SUMMARY
Osteomyelitis is an inflammatory bone disease that is caused by an infecting microorganism and leads to progressive bone destruction and loss. The most common causative species are the usually commensal staphylococci, with Staphylococcus aureus and Staphylococcus epidermidis responsible for the majority of cases. Staphylococcal infections are becoming an increasing global concern, partially due to the resistance mechanisms developed by staphylococci to evade the host immune system and antibiotic treatment. In addition to the ability of staphylococci to withstand treatment, surgical intervention in an effort to remove necrotic and infected bone further exacerbates patient impairment. Despite the advances in current health care, osteomyelitis is now a major clinical challenge, with recurrent and persistent infections occurring in approximately 40% of patients. This review aims to provide information about staphylococcus-induced bone infection, covering the clinical presentation and diagnosis of osteomyelitis, pathophysiology and complications of osteomyelitis, and future avenues that are being explored to treat osteomyelitis.
KEYWORDS: Staphylococcus aureus, Staphylococcus epidermidis, antibiotic, joint infections, nonantibiotic, osteomyelitis
INTRODUCTION
Osteomyelitis, translated from Greek, means inflammation of the bone marrow (osteon, bone; myelos, marrow; and itis, inflammation) (1). The disease can be restricted to a single portion of the bone or affect several regions, such as the marrow, cortex, periosteum, and/or surrounding soft tissue (Fig. 1) (2). Osteomyelitis is often classified by the location within the bone, extent of dispersion, and source of infection. Although it can be caused by a variety of pathogens, it is most commonly caused by the opportunistic Gram-positive staphylococci (approximately 75% of cases, collectively) (3), which can originate from the blood (hematogenous source) or contiguously.
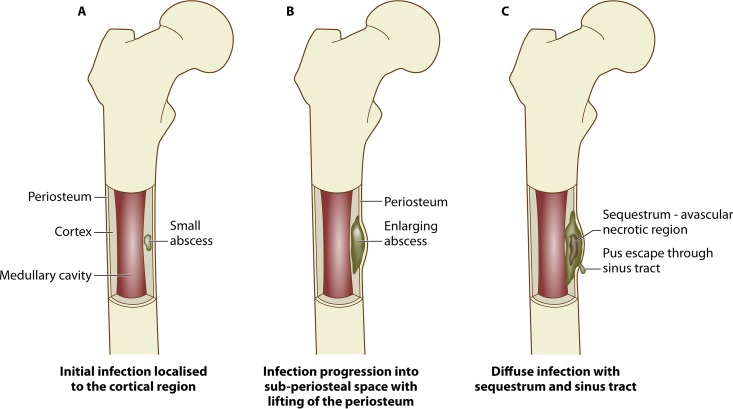
FIG 1.
Progression of osteomyelitis. An abscess develops from a localized infection that constricts the blood flow to the area (A), resulting in an avascular region of necrotic bone tissue called the sequestrum (B), followed by development of new bone surrounding the sequestrum, termed the involucrum, which may also have a sinus tract through which purulence can escape (C).
Microbiology of Staphylococci
Staphylococci are Gram-positive bacteria that have a round morphology and a diameter of 0.5 to 1.8 μm. The cell wall is what attributes the term “Gram positive” to staphylococci and is composed of layers of peptidoglycan, lipoteichoic acids, and teichoic acids (4). There are more than 20 different staphylococcal species described in Bergey's Manual of Systematic Bacteriology (5); however, Staphylococcus aureus and S. epidermidis are the most significant in regard to human interactions (6). S. aureus and S. epidermidis are usually commensal inhabitants of the skin microflora and mucosal surfaces. Approximately 20% of healthy individuals are permanently colonized asymptomatically by S. aureus, with 70% of individuals either transiently or not colonized (7). However, S. aureus has adapted to become a perilous human pathogen causing a variety of diseases, ranging from suppurative infections, such as boils, to more life-threatening infections, such as septicemia (8). In addition to a thick cell wall, around 80 to 90% of S. aureus strains possess a capsule which provides protection for the bacterium, as it has antiphagocytic properties due to the host's inability to recognize the invading microorganisms (9). Notably, S. aureus strains that are capsule negative have been shown to induce chronic infection in mouse models due to their ability to survive intracellularly (10). The ability of S. aureus and S. epidermidis to colonize and cause host infection is attributed primarily to the presence of various cell wall-anchored (CWA) proteins and extracellular factors. Several of these proteins can adhere to host cells and/or extracellular matrix (ECM) molecules and have been termed microbial surface components recognizing adhesive matrix molecules (MSCRAMM) (9). Examples of MSCRAMMs include fibronectin binding proteins (FnBP) and collagen adhesin (Cna) (11). Once colonized, staphylococci can secrete toxins which aid in invasion and dissemination throughout the host. Nearly all strains of S. aureus and S. epidermidis secrete the four hemolysins (alpha, beta, gamma, and delta), lipases, proteases, hyaluronidase, nucleases, and collagenase. The main functions of these toxins are to break down the host tissue and provide nutrients for bacterial survival and growth (12, 13).
MODES OF BONE INFECTION
There are many contributing factors that predispose a patient to developing osteomyelitis, including age, diabetes, peripheral vascular disease, intravenous (i.v.) drug use, surgical implants, and immunodeficiency due to disease or immunosuppressant drugs (14). The causative organisms in osteomyelitis can originate from either hematogenous or contiguously spread sources, often referred to as endogenous or exogenous sources, respectively (15).
Hematogenous Osteomyelitis
Hematogenous osteomyelitis is usually monomicrobial (16). It occurs most commonly in patients lacking any prior risk factors or infection; however, it can also be caused by the seeding of circulating pathogens in the blood, which can arise from an existing infection. Hematogenous osteomyelitis represents just 20% of all osteomyelitis infections; however, the majority of osteomyelitis cases in children are hematogenous (85% of cases for patients under 17 years of age) (15).
Contiguous Spread of Infection
In contrast to hematogenous osteomyelitis, contiguous spread of infection is most often polymicrobial and most commonly affects adults (17–19). Contiguously spread osteomyelitis can originate from trauma, direct inoculation during operative procedures, or surrounding infected soft tissues.
It is estimated that half of osteomyelitis cases in adults are due to trauma (20). Trauma can result in either open or closed fractures (presence or absence of exposed bone). Damaged connective tissues, including skin, muscle, and bone, expose proteins to which bacteria readily bind, such as collagen and fibronectin, increasing the chance of inoculation (21). In a clinical study carried out by Merritt, up to 1 in 5 patients who acquired open fractures were reported to have developed infections (22).
People with soft tissue infections who develop underlying infection of the bone are most commonly over the age of 40 and have diabetes mellitus (23). Osteomyelitis spreading from diabetic ulcers due to neuropathy and vascular insufficiency most commonly occurs in the bones of the feet: the toes, metatarsal heads, and calcaneum (24). According to Malhotra et al. and Lavery et al., 12 to 20% of those with diabetic foot ulcers develop an infection of the underlying bone (25, 26), and in severe cases of foot ulcers this prevalence can be higher than 66% (27).
DEVELOPMENT OF ACUTE AND CHRONIC BONE INFECTIONS
The pathology of osteomyelitis is characterized by severe inflammation, impairment of vasculature, and localized bone loss and destruction. In an attempt to overcome the infective microorganisms, leukocytes produce inflammatory cytokines and enzymes that break down the infected and surrounding tissue (28). Purulence consisting of dead leukocytes and host/bacterial cells can fill intercellular spaces around the infection and form an abscess. In chronic infection, abscesses can impair blood flow and strip the periosteum, creating an area of vascularized, necrotic bone called a sequestrum (29). Vascular impairment makes the foci of chronic infection impervious to the immune system and systemic antibiotics. The sequestrum is indicative of a chronic infection and compromises the bone's integrity. Often the formation of new bone—an involucrum—occurs, which forms from remaining intact fragments of the periosteum and functions to provide axial support to weight-bearing bones and prevent pathological fracture (14, 30). Exudate or purulence from the infection may escape through an opening in the bone called a sinus tract (Fig. 1).
CLINICAL PRESENTATION AND DIAGNOSIS
Diagnosing osteomyelitis is often a difficult challenge, as there are vast variations in clinical presentation. Early diagnosis is the key to the successful treatment of osteomyelitis. Schmidt et al. developed a diagnostic tool for osteomyelitis that uses a scoring system based on clinical, laboratory, and technical information (31). The scoring system is based on (i) clinical history and risk factors; (ii) clinical examination and laboratory test results, including leukocyte counts and detection of inflammatory markers, such as via the erythrocyte sedimentation rate (ESR) and the C-reactive protein (CRP) level; (iii) diagnostic imaging, such as ultrasound, radiology, computed tomography (CT), or magnetic resonance imaging (MRI); (iv) microbiology analysis; and (v) histopathology. Unfortunately, many of these individual diagnostic methods lack specificity and sensitivity and are associated with many issues, as Tiemann et al. outlined (32). Lab test results involving leukocyte counts and inflammatory markers are often not reliable. For example, in a review by Scott et al., 41% of patients who presented with acute hematogenous osteomyelitis presented with a leukocyte count of <10,500, which is within the normal range of ∼4,500 to 11,000 (33). In up to 40% of osteomyelitis cases, microbiological tests produce false-negative results. This may be due to the difficulty in culturing the causative organism secondary to location, inability of the patient to undergo surgical intervention, or the fact that the patient may have been started on antibiotics prior to the collection of a specimen for culture, thus altering the results of laboratory testing. In addition, diagnosing osteomyelitis through imaging methods is often delayed because bone necrosis is difficult to detect by plain radiography until up to week 3 of infection, with a reported positive diagnosis rate of only 20% after 2 weeks (21).
OSTEOMYELITIS CLASSIFICATION
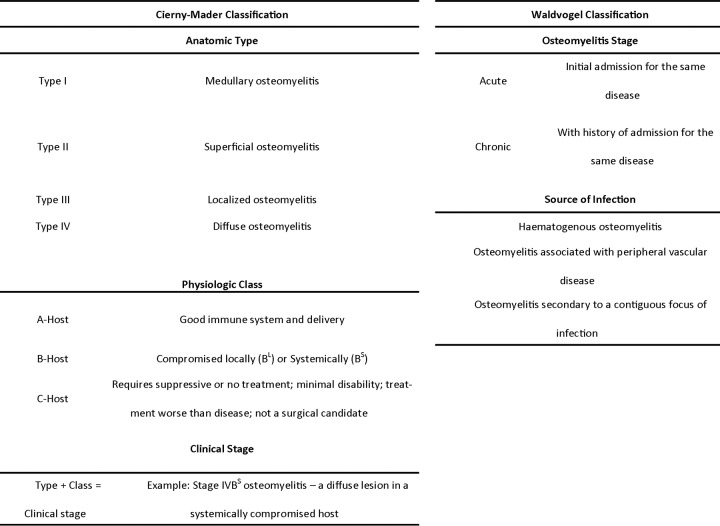
As osteomyelitis is a heterogeneous disease, the large variation in patient populations along with a number of factors critical for guiding an appropriate treatment strategy has resulted in more than 12 different classifications. While none of the classifications are ubiquitously accepted, two classifications are widely used because they provide information on the nature and origin of the disease while taking into account the patient's physiological status, parameters deemed critical in osteomyelitis. Any type of osteomyelitis can develop from the acute stage and continue into the chronic stage of the disease (34). Prescription of treatment for osteomyelitis in the clinical setting largely depends on the classification as either “acute” or “chronic.” Although there is often much difficulty in this classification, the degree of tissue injury is generally directly correlated with the disease stage (35). Throughout the literature, there are a number of detailed guidelines published to classify the infection, the most highly cited of which are the Waldvogel system and the Cierny-Mader system (16, 36).
Waldvogel Classification
The Waldvogel classification system (Table 1) defines the infection as either acute or chronic based on the persistence of infection, and the infection is subsequently classified based on the source of infection (16). Waldvogel et al. found that this definition not only showed evidence of differences in clinical presentation but also improved the disease cure rate.
TABLE 1.
Major classification systems used for diagnosis of osteomyelitisa
Cierny-Mader Classification
The Cierny-Mader classification system (Table 1) is based on four key factors: the condition of the host, the functional impairment caused by the disease, the site of involvement, and the extent of bony necrosis. It does not deem it necessary to distinguish between acute and chronic infections. In this classification system, the anatomic type of osteomyelitis (I to IV) is added to the physiologic class of the patient (A, B, or C), which results in one of the 12 clinical staging systems of adult osteomyelitis (IA,B,C, IIA,B,C, IIIA,B,C, and IVA,B,C). From this staging system, the osteomyelitis treatment is derived, including debridement strategies, dead space management, and antibiotic administration. Cierny et al. state that using these four key factors allows comparison of new treatment protocols and the effectiveness of new therapeutic modalities (36).
PATHOPHYSIOLOGY OF OSTEOMYELITIS
Bone as a Target Organ
Bone is a dynamic connective tissue that is constantly being remodeled and renewed under the governance of three main bone cells: osteoblasts, osteocytes, and osteoclasts. Osteoblasts are the bone-forming cells, derived from mesenchymal stem cells (MSC) in the bone marrow, and are responsible for producing the main organic extracellular matrix (ECM) components of bone. When osteoblasts are fully mature cells, they produce osteoid—unmineralized organic bone matrix—in the form of a membrane-bound vesicle (37). Osteoid consists of collagenous and noncollagenous proteins. Collagen type I makes up 90% of the osteoid, with the remainder comprised of proteins, such as proteoglycans (38) and glycoproteins. Common glycoproteins found in the ECM include fibronectin, osteonectin, osteopontin, bone sialoprotein, and osteocalcin (39, 40). When osteoblasts generate and fully immerse themselves in ECM, they become osteocytes—terminally differentiated osteoblasts. Osteocytes have been implicated in directing the bone remodeling process through their ability to respond to bone loading and detection of microcracks. Osteoclasts are the bone-resorbing cells, which operate by decalcifying hydroxyapatite and degrading organic ECM. Osteoclasts work in harmony with osteoblasts to retain bone remodeling homeostasis. Notably, an imbalance in the activity between these cells can result in altered bone morphology and pathological bone (41–43). When bone is exposed to the external environment, bone cells and the ECM are ideal colonizing targets of microbes, in particular staphylococci, which have the MSCRAMMs and anchoring proteins to colonize bone (44).
