, 290, 291), and exposure to colistin has been identified as a risk factor for infections due to colistin-resistant CPE (288). More recently, plasmid-mediated resistance (mediated by mcr genes) was discovered (292). While the association between mcr genes and carbapenemase production is anecdotal so far (293–298), the association of these genes with successful mobile genetic elements or clones, together with the use of colistin in veterinary and human medicine leading to increased selection pressure, is a cause for concern. Furthermore, susceptibility testing with colistin is problematic: broth microdilution methods are recommended because diffusion tests are not reliable (66), and semiautomated methods may cause very major errors (as explained at http://www.eucast.org/ast_of_bacteria/warnings/ [accessed 22 October 2017]). The EUCAST breakpoint for colistin susceptibility (which is also the epidemiological cutoff value) is ≤2 mg/liter (66); CLSI does not provide breakpoints for polymyxins and Enterobacteriaceae (67).
In summary, polymyxins are still frequently key drugs for the treatment of CRE, but their actual efficacy and optimal dosing are not well defined; combination therapy is probably beneficial for high-risk patients.
Tigecycline
Tigecycline frequently remains active against CRE in vitro (113, 119, 145, 148–150). As mentioned in the section on treatments for infections with ESBL- and AmpC-producing Enterobacteriaceae, tigecycline is recommended only when other options are unavailable or unsuitable, which is often the case for infections due to CRE. A recent meta-analysis reviewed 21 studies comparing outcomes associated with tigecycline versus other antimicrobial agents used for treatment of CRE infections (299). No significant differences in patient mortality were found between patients treated with tigecycline and those treated with other antibiotics. In subgroup analyses, tigecycline in combination was associated with lower mortality. The analysis was limited by the heterogeneity of the studies, types of infection, and comparators.
The problem of the lower efficacy of tigecycline has been linked to dosage (396). The concentrations reached at sites of infection may be lower than desired with the standard dose (100-mg loading dose and then 50 mg/12 h) (202), particularly in cases of HAP (300) and despite the fact that the drug is concentrated in the tissues. Tigecycline concentrations in blood are also low (113), which raised doubts early on about its efficacy in bacteremic infections. A meta-analysis including mostly observational studies found no significant differences in mortality and higher rates of clinical cure for patients with BSI treated with tigecycline than those treated with other regimens, although the studies were heterogenenous with respect to design, type of infection, microorganism, comparators, and dosing (301). In subgroup analysis, monotherapy wth tigecycline was associated with higher mortality than that with combination therapy.
In a phase 2 randomized trial for treatment of HAP, patients were randomized to receive 150 mg of tigecycline followed by 75 mg/12 h (36 patients), 200 mg of tigecycline followed by 100 mg/12 h (35 patients), or imipenem-cilastatin at 1 g/8 h (34 patients) (302). The clinical cure rate was higher for patients receiving the highest dose of tigecycline than those receiving the lower dose, but the study was underpowered to detect superiority. The rates of serious adverse events were similar across groups, but diarrhea, nausea, and vomiting were more frequent at the highest tigecycline dose. A systematic review carried out in 2014 found three other observational studies that compared the outcomes for patients with infections caused by Gram-negative pathogens (mostly MDR) who received the standard dose of 50 mg/12 h and those receiving 100 mg/12 h (303). Two of these studies, performed on ICU patients, found better results with the high-dose regimen, and for the subset of patients with VAP in one of them (304, 305), and one did not (306). A recent case series of ICU patients showed a decrease in plasma fibrinogen levels and a prolongation of international normalized ratio (INR) and activated partial thromboplastin time (aPTT) values during high-dose tigecycline treatment in ICU patients (307).
Tigecycline concentrations in urine are also low, and the drug has not been evaluated in an RCT for UTI. Since UTI is a frequent type of infection among patients with CRE (308), tigecycline has been used anecdotally, sometimes with apparently good results (309). However, it is associated with a lower rate of clearance of carbapenem-resistant K. pneumoniae in patients with bacteriuria or UTI than that with aminoglycosides (310, 311) and therefore does not seem to be the best option for this type of infection.
Since tigecycline remains active against a significant proportion of CRE isolates, it might be useful as part of the treatment regimens against many infections caused by these pathogens. Higher doses may be considered for severe infections with very limited options, particularly pneumonia and BSI.
Aminoglycosides
General aspects of aminoglycoside use were discussed previously, in the section on ESBL- and AmpC-producing Enterobacteriaceae. A variable, occasionally large proportion of CRE isolates are susceptible to some members of the aminoglycoside family, except for isolates producing 16S rRNA methyltransferases, which confer resistance to all aminoglycosides (312). These acquired enzymes are particularly frequent among NDM producers and are increasingly being described for KPC producers (312–314).
Aminoglycosides have been used both alone and in combination (more frequently) in the management of infections caused by CRE; indeed, aminoglycosides are often part of the combination therapies listed in the studies in Tables 4 and 5. The aminoglycosides have been found to be independently associated with higher rates of clearance of carbapenem-resistant K. pneumoniae from urine than those for tigecycline and polymyxin B (310); it should be noted that concentrations of polymyxin B in urine are low (279), while colistimethate is eliminated in part in the urine, where it is converted to colistin. Studies comparing outcomes for patients treated with and without aminoglycosides are scarce. One study investigated the outcomes for 157 patients with physician-diagnosed UTI due to carbapenem-resistant K. pnuemoniae (mostly KPC producers); treatment with aminoglycosides was associated with a lower probability of failure (other drugs were colistin, tigecycline, trimethoprim-sulfamethoxazole, and fosfomycin) in an adjusted analysis (311). In that study, amikacin was active against 83% of the isolates. A study carried out in Spain included 50 patients with sepsis due to colistin-resistant, clonally related KPC-producing K. pneumoniae isolates and compared 30-day mortality for patients treated with gentamicin (29 patients) and without gentamicin (21 patients) (315). Overall, 48% of patients had HAP and 20% had UTI. In a multivariate analysis, treatment with gentamicin (particularly for isolates with MICs of ≤2 mg/liter) was associated with lower mortality. It should be noted, reflecting the extensive resistance of the isolates, that most patients not receiving gentamicin were considered to have received suboptimal treatment (meaning that the treatment regimen included only drugs with intermediate susceptibility). Crude mortality rates were 7.7% (1/13 patients) and 31.2% (5/16 patients) for isolates with gentamicin MICs of ≤2 mg/liter (susceptible, according to EUCAST) and 4 mg/liter (intermediate), respectively. The gentamicin dose was 4 to 5 mg/kg, with dose adjustment based on therapeutic drug monitoring. It is important that resistance to gentamicin among KPC producers is high in many areas (311).
As with other drugs used in the treatment of CRE infections, the adequacy of the generally recommended dosage for aminoglycosides (5 to 7 mg/kg/day for gentamicin and tobramycin; 15 to 20 mg/kg/day for amikacin) has also been questioned. A study of patients with severe sepsis or septic shock who were treated with amikacin at 25 mg/kg/day showed that only 70% reached peak concentrations of >64 mg/liter, which would be ≥8 times the susceptibility breakpoint against Enterobacteriaceae according to EUCAST (8 mg/liter) (316); the same dose, however, should be enough for isolates with a MIC of 4 mg/liter to reach the same target (317). An initial dose of 2,500 mg followed by therapeutic drug monitoring has been suggested for patients with body weights of >40 kg (318, 319). Importantly, the peak concentration/MIC ratio has been challenged as the only pharmacokinetic-pharmacodynamic target to be considered for aminoglycosides (320). Use of even higher doses in patients with high-flow hemofiltration has been explored for MDR Gram-negative infections with very limited options (321). To investigate the impact of the maximum concentration of drug in serum (Cmax) on mortality, a retrospective observational study including 110 patients with septic shock who received amikacin at 30 mg/kg/day was carried out in two ICUs; around half the patients had infections caused by Enterobacteriaceae (322). Mortality rates were 28.3% for 46 patients reaching Cmax values of 60 to 80 mg/liter, 40% for 20 patients reaching <60 mg/liter, and 58.8% for 44 patients reaching >80 mg/liter. In a multivariate analysis, Cmax values of >80 mg/liter were independently associated with increased mortality (OR = 3.96; 95% CI = 1.54 to 10.2). A randomized trial would be needed to compare the efficacies and safeties of higher doses of aminoglycosides. At this time, we are cautious about recommending them except for patients with septic shock due to CRE infection with very few other available alternatives.
Fosfomycin
Fosfomycin was also reviewed in the section on ESBL- and AmpC-producing Enterobacteriaceae, so only specific information about CRE infections is added here. Fosfomycin is active against a significant proportion of CRE isolates (121, 122, 153, 323) and was therefore frequently included as part of combination therapy in the studies listed in Tables 4 and 5. Since we found no studies with sufficient numbers of patients to compare the outcomes for patients treated with and without fosfomycin, its role as an individual drug is difficult to ascertain. Development of resistance has been described even for its use in combination for infections caused by KPC producers (324). A multicenter case series analyzing 48 patients admitted to the ICU and treated with fosfomycin for XDR, fosfomycin-susceptible pathogens has been reported (325). The predominant infections were VAP and BSI, the median dose was 24 g per day, and the most frequent accompanying drugs were colistin and tigecycline. The 28-day mortality rate was 37.5%, and clinical outcomes were considered successful at day 14 for 54.2% of patients, with failure in 33.3% of patients. Resistance development occurred in 3 cases.
Because of the scarcity of information, fosfomycin is not a first option against serious CRE infections when other active drugs are available, but it may be needed in some patients with scarce options. In such cases, a fosfomycin dose of 16 to 24 g per day in combination is recommended.
β-Lactams Other than Carbapenems: Temocillin for KPC Producers, Aztreonam for MBL Producers, and Cephalosporins for OXA-48 Producers
Temocillin is active against a small proportion of KPC producers, using British Society for Antimicrobial Chemotherapy breakpoints (≤8 mg/liter; ≤32 mg/liter for UTI) (153, 326), and against CRE isolates with combinations of impermeability and ESBL or AmpC production (153). Promising results with temocillin were found in a murine model of intra-abdominal infection against KPC-producing E. coli isolates with temocillin MICs of ≤16 mg/liter (327). Unfortunately, we found no published clinical experiences. OXA-48 producers show high resistance to temocillin, which has been proposed as a diagnostic marker for these enzymes (328, 329).
Aztreonam is not efficiently hydrolyzed by MBLs (145, 149, 150). In an in vitro model, it showed slow bactericidal activity against VIM-1-producing K. pneumoniae (330). Animal model studies showed efficacy against susceptible isolates producing NDM and VIM MBLs (331, 332). The problem here is that a large proportion of MBL producers coproduce ESBLs, thus making them aztreonam resistant (333). Clinical experience is lacking in any case.
The case of OXA-48 is somewhat similar. This enzyme has low hydrolytic activity against cephalosporins and does not confer cephalosporin resistance (334), but most OXA-48 producers are resistant because they are also ESBL producers (242, 335). However, since some OXA-48 producers also show low carbapenem MICs, they may not be detected, particularly if they do not also coproduce an ESBL. Ceftazidime showed significant antibacterial activity in animal models against OXA-48 producers lacking ESBLs or AmpC (336, 337). Again, no clinical experience has been published.
Ceftazidime-Avibactam
As previously reviewed in the section on ESBL- and AmpC-producing Enterobacteriaceae, ceftazidime-avibactam is active in vitro against most KPC- and OXA-48-producing Enterobacteriaceae and some carbapenem-resistant strains due to a loss of impermeability or ESBL or AmpC production at the EUCAST and FDA susceptibility breakpoint (≤8/4 mg/liter) (61, 338–342). Activity against KPC and OXA-48 producers has been confirmed in animal studies (343, 344). Curiously, in a murine thigh infection model, ceftazidime-avibactam (but not ceftazidime alone) also showed efficacy against ESBL- and NDM-producing E. coli and K. pneumoniae isolates highly resistant to ceftazidime-avibactam, suggesting that ceftazidime resistance was due mostly to the ESBL (345). These results were replicated in a murine lung infection model with NDM-, OXA-48-, and CTX-M-producing K. pneumoniae isolates (346). However, data for patients are lacking, and therefore we would not recommend ceftazidime-avibactam for patients infected with MBL-producing Enterobacteriaceae.
There are some published experiences of the treatment of CRE because this combination has been tested in compassionate use programs for the treatment of infections caused by XDR Enterobacteriaceae and recently received approval. We found 7 case series or cohort studies that included 6 to 60 patients with CRE infections who were treated with ceftazidime-avibactam (Table 6) (347–353). Some patients may have been included in more than one of these series. Ceftazidime-avibactam was used as targeted therapy, sometimes as salvage therapy after failure with other drugs. It was administered in combination with other active drugs in 30 to 100% of cases. Mortality rates ranged from 7.6% to 39% for patients with BSI and from 8% to 50% when total infections were considered in each study. Overall, there were no obvious differences in mortality or clinical response between patients treated with monotherapy or a drug combination. In three studies, there was a comparison with patients not treated with ceftazidime-avibactam. The first study included hematological patients with BSI due to CRE and compared 8 patients treated with this combination with 23 treated with other regimens (347). In the crude analysis, clinical cure (but not mortality) was more frequent with ceftazidime-avibactam, although the small patient numbers precluded multivariate analysis. In another study of patients with BSI due to CRE, 13 patients treated with ceftazidime-avibactam were compared to those receiving other regimens (348). Clinical response was more frequent with ceftazidime-avibactam in the adjusted analysis, which was clearly limited because of small numbers. Finally, van Duin et al. used the CRAKCLE prospective cohort data to compared the outcomes for patients with diverse types of infections due to CRE (>95% KPC-producing K. pneumoniae isolates) and treated with ceftazidime-avibactam (38 patients; 39% had BSI and 24% had HAP) or colistin (99 patients; 48% had BSI and 21% had HAP); combination therapy was used in 63% and 94% of patients treated with ceftazidime-avibactam and colistin, respectively (353). Inverse probabilities of treatment weighting-adjusted mortality were 9% and 32%, respectively (absolute difference, 23%; 95% CI, 9 to 35%). At day 30, patients treated with ceftazidime-avibactam had a 64% (95% CI, 57 to 71%) adjusted probability of a better outcome.
TABLE 6.
Case series and cohort studies providing outcome information for infections due to carbapenem-resistant Enterobacteriaceae treated with ceftazidime-avibactama
| Reference | Design, no. of sites; inclusion criteria | n | Types of infections and pathogens | No. of patients treated with CAZ-AVI | No. of patients treated with other regimens | Mortality definition (no. of deaths/no. of patients treated [%] [CAZ-AVI vs other regimens]) | Clinical cure (no. of patients with cure/no. of patients treated [%] [CAZ-AVI vs other regimens]) |
|---|---|---|---|---|---|---|---|
| 346 | Retrospective cohort, hematological patients, 4 sites; BSI due to CRE, ≥48 h of therapy | 31 | ≈85% K. pneumoniae; ≈60% OXA-48 producers and 40% KPC producers; sources: 14 (45.1%) primary, 6 (19.3%) HAP | 8 (all in combination); all isolates susceptible to CAZ-AVI | 23 (17 [94.4%] in combination) | 30-day; 2/8 (25) vs 12/23 (52.2); P = 0.24 | Day 14; 6/8 (75) vs 8/23 (34.8); P = 0.03 |
| 347 | Retrospective cohort, 1 site; BSI due to CR K. pneumoniae, ≥3 days of therapy | 109 | All K. pneumoniae; 97% KPC; 50% in ICU; Source: 50 (45.8%) IAI, 28 (25.6%) primary BSI | 13 (5 [38.5%] in combination); all isolates susceptible to CAZ-AVI | 96 (27 [28.2%] in combination) | 30-day; 1/13 (7.6) vs 30/96 (31.2) | Day 30; 11/13 (85) vs 30/96 (40.6); P = 0.006; adjusted OR = 8.64 (95% CI = 1.61–43.39) |
| 348 | Retrospective, 1 site; CRE infections treated with CAZ-AVI | 37 | 84% K. pneumoniae; 78.3% KPC | 37 (11 [30%] in combination) | Not included | 30-day; 9/37 (24.3) | 23/37 (62); for monotherapy, 58%; for combination therapy, 64%; 10 (27%) recurrences, with 3 isolates developing resistance |
| 349 | Retrospective, 1 site; CRE infections treated with CAZ-AVI | 6 | All K. pneumoniae, KPC; all susceptible to CAZ-AVI | 6 (4 [66.6%] in combination) | Not included | In-hospital; 3/6 (50) | 4/6 (66.6); 2 relapses, no development of resistance |
| 350 | Retrospective cohort, 15 sites; CRE infections treated with CAZ-AVI, salvage therapy | 38 | 34 K. pneumoniae; 23 KPC, 13 OXA-48; type of infection: 15 (39.4%) IAI, 7 (18.4%) HAP | 38 (25 [65.8%] in combination) | Not included | In-hospital; overall, 15/38 (39.5); for IAI, 6/15 (40); for HAP, 5/7 (71.4) | 28 (73.7); for monotherapy, 69.2%; for combination therapy, 76%; 2 relapses, no resistance detected |
| 351 | Retrospective cohort, 9 health care systems in USA; CRE infections treated with CAZ-AVI for ≥24 h | 60 | 83% K. pneumoniae; type of infection: 38% BSI, 28% UTI, 27% HAP | 60 (33 [55%] in combination); of 36 isolates tested, 23 susceptible to CAZ-AVI; treatment started a median of 8 (IQR, 5–22) days after infection | Not included | In-hospital; overall, 19/60 (32); for monotherapy, 30%; for combination therapy, 33%; for BSI, 39%; for UTI, 12%; for pneumonia, 56% | 39/60 (65); for monotherapy, 67%; for combination therapy, 63% |
| 352 | Prospective cohort, 18 hospitals in USA; CRE infections | 137 | 97% K. pneumoniae, 96% KPC-producers; type of infection: 46% BSI, 22% HAP, 14% UTI | 38 (63% in combination) | 99 (94% in combination) | 30-day, adjusted; 8% vs 32% (difference, 23%; 95% CI, 9–35%) | 30-day adjusted probability of better outcome (using desirable outcome ranking), 64% (95% CI, 57–71%) with ceftazidime-avibactam |
CAZ-AVI, ceftazidime-avibactam; BSI, bloodstream infection; CRE, carbapenem-resistant Enterobacteriaceae; HAP, hospital-acquired pneumonia; CR, carbapenem resistant; IAI, intra-abdominal infection; UTI, urinary tract infection; IQR, interquartile range.
