SUMMARY
The obligate intracellular bacterium Orientia tsutsugamushi is the causative agent of scrub typhus in humans, a serious mite-borne disease present in a widespread area of endemicity, which affects an estimated 1 million people every year. This disease may exhibit a broad range of presentations, ranging from asymptomatic to fatal conditions, with the latter being due to disseminated endothelial infection and organ injury. Unique characteristics of the biology and host-pathogen interactions of O. tsutsugamushi, including the high antigenic diversity among strains and the highly variable, short-lived memory responses developed by the host, underlie difficulties faced in the pursuit of an effective vaccine, which is an imperative need. Other factors that have hindered scientific progress relative to the infectious mechanisms of and the immune response triggered by this bacterium in vertebrate hosts include the limited number of mechanistic studies performed on animal models and the lack of genetic tools currently available for this pathogen. However, recent advances in animal model development are promising to improve our understanding of host-pathogen interactions. Here, we comprehensively discuss the recent advances in and future perspectives on host-pathogen interactions and the modulation of immune responses related to this reemerging disease, highlighting the role of animal models.
KEYWORDS: Orientia tsutsugamushi, scrub typhus, immunity, immune evasion
INTRODUCTION
Orientia tsutsugamushi is a Gram-negative, obligate intracellular bacterium known as the etiological agent of scrub typhus, a mite-borne disease with endemic presentation in vast regions of Asia and insular territories of the Pacific and Indian Oceans (1, 2). While areas of endemicity continue to expand in Asian regions (3, 4), strong molecular, serological, and clinical evidence of transmission of O. tsutsugamushi in Africa and South America has been reported (5–7), suggesting that the global health burden of this severe infection may currently be underestimated. The Orientia genus belongs to the Rickettsiales order, Rickettsiaceae family, and is therefore closely related to the Rickettsia genus. Both genera have in common that infectious particles are located free in the cell cytoplasm; however, the absence of peptidoglycan, lipopolysaccharide (LPS), and genes involved in the lipid A biosynthetic pathway is a feature that differentiates this pathogen from the Rickettsia genus. Interestingly, the presence of peptidoglycan-like structures in Orientia was recently reported, turning this into an interesting field of debate (8–11). These molecular features may shape the immune response to different O. tsutsugamushi strains, since the presence of classical LPS or peptidoglycans usually contributes to the generation of cross-protective responses. According to the antigenic variation of the major outer membrane protein 56-kDa type-specific antigen (TSA56), several strains of O. tsutsugamushi have been described (12, 13). Nevertheless, to date, the genomes of only two strains, Ikeda and Boryong, have been reported, revealing high levels of sequence variation among surface genes. The circular genome of O. tsutsugamushi has a length of 2.0 to 2.1 Mb, showing high levels of sequence repeat density and complexity, making this species one of the most remarkable species, in genomic terms, within the Rickettsiaceae family. Genes encoding proteins that mediate interactions with host cells and constituents of the conjugative type IV secretion system (T4SS) have undergone massive proliferation, while the intensive amplification of several mobile elements reveals evolutionarily recent genome shuffling (9, 12, 14).
Scrub typhus is described as an acute affection, usually febrile, with a wide range of clinical manifestations, including nonspecific signs such as headache, fever, rash, breathlessness, cough, nausea, vomiting, myalgia, and regional lymphadenopathy, which are observable after a 6- to 21-day incubation period. A highly variable percentage of patients may develop a distinctive eschar at the inoculation site. Despite self-limiting clinical conditions being commonly reported, fatal multiple-organ involvement may be observed in an important percentage of cases (7 to 15%). In these cases, a syndrome of acute respiratory distress may occur, related to lung injury, hepatitis, renal failure, myocarditis, encephalitis, and central nervous system involvement. Usually, these severe manifestations develop 2 weeks after infection and are frequently related to inappropriate or absent antibiotic treatment (13, 15–20). Moreover, antibiotic resistance has been reported, which sums to the natural resistance of the pathogen to fluoroquinolones and beta-lactam drugs (1, 21–23). The broad range of unspecific manifestations may lead to the late establishment of a diagnosis, contributing to aggravation of the clinical condition. Several other factors may be involved in misdiagnosis or delayed diagnosis, such as the suboptimal capacity of available serological tests, the high number of strains of the pathogen, the presence of serum IgG and IgM from previous infections, the time of generation of new detectable antibodies, and the difficult access to rural medical attention within zones of endemicity (24, 25). To complement serological assays and overcome the difficulties in diagnosis, the routine use of serum- or biopsy specimen-based molecular assays for scrub typhus diagnosis, including PCR, quantitative PCR (qPCR), and loop-mediated isothermal PCR (26–34), is hopefully increasing.
Although the physiopathology of this disease is not fully understood, immune-mediated processes are thought to contribute, along with direct bacterial damage, to cause local or disseminated vasculitis that drives the pathological mechanism (20, 35). Humans acquire the pathogen during the feeding of trombiculid mite larvae (chiggers), which host Orientia tsutsugamushi in salivary glands (36). Humans are incidental hosts of Leptotrombidium species and dead-end hosts of O. tsutsugamushi. Transovarian transmission between mites and parasitism of the larvae on infected animals maintain the bacterium in its natural cycle (36–38). In rural areas of endemicity, more than 20% of hospitalized patients that present with acute undifferentiated fever are diagnosed with this infection, and 1 million cases are detected every year, with an estimated 1 billion people being at risk (24, 39, 40). Outbreaks of scrub typhus and Orientia reinfections are frequent, possibly due to the heterogeneity of the organism and the suboptimal development of immunity from natural infections (15, 41). Despite decades of research efforts, the development of an effective vaccine eliciting long-lasting protection against O. tsutsugamushi has not been achieved. The urgent need for this vaccine is supported by several reasons, such as (i) the high public health burden caused by this pathogen, (ii) the expansion of anthropic environmental modifications that impact the epidemiology of the disease, (iii) the ever-growing geographical areas of endemicity where cases are reported, (iv) the deficient natural immune response that lacks cross-protection, (v) the challenges underlying the achievement of early diagnosis, (vi) reports of antibiotic resistance, and (vii) the mortality rate related to systemic infections, among others (24, 42, 43). Currently, in order to achieve a comprehensive understanding of host-pathogen interactions and immune responses, promising advances are opening alternatives to carry out profound mechanistic studies, including the development of accurate animal models that resemble the natural inoculation routes, the dissemination of the pathogen into the vertebrate host, or the human-pathological characteristics of the disease (43–49). Thus, the aim of this review is to present an update on studies of the O. tsutsugamushi-host interplay and immunological responses to this pathogen, which constitute a basis for the development of disease control strategies, highlighting the current state of the art and future perspectives on animal model employment.
HOST-PATHOGEN INTERPLAY IN ORIENTIA TSUTSUGAMUSHI INFECTION
Target Cells
To accomplish its replicative cycle, Orientia tsutsugamushi may infect a wide range of cells, displaying differential tropism depending on the phase of infection and the affected tissue. After initial inoculation in the dermal layer, dermal dendritic cells (DCs) and activated monocytes constitute the main target cells, as reported in an ex vivo study of human eschar biopsy specimens (50). Infection of monocytes has also been observed in studies based on isolated cells from healthy human donors both ex vivo and in vitro (51). Activated DCs and monocytes may constitute a rapid, potential dissemination vehicle for the pathogen while circulating to lymph nodes (50). The accumulation of O. tsutsugamushi in these organs in a sublethal, footpad-injected BALB/c mouse model has been reported. Posterior systemic dissemination along with macrophage tropism are characteristic of this model, instead of disseminated endothelial infection (44), which constitutes a hallmark of lethal infection in humans (52). Endothelial infection involves several organs, including skin, heart, lung, kidney, pancreas, and brain, with additional infection of macrophages in the liver and spleen, as reported in postmortem analyses of human patients (52). In addition, endothelial cell (EC) infection has been observed in several rodent models of Orientia infection, including intradermal (i.d.) and intravenous (i.v.) models (45, 46, 48, 49). Also, mouse fibroblasts (51, 53), cultured polymorphonuclear leukocytes (PMNs) of guinea pigs, and neutrophils in intraperitoneal (i.p.) infection in BALB/c mice are reported target cells (54, 55).