Staphylococcal Colonization of Bone
Once staphylococci have accessed the bone, the first step to colonization is primary attachment. Attachment is facilitated by the presence of MSCRAMMs and other cell wall-anchored proteins on staphylococci (Fig. 2). Colonization of bone can occur through direct interaction with the bone cells, plasma proteins, or the ECM. Once colonized, staphylococci can produce a biofilm, which facilitates persistence of the infection (45, 46). Biofilms are organized communities of microorganisms enveloped in an extracellular matrix attached to a surface (47–49). Additionally, staphylococci can also produce toxins, many of which facilitate dissemination throughout the host, allowing recolonization and reinfection (50).
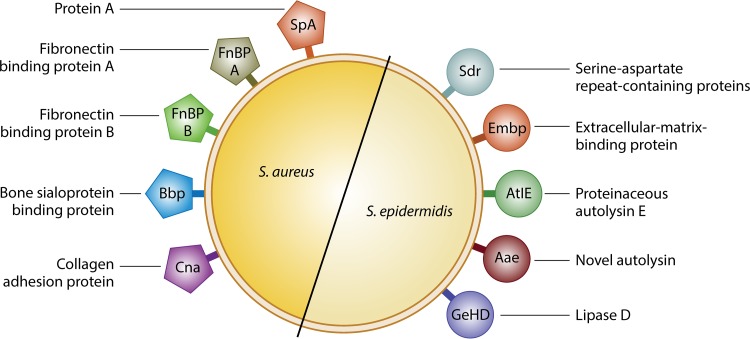
FIG 2.
Staphylococcus aureus and Staphylococcus epidermidis cell surface proteins, known as microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), that are involved in interacting with bone and the bone ECM.
Staphylococcus aureus.
In S. aureus, there are multiple MSCRAMMS and CWA proteins important for the pathogenicity of infection, including protein A (SpA), fibronectin binding proteins A and B (FnBP A/B), bone sialoprotein binding protein (Bbp), and collagen adhesion protein (Cna) (Table 2). In addition to being anchored to S. aureus's cell wall, SpA can also be secreted. Although the primary function of SpA is immune evasion, studies have documented its direct role in bone infection. It was shown that SpA can directly bind to osteoblasts, mediating cell death, inhibition of bone formation (osteogenesis), and induction of bone resorption (osteoclastogenesis) (51–54). The importance of osteoclastic activity in osteomyelitis is becoming evident, and therefore many studies have emerged to examine the effects of S. aureus in promoting osteoclastogenesis and osteoclastic activity. Osteoblast inhibition and osteoclast activation were also described by Kim et al., who demonstrated an induction of proinflammatory cytokines by activation of Toll-like receptor 2 (TLR2) in osteoblasts, resulting in production of RANKL. This in turn activated osteoclast differentiation, facilitating bone resorption in mice lacking TLR2 and demonstrating the hallmark presentations seen in osteomyelitis (44). Activation of osteoclasts through various cellular pathways was also recently documented, with protein A once again being a key player in this process (54, 55). If S. aureus does not interact directly with the cell, its FnBPs facilitate binding to host plasma proteins, such as fibronectin and fibrinogen, which can act as bridging molecules between the bacterium and the host cell receptors (56, 57). Additionally, when these FnBPs, specifically FnBPA and FnBPB, interact with fibronectin, it can cause internalization via the α5β1 receptor on osteoblasts (58–60). Activation of this integrin results in the recruitment of molecules, such as tensin and talin, which interact directly with the cellular cytoskeleton. These molecules in turn cause the recruitment of tyrosine kinases, which initiate phosphorylation of the cytoskeleton and thus uptake of the bacteria (61). Internalization can lead to two outcomes: apoptosis of the cell or persistence of infection intracellularly. Apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) occurs due to it binding to its receptor on the osteoblast membrane. There are 5 known receptors of TRAIL: death receptors DR5 and DR4, decoy receptors DcR1 and DcR2, and soluble receptor OPG. Binding of TRAIL to these receptors leads to the activation of caspases 8 and 10 (62). When activated, these then cleave caspase 3, which results in cellular apoptosis via mitochondrial dysregulation (63). Additionally, intracellular S. aureus can activate interleukin-6 (IL-6), IL-12, and colony-stimulating factor (CSF), further contributing to bone destruction (64, 65). If S. aureus persists intracellularly, it will not activate this pathway, as discussed in more detail in the sections on complications of osteomyelitis. Internalization is not unique to osteoblasts and is generally seen as a mechanism of immune evasion. However, studies have demonstrated that S. aureus can interact with cells and not cause cell death but become internalized by bone marrow-derived macrophages, causing differentiation into mature osteoclasts as well as activation of noninfected osteoclasts (66). S. aureus is also equipped to interact with the bone ECM through Cna and Bbp. Notably, Cna is the only identified S. aureus cell surface protein that binds to collagen, whereas Bbp has been documented to bind both bone sialoprotein (BSP) and fibrinogen (67, 68). Note that it has been shown that MSCRAMMs give S. aureus the ability to invade various mammalian cells in addition to bone cells (58, 69–73).
TABLE 2.
Protein interactions involved in progression and pathogenicity of staphylococcal infection
| Organism | MSCRAMM(s) | Matrix/bridge protein(s) | Receptora | Functional response | Reference(s) |
|---|---|---|---|---|---|
| S. aureus | Collagen adhesion (CNA) | Collagen | NA | Colonization | 68, 201 |
| Bone sialoprotein (Bbp) | Fibrinogen | Bone sialoprotein | Unknown | 67, 202 | |
| Fibronectin binding proteins (FnBP) | Fibronectin | α5β1 | Internalization | 58–60 | |
| Staphylococcal protein A (SpA) | TNFR1 | Induction of bone loss (apoptosis) and bone destruction (osteoclastogenesis), inhibits mineralization | 51–54 | ||
| S. epidermidis | Serine-aspartate repeat-containing proteins (Sdr) | Fibrinogen, collagen | NA | Colonization | 13, 78, 79, 82 |
| Extracellular matrix binding protein (Embp) | Fibronectin | NA | Colonization | 83, 203, 204 | |
| Autolysin E (AtlE) | Vitronectin | NA | Colonization | 84 | |
| Autolysin adhesion protein (Aae) | Vitronectin | NA | Colonization | 85 | |
| GehD lipase | Collagen | NA | Colonization | 86, 205 |
NA, not available.
Toxins play a major role in the progression and pathogenesis of osteomyelitis. S. aureus produces many toxins; however, toxic shock syndrome toxin 1 (TSST-1), hemolysins (Hla), Panton-Valentine leukocidin (PVL), coagulase, and phenol-soluble modulins (PSMs) are known to contribute to the severity of bone infection (Table 3). TSST-1 is known as a superantigen whose primary function is to inhibit the host immune response. It was recently shown to activate osteoclasts, increasing bone resorption through an unknown novel mechanism and contributing to the weakening of the bone (74). Interestingly, however, this superantigen was not shown to be cytotoxic to osteoclasts. Hla is one of the most studied cytotoxins produced by S. aureus due to its prevalence among different strains and its toxicity toward a wide range of mammalian cells. Hla lyses red blood cells by forming pores in the cell membrane, facilitating the spread and dissemination of infection through tissues. In persistent bone infection, hla is downregulated, therefore contributing to the quiescent and latent nature of recurrent osteomyelitis (75). PVL is produced by only a small percentage of S. aureus strains (approximately 2 to 3%) but is associated with persistence and rapid extension of osteomyelitis in murine models, leading to extensive spread of the infection (76). The primary role of coagulase is to convert fibrinogen to fibrin, thus providing a fibrin coating on the surface of S. aureus, protecting it from the host immune response. Coagulase also aggravates bone destruction and bone loss in mouse models of osteomyelitis by reducing osteoblast proliferation, inducing apoptosis, and decreasing mineralization (77).
TABLE 3.
Toxins and exoproteins involved in progression and pathogenicity of staphylococcal infection
| Organism | Toxin(s) or exoprotein(s) | Functional response | Reference(s) |
|---|---|---|---|
| S. aureus | Toxic shock syndrome toxin 1 (TSST-1) | Activates osteoclastogenesis | 74 |
| Alpha-hemolysin (Hla) | Osteoblast/osteoclast cell death | 75 | |
| Panton-Valentine leukocidin (PVL) | Persistence of infection | 76, 206 | |
| Canonical coagulase (Coa) | Inhibits osteoblast function | 77 | |
| Phenol-soluble modulins (PSMs) | Osteoblast cytotoxicity | 207–209 | |
| S. epidermidis | PSMs | Osteoblast cytotoxicity and biofilm dispersal | 90, 210 |
Staphylococcus epidermidis.
S. epidermidis has not been studied as extensively as S. aureus; hence, only a limited number of MSCRAMMs have been identified in this species, to date. These are the serine-aspartate repeat-containing (Sdr) proteins, extracellular matrix-binding protein (Embp), proteinaceous autolysin E (AtlE), novel autolysin (Aae), and lipase D (GehD) (13) (Table 2). The most extensively studied cell wall protein in S. epidermidis is SdrG, which binds fibrinogen (78) and is known to bind to osteoblasts (79). SdrG is part of the Sdr family, which is also composed of SdrF and SdrH, which are expressed in most strains (80). SdrF has been shown to facilitate binding to collagen and is thought to be expressed in isolates from medical device infections (81). SdrG binds to fibrinogen (78, 82), Embp binds to fibronectin (83), AtlE and Aae bind to vitronectin (84, 85), and GehD and SdrF bind to collagen, facilitating the interactions between bone ECM/cells and bacteria (81, 86).
In addition to the cell surface-associated virulence factors, staphylococci also secrete exoproteins, which can be cytotoxic, to aid in infection and dissemination (Table 3). S. epidermidis is traditionally known to form biofilms rather than to secrete exotoxin, with toxin production mostly limited to PSMs. PSMs are short, amphipathic, detergent-like molecules that have a proinflammatory and sometimes cytolytic function (13, 87). Biofilms are surface-attached agglomerates of bacteria embedded in a sticky extracellular matrix that is highly resistant to the host immune response and antibiotics. S. epidermidis is well known to form biofilms on medical device implants, allowing for the persistence of infection. These device-related infections are commonly seen in orthopedic implants, with removal of the device often required to remove the infection (88, 89). Notably, the activation of biofilm production is conversely related to PSM production, suggesting that PSM-negative strains readily form biofilms (90).
COMPLICATIONS IN OSTEOMYELITIS TREATMENT
Persistent and Recurring Infections in Osteomyelitis
Staphylococcal biofilm development.
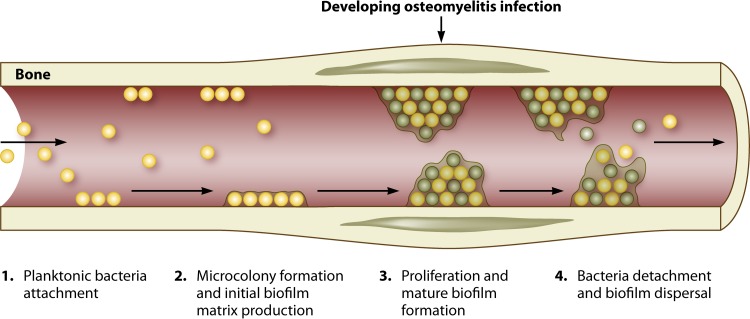
The ability of bacteria to form biofilms is a natural mechanism. The stages of biofilm development are attachment, accumulation, and dispersal (Fig. 3). A number of factors mediate attachment, including Atl, teichoic acids, and MSCRAMMs (91), which allow positioning of the premature biofilm. The presence of human serum proteins alone enhances the expression of MSCRAMMs that promote biofilm formation (92). Once attached, the bacterial cells within the matrix multiply and accumulate, shaping the matrix surrounding them to include complexities such as water channels for nutrient and waste diffusion. It is thought that through quorum sensing governed by the agr system, bacteria are able to sense their environment and can disperse from the mature biofilm matrix and spread to other areas (49, 93). At present, there are two types of biofilm: (i) polysaccharide intracellular adhesion (PIA)/polymeric N-acetylglucosamine (PNAG)-mediated biofilm and (ii) a proteinaceous biofilm mediated predominantly by FnBPs and the major Atl protein (94, 95). In regard to S. aureus, methicillin-susceptible S. aureus (MSSA) isolates have previously been shown to produce PIA biofilm, with fewer invasive methicillin-resistant S. aureus (MRSA) isolates documented to produce the proteinaceous matrix due to the downregulation of the accessory gene regulator (Agr) system associated with expression of the methicillin resistance gene in MRSA isolates (95–97). Additionally, extracellular DNA (eDNA) released from both S. aureus and S. epidermidis is important for the adherence and accumulation of biofilms. Abolishment of AtlE, involved in eDNA release, resulted in a reduced capacity of the bacteria to form biofilms (18).
FIG 3.
Stages of biofilm development (214). The first stage of biofilm formation in bone is attachment. Once attached, the bacteria begin to accumulate and produce a sticky matrix, which is the initial biofilm. This accumulation results in the formation of biofilm microcolonies and development of mature biofilm. The biofilm may then finally break down and release the bacteria from within, causing dissemination throughout the host.