In one study, ceftazidime-avibactam resistance developed in 3 of 10 isolates recovered from recurrent infections (349) due to mutations in the blaKPC-3 gene (354). This was also described in another case (355). Curiously, the same mutation restored carbapenem susceptibility in some isolates (354, 355). Acquisition of resistance due to mutations in the blaCTM-M-14 gene conferring augmented ceftazidime hydrolysis has also been described for a K. pneumoniae isolate coproducing OXA-48 (356). The development of resistance to ceftazidime-avibactam should therefore be checked in all cases by performing follow-up cultures. Studies are needed to evaluate the overall rate of this phenomenon and whether it is associated with monotherapy, dosing, or high-inoculum infections.
The lack of in vitro activity of ceftazidime-avibactam against MBL producers and the fact that many MBL producers also coproduce other β-lactamases (such as ESBLs, AmpC, OXA-48, etc.) have attracted some attention to the potential effect of combining ceftazidime-avibactam with aztreonam. Synergistic effects have been seen in in vitro and in vivo studies (346, 357), and indeed, some patients were successfully treated with this combination (208, 397). This raises the possibility of using atypical combinations of BLBLIs and other β-lactams, such as piperacillin-tazobactam plus aztreonam, against MBL and ESBL producers, although many of these should be tested in vitro, in vivo, and on patients before any recommendations can be provided. As stated below, aztreonam-avibactam is undergoing clinical development.
Although more data are needed, ceftazidime-avibactam may already be considered the new keystone in the treatment of severe infections due to KPC- and OXA-48-producing Enterobacteriaceae.
Meropenem-Vaborbactam
Vaborbactam is another new β-lactamase inhibitor which has been shown to restore the ativity of meropenem against KPC producers; however, it does not enhance the activity of meropenem against MBL producers (NDM or VIM) or OXA-48 producers (358, 359). It was recently approved by the FDA for the treatment of cUTI due to susceptible enterobacteria, based on the data from a phase 3 trial in which meropenem-vaborbactam showed noninferiority to piperacillin-tazobactam (398). The preliminary results of a small phase 3 trial including diverse types of infections caused by CRE showed higher rates of clinical cure with meropenem-vaborbactam than with the best available therapy (57.1% of 28 patients versus 26.7% of 15 patients; absolute difference, 30.5%; 95% CI, 1.5 to 59.4%), as well as lower rates of nephrotoxicity (360); recruitment was stopped because of the superiority of meropenem-vaborbactam. Despite the limited information available, limitations of the study include heterogeneous infections (with a majority of cUTI) and very diverse treatment regimens in the control arm, some of which might have been substandard.
Pipeline of Drugs against CRE
Plazomicin is a new aminoglycoside undergoing clinical development. It escapes the activity of aminoglycoside-modifying enzymes and is therefore active against a greater proportion of CRE than those with gentamicin, tobramycin, and amikacin. Nonetheless, like all other aminoglycosides, it is affected by 16S rRNA methyltransferases (111, 361–363). The results of a phase 3 randomized trial comparing plazomicin (15 mg/kg/day) and meropenem (1 g/8 h) for treatment of cUTI, including pyelonephritis, have been reported; 388 patients were included in the microbiological modified intention-to-treat (mMITT) population, and plazomicin showed a higher rate of microbiological response (87.4% versus 72.1%) (364). The rates and severities of adverse events were similar. The results of an open-label phase 3 trial of patients with BSI or HAP/VAP caused by CRE, comparing plazomicin (17 patients) and colistin (20 patients) in combination with tigecycline or meropenem, have also been reported. Mortality rates were 11.8% and 40%, respectively (difference, 28%; 95% CI, 0.7 to 52.5%). Renal toxicity was less frequent with plazomicin (365).
Eravacycline is a novel fluorocycline antibiotic with in vitro activity against MDR Gram-positive and Gram-negative pathogens, including carbapenemase-producing Enterobacteriaceae (366–368). The drug has demonstrated noninferiority to ertapenem in the treatment of cIAI in a phase 3 trial (369). Another RCT involving comparison with meropenem in cIAI is being developed, and trials against cUTI are planned.
Cefiderocol is a new siderophore cephalosporin that is active against MDR Gram-negative organisms, including carbapenemase-producing Enterobacteriaceae (370–375). At a dose of 2 g every 8 h, it reaches >50% time above the MIC for MICs of up to 8 mg/liter (376). It has also been shown to be effective against KPC- and NDM-producing K. pneumoniae in a rat respiratory tract infection model, particularly when administered over 3 h (377). Preliminary results of a phase 3 trial against cUTI have reported noninferiority to imipenem, and the results are consistent with superiority (378).
Aztreonam-avibactam is an interesting combination because of the ability of avibactam to inhibit ESBLs, AmpC, KPC, and OXA-48 enzymes and the stability of aztreonam against MBLs. Therefore, this compound is active against many CPE isolates, regardless of the carbapenemase produced (242, 332, 379–388), and is undergoing phase II trials against intra-abdominal infections, in association with metronidazole.
Relebactam is another β-lactamase inhibitor with activity against KPC and ESBL producers (KPC producers with major OmpK36 mutations affecting permeability have higher MICs). It is less active against OXA-48 and not active against MBLs (389–395). It is being studied in combination with imipenem in phase 3 studies of cIAI and cUTI.
ACKNOWLEDGMENTS
This study was funded by the Plan Nacional de I+D+i 2013–2016, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (grants RD16/0016/0001 and RD16/0016/0008), cofinanced by the European Development Regional Fund “A Way to Achieve Europe” and the Operational Program for Smart Growth 2014–2020. We also received funds for research from the Innovative Medicines Initiative (IMI) and the European Union's Seventh Framework Programme (grant FP7/2007-2013) and in-kind contributions from EFPIA companies (COMBACTE-CARE project; agreement 115620).
J.R.-B. has received honoraria from Merck for participating in accredited educational activities and from AstraZeneca for coordinating a research project. A.P. has received honoraria from Merck for participating in accredited educational activities. B.G.-G. and I.M. have no conflicts to declare.
Biographies

Jesús Rodríguez-Baño, M.D., Ph.D., is a specialist in internal medicine. He is Head of the Infectious Diseases Division at the Hospital Universitario Virgen Macarena and Professor of Medicine in the Department of Medicine of the Universidad de Sevilla (Seville, Spain), as well as Scientific Coordinator of the Spanish Network for Research in Infectious Diseases (REIPI), funded by the Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness; President-Elect of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID); and member of the scientific advisory committee for the Joint Programme Initiative for Antimicrobial Resistance, European Union.

Belén Gutiérrez-Gutiérrez, M.D., Ph.D., obtained her medical degree from the University of Granada and her Ph.D. from the University of Seville. She is a specialist in internal medicine and a faculty member of the Infectious Diseases Division at the Hospital Universitario Virgen Macarena in Seville, Spain. She has an expert degree in epidemiology and clinical research. Her research interests have focused on the treatment of patients with multidrug-resistant Gram-negative infections. She is involved in various international research projects on antimicrobial resistance.

Isabel Machuca, M.D., is a specialist in internal medicine and a faculty member of the Infectious Diseases Division at the Hospital Universitario Reina Sofía in Córdoba, Spain. She is developing her Ph.D. thesis about treatment of infections caused by carbapenemase-producing Enterobacteriaceae.

Alvaro Pascual obtained his M.D. and Ph.D. from the University of Seville (Spain). He completed his training in the Department of Microbiology at University of Utrecht, The Netherlands (1983–1984), the Mayo Clinic, USA (1986), the University of Minnesota, USA (1986), and the University of North Carolina at Chapel Hill, USA (1998–1999). He is currently Head of the Clinical Microbiology Laboratory at the Hospital Universitario Virgen Macarena and Full Professor of Microbiology at the University of Seville (Spain). His research areas are the molecular basis of antimicrobial resistance in bacteria and the epidemiology of infections caused by multidrug-resistant bacteria, particularly Gram-negative bacteria. He is an author of around 350 articles in indexed scientific journals and the principal investigator on more than 50 research projects with competitive funding at the national and international levels.
REFERENCES
- 1.ECDC/EMEA. 2009. ECDC/EMEA joint technical report. The bacterial challenge: time to react. A call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. European Centre for Disease Prevention and Control, Stockholm, Sweden: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf. [Google Scholar]
- 2.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 3.Paterson DL, Bonomo R. 2005. Extended-spectrum β-lactamase: a clinical update. Clin Microbiol Rev 18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitout JD, Laupland KB. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 5.Jacoby GA. 2009. AmpC beta-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. 2012. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother 67:2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 7.Lee B, Kang SY, Kang HM, Yang NR, Kang HG, Ha IS, Cheong HI, Lee HJ, Choi EH. 2013. Outcome of antimicrobial therapy of pediatric urinary tract infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae. Infect Chemother 45:415–421. doi: 10.3947/ic.2013.45.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PN, Wei JY, Shen AW, Abdile AA, Paynter S, Huxley RR, Pandeya N, Doi Y, Huh K, O'Neal CS, Talbot TR, Paterson DL. 2016. Carbapenems versus alternative antibiotics for the treatment of bloodstream infections caused by Enterobacter, Citrobacter or Serratia species: a systematic review with meta-analysis. J Antimicrob Chemother 71:296–306. doi: 10.1093/jac/dkv346. [DOI] [PubMed] [Google Scholar]
- 9.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R. 2014. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Baño J, Pascual A. 2008. Clinical significance of extended-spectrum β-lactamases. Expert Rev Anti Infect Ther 6:671–683. doi: 10.1586/14787210.6.5.671. [DOI] [PubMed] [Google Scholar]
- 11.Kaniga K, Flamm R, Tong SY, Lee M, Friedland I, Redman R. 2010. Worldwide experience with the use of doripenem against extended-spectrum-beta-lactamase-producing and ciprofloxacin-resistant Enterobacteriaceae: analysis of six phase 3 clinical studies. Antimicrob Agents Chemother 54:2119–2124. doi: 10.1128/AAC.01450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolau DP, Carmeli Y, Crank CW, Goff DA, Graber CJ, Lima AL, Goldstein EJ. 2012. Carbapenem stewardship: does ertapenem affect Pseudomonas susceptibility to other carbapenems. A review of the evidence. Int J Antimicrob Agents 39:11–15. doi: 10.1016/j.ijantimicag.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Lim CL, Lee W, Lee AL, Liew LT, Nah SC, Wan CN, Chlebicki MP, Kwa AL. 2013. Evaluation of ertapenem use with impact assessment on extended-spectrum beta-lactamases (ESBL) production and gram-negative resistance in Singapore General Hospital (SGH). BMC Infect Dis 13:523. doi: 10.1186/1471-2334-13-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee NY, Huang WH, Tsui KC, Hsueh PR, Ko WC. 2011. Carbapenem therapy for bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae. Diagn Microbiol Infect Dis 70:150–153. doi: 10.1016/j.diagmicrobio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Wu UI, Chen WC, Yang CS, Wang JL, Hu FC, Chang SC, Chen YC. 2012. Ertapenem in the treatment of bacteremia caused by extended-spectrum beta-lactamase-producing Escherichia coli: a propensity score analysis. Int J Infect Dis 16:e47–e52. doi: 10.1016/j.ijid.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Collins VL, Marchaim D, Pogue JM, Moshos J, Bheemreddy S, Sunkara B, Shallal A, Chugh N, Eiseler S, Bhargava P, Blunden C, Lephart PR, Memon BI, Hayakawa K, Abreu-Lanfranco O, Chopra T, Munoz-Price LS, Carmeli Y, Kaye KS. 2012. Efficacy of ertapenem for treatment of bloodstream infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 56:2173–2177. doi: 10.1128/AAC.05913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. 2012. Carbapenem therapy for bacteremia due to extended-spectrum-beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae: implications of ertapenem susceptibility. Antimicrob Agents Chemother 56:2888–2893. doi: 10.1128/AAC.06301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutiérrez-Gutiérrez B, Bonomo RA, Carmeli Y, Paterson DL, Almirante B, Martínez-Martínez L, Oliver A, Calbo E, Peña C, Akova M, Pitout J, Origüen J, Pintado V, García-Vázquez E, Gasch O, Hamprecht A, Prim N, Tumbarello M, Bou G, Viale P, Tacconelli E, Almela M, Pérez F, Giamarellou H, Cisneros JM, Schwaber MJ, Venditti M, Lowman W, Bermejo J, Hsueh PR, Mora-Rillo M, Gracia-Ahulfinger I, Pascual A, Rodríguez-Baño J. 2016. Ertapenem for the treatment of bloodstream infections due to ESBL-producing Enterobacteriaceae: a multinational pre-registered cohort study. J Antimicrob Chemother 71:1672–1680. doi: 10.1093/jac/dkv502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkhardt O, Kumar V, Katterwe D, Majcher-Peszynska J, Drewelow B, Derendorf H, Welte T. 2007. Ertapenem in critically ill patients with early-onset ventilator-associated pneumonia: pharmacokinetics with special consideration of free-drug concentration. J Antimicrob Chemother 59:277–284. doi: 10.1093/jac/dkl485. [DOI] [PubMed] [Google Scholar]
- 20.Bassetti M, Righi E, Fasce R, Molinari MP, Rosso R, Di Biagio A, Mussap M, Pallavicini FB, Viscoli C. 2007. Efficacy of ertapenem in the treatment of early ventilator-associated pneumonia caused by extended-spectrum beta-lactamase-producing organisms in an intensive care unit. J Antimicrob Chemother 60:433–435. doi: 10.1093/jac/dkm180. [DOI] [PubMed] [Google Scholar]
- 21.Rattanaumpawan P, Werarak P, Jitmuang A, Kiratisin P, Thamlikitkul V. 2017. Efficacy and safety of de-escalation therapy to ertapenem for treatment of infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae: an open-label randomized controlled trial. BMC Infect Dis 17:183. doi: 10.1186/s12879-017-2284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalgic N, Sancar M, Bayraktar B, Dincer E, Pelit S. 2011. Ertapenem for the treatment of urinary tract infections caused by extended-spectrum β-lactamase-producing bacteria in children. Scand J Infect Dis 43:339–343. doi: 10.3109/00365548.2011.553241. [DOI] [PubMed] [Google Scholar]
- 23.Karaaslan A, Kadayifci EK, Atici S, Akkoc G, Yakut N, Öcal Demir S, Soysal A, Bakir M. 2015. The clinical efficacy and safety of ertapenem for the treatment of complicated urinary tract infections caused by ESBL-producing bacteria in children. Int J Nephrol 2015:595840. doi: 10.1155/2015/595840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazaz R, Chapman AL, Winstanley TG. 2010. Ertapenem administered as outpatient parenteral antibiotic therapy for urinary tract infections caused by extended-spectrum-beta-lactamase-producing Gram-negative organisms. J Antimicrob Chemother 65:1510–1513. doi: 10.1093/jac/dkq152. [DOI] [PubMed] [Google Scholar]
- 25.Forestier E, Gros S, Peynaud D, Levast M, Boisseau D, Ferry-Blanco C, Labe A, Lecomte C, Rogeaux O. 2012. Ertapenem intraveineux ou sous-cutane pour le traitement des infections urinaires a enterobacterie secretrice de BLSE. Med Mal Infect 42:440–443. doi: 10.1016/j.medmal.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Song S, Kim C, Lim D. 2014. Clinical efficacy of ertapenem for recurrent cystitis caused by multidrug-resistant extended-spectrum β-lactamase-producing Escherichia coli in female outpatients. Korean J Urol 55:270–275. doi: 10.4111/kju.2014.55.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trad MA, Zhong LH, Llorin RM, Tan SY, Chan M, Archuleta S, Sulaiman Z, Tam VH, Lye DC, Fisher DA. 2017. Ertapenem in outpatient parenteral antimicrobial therapy for complicated urinary tract infections. J Chemother 29:25–29. doi: 10.1080/1120009X.2016.1158937. [DOI] [PubMed] [Google Scholar]
- 28.Veve MP, Wagner JL, Kenney RM, Grunwald JL, Davis SL. 2016. Comparison of fosfomycin to ertapenem for outpatient or step-down therapy of extended-spectrum β-lactamase urinary tract infections. Int J Antimicrob Agents 48:56–60. doi: 10.1016/j.ijantimicag.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Zhanel GG, Denisuik A, Vashisht S, Yachison C, Adam HJ, Hoban DJ. 2014. Pharmacodynamic activity of ertapenem versus genotypically characterized extended-spectrum β-lactamase (ESBL)-, KPC- or NDM-producing Escherichia coli with reduced susceptibility or resistance to ertapenem using an in vitro model. J Antimicrob Chemother 69:2448–2452. doi: 10.1093/jac/dku149. [DOI] [PubMed] [Google Scholar]
- 30.Ungphakorn W, Tängdén T, Sandegren L, Nielsen EI. 2016. A pharmacokinetic-pharmacodynamic model characterizing the emergence of resistant Escherichia coli subpopulations during ertapenem exposure. J Antimicrob Chemother 71:2521–2533. doi: 10.1093/jac/dkw205. [DOI] [PubMed] [Google Scholar]
- 31.Elliott E, Brink AJ, van Greune J, Els Z, Woodford N, Turton J, Warner M, Livermore DM. 2006. In vivo development of ertapenem resistance in a patient with pneumonia caused by Klebsiella pneumoniae with an extended-spectrum beta-lactamase. Clin Infect Dis 42:e95–e98. doi: 10.1086/503264. [DOI] [PubMed] [Google Scholar]