Cell Invasion and Intracellular Life Cycle
Although a precise understanding of host cell invasion mechanisms remains to be elucidated, some molecular interactions concerning the attachment and entry of Orientia have been described. The internalization of intracellular bacteria requires initial attachment to the extracellular matrix (ECM) and host cells, mediated by high-affinity binding to host receptors. Fibronectin, a glycoprotein of the ECM involved in the binding and entry of several intracellular bacteria, takes part in this process by interacting with the Orientia TSA56 antigen (56) and potentially with one of the autotransporter proteins, ScaC (57). The interaction of fibronectin with an extracellular binding region of TSA56, formed by amino acids (aa) 312 to 341, decreases the invasion of the bacteria in L929 cells (56, 58). Moreover, the potential interaction of ScaC with fibronectin may be responsible for the demonstrated adherence to nonphagocytic cells mediated by this antigen (57). Additionally, Orientia ScaA, another autotransporter protein, mediates bacterial adhesion to nonphagocytic cells, since adhesion is enhanced by the recombinant expression of this antigen in Escherichia coli, while treatment with anti-ScaA antibody neutralizes it. However, an interacting host protein has not been reported for this antigen (59). Heparan sulfate (HS) proteoglycans (HSPGs), which are molecules expressed on cell surfaces and the ECM, are also involved in the attachment of viruses and the uptake of intracellular bacteria. Low expression levels of HSPGs in mutant Chinese hamster ovarian cell lines lead to a diminished infection capacity of O. tsutsugamushi. Specifically, HS and heparin may be involved in the infectious process, since invasion of L929 cells is inhibited with HS or heparin pretreatment (60).
Other HSPGs involved in Orientia interaction are syndecans, molecules widely distributed in mammalian cells. Syndecan-4 is the most ubiquitous (61) and participates in the early attachment of Orientia, as demonstrated by experiments with rat embryo fibroblasts, where bacterial invasion is correlated with the expression levels of this transmembrane molecule and is specifically reduced by the addition of a recombinant core protein of syndecan-4 (62). However, a bacterial ligand for syndecan-4 has not yet been reported, and the role of this molecule in the invasion mechanism requires further investigation (62).
Integrins, a family of transmembrane glycoproteins that usually associate with fibronectin in the bacterial entry process, are also involved in cell invasion by O. tsutsugamushi. The early colocalization of O. tsutsugamushi with integrin α5β1 has been related to the activation of integrin-activated signal transduction pathways (56). Focal adhesion (FAK) and Src kinases as well as RhoA GTPase act as integrin downstream signaling molecules in nonphagocytic cells, promoting the local induction of focal adhesion points and the subsequent induction of actin cytoskeleton rearrangement. Talin and paxillin are also upregulated and mediate this process (56). The role of integrin α5β1 is also supported by a report showing that the administration of anti-integrin α5β1 antibodies decreased L929 cell invasion (58).
In summary, it seems that the O. tsutsugamushi strategy of manipulating these widely distributed cell receptor families and molecules has allowed attachment to host cells not specialized in phagocytosis, thereby providing a mechanism of active internalization in target cells.
The internalization of Orientia in human epithelial cells and fibroblasts exploits the endocytic pathway, particularly clathrin-mediated endocytosis. This has been shown by the colocalization of the bacterium with clathrin and adaptor protein 2 and confirmed by inhibition assays with chlorpromazine hydrochloride, monodansylcadaverine, and sucrose. A similar approach, this time with filipin III inhibition assays, discarded the utilization of caveola-mediated endocytosis in human epithelial cells and fibroblasts. Colocalization with early and late endosomal markers has been observed in nonphagocytic cells at between 1 and 2 h postinfection (hpi). After this time, visualization of free Orientia bacteria in the cytoplasm revealed the early escape of the bacteria from the phagosomal compartment.
Once in the cytoplasm, Orientia can replicate by binary fusion (36, 63), after moving to a perinuclear region. This is accomplished within the first 2 hpi through specific interactions with microtubules and the dynein-dynactin motor protein complex (64). Evidence of replication at this region has been observed in nonphagocytic cells (64) and phagocytic cells, including macrophages and dermal DCs (50, 65). Although the whole replicative cycle can be fulfilled within the cytoplasm, an intranuclear location has been reported (53).
The early escape from the phagosomal compartment allows this bacterium to subvert an important innate defense mechanism as well as to establish its intracytoplasmic life cycle. Unfortunately, the mechanisms underlying this process are poorly characterized. Blocking the acidification of the endocytic compartment through NH4Cl or bafilomycin A treatment impairs Orientia escape, thereby suggesting the requirement of this metabolic acidification process (66). However, a detailed mechanism involved in this escape is lacking. Studies have revealed the presence of a hemolysin gene and a potential phospholipase D gene in the Orientia genome, along with the expression of the corresponding protein in cultured mouse fibroblasts, but whether they have a role in the Orientia replicative cycle or not has not been explored (9, 67). Considering that the Listeria monocytogenes listeriolysin and phospholipase C proteins have a role in phagosomal escape by this intracellular bacterium, some authors have speculated about their potential involvement (68–70). In accordance with the early escape from phagosome observed for Orientia, it has been reported that nitric oxide (NO), a potent free radical involved in the killing of engulfed microbes, enhances the cytosolic replication of O. tsutsugamushi in RAW264.7 murine macrophages through a mechanism that is not currently elucidated (71).
Infected cells may harbor a high density of Orientia bacteria despite the slow growth reported at 37°C, reaching a density of 103 bacteria in a single cell (72–74). The release of infective bacteria from host cells involves budding from the cell surface with the acquisition of a membrane coat (63). Membrane lipid rafts may participate in this process, since an Orientia protein, 47-kDa high-temperature requirement A, colocalizes at 72 hpi with the caveolin raft protein in an endothelial cell line. At this time, Orientia moves to the cell surface and starts the release process (75). The main events reported in Orientia infection of nonphagocytic cells are depicted in Fig. 1. Despite reports of budding-like release, BHK-21 cell line infection results in few extracellular membrane-coated Orientia bacteria (54). Previous studies suggested that after being released from an infected cell, Orientia may employ different mechanisms to infect subsequent cells, which may require the bacteria either to detach from the surrounding membrane to contact further cells or to involve phagocytosis of membrane-coated Orientia bacteria by host cells (54, 63, 70), as reported for mouse peritoneal mesothelial cells (63).
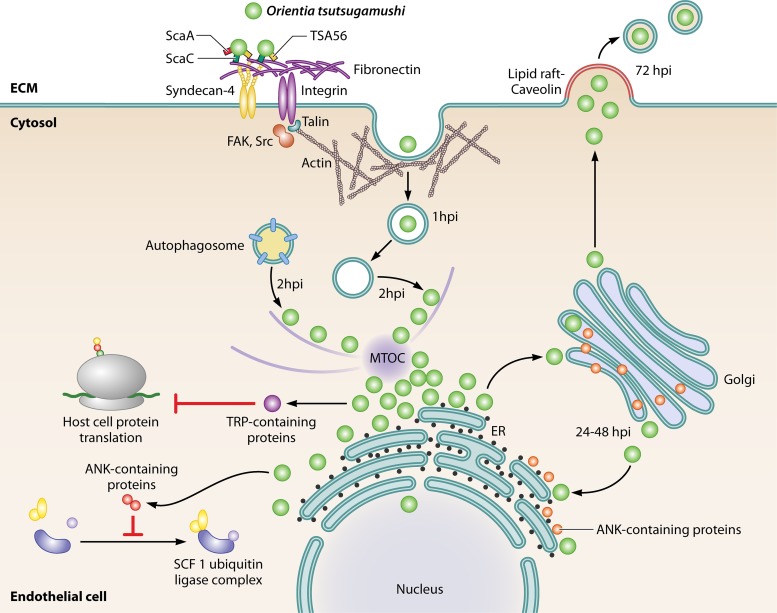
FIG 1.