In chronic osteomyelitis, the ability of staphylococci to persist and reinfect is partially attributed to the development of biofilms. The presence of biofilms has been suggested as the main cause of clinical quiescence of chronic osteomyelitis. Biofilms can provide protection from the antibiotic arsenal, the host immune response, and shear stresses. Biofilms further enhance the survival of the staphylococci residing within them by functioning to seize and concentrate important environmental nutrients (18, 98).
As with most cases of chronic osteomyelitis, surgical intervention is usually required for removal of the sequestrum. The sequestrum has a decreased vascularity and oxygen tension, providing optimum conditions for bacterial attachment and biofilm formation. Debridement of the infected area would also include removal of the sequestra, as antibiotic therapy alone is unable to sufficiently penetrate the biofilm matrix and eradicate the infection within. Surgical revisions can result in infection relapse in up to 40% of cases; however, if the sequestrum remains present in the bone, it will facilitate spreading of the infection throughout the bone. Spreading of the infection will eventually result in the need for radical debridement and possible limb amputation (99, 100).
Persistent SCV staphylococci.
In conjunction with the biofilm matrix, which provides protection for the bacteria within it, alterations of the bacterial metabolic activity which confer resistance to antibiotics are also observed. Persister cells and small-colony variants (SCVs) are found within biofilms and have been investigated extensively in the staphylococcal species (101, 102). SCVs have been described for osteomyelitis cases and have been deemed responsible for the recurrent infection associated with the disease due to their ability to survive intracellularly in a dormant state for many years, to then remerge as the parent strain and cause reinfection (103). Invasion and persistence of S. aureus in naturally nonphagocytic cells have been described for a range of cell types, including endothelial cells and keratinocytes (104, 105). One family of surface proteins found across the majority of S. aureus species are the FnBPs (e.g., FnBPA and FnBPB), which bind to the extracellular matrix protein fibronectin (106). It has been shown that these proteins not only can promote adhesion to surfaces but also can interact with naturally nonphagocytic cells and encourage uptake into the cell. Notably, Cna and Bbp favor FnBP internalization into nonprofessional phagocytic cells (44). This internalization has two possible outcomes: either the S. aureus invader activates production of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which in turn causes osteoblast apoptosis, or it can persist intracellularly as an SCV and cause recurrent infection months or years later (107, 108).
Antibiotic resistance.
Antibiotic resistance is an international issue that affects both developed and developing countries. The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) estimate that in both the European Union and the United States, more than 23,000 people die annually as a result of antimicrobial resistance, with S. aureus responsible for nearly 50% of those deaths. Antibiotic resistance can exacerbate staphylococcal infections by making them increasingly difficult to treat with antibiotics. There are three main mechanisms by which bacteria confer resistance: (i) changes in the membrane permeability/efflux of the antimicrobial, (ii) destruction of the antimicrobial compound, and (iii) alteration of the bacterial protein which is a target of the antimicrobial (109, 110). Efflux pumps present in bacteria can confer a natural resistance to antibiotics. These pumps are seen across both Gram-negative and Gram-positive bacteria, including Escherichia coli and S. aureus. When overexpressed, these pumps have the ability to transfer unwanted molecules from the cell (111, 112).
The direct inactivation of antibiotics via enzymatic strategies has been a major mechanism of antibiotic resistance since penicillin resistance emerged in the 1940s. Penicillinase, or β-lactamase, was shown to directly inactivate penicillin via hydrolysis of the β-lactam ring of the compound (113, 114). Since then, a multitude of enzymes have been identified that can degrade various classes of antibiotics, including β-lactams, aminoglycosides, phenicols, and macrolides (114).
Alteration of the bacterial target to prevent the interaction with the antibiotic is another mechanism by which resistance is conferred. There are two ways that this is possible: via a mutational change in the target protein or by a nonmutational modification of the target. An example of target change includes the acquisition of a gene homologous to the original target, such as that seen in S. aureus and S. epidermidis. In an effort to overcome the arsenal of β-lactams, S. aureus and S. epidermidis acquired a methicillin resistance gene, mecA, which is carried on a mobile heterogeneous genetic element called the staphylococcal cassette chromosome (SCC). The mecA gene encodes a penicillin binding protein, PBP2a, which displays decreased affinity for β-lactam antibiotics, allowing cell wall synthesis to occur as normal in the presence of the antibiotic. This can lead to the emergence of MRSA (115–118). MRSA is often isolated from bone infections and is usually treated with vancomycin, a glycopeptide that inhibits cells wall synthesis of S. aureus in a manner different from that for β-lactams. However, vancomycin-resistant S. aureus (VRSA) was isolated in Japan in 1997, instilling concerns over the treatment of these infections globally (119).
Noninfectious Complications of Osteomyelitis
With the onset of infection, there are various complications related to the bone that are not directly related to the infection but are a result of the infection. As previously described, the presence of infection can result in the production of cytokines which activate the bone-resorbing osteoclasts. Additionally, the presence of infection causes osteoblast cell death, thus preventing new bone formation (51, 53). This weakens the bone, which can result in pathological bone fractures, further compounding the issue (120). Moreover, surgical debridement of the bone can also result in weakening of the bone, which may further result in bone fractures if the bone is not supported sufficiently or is loaded prematurely. In the case of vertebral osteomyelitis, neurological compromise has been described. This includes documentation of motor weakness, paraparesis, and even paralysis, all caused by abscess formation compressing various parts of the spine, such as the spinal cord and nerve root (121). In children, osteomyelitis at the growth plates of long bones may interrupt normal growth. Patients with a chronic draining osteomyelitic sinus are also at increased risk of development of a squamous cell carcinoma (122).
CURRENT TREATMENT STRATEGIES
Osteomyelitis therapy requires an interdisciplinary approach involving a combination of patient evaluation, antibiotic therapy, and surgical intervention (123–125). Once the diagnosis of staphylococcal osteomyelitis is established, there are several factors that need to be considered for effective treatment. Successful treatment will almost certainly depend on debridement of infected tissue and the surgical resection of any necrotic bone or prosthetic material. Fundamentally, necrotic bone is the hallmark of chronic osteomyelitis, and its presence necessitates surgical debridement prior to any successful antimicrobial treatment. The management of prosthetic joint infection is beyond the scope of this review, but this is well covered elsewhere (126). Fracture fixation may also be required. When surgery is not possible, the patient may require long-term (usually oral) antimicrobial suppression of the infection.
Osteomyelitis Treatment Guidelines
The 2013 Cochrane review of chronic osteomyelitis examined all randomized and quasi-randomized trials of different antibiotic regimens given after surgical debridement of chronic osteomyelitis and found only eight small applicable trials, with a total of just 282 patients (127). Most trials were over 20 years old and do not reflect the emerging prevalence of antimicrobial-resistant pathogens, which are becoming more and more commonplace in modern health care settings. The authors concluded that the quality and reporting of these trials were often inadequate.
The level of evidence for treatment of acute osteomyelitis in adults is even worse. There is little objective evidence for the accepted precepts of treatment, and large, high-quality trials are lacking. There are various pieces of advice on the duration and route of treatment, and confusion exists regarding the superiority of intravenous/parenteral treatment over oral treatment. There are no Cochrane reviews for the treatment of acute osteomyelitis in adults. There are no UK or ECCMID guidelines for the treatment of acute osteomyelitis in adults, although the Bone Joint Infection Committee for the Italian Society of Infectious Tropical Diseases (SIMIT) guidelines are published in English and can provide useful guidance to clinicians (128). There are widely accepted and used Infectious Diseases Society of America (IDSA) treatment practice guidelines for the treatment of prosthetic joint infection and vertebral osteomyelitis, but dedicated treatment guidelines for acute osteomyelitis are still awaited. The treatment of acute osteomyelitis can be difficult and is largely based on expert opinion.
Although S. aureus and S. epidermidis remain the commonest etiological agents of native bone and joint infections, empirical treatment of osteomyelitis should be delayed (where possible) until samples for culture are obtained to allow for optimal antimicrobial selection (129). The gold standard for diagnosis is bone biopsy (130). Having found an organism to treat, the results of susceptibility testing can then inform the choice of the optimal agent, route, and duration of treatment.
Antibiotic Selection
The agent selected for treatment should be guided by the antimicrobial susceptibility testing results. The most important susceptibility distinction is the oxacillin/methicillin susceptibility result, which defines whether methicillin-susceptible or -resistant S. aureus or S. epidermidis (MSSA/MSSE or MRSA/MRSE) is involved. If the organism has not been cultured but is detected by 16S rRNA gene PCR or another molecular method, then the susceptibility testing results may not be available, and treatment has to be planned on the basis of the resistance patterns detected from the staphylococci cultured from the patient's other sites or local epidemiology. One day, genome sequencing may possibly be used to provide a genotypic prediction of the organism's susceptibility pattern (131), but this is expensive and not available outside research labs at present. An indication of the success of the selected treatment method may be given by reductions in the erythrocyte sedimentation rate (ESR) and the C-reactive protein (CRP) level. The main treatment choices for both methicillin-susceptible and -resistant S. aureus and S. epidermidis all achieve therapeutic levels of bone penetration (132) and are shown in Table 4 (133, 134).
TABLE 4.
Therapeutic options for treatment of S. aureus and S. epidermidis osteomyelitisa
| Agent (class) | Dose | Methicillin susceptibility status | Interactions | Side effects | Comments |
|---|---|---|---|---|---|
| Recommended i.v. agents for treatment of S. aureus and S. epidermidis osteomyelitis | |||||
| Flucloxacillin (penicillin) | 2 g q6h | MSSA/MSSE | No significant interactions | Rash, nausea, vomiting, diarrhea, cholestatic hepatitis | First-line treatment for MSSA/MSSE infection |
| Nafcillin (penicillin) | 2 g q4h | MSSA/MSSE | Tetracyclines, warfarin | Phlebitis, rash, neutropenia, interstitial nephritis | First-line treatment for MSSA/MSSE infection |
| Oxacillin (penicillin) | 2 g q4h | MSSA/MSSE | Tetracyclines | Phlebitis, rash, hepatitis | First-line treatment for MSSA/MSSE infection |
| Cefazolin (cephalosporin) | 2 g q8h | MSSA/MSSE | Probenecid (increase in cephalosporin serum concn), warfarin | Phlebitis, rash, fever, eosinophilia | Convenient for OPAT |
| Ceftriaxone (cephalosporin) | 2 g q24h | MSSA/MSSE | Calcium-containing solutions, probenecid (as described above), warfarin, lansoprazole | Pseudocholelithiasis, phlebitis, rash, fever | Convenient for OPAT |
| Vancomycin (glycopeptide) | 15 mg/kg of body wt q12h | MRSA/MRSE | Nondepolarizing muscle relaxants, nephrotoxic agents | Nephrotoxicity, ototoxicity, thrombocytopenia, red man syndrome | Target trough of 15–20 mg/liter, consider combination therapy, may be less effective against strains with MICs of 1–2 μg/ml |
| Teicoplanin (glycopeptide) | 12 mg/kg q24h | MRSA/MRSE | Nephrotoxic agents, ototoxic agents | Thrombophlebitis, rash, neutropenia, eosinophilia, ototoxicity | Target trough of >20 μg/ml |
| Daptomycin (cyclic lipopeptide) | 6 mg/kg q24h | MRSA/MRSE | Statins | CK elevation, eosinophilic pneumonia | Monitor CK, convenient for OPAT |
| Oral treatment options for either MSSA/MSSE or MRSA/MRSE osteomyelitis (if isolates are susceptible) | |||||
| Levofloxacin (fluoroquinolone) | 750 mg q24h | MSSA/MSSE, MRSA/MRSE | QTc-prolonging agents, warfarin | Diarrhea, phototoxicity, QTc prolongation, tendon rupture, seizures | Use combination therapy |
| Trimethoprim-sulfamethoxazole (antifolate) | DS 2 tabs q12h | MSSA/MSSE, MRSA/MRSE | ACE inhibitors, azathioprine, cyclosporine, folinic acid, para-aminobenzoic acid, phenytoin, sulfonylureas, oral contraceptives, warfarin | Nausea, vomiting, rash, hyperkalemia, bone marrow suppression | Consider combination therapy |
| Doxycycline (tetracycline) | 100 mg q12h | MSSA/MSSE, MRSA/MRSE | Acitretin, barbiturates, bismuth salts, carbamazepine, digoxin, oral contraceptives, penicillins, warfarin | GI intolerance, photosensitivity, dental deposition | |
| Minocycline (tetracycline) | 100 mg q12h | MSSA/MSSE, MRSA/MRSE | Acitretin, barbiturates, bismuth salts, carbamazepine, digoxin, oral contraceptives, penicillins, warfarin | Vertigo, ataxia, hypersensitivity pneumonitis, rash, GI intolerance, photosensitivity, dental deposition | Consider combination therapy |
| Linezolid (oxazolidinone) | 600 mg q12h | MSSA/MSSE, MRSA/MRSE | SSRIs, MAOIs, tricyclic antidepressants, adrenergic agents, rifampin | Thrombocytopenia, anemia, optic neuropathy, peripheral neuropathy | Reserve for use when alternatives not available, monitor FBC |
| Clindamycin (lincosamide) | 600 mg q6h (i.v.), 450 mg q6h (p.o.) | MSSA/MSSE, MRSA/MRSE | Erythromycin, kaolin-pectin, loperamide, nondepolarizing muscle relaxants | Diarrhea, nausea, vomiting, anorexia, rash | Check for inducible clindamycin resistance if erythromycin resistant |
| Rifampin (rifamycin) | 300–450 mg q12h or 600 mg q24h | MSSA/MSSE, MRSA/MRSE | Numerous—check interactions when prescribing | Orange discoloration of urine, tears, and sweat, hepatitis, GI intolerance, flu-like syndrome | Use in combination therapy only, as S. aureus resistance develops quickly in response to monotherapy; particularly effective in treatment of biofilms and infected prosthetic material |
| Fusidic acid (fusidane) | 500 mg q6h | MSSA/MSSE, MRSA/MRSE | Statins, ritonavir | Phlebitis, nausea, vomiting, diarrhea, elevated bilirubin | Use in combination therapy only, as S. aureus resistance develops quickly in response to monotherapy |
| Newer i.v. agents with unproven but potential future role in treatment of MRSA osteomyelitis | |||||
| Ceftaroline (cephalosporin) | 600 mg q8h | MRSA/MRSE | No significant interactions | Nausea, vomiting, diarrhea, crystalluria, elevated transaminases | Limited data, new agent with activity against MRSA/MRSE |
| Tigecycline (glycylcycline) | 100-mg load, then 50 mg q12h | MRSA/MRSE | Oral contraceptives | Nausea, vomiting, hepatic failure, pancreatitis | Limited data, new agent with activity against MRSA/MRSE, spectrum may be excessively broad |
| Telavancin (lipoglycopeptide) | 10 mg/kg q24h | MRSA/MRSE | QTc-prolonging agents, nephrotoxic agents | Nephrotoxicity, QTc prolongation, taste disturbances, nausea, vomiting | Limited data, new agent with activity against MRSA/MRSE |
| Dalbavancin | 1,000–1,500-mg first dose, then 500 mg once a week | MRSA/MRSE | Unknown | Diarrhea, headache, nausea, abdominal pain, blood disorders, Clostridium difficile colitis, constipation, cough, fungal infection, oral candidiasis, phlebitis, pruritus, rash, urticaria, vomiting, vulvovaginal mycotic infection, red man syndrome | Limited data, new agent with activity against MRSA/MRSE |
Data are from references 133, 134, 137, and 211 to 213. Abbreviations: ACE, angiotensin-converting enzyme; CK, creatine kinase; FBC, full blood count; GI, gastrointestinal; i.v., intravenous; MAOI, monoamine oxidase inhibitor; MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis; MSSA, methicillin-susceptible Staphylococcus aureus; MSSE, methicillin-susceptible Staphylococcus epidermidis; OPAT, outpatient parenteral antimicrobial therapy; p.o., per os; SSRI, selective serotonin reuptake inhibitor; q6h, every 6 h.