- 32.Oteo J, Delgado-Iribarren A, Vega D, Bautista V, Rodríguez MC, Velasco M, Saavedra JM, Pérez-Vázquez M, García-Cobos S, Martínez-Martínez L, Campos J. 2008. Emergence of imipenem resistance in clinical Escherichia coli during therapy. Int J Antimicrob Agents 32:534–537. doi: 10.1016/j.ijantimicag.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Skurnik D, Lasocki S, Bremont S, Muller-Serieys C, Kitzis MD, Courvalin P, Andremont A, Montravers P. 2010. Development of ertapenem resistance in a patient with mediastinitis caused by Klebsiella pneumoniae producing an extended-spectrum beta-lactamase. J Med Microbiol 59:115–119. doi: 10.1099/jmm.0.012468-0. [DOI] [PubMed] [Google Scholar]
- 34.Mena A, Plasencia V, García L, Hidalgo O, Ayestarán JI, Alberti S, Borrell N, Pérez JL, Oliver A. 2006. Characterization of a large outbreak by CTX-M-1-producing Klebsiella pneumoniae and mechanisms leading to in vivo carbapenem resistance development. J Clin Microbiol 44:2831–2837. doi: 10.1128/JCM.00418-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pérez F, Bonomo RA. 2012. Can we really use β-lactam/β-lactam inhibitor combinations for the treatment of infections caused by extended-spectrum β-lactamase-producing bacteria? Clin Infect Dis 54:175–177. doi: 10.1093/cid/cir793. [DOI] [PubMed] [Google Scholar]
- 36.Karlowsky JA, Hoban DJ, Hackel MA, Lob S, Sahm DF. 2017. Antimicrobial susceptibility of Gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Asia-Pacific countries: SMART 2013–2015. J Med Microbiol 66:61–69. doi: 10.1099/jmm.0.000421. [DOI] [PubMed] [Google Scholar]
- 37.Pitout JD, Gregson DB, Campbell L, Laupland KB. 2009. Molecular characteristics of extended-spectrum-beta-lactamase-producing Escherichia coli isolates causing bacteremia in the Calgary Health Region from 2000 to 2007: emergence of clone ST131 as a cause of community-acquired infections. Antimicrob Agents Chemother 53:2846–2851. doi: 10.1128/AAC.00247-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitout JD, Le P, Church DL, Gregson DB, Laupland KB. 2008. Antimicrobial susceptibility of well-characterised multiresistant CTX-M-producing Escherichia coli: failure of automated systems to detect resistance to piperacillin/tazobactam. Int J Antimicrob Agents 32:333–338. doi: 10.1016/j.ijantimicag.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 39.López-Cerero L, Picón E, Morillo C, Hernández JR, Docobo F, Pachón J, Rodríguez-Baño J, Pascual A. 2010. Comparative assessment of inoculum effects on the antimicrobial activity of amoxycillin-clavulanate and piperacillin-tazobactam with extended-spectrum beta-lactamase-producing and extended-spectrum beta-lactamase-non-producing Escherichia coli isolates. Clin Microbiol Infect 16:132–136. doi: 10.1111/j.1469-0691.2009.02893.x. [DOI] [PubMed] [Google Scholar]
- 40.Rice LB, Carias LL, Shlaes DM. 1994. In vivo efficacies of β-lactam-β-lactamase inhibitor combinations against a TEM-26-producing strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 38:2663–2664. doi: 10.1128/AAC.38.11.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thauvin-Eliopoulos C, Tripodi MF, Moellering RC Jr, Eliopoulos GM. 1997. Efficacies of piperacillin-tazobactam and cefepime in rats with experimental intra-abdominal abscesses due to an extended-spectrum beta-lactamase-producing strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 41:1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambrose PG, Bhavnani SM, Jones RN. 2003. Pharmacokinetics-pharmacodynamics of cefepime and piperacillin-tazobactam against Escherichia coli and Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases: report from the ARREST program. Antimicrob Agents Chemother 47:1643–1646. doi: 10.1128/AAC.47.5.1643-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Docobo-Pérez F, López-Cerero L, López-Rojas R, Egea P, Domínguez-Herrera J, Rodríguez-Baño J, Pascual A, Pachón J. 2013. Inoculum effect on the efficacies of amoxicillin-clavulanate, piperacillin-tazobactam, and imipenem against extended-spectrum β-lactamase (ESBL)-producing and non-ESBL-producing Escherichia coli in an experimental murine sepsis model. Antimicrob Agents Chemother 57:2109–2113. doi: 10.1128/AAC.02190-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harada Y, Morinaga Y, Kaku N, Nakamura S, Uno N, Hasegawa H, Izumikawa K, Kohno S, Yanagihara K. 2014. In vitro and in vivo activities of piperacillin-tazobactam and meropenem at different inoculum sizes of ESBL-producing Klebsiella pneumoniae. Clin Microbiol Infect 20:O831–O839. doi: 10.1111/1469-0691.12677. [DOI] [PubMed] [Google Scholar]
- 45.Zimhony O, Chmelnitsky I, Bardenstein R, Goland S, Hammer Muntz O, Navon Venezia S, Carmeli Y. 2006. Endocarditis caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae: emergence of resistance to ciprofloxacin and piperacillin-tazobactam during treatment despite initial susceptibility. Antimicrob Agents Chemother 50:3179–3182. doi: 10.1128/AAC.00218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Pascual Á. 2012. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis 54:167–174. doi: 10.1093/cid/cir790. [DOI] [PubMed] [Google Scholar]
- 47.Shiber S, Yahav D, Avni T, Leibovici L, Paul M. 2015. β-Lactam/β-lactamase inhibitors versus carbapenems for the treatment of sepsis: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 70:41–47. doi: 10.1093/jac/dku351. [DOI] [PubMed] [Google Scholar]
- 48.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE. 2015. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis 60:1319–1325. doi: 10.1093/cid/civ003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai HY, Chen YH, Tang HJ, Huang CC, Liao CH, Chu FY, Chuang YC, Sheng WH, Ko WC, Hsueh PR. 2014. Carbapenems and piperacillin/tazobactam for the treatment of bacteremia caused by extended-spectrum β-lactamase-producing Proteus mirabilis. Diagn Microbiol Infect Dis 80:222–226. doi: 10.1016/j.diagmicrobio.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Harris PN, Yin M, Jureen R, Chew J, Ali J, Paynter Paterson DL, Tambyah PA. 2015. Comparable outcomes for β-lactam/β-lactamase inhibitor combinations and carbapenems in definitive treatment of bloodstream infections caused by cefotaxime-resistant Escherichia coli or Klebsiella pneumoniae. Antimicrob Resist Infect Control 4:14. doi: 10.1186/s13756-015-0055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, de Cueto M, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, Martínez-Martínez L, Pitout J, Akova M, Peña C, Molina J, Hernández A, Venditti M, Prim N, Origüen J, Bou G, Tacconelli E, Tumbarello M, Hamprecht A, Giamarellou H, Almela M, Pérez F, Schwaber MJ, Bermejo J, Lowman W, Hsueh PR, Mora-Rillo M, Natera C, Souli M, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J. 2016. A multinational, preregistered cohort study of β-lactam/β-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:4159–4169. doi: 10.1128/AAC.00365-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ng TM, Khong WX, Harris PN, De PP, Chow A, Tambyah PA, Lye DC. 2016. Empiric piperacillin-tazobactam versus carbapenems in the treatment of bacteraemia due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. PLoS One 11:e0153696. doi: 10.1371/journal.pone.0153696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gudiol C, Royo-Cebrecos C, Abdala E, Akova M, Álvarez R, Maestro-de la Calle G, Cano A, Cervera C, Clemente WT, Martín-Dávila P, Freifeld A, Gómez L, Gottlieb T, Gurguí M, Herrera F, Manzur A, Maschmeyer G, Meije Y, Montejo M, Peghin M, Rodríguez-Baño J, Ruiz-Camps I, Sukiennik TC, Tebe C, Carratalà J. 2017. Efficacy of β-lactam/β-lactamase inhibitor combinations for the treatment of bloodstream infection due to extended-spectrum-β-lactamase-producing Enterobacteriaceae in hematological patients with neutropenia. Antimicrob Agents Chemother 61:e00164-17. doi: 10.1128/AAC.00164-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dizbay M, Özger HS, Karaşahin Ö, Karaşahin EF. 2016. Treatment efficacy and superinfection rates in complicated urinary tract infections treated with ertapenem or piperacillin tazobactam. Turk J Med Sci 46:1760–1764. doi: 10.3906/sag-1506-157. [DOI] [PubMed] [Google Scholar]
- 55.Yoon YK, Kim JH, Sohn JW, Yang KS, Kim MJ. 2017. Role of piperacillin/tazobactam as a carbapenem-sparing antibiotic for treatment of acute pyelonephritis due to extended-spectrum β-lactamase-producing Escherichia coli. Int J Antimicrob Agents 49:410–415. doi: 10.1016/j.ijantimicag.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 56.Seo YB, Lee J, Kim YK, Lee SS, Lee JA, Kim HY, Uh Y, Kim HS, Song W. 2017. Randomized controlled trial of piperacillin-tazobactam, cefepime and ertapenem for the treatment of urinary tract infection caused by extended-spectrum beta-lactamase-producing Escherichia coli. BMC Infect Dis 17:404. doi: 10.1186/s12879-017-2502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Retamar P, López-Cerero L, Muniain MA, Pascual Á, Rodríguez-Baño J. 2013. Impact of the MIC of piperacillin-tazobactam on the outcome of patients with bacteremia due to extended-spectrum-β-lactamase-producing Escherichia coli. Antimicrob Agents Chemother 57:3402–3404. doi: 10.1128/AAC.00135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris PN, Peleg AY, Iredell J, Ingram PR, Miyakis S, Stewardson AJ, Rogers BA, McBryde ES, Roberts JA, Lipman J, Athan E, Paul SK, Baker P, Harris-Brown T, Paterson DL. 2015. Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections due to ceftriaxone non-susceptible Escherichia coli and Klebsiella spp (the MERINO trial): study protocol for a randomised controlled trial. Trials 16:24. doi: 10.1186/s13063-014-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang H, Zhang C, Zhou Q, Wang Y, Chen L. 2015. Clinical outcomes with alternative dosing strategies for piperacillin/tazobactam: a systematic review and meta-analysis. PLoS One 10:e0116769. doi: 10.1371/journal.pone.0116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng L, Nelson BC, Mehta M, Seval N, Park S, Giddins MJ, Shi Q, Whittier S, Gomez-Simmonds A, Uhlemann AC. 2017. Piperacillin-tazobactam versus other antibacterial agents for treatment of bloodstream infections due to AmpC β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 61:e00276-17. doi: 10.1128/AAC.00276-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Popejoy MW, Paterson DL, Cloutier D, Huntington JA, Miller B, Bliss CA, Steenbergen JN, Hershberger E, Umeh O, Kaye KS. 2017. Efficacy of ceftolozane/tazobactam against urinary tract and intra-abdominal infections caused by ESBL-producing Escherichia coli and Klebsiella pneumoniae: a pooled analysis of phase 3 clinical trials. J Antimicrob Chemother 72:268–272. doi: 10.1093/jac/dkw374. [DOI] [PubMed] [Google Scholar]
- 63.Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, Yates K, Gasink LB. 2016. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis 63:754–762. doi: 10.1093/cid/ciw378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, Llorens L, Newell P, Pachl J. 2016. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 62:1380–1389. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carmeli Y, Armstrong J, Laud PJ, Newell P, Stone G, Wardman A, Gasink LB. 2016. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 16:661–673. doi: 10.1016/S1473-3099(16)30004-4. [DOI] [PubMed] [Google Scholar]
- 66.European Committee on Antimicrobial Susceptibility Testing. 2017. Breakpoint tables for interpretation of MICs and zone diameters. Version 7.0. http://www.eucast.org.
- 67.Clinical and Laboratory Standards Institute (CLSI). 2017. Performance standards for antimicrobial susceptibility testing, 27th ed CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 68.Rodríguez-Baño J, Picón E, Navarro MD, López-Cerero L, Pascual A. 2012. Impact of changes in CLSI and EUCAST breakpoints for susceptibility in bloodstream infections due to extended-spectrum β-lactamase-producing Escherichia coli. Clin Microbiol Infect 18:894–900. doi: 10.1111/j.1469-0691.2011.03673.x. [DOI] [PubMed] [Google Scholar]
- 69.Huang CC, Chen YS, Toh HS, Lee YL, Liu YM, Ho CM, Lu PL, Liu CE, Chen YH, Wang JH, Tang HJ, Yu KW, Liu YC, Chuang YC, Xu Y, Ni Y, Ko WC, Hsueh PR. 2012. Impact of revised CLSI breakpoints for susceptibility to third-generation cephalosporins and carbapenems among Enterobacteriaceae isolates in the Asia-Pacific region: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2002–2010. Int J Antimicrob Agents 40(Suppl):S4–S10. doi: 10.1016/S0924-8579(12)70003-1. [DOI] [PubMed] [Google Scholar]
- 70.Park KH, Chong YP, Kim SH, Lee SO, Lee MS, Sung H, Kim MN, Kim YS, Woo JH, Choi SH. 2017. Impact of revised broad-spectrum cephalosporin Clinical and Laboratory Standards Institute breakpoints on susceptibility in Enterobacteriaceae producing AmpC β-lactamase. Infect Chemother 49:62–67. doi: 10.3947/ic.2017.49.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paterson DL, Ko WC, Von Gottberg A, Casellas JM, Mulazimoglu L, Klugman KP, Bonomo RA, Rice LB, McCormack JG, Yu VL. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum β-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 39:2206–2212. doi: 10.1128/JCM.39.6.2206-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SY, Kuti JL, Nicolau DP. 2007. Cefepime pharmacodynamics in patients with extended spectrum beta-lactamase (ESBL) and non-ESBL infections. J Infect 54:463–468. doi: 10.1016/j.jinf.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 73.McGowan A. 2008. Breakpoints for extended-spectrum beta-lactamase-producing Enterobacteriaceae: pharmacokinetic/pharmacodynamic considerations. Clin Microbiol Infect 14(Suppl 1):S166–S168. [DOI] [PubMed] [Google Scholar]
- 74.Goethaert K, Van Looveren M, Lammens C, Jansens H, Baraniak A, Gniadkowski M, Van Herck K, Jorens PG, Demey HE, Ieven M, Bossaert L, Goossens H. 2006. High-dose cefepime as an alternative treatment for infections causedby TEM-24 ESBL-producing Enterobacter aerogenes in severely-ill patients. Clin Microbiol Infect 12:56–62. doi: 10.1111/j.1469-0691.2005.01290.x. [DOI] [PubMed] [Google Scholar]
- 75.Bin C, Hui W, Renyuan Z, Yongzhong N, Xiuli X, Yingchun X, Yuanjue Z, Minjun C. 2006. Outcome of cephalosporin treatment of bacteremia due to CTX-M-type extended-spectrum beta-lactamase-producing Escherichia coli. Diagn Microbiol Infect Dis 56:351–357. doi: 10.1016/j.diagmicrobio.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 76.Chopra T, Marchaim D, Veltman J, Johnson P, Zhao JJ, Tansek R, Hatahet D, Chaudhry K, Pogue JM, Rahbar H, Chen TY, Truong T, Rodriguez V, Ellsworth J, Bernabela L, Bhargava A, Yousuf A, Alangaden G, Kaye KS. 2012. Impact of cefepime therapy on mortality among patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother 56:3936–3942. doi: 10.1128/AAC.05419-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. 2013. Cefepime therapy for monomicrobial bacteremia caused by cefepime-susceptible extended-spectrum beta-lactamase-producing Enterobacteriaceae: MIC matters. Clin Infect Dis 56:488–495. doi: 10.1093/cid/cis916. [DOI] [PubMed] [Google Scholar]
- 78.Wang R, Cosgrove SE, Tschudin-Sutter S, Han JH, Turnbull AE, Hsu AJ, Avdic E, Carroll KC, Tamma PD. 2016. Cefepime therapy for cefepime-susceptible extended-spectrum β-lactamase-producing Enterobacteriaceae bacteremia. Open Forum Infect Dis 3:ofw132. doi: 10.1093/ofid/ofw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee NY, Lee CC, Li CW, Li MC, Chen PL, Chang CM, Ko WC. 2015. Cefepime therapy for monomicrobial Enterobacter cloacae bacteremia: unfavorable outcomes in patients infected by cefepime-susceptible dose-dependent isolates. Antimicrob Agents Chemother 59:7558–7563. doi: 10.1128/AAC.01477-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsieh CC, Lee CH, Li MC, Hong MY, Chi CH, Lee CC. 2016. Empirical third-generation cephalosporin therapy for adults with community-onset Enterobacteriaceae bacteraemia: impact of revised CLSI breakpoints. Int J Antimicrob Agents 47:297–303. doi: 10.1016/j.ijantimicag.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 81.Bedenić B, Beader N, Zagar Z. 2001. Effect of inoculum size on the antibacterial activity of cefpirome and cefepime against Klebsiella pneumoniae strains producing SHV extended-spectrum beta-lactamases. Clin Microbiol Infect 7:626–635. doi: 10.1046/j.1198-743x.2001.x. [DOI] [PubMed] [Google Scholar]
- 82.Thomson KS, Moland ES. 2001. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 45:3548–3554. doi: 10.1128/AAC.45.12.3548-3554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu N, Chen BY, Tian SF, Chu YZ. 2014. The inoculum effect of antibiotics against CTX-M-extended-spectrum β-lactamase-producing Escherichia coli. Ann Clin Microbiol Antimicrob 13:45. doi: 10.1186/s12941-014-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Costa Ramos JM, Stein C, Pfeifer Y, Brandt C, Pletz MW, Makarewicz O. 2015. Mutagenesis of the CTX-M-type ESBL—is MIC-guided treatment according to the new EUCAST recommendations a safe approach? J Antimicrob Chemother 70:2528–2535. doi: 10.1093/jac/dkv153. [DOI] [PubMed] [Google Scholar]
- 85.Tamma PD, Girdwood SC, Gopaul R, Tekle T, Roberts AA, Harris AD, Cosgrove SE, Carroll KC. 2013. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis 57:781–788. doi: 10.1093/cid/cit395. [DOI] [PubMed] [Google Scholar]
- 86.Kang CI, Pai H, Kim SH, Kim HB, Kim EC, Oh MD, Choe KW. 2004. Cefepime and the inoculum effect in tests with Klebsiella pneumoniae producing plasmid-mediated AmpC-type beta-lactamase. J Antimicrob Chemother 54:1130–1133. doi: 10.1093/jac/dkh462. [DOI] [PubMed] [Google Scholar]
- 87.Pichardo C, Rodríguez-Martínez JM, Pachón-Ibañez ME, Conejo C, Ibáñez-Martínez J, Martínez-Martínez L, Pachón J, Pascual A. 2005. Efficacy of cefepime and imipenem in experimental murine pneumonia caused by porin-deficient Klebsiella pneumoniae producing CMY-2 beta-lactamase. Antimicrob Agents Chemother 49:3311–3316. doi: 10.1128/AAC.49.8.3311-3316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Betriu C, Salso S, Sánchez A, Culebras E, Gómez M, Rodríguez-Avial I, Picazo JJ. 2006. Comparative in vitro activity and the inoculum effect of ertapenem against Enterobacteriaceae resistant to extended-spectrum cephalosporins. Int J Antimicrob Agents 28:1–5. [DOI] [PubMed] [Google Scholar]