Reported events related to the replication cycle of Orientia tsutsugamushi in nonphagocytic cells. Fibronectin in the ECM interacts with the Orientia antigen TSA56, and potentially with ScaC, for the attachment of bacteria to the host cell. This results in integrin-mediated signaling, involving FAK, Src, and Rho GTPase, with consequent actin cytoskeleton rearrangement, talin/paxillin recruitment, and clathrin-mediated endocytosis. The Orientia antigen ScaA and transmembrane syndecan-4 also mediate bacterial adhesion to nonphagocytic cells. At 1 hpi, Orientia colocalizes with early endosomal markers but at 2 hpi escapes from phagosomes through an unknown mechanism that requires compartment acidification and also evades cellular autophagy. It reaches the perinuclear region after moving through microtubules associated with the dynein-dynactin protein complex, where it replicates via binary fission. The secretion of effector cells participates as a virulence factor, probably via the T1SS and T4SS, disrupting the SCF1 ubiquitin ligase complex, degrading EF1α, and impairing protein translation. Also, Orientia colocalizes with the Golgi complex and moves backwards to the ER, producing membrane instability mediated by the effector protein Ank9. The release of Orientia particles occurs in a way similar to virus budding at 72 hpi and has been associated with lipid rafts and caveolin colocalization. MTOC, microtubule organizing center.
Apoptosis Modulation and Subversion of Autophagy
In a cell infection context, apoptosis may be a host defense mechanism aimed at limiting pathogen replication. Whether this process is detrimental or advantageous to the host depends on several factors, such as the identity of the infected cell, the stage of infection, and the nature of the pathogen. This highly regulated cell death process has a significant role in the infected cell-pathogen interplay, especially in the life cycle of obligate intracellular pathogens. Interestingly, events taking part in this process are often the target of extensive modulation by the infectious agent (76, 77). Studies on O. tsutsugamushi have revealed either the induction or inhibition of cell apoptosis after infection, with this duality being relative to the strain or host cell/animal model studied. Most importantly, mechanisms of modulation of apoptosis have been scarcely explored to date. On the human monocyte cell line THP-1, apoptosis induction by beauvericin treatment was inhibited by both heat-killed and live Orientia bacteria, with the former showing greater antiapoptotic activity, thereby suggesting a role for heat-stable molecules. In general, extensive calcium release from the endoplasmic reticulum (ER) mediates apoptosis induction triggered by this drug; however, in O. tsutsugamushi-infected cells, a reduced, retarded cytosolic calcium redistribution may be responsible for the inhibition of apoptosis (78). Experimental modulation of NF-κB levels did not influence apoptotic effects, suggesting that this transcription factor is not implicated in this inhibition pathway (78). That observation contrasts with the reported NF-κB-dependent antiapoptotic effect on cells elicited by Rickettsia rickettsii (79).
In contrast, apoptotic effects of Orientia have been observed for various cell types and organs. In O. tsutsugamushi-infected BALB/c mice, nucleus fragmentation has been observed in a strain-specific manner in lymphocytes located in spleens and lymph nodes. This effect was observed 10 days after lethal Karp strain challenge but not after nonlethal Gilliam strain challenge (80). Moreover, apoptosis of the cultured endothelial cell line ECV304 has been reported (81, 82). Detachment from the substrate and DNA fragmentation were observed following infection with the Boryong strain at a multiplicity of infection (MOI) of 20:1, accompanied by the disruption of focal adhesions and changes in the cytoskeleton, which may be related to the apoptotic mechanism (81). It has been suggested that the replicative capacity of Orientia is associated with the extent of apoptosis induction, since live bacteria produced apoptosis of 18% and 8% ± 1% of human monocyte-derived macrophages and naive monocytes, respectively, with naive monocytes being less suitable for Orientia strain Kato replication (83, 84). More recently, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) immunochemistry performed on mouse kidneys revealed apoptotic effects on ECs after lethal infection with the Karp strain. A large number of apoptotic cells along with the reduced transcription of antiapoptotic Bcl-2 were observed in wild-type (WT) mice in comparison to interleukin-33 (IL-33)−/− mice, suggesting a role for this interleukin in promoting EC stress and apoptosis (49).
Considering these results, it may be hypothesized that the existence of differential pro- and antiapoptotic effects may vary within different stages of target cell infection or host dissemination to decrease the spread of O. tsutsugamushi. However, to unveil the mechanisms and objectives of apoptosis modulation in an infection context, integrative studies on susceptible cell lines and animal models should be performed.
Another defense strategy that counters intracellular pathogen growth is autophagy, a regulated catabolic process aimed at degrading cytosolic components. To accomplish this, endomembrane-based organelles, called autophagosomes, engulf components targeted for degradation, which is achieved by the fusion of the organelle with other endosomal and lysosomal compartments (85, 86). One of the first studies addressing the role of autophagy showed increased autophagosomal formation on Orientia tsutsugamushi-infected PMNs (87). Subsequently, immunoblot and immunofluorescence confocal microscopy studies employing phagocytic and nonphagocytic cell lines revealed the formation of autophagosomes within 1 h after Boryong strain infection, as identified by LC3B marker recruitment (86). Later, at 2 hpi, escape from autophagosomes was observed in HeLa cells, revealed by the scarce colocalization of the bacterial particles with LC3B. This evasion was evident even in the presence of rapamycin, an autophagy inducer. It is noteworthy that the induction of autophagy did not impair bacterial growth (86). Moreover, phagosome formation also takes place with live and UV-inactivated Boryong strain bacteria in bone marrow-derived DCs (bmDCs), with only live bacteria showing an ability to evade the autophagic system (65). An elucidation of virulence factors that impair the phagosome degradation of O. tsutsugamushi has not yet been described, but it has been proposed that this bacterium may carry several factors involved in this process. Finally, since autophagy may have a role in inflammasome activation and naive T cell antigen presentation (85, 88), the extent and mechanisms of autophagy subversion should be further explored in order to understand the mechanisms underlying the impaired adaptive responses related to human O. tsutsugamushi infection.
Secretion Systems and Effectors
Genome sequencing of the Boryong strain of O. tsutsugamushi revealed the presence of 79 genomic sites, encompassing 359 tra genes related to constituents of conjugative T4SSs, a strikingly high number in comparison to those of other intracellular bacteria and the Rickettsia genus (9, 89, 90). Several bacteria and some archaea use T4SSs, which are large protein complexes that allow the translocation of DNA and protein substrates through a channel assembly that connects two cells. These structures participate in horizontal gene transfer and in delivering virulence factors to the cytosol of infected cells, enabling bacteria to subvert host cell processes (91, 92). Strain Ikeda also exhibits an important set of genes of the T4SS. Genome analysis of T4SSs in Rickettsia and Orientia revealed a highly conserved gene organization, as observed for the Anaplasma genus, which suggests an essential role for this secretion system in the replicative cycle of these intracellular pathogens (14). The Orientia genome also harbors a high number of genes encoding host cell interaction and signaling proteins, such as histidine kinases, ankyrin repeat proteins, and tetratricopeptide repeat proteins (TRPs) (9).
The tetratricopeptide repeat structural motif, present in prokaryote and eukaryote organisms, functions as a scaffold for assembling multiprotein complexes. Every TPR fold structure may participate in several protein-protein interactions and therefore impact different facets of cellular metabolism, including the functional modulation of steroid receptors, gene regulation, cell cycle control, protein folding, and protein transport (93, 94). Importantly, they serve as virulence factors in several bacterial pathogens, such as Francisella tularensis, Francisella novicida, Mycobacterium tuberculosis, Shigella spp., Pseudomonas, and Yersinia (95–103). In O. tsutsugamushi, this family of proteins may interfere with host cell metabolism through interactions with DDX3, an RNA helicase that belongs to the multifunctional DEAD box family of proteins (104, 105). Proteins encoded by trp genes are expressed after L929 cell infection and interact with DDX3, as revealed by pulldown assays with ECV304 cell lysates and immunoblot assays with specific antibodies. In vitro translation assays employing reticulocyte cell lysates have shown dose-dependent translation impairment of a reporter by the TRP43 and TRP46 proteins (94).