Route and Duration of Treatment
Since the paper of Waldvogel et al. in the New England Journal of Medicine in 1970 (135), a treatment duration of at least 4 weeks has commonly been advocated. This is based on Waldvogel et al.'s comparison of patient outcomes between two groups treated with “intensive” (more than 4 weeks) and limited therapy regimens. This rationale has been reiterated in recent years based on similar case series. Zimmerli published a meta-analysis of vertebral osteomyelitis trials and found no significant difference in outcomes for 22 different treatment regimens (136). Seven trials in S. aureus osteomyelitis from 1987 to 1999 showed no difference in outcomes between parenteral and oral antibiotics, but he noted that emerging resistance trends may render these outcomes clinically meaningless. He concluded that “although control trials are lacking, a treatment duration of 6 weeks is generally recommended.”
However, antimicrobial choice should also be determined by the reported penetration of the chosen agent into bone. Extant data are drawn from animal models comparing bone and serum levels of drugs, but there is a lack of standardized methodology and standard assays, and performances may differ from animal bone to human bone and between diseased and healthy tissues (130). Further, antibiotic levels may differ between healthy/experimental tissue and diseased human bone due to the differences in the pH and oxidative microenvironment of infection (136). The commonly used animal models were first developed by Norden et al. in the 1960s, and these have contributed to our understanding of bone revascularization and remodeling in response to infection and debridement, but some of the drugs used in humans are toxic to animals or have a poor correlation between animal and human efficacies, and vancomycin (which is a commonly used agent in human treatment) performs poorly in rabbit models (137). Lew and Waldvogel (2) reviewed the treatment of acute osteomyelitis, and while they concluded that antibiotics should be given for 4 to 6 weeks and “if possible by the intravenous route,” they did caution against the complications and risks associated with long-term intravenous catheters and a prolonged hospital stay. They concluded that “parenteral therapy remains the approach of choice until more comparative studies are completed” (16). However, there is an emerging body of opinion and evidence to challenge the dogma of 6 weeks of parenteral treatment. Spellberg and Lipsky questioned Waldvogel et al.'s case series and described it as retrospective, uncontrolled, heterogeneous, and based only on using penicillins as the treating agent (132). They stated that many oral agents now available can penetrate bone well and achieve levels in excess of the MICs, including agents with some action against susceptible strains of MRSA. They concluded that oral therapy is acceptable and simple, that any preference for parenteral treatment may be based “more on custom than evidence,” and that no strong evidence supports 4 to 6 weeks of treatment. Daver et al. retrospectively reviewed a cohort of adults with S. aureus osteomyelitis and compared those who received more than 4 weeks of intravenous treatment (median treatment duration of 60 days) to a group receiving less than 4 weeks of treatment (median intravenous treatment of 12 days followed by 42 days of oral treatment) (138). The overall cure rate was 74%, with no significant difference between the groups. Several other studies have shown equivalent results between intravenous treatment and highly bioavailable oral treatment (127, 139, 140).
Clinicians are eagerly awaiting full publication of the OVIVA trial (oral versus i.v. antibiotics for bone and joint infection) (139). This large multicenter trial (>1,000 patients from >20 UK centers) is a randomized, noninferiority trial comparing oral and i.v. antibiotics for the duration of the patient's osteomyelitis treatment. Preliminary results presented at ECCMID 2017 demonstrated equipoise, reflecting the strongest evidence, to date, that carefully selected, highly bioavailable agents with good bone penetration are an appropriate therapy for bone and joint infections, relieving physicians of the long-held dogmas that intravenous therapy is paramount in the treatment of these infections. As well as facilitating early discharge from hospital, the oral route obviously avoids the potential complications of long-term indwelling venous access catheters.
Dead Space Management
After debridement of the infected site, there is an area left that is termed dead space. Dead space management typically involves harvesting autologous or autogenous bone grafts, most often from the pelvic iliac crest, followed by implantation into the defect site. Autologous bone grafts remain the gold standard for promoting healing, with almost 2.2 million procedures estimated per annum (133, 141). Grafts of this kind have optimal biological performance in terms of osteogenicity, osteoinductivity, and osteoconductivity (142). However, the use of autologous bone grafts is limited by considerable donor site morbidity, postoperative pain, and risk of infection and the lack of available tissue. Allogeneic bone grafts can also be employed, most commonly by transplantation of sterilized cadaverous bone. However, this is also restricted due to viral transmission and immune rejection issues (15, 143). Another method used to manage dead space is the use of muscle flaps. This method has several advantages, such as malleability, a dense capillary network, and encouragement of rapid collagen deposition. One study by Anthony et al. demonstrated a 96% success rate in 34 patients by use of this strategy (144). Drawbacks, however, include recurrent infection in cases of chronic osteomyelitis, which can result in infection of the muscle flap (145).
Local Antibiotic Delivery Strategies
The systemic administration of a sufficiently high dose of antibiotics to reach the necrotic region and clear the infection often results in toxicity. Therefore, a number of products focused on the local delivery of antibiotics to the site of infection while simultaneously regenerating bone have emerged in recent years (146–151). There are a range of products currently on the market (Table 5), which are typically classified according to the degree of biodegradability of the carrier and which vary with regard to material type, antibiotic type, and delivery method. Each technique ultimately aims to reduce the dependence on systemic antibiotics, decrease hospitalization costs, and, importantly, prevent late relapse, which is common in chronic osteomyelitis.
TABLE 5.
Commercially available bone-regenerative biomaterials, including collagen-based sponges and bone cement/beads, loaded with antimicrobials and used for treatment of osteomyelitis
| Product | Company | Description | Indications |
|---|---|---|---|
| Collagen-based sponges | |||
| Collatamp G/EG | EUSA Pharma | Resorbable collagen implant impregnated with gentamicin | Prevent and treat surgical site infections through local antibiotic delivery |
| Genta-Coll | Resorba | Hemostyptic collagen sponge containing gentamicin | Hemostasis in wounds when there is high risk of infection (including in osteomyelitis) |
| Septocoll E | Biomet UK Ltd. | Resorbable equine collagen fleece containing 2 forms of gentamicin (gentamicin sulfate [rapid release] and gentamicin crobefate [protracted release]) | Potentially contaminated/contaminated wounds; revision operations in septic surgery |
| Bone cement/beads | |||
| Septopal | Biomet UK Ltd. | PMMA chains loaded with gentamicin sulfate | Local drug delivery after surgical debridement |
| Stimulan | Biocomposites | Calcium sulfate (can mix with gentamicin, vancomycin, and tobramycin) | Complements dead space and infection management strategies (e.g., infected nonunions, osteomyelitis, and periprosthetic joint infection) |
| Palacos | Heraeus Medical | Bone cement (available with gentamicin) | Orthopedic replacement procedures |
Nonbiodegradable antibiotic delivery systems are based on the acrylic material polymethylmethacrylate (PMMA), in the form of either cement (Palacos) or beads (Septopal). These can be combined with a number of antibiotics and have been used extensively in surgery to locally deliver antibiotics for the treatment of various musculoskeletal infections. Notably, this treatment is limited due to toxicity and the requirement for a thermally stable antibiotic (152). Additionally, PMMA products require removal, giving rise to the risk of reinfection. This drawback can be overcome by the use of biodegradable antimicrobial products.
Biodegradable delivery systems using calcium sulfate beads and collagen sponges with antibiotics have been in use for the past decade. These biodegradable delivery systems allow for the local delivery of antibiotics to the site of infection while providing a scaffold for the repair and regeneration of bone. Such products include Stimulan beads, which can be combined with a number of antibiotics, Collatamp G/EG (EUSA Pharma), and Genta-Coll (Resorba).
Nonantibiotic Antimicrobial Therapies
Current treatment strategies are continuously being researched and optimized, with many therapies, such as the Collatamp G/EG and Stimulan products mentioned above, reaching clinical settings. However, there are various limitations to these treatments, in particular targeting the infection. Thus, research into new and emerging technologies, such as nonantibiotic compounds, is an area of growing interest. A wide range of nonantibiotic materials, such as metals, polymers, and peptides, demonstrate antimicrobial activity (153–155). To date, these materials have been delivered by a variety of methods, including topically to the skin in the form of creams or bandages, as a coating on the surfaces of medical devices, or combined with other natural scaffolding materials and delivered locally to the site of infection, often reducing or even negating the use of antibiotics. Many of these nonantibiotic antimicrobial therapies are either clinically available or on the regulatory path toward product approval.
Metals.
A number of metals, e.g., silver (156–158), iron (159), mercury (160), tellurium (161, 162), copper (163, 164), zinc (21, 165, 166), and lead (167), have been shown to possess antimicrobial properties. In contrast to antibiotics, metals do not pose the risk of decomposition and can usually be processed at high temperatures (168). The mechanisms by which metals target microbes are only partially known; it is thought that some metals kill microbes by ion penetration, which inactivates microbial enzymes, while others impair membrane function or produce reactive oxygen species (167, 169). They have even been shown to be potential antimicrobial agents against drug-resistant bacteria, including MRSA and MRSE (170).
Polymers (chitosan).
Chitosan is a positively charged linear polysaccharide that is found naturally, most commonly derived from the shells of crustaceans. It is biodegradable, biocompatible, and nontoxic and displays antimicrobial activity (171). The molecular weight and degree of deacetylation of chitosan are said to affect its antimicrobial activity (172, 173). Chitosan also has excellent metal binding properties, as it is a chelating agent, and it is often combined with metal ions, such as the ions discussed above, to increase its antimicrobial activity against bacteria, including S. aureus (including MRSA) and S. epidermidis (174, 175).
Peptides.
Antimicrobial peptides (AMPs) are short proteins (<50 amino acids) that form part of the human innate immune system and are secreted by leukocytes, epithelial layers in the skin, and various mucosal membranes (176, 177). Some antimicrobial peptides, e.g., LL-37, demonstrate broad antimicrobial activity along with the promotion of bone regeneration (178, 179). LL-37 has also been shown to inhibit both the binding and biofilm-forming abilities of S. epidermidis (180) and has demonstrated effectiveness against both extracellular and intracellular S. aureus isolates (181). There are currently 2,707 peptides in the Antimicrobial Peptide Database reported to have antimicrobial activity derived from a variety of sources, including bacteria, archaea, protists, fungi, plants, and animals (182).
CONCLUSIONS AND FUTURE PERSPECTIVES
Staphylococcus-induced osteomyelitis is a major clinical challenge, as current treatment strategies are suboptimal for tackling both the infection and restoration of the affected bone. The pathogenesis of this disease is a double-edged sword whereby not only can staphylococci utilize bone for colonization, but bone itself can facilitate infection progression. Moreover, in addition to the ability of staphylococci to adapt to and evade the immune response by using the host's own machinery, they have also acquired resistance mechanisms to survive a plethora of antibiotic treatments available today. This, in conjunction with the need for surgical intervention, has led to new, exciting approaches in the field. For example, there has been a shift toward developing bifunctional bone-regenerative biomaterials whose degradation matches the native bone regeneration rate, combined with local delivery of antibiotics (183–185). Controlling the release of antimicrobials, which functions both to minimize systemic toxicity and to reduce the risk of inducing antibiotic resistance by ensuring that the release dose and rate are above minimum bactericidal concentrations sufficient for total infection clearance, has also become a hot topic in the drug delivery field. This may be achieved through methods such as microparticle incorporation or surface adsorption, with an on-demand release responsive to infection development (pH change, presence of bacterial toxins, or raised temperature) possible (186–189). Although nonantibiotic antimicrobials may be second to antibiotics at infection clearance, they do have the added advantage of overcoming some of the resistance mechanisms developed by bacteria (190–192). These nonantibiotic antimicrobial-loaded materials may be used for infection prophylaxis, perhaps after orthopedic procedures, which may be lengthy, post-implant removal, or following bone debridement if there is an infection risk.