- 89.Pangon B, Bizet C, Buré AA, Pichon F, Philippon A, Regnier B, Gutmann L. 1989. In vivo selection of a cephamycin-resistant, porin-deficient mutant of Klebsiella pneumoniae producing a TEM-3 beta-lactamase. J Infect Dis 159:1005–1006. doi: 10.1093/infdis/159.5.1005. [DOI] [PubMed] [Google Scholar]
- 90.Siu LK, Lu PL, Hsueh PR, Lin FM, Chang SC, Luh KT, Ho M, Lee CY. 1999. Bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric oncology ward: clinical features and identification of different plasmids carrying both SHV-5 and TEM-1 genes. J Clin Microbiol 37:4020–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee CH, Su LH, Tang YF, Liu JW. 2006. Treatment of ESBL-producing Klebsiella pneumoniae bacteraemia with carbapenems or flomoxef: a retrospective study and laboratory analysis of the isolates. J Antimicrob Chemother 58:1074–1077. doi: 10.1093/jac/dkl381. [DOI] [PubMed] [Google Scholar]
- 92.Yang CC, Li SH, Chuang FR, Chen CH, Lee CH, Chen JB, Wu CH, Lee CT. 2012. Discrepancy between effects of carbapenems and flomoxef in treating nosocomial hemodialysis access-related bacteremia secondary to extended spectrum beta-lactamase producing Klebsiella pneumoniae in patients on maintenance hemodialysis. BMC Infect Dis 12:206. doi: 10.1186/1471-2334-12-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doi A, Shimada T, Harada S, Iwata K, Kamiya T. 2013. The efficacy of cefmetazole against pyelonephritis caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Int J Infect Dis 17:e159–e163. doi: 10.1016/j.ijid.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 94.Pilmis B, Parize P, Zahar JR, Lortholary O. 2014. Alternatives to carbapenems for infections caused by ESBL-producing Enterobacteriaceae. Eur J Clin Microbiol Infect Dis 33:1263–1265. doi: 10.1007/s10096-014-2094-y. [DOI] [PubMed] [Google Scholar]
- 95.Matsumura Y, Yamamoto M, Nagao M, Komori T, Fujita N, Hayashi A, Shimizu T, Watanabe H, Doi S, Tanaka M, Takakura S, Ichiyama S. 2015. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-β-lactamase-producing Escherichia coli bacteremia. Antimicrob Agents Chemother 59:5107–5113. doi: 10.1128/AAC.00701-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee CH, Su LH, Chen FJ, Tang YF, Li CC, Chien CC, Liu JW. 2015. Comparative effectiveness of flomoxef versus carbapenems in the treatment of bacteraemia due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae with emphasis on minimum inhibitory concentration of flomoxef: a retrospective study. Int J Antimicrob Agents 46:610–615. doi: 10.1016/j.ijantimicag.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 97.Fukuchi T, Iwata K, Kobayashi S, Nakamura T, Ohji G. 2016. Cefmetazole for bacteremia caused by ESBL-producing Enterobacteriaceae comparing with carbapenems. BMC Infect Dis 16:427. doi: 10.1186/s12879-016-1770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Livermore DM, Hope R, Fagan EJ, Warner M, Woodford N, Potz N. 2006. Activity of temocillin against prevalent ESBL- and AmpC-producing Enterobacteriaceae from South-East England. J Antimicrob Chemother 57:1012–1014. doi: 10.1093/jac/dkl043. [DOI] [PubMed] [Google Scholar]
- 99.Giamarellou H. 2006. Treatment options for multi drug-resistant bacteria. Expert Rev Anti Infect Ther 4:601–618. doi: 10.1586/14787210.4.4.601. [DOI] [PubMed] [Google Scholar]
- 100.Soubirou JF, Rossi B, Couffignal C, Ruppé E, Chau F, Massias L, Lepeule R, Mentre F, Fantin B. 2015. Activity of temocillin in a murine model of urinary tract infection due to Escherichia coli producing or not producing the ESBL CTX-M-15. J Antimicrob Chemother 70:1466–1472. doi: 10.1093/jac/dku542. [DOI] [PubMed] [Google Scholar]
- 101.Balakrishnan I, Awad-El-Kariem FM, Aali A, Kumari P, Mulla R, Tan B, Brudney D, Ladenheim D, Ghazy A, Khan I, Virgincar N, Iyer S, Carryn S, Van de Velde S. 2011. Temocillin use in England: clinical and microbiological efficacies in infections caused by extended-spectrum and/or derepressed AmpC beta-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother 66:2628–2631. doi: 10.1093/jac/dkr317. [DOI] [PubMed] [Google Scholar]
- 102.Laterre PF, Wittebole X, Van de Velde S, Muller AE, Mouton JW, Carryn S, Tulkens PM, Dugernier T. 2015. Temocillin (6 g daily) in critically ill patients: continuous infusion versus three times daily administration. J Antimicrob Chemother 70:891–898. doi: 10.1093/jac/dku465. [DOI] [PubMed] [Google Scholar]
- 103.Vidal L, Gafter-Vili Borok S, Fraser A, Leibovici L, Paul M. 2007. Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 60:247–257. doi: 10.1093/jac/dkm193. [DOI] [PubMed] [Google Scholar]
- 104.Paul M, Lador A, Grozinsky-Glasberg S, Leibovici L. 2014. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2014:CD003344. doi: 10.1002/14651858.CD003344.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ong DSY, Frencken JF, Klein Klouwenberg PMC, Juffermans N, van der Poll T, Bonten MJM, Cremer OL. 2017. Short-course adjunctive gentamicin as empirical therapy in patients with severe sepsis and septic shock: a prospective observational cohort study. Clin Infect Dis 64:1731–1736. doi: 10.1093/cid/cix186. [DOI] [PubMed] [Google Scholar]
- 106.Palacios-Baena ZR, Gutiérrez-Gutiérrez B, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, Martínez-Martínez L, Pitout J, Akova M, Peña C, Molina Gil-Bermejo J, Hernández A, Venditti M, Prim N, Bou G, Tacconelli E, Tumbarello M, Hamprecht A, Giamarellou H, Almela M, Pérez F, Schwaber MJ, Bermejo J, Lowman W, Hsueh PR, Paño-Pardo JR, Torre-Cisneros J, Souli M, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J. 2017. Empiric therapy with carbapenem-sparing regimens for bloodstream infections due to extended-spectrum β-lactamase-producing Enterobacteriaceae: results from the INCREMENT cohort. Clin Infect Dis 65:1615–1623. doi: 10.1093/cid/cix606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gudiol C, Calatayud L, Garcia-Vidal C, Lora-Tamayo J, Cisnal M, Duarte R, Arnan M, Marin M, Carratalà J, Gudiol F. 2010. Bacteraemia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother 65:333–341. doi: 10.1093/jac/dkp411. [DOI] [PubMed] [Google Scholar]
- 108.Han SB, Jung SW, Bae EY, Lee JW, Lee DG, Chung NG, Jeong DC, Cho B, Kang JH, Kim HK, Park YJ. 2015. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae bacteremia in febrile neutropenic children. Microb Drug Resist 21:244–251. doi: 10.1089/mdr.2014.0092. [DOI] [PubMed] [Google Scholar]
- 109.Zavascki AP, Klee BO, Bulitta JB. 2017. Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: the pitfalls of aminoglycoside susceptibility. Expert Rev Anti Infect Ther 15:519–526. doi: 10.1080/14787210.2017.1316193. [DOI] [PubMed] [Google Scholar]
- 110.Jean SS, Coombs G, Ling T, Balaji V, Rodrigues C, Mikamo H, Kim MJ, Rajasekaram DG, Mendoza M, Tan TY, Kiratisin P, Ni Y, Weinman B, Xu Y, Hsueh PR. 2016. Epidemiology and antimicrobial susceptibility profiles of pathogens causing urinary tract infections in the Asia-Pacific region: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2010–2013. Int J Antimicrob Agents 47:328–334. doi: 10.1016/j.ijantimicag.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 111.Haidar G, Alkroud A, Cheng S, Churilla TM, Churilla BM, Shields RK, Doi Y, Clancy CJ, Nguyen MH. 2016. Association between the presence of aminoglycoside-modifying enzymes and in vitro activity of gentamicin, tobramycin, amikacin, and plazomicin against Klebsiella pneumoniae carbapenemase- and extended-spectrum-β-lactamase-producing Enterobacter species. Antimicrob Agents Chemother 60:5208–5214. doi: 10.1128/AAC.00869-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.López-Diaz MD, Culebras E, Rodríguez-Avial I, Rios E, Viñuela-Prieto JM, Picazo JJ, Rodríguez-Avial C. 2017. Plazomicin activity against 346 extended-spectrum-β-lactamase/AmpC-producing Escherichia coli urinary isolates in relation to aminoglycoside-modifying enzymes. Antimicrob Agents Chemother 61:e02454-16. doi: 10.1128/AAC.02454-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peterson LR. 2008. A review of tigecycline—the first glycylcycline. Int J Antimicrob Agents 32(Suppl 4):S215–S222. doi: 10.1016/S0924-8579(09)70005-6. [DOI] [PubMed] [Google Scholar]
- 114.Tasina E, Haidich AB, Kokkali S, Arvanitidou M. 2011. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 11:834–844. doi: 10.1016/S1473-3099(11)70177-3. [DOI] [PubMed] [Google Scholar]
- 115.Yahav D, Lador A, Paul M, Leibovici L. 2011. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 66:1963–1971. doi: 10.1093/jac/dkr242. [DOI] [PubMed] [Google Scholar]
- 116.Cai Y, Wang R, Liang B, Bai N, Liu Y. 2011. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 55:1162–1172. doi: 10.1128/AAC.01402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shen F, Han Q, Xie D, Fang M, Zeng H, Deng Y. 2015. Efficacy and safety of tigecycline for the treatment of severe infectious diseases: an updated meta-analysis of RCTs. Int J Infect Dis 39:25–33. doi: 10.1016/j.ijid.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 118.Vasilev K, Reshedko G, Orasan R, Sanchez M, Teras J, Babinchak T, Dukart G, Cooper A, Dartois N, Gandjini H, Orrico R, Ellis-Grosse E. 2008. A phase 3, open-label, non-comparative study of tigecycline in the treatment of patients with selected serious infections due to resistant Gram-negative organisms including Enterobacter species, Acinetobacter baumannii and Klebsiella pneumoniae. J Antimicrob Chemother 62(Suppl 1):i29–i40. doi: 10.1093/jac/dkn249. [DOI] [PubMed] [Google Scholar]
- 119.Poulakou G, Kontopidou FV, Paramythiotou E, Kompoti M, Katsiari M, Mainas E, Nicolaou C, Yphantis D, Antoniadou A, Trikka-Graphakos E, Roussou Z, Clouva P, Maguina N, Kanellakopoulou K, Armaganidis A, Giamarellou H. 2009. Tigecycline in the treatment of infections from multi-drug resistant gram-negative pathogens. J Infect 58:273–284. doi: 10.1016/j.jinf.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 120.Eckmann C, Heizmann WR, Leitner E, von Eiff C, Bodmann KF. 2011. Prospective, non-interventional, multicentre trial of tigecycline in the treatment of severely ill patients with complicated infections: new insights into clinical results and treatment practice. Chemotherapy 57:275–284. doi: 10.1159/000329406. [DOI] [PubMed] [Google Scholar]
- 121.Falagas ME, Maraki S, Karageorgopoulos DE, Katoris AC, Mavromanolakis E, Samonis G. 2010. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents 35:240–243. doi: 10.1016/j.ijantimicag.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 122.Vardakas KZ, Legakis NJ, Triarides N, Falagas ME. 2016. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents 47:269–285. doi: 10.1016/j.ijantimicag.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 123.Pullukcu H, Tasbakan M, Sipahi OR, Yamazhan T, Aydemir S, Ulusoy S. 2007. Fosfomycin in the treatment of extended spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infections. Int J Antimicrob Agents 29:62–65. doi: 10.1016/j.ijantimicag.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 124.Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, Tórtola T, Mirelis B, Navarro G, Cuenca M, Esteve M, Peña C, Llanos AC, Cantón R, Pascual A. 2008. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med 168:1897–1902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 125.Senol S, Tasbakan M, Pullukcu H, Sipahi OR, Sipahi H, Yamazhan T, Arda B, Ulusoy S. 2010. Carbapenem versus fosfomycin tromethanol in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related complicated lower urinary tract infection. J Chemother 22:355–357. doi: 10.1179/joc.2010.22.5.355. [DOI] [PubMed] [Google Scholar]
- 126.Falagas ME, Kastoris AC, Kapaskelis AM, Karagoergopoulos DE. 2010. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis 10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 127.Grabein B, Graninger W, Rodríguez Baño J, Dinh A, Liesenfeld DB. 2017. Intravenous fosfomycin—back to the future. Systematic review and meta-analysis of the clinical literature. Clin Microbiol Infect 23:363–372. doi: 10.1016/j.cmi.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 128.Karageorgopoulos DE, Wang R, Yu XY, Falagas ME. 2012. Fosfomycin: evaluation of the published evidence on the emergence of antimicrobial resistance in Gram-negative pathogens. J Antimicrob Chemother 67:255–268. doi: 10.1093/jac/dkr466. [DOI] [PubMed] [Google Scholar]
- 129.Docobo-Pérez F, Drusano GL, Johnson A, Goodwin J, Whalley S, Ramos-Martín V, Ballestero-Tellez M, Rodriguez-Martinez JM, Conejo MC, van Guilder M, Rodríguez-Baño J, Pascual A, Hope WW. 2015. Pharmacodynamics of fosfomycin: insights into clinical use for antimicrobial resistance. Antimicrob Agents Chemother 59:5602–5610. doi: 10.1128/AAC.00752-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.VanScoy BD, McCauley J, Ellis-Grosse EJ, Okusanya OO, Bhavnani SM, Forrest A, Ambrose PG. 2015. Exploration of the pharmacokinetic-pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob Agents Chemother 59:7170–7177. doi: 10.1128/AAC.04955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaye KS, Rice LB, Dane A, Stus V, Sagan O, Fedosiuk E, Das A, Skarinsky D, Eckburg PB, Ellis-Grosse EJ. 2017. Intravenous fosfomycin (ZTI-01) for the treatment of complicated urinary tract infections (cUTI) including acute pyelonephritis (AP): results from a multi-center, randomized, double-blind phase 2/3 study in hospitalized adults (ZEUS), abstr 1845. IDWeek. [Google Scholar]
- 132.Rosso-Fernández C, Sojo-Dorado J, Barriga A, Lavín-Alconero L, Palacios Z, López-Hernández I, Merino V, Camean M, Pascual A, Rodríguez-Baño J. 2015. Fosfomycin versus meropenem in bacteraemic urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli (FOREST): study protocol for an investigator-driven randomised controlled trial. BMJ Open 5:e007363. doi: 10.1136/bmjopen-2014-007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rodríguez-Martínez JM, Machuca J, Cano ME, Calvo J, Martínez-Martínez L, Pascual A. 2016. Plasmid-mediated quinolone resistance: two decades on. Drug Resist Updat 29:13–29. doi: 10.1016/j.drup.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 134.Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, Citton R, D'Inzeo T, Fadda G, Cauda R, Spanu T. 2007. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 51:1987–1994. doi: 10.1128/AAC.01509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Endimiani A, Luzzaro F, Perilli M, Lombardi G, Colì A, Tamborini A, Amicosante G, Toniolo A. 2004. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofloxacin. Clin Infect Dis 38:243–251. doi: 10.1086/380645. [DOI] [PubMed] [Google Scholar]
- 136.Rodríguez-Martínez JM, Pichardo C, García I, Pachón-Ibañez ME, Docobo-Pérez F, Pascual A, Pachón J, Martínez-Martínez L. 2008. Activity of ciprofloxacin and levofloxacin in experimental pneumonia caused by Klebsiella pneumoniae deficient in porins, expressing active efflux and producing QnrA1. Clin Microbiol Infect 14:691–697. doi: 10.1111/j.1469-0691.2008.02020.x. [DOI] [PubMed] [Google Scholar]
- 137.Allou N, Cambau E, Massias L, Chau F, Fantin B. 2009. Impact of low-level resistance to fluoroquinolones due to qnrA1 and qnrS1 genes or a gyrA mutation on ciprofloxacin bactericidal activity in a murine model of Escherichia coli urinary tract infection. Antimicrob Agents Chemother 53:4292–4297. doi: 10.1128/AAC.01664-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Briales A, Rodríguez-Martínez JM, Velasco C, Díaz de Alba P, Domínguez-Herrera J, Pachón J, Pascual A. 2011. In vitro effect of qnrA1, qnrB1, and qnrS1 genes on fluoroquinolone activity against isogenic Escherichia coli isolates with mutations in gyrA and parC. Antimicrob Agents Chemother 55:1266–1269. doi: 10.1128/AAC.00927-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jakobsen L, Cattoir V, Jensen KS, Hammerum AM, Nordmann P, Frimodt-Møller N. 2012. Impact of low-level fluoroquinolone resistance genes qnrA1, qnrB19 and qnrS1 on ciprofloxacin treatment of isogenic Escherichia coli strains in a murine urinary tract infection model. J Antimicrob Chemother 67:2438–2444. doi: 10.1093/jac/dks224. [DOI] [PubMed] [Google Scholar]
- 140.Domínguez-Herrera J, Velasco C, Docobo-Pérez F, Rodríguez-Martínez JM, López-Rojas R, Briales A, Pichardo C, Díaz-de-Alba P, Rodríguez-Baño J, Pascual A, Pachón J. 2013. Impact of qnrA1, qnrB1 and qnrS1 on the efficacy of ciprofloxacin and levofloxacin in an experimental pneumonia model caused by Escherichia coli with or without the GyrA mutation Ser83Leu. J Antimicrob Chemother 68:1609–1615. doi: 10.1093/jac/dkt063. [DOI] [PubMed] [Google Scholar]
- 141.Daoud Z, Sokhn ES, Azar E, Masri K, Doron S. 2014. Mutant prevention concentrations of ciprofloxacin against urinary isolates of Escherichia coli and Klebsiella pneumoniae. J Infect Dev Ctries 8:154–159. doi: 10.3855/jidc.3164. [DOI] [PubMed] [Google Scholar]
- 142.Liao CH, Hsueh PR, Jacoby GA, Hooper DC. 2013. Risk factors and clinical characteristics of patients with qnr-positive Klebsiella pneumoniae bacteraemia. J Antimicrob Chemother 68:2907–2914. doi: 10.1093/jac/dkt295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee CC, Lui G, Ip M, Ling TK, Lee N. 2012. Frequent detection of plasmid-mediated quinolone resistance (qnr) genes in multidrug-resistant Enterobacteriaceae blood isolates, Hong Kong. Eur J Clin Microbiol Infect Dis 31:3183–3189. doi: 10.1007/s10096-012-1683-x. [DOI] [PubMed] [Google Scholar]