Another important family of molecules employed by intracellular organisms to accomplish their infectious cycle is the family of Ank-containing proteins. These proteins mediate various protein-protein interactions aimed at impeding normal processes of eukaryotic cells (106–108). In this regard, the Ikeda strain carries 38 ank open reading frames and 9 ank pseudogenes (14). L929 cells respond to Orientia infection by upregulating the transcription of type I secretion systems (T1SSs), T4SSs, and several ank genes (109, 110). The products of these ank genes may be T1SS substrates, as revealed by the examination of C termini of Ank-containing proteins. It is noteworthy that after their ectopic expression in HeLa cells, Ank-containing proteins are distributed in diverse cellular locations, suggesting a vast potential to modulate many host cellular functions (109, 110). Particularly, the interaction of these proteins with core components of the SCF1 ubiquitin ligase complex has been documented in two reports. Interactions with Cullin-1 in ECV304 cell lysates have been revealed by using glutathione S-transferase (GST) pulldown assays with E. coli-purified GST fusion proteins, while immunoblot analysis revealed interactions with Skp1 (109, 111). F-box-like motifs, homologous to those found in eukaryotes and poxvirus, are present in Ank-containing proteins, as revealed by in silico and manual sequence analyses (109, 111). This F-box motif is required for the interaction of the Ank-containing proteins with Skp1, as demonstrated in transfected HeLa cells by coimmunoprecipitation of FLAG-tagged Ank-containing proteins with GST-tagged Skp1 and GST-Skp1 pulldown analyses performed with FLAG-tagged Ank-containing proteins with a deletion of their F-box motifs. In addition to this recombinant-protein approach, the expression of the Ank9 protein and interaction with Skp1 also occur after Orientia infection of mammalian host cells, which were observed by antiserum recognition of the Ank9 protein coprecipitated with GST-Skp1. This interaction is due to residues L384, I392, and E400 in the F-box-like motif of Ank9 (111).
Furthermore, an Orientia mechanism for targeting the eukaryotic secretory pathway was recently proposed, where Ank9 plays a key role (112). Experiments with Ank9 ectopic expression in HeLa cells allowed the recognition of a novel GRIP-like motif in Ank9, which is necessary and sufficient for the protein to localize within the Golgi complex, to reach the ER by retrograde trafficking, and to generate instability in both compartments. The binding of coatomer protein complex subunit beta 2, involved in vesicular trafficking, Golgi budding, and Golgi-to-ER trafficking, may be a factor contributing to the instability of the Golgi complex. As a result of the interaction of Ank9 and coatomer protein complex subunit beta 2, ER stress and the impairment of eukaryotic cell protein secretion are generated. In addition, Golgi instability, the impairment of host cell protein secretion, and the enhanced replication of bacteria have also been observed during O. tsutsugamushi infection (112), highlighting the importance of Ank-containing proteins as virulence factors in O. tsutsugamushi. Moreover, GST pulldown assays and immunoblot analyses have confirmed the interaction of several of these proteins with eukaryotic elongation factor 1α (EF1α). In a functional analysis of transfected HeLa cells expressing of AnkU5 and AnkD, colocalization of AnkU5 with Cullin and EF1α was observed mainly in nuclei by using immunofluorescence confocal microscopy. This interaction mediated a reduction of the host protein level via polyubiquitination, as revealed by in vitro ubiquitination reactions. Following Orientia infection, a reduction in EF1α levels in endothelial, epithelial, and monocytic cell lines was observed at 2 days postinfection (dpi) by using a specific anti-EF1α antibody, with this reduction being triggered by posttranscriptional proteosomal degradation (109). Although the EF1α function is canonically related to the cytosolic loading of aminoacyl-tRNAs, there may be several other functions related to this protein in physiological and pathological scenarios (113, 114). Nonetheless, the impact of EF1α degradation on O. tsutsugamushi infection has not yet been assessed.
Unveiling the potential roles of virulence factors and pathways related to secretion systems, repeat-containing proteins, and effector proteins in general constitutes an exciting area of study that should be promptly explored. Considering the range of effects of a unique Ank-containing protein, Ank9, it may be speculated that the entire repertoire of Ank-containing proteins may potentially exert a vast effect on host cell biology.
INNATE IMMUNITY CELLS: EARLY RESPONSES AND EFFECTOR ROLES
Dendritic Cell Responses to Orientia Infection
With the objective of surviving and perpetuating in a host, several pathogens prevent or modulate the normal function of cells involved in innate immune responses (115, 116). Considering that the main infection targets of O. tsutsugamushi are DCs, monocytes, and ECs (50, 52), it is of major importance to understand the response of these cells and host immunity to infection.
In vitro studies have revealed that Orientia infection of monocyte-derived DCs (moDCs) or bmDCs drives the activation of these cells, but some of their functions may be severely impaired (65, 117, 118). Upregulated expression of major histocompatibility complex (MHC) class II and CD40, CD80, CD86 (65), and CD83 costimulatory molecules (117, 118) has been reported prior to 24 hpi, revealing DC activation. Interestingly, no differences were found in the expression levels of costimulatory molecules between live and heat-inactivated bacteria, suggesting the involvement of heat-stable molecules in the activation of DCs (117). A reduction of endocytic activity is also associated with the maturation of DCs and has been reported for Orientia-infected moDCs in an in vitro assay of fluorescein isothiocyanate (FITC)-coupled albumin uptake (65). The upregulation of a proinflammatory profile, including tumor necrosis factor alpha (TNF-α), IL-6, IL-8, IL-12p70, and the chemoattractant molecules CCL3 and CCL5, provides additional evidence of Orientia-infected DC activation (65, 117) and suggests that these sentinel cells participate in lymphocyte and monocyte recruitment. Orientia infection also upregulates the antiviral type I interferon (IFN) pathway, enhancing the phosphorylation of p38 mitogen-activated protein kinase (MAPK) and IFN-β production, a feature also observed in other intracellular pathogens after being sensed in the cytosolic compartment of dendritic cells, such as Listeria and Francisella (119–121). Once activated, DCs must move toward lymph nodes to prime naive T cells in a tightly regulated event required for the proper development of adaptive immunity (122, 123). In vitro assays indicate that infected moDCs retain activity to activate CD4+ T cells, as revealed by lymphocyte IFN-γ secretion (117). However, the migration process seems to be impaired in O. tsutsugamushi infection, as demonstrated by a migration assay in a three-dimensional collagen matrix using the chemoattractant CCL19, where Orientia-infected DCs exhibited a diminished chemotactic response, in a way similar to that exhibited by immature DCs. This diminished chemotactic response was not related to decreased levels of CCR7 (65), which is the receptor for the CCL19 and CCL21 chemoattractant molecules (124), and was confirmed by ex vivo and in vivo assays. Therefore, Orientia exhibits the potential to interfere with the migration of DCs while harnessing this niche for replication.
Taking these data in consideration, along with the reported accumulation of DCs in eschars of infected human patients (50), we recommend performing future in vivo studies with rodent animal models for characterizing dendritic cell dynamics employing i.d. (47, 48, 125) or laboratory-reared chigger infection models that closely resemble the natural acquisition of this pathogen (126, 127).
Monocyte/Macrophage Responses to Orientia Infection
As mentioned above, monocytes are either present at or recruited to the inoculation site in dermal tissue, where they contribute to early inflammatory responses and serve as a niche for bacterial replication (Fig. 2) (50, 83, 84, 128–131). Moreover, they may contribute to Orientia dissemination toward the bloodstream and lymph nodes as well as mononuclear cell recruitment at the infection site (50). Early insights into the importance of monocytes and macrophages in combating initial phases of Orientia infection were reported in challenges of mice by i.p. inoculation (132, 133), a route that does not mimic natural inoculation but nevertheless may enlighten us about the activities of these cells during infection. Additional evidence of monocyte/macrophage activation comes from markers detected in South Indian scrub typhus patients. Some of these markers have been associated with severe disease presentation, including sCD163 and sCD14, and even with host lethality in the case of macrophage migration inhibitory factor (MIF) and tyrosine-lysine-leucine-40 (YKL-40) (134).