Another exciting research avenue is the development of new methods to target infection by using a more tailored approach. One such area is the use of clustered regularly interspaced palindromic repeats (CRISPR). CRISPR technology has gained much attention for its gene editing abilities, mainly in mammalian cells (193, 194). However, there has been considerable research into the use of CRISPR for the treatment of infectious diseases (195). Seminal research by Bikard et al. demonstrated the potential to use CRISPR/Cas9 in targeting staphylococcal infection by targeting the methicillin resistance gene in S. aureus, making a MRSA isolate susceptible to methicillin once again (196). Moreover, when the technology was delivered in vivo, there was a moderate, albeit significant reduction in infection in mouse models of S. aureus infection. This research demonstrates the potential use of CRISPR/Cas9 in vivo, advancing the field toward a more targeted and selective approach to treat infections.
Currently, the majority of biological processes understood today are conducted in a two-dimensional (2D) setting. However, there is an increasing need for more physiologically relevant models (197). Studies using three-dimensional (3D) models over the past 2 decades have bridged the gap between 2D cell culture and in vivo culture (198, 199). The development of collagen-based scaffolds for tissue regeneration has presented a new focus for studying bone infection. Research from our group has demonstrated that staphylococcus-induced bone infection results in hypermineralization of the osteoblasts, correlating with increased metabolic activity, when the bacteria are cultured in a 3D bone matrix (N. Kavanagh, F. J. O'Brien, and S. W. Kerrigan, submitted for publication). This has not been demonstrated previously, therefore highlighting the importance of using more physiologically representative models to study infection. Using such 3D models will help us to elucidate and understand disease progression and thus inform our decisions for translating into in vivo models.
To conclude, staphylococcus-induced bone infection requires extensive research, with a particular focus on the molecular mechanism adopted by staphylococci to cause infection. Development of physiologically relevant models, such as the 3D model developed by our group, is an important part of driving knowledge forward within the field. As a result, incorporating new emerging technologies into the scaffold, such as CRISPR, to treat the infection provides an exciting new platform for not only regenerating the affected area but also treating the infection in a tailored and selective manner, avoiding the perils of antibiotic-based treatments currently seen in osteomyelitis patients.
ACKNOWLEDGMENTS
We are funded by the Irish Research Council (IRC) (projects GOIPG/2015/3044 [E.J.R.] and GOIPG/2013/1171 [S.W.K. and N.K.]) and the European Research Council (ERC) and cofunded by Enterprise Ireland and the European Regional Development Fund (ERDF) under the National Strategic Reference Framework (NSRF) 2007–2013. For funding, C.J.K. acknowledges an RCSI Office of Research and Innovation Seed Fund award (grant GR 14-0963), a Science Foundation Ireland (SFI) grant (grant SFI/12/RC/2278), and the European Union for a Marie Curie European reintegration grant under H2020 (project 659715) and an ERC starting grant (project 758064).
Biographies

Nicola Kavanagh received a B.S. in microbiology from University College Dublin in 2013. She is currently completing her Ph.D. at the Royal College of Surgeons in Ireland. Her research focus is the development of improved models for studying mechanisms of bone infection and novel treatment therapies for osteomyelitis.

Emily J. Ryan received a B.Eng. degree from the National University of Ireland, Galway, Ireland, in 2014. She then worked in biomedical engineering as a research and design engineer until 2015. She is currently completing her Ph.D. at the Royal College of Surgeons in Ireland, Dublin, Ireland. Her research focuses on the development of biodegradable, nonantibiotic antimicrobial scaffolds for the treatment of infection and regeneration of bone during staphylococcal osteomyelitis.

Amro Widaa, Ph.D, received a B.Sc. degree from the National University of Ireland, Maynooth, Ireland, in 2007 and an M.Sc. degree from the National University of Ireland, Galway, Ireland, in 2008. He carried out his Ph.D. and postdoctoral work at the Royal College of Surgeons in Ireland in 2008 to 2015. His research focuses on the molecular interactions that result from staphylococcus-induced osteomyelitis.

Gillian Sexton received a B.A. degree in the natural sciences from the Trinity College Dublin in 2013. She then went on to receive an M.Sc. in regenerative medicine at the National University of Ireland, Galway, Ireland, in 2015. She then moved to Dublin, where she works as a research assistant at the Royal College of Surgeons in Ireland. Her research focuses on biodegradable and antimicrobial scaffolds for the treatment of osteomyelitis.

Jérôme Fennell, M.D., is a consultant microbiologist at Tallaght, Naas, and Beamount Hospitals in Dublin, Ireland. Dr. Fennell is also a clinical microbiology lecturer at Trinity College Dublin. Dr. Fennell's research interests include orthopedic infections, glycopeptide dosing, urinary tract infections, and carbapenemase-producing Enterobacteriaceae.

Sadhbh O'Rourke, M.D., is a clinical microbiologist working at Tallaght Hospital, Dublin, Ireland. She is a member of the Royal College of Physicians, Ireland, and an associate member of the Royal College of Pathologists. Dr. O'Rourke is completing her training as a specialist registrar in clinical microbiology with the Royal College of Physicians, Ireland, and her research focus involves orthopedic infections.

Kevin Cahill, M.D., is a senior specialist registrar in plastic and reconstructive surgery at St. James's Hospital, Dublin, Ireland. His research interests include surgical infections, trauma, and microvascular reconstructive surgery, including orthoplastic lower limb reconstruction.

Cathal Kearney, Ph.D., is a biomedical engineer with a research focus on controlled drug delivery from tissue engineering scaffolds for a variety of applications, including simultaneous regeneration and infection treatment. He is a lecturer in the Anatomy Department at the Royal College of Surgeons in Ireland and a research fellow at the AMBER Centre. He obtained his B.A. and B.A.I. in mechanical and manufacturing engineering from Trinity College Dublin, Ireland, and his S.M. in mechanical engineering (2006) and Ph.D. (2011) from the Harvard/MIT Division of Health Sciences and Technology at the Massachusetts Institute of Technology, Cambridge, MA.

Fergal J. O'Brien, Ph.D., is Chair of Bioengineering & Regenerative Medicine and Head of the Tissue Engineering Research Group at the Royal College of Surgeons in Ireland and a principal investigator and Deputy Director of Advanced Materials and Bioengineering Research at the AMBER Centre. He is a leading innovator in the development of advanced biomaterials for regenerative medicine. He is a member of the World Council of Biomechanics and previously served as Biomaterials Topic Chair for the Orthopaedic Research Society and as an EU Council Member of the Tissue Engineering and Regenerative Medicine International Society. His research focuses on the development and clinical translation of scaffold-based therapeutics for tissue engineering, with a major focus on functionalizing these scaffolds as systems to deliver biomedicines and as advanced 3D pathophysiology in vitro systems for drug development and for studying cellular cross talk in cocultures and understanding disease states in cancer, angiogenesis, immunology, and infection.

Steven W. Kerrigan, PhD., is Head of the Cardiovascular Infection Research Group and Principal Investigator in the Tissue Engineering Research Group at the Royal College of Surgeons in Ireland. Professor Kerrigan obtained his Ph.D. in infectious diseases from the Royal College of Surgeons in Ireland in 2001 and carried out postdoctoral research at the University of California, San Francisco. Professor Kerrigan's main research interests are developing and investigating host-microbe interactions in both 2D and 3D ex vivo model systems of bloodstream infections (bacteremia and sepsis) and elucidating the mechanisms that lead to metastatic spread to distant sites, such as the bone. Professor Kerrigan's research focuses primarily on the opportunistic pathogens Staphylococcus aureus and Escherichia coli.
REFERENCES
- 1.Beck-Broichsitter BE, Smeets R, Heiland M. 2015. Current concepts in pathogenesis of acute and chronic osteomyelitis. Curr Opin Infect Dis 28:240–245. doi: 10.1097/QCO.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 2.Lew DP, Waldvogel FA. 2004. Osteomyelitis. Lancet 364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 3.Walter G, Kemmerer M, Kappler C, Hoffmann R. 2012. Treatment algorithms for chronic osteomyelitis. Dtsch Arztebl Int 109:257–264. doi: 10.3238/arztebl.2012.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schleifer KH, Kandler O. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36:407–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrity GM, Boone DR, Castenholz RW. 2005. Bergey's manual of systematic bacteriology, 2nd ed, vol 2 Springer, New York, NY. [Google Scholar]
- 6.Kornacki J, Doyle MP. 2010. Principles of microbiological troubleshooting in the industrial food processing environment, 1st ed Springer, New York, NY. doi: 10.1007/978-1-4419-5518-0. [DOI] [Google Scholar]
- 7.Otto M. 2010. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol 5:183–195. doi: 10.1586/edm.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madigan M, Martinko J, Stahl D, Clark D. 2012. Brock biology of microorganisms, 13th ed Benjamin Cummings, San Francisco, CA. [Google Scholar]
- 9.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 10.Tuchscherr LP, Buzzola FR, Alvarez LP, Caccuri RL, Lee JC, Sordelli DO. 2005. Capsule-negative Staphylococcus aureus induces chronic experimental mastitis in mice. Infect Immun 73:7932–7937. doi: 10.1128/IAI.73.12.7932-7937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster TJ, Geoghegan JA, Ganesh VK, Hook M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13:16–34. doi: 10.1128/CMR.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otto M. 2012. Molecular basis of Staphylococcus epidermidis infections. Semin Immunopathol 34:201–214. doi: 10.1007/s00281-011-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calhoun JH, Manring MM, Shirtliff M. 2009. Osteomyelitis of the long bones. Semin Plast Surg 23:59–72. doi: 10.1055/s-0029-1214158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oryan A, Alidadi S, Moshiri A, Maffulli N. 2014. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 9:18. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waldvogel FA, Medoff G, Swartz MN. 1970. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med 282:198–322. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- 17.Pichichero ME, Friesen HA. 1982. Polymicrobial osteomyelitis: report of three cases and review of the literature. Rev Infect Dis 4:86–96. doi: 10.1093/clinids/4.1.86. [DOI] [PubMed] [Google Scholar]
- 18.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. 2008. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol 52:13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 19.Alguire PC. 2009. Internal medicine essentials for clerkship students 2. American College of Physicians, Philadelphia, PA. [Google Scholar]
- 20.Lima AL, Oliveira PR, Carvalho VC, Cimerman S, Savio E. 2014. Recommendations for the treatment of osteomyelitis. Braz J Infect Dis 18:526–534. doi: 10.1016/j.bjid.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquet J, Chevalier Y, Couval E, Bouvier D, Bolzinger MA. 2015. Zinc oxide as a new antimicrobial preservative of topical products: interactions with common formulation ingredients. Int J Pharm 479:88–95. doi: 10.1016/j.ijpharm.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Merritt K. 1988. Factors increasing the risk of infection in patients with open fractures. J Trauma 28:823–827. doi: 10.1097/00005373-198806000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong DG, Lavery LA, Harkless LB. 1998. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 21:855–859. [DOI] [PubMed] [Google Scholar]
- 24.Berendt AR, Peters EJ, Bakker K, Embil JM, Eneroth M, Hinchliffe RJ, Jeffcoate WJ, Lipsky BA, Senneville E, Teh J, Valk GD. 2008. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev 24(Suppl 1):S145–S161. doi: 10.1002/dmrr.836. [DOI] [PubMed] [Google Scholar]
- 25.Lavery LA, Armstrong DG, Peters EJ, Lipsky BA. 2007. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care 30:270–274. doi: 10.2337/dc06-1572. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra R, Chan CS, Nather A. 2014. Osteomyelitis in the diabetic foot. Diabet Foot Ankle 5:24445. doi: 10.3402/dfa.v5.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. 1995. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 273:721–723. [PubMed] [Google Scholar]
- 28.Vigorita VJ, Ghelman B, Mintz D. 2007. Orthopaedic pathology, 2nd ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 29.Healy B, Freedman A. 2006. Infections. BMJ 332:838–841. doi: 10.1136/bmj.332.7545.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar V, Abbas A, Fausto N, Mitchell R. 2012. Robbins basic pathology, 9th ed Elsevier Health Sciences, Philadelphia, PA. [Google Scholar]