- 144.Chong YP, Choi SH, Kim ES, Song EH, Lee EJ, Park KH, Cho OH, Kim SH, Lee SO, Kim MN, Jeong JY, Woo JH, Kim YS. 2010. Bloodstream infections caused by qnr-positive Enterobacteriaceae: clinical and microbiologic characteristics and outcomes. Diagn Microbiol Infect Dis 67:70–77. doi: 10.1016/j.diagmicrobio.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 145.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pitout JD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee CR, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. 2016. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context treatment options, and detection methods. Front Microbiol 7:895. doi: 10.3389/fmicb.2016.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rodríguez-Baño J, Cisneros JM, Gudiol C, Martínez JA. 2014. Treatment of infections caused by carbapenemase-producing Enterobacteriaceae. Enferm Infecc Microbiol Clin 32(Suppl 4):S49–S55. doi: 10.1016/S0213-005X(14)70174-0. [DOI] [PubMed] [Google Scholar]
- 150.Delgado-Valverde M, Sojo-Dorado J, Pascual A, Rodríguez-Baño J. 2013. Clinical management of infections caused by multidrug-resistant Enterobacteriaceae. Ther Adv Infect Dis 1:49–69. doi: 10.1177/2049936113476284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. 2015. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Falagas ME, Karageorgopoulos DE, Nordmann P. 2011. Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiol 6:653–666. doi: 10.2217/fmb.11.49. [DOI] [PubMed] [Google Scholar]
- 153.Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. 2011. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents 37:415–419. doi: 10.1016/j.ijantimicag.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 154.Ahn C, Syed A, Hu F, O'Hara JA, Rivera JI, Doi Y. 2014. Microbiological features of KPC-producing Enterobacter isolates identified in a U.S. hospital system. Diagn Microbiol Infect Dis 80:154–158. doi: 10.1016/j.diagmicrobio.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mediavilla JR, Patrawalla A, Chen L, Chavda KD, Mathema B, Vinnard C, Dever LL, Kreiswirth BN. 2016. Colistin- and carbapenem-resistant Escherichia coli harboring mcr-1 and blaNDM-5, causing a complicated urinary tract infection in a patient from the United States. mBio 7:e01191-16. doi: 10.1128/mBio.01191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Murri R, Fiori B, Spanu T, Mastrorosa I, Giovannenze F, Taccari F, Palazzolo C, Scoppettuolo G, Ventura G, Sanguinetti M, Cauda R, Fantoni M. 2017. Trimethoprim-sulfamethoxazole therapy for patients with carbapenemase-producing Klebsiella pneumoniae infections: retrospective single-center case series. Infection 45:209–213. doi: 10.1007/s15010-016-0968-x. [DOI] [PubMed] [Google Scholar]
- 157.Perez F, El Chakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. 2016. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply “precision medicine” to antimicrobial chemotherapy? Expert Opin Pharmacother 17:761–781. doi: 10.1517/14656566.2016.1145658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cobo J, Morosini MI, Pintado V, Tato M, Samaranch N, Baquero F, Cantón R. 2008. Use of tigecycline for the treatment of prolonged bacteremia due to a multiresistant VIM-1 and SHV-12 beta-lactamase-producing Klebsiella pneumoniae epidemic clone. Diagn Microbiol Infect Dis 60:319–322. doi: 10.1016/j.diagmicrobio.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 159.Tascini C, Urbani L, Biancofiore G, Rossolini GM, Leonildi A, Gemignani G, Bindi ML, Mugnaioli C, Filipponi F, Menichetti F. 2008. Colistin in combination with rifampin and imipenem for treating a blaVIM-1 metallo-beta-lactamase-producing Enterobacter cloacae disseminated infection in a liver transplant patient. Minerva Anestesiol 74:47–49. [PubMed] [Google Scholar]
- 160.Souli M, Rekatsina PD, Chryssouli Z, Galani I, Giamarellou H, Kanellakopoulou K. 2009. Does the activity of the combination of imipenem and colistin in vitro exceed the problem of resistance in metallo-beta-lactamase-producing Klebsiella pneumoniae isolates? Antimicrob Agents Chemother 53:2133–2135. doi: 10.1128/AAC.01271-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Souli M, Galani I, Boukovalas S, Gourgoulis MG, Chryssouli Z, Kanellakopoulou K, Panagea T, Giamarellou H. 2011. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrob Agents Chemother 55:2395–2397. doi: 10.1128/AAC.01086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wiskirchen DE, Koomanachai P, Nicasio AM, Nicolau DP, Kuti JL. 2011. In vitro pharmacodynamics of simulated pulmonary exposures of tigecycline alone and in combination against Klebsiella pneumoniae isolates producing a KPC carbapenemase. Antimicrob Agents Chemother 55:1420–1427. doi: 10.1128/AAC.01253-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Elemam A, Rahimian J, Doymaz M. 2010. In vitro evaluation of antibiotic synergy for polymyxin B-resistant carbapenemase-producing Klebsiella pneumoniae. J Clin Microbiol 48:3558–3562. doi: 10.1128/JCM.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bulik CC, Nicolau DP. 2010. In vivo efficacy of simulated human dosing regimens of prolonged-infusion doripenem against carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 54:4112–4115. doi: 10.1128/AAC.00026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Le J, McKee B, Srisupha-Olarn W, Burgess DS. 2011. In vitro activity of carbapenems alone and in combination with amikacin against KPC-producing Klebsiella pneumoniae. J Clin Med Res 3:106–110. doi: 10.4021/jocmr551w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Berçot B, Poirel L, Dortet L, Nordmann P. 2011. In vitro evaluation of antibiotic synergy for NDM-1-producing Enterobacteriaceae. J Antimicrob Chemother 66:2295–2297. doi: 10.1093/jac/dkr296. [DOI] [PubMed] [Google Scholar]
- 167.Samonis G, Maraki S, Karageorgopoulos DE, Vouloumanou EK, Falagas ME. 2012. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur J Clin Microbiol Infect Dis 31:695–701. doi: 10.1007/s10096-011-1360-5. [DOI] [PubMed] [Google Scholar]
- 168.Pankey GA, Ashcraft DS. 2011. Detection of synergy using the combination of polymyxin B with either meropenem or rifampin against carbapenemase-producing Klebsiella pneumoniae. Diagn Microbiol Infect Dis 70:561–564. doi: 10.1016/j.diagmicrobio.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 169.Jernigan MG, Press EG, Nguyen MH, Clancy CJ, Shields RK. 2012. The combination of doripenem and colistin is bactericidal and synergistic against colistin-resistant, carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 56:3395–3398. doi: 10.1128/AAC.06364-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hirsch EB, Guo B, Chang KT, Cao H, Ledesma KR, Singh M, Tam VH. 2013. Assessment of antimicrobial combinations for Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. J Infect Dis 207:786–793. doi: 10.1093/infdis/jis766. [DOI] [PubMed] [Google Scholar]
- 171.Tascini C, Tagliaferri E, Giani T, Leonildi A, Flammini S, Casini B, Lewis R, Ferranti S, Rossolini GM, Menichetti F. 2013. Synergistic activity of colistin plus rifampin against colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 57:3990–3993. doi: 10.1128/AAC.00179-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee GC, Burgess DS. 2013. Polymyxins and doripenem combination against KPC-producing Klebsiella pneumoniae. J Clin Med Res 5:97–100. doi: 10.4021/jocmr1220w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hong JH, Clancy CJ, Cheng S, Shields RK, Chen L, Doi Y, Zhao Y, Perlin DS, Kreiswirth BN, Nguyen MH. 2013. Characterization of porin expression in Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae identifies isolates most susceptible to the combination of colistin and carbapenems. Antimicrob Agents Chemother 57:2147–2153. doi: 10.1128/AAC.02411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nastro M, Rodríguez CH, Monge R, Zintgraff J, Neira L, Rebollo M, Vay C, Famiglietti A. 2014. Activity of the colistin-rifampicin combination against colistin-resistant, carbapenemase-producing Gram-negative bacteria. J Chemother 26:211–216. doi: 10.1179/1973947813Y.0000000136. [DOI] [PubMed] [Google Scholar]
- 175.Demiraslan H, Dinc G, Ahmed SS, Elmali F, Metan G, Alp E, Doganay M. 2014. Carbapenem-resistant Klebsiella pneumoniae sepsis in corticosteroid receipt mice: tigecycline or colistin monotherapy versus tigecycline/colistin combination. J Chemother 26:276–281. doi: 10.1179/1973947813Y.0000000143. [DOI] [PubMed] [Google Scholar]
- 176.Michail G, Labrou M, Pitiriga V, Manousaka S, Sakellaridis N, Tsakris A, Pournaras S. 2013. Activity of tigecycline in combination with colistin, meropenem, rifampin, or gentamicin against KPC-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother 57:6028–6033. doi: 10.1128/AAC.00891-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gaibani P, Lombardo D, Lewis RE, Mercuri M, Bonora S, Landini MP, Ambretti S. 2014. In vitro activity and post-antibiotic effects of colistin in combination with other antimicrobials against colistin-resistant KPC-producing Klebsiella pneumoniae bloodstream isolates. J Antimicrob Chemother 69:1856–1865. doi: 10.1093/jac/dku065. [DOI] [PubMed] [Google Scholar]
- 178.Clancy CJ, Hao B, Shields RK, Chen L, Perlin DS, Kreiswirth BN, Nguyen MH. 2014. Doripenem, gentamicin, and colistin, alone and in combinations, against gentamicin-susceptible, KPC-producing Klebsiella pneumoniae strains with various ompK36 genotypes. Antimicrob Agents Chemother 58:3521–3525. doi: 10.1128/AAC.01949-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tängdén T, Hickman RA, Forsberg P, Lagerbäck P, Giske CG, Cars O. 2014. Evaluation of double- and triple-antibiotic combinations for VIM- and NDM-producing Klebsiella pneumoniae by in vitro time-kill experiments. Antimicrob Agents Chemother 58:1757–1762. doi: 10.1128/AAC.00741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chua NG, Zhou YP, Tan TT, Lingegowda PB, Lee W, Lim TP, Teo J, Cai Y, Kwa AL. 2015. Polymyxin B with dual carbapenem combination therapy against carbapenemase-producing Klebsiella pneumoniae. J Infect 70:309–311. doi: 10.1016/j.jinf.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 181.Maraki S, Papadakis IS. 2015. Evaluation of antimicrobial combinations against colistin-resistant carbapenemase (KPC)-producing Klebsiella pneumoniae. J Chemother 27:348–352. doi: 10.1179/1973947814Y.0000000218. [DOI] [PubMed] [Google Scholar]
- 182.Toledo PV, Tuon FF, Arend L, Aranha AA Jr. 2014. Efficacy of tigecycline, polymyxin, gentamicin, meropenem and associations in experimental Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae non-lethal sepsis. Braz J Infect Dis 18:574–575. doi: 10.1016/j.bjid.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Krishnappa LG, Marie MA, Al Sheikh YA. 2015. Characterization of carbapenem resistance mechanisms in Klebsiella pneumoniae and in vitro synergy of the colistin-meropenem combination. J Chemother 27:277–282. doi: 10.1179/1973947814Y.0000000197. [DOI] [PubMed] [Google Scholar]
- 184.Oliva A, Mascellino MT, Cipolla A, D'Abramo A, De Rosa A, Savinelli S, Ciardi MR, Mastroianni CM, Vullo V. 2015. Therapeutic strategy for pandrug-resistant Klebsiella pneumoniae severe infections: short-course treatment with colistin increases the in vivo and in vitro activity of double carbapenem regimen. Int J Infect Dis 33:132–134. doi: 10.1016/j.ijid.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 185.Shields RK, Nguyen MH, Potoski BA, Press EG, Chen L, Kreiswirth BN, Clarke LG, Eschenauer GA, Clancy CJ. 2015. Doripenem MICs and ompK36 porin genotypes of sequence type 258, KPC-producing Klebsiella pneumoniae may predict responses to carbapenem-colistin combination therapy among patients with bacteremia. Antimicrob Agents Chemother 59:1797–1801. doi: 10.1128/AAC.03894-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ji S, Lv F, Du X, Wei Z, Fu Y, Mu X, Jiang Y, Yu Y. 2015. Cefepime combined with amoxicillin/clavulanic acid: a new choice for the KPC-producing K. pneumoniae infection. Int J Infect Dis 38:108–114. doi: 10.1016/j.ijid.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 187.Stein C, Makarewicz O, Bohnert JA, Pfeifer Y, Kesselmeier M, Hagel S, Pletz MW. 2015. Three dimensional checkerboard synergy analysis of colistin, meropenem, tigecycline against multidrug-resistant clinical Klebsiella pneumoniae isolates. PLoS One 10:e0126479. doi: 10.1371/journal.pone.0126479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nakamura I, Sakamoto N, Ida Y, Imai R, Aoki K, Ando R, Yamaguchi T, Matsumura H, Matsumoto T. 2015. Combination therapy against polymicrobial infection, including by NDM-1-producing Enterobacteriaceae resistant to colistin. Antimicrob Agents Chemother 59:5092–5093. doi: 10.1128/AAC.00746-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Abdul Rahim N, Cheah SE, Johnson MD, Yu H, Sidjabat HE, Boyce J, Butler MS, Cooper MA, Fu J, Paterson DL, Nation RL, Bergen PJ, Velkov T, Li J. 2015. Synergistic killing of NDM-producing MDR Klebsiella pneumoniae by two ‘old’ antibiotics—polymyxin B and chloramphenicol. J Antimicrob Chemother 70:2589–2597. doi: 10.1093/jac/dkv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Salloum NA, Kissoyan KA, Fadlallah S, Cheaito K, Araj GF, Wakim R, Kanj S, Kanafani Z, Dbaibo G, Matar GM. 2015. Assessment of combination therapy in BALB/c mice injected with carbapenem-resistant Enterobacteriaceae strains. Front Microbiol 6:999. doi: 10.3389/fmicb.2015.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rodríguez-Avial I, Pena I, Picazo JJ, Rodríguez-Avial C, Culebras E. 2015. In vitro activity of the next-generation aminoglycoside plazomicin alone and in combination with colistin, meropenem, fosfomycin or tigecycline against carbapenemase-producing Enterobacteriaceae strains. Int J Antimicrob Agents 46:616–621. doi: 10.1016/j.ijantimicag.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 192.Albur MS, Noel A, Bowker K, MacGowan A. 2015. The combination of colistin and fosfomycin is synergistic against NDM-1-producing Enterobacteriaceae in vitro pharmacokinetic/pharmacodynamic model experiments. Int J Antimicrob Agents 46:560–567. doi: 10.1016/j.ijantimicag.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 193.Gagetti P, Pasteran F, Martinez MP, Fatouraei M, Gu J, Fernandez R, Paz L, Rose WE, Corso A, Rosato AE. 2016. Modeling meropenem treatment, alone and in combination with daptomycin, for KPC-producing Klebsiella pneumoniae strains with unusually low carbapenem MICs. Antimicrob Agents Chemother 60:5047–5050. doi: 10.1128/AAC.00168-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Albiero J, Sy SK, Mazucheli J, Caparroz-Assef SM, Costa BB, Alves JL, Gales AC, Tognim MC. 2016. Pharmacodynamic evaluation of the potential clinical utility of fosfomycin and meropenem in combination therapy against KPC-2-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 60:4128–4139. doi: 10.1128/AAC.03099-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ni W, Wei C, Zhou C, Zhao J, Liang B, Cui J, Wang R, Liu Y. 2016. Tigecycline-amikacin combination effectively suppresses the selection of resistance in clinical isolates of KPC-producing Klebsiella pneumoniae. Front Microbiol 7:1304. doi: 10.3389/fmicb.2016.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Laishram S, Anandan S, Devi BY, Elakkiya M, Priyanka B, Bhuvaneshwari T, Peter JV, Subramani K, Balaji V. 2016. Determination of synergy between sulbactam, meropenem and colistin in carbapenem-resistant Klebsiella pneumoniae and Acinetobacter baumannii isolates and correlation with the molecular mechanism of resistance. J Chemother 28:297–303. doi: 10.1080/1120009X.2016.1143261. [DOI] [PubMed] [Google Scholar]
- 197.Lagerbäck P, Khine WW, Giske CG, Tängdén T. 2016. Evaluation of antibacterial activities of colistin, rifampicin and meropenem combinations against NDM-1-producing Klebsiella pneumoniae in 24 h in vitro time-kill experiments. J Antimicrob Chemother 71:2321–2325. doi: 10.1093/jac/dkw213. [DOI] [PubMed] [Google Scholar]
- 198.Sharma R, Patel S, Abboud C, Diep J, Ly NS, Pogue JM, Kaye KS, Li J, Rao GG. 2017. Polymyxin B in combination with meropenem against carbapenemase-producing Klebsiella pneumoniae: pharmacodynamics and morphological changes. Int J Antimicrob Agents 49:224–232. doi: 10.1016/j.ijantimicag.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.de Maio Carrillho CM, Gaudereto JJ, Martins RC, de Castro Lima VA, de Oliveira LM, Urbano MR, Perozin JS, Levin AS, Costa SF. 2017. Colistin-resistant Enterobacteriaceae infections: clinical and molecular characterization and analysis of in vitro synergy. Diagn Microbiol Infect Dis 87:253–257. doi: 10.1016/j.diagmicrobio.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 200.Diep JK, Jacobs DM, Sharma R, Covelli J, Bowers DR, Russo TA, Rao GG. 2017. Polymyxin B in combination with rifampin and meropenem against polymyxin B-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 61:e02121-16. doi: 10.1128/AAC.02456-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ozbek B, Mataracı-Kara E, Er S, Ozdamar M, Yilmaz M. 2015. In vitro activities of colistin, tigecycline and tobramycin, alone or in combination, against carbapenem-resistant Enterobacteriaceae strains. J Glob Antimicrob Resist 3:278–282. doi: 10.1016/j.jgar.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 202.Huang D, Yu B, Diep JK, Sharma R, Dudley M, Monteiro J, Kaye KS, Pogue JM, Abboud CS, Rao GG. 2017. In vitro assessment of combined polymyxin B and minocycline therapy against Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae. Antimicrob Agents Chemother 61:e00073-17. doi: 10.1128/AAC.00073-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oliva A, Scorzolini L, Cipolla A, Mascellino MT, Cancelli F, Castaldi D, D'Abramo A, D'Agostino C, Russo G, Ciardi MR, Mastroianni CM, Vullo V. 2017. In vitro evaluation of different antimicrobial combinations against carbapenemase-producing Klebsiella pneumoniae: the activity of the double-carbapenem regimen is related to meropenem MIC value. J Antimicrob Chemother 72:1981–1984. doi: 10.1093/jac/dkx084. [DOI] [PubMed] [Google Scholar]
- 204.Zhou YF, Tao MT, Feng Y, Yang RS, Liao XP, Liu YH, Sun J. 2017. Increased activity of colistin in combination with amikacin against Escherichia coli co-producing NDM-5 and MCR-1. J Antimicrob Chemother 72:1723–1730. doi: 10.1093/jac/dkx038. [DOI] [PubMed] [Google Scholar]