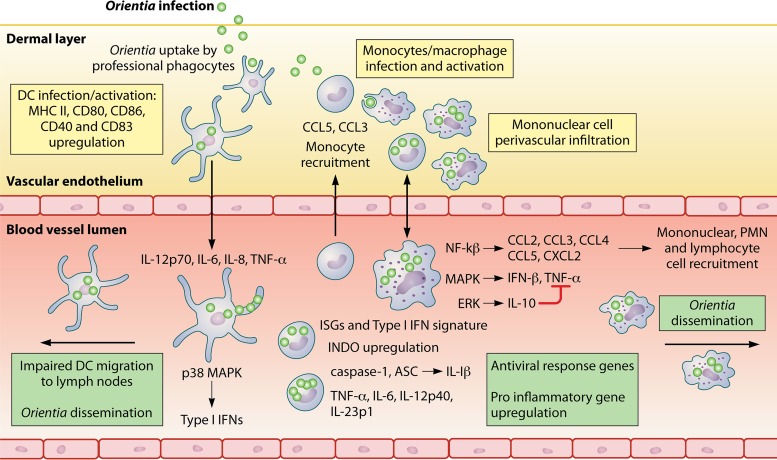
FIG 2.
Early events following Orientia tsutsugamushi infection. An initial tropism for dendritic cells (DCs) and monocytes at the inoculation zone induces infection and maturation of these cell populations. Infection of DCs impairs their migration, but this promotes rickettsial dissemination nonetheless. Additionally, DCs secrete proinflammatory cytokines and activate the type I IFN response. Monocyte recruitment occurs, which results in mononuclear cell perivascular infiltration and the activation of several transcriptional programs and pathways ending in the upregulation of proinflammatory genes and of the antiviral response and the recruitment of other cell subsets. In summary, by exploiting immune cells, early infection events may favor rickettsial dissemination and an inflammatory profile in Orientia tsutsugamushi infection.
Except for those reports, most subsequent studies have been performed by employing murine cell lines. Infection of the J774A.1 mouse macrophage cell line with either live or heat-inactivated Orientia bacteria triggered similar expression levels of CCL2, CCL3, CCL4, CCL5, and CXCL2 transcripts without the need for de novo protein synthesis and mostly within 0.5 hpi. The NF-κB transcription factor may be responsible for the induction of these chemokines, since NF-κB nuclear translocation was observed, while the inhibition of its activation resulted in the downregulation of chemokine expression (128). Additionally, live, but not heat-inactivated, Orientia bacteria also upregulated IFN-β. MAPK pathways were required for this upregulation, as suggested by the reduced levels of IFN-β transcripts resulting from extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 phosphorylation inhibition (129). Moreover, the MAPK pathways are also involved in the upregulation of TNF-α at the transcriptional and posttranscriptional levels (131). Consistent with the lack of LPS (8, 14), both pharmacological blockade of LPS, which did not affect TNF-α production, and Toll-like receptor 4 (TLR4) mutation, which rescued its production only slightly, suggest that TNF-α production may be independent of TLR4 and LPS (131). In contrast, a very weak stimulation of TNF-α expression, along with a repression of its production in LPS-stimulated J774A.1 cells, has been reported after infection with the Boryong strain, without affecting NF-κB activation. Similar inhibitory effects were seen with culture medium stimulation of J774A.1 cells, which contained high levels of an uncharacterized, potent IL-10-inducing factor, suggesting that Orientia increases the production of this immunosuppressive molecule in order to inhibit TNF-α production and enhance bacterial survival inside phagocytic cells (135). Therefore, Orientia may exploit a strategy similar to those observed for several bacterial intracellular pathogens (136, 137), including Mycobacterium tuberculosis (138), Coxiella burnetii (139), and Yersinia enterocolitica (140).
The discrepancies among data from studies of TNF-α expression in monocytes may be attributed to several factors, including the strain and dose of Orientia as well as the nature of the infected cell line, tissue, or animal model employed. A recent study revealed differential responses of THP-1 macrophages to different doses of the highly virulent Karp strain (141), in contrast to previous reports, where a low-virulence Boryong strain was used on murine macrophages (131, 135, 142, 143). NF-κB/TNF-α pathway inhibition via STAT3 as well as high-level IL-10 expression via ERK/IL-10 were observed with low-dose infection, promoting bacterial replication. An opposite response was observed with high-dose infection, characterized by the upregulation of NF-κB/TNF-α along with diminished IL-10 production. Moreover, pretreatment of macrophages with this cytokine completely suppressed IL-1β, IL-6, and TNF-α production, even during high-dose infection, suggesting that this interleukin contributes to the regulation of the inflammatory response. MicroRNAs (miRNAs) may participate in this process, with miRNA-155 being a molecule that acts as a fine-tuner of the inflammatory response, acting with IL-10 to prevent cytokine hyperproduction. In response to Karp strain challenge, peripheral blood mononuclear cells obtained from patients who recovered from severe scrub typhus and a cytokine storm were low producers of IL-10 and miRNA-155 but high producers of proinflammatory TNF-α, IL-1β, and IL-6 in comparison to patients who recovered from mild scrub typhus. These observations support the role of IL-10 and miRNA-155 cross talk in modulating cytokine production (141). In addition, an early peak from day 9 postinfection, followed by elevated IL-10 production for 8 days, was observed in a sublethal i.d. mouse model, which was characterized by bacterial persistence in several tissues and enhanced, but nonlethal, pathology (48). In this model, lung and spleen histological lesions did not resolve before 60 dpi, suggesting that early, sustained IL-10 production contributed to ameliorating tissue injury (48). In summary, these results suggest a significant role for this cytokine in the regulation of widespread tissue damage and in the modulation of proinflammatory cytokine levels.
Moreover, cytokine overproduction has also been observed in rodent models of i.p. infection, where the susceptibility of C3H/HeN and BALB/c mice to Gilliam and Karp strain challenges, respectively, was accompanied by the overexpression of chemokines and cytokines that clearly correlated with inflammatory cell infiltration. This was otherwise not observed in BALB/c mice resistant to the Gilliam strain (130). Moreover, analyses of human clinical samples revealed increased IFN-γ, IL-1β, IL12p40, TNF-α, and IL-10 levels and, interestingly, a significant correlation between TNF-α and disease severity in early phases of infection (144–146). Those results highlight the inflammatory response program elicited after Orientia infection and its impact on disease susceptibility and development. Gene expression analysis following Orientia strain Kato infection in human naive monocytes revealed a massive modification of transcriptional programs, with both downregulation and upregulation of several groups of genes. Particularly, immune response, inflammatory response, cell-cell signaling, and chemotaxis-related genes showed 20% transcriptional enrichment, while genes involved in the antiviral state reached up to 40% upregulation (84). The upregulation of genes that encode IFN-β, IFN-α, and several interferon-stimulated genes has been observed after inoculation with live, but not heat-killed, bacteria, along with a marked type I IFN signature characterized by IFN-α8, IFN-β, MX1, and OAS1 expression. In contrast, proinflammatory cytokine genes were upregulated by both live and heat-killed bacteria, including IL-1β, IL-6, IL-12p40, IL-23p19, and TNF-α. However, infected cells showed reduced TNF-α production and an inability to produce IL-1β when infected with heat-killed bacteria. Indoleaminepyrrole 2,3-dioxygenase (INDO), a protein involved in the killing of intracellular bacteria and several other functions, was upregulated, and an M1-specific prolife of monocyte activation was, in summary, evident in the presence of both live and heat-killed bacteria. These in vitro results correlated with IFN-related genes and certain characteristics of M1 polarization that occur in human patients (84). Furthermore, Kato strain infection of macrophage-derived monocytes may also trigger the enhanced transcription of genes that participate in inflammation, the type I IFN response, and M1 activation. Differences regarding bacterial viability were also reported, with a type I IFN response being present only in response to live organisms and a reduced inflammatory response being observed after infection by heat-killed bacteria (83, 84).