- 31.Schmidt HG, Tiemann AH, Braunschweig R, Diefenbeck M, Buhler M, Abitzsch D, Haustedt N, Walter G, Schoop R, Heppert V, Hofmann GO, Glombitza M, Grimme C, Gerlach UJ, Flesch I. 2011. Definition of the diagnosis osteomyelitis—osteomyelitis diagnosis score (ODS). Z Orthop Unfall 149:449–460. doi: 10.1055/s-0030-1270970. [DOI] [PubMed] [Google Scholar]
- 32.Tiemann A, Hofmann GO, Krukemeyer MG, Krenn V, Langwald S. 2014. Histopathological osteomyelitis evaluation score (HOES)—an innovative approach to histopathological diagnostics and scoring of osteomyelitis. GMS Interdiscip Plast Reconstr Surg DGPW 3:Doc08. doi: 10.3205/iprs000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott RJ, Christofersen MR, Robertson WW Jr, Davidson RS, Rankin L, Drummond DS. 1990. Acute osteomyelitis in children: a review of 116 cases. J Pediatr Orthop 10:649–652. doi: 10.1097/01241398-199009000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Tyrrell PN, Cassar-Pullicino VN, McCall IW. 1999. Spinal infection. Eur J Radiol 9:1066–1077. doi: 10.1007/s003300050793. [DOI] [PubMed] [Google Scholar]
- 35.Ziran BH. 2007. Osteomyelitis. J Trauma 62:S59–S60. doi: 10.1097/TA.0b013e318065abbd. [DOI] [PubMed] [Google Scholar]
- 36.Cierny G, Mader JT, Penninck JJ. 2003. The classic: a clinical staging system for adult osteomyelitis. Clin Orthop Relat Res 414:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 37.Bilezikian JP, Raisz LG, Martin TJ. 2008. Principles of bone biology, 3rd ed Elsevier, Amsterdam, Netherlands. [Google Scholar]
- 38.Keene DR, San Antonio JD, Mayne R, McQuillan DJ, Sarris G, Santoro SA, Iozzo RV. 2000. Decorin binds near the C terminus of type I collagen. J Biol Chem 275:21801–21804. doi: 10.1074/jbc.C000278200. [DOI] [PubMed] [Google Scholar]
- 39.Blair HC. 1998. How the osteoclast degrades bone. Bioessays 20:837–846. doi:. [DOI] [PubMed] [Google Scholar]
- 40.Mackie E. 2003. Osteoblasts: novel roles in orchestration of skeletal architecture. Int J Biochem Cell Biol 35:1301–1305. doi: 10.1016/S1357-2725(03)00107-9. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura H. 2007. Morphology, function, and differentiation of bone cells. J Hard Tissue Biol 16:15–22. doi: 10.2485/jhtb.16.15. [DOI] [Google Scholar]
- 42.Freemont AJ. 1993. Basic bone cell biology. Int J Clin Exp Pathol 74:411–416. [PMC free article] [PubMed] [Google Scholar]
- 43.Bruzzaniti A, Baron R. 2006. Molecular regulation of osteoclast activity. Rev Endocr Metab Disord 7:123–139. doi: 10.1007/s11154-006-9009-x. [DOI] [PubMed] [Google Scholar]
- 44.Josse J, Velard F, Gangloff SC. 2015. Staphylococcus aureus vs. osteoblast: relationship and consequences in osteomyelitis. Front Cell Infect Microbiol 5:85. doi: 10.3389/fcimb.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 46.Stewart PS. 2002. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol 292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 47.Branda SS, Vik Å, Friedman L, Kolter R. 2005. Biofilms: the matrix revisited. Trends Microbiol 13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Donlan RM. 2001. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 33:1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 49.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 50.Patti JM, Allen BL, McGavin MJ, Hook M. 1994. Mscramm-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol 48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 51.Claro T, Widaa A, O'Seaghdha M, Miajlovic H, Foster TJ, O'Brien FJ, Kerrigan SW. 2011. Staphylococcus aureus protein A binds to osteoblasts and triggers signals that weaken bone in osteomyelitis. PLoS One 6:e18748. doi: 10.1371/journal.pone.0018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Widaa A, Claro T, Foster TJ, O'Brien FJ, Kerrigan SW. 2012. Staphylococcus aureus protein a plays a critical role in mediating bone destruction and bone loss in osteomyelitis. PLoS One 7:e40586. doi: 10.1371/journal.pone.0040586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Claro T, Widaa A, McDonnell C, Foster TJ, O'Brien FJ, Kerrigan SW. 2013. Staphylococcus aureus protein A binding to osteoblast tumour necrosis factor receptor 1 results in activation of nuclear factor kappa B and release of interleukin-6 in bone infection. Microbiology 159:147–154. doi: 10.1099/mic.0.063016-0. [DOI] [PubMed] [Google Scholar]
- 54.Mendoza Bertelli A, Delpino MV, Lattar S, Giai C, Llana MN, Sanjuan N, Cassat JE, Sordelli D, Gómez MI. 2016. Staphylococcus aureus protein A enhances osteoclastogenesis via TNFR1 and EGFR signaling. Biochim Biophys Acta 1862:1975–1983. doi: 10.1016/j.bbadis.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Liu X, Dou C, Cao Z, Liu C, Dong S, Fei J. 2017. Staphylococcal protein A promotes osteoclastogenesis through MAPK signaling during bone infection. J Cell Physiol 232:2396–2406. doi: 10.1002/jcp.25774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massey RC, Kantzanou MN, Fowler T, Day NP, Schofield K, Wann ER, Berendt AR, Höök M, Peacock SJ. 2001. Fibronectin binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell Microbiol 3:839–851. doi: 10.1046/j.1462-5822.2001.00157.x. [DOI] [PubMed] [Google Scholar]
- 57.Wann ER, Gurusiddappa S, Höök M. 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J Biol Chem 275:13863–13871. doi: 10.1074/jbc.275.18.13863. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed S, Meghji S, Williams RJ, Henderson B, Brock JH, Nair SP.. 2001. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect Immun 69:2872–2877. doi: 10.1128/IAI.69.5.2872-2877.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edwards AM, Potts JR, Josefsson E, Massey RC. 2010. Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within FnBPA. PLoS Pathog 6:e1000964. doi: 10.1371/journal.ppat.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shinji H, Yosizawa Y, Tajima A, Iwase T, Sugimoto S, Seki K, Mizunoe Y. 2011. Role of fibronectin-binding proteins A and B in in vitro cellular infections and in vivo septic infections by Staphylococcus aureus. Infect Immun 79:2215–2223. doi: 10.1128/IAI.00133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agerer F, Lux S, Michel A, Rohde M, Ohlsen K, Hauck CR. 2005. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J Cell Sci 118:2189–2200. doi: 10.1242/jcs.02328. [DOI] [PubMed] [Google Scholar]
- 62.Mahalingam D, Szegezdi E, Keane M, de Jong S, Samali A. 2009. TRAIL receptor signalling and modulation: are we on the right TRAIL? Cancer Treat Rev 35:280–288. doi: 10.1016/j.ctrv.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Wang S, El-Deiry WS. 2003. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 64.Bost KL, Bento JL, Ellington JK, Marriott I, Hudson MC. 2000. Induction of colony-stimulating factor expression following staphylococcus or salmonella interaction with mouse or human osteoblasts. Infect Immun 68:5075–5083. doi: 10.1128/IAI.68.9.5075-5083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bost KL, Ramp WK, Nicholson NC, Bento JL, Marriott I, Hudson MC. 1999. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J Infect Dis 180:1912–1920. doi: 10.1086/315138. [DOI] [PubMed] [Google Scholar]
- 66.Trouillet-Assant S, Gallet M, Nauroy P, Rasigade JP, Flammier S, Parroche P, Marvel J, Ferry T, Vandenesch F, Jurdic P, Laurent F. 2015. Dual impact of live Staphylococcus aureus on the osteoclast lineage, leading to increased bone resorption. J Infect Dis 211:571–581. doi: 10.1093/infdis/jiu386. [DOI] [PubMed] [Google Scholar]
- 67.Vazquez V, Liang X, Horndahl JK, Ganesh VK, Smeds E, Foster TJ, Hook M. 2011. Fibrinogen is a ligand for the Staphylococcus aureus microbial surface components recognizing adhesive matrix molecules (MSCRAMM) bone sialoprotein-binding protein (Bbp). J Biol Chem 286:29797–29805. doi: 10.1074/jbc.M110.214981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elasri MO, Thomas JR, Skinner RA, Blevins JS, Beenken KE, Nelson CL, Smeltzer MS. 2002. Staphylococcus aureus collagen adhesin contributes to the pathogenesis of osteomyelitis. Bone 30:275–280. doi: 10.1016/S8756-3282(01)00632-9. [DOI] [PubMed] [Google Scholar]
- 69.Garzoni C, Kelley WL. 2009. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol 17:59–65. doi: 10.1016/j.tim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 70.von Eiff C. 2008. Staphylococcus aureus small colony variants: a challenge to microbiologists and clinicians. Int J Antimicrob Agents 31:507–510. doi: 10.1016/j.ijantimicag.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 71.Ellington JK, Harris M, Webb L, Smith B, Smith T, Tan K, Hudson M. 2003. Intracellular Staphylococcus aureus: a mechanism for the indolence of osteomyelitis. J Bone Joint Surg Br 85:918–921. [PubMed] [Google Scholar]
- 72.von Eiff C, Heilmann C, Proctor RA, Woltz C, Peters G, Götz F. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J Bacteriol 179:4706–4712. doi: 10.1128/jb.179.15.4706-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Almeida RA, Matthews KR, Cifrian E, Guidry AJ, Oliver SP.. 1996. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci 79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 74.Flammier S, Rasigade J-P, Badiou C, Henry T, Vandenesch F, Laurent F, Trouillet-Assant S. 2016. Human monocyte-derived osteoclasts are targeted by staphylococcal pore-forming toxins and superantigens. PLoS One 11:e0150693. doi: 10.1371/journal.pone.0150693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trouillet-Assant S, Lelièvre L, Martins-Simões P, Gonzaga L, Tasse J, Valour F, Rasigade JP, Vandenesch F, Muinz Guedes RL, Ribeiro de Vasconcelos AT, Caillon J, Lustig S, Ferry T, Jacqueline C, Loss de Morais G, Laurent F. 2016. Adaptive processes of Staphylococcus aureus isolates during the progression from acute to chronic bone and joint infections in patients. Cell Microbiol 18:1405–1414. doi: 10.1111/cmi.12582. [DOI] [PubMed] [Google Scholar]
- 76.Badiou C, Dumitrescu O, George N, Forbes AR, Drougka E, Chan KS, Ramdani-Bouguessa N, Meugnier H, Bes M, Vandenesch F, Etienne J, Hsu LY, Tazir M, Spiliopoulou I, Nimmo GR, Hulten KG, Lina G. 2010. Rapid detection of Staphylococcus aureus Panton-Valentine leukocidin in clinical specimens by enzyme-linked immunosorbent assay and immunochromatographic tests. J Clin Microbiol 48:1384–1390. doi: 10.1128/JCM.02274-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin T, Zhu YL, Li J, Shi J, He XQ, Ding J, Xu YQ. 2013. Staphylococcal protein A, Panton-Valentine leukocidin and coagulase aggravate the bone loss and bone destruction in osteomyelitis. Cell Physiol Biochem 32:322–333. doi: 10.1159/000354440. [DOI] [PubMed] [Google Scholar]
- 78.Hartford O, O'Brien L, Schofield K, Wells J, Foster TJ. 2001. The Fbe (SdrG) protein of Staphylococcus epidermidis HB promotes bacterial adherence to fibrinogen. Microbiology 147:2545. doi: 10.1099/00221287-147-9-2545. [DOI] [PubMed] [Google Scholar]
- 79.Claro T, Kavanagh N, Foster TJ, O'Brien FJ, Kerrigan SW. 2015. Staphylococcus epidermidis serine-aspartate repeat protein G (SdrG) binds to osteoblast integrin alpha V beta 3. Microbes Infect 17:395–401. doi: 10.1016/j.micinf.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 80.McCrea KW, Hartford O, Davis S, Eidhin DN, Lina G, Speziale P, Foster TJ, Höök M. 2000. The serine-aspartate repeat (Sdr) protein family in Staphylococcus epidermidis. Microbiology 146:1535. doi: 10.1099/00221287-146-7-1535. [DOI] [PubMed] [Google Scholar]
- 81.Arrecubieta C, Lee MH, Macey A, Foster TJ, Lowy FD. 2007. SdrF, a Staphylococcus epidermidis surface protein, binds type I collagen. J Biol Chem 282:18767–18776. doi: 10.1074/jbc.M610940200. [DOI] [PubMed] [Google Scholar]
- 82.Davis SL, Gurusiddappa S, McCrea KW, Perkins S, Höök M. 2001. SdrG, a fibrinogen-binding bacterial adhesin of the microbial surface components recognizing adhesive matrix molecules subfamily from Staphylococcus epidermidis, targets the thrombin cleavage site in the Bbeta chain. J Biol Chem 276:27799–27805. doi: 10.1074/jbc.M103873200. [DOI] [PubMed] [Google Scholar]
- 83.Williams RJ, Henderson B, Sharp LJ, Nair SP.. 2002. Identification of a fibronectin-binding protein from Staphylococcus epidermidis. Infect Immun 70:6805. doi: 10.1128/IAI.70.12.6805-6810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heilmann C, Hussain M, Peters G, Götz F. 1997. Evidence for autolysin mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol 24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 85.Heilmann C, Thumm G, Chhatwal GS, Hartleib J, Uekötter A, Peters G. 2003. Identification and characterization of a novel autolysin (Aae) with adhesive properties from Staphylococcus epidermidis. Microbiology 149:2769. doi: 10.1099/mic.0.26527-0. [DOI] [PubMed] [Google Scholar]
- 86.Bowden MG, Visai L, Longshaw CM, Holland KT, Speziale P, Höök M. 2002. Is the GehD lipase from Staphylococcus epidermidis a collagen binding adhesin? J Biol Chem 277:43017–43023. doi: 10.1074/jbc.M207921200. [DOI] [PubMed] [Google Scholar]
- 87.Vuong C, Dürr M, Carmody AB, Peschel A, Klebanoff SJ, Otto M. 2004. Regulated expression of pathogen-associated molecular pattern molecules in Staphylococcus epidermidis: quorum-sensing determines pro-inflammatory capacity and production of phenol-soluble modulins. Cell Microbiol 6:753–759. doi: 10.1111/j.1462-5822.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 88.Valour F, Trouillet-Assant S, Rasigade JP, Lustig S, Chanard E, Meugnier H, Tigaud S, Vandenesch F, Etienne J, Ferry T, Laurent F. 2013. Staphylococcus epidermidis in orthopedic device infections: the role of bacterial internalization in human osteoblasts and biofilm formation. PLoS One 8:e67240. doi: 10.1371/journal.pone.0067240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song Z, Borgwardt L, Høiby N, Wu H, Sørensen TS, Borgwardt A. 2013. Prosthesis infections after orthopedic joint replacement: the possible role of bacterial biofilms. Orthop Rev 5:e14. doi: 10.4081/or.2013.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao Y, Sturdevant DE, Otto M. 2005. Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J Infect Dis 191:289–298. doi: 10.1086/426945. [DOI] [PubMed] [Google Scholar]