- 205.Tseng SP, Wang SF, Ma L, Wang TY, Yang TY, Siu LK, Chuang YC, Lee PS, Wang JT, Wu TL, Lin JC, Lu PL. 2017. The plasmid-mediated fosfomycin resistance determinants and synergy of fosfomycin and meropenem in carbapenem-resistant Klebsiella pneumoniae isolates in Taiwan. J Microbiol Immunol Infect 50:653–661. doi: 10.1016/j.jmii.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 206.Yu W, Zhou K, Guo L, Ji J, Niu T, Xiao T, Shen P, Xiao Y. 2017. In vitro pharmacokinetics/pharmacodynamics evaluation of fosfomycin combined with amikacin or colistin against KPC2-producing Klebsiella pneumoniae. Front Cell Infect Microbiol 7:246. doi: 10.3389/fcimb.2017.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yu W, Shen P, Bao Z, Zhou K, Zheng B, Ji J, Guo L, Huang C, Xiao Y. 2017. In vitro antibacterial activity of fosfomycin combined with other antimicrobials against KPC-producing Klebsiella pneumoniae. Int J Antimicrob Agents 50:237–241. doi: 10.1016/j.ijantimicag.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 208.Wenzler E, Deraedt MF, Harrington AT, Danizger LH. 2017. Synergistic activity of ceftazidime-avibactam and aztreonam against serine and metallo-β-lactamase-producing gram-negative pathogens. Diagn Microbiol Infect Dis 88:352–354. doi: 10.1016/j.diagmicrobio.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 209.Fredborg M, Sondergaard TE, Wang M. 2017. Synergistic activities of meropenem double and triple combinations against carbapenemase-producing Enterobacteriaceae. Diagn Microbiol Infect Dis 88:355–360. doi: 10.1016/j.diagmicrobio.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 210.Zhao M, Bulman ZP, Lenhard JR, Satlin MJ, Kreiswirth BN, Walsh TJ, Marrocco A, Bergen PJ, Nation RL, Li J, Zhang J, Tsuji BT. 2017. Pharmacodynamics of colistin and fosfomycin: a ‘treasure trove’ combination combats KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother 72:1985–1990. doi: 10.1093/jac/dkx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. 2013. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Balkan II, Aygün G, Aydın S, Mutcalı SI, Kara Z, Kuşkucu M, Midilli K, Şemen V, Aras S, Yemişen M, Mete B, Özaras R, Saltoğlu N, Tabak F, Öztürk R. 2014. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis 26:51–56. doi: 10.1016/j.ijid.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 213.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP, Tarasi A, Parisi G, Lappa A, Carattoli A, Petrosillo N, SEERBIO-GRAB Network. 2013. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect 19:E23–E30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 214.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.de Oliveira MS, de Assis DB, Freire MP, Boas do Prado GV, Machado AS, Abdala E, Pierrotti LC, Mangini C, Campos L, Caiaffa Filho HH, Levin AS. 2015. Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect 21:179.e1–179.e7. doi: 10.1016/j.cmi.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 216.Gomez-Simmonds A, Nelson B, Eiras DP, Loo A, Jenkins SG, Whittier S, Calfee DP, Satlin MJ, Kubin CJ, Furuya EY. 2016. Combination regimens for treatment of carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 60:3601–3607. doi: 10.1128/AAC.03007-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Cantón R, Doi Y, Tuon FF, Karaiskos I, Pérez-Nadales E, Schwaber MJ, Azap Ö, Souli KM, Roilides E, Pournaras S, Akova M, Pérez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J, REIPI/ESGBIS/INCREMENT Investigators. 2017. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 17:726–734. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 218.Machuca I, Gutiérrez-Gutiérrez B, Gracia-Ahufinger I, Rivera Espinar F, Cano Á, Guzmán-Puche J, Pérez-Nadales E, Natera C, Rodríguez M, León R, Castón JJ, Rodríguez-López F, Rodríguez-Baño J, Torre-Cisneros J. 2017. Mortality associated with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance: importance of combination therapy without colistin and carbapenems. Antimicrob Agents Chemother 61:e00406-17. doi: 10.1128/AAC.00406-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nabarro LEB, Shankar C, Pragasam AK, Mathew G, Jeyaseelan V, Veeraraghavan B, Verghese VP. 2017. Clinical and bacterial risk factors for mortality in children with carbapenem-resistant Enterobacteriaceae bloodstream infections in India. Pediatr Infect Dis J 36:161–166. doi: 10.1097/INF.0000000000001499. [DOI] [PubMed] [Google Scholar]
- 220.Navarro-San Francisco C, Mora-Rillo M, Romero-Gómez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, Gómez-Gil R, Arribas-López JR, Mingorance J, Paño-Pardo JR. 2013. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect 19:E72–E79. doi: 10.1111/1469-0691.12091. [DOI] [PubMed] [Google Scholar]
- 221.Papadimitriou-Olivgeris M, Fligou F, Bartzavali C, Zotou A, Spyropoulou A, Koutsileou K, Vamvakopoulou S, Sioulas N, Karamouzos V, Anastassiou ED, Spiliopoulou I, Christofidou M, Marangos M. 2017. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: risk factors and predictors of mortality. Eur J Clin Microbiol Infect Dis 36:1125–1131. doi: 10.1007/s10096-017-2899-6. [DOI] [PubMed] [Google Scholar]
- 222.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Satlin MJ, Chen L, Patel G, Gomez-Simmonds A, Weston G, Kim AC, Seo SK, Rosenthal ME, Sperber SJ, Jenkins SG, Hamula CL, Uhlemann AC, Levi MH, Fries BC, Tang YW, Juretschko S, Rojtman AD, Hong T, Mathema B, Jacobs MR, Walsh TJ, Bonomo RA, Kreiswirth BN. 2017. Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant Enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrob Agents Chemother 61:e02349-16. doi: 10.1128/AAC.02349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tofas P, Skiada A, Angelopoulou M, Sipsas N, Pavlopoulou I, Tsaousi S, Pagoni M, Kotsopoulou M, Perlorentzou S, Antoniadou A, Pirounaki M, Skoutelis A, Daikos GL. 2016. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections in neutropenic patients with haematological malignancies or aplastic anaemia: analysis of 50 cases. Int J Antimicrob Agents 47:335–339. doi: 10.1016/j.ijantimicag.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 225.Trecarichi EM, Pagano L, Martino B, Candoni A, Di Blasi R, Nadali G, Fianchi L, Delia M, Sica S, Perriello V, Busca A, Aversa F, Fanci R, Melillo L, Lessi F, Del Principe MI, Cattaneo C, Tumbarello M, Haematologic Malignancies Associated Bloodstream Infections Surveillance (HEMABIS) Registry-Sorveglianza Epidemiologica Infezioni Funginein Emopatie Maligne (SEIFEM) Group, Italy. 2016. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol 91:1076–1081. doi: 10.1002/ajh.24489. [DOI] [PubMed] [Google Scholar]
- 226.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 227.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 228.Villegas MV, Pallares CJ, Escandón-Vargas K, Hernández-Gómez C, Correa A, Álvarez C, Rosso F, Matta L, Luna C, Zurita J, Mejía-Villatoro C, Rodríguez-Noriega E, Seas C, Cortesía M, Guzmán-Suárez A, Guzmán-Blanco M. 2016. Characterization and clinical impact of bloodstream infection caused by carbapenemase-producing Enterobacteriaceae in seven Latin American countries. PLoS One 11:e0154092. doi: 10.1371/journal.pone.0154092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 17:1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 230.Chang YY, Chuang YC, Siu LK, Wu TL, Lin JC, Lu PL, Wang JT, Wang LS, Lin YT, Huang LJ, Fung CP. 2015. Clinical features of patients with carbapenem nonsusceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J Microbiol Immunol Infect 48:219–225. doi: 10.1016/j.jmii.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 231.de Maio Carrilho CM, de Oliveira LM, Gaudereto J, Perozin JS, Urbano MR, Camargo CH, Grion CM, Levin AS, Costa SF. 2016. A prospective study of treatment of carbapenem-resistant Enterobacteriaceae infections and risk factors associated with outcome. BMC Infect Dis 16:629. doi: 10.1186/s12879-016-1979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Díaz A, Ortiz DC, Trujillo M, Garcés C, Jaimes F, Restrepo AV. 2016. Clinical characteristics of carbapenem-resistant Klebsiella pneumoniae infections in ill and colonized children in Colombia. Pediatr Infect Dis J 35:237–241. doi: 10.1097/INF.0000000000000987. [DOI] [PubMed] [Google Scholar]
- 233.Freire MP, Pierrotti LC, Filho HH, Ibrahim KY, Magri AS, Bonazzi PR, Hajar L, Diz MP, Pereira J, Hoff PM, Abdala E. 2015. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae in cancer patients. Eur J Clin Microbiol Infect Dis 34:277–286. doi: 10.1007/s10096-014-2233-5. [DOI] [PubMed] [Google Scholar]
- 234.Katsiari M, Panagiota G, Likousi S, Roussou Z, Polemis M, Alkiviadis Vatopoulos C, Evangelia Platsouka D, Maguina A. 2015. Carbapenem-resistant Klebsiella pneumoniae infections in a Greek intensive care unit: molecular characterisation and treatment challenges. J Glob Antimicrob Resist 3:123–127. doi: 10.1016/j.jgar.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 235.Lowman W, Schleicher G. 2015. Antimicrobial treatment and outcomes of critically ill patients with OXA-48like carbapenemase-producing Enterobacteriaceae infections. Diagn Microbiol Infect Dis 81:138–140. doi: 10.1016/j.diagmicrobio.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 236.Tuon FF, Graf ME, Merlini A, Rocha JL, Stallbaum S, Arend LN, Pecoit-Filho R. 2017. Risk factors for mortality in patients with ventilator-associated pneumonia caused by carbapenem-resistant Enterobacteriaceae. Braz J Infect Dis 21:1–6. doi: 10.1016/j.bjid.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. 2014. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother 58:654–663. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zusman O, Altunin S, Koppel F, Dishon Benattar Y, Gedik H, Paul M. 2017. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: systematic review and meta-analysis. J Antimicrob Chemother 72:29–39. doi: 10.1093/jac/dkw377. [DOI] [PubMed] [Google Scholar]
- 239.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Pintado V, Doi Y, Tuon FF, Karaiskos I, Machuca I, Schwaber MJ, Azap Ö, Souli KM, Roilides E, Pournaras S, Akova M, Pérez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J, REIPI/ESGBIS/INCREMENT Investigators. 2016. A predictive model of mortality in patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae. Mayo Clin Proc 91:1362–1371. doi: 10.1016/j.mayocp.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 240.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, Bassetti M, Malacarne P, Petrosillo N, Galdieri N, Mocavero P, Corcione A, Viscoli C, Zarrilli R, Gallo C, Utili R. 2013. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 57:349–358. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 241.Cuzon G, Naas T, Truong H, Villegas MV, Wisell KT, Carmeli Y, Gales AC, Venezia SN, Quinn JP, Nordmann P. 2010. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg Infect Dis 16:1349–1356. doi: 10.3201/eid1609.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dautzenberg MJ, Ossewaarde JM, de Kraker ME, van der Zee A, van Burgh S, de Greeff SC, Bijlmer HA, Grundmann H, Cohen Stuart JW, Fluit AC, Troelstra A, Bonten MJ. 2014. Successful control of a hospital-wide outbreak of OXA-48 producing Enterobacteriaceae in the Netherlands, 2009 to 2011. Euro Surveill 19:20723. doi: 10.2807/1560-7917.ES2014.19.9.20723. [DOI] [PubMed] [Google Scholar]
- 243.Kuti JL, Dandekar PK, Nightingale CH, Nicolau DP. 2003. Use of Monte Carlo simulation to design an optimized pharmacodynamic dosing strategy for meropenem. J Clin Pharmacol 43:1116–1123. doi: 10.1177/0091270003257225. [DOI] [PubMed] [Google Scholar]
- 244.Daikos GL, Markogiannakis A. 2011. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 17:1135–1141. doi: 10.1111/j.1469-0691.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 245.Giannella M, Trecarichi EM, Giacobbe DR, De FG, Bassetti M, Bartoloni A, Bartoletti M, Losito AR, Del Bono V, Corcione S, Tedeschi S, Raffaelli F, Saffioti C, Spanu T, Rossolini GM, Marchese A, Ambretti S, Cauda R, Viscoli C, Lewis RE, Viale P, Tumbarello M, Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva (ISGRI-SITA). 2017. Effect of combination therapy containing a high dose carbapenem on mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents 2017:S0924-8579(17)30311-4. doi: 10.1016/j.ijantimicag.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 246.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2013. Efficacy of humanized carbapenem exposures against New Delhi metallo-β-lactamase (NDM-1)-producing Enterobacteriaceae in a murine infection model. Antimicrob Agents Chemother 57:3936–3940. doi: 10.1128/AAC.00708-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hagihara M, Crandon JL, Urban C, Nicolau DP. 2013. Efficacy of doripenem and ertapenem against KPC-2-producing and non-KPC-producing Klebsiella pneumoniae with similar MICs. J Antimicrob Chemother 68:1616–1618. doi: 10.1093/jac/dkt056. [DOI] [PubMed] [Google Scholar]
- 248.Anderson KF, Lonsway DR, Rasheed JK, Biddle J, Jensen B, McDougal LK, Carey RB, Thompson A, Stocker S, Limbago B, Patel JB. 2007. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 45:2723–2725. doi: 10.1128/JCM.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bulik CC, Nicolau DP. 2011. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 55:3002–3004. doi: 10.1128/AAC.01420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Oliva A, D'Abramo A, D'Agostino C, Iannetta M, Mascellino MT, Gallinelli C, Mastroianni CM, Vullo V. 2014. Synergistic activity and effectiveness of a double-carbapenem regimen in pandrug-resistant Klebsiella pneumoniae bloodstream infections. J Antimicrob Chemother 69:1718–1720. doi: 10.1093/jac/dku027. [DOI] [PubMed] [Google Scholar]
- 251.Oliva A, Gizzi F, Mascellino MT, Cipolla A, D'Abramo A, D'Agostino C, Trinchieri V, Russo G, Tierno F, Iannetta M, Mastroianni CM, Vullo V. 2016. Bactericidal and synergistic activity of double-carbapenem regimen for infections caused by carbapenemase-producing Klebsiella pneumoniae. Clin Microbiol Infect 22:147–153. doi: 10.1016/j.cmi.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 252.Cprek JB, Gallagher JC. 2015. Ertapenem-containing double-carbapenem therapy for treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother 60:669–673. doi: 10.1128/AAC.01569-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Souli M, Karaiskos I, Masgala A, Galani L, Barmpouti E, Giamarellou H. 2017. Double-carbapenem combination as salvage therapy for untreatable infections by KPC-2-producing Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis 36:1305–1315. doi: 10.1007/s10096-017-2936-5. [DOI] [PubMed] [Google Scholar]
- 254.De Pascale G, Martucci G, Montini L, Panarello G, Cutuli SL, Di Carlo D, Di Gravio V, Di Stefano R, Capitanio G, Vallecoccia MS, Polidori P, Spanu T, Arcadipane A, Antonelli M. 2017. Double carbapenem as a rescue strategy for the treatment of severe carbapenemase-producing Klebsiella pneumoniae infections: a two-center, matched case-control study. Crit Care 21:173. doi: 10.1186/s13054-017-1769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 256.Zavascki AP, Goldani LZ, Li J, Nation RL. 2007. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother 60:1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- 257.Landman D, Georgescu C, Martin DA, Quale J. 2008. Polymyxins revisited. Clin Microbiol Rev 21:449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yahav D, Farbman L, Leibovici L, Paul M. 2012. Colistin: new lessons on an old antibiotic. Clin Microbiol Infect 18:18–29. doi: 10.1111/j.1469-0691.2011.03734.x. [DOI] [PubMed] [Google Scholar]
- 259.Rosso-Fernández C, Garnacho-Montero J, Antonelli M, Dimopoulos G, Cisneros JM, MagicBullet Study Group. 2015. Safety and efficacy of colistin versus meropenem in the empirical treatment of ventilator-associated pneumonia as part of a macro-project funded by the Seventh Framework Program of the European Commission studying off-patent antibiotics: study protocol for a randomized controlled trial. Trials 16:102. doi: 10.1186/s13063-015-0614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hirsch EB, Tam VH. 2010. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother 65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 261.Dickstein Y, Leibovici L, Yahav D, Eliakim-Raz N, Daikos GL, Skiada A, Antoniadou A, Carmeli Y, Nutman A, Levi I, Adler A, Durante-Mangoni E, Andini R, Cavezza G, Mouton JW, Wijma RA, Theuretzbacher U, Friberg LE, Kristoffersson AN, Zusman O, Koppel F, Dishon Benattar Y, Altunin S, Paul M, AIDA Consortium. 2016. Multicentre open-label randomised controlled trial to compare colistin alone with colistin plus meropenem for the treatment of severe infections caused by carbapenem-resistant Gram-negative infections (AIDA): a study protocol. BMJ Open 6:e009956. doi: 10.1136/bmjopen-2015-009956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bergen PJ, Li J, Nation RL. 2011. Dosing of colistin—back to basic PK/PD. Curr Opin Pharmacol 11:464–469. doi: 10.1016/j.coph.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. 2012. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 56:4241–4249. doi: 10.1128/AAC.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Karaiskos I, Friberg LE, Pontikis K, Ioannidis K, Tsagkari V, Galani L, Kostakou E, Baziaka F, Paskalis C, Koutsoukou A, Giamarellou H. 2015. Colistin population pharmacokinetics after application of a loading dose of 9 MU colistin methanesulfonate in critically ill patients. Antimicrob Agents Chemother 59:7240–7248. doi: 10.1128/AAC.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.EMA. 2017. European Medicines Agency completes review of polymyxin-based medicines. Recommendations issued for safe use in patients with serious infections resistant to standard antibiotics. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Polymyxin_31/WC500176333.pdf.
- 268.FDA. 2017. Approved drug products. Label and approval history for Coly-Mycin M, NDA 050108. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050108s030lbl.pdf.