The generation of active IL-1β requires a first signal derived from the activation of pattern recognition receptors (PRRs) and a second one involving nucleotide-binding oligomerization domain-containing protein (NOD)-like receptor (NLR) inflammasomes. These signals lead to pro-IL-1β transcription and the cleavage of the IL-1β precursor, respectively, to produce active interleukin (147, 148). In Orientia infection of bone marrow-derived macrophages, the secretion and processing of IL-1β require the internalization of live bacteria and phagosome maturation, including the acidification of the endosomal compartment. The NLR involved in the recognition of live cytosolic Orientia bacteria and the triggering of this process remains to be characterized, since LPS-stimulated macrophages from Nlrp3-, Nlrc4-, or Aim2-deficient mice challenged with Orientia tsutsugamushi were able to produce IL-1β and the caspase-1 p10 subunit, which is a hallmark of caspase-1 activation (149), in a way similar to that of macrophages from WT C57BL/6 mice (143). Furthermore, rip2-deficient macrophages, which do not produce the RIP2 downstream effector of NOD1 or NOD2, also showed IL-1β production similar to that of WT mice, suggesting that caspase-1 production does not depend on signaling from these PRRs (143). The apoptosis-associated speck-like protein containing a CARD (ASC) adaptor protein is present in all inflammasomes, connecting with the sensor molecule of the inflammasome via a pyrin domain. It possess an activation-and-recruitment domain, which participates in the activation of caspase-1 (150). The inability of Asc-deficient macrophages to produce active IL-1β after Orientia infection supports the role of this adaptor molecule in the activation of caspase-1 (143). Finally, data from in vivo studies with C57BL/6 mice support evidence of the role of the downstream signaling of IL-1 receptors (IL-1Rs) in protection against O. tsutsugamushi infection, as revealed by an elevated pathogen load within blood and spleen of IL-1R-deficient animals at 6 and 19 dpi in comparison to WT mice (143). However, supporting data for the role of this receptor family in human scrub typhus, from either clinical samples or in vitro studies, are scarce, and a study of scrub typhus patient samples revealed that neither a correlation between IL-1β and serum bacterial loads nor differences in bacterial loads between the acute and posttreatment phases were evident (146).
Data from studies addressing the impact of the type I IFN response do not support a clear protective role for this innate mechanism. The administration of IFN-α and IFN-β showed a modest or even absent capacity to reduce the bacterial replication of the Karp, Gilliam, and TA716 strains on fibroblasts of C3H and BALB/c mice, suggesting that the inhibitory effect elicited by the type I IFN response is strain and host cell specific but may not be a strong factor in bacterial clearance (151). It is noteworthy that the effects of type I IFN induction may be related to several factors, which include the time after infection, the specific intracellular pathogen involved, and the local levels of mediators produced, thereby showing various outcomes of analyses of these variables (152). Also, various and negative effects on host cells and in vivo models of intracellular pathogen infections have been widely documented in several reviews (152–154). Thus, elucidating the impact of this innate defense mechanism requires exhaustive research using different models of infection.
Endothelial Cell Responses to Orientia Infection
Endothelial activation plays a substantial role in processes leading to pathogen clearance, through the development of self-proinflammatory, prothrombotic, and permeable conditions. Endothelial cells are greatly influenced by cytokine signaling, which controls most of the physiological and pathological processes in which they participate. The expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and other adhesion molecules mediates leukocyte adhesion and trafficking in cooperation with chemokines. The expression of procoagulant factors participates in the containment of the pathogen in local foci. In addition, the detection of molecules involved in endothelial activation may serve as biomarkers of clinical prognosis and disease severity. However, as a negative effect of these molecules, sustained, widespread endothelial activation and dysregulation may enhance the severity of disease (155, 156).
Once the pathogen has disseminated, ECs constitute an important target in human Orientia infection (Fig. 3), as observed in human autopsy tissues (52) and diverse rodent models of lethal and sublethal infection recently reported. These models include i.v. and i.d. C57BL/6 mouse challenge models of Gilliam and Karp strain infections that, interestingly, reflect several aspects of Orientia infection in humans, including Orientia dissemination to several organs, namely, brain, lung, liver, and kidney (43, 45, 46, 48, 49). Activation markers of ECs, including activated leukocyte cell adhesion molecule (ALCAM) and VCAM-1, have been linked to scrub typhus disease severity (134). Moreover, early activation and dysregulation of ECs have been observed in a mechanistic study of a mouse model of lethal i.v. inoculation of the Karp strain, suggesting a role of EC activation in the pathogenesis of scrub typhus (46). In this model, two regulators of EC function were analyzed: angiopoietin 1 (Ang-1), a constitutively expressed regulator of activation, and Ang-2, which is normally kept in ECs but is released after inflammatory signals to trigger endothelial activation (155, 156). An augmented Ang-2/Ang1 ratio due to altered levels of both molecules was observed in this model (46), a pattern that was previously observed for septic shock, streptococcal toxic shock, and cerebral malaria (155, 157–160). This elevated ratio along with the enhanced production of endothelin 1 and endothelial nitric oxide synthase (eNOS) were observed at 2 dpi in a lethal model of Karp strain inoculation in C57BL/6 mice, where renal pathology, including cellular infiltration in and around the glomeruli and in the intertubular region, was evident. Interestingly, the upregulation of IL-33 and its receptor was observed in livers and kidneys of challenged WT mice, whereas IL-33−/− mice showed marked decreases in endothelium stress and renal pathology (49). This interleukin is a nuclear factor belonging to the IL-1 family that may modulate inflammatory responses in either a pro- or an anti-inflammatory way and has a well-supported role in the development of acute or chronic kidney injury and disease (161–163). In this lethal Karp strain infection model, a tissue-specific pathogenic role was attributed to IL-33 promoting EC activation and dysregulation as well as extensive tissue damage (49). Additionally, an apoptotic response of ECs that was present in WT mice but greatly diminished in IL-33−/− mice provided further evidence of the proapoptotic effects of Orientia observed previously (81, 82) but suggested that apoptotic effects may also be dependent on the tissue and infection model, highlighting the importance of dissecting its underlying mechanisms.
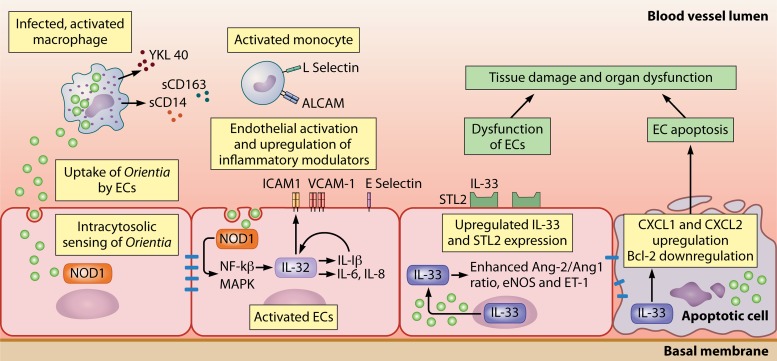
FIG 3.
Model for endothelial infection and renal injury in severe, disseminated O. tsutsugamushi infection. Orientia tsutsugamushi uptake in ECs is followed by cytosolic sensing, where a confirmed role for NOD1 has been described. This leads to the activation of the infected endothelium, with the IL-32-mediated upregulation of inflammatory (IL-1β, IL-6, and IL-8) and adhesion (ICAM-1, VCAM-1, and E selectin) molecules. The increased expression of adhesion molecules in other cell subsets may be involved, as suggested by studies of human patients. The activation of ECs may present a dysfunctional phenotype, with an increased Ang2/Ang1 ratio and altered levels of endothelial nitric oxide synthase (eNOS) and endothelin (ET-1), in which the intranuclear expression and liberation of the alarmin IL-33, along with the upregulated expression of its receptor STL2, play a significant role. Renal tissue damage is enhanced by endothelial apoptosis, where the recruitment of cellular components by chemokine expression and the downregulation of antiapoptotic Bcl-2 may also be involved.
Another interleukin that may be involved in the response of ECs to Orientia infection is IL-32, a constitutively expressed regulator of ICAM-1 expression and endothelial inflammation, along with IL-1β. This interleukin has been related to a protective effect in bacterial control, especially against Mycobacterium, but also to the immunopathogenesis of some infectious and noninfectious diseases (164, 165). Via NF-κB and p38 MAPK pathways, IL-32 induces several proinflammatory molecules. The role of IL-32 in EC activation has been studied by Boryong strain infection of the ECV304 cell line, where the production and secretion of ICAM-1, IL-1β, IL-6, and IL-8 occur after the activation of an upstream NOD1–IL-32 pathway, as revealed by the diminished production of IL-32 and these cytokines after treatment with specific NOD1 short interfering RNA and by the reversion of this phenotype after exogenous IL-32 treatment (142). This role has not yet been addressed in mechanistic studies, which are required to draw conclusive results.