- 91.Raad I, Hanna H, Maki D. 2007. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. Lancet Infect Dis 7:645–657. doi: 10.1016/S1473-3099(07)70235-9. [DOI] [PubMed] [Google Scholar]
- 92.Cardile AP, Sanchez CJ, Samberg ME, Romano DR, Hardy SK, Wenke JC, Murray CK, Akers KS. 2014. Human plasma enhances the expression of staphylococcal microbial surface components recognizing adhesive matrix molecules promoting biofilm formation and increases antimicrobial tolerance in vitro. BMC Res Notes 7:457. doi: 10.1186/1756-0500-7-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yarwood JM, Bartels DJ, Volper EM, Greenberg EP. 2004. Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol 186:1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67:5427–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Houston P, Rowe SE, Pozzi C, Waters EM, O'Gara JP. 2011. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect Immun 79:1153–1165. doi: 10.1128/IAI.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bui LMG, Turnidge JD, Kidd SP.. 2015. The induction of Staphylococcus aureus biofilm formation or small colony variants is a strain-specific response to host-generated chemical stresses. Microbes Infect 17:77–82. doi: 10.1016/j.micinf.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 97.McCarthy H, Rudkin JK, Black NS, Gallagher L, O'Neill E, O'Gara JP. 2015. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front Cell Infect Microbiol 5:1–9. doi: 10.3389/fcimb.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. 1999. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moralle MR, Stekas ND, Reilly MC, Sirkin MS, Adams MR. 2016. Salvage of a below knee amputation utilizing rotationplasty principles in a patient with chronic tibial osteomyelitis. J Orthop Case Rep 6:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh R, Ray P, Das A, Sharma M. 2009. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J Med Microbiol 58:1067–1073. doi: 10.1099/jmm.0.009720-0. [DOI] [PubMed] [Google Scholar]
- 102.Wilde AD, Snyder DJ, Putnam NE, Valentino MD, Hammer ND, Lonergan ZR, Hinger SA, Aysanoa EE, Blanchard C, Dunman PM, Wasserman GA, Chen J, Shopsin B, Gilmore MS, Skaar EP, Cassat JE. 2015. Bacterial hypoxic responses revealed as critical determinants of the host-pathogen outcome by TnSeq analysis of Staphylococcus aureus invasive infection. PLoS Pathog 11:e1005341. doi: 10.1371/journal.ppat.1005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 104.Mempel M, Schnopp C, Hojka M, Fesq H, Weidinger S, Schaller M, Korting HC, Ring J, Abeck D. 2002. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br J Dermatol 146:943–951. doi: 10.1046/j.1365-2133.2002.04752.x. [DOI] [PubMed] [Google Scholar]
- 105.Vesga O, Groeschel MC, Otten MF, Brar DW, Vann JM, Proctor RA. 1996. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J Infect Dis 173:739–742. doi: 10.1093/infdis/173.3.739. [DOI] [PubMed] [Google Scholar]
- 106.Brouillette E, Talbot BG, Malouin F. 2003. The fibronectin-binding proteins of Staphylococcus aureus may promote mammary gland colonization in a lactating mouse model of mastitis. Infect Immun 71:2292–2295. doi: 10.1128/IAI.71.4.2292-2295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia LG, Lemaire S, Kahl BC, Becker K, Proctor RA, Denis O, Tulkens PM, Van Bambeke F. 2013. Antibiotic activity against small-colony variants of Staphylococcus aureus: review of in vitro, animal and clinical data. J Antimicrob Chemother 68:1455–1464. doi: 10.1093/jac/dkt072. [DOI] [PubMed] [Google Scholar]
- 108.Alexander EH, Bento JL, Hughes FM Jr, Marriott I, Hudson MC, Bost KL. 2001. Staphylococcus aureus and Salmonella enterica serovar Dublin induce tumor necrosis factor-related apoptosis-inducing ligand expression by normal mouse and human osteoblasts. Infect Immun 69:1581–1586. doi: 10.1128/IAI.69.3.1581-1586.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. 2015. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 110.Dever LA, Dermody TS. 1991. Mechanisms of bacterial resistance to antibiotics. Arch Intern Med 151:886–895. [PubMed] [Google Scholar]
- 111.Floyd JL, Smith KP, Kumar SH, Floyd JT, Varela MF. 2010. Lmrs is a multidrug efflux pump of the major facilitator superfamily from Staphylococcus aureus. Antimicrob Agents Chemother 54:5406–5412. doi: 10.1128/AAC.00580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wozniak RAF, Waldor MK. 2010. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat Rev Microbiol 8:552–563. doi: 10.1038/nrmicro2382. [DOI] [PubMed] [Google Scholar]
- 113.Abraham EP, Chain E. 1988. An enzyme from bacteria able to destroy penicillin. 1940. Rev Infect Dis 10:677–678. doi: 10.1093/clinids/10.4.677. [DOI] [PubMed] [Google Scholar]
- 114.Livermore DM. 2008. Defining an extended-spectrum β-lactamase. Clin Microbiol Infect 14:3–10. [DOI] [PubMed] [Google Scholar]
- 115.Katayama Y, Ito T, Hiramatsu K. 2000. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 44:1549–1555. doi: 10.1128/AAC.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Storrs MJ, Courvalin P, Foster TJ. 1988. Genetic analysis of gentamicin resistance in methicillin- and gentamicin-resistant strains of Staphylococcus aureus isolated in Dublin hospitals. Antimicrob Agents Chemother 32:1174–1181. doi: 10.1128/AAC.32.8.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wielders CLC, Vriens MR, Brisse S, de Graaf-Miltenburg LAM, Troelstra A, Fleer A, Schmitz FJ, Verhoef J, Fluit AC. 2001. Evidence for in-vivo transfer of mecA DNA between strains of Staphylococcus aureus. Lancet 357:1674–1675. doi: 10.1016/S0140-6736(00)04832-7. [DOI] [PubMed] [Google Scholar]
- 118.Ubukata K, Nonoguchi R, Matsuhashi M, Konno M. 1989. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol 171:2882–2885. doi: 10.1128/jb.171.5.2882-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Conly JM, Johnston BL. 2002. VISA, hetero-VISA and VRSA: the end of the vancomycin era? Can J Infect Dis Med Microbiol 13:282–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Belthur MV, Birchansky SB, Verdugo AA, Mason EO Jr, Hulten KG, Kaplan SL, Smith EO, Phillips WA, Weinberg J. 2012. Pathologic fractures in children with acute Staphylococcus aureus osteomyelitis. J Bone Joint Surg Am 94:34–42. doi: 10.2106/JBJS.J.01915. [DOI] [PubMed] [Google Scholar]
- 121.McHenry MC, Easley KA, Locker GA. 2002. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis 34:1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 122.Li Q, Cui H, Dong J, He Y, Zhou D, Zhang P, Liu P. 2015. Squamous cell carcinoma resulting from chronic osteomyelitis: a retrospective study of 8 cases. Int J Clin Exp Pathol 8:10178–10184. [PMC free article] [PubMed] [Google Scholar]
- 123.Hatzenbuehler J, Pulling TJ. 2011. Diagnosis and management of osteomyelitis. Am Fam Physician 9:1027–1033. [PubMed] [Google Scholar]
- 124.Parsons B, Strauss E. 2004. Surgical management of chronic osteomyelitis. Am J Surg 188(1A Suppl):57–66. [DOI] [PubMed] [Google Scholar]
- 125.Fritz JM, McDonald JR. 2008. Osteomyelitis: approach to diagnosis and treatment. Phys Sportsmed 36:nihpa116823. doi: 10.3810/psm.2008.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 127.Conterno LO, da Silva Filho CR. 2009. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst Rev 2009:CD004439. doi: 10.1002/14651858.CD004439.pub2. [DOI] [PubMed] [Google Scholar]
- 128.Esposito S, Leone S, Bassetti M, Borre S, Leoncini F, Meani E, Venditti M, Mazzotta F. 2009. Italian guidelines for the diagnosis and infectious disease management of osteomyelitis and prosthetic joint infections in adults. Infection 37:478–496. doi: 10.1007/s15010-009-8269-2. [DOI] [PubMed] [Google Scholar]
- 129.Kaplan SL. 2014. Recent lessons for the management of bone and joint infections. J Infect 68(Suppl 1):S51–S56. doi: 10.1016/j.jinf.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 130.Johnston B, Conly J. 2007. Osteomyelitis management: more art than science? Can J Infect Dis Med Microbiol 18:115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gordon NC, Price JR, Cole K, Everitt R, Morgan M, Finney J, Kearns AM, Pichon B, Young B, Wilson DJ, Llewelyn MJ, Paul J, Peto TE, Crook DW, Walker AS, Golubchik T. 2014. Prediction of Staphylococcus aureus antimicrobial resistance by whole-genome sequencing. J Clin Microbiol 52:1182–1191. doi: 10.1128/JCM.03117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spellberg B, Lipsky BA. 2012. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 54:393–407. doi: 10.1093/cid/cir842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Talan M JRDA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 134.Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM III, Petermann GW, Osmon DR. 2015. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 61:e26–e46. doi: 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- 135.Waldvogel FA, Medoff G, Swartz MN. 1970. Treatment of osteomyelitis. N Engl J Med 283:822–823. doi: 10.1056/NEJM197010082831523. [DOI] [PubMed] [Google Scholar]
- 136.Zimmerli W. 2010. Clinical practice. Vertebral osteomyelitis. N Engl J Med 362:1022–1029. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
- 137.Fraimow HS. 2009. Systemic antimicrobial therapy in osteomyelitis. Semin Plast Surg 23:90–99. doi: 10.1055/s-0029-1214161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Daver NG, Shelburne SA, Atmar RL, Giordano TP, Stager CE, Reitman CA, White AC Jr. 2007. Oral step-down therapy is comparable to intravenous therapy for Staphylococcus aureus osteomyelitis. J Infect 54:539–544. doi: 10.1016/j.jinf.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 139.Li HK, Scarborough M, Zambellas R, Cooper C, Rombach I, Walker AS, Lipsky BA, Briggs A, Seaton A, Atkins B, Woodhouse A, Berendt A, Byren I, Angus B, Pandit H, Stubbs D, McNally M, Thwaites G, Bejon P. 2015. Oral versus intravenous antibiotic treatment for bone and joint infections (OVIVA): study protocol for a randomised controlled trial. Trials 16:583. doi: 10.1186/s13063-015-1098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Long WG, Einhorn TA, Koval K, McKee M, Smith W, Sanders R, Watson T. 2007. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg 89:649–658. [DOI] [PubMed] [Google Scholar]
- 141.Giannoudis PV, Dinopoulos H, Tsiridis E. 2005. Bone substitutes: an update. Injury 36(Suppl 3):S20–S27. [DOI] [PubMed] [Google Scholar]
- 142.Bauer TW, Muschler GF. 2000. Bone graft materials—an overview of the basic science. Clin Orthop Relat Res 371:10–27. doi: 10.1097/00003086-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 143.Roberts TT, Rosenbaum AJ. 2012. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis 8:114–124. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Anthony JP, Mathes SJ, Alpert BS. 1991. The muscle flap in the treatment of chronic lower extremity osteomyelitis: results in patients over 5 years after treatment. Plast Reconstr Surg 88:311–318. doi: 10.1097/00006534-199108000-00023. [DOI] [PubMed] [Google Scholar]
- 145.Klebuc M, Menn Z. 2013. Muscle flaps and their role in limb salvage. Methodist Debakey Cardiovasc J 9:95–99. doi: 10.14797/mdcj-9-2-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kearney CJ, Mooney DJ. 2013. Macroscale delivery systems for molecular and cellular payloads. Nat Mater 12:1004–1017. doi: 10.1038/nmat3758. [DOI] [PubMed] [Google Scholar]
- 147.Gao P, Nie X, Zou M, Shi Y, Cheng G. 2011. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot 64:625–634. doi: 10.1038/ja.2011.58. [DOI] [PubMed] [Google Scholar]
- 148.Gimeno M, Pinczowski P, Pérez M, Giorello A, Martínez MÁ Santamaría J, Arruebo M, Luján L. 2015. A controlled antibiotic release system to prevent orthopedic-implant associated infections: an in vitro study. Eur J Pharm Biopharm 96:264–271. doi: 10.1016/j.ejpb.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Adams SB, Shamji MF, Nettles DL, Hwang P, Setton LA. 2009. Sustained release of antibiotics from injectable and thermally responsive polypeptide depots. J Biomed Mater Res B Appl Biomater 90:67–74. doi: 10.1002/jbm.b.31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Adams CS, Antoci V Jr, Harrison G, Patal P, Freeman TA, Shapiro IM, Parvizi J, Hickok NJ, Radin S, Ducheyne P. 2009. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J Orthop Res 27:701–709. doi: 10.1002/jor.20815. [DOI] [PubMed] [Google Scholar]
- 151.El-Ghannam A, Ahmed K, Omran M. 2005. Nanoporous delivery system to treat osteomyelitis and regenerate bone: gentamicin release kinetics and bactericidal effect. J Biomed Mater Res B Appl Biomater 73:277–284. doi: 10.1002/jbm.b.30209. [DOI] [PubMed] [Google Scholar]
- 152.Bistolfi A, Massazza G, Verne E, Masse A, Deledda D, Ferraris S, Miola M, Galetto F, Crova M. 2011. Antibiotic-loaded cement in orthopedic surgery: a review. ISRN Orthop 2011:290851. doi: 10.5402/2011/290851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M. 2015. Silver nanoparticles as potential antibacterial agents. Molecules 20:8856. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hancock REW, Sahl H-G. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 155.Lam SJ, O'Brien-Simpson NM, Pantarat N, Sulistio A, Wong EHH, Chen Y-Y, Lenzo JC, Holden JA, Blencowe A, Reynolds EC, Qiao GG. 2016. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat Microbiol 1:16162. doi: 10.1038/nmicrobiol.2016.162. [DOI] [PubMed] [Google Scholar]