- 269.Deryke CA, Crawford AJ, Uddin N, Wallace MR. 2010. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrob Agents Chemother 54:4503–4505. doi: 10.1128/AAC.01707-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gibson GA, Bauer SR, Neuner EA, Bass SN, Lam SW. 2015. Influence of colistin dose on global cure in patients with bacteremia due to carbapenem-resistant Gram-negative bacilli. Antimicrob Agents Chemother 60:431–436. doi: 10.1128/AAC.01414-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Elefritz JL, Bauer KA, Jones C, Mangino JE, Porter K, Murphy CV. 2017. Efficacy and safety of a colistin loading dose, high-dose maintenance regimen in critically ill patients with multidrug-resistant Gram-negative pneumonia. J Intensive Care Med 32:487–493. doi: 10.1177/0885066616646551. [DOI] [PubMed] [Google Scholar]
- 272.Benattar YD, Omar M, Zusman O, Yahav D, Zak-Doron Y, Altunin S, Elbaz M, Daitch V, Granot M, Leibovici L, Paul M. 2016. The effectiveness and safety of high-dose colistin: prospective cohort study. Clin Infect Dis 63:1605–1612. doi: 10.1093/cid/ciw684. [DOI] [PubMed] [Google Scholar]
- 273.Dalfino L, Puntillo F, Ondok MJ, Mosca A, Monno R, Coppolecchia S, Spada ML, Bruno F, Brienza N. 2015. Colistin-associated acute kidney injury in severely ill patients: a step toward a better renal care? A prospective cohort study. Clin Infect Dis 61:1771–1777. doi: 10.1093/cid/civ717. [DOI] [PubMed] [Google Scholar]
- 274.Lee YJ, Wi YM, Kwon YJ, Kim SR, Chang SH, Cho S. 2015. Association between colistin dose and development of nephrotoxicity. Crit Care Med 43:1187–1193. doi: 10.1097/CCM.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 275.Omrani AS, Alfahad WA, Shoukri MM, Baadani AM, Aldalbahi S, Almitwazi AA, Albarrak AM. 2015. High dose intravenous colistin methanesulfonate therapy is associated with high rates of nephrotoxicity; a prospective cohort study from Saudi Arabia. Ann Clin Microbiol Antimicrob 14:3. doi: 10.1186/s12941-015-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Vicari G, Bauer SR, Neuner EA, Lam SW. 2013. Association between colistin dose and microbiologic outcomes in patients with multidrug-resistant gram-negative bacteremia. Clin Infect Dis 56:398–404. doi: 10.1093/cid/cis909. [DOI] [PubMed] [Google Scholar]
- 277.Ordooei Javan A, Shokouhi S, Sahraei Z, Salamzadeh J, Azad Armaki S. 2017. Nephrotoxicity of high and conventional dosing regimens of colistin: a randomized clinical trial. Iran J Pharm Res 16:781–790. [PMC free article] [PubMed] [Google Scholar]
- 278.Nelson BC, Eiras DP, Gomez-Simmonds A, Loo AS, Satlin MJ, Jenkins SG, Whittier S, Calfee DP, Furuya EY, Kubin CJ. 2015. Clinical outcomes associated with polymyxin B dose in patients with bloodstream infections due to carbapenem-resistant Gram-negative rods. Antimicrob Agents Chemother 59:7000–7006. doi: 10.1128/AAC.00844-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhão RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. 2013. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57:524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 280.Zavascki AP, Nation RL. 2017. Nephrotoxicity of polymyxins: is there any difference between colistimethate and polymyxin B? Antimicrob Agents Chemother 61:e02319-16. doi: 10.1128/AAC.02319-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Vardakas KZ, Falagas ME. 2017. Colistin versus polymyxin B for the treatment of patients with multidrug-resistant Gram-negative infections: a systematic review and meta-analysis. Int J Antimicrob Agents 49:233–238. doi: 10.1016/j.ijantimicag.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 282.Kassamali Z, Danziger L. 2015. To B or not to B, that is the question: is it time to replace colistin with polymyxin B? Pharmacotherapy 35:17–21. doi: 10.1002/phar.1510. [DOI] [PubMed] [Google Scholar]
- 283.Abdelraouf K, He J, Ledesma KR, Hu M, Tam VH. 2012. Pharmacokinetics and renal disposition of polymyxin B in an animal model. Antimicrob Agents Chemother 56:5724–5727. doi: 10.1128/AAC.01333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Thamlikitkul V, Dubrovskaya Y, Manchandani P, Ngamprasertchai T, Boonyasiri A, Babic JT, Tam VH. 2017. Dosing and pharmacokinetics of polymyxin B in patients with renal insufficiency. Antimicrob Agents Chemother 61:e01337-16. doi: 10.1128/AAC.01337-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.López-Cerero L, Egea P, Gracia-Ahufinger I, González-Padilla M, Rodríguez-López F, Rodríguez-Baño J, Pascual A. 2014. Characterisation of the first ongoing outbreak due to KPC-3-producing Klebsiella pneumoniae (ST512) in Spain. Int J Antimicrob Agents 44:538–540. doi: 10.1016/j.ijantimicag.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 286.Rossi Gonçalves I, Ferreira ML, Araujo BF, Campos PA, Royer S, Batistão DW, Souza LP, Brito CS, Urzedo JE, Gontijo-Filho PP, Ribas RM. 2016. Outbreaks of colistin-resistant and colistin-susceptible KPC-producing Klebsiella pneumoniae in a Brazilian intensive care unit. J Hosp Infect 94:322–329. doi: 10.1016/j.jhin.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 287.Mavroidi A, Katsiari M, Likousi S, Palla E, Roussou Z, Nikolaou C, Maguina A, Platsouka ED. 2016. Characterization of ST258 colistin-resistant, blaKPC-producing Klebsiella pneumoniae in a Greek hospital. Microb Drug Resist 22:392–398. doi: 10.1089/mdr.2015.0282. [DOI] [PubMed] [Google Scholar]
- 288.Giacobbe DR, Del Bono V, Trecarichi EM, De Rosa FG, Giannella M, Bassetti M, Bartoloni A, Losito AR, Corcione S, Bartoletti M, Mantengoli E, Saffioti C, Pagani N, Tedeschi S, Spanu T, Rossolini GM, Marchese A, Ambretti S, Cauda R, Viale P, Viscoli C, Tumbarello M, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). 2015. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect 21:1106.e1–1106.e8. doi: 10.1016/j.cmi.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 289.Weterings V, Zhou K, Rossen JW, van Stenis D, Thewessen E, Kluytmans J, Veenemans J. 2015. An outbreak of colistin-resistant Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae in the Netherlands (July to December 2013), with inter-institutional spread. Eur J Clin Microbiol Infect Dis 34:1647–1655. doi: 10.1007/s10096-015-2401-2. [DOI] [PubMed] [Google Scholar]
- 290.Falcone M, Russo A, Iacovelli A, Restuccia G, Ceccarelli G, Giordano A, Farcomeni A, Morelli A, Venditti M. 2016. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect 22:444–450. doi: 10.1016/j.cmi.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 291.Rojas LJ, Salim M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Marshall S, Rudin SD, Domitrovic TN, Hujer AM, Hujer KM, Doi Y, Kaye KS, Evans S, Fowler VG Jr, Bonomo RA, van Duin D, Antibacterial Resistance Leadership Group. 2017. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: laboratory detection and impact on mortality. Clin Infect Dis 64:711–718. doi: 10.1093/cid/ciw805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 293.Di Pilato V, Arena F, Tascini C, Cannatelli A, Henrici De Angelis L, Fortunato S, Giani T, Menichetti F, Rossolini GM. 2016. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother 60:5612–5615. doi: 10.1128/AAC.01075-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Delgado-Blas JF, Ovejero CM, Abadia-Patiño L, Gonzalez-Zorn B. 2016. Coexistence of mcr-1 and blaNDM-1 in Escherichia coli from Venezuela. Antimicrob Agents Chemother 60:6356–6358. doi: 10.1128/AAC.01319-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mediavilla JR, Patrawalla A, Chen L, Chavda KD, Mathema B, Vinnard C, Dever LL, Kreiswirth BN. 2016. Colistin- and carbapenem-resistant Escherichia coli harboring mcr-1 and blaNDM-5, causing a complicated urinary tract infection in a patient from the United States. mBio 7:e1191-16. doi: 10.1128/mBio.01191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhang R, Liu L, Zhou H, Chan EW, Li J, Fang Y, Li Y, Liao K, Chen S. 2017. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine 19:98–106. doi: 10.1016/j.ebiom.2017.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Beyrouthy R, Robin F, Lessene A, Lacombat I, Dortet L, Naas T, Ponties V, Bonnet R. 2017. MCR-1 and OXA-48 in vivo acquisition in KPC-producing Escherichia coli after colistin treatment. Antimicrob Agents Chemother 61:e02540-16. doi: 10.1128/AAC.02540-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Huang TD, Bogaerts P, Berhin C, Hoebeke M, Bauraing C, Glupczynski Y. 2017. Increasing proportion of carbapenemase-producing Enterobacteriaceae and emergence of a MCR-1 producer through a multicentric study among hospital-based and private laboratories in Belgium from September to November 2015. Euro Surveill 22:30530. doi: 10.2807/1560-7917.ES.2017.22.19.30530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Ni W, Han Y, Liu J, Wei C, Zhao J, Cui J, Wang R, Liu Y. 2016. Tigecycline treatment for carbapenem-resistant Enterobacteriaceae infections: a systematic review and meta-analysis. Medicine (Baltimore, MD) 95:e3126. doi: 10.1097/MD.0000000000003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Burkhardt O, Rauch K, Kaever V, Hadem J, Kielstein JT, Welte T. 2009. Tigecycline possibly underdosed for the treatment of pneumonia: a pharmacokinetic viewpoint. Int J Antimicrob Agents 34:101–102. doi: 10.1016/j.ijantimicag.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 301.Wang J, Pan Y, Shen J, Xu Y. 2017. The efficacy and safety of tigecycline for the treatment of bloodstream infections: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 16:24. doi: 10.1186/s12941-017-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. 2013. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 57:1756–1762. doi: 10.1128/AAC.01232-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Falagas ME, Vardakas KZ, Tsiveriotis KP, Triarides NA, Tansarli GS. 2014. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents 44:1–7. doi: 10.1016/j.ijantimicag.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 304.Di Carlo P, Gulotta G, Casuccio A, Pantuso G, Raineri M, Farulla CA, Bonventre S, Guadagnino G, Ingrassia D, Cocorullo G, Mammina C, Giarratano A. 2013. KPC-3 Klebsiella pneumoniae ST258 clone infection in postoperative abdominal surgery patients in an intensive care setting: analysis of a case series of 30 patients. BMC Anesthesiol 13:13. doi: 10.1186/1471-2253-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.De Pascale G, Montini L, Pennisi M, Bernini V, Maviglia R, Bello G, Spanu T, Tumbarello M, Antonelli M. 2014. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 18:R90. doi: 10.1186/cc13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Balandin Moreno B, Fernández Simón I, Pintado García V, Sánchez Romero I, Isidoro Fernández B, Romera Ortega MA, Alcántara Carmona S, Pérez Redondo M, Galdos Anuncibay P. 2014. Tigecycline therapy for infections due to carbapenemase-producing Klebsiella pneumoniae in critically ill patients. Scand J Infect Dis 46:175–180. doi: 10.3109/00365548.2013.861608. [DOI] [PubMed] [Google Scholar]
- 307.Routsi C, Kokkoris S, Douka E, Ekonomidou F, Karaiskos I, Giamarellou H. 2015. High-dose tigecycline-associated alterations in coagulation parameters in critically ill patients with severe infections. Int J Antimicrob Agents 45:90–93. doi: 10.1016/j.ijantimicag.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 308.Palacios-Baena ZR, Oteo J, Conejo C, Larrosa MN, Bou G, Fernández-Martínez M, González-López JJ, Pintado V, Martínez-Martínez L, Merino M, Pomar V, Mora-Rillo M, Rivera MA, Oliver A, Ruiz-Carrascoso G, Ruiz-Garbajosa P, Zamorano L, Bautista V, Ortega A, Morales I, Pascual Á, Campos J, Rodríguez-Baño J, GEIH-GEMARA (SEIMC) and REIPI Group for CPE. 2016. Comprehensive clinical and epidemiological assessment of colonisation and infection due to carbapenemase-producing Enterobacteriaceae in Spain. J Infect 72:152–160. doi: 10.1016/j.jinf.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 309.Brust K, Evans A, Plemmons R. 2014. Tigecycline in treatment of multidrug-resistant Gram-negative bacillus urinary tract infections: a systematic review. J Antimicrob Chemother 69:2606–2610. doi: 10.1093/jac/dku189. [DOI] [PubMed] [Google Scholar]
- 310.Satlin MJ, Kubin CJ, Blumenthal JS, Cohen AB, Furuya EY, Wilson SJ, Jenkins SG, Calfee DP. 2011. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 55:5893–5899. doi: 10.1128/AAC.00387-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.van Duin D, Cober E, Richter SS, Perez F, Kalayjian RC, Salata RA, Evans S, Fowler VG Jr, Kaye KS, Bonomo RA. 2015. Impact of therapy and strain type on outcomes in urinary tract infections caused by carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother 70:1203–1211. doi: 10.1093/jac/dku495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Doi Y, Wachino JI, Arakawa Y. 2016. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin North Am 30:523–537. doi: 10.1016/j.idc.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Li JJ, Sheng ZK, Deng M, Bi S, Hu FS, Miao HF, Ji ZK, Sheng JF, Li LJ. 2012. Epidemic of Klebsiella pneumoniae ST11 clone coproducing KPC-2 and 16S rRNA methylase RmtB in a Chinese university hospital. BMC Infect Dis 12:373. doi: 10.1186/1471-2334-12-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mezzatesta ML, Gona F, Caio C, Adembri C, Dell'utri P, Santagati M, Stefani S. 2013. Emergence of an extensively drug-resistant ArmA- and KPC-2-producing ST101 Klebsiella pneumoniae clone in Italy. J Antimicrob Chemother 68:1932–1934. doi: 10.1093/jac/dkt116. [DOI] [PubMed] [Google Scholar]
- 315.Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, Pontes-Moreno A, López-Cerero L, Pascual A, Natera C, Rodríguez M, Salcedo I, Rodríguez-López F, Rivero A, Rodríguez-Baño J. 2015. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother 70:905–913. doi: 10.1093/jac/dku432. [DOI] [PubMed] [Google Scholar]
- 316.Taccone FS, Laterre PF, Spapen H, Dugernier T, Delattre I, Layeux B, De Backer D, Wittebole X, Wallemacq P, Vincent JL, Jacobs F. 2010. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care 14:R53. doi: 10.1186/cc8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kato H, Hagihara M, Hirai J, Sakanashi D, Suematsu H, Nishiyama N, Koizumi Y, Yamagishi Y, Matsuura K, Mikamo H. 2017. Evaluation of amikacin pharmacokinetics and pharmacodynamics for optimal initial dosing regimen. Drugs R D 17:177–187. doi: 10.1007/s40268-016-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Drusano GL, Louie A. 2011. Optimization of aminoglycoside therapy. Antimicrob Agents Chemother 55:2528–2531. doi: 10.1128/AAC.01314-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.White BP, Lomaestro B, Pai MP. 2015. Optimizing the initial amikacin dosage in adults. Antimicrob Agents Chemother 59:7094–7096. doi: 10.1128/AAC.01032-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zazo H, Martín-Suárez A, Lanao JM. 2013. Evaluating amikacin dosage regimens in intensive care unit patients: a pharmacokinetic/pharmacodynamic analysis using Monte Carlo simulation. Int J Antimicrob Agents 42:155–160. doi: 10.1016/j.ijantimicag.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 321.Brasseur A, Hites M, Roisin S, Cotton F, Vincent JL, De Backer D, Jacobs F, Taccone FS. 2016. A high-dose aminoglycoside regimen combined with renal replacement therapy for the treatment of MDR pathogens: a proof-of-concept study. J Antimicrob Chemother 71:1386–1394. doi: 10.1093/jac/dkv491. [DOI] [PubMed] [Google Scholar]
- 322.Allou N, Bouteau A, Allyn J, Snauwaert A, Valance D, Jabot J, Bouchet B, Galliot R, Corradi L, Montravers P, Augustin P. 2016. Impact of a high loading dose of amikacin in patients with severe sepsis or septic shock. Ann Intensive Care 6:106. doi: 10.1186/s13613-016-0211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Choudhury S, Yeng JL, Krishnan PU. 2015. In vitro susceptibilities of clinical isolates of carbapenemase-producing Enterobacteriaceae to fosfomycin and tigecycline. Clin Microbiol Infect 21:e75–e76. doi: 10.1016/j.cmi.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 324.Karageorgopoulos DE, Miriagou V, Tzouvelekis LS, Spyridopoulou K, Daikos GL. 2012. Emergence of resistance to fosfomycin used as adjunct therapy in KPC Klebsiella pneumoniae bacteraemia: report of three cases. J Antimicrob Chemother 67:2777–2779. doi: 10.1093/jac/dks270. [DOI] [PubMed] [Google Scholar]
- 325.Pontikis K, Karaiskos I, Bastani S, Dimopoulos G, Kalogirou M, Katsiari M, Oikonomou A, Poulakou G, Roilides E, Giamarellou H. 2014. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents 43:52–59. doi: 10.1016/j.ijantimicag.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 326.Adams-Haduch JM, Potoski BA, Sidjabat HE, Paterson DL, Doi Y. 2009. Activity of temocillin against KPC-producing Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother 53:2700–2701. doi: 10.1128/AAC.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Alexandre K, Chau F, Guérin F, Massias L, Lefort A, Cattoir V, Fantin B. 2016. Activity of temocillin in a lethal murine model of infection of intra-abdominal origin due to KPC-producing Escherichia coli. J Antimicrob Chemother 71:1899–1904. doi: 10.1093/jac/dkw066. [DOI] [PubMed] [Google Scholar]
- 328.van Dijk K, Voets GM, Scharringa J, Voskuil S, Fluit AC, Rottier WC, Leverstein-Van Hall MA, Cohen Stuart JW. 2014. A disc diffusion assay for detection of class A, B and OXA-48 carbapenemases in Enterobacteriaceae using phenyl boronic acid, dipicolinic acid and temocillin. Clin Microbiol Infect 20:345–349. doi: 10.1111/1469-0691.12322. [DOI] [PubMed] [Google Scholar]
- 329.Woodford N, Pike R, Meunier D, Loy R, Hill R, Hopkins KL. 2014. In vitro activity of temocillin against multidrug-resistant clinical isolates of Escherichia coli, Klebsiella spp. and Enterobacter spp., and evaluation of high-level temocillin resistance as a diagnostic marker for OXA-48 carbapenemase. J Antimicrob Chemother 69:564–567. doi: 10.1093/jac/dkt383. [DOI] [PubMed] [Google Scholar]
- 330.Panagiotakopoulou A, Daikos GL, Miriagou V, Loli A, Tzelepi E, Tzouvelekis LS. 2007. Comparative in vitro killing of carbapenems and aztreonam against Klebsiella pneumoniae producing VIM-1 metallo-beta-lactamase. Int J Antimicrob Agents 29:360–362. doi: 10.1016/j.ijantimicag.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 331.Souli M, Konstantinidou E, Tzepi I, Tsaganos T, Pefanis A, Chryssouli Z, Galani I, Giamarellos-Bourboulis E, Giamarellou H. 2011. Efficacy of carbapenems against a metallo-β-lactamase-producing Escherichia coli clinical isolate in a rabbit intra-abdominal abscess model. J Antimicrob Chemother 66:611–617. doi: 10.1093/jac/dkq470. [DOI] [PubMed] [Google Scholar]
- 332.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-β-lactamase producers. Antimicrob Agents Chemother 57:3299–3306. doi: 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Psichogiou M, Tassios PT, Avlamis A, Stefanou I, Kosmidis C, Platsouka E, Paniara O, Xanthaki A, Toutouza M, Daikos GL, Tzouvelekis LS. 2008. Ongoing epidemic of blaVIM-1-positive Klebsiella pneumoniae in Athens, Greece: a prospective survey. J Antimicrob Chemother 61:59–63. doi: 10.1093/jac/dkm443. [DOI] [PubMed] [Google Scholar]