In summary, the studies discussed above highlight the known importance of ECs in their cross talk with immune cells and as key mediators of inflammatory responses and immunopathology. Most importantly, these studies strongly suggest that ECs and the induced vasculitis are protagonists in Orientia-induced pathogenesis and provide evidence of the importance of mouse models in characterizing molecular interactions regarding Orientia infection.
Bacterial Recognition by PRRs and Activation of Inflammatory Responses
A major gap in understanding the Orientia-host interplay and immune responses to this infection is the limited knowledge of bacterial antigens and molecules sensed by PRRs. This information gap, partially explained by the unavailability of appropriate genetic tools to manipulate Orientia and the scarce mechanistic studies performed on animal models, has been delaying the development and testing of efficient, long-lasting, cross-protective vaccines. The recognition of ligands, receptors, and pathways involved in the host response to Orientia infection is of vital importance for developing therapeutic and vaccine formulations. For instance, the identification of immunodominant antigens and epitopes is the basis of epitope-focused vaccinology, aimed at designing safe and effective vaccines through appropriately delivering selected molecules that are able to generate a protective response in the host (166, 167). Finding conserved epitopes is particularly relevant for pathogens that display antigens with high genetic diversity, such as O. tsutsugamushi. Likewise, the selection of proper vaccine adjuvants, which are molecules that act as agonists of PRRs, may enhance the magnitude and quality of innate and adaptive responses and therefore may shape an improved response to vaccination (168, 169).
There is no clarity about the ligand-receptor interactions that elicit a type I IFN response in either DCs or monocytes. Moreover, the Orientia molecules involved in NOD1 activation are still not identified, but current genomic and structural data about this pathogen are shedding light on the possible mechanism. Considering (i) that γ-d-glutamyl-meso-diaminopimelic acid of peptidoglycan is responsible for NLR activation (170, 171), (ii) the existence of metabolic pathways that may be able to synthesize peptidoglycan from UDP N-acetyl-d-glucosamine revealed by genome analysis (172), and (iii) the presence of both diaminopimelic acid and peptidoglycan-like structures in O. tsutsugamushi (11), it is possible that a unique, still undetermined ligand of this peptidoglycan-like structure may be responsible for triggering some PRRs or at least the NOD1 receptor. Furthermore, the role for TLR signaling in human Orientia infection remains poorly characterized, but initial insights come from studies using murine and human cell lines and rodent models of infection. Toll-like receptors 2 and 4 have been related to protective roles in mouse bacterial and fungal infections, including rickettsial (Rickettsia akari and R. conorii) infections. On the other hand, some studies have linked the activity of TLR2 to increased susceptibility to infection or enhanced pathology (173, 174). Recently, a role for TLR2 in recognizing an Orientia ligand and enhancing susceptibility to disease in a mouse model has been described (47). In HEK293 cells, heat-stable Orientia molecules are recognized by TLR2 but not TLR4, while TNF-α and IL-6 secretion in infected bmDCs obtained from C57BL/6 mice requires TLR2 (47), results which are in accordance with data from a previous study suggesting that TNF-α production was independent of ligand recognition by TLR4 (131). Interestingly, TLR2-deficient and WT C57BL/6 mice showed similar abilities to restrict bacterial growth after i.d. challenge with the Karp strain and also were more resistant than WT mice during the convalescent phase of a lethal i.p. challenge, suggesting that TLR2 is not necessary for protective immunity against O. tsutsugamushi in this model. Accelerated clearance of Orientia in affected tissues and sustained TNF-α and IL-6 production at between 15 and 18 dpi in peritoneal tissue, which is the primary target following i.p. inoculation, were observed in TLR2-competent mice, suggesting that neither elevated transcription levels of proinflammatory molecules nor a diminished ability to minimize bacterial growth was associated with a lethal outcome in this model. The differential responses in the convalescent phase suggest that adaptive immunity responses enhanced by TLR2 signaling may be responsible for these differences in susceptibility (47).
Possible Role for Mucosa-Associated Invariant T Cells
Mucosa-associated invariant T (MAIT) cells are a unique, abundant subset of T cells expressing a semi-invariant T cell receptor restricted to interactions with the MHC-related protein, or MR1 (175–177). Due to the highly conserved and nonpolymorphic nature of MR1, these cells are referred to as T cells with an innate-like phenotype. They sense infection through the riboflavin synthesis pathway, which is present only in bacteria and yeast, and have unique, important roles in antibacterial immunity against pulmonary pathogens and E. coli. After the recognition of antigens, they secrete proinflammatory cytokines and directly kill infected cells. Regarding this, a reduced count of circulating MAIT cells has been observed in pneumonia and tuberculosis sufferers (175, 176, 178–181). Indirect activation after chronically established viral infections through the IL-12/IL-18 pathway has also been reported (182).
The role of this cell subset in rickettsial infection has barely been explored, but a recent study of scrub typhus patients reported MAIT cell activation after Orientia infection and suggested that this cell subset constitutes an important arm of cell-mediated immunity (CMI). Moreover, MAIT cells may be related to disease severity after their dysregulation or deficiency (183). Diminished MAIT cell levels in peripheral blood were revealed when scrub typhus patients (0.69%) were compared to healthy controls (1.37%). These cells also show diminished production of TNF-α in comparison to those of healthy donors but increased production in the remission stage of the disease (183). Performing future studies on MAIT cells may shed light on their role in rickettsial infections and their potential for the development of immunotherapies based on cell immunity. Although they display phenotypic differences, mouse MAIT cells closely resemble human ones (184), and therefore, mouse models have interesting potential for the study of the physiological and pathological roles of these cells.
ADAPTIVE IMMUNITY: PROTECTIVE ROLES AND KEY LIMITATIONS
Acquisition of Natural Heterologous and Homologous Immune Responses
One of the most remarkable traits of adaptive immunity is the development of highly specific immunological memory after encountering a pathogen. This specificity aims to safeguard the host from successive infections by the same pathogen, a feature known as homologous protection. Nevertheless, the generated immunological memory may influence immune responses to other pathogens, which is known as heterologous immunity. Although usually associated with closely related pathogens (i.e., different strains of the same bacterial species), it is also observed between unrelated taxa and may either result in boosting protective responses or favor immunopathology (185). Observations from studies performed with human volunteers more than 50 years ago revealed that natural heterologous immunity acquired after O. tsutsugamushi infection is strikingly short-lived, whereas homologous protection may last some years (186, 187), observations which are further supported by data from studies performed with nonhuman primates (NHPs) (188). Experimental subcutaneous (s.c.) inoculation of the Gilliam strain in human volunteers who were naturally infected with Orientia 11 to 24 months before the experiment resulted in clinical disease. On the contrary, infection of patients who were naturally infected 1 to 2 months previously did not result in clinical disease (186). As discussed by those authors, most of those challenges were probably heterologous. A different picture comes from homologous challenges, where only an erythematous lesion surrounding the Gilliam strain inoculation site was observed in a patient who was infected with the same strain 3 years prior to the study. Additionally, complete protection was observed in another homologous challenge with a volunteer who was naturally infected more than 3 years earlier (186, 187). Similar results were observed in NHPs, where susceptibility to experimental homologous reinfection was observed 6 years after the initial infection, in contrast to the resistance otherwise observed when reinfection was performed 8 months after the initial infection (188). Previous evidence for the lack of heterologous protection came from studies with formalin-killed vaccine preparations, where lethal i.p. homologous challenge resulted in the survival of mice, whereas heterologous challenge resulted in various outcomes, from an absolute lack of protection to significant protection. This was observed only when immunization was done by the same administration route and not when the subcutaneous route was used, which resulted in a lack of protection even with homologous challenge (189). However, when live O. tsutsugamushi bacteria were used for s.c. immunization, homologous protection was observed even when mice were challenged by a different route (190). Interestingly, those early studies suggested high antigen diversity characterizing Orientia strains. Additionally, those studies revealed that both the viability of vaccine organisms and the immunization/challenge routes differentially influence the host immune response.