- 156.Randall CP, Oyama LB, Bostock JM, Chopra I, O'Neill AJ. 2013. The silver cation (Ag+): antistaphylococcal activity, mode of action and resistance studies. J Antimicrob Chemother 68:131–138. doi: 10.1093/jac/dks372. [DOI] [PubMed] [Google Scholar]
- 157.Chernousova S, Epple M. 2013. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl 52:1636–1653. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- 158.Choi O, Deng KK, Kim NJ, Ross L Jr, Surampalli RY, Hu Z. 2008. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res 42:3066–3074. doi: 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 159.Arakha M, Pal S, Samantarrai D, Panigrahi TK, Mallick BC, Pramanik K, Mallick B, Jha S. 2015. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci Rep 5:14813. doi: 10.1038/srep14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Morrier JJ, Suchett-Kaye G, Nguyen D, Rocca JP, Blanc-Benon J, Barsotti O. 1998. Antimicrobial activity of amalgams, alloys and their elements and phases. Dent Mater 14:150–157. doi: 10.1016/S0109-5641(98)00022-0. [DOI] [PubMed] [Google Scholar]
- 161.Zhong CL, Qin BY, Xie XY, Bai Y. 2013. Antioxidant and antimicrobial activity of tellurium dioxide nanoparticles sols. J Nano Res 25:8–15. doi: 10.4028/www.scientific.net/JNanoR.25.8. [DOI] [Google Scholar]
- 162.Zonaro E, Lampis S, Turner RJ, Qazi SJ, Vallini G. 2015. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front Microbiol 6:584. doi: 10.3389/fmicb.2015.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Azam A, Ahmed AS, Oves M, Khan MS, Memic A. 2012. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int J Nanomed (Lond) 7:3527–3535. doi: 10.2147/IJN.S29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Beeton ML, Aldrich-Wright JR, Bolhuis A. 2014. The antimicrobial and antibiofilm activities of copper(II) complexes. J Inorg Biochem 140:167–172. doi: 10.1016/j.jinorgbio.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 165.Pasquet J, Chevalier Y, Pelletier J, Couval E, Bouvier D, Bolzinger M-A. 2014. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf A Physicochem Eng Asp 457:263–274. doi: 10.1016/j.colsurfa.2014.05.057. [DOI] [Google Scholar]
- 166.Raghupathi KR, Koodali RT, Manna AC. 2011. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27:4020–4028. doi: 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- 167.Lemire JA, Harrison JJ, Turner RJ. 2013. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 168.Mouriño V, Cattalini JP, Boccaccini AR. 2012. Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments. J R Soc Interface 9:401–419. doi: 10.1098/rsif.2011.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim T, Feng Q, Kim J, Wu J, Wang H, Chen G, Cui F. 1998. Antimicrobial effects of metal ions (Ag1, Cu21, Zn21) in hydroxyapatite. J Mater Sci Mater Med 3:129–134. doi: 10.1023/A:1008811501734. [DOI] [PubMed] [Google Scholar]
- 170.Allahverdiyev AM, Abamor ES, Bagirova M, Rafailovich M. 2011. Antimicrobial effects of TiO(2) and Ag(2)O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol 6:933–940. doi: 10.2217/fmb.11.78. [DOI] [PubMed] [Google Scholar]
- 171.Ma Y, Zhou T, Zhao C. 2008. Preparation of chitosan-nylon-6 blended membranes containing silver ions as antibacterial materials. Carbohydr Res 343:230–237. doi: 10.1016/j.carres.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 172.Kong M, Chen XG, Xing K, Park HJ. 2010. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 173.Goy RC, de Britto D, Assis OBG. 2009. A review of the antimicrobial activity of chitosan. Polímeros 19:241–247. [Google Scholar]
- 174.Wang X, Du Y, Fan L, Liu H, Hu Y. 2005. Chitosan-metal complexes as antimicrobial agent: synthesis, characterization and structure-activity study. Polym Bull 55:105–113. doi: 10.1007/s00289-005-0414-1. [DOI] [Google Scholar]
- 175.Anisha BS, Biswas R, Chennazhi KP, Jayakumar R. 2013. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int J Biol Macromol 62:310–320. doi: 10.1016/j.ijbiomac.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 176.Gordon YJ, Romanowski EG. 2005. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res 30:505–515. doi: 10.1080/02713680590968637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ganz T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 178.Kittaka M, Shiba H, Kajiya M, Fujita T, Iwata T, Rathvisal K, Ouhara K, Takeda K, Fujita T, Komatsuzawa H, Kurihara H. 2013. The antimicrobial peptide LL37 promotes bone regeneration in a rat calvarial bone defect. Peptides 46:136–142. doi: 10.1016/j.peptides.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 179.Chereddy KK, Her CH, Comune M, Moia C, Lopes A, Porporato PE, Vanacker J, Lam MC, Steinstraesser L, Sonveaux P, Zhu H, Ferreira LS, Vandermeulen G, Preat V. 2014. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. J Control Release 194:138–147. doi: 10.1016/j.jconrel.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 180.Hell E, Giske CG, Nelson A, Römling U, Marchini G. 2010. Human cathelicidin peptide LL37 inhibits both attachment capability and biofilm formation of Staphylococcus epidermidis. Lett Appl Microbiol 50:211–215. doi: 10.1111/j.1472-765X.2009.02778.x. [DOI] [PubMed] [Google Scholar]
- 181.Noore J, Noore A, Li B. 2013. Cationic antimicrobial peptide LL-37 is effective against both extra- and intracellular Staphylococcus aureus. Antimicrob Agents Chemother 57:1283–1290. doi: 10.1128/AAC.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang Z, Wang G. 2004. APD: the Antimicrobial Peptide Database. Nucleic Acids Res 32:D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang Y, Zhang M. 2002. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J Biomed Mater Res 62:378–386. doi: 10.1002/jbm.10312. [DOI] [PubMed] [Google Scholar]
- 184.McLaren JS, White LJ, Cox HC, Ashraf W, Rahman CV, Blunn GW, Goodship AE, Quirk RA, Shakesheff KM, Bayston R, Scammell BE. 2014. A biodegradable antibiotic-impregnated scaffold to prevent osteomyelitis in a contaminated in vivo bone defect model. Eur Cell Mater 27:332–349. doi: 10.22203/eCM.v027a24. [DOI] [PubMed] [Google Scholar]
- 185.Li B, Brown KV, Wenke JC, Guelcher SA. 2010. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J Control Release 145:221–230. doi: 10.1016/j.jconrel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 186.Ambrose CG, Clyburn TA, Mikos AG. March 2015. Antibiotic microspheres for treatment and prevention of osteomyelitis and enhancement of bone regrowth. US patent 8986737 B2.
- 187.Peng KT, Chen CF, Chu IM, Li YM, Hsu WH, Hsu RW, Chang PJ. 2010. Treatment of osteomyelitis with teicoplanin-encapsulated biodegradable thermosensitive hydrogel nanoparticles. Biomaterials 31:5227–5236. doi: 10.1016/j.biomaterials.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 188.Pornpattananangkul D, Zhang L, Olson S, Aryal S, Obonyo M, Vecchio K, Huang CM, Zhang L. 2011. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J Am Chem Soc 133:4132–4139. doi: 10.1021/ja111110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Karimi M, Sahandi Zangabad P, Ghasemi A, Amiri M, Bahrami M, Malekzad H, Ghahramanzadeh Asl H, Mahdieh Z, Bozorgomid M, Ghasemi A, Rahmani Taji Boyuk MR, Hamblin MR. 2016. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: applications and recent advances. ACS Appl Mater Interfaces 8:21107–21133. doi: 10.1021/acsami.6b00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mohiti-Asli M, Molina C, Diteepeng T, Pourdeyhimi B, Loboa EG. 2016. Evaluation of silver ion-releasing scaffolds in a 3D coculture system of MRSA and human adipose-derived stem cells for their potential use in treatment or prevention of osteomyelitis. Tissue Eng Part A 22:1258–1263. doi: 10.1089/ten.tea.2016.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eom SH, Kang SK, Lee DS, Myeong JI, Lee J, Kim HW, Kim KH, Je JY, Jung WK, Kim YM. 2016. Synergistic antibacterial effect and antibacterial action mode of chitosan-ferulic acid conjugate against methicillin-resistant Staphylococcus aureus. J Microbiol Biotechnol 26:784–789. doi: 10.4014/jmb.1511.11046. [DOI] [PubMed] [Google Scholar]
- 192.Costa EM, Silva S, Tavaria FK, Pintado MM. 2017. Insights into chitosan antibiofilm activity against methicillin-resistant Staphylococcus aureus. J Appl Microbiol 122:1547–1557. doi: 10.1111/jam.13457. [DOI] [PubMed] [Google Scholar]
- 193.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sander JD, Joung JK. 2014. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Doerflinger M, Forsyth W, Ebert G, Pellegrini M, Herold MJ. 2017. CRISPR/Cas9—the ultimate weapon to battle infectious diseases? Cell Microbiol 19:1–10. doi: 10.1111/cmi.12693. [DOI] [PubMed] [Google Scholar]
- 196.Bikard D, Euler CW, Jiang W, Nussenzweig PM, Goldberg GW, Duportet X, Fischetti VA, Marraffini LA. 2014. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol 32:1146–1150. doi: 10.1038/nbt.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mazzoleni G, Di Lorenzo D, Steimberg N. 2009. Modelling tissues in 3D: the next future of pharmaco-toxicology and food research? Genes Nutr 4:13–22. doi: 10.1007/s12263-008-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pampaloni F, Reynaud EG, Stelzer EH. 2007. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 199.Baker BM, Chen CS. 2012. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci 125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rendueles O, Ghigo J-M. 2012. Multi-species biofilms: how to avoid unfriendly neighbors. FEMS Microbiol Rev 36:972–989. doi: 10.1111/j.1574-6976.2012.00328.x. [DOI] [PubMed] [Google Scholar]
- 201.Xu Y, Rivas JM, Brown EL, Liang X, Höök M. 2004. Virulence potential of the staphylococcal adhesin CNA in experimental arthritis is determined by its affinity for collagen. J Infect Dis 189:2323–2333. doi: 10.1086/420851. [DOI] [PubMed] [Google Scholar]
- 202.Tung HS, Guss B, Hellman U, Persson L, Rubin K, Rydén C. 2000. A bone sialoprotein-binding protein from Staphylococcus aureus: a member of the staphylococcal Sdr family. Biochem J 345:611–619. [PMC free article] [PubMed] [Google Scholar]
- 203.Linnes JC, Ma H, Bryers JD. 2013. Giant extracellular matrix binding protein expression in Staphylococcus epidermidis is regulated by biofilm formation and osmotic pressure. Curr Microbiol 66:627–633. doi: 10.1007/s00284-013-0316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Christner M, Franke GC, Schommer NN, Wendt U, Wegert K, Pehle P, Kroll G, Schulze C, Buck F, Mack D, Aepfelbacher M, Rohde H. 2010. The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin. Mol Microbiol 75:187–207. doi: 10.1111/j.1365-2958.2009.06981.x. [DOI] [PubMed] [Google Scholar]
- 205.Longshaw CM, Farrell AM, Wright JD, Holland KT. 2000. Identification of a second lipase gene, gehD, in Staphylococcus epidermidis: comparison of sequence with those of other staphylococcal lipases. Microbiology 146:1419–1427. doi: 10.1099/00221287-146-6-1419. [DOI] [PubMed] [Google Scholar]
- 206.Cremieux AC, Dumitrescu O, Lina G, Vallee C, Cote JF, Muffat-Joly M, Lilin T, Etienne J, Vandenesch F, Saleh-Mghir A. 2009. Panton-Valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS One 4:e7204. doi: 10.1371/journal.pone.0007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Loughran AJ, Gaddy D, Beenken KE, Meeker DG, Morello R, Zhao H, Byrum SD, Tackett AJ, Cassat JE, Smeltzer MS. 2016. Impact of sarA and phenol-soluble modulins on the pathogenesis of osteomyelitis in diverse clinical isolates of Staphylococcus aureus. Infect Immun 84:2586–2594. doi: 10.1128/IAI.00152-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rasigade JP, Trouillet-Assant S, Ferry T, Diep BA, Sapin A, Lhoste Y, Ranfaing J, Badiou C, Benito Y, Bes M, Couzon F, Tigaud S, Lina G, Etienne J, Vandenesch F, Laurent F. 2013. PSMs of hypervirulent Staphylococcus aureus act as intracellular toxins that kill infected osteoblasts. PLoS One 8:e63176. doi: 10.1371/journal.pone.0063176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cassat JE, Hammer ND, Campbell JP, Benson MA, Perrien DS, Mrak LN, Smeltzer MS, Torres VJ, Skaar EP. 2013. A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell Host Microbe 13:759–772. doi: 10.1016/j.chom.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cheung GYC, Joo H-S, Chatterjee SS, Otto M. 2014. Phenol-soluble modulins—critical determinants of staphylococcal virulence. FEMS Microbiol Rev 38:698–719. doi: 10.1111/1574-6976.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Moenster RP, Linneman TW, Call WB, Kay CL, McEvoy TA, Sanders JL. 2013. The potential role of newer gram-positive antibiotics in the setting of osteomyelitis of adults. J Clin Pharm Ther 38:89–96. doi: 10.1111/jcpt.12030. [DOI] [PubMed] [Google Scholar]
- 212.Dunne MW, Puttagunta S, Sprenger CR, Rubino C, Van Wart S, Baldassarre J. 2015. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother 59:1849–1855. doi: 10.1128/AAC.04550-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E. 2012. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 54:e132–e173. doi: 10.1093/cid/cis346. [DOI] [PubMed] [Google Scholar]
- 214.Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L. 2015. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti Infect Ther 13:1499–1516. doi: 10.1586/14787210.2015.1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]