- 334.Poirel L, Héritier C, Tolün V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother 48:15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Paño-Pardo JR, Ruiz-Carrascoso G, Navarro-San Francisco C, Gómez-Gil R, Mora-Rillo M, Romero-Gómez MP, Fernández-Romero N, García-Rodríguez J, Pérez-Blanco V, Moreno-Ramos F, Mingorance J. 2013. Infections caused by OXA-48-producing Klebsiella pneumoniae in a tertiary hospital in Spain in the setting of a prolonged, hospital-wide outbreak. J Antimicrob Chemother 68:89–96. doi: 10.1093/jac/dks364. [DOI] [PubMed] [Google Scholar]
- 336.Mimoz O, Grégoire N, Poirel L, Marliat M, Couet W, Nordmann P. 2012. Broad-spectrum β-lactam antibiotics for treating experimental peritonitis in mice due to Klebsiella pneumoniae producing the carbapenemase OXA-48. Antimicrob Agents Chemother 56:2759–2760. doi: 10.1128/AAC.06069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2014. Efficacy of humanized carbapenem and ceftazidime regimens against Enterobacteriaceae producing OXA-48 carbapenemase in a murine infection model. Antimicrob Agents Chemother 58:1678–1683. doi: 10.1128/AAC.01947-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Livermore DM, Mushtaq S, Warner M, Miossec C, Woodford N. 2008. NXL104 combinations versus Enterobacteriaceae with CTX-M extended-spectrum beta-lactamases and carbapenemases. J Antimicrob Chemother 62:1053–1056. doi: 10.1093/jac/dkn320. [DOI] [PubMed] [Google Scholar]
- 339.Stachyra T, Levasseur P, Péchereau MC, Girard AM, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the beta-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother 64:326–329. doi: 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Endimiani A, Choudhary Y, Bonomo RA. 2009. In vitro activity of NXL104 in combination with beta-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob Agents Chemother 53:3599–3601. doi: 10.1128/AAC.00641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Aktaş Z, Kayacan C, Oncul O. 2012. In vitro activity of avibactam (NXL104) in combination with β-lactams against Gram-negative bacteria, including OXA-48 β-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents 39:86–89. doi: 10.1016/j.ijantimicag.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 343.Endimiani A, Hujer KM, Hujer AM, Pulse ME, Weiss WJ, Bonomo RA. 2011. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 55:82–85. doi: 10.1128/AAC.01198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. 2014. In vivo efficacy of humanized exposures of ceftazidime-avibactam in comparison with ceftazidime against contemporary Enterobacteriaceae isolates. Antimicrob Agents Chemother 58:6913–6919. doi: 10.1128/AAC.03267-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. 2014. Unexpected in vivo activity of ceftazidime alone and in combination with avibactam against New Delhi metallo-β-lactamase-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother 58:7007–7009. doi: 10.1128/AAC.02662-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Monogue ML, Abbo LM, Rosa R, Camargo JF, Martinez O, Bonomo RA, Nicolau DP. 2017. In vitro discordance with in vivo activity: humanized exposures of ceftazidime-avibactam, aztreonam, and tigecycline alone and in combination against New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 61:e00486-17. doi: 10.1128/AAC.00486-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Castón JJ, Lacort-Peralta I, Martín-Dávila P, Loeches B, Tabares S, Temkin L, Torre-Cisneros J, Paño-Pardo JR. 2017. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int J Infect Dis 59:118–123. doi: 10.1016/j.ijid.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 348.Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. 2017. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883-17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Krapp F, Grant JL, Sutton SH, Ozer EA, Barr VO. 2017. Treating complicated carbapenem-resistant Enterobacteriaceae infections with ceftazidime/avibactam: a retrospective study with molecular strain characterisation. Int J Antimicrob Agents 49:770–773. doi: 10.1016/j.ijantimicag.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 351.Temkin E, Torre-Cisneros J, Beovic B, Benito N, Giannella M, Gilarranz R, Jeremiah C, Loeches B, Machuca I, Jiménez-Martín MJ, Martínez JA, Mora-Rillo M, Navas E, Osthoff M, Pozo JC, Ramos Ramos JC, Rodriguez M, Sánchez-García M, Viale P, Wolff M, Carmeli Y. 2017. Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother 61:e01964-16. doi: 10.1128/AAC.01964-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.King M, Heil E, Kuriakose S, Bias T, Huang V, El-Beyrouty C, McCoy D, Hiles J, Richards L, Gardner J, Harrington N, Biason K, Gallagher JC. 2017. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 61:e00449-17. doi: 10.1128/AAC.00449-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, Kaye KS, Fowler VG Jr, Paterson DL, Bonomo RA, Evans S, Antibacterial Resistance Leadership Group. 2018. Colistin vs. ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 66:163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: a case report and review of literature. Open Forum Infect Dis 4:ofx101. doi: 10.1093/ofid/ofx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Both A, Büttner H, Huang J, Perbandt M, Belmar Campos C, Christner M, Maurer FP, Kluge S, König C, Aepfelbacher M, Wichmann D, Rohde H. 2017. Emergence of ceftazidime/avibactam non-susceptibility in an MDR Klebsiella pneumoniae isolate. J Antimicrob Chemother doi: 10.1093/jac/dkx179. [DOI] [PubMed] [Google Scholar]
- 357.Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, Hujer KM, Marshall EK, Rudin SD, Perez F, Wilson BM, Wasserman RB, Chikowski L, Paterson DL, Vila AJ, van Duin D, Kreiswirth BN, Chambers HF, Fowler VG Jr, Jacobs MR, Pulse ME, Weiss WJ, Bonomo RA. 2017. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 61:e02243-16. doi: 10.1128/AAC.02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Castanheira M, Rhomberg PR, Flamm RK, Jones RN. 2016. Effect of the β-lactamase inhibitor vaborbactam combined with meropenem against serine carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:5454–5458. doi: 10.1128/AAC.00711-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Castanheira M, Huband MD, Mendes RE, Flamm RK. 2017. Meropenem-vaborbactam tested against contemporary Gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 61:e00567-17. doi: 10.1128/AAC.00567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Kaye K, Vazquez J, Mathers A, Daikos G, Alexander E, Loutit J, Zhang S, Dudley M, Cornely O. 2017. Clinical outcomes of serious infections due to carbapenem-resistant Enterobacteriaceae (CRE) in TANGO II, a phase 3, randomized, multi-national, open-label trial of meropenem-vaborbactam (M-V) versus best available therapy (BAT), poster 1862. IDWeek. [Google Scholar]
- 361.Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. 2011. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother 66:48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- 362.Zhanel GG, Lawson CD, Zelenitsky S, Findlay B, Schweizer F, Adam H, Walkty A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2012. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev Anti Infect Ther 10:459–473. doi: 10.1586/eri.12.25. [DOI] [PubMed] [Google Scholar]
- 363.Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Cloutier D, Miller L, Komirenko A, Cebrik D, Krause K, Keepers T, Connolly L, Wagenlehner F. 2017. Plazomicin versus meropenem for the treatment of complicated urinary tract infection (cUTI) and acute pyelonephritis (AP): results of the EPIC Study, abstr OS0250E. Abstr 27th Eur Congr Clin Microbiol Infect Dis. https://www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=43219. [Google Scholar]
- 365.Connolly L, Jubb A, O'Keeffe B, Serio A, Smith A, Gall J, Riddle V, Krause K, Mckinnell J, Zakynthinos E, Daikos GL. 2017. Plazomicin (PLZ) associated with improved survival and safety compared to colistin (CST) in serious carbapenem-resistant Enterobacteriaceae (CRE) infections: results of the CARE Study, abstr OS0250F. Abstr 27th Eur Congr Clin Microbiol Infect Dis. https://www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=43221. [Google Scholar]
- 366.Sutcliffe JA, O'Brien W, Fyfe C, Grossman TH. 2013. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother 57:5548–5558. doi: 10.1128/AAC.01288-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Livermore DM, Mushtaq S, Warner M, Woodford N. 2016. In vitro activity of eravacycline against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob Agents Chemother 260:3840–3844. doi: 10.1128/AAC.00436-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Zhang Y, Lin X, Bush K. 2016. In vitro susceptibility of β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE) to eravacycline. J Antibiot (Tokyo) 69:600–604. doi: 10.1038/ja.2016.73. [DOI] [PubMed] [Google Scholar]
- 369.Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, Tsai L, Sutcliffe JA, Horn P. 2017. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg 152:224–232. doi: 10.1001/jamasurg.2016.4237. [DOI] [PubMed] [Google Scholar]
- 370.Kohira N, West J, Ito A, Ito-Horiyama T, Nakamura R, Sato T, Rittenhouse S, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 60:729–734. doi: 10.1128/AAC.01695-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ito-Horiyama T, Ishii Y, Ito A, Sato T, Nakamura R, Fukuhara N, Tsuji M, Yamano Y, Yamaguchi K, Tateda K. 2016. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 60:4384–4386. doi: 10.1128/AAC.03098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Falagas ME, Skalidis T, Vardakas KZ, Legakis NJ, Hellenic Cefiderocol Study Group. 2017. Activity of cefiderocol (S-649266) against carbapenem-resistant Gram-negative bacteria collected from inpatients in Greek hospitals. J Antimicrob Chemother 72:1704–1708. doi: 10.1093/jac/dkx049. [DOI] [PubMed] [Google Scholar]
- 373.Kanazawa S, Sato T, Kohira N, Ito-Horiyama T, Tsuji M, Yamano Y. 2017. Susceptibility of imipenem-susceptible but meropenem-resistant bla(IMP-6)-carrying Enterobacteriaceae to various antibacterials, including the siderophore cephalosporin cefiderocol. Antimicrob Agents Chemother 61:e00576-17. doi: 10.1128/AAC.00576-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2017. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant Gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 Study). Antimicrob Agents Chemother 61:e00093-17. doi: 10.1128/AAC.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Dobias J, Dénervaud-Tendon V, Poirel L, Nordmann P. 2017. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis 36:2319–2327. doi: 10.1007/s10096-017-3063-z. [DOI] [PubMed] [Google Scholar]
- 376.Katsube T, Wajima T, Ishibashi T, Arjona Ferreira JC, Echols R. 2017. Pharmacokinetic/pharmacodynamic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, for dose adjustment based on renal function. Antimicrob Agents Chemother 61:e01381-16. doi: 10.1128/AAC.01381-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Matsumoto S, Singley CM, Hoover J, Nakamura R, Echols R, Rittenhouse S, Tsuji M, Yamano Y. 2017. Efficacy of cefiderocol against carbapenem-resistant Gram-negative bacilli in immunocompetent rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother 61:e00700-17. doi: 10.1128/AAC.00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Arjona Ferreira JC, Ariyasu M, Nagata T. 2017. Cefiderocol compared with imipenem/cirastatin in the treatment of adults with complicated urinary tract infections with or without pyelonephritis or acute uncomplicated pyelonephritis: results from a multicenter, double-blind, randomized study, abstr OS0250D. Abstr 27th Eur Congr Clin Microbiol Infect Dis. https://www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=43213. [Google Scholar]
- 379.Wang X, Zhang F, Zhao C, Wang Z, Nichols WW, Testa R, Li H, Chen H, He W, Wang Q, Wang H. 2014. In vitro activities of ceftazidime-avibactam and aztreonam-avibactam against 372 Gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob Agents Chemother 58:1774–1778. doi: 10.1128/AAC.02123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Yoshizumi A, Ishii Y, Aoki K, Testa R, Nichols WW, Tateda K. 2015. In vitro susceptibility of characterized β-lactamase-producing Gram-negative bacteria isolated in Japan to ceftazidime-, ceftaroline-, and aztreonam-avibactam combinations. J Infect Chemother 21:148–151. doi: 10.1016/j.jiac.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 381.Li H, Estabrook M, Jacoby GA, Nichols WW, Testa RT, Bush K. 2015. In vitro susceptibility of characterized β-lactamase-producing strains tested with avibactam combinations. Antimicrob Agents Chemother 59:1789–1793. doi: 10.1128/AAC.04191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Papp-Wallace KM, Bajaksouzian S, Abdelhamed AM, Foster AN, Winkler ML, Gatta JA, Nichols WW, Testa R, Bonomo RA, Jacobs MR. 2015. Activities of ceftazidime, ceftaroline, and aztreonam alone and combined with avibactam against isogenic Escherichia coli strains expressing selected single β-lactamases. Diagn Microbiol Infect Dis 82:65–69. doi: 10.1016/j.diagmicrobio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Biedenbach DJ, Kazmierczak K, Bouchillon SK, Sahm DF, Bradford PA. 2015. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob Agents Chemother 59:4239–4248. doi: 10.1128/AAC.00206-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Vasoo S, Cunningham SA, Cole NC, Kohner PC, Menon SR, Krause KM, Harris KA, De PP, Koh TH, Patel R. 2015. In vitro activities of ceftazidime-avibactam, aztreonam-avibactam, and a panel of older and contemporary antimicrobial agents against carbapenemase-producing Gram-negative bacilli. Antimicrob Agents Chemother 59:7842–7846. doi: 10.1128/AAC.02019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Dupont H, Gaillot O, Goetgheluck AS, Plassart C, Emond JP, Lecuru M, Gaillard N, Derdouri S, Lemaire B, Girard de Courtilles M, Cattoir V, Mammeri H. 2015. Molecular characterization of carbapenem-nonsusceptible enterobacterial isolates collected during a prospective interregional survey in France and susceptibility to the novel ceftazidime-avibactam and aztreonam-avibactam combinations. Antimicrob Agents Chemother 60:215–221. doi: 10.1128/AAC.01559-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Sy SK, Beaudoin ME, Zhuang L, Löblein KI, Lux C, Kissel M, Tremmel R, Frank C, Strasser S, Heuberger JA, Mulder MB, Schuck VJ, Derendorf H. 2016. In vitro pharmacokinetics/pharmacodynamics of the combination of avibactam and aztreonam against MDR organisms. J Antimicrob Chemother 71:1866–1880. doi: 10.1093/jac/dkw082. [DOI] [PubMed] [Google Scholar]
- 387.Kazmierczak KM, Biedenbach DJ, Hackel M, Rabine S, de Jonge BL, Bouchillon SK, Sahm DF, Bradford PA. 2016. Global dissemination of blaKPC into bacterial species beyond Klebsiella pneumoniae and in vitro susceptibility to ceftazidime-avibactam and aztreonam-avibactam. Antimicrob Agents Chemother 60:4490–4500. doi: 10.1128/AAC.00107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Mischnik A, Baumert P, Hamprecht A, Rohde A, Peter S, Feihl S, Knobloch J, Gölz H, Kola A, Obermann B, Querbach C, Willmann M, Gebhardt F, Tacconelli E, Gastmeier P, Seifert H, Kern WV, DZIF-ATHOS Study Group. 2017. Susceptibility to cephalosporin combinations and aztreonam/avibactam among third-generation cephalosporin-resistant Enterobacteriaceae recovered on hospital admission. Int J Antimicrob Agents 49:239–242. doi: 10.1016/j.ijantimicag.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 389.Hirsch EB, Ledesma KR, Chang KT, Schwartz MS, Motyl MR, Tam VH. 2012. In vitro activity of MK-7655, a novel β-lactamase inhibitor, in combination with imipenem against carbapenem-resistant Gram-negative bacteria. Antimicrob Agents Chemother 56:3753–3757. doi: 10.1128/AAC.05927-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Livermore DM, Warner M, Mushtaq S. 2013. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother 68:2286–2290. doi: 10.1093/jac/dkt178. [DOI] [PubMed] [Google Scholar]
- 391.Lapuebla A, Abdallah M, Olafisoye O, Cortes C, Urban C, Landman D, Quale J. 2015. Activity of imipenem with relebactam against Gram-negative pathogens from New York City. Antimicrob Agents Chemother 59:5029–5031. doi: 10.1128/AAC.00830-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Lob SH, Hackel MA, Kazmierczak KM, Hoban DJ, Young K, Motyl MR, Karlowsky JA, Sahm DF. 2017. In vitro activity of imipenem-relebactam against gram-negative bacilli isolated from patients with lower respiratory tract infections in the United States in 2015—results from the SMART global surveillance program. Diagn Microbiol Infect Dis 88:171–176. doi: 10.1016/j.diagmicrobio.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 393.Lob SH, Hackel MA, Kazmierczak KM, Young K, Motyl MR, Karlowsky JA, Sahm DF. 2017. In vitro activity of imipenem-relebactam against Gram-negative ESKAPE pathogens isolated by clinical laboratories in the United States in 2015 (results from the SMART global surveillance program). Antimicrob Agents Chemother 61:e02209-16. doi: 10.1128/AAC.02209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Haidar G, Clancy CJ, Chen L, Samanta P, Shields RK, Kreiswirth BN, Nguyen MH. 2017. Identifying spectra of activity and therapeutic niches for ceftazidime-avibactam and imipenem-relebactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 61:e00642-17. doi: 10.1128/AAC.00642-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Hecker SJ, Reddy KR, Totrov M, Hirst GC, Lomovskaya O, Griffith DC, King P, Tsivkovski R, Sun D, Sabet M, Tarazi Z, Clifton MC, Atkins K, Raymond A, Potts KT, Abendroth J, Boyer SH, Loutit JS, Morgan EE, Durso S, Dudley MN. 2015. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class A serine carbapenemases. J Med Chem 58:3682–3692. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- 396.Giamarellou H, Poulakou G. 2011. Pharmacokinetic and pharmacodynamic evaluation of tigecycline. Expert Opin Drug Metab Toxicol 7:1459–1470. doi: 10.1517/17425255.2011.623126. [DOI] [PubMed] [Google Scholar]
- 397.Davido B, Fellous L, Lawrence C, Maxime V, Rottman M, Dinh A. 2017. Ceftazidime-avibactam and aztreonam an interesting strategy to overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 61:e01008-17. doi: 10.1128/AAC.01008-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Bidair M, Zervos M, Sagan O, Zaitsev V, Loutit JS, Dudley MN, Vazquez J. 2017. Clinical outcomes in adults with complicated urinary tract infections (cUTI), including acute pyelonephritis (AP) in TANGO 1, a phase 3 randomized, double-blind, double-dummy trial comparing meropenem-vaborbactam (M-V) with piperacillin-tazobactam (P-T), abstr P1289. Abstr 27th Eur Congr Clin Microbiol Infect Dis. https://www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=40121.