Antigen Diversity
More recent studies on O. tsutsugamushi surface molecules and antigens confirmed the previously suspected heterogeneity of surface antigens. Despite these studies, we are still far from having a comprehensive understanding of the diversity of Orientia antigens and their relevance in immunity. To date, five immunodominant proteins have been detected by Western blotting, with molecular masses of 22, 47, 56, 58, and 110 kDa (191–195). Some of these antigens show discrete variability: the 22-kDa antigen is well conserved among several strains (>95% similarity), whereas the 47-kDa antigen is a conserved transmembrane protein that also presents little contribution to the antigenic variability of Orientia (196, 197). On the other hand, higher variability is observed for the sequences of the 56- and 110-kDa antigens. TSA56 is the most well-characterized antigen and the major variable immunogenic surface protein; it contains four major variable domains (VDs) but also conserved epitopes and is the most frequently recognized antigen in human and animal infections (192, 198–201). This protein has been the subject of several vaccine studies due to its high immunogenicity; some of these studies are discussed below. The 110-kDa antigen is less abundant in outer membranes but also presents strain-specific epitopes and is recognized in natural human infection. Its study as an immunogen is limited to one report, where recombinant plasmid expression of this antigen provided partial protection in a homologous challenge of Swiss CD-1 mice (42).
In many studies performed in several regions of endemicity in Asia examining rodent, arthropod, and human samples, high antigenic diversity is indeed observed. However, analysis of Orientia genetic diversity reveals a more complex diversity, where a variable frequency of genotypes is frequently found either within or between regions of endemicity (202–211). Moreover, molecular analyses of field samples, which are commonly based on analyses of outer membrane proteins, mainly the 56-kDa protein, reveal that common genotypes found in human patients do not reliably represent the prototype strains widely used in diagnostic assays and vaccine studies but fall into strain-related genotypes that show higher genotypic diversity (205–207, 209, 210, 212, 213). Thus, the O. tsutsugamushi diversity observed in regions of endemicity represents a major barrier that is difficult for the development of accurate diagnostic tools and adequate cross-protection through vaccination. This heterogeneity leads to the ineffective development of both effector and memory cross-protective immunity due to the antigen-specific nature of humoral and cell-mediated responses. Although testing of either heat-killed, irradiated, or live Orientia vaccines revealed that several subunit vaccines may provide strong homologous protection (42), on the contrary, the development of heterologous protection remains a major challenge. Since immunodominant, variable antigens have failed to provide long-lasting heterologous protection, it has been proposed that sustained, cross-protective immunization may be reached by testing vaccines that include a combination of nonimmunodominant, widely conserved antigens displayed in an adequate context that favors the development of both humoral and cellular responses (24). However, candidate antigens remain to be identified.
Humoral Immunity: Protective Roles and Related Problems
In human patients, levels of specific antibodies against O. tsutsugamushi are maintained during a short time, as observed in serological surveillance studies. Orientia-specific titers rise from the second week postinfection (214), reaching a mean peak titer of 1:499, but quickly decrease afterwards, with a mean reversion time of ∼49 weeks, reaching undetectable levels after 40 months (215). The short duration of serum antibodies has also been observed in NHPs, where detectable levels disappear within 1 year after generation (188). Complement fixation studies on human strain-specific antisera show that sample titration results in significantly higher endpoint titers when tested against homologous strains than when tested against heterologous strains (189, 216).
Those observations are clearly reflected in the characteristics of the natural immune response acquired by naturally infected individuals, which are discussed above. The kinetics of antibody production have scarcely been reported in mouse inoculation studies that resemble human disease, where the duration of significant serum antibody levels was not measured. In a Karp strain i.v. infection model, animals suffering from acute lethal disease presented higher levels of IgG2c at 10 dpi than did their uninfected counterparts, whereas levels of IgG1 antibodies did not differ between the two groups. Additionally, a marked Th1 cytokine profile was evident in animals presenting serious disease, giving hints of the importance of this secretory profile in disease development. Additionally, higher IgM levels were detected by an enzyme-linked immunosorbent assay (ELISA) at 10 dpi in the lethal challenge group than in uninfected control mice (46). That same research group reported antibody responses in a sublethal challenge with the Gilliam strain, where the kinetics of seroconversion were dependent on the dose of the pathogen and the inoculation route. An overall predominance of IgG2c over IgG1 isotypes was reported, suggesting a more significant Th1 response. However, significant levels of IgG1 were also reported, suggesting that a Th2 response was present, possibly contributing to the improvement of immune homeostasis leading to a sublethal outcome rather than a lethal one (43). When considering studies of human sera, one study reported that the kinetics and levels of antibody responses differ between conserved and variable antigens; specifically, the variable 56-kDa protein produced early, robust, and long-lasting antibody responses, while those elicited by the 47-kDa antigen were detectable later and were less pronounced (191).
Although a complete understanding of the robustness of the antibody response requires further research efforts, the results discussed above suggest that the kinetics and isotypes implicated in the humoral response may be modulated by several factors and may impact the general immune response to Orientia and the pathogenesis of scrub typhus. Despite the short duration of antibody levels in human patients, protective roles of antibodies were observed in studies reported decades ago, through the passive transfer of sera from immunized animals to challenged mice. In one study, complete protection or delayed death was observed after a lethal i.p. challenge when serum transfer was performed within the first week of infection (217), whereas complete protection against acute disease and enhanced mouse survival were reported in another study after the transfer of immune sera previously incubated with Orientia in vitro (218). Protection by antibody neutralization was observed in another report where strain-specific differences were studied. Particularly, complete protection was achieved only with homologous strain challenges, whereas no protection was observed with heterologous strain challenges (219). Hyperimmune serum inhibited infection by Orientia in suspended chicken cells by blocking a strain-specific surface antigen. Additional studies have shown that strain-specific antibodies enhance the intracellular uptake of the bacteria by professional phagocytes (54, 133). Guinea pig PMNs enhanced the uptake of antibody-bound Orientia bacteria, which were subsequently released from phagosomes to the cytosol, where these antibodies inhibited the translocation of Orientia to areas where free bacteria usually move (54). When the effect of immune serum on Gilliam strain infection of resident BALB/c mouse peritoneal macrophages was evaluated, a 50% reduction in the number of infected macrophages was achieved. This was improved to a 75% reduction when infection was evaluated in cytokine-activated macrophages, which also presented enhanced growth suppression of opsonized Orientia bacteria (133). Nonetheless, complete suppression of infection by macrophages was not observed when considering these treatments separately, indicating that the humoral response may not be able to fully subvert the infective process.
Antigens inducing neutralizing antibodies were not identified in those previous studies. However, other studies reported that sera obtained from human patients show reactivity with the 56-kDa protein (220, 221), and some important epitopes of this protein have been identified. These epitopes were mapped in a study where mouse monoclonal antibodies and sera obtained from naturally infected humans and mice immunized with the 56-kDa antigen from the Boryong strain (Bor56) were used to characterize antibody-binding domains through the expression of deletion constructs of Bor56 recombinantly expressed in E. coli. Antigenic domains (ADs) I (aa 19 to 103), II (aa 142 to 203), and III (aa 243 to 328) were identified as being important immunogenic regions (222). Moreover, in a similar study, sera obtained from Boryong-, Gilliam-, Karp-, or Kato-immunized mice were employed to analyze homotypic and heterotypic antibody responses, revealing that different epitopes on the 56-kDa antigen were involved in distinct antibody responses. Particularly, it was concluded that ADs II and III and VD IV were relevant for heterotypic antibody responses, whereas VD I defined homotypic antibody responses (223). Those results suggested that the 56-kDa antigen plays a significant role in generating protective humoral responses. In fact, C3H/HeDub mice immunized with recombinant Bor56, but not control mice, showed a triggering of the secretion of neutralizing antibodies, which conferred enhanced resistance to lethal challenge. These immunized mice also secreted Th1 cytokines when stimulated with irradiated strain Boryong bacteria and displayed dose-dependent lymphocytic proliferation, thereby shedding light on the protective responses achieved by targeting this antigen (224). Additionally, high antibody titers were also observed, which, in other studies, showed the ability to neutralize infection in an i.p. challenge with a homologous strain (222, 225). Also, immunization of cynomolgus monkeys 1 month prior to challenge with a recombinant truncated fragment of this antigen elicited significant humoral and cellular immune responses and diminished clinical disease but did not prevent rickettsemia (42).
The reports discussed above support the idea that humoral immunity has a significant role in providing protection against homologous infections. On the contrary, a protective role in the development of heterologous protection is otherwise less well supported. Several aspects of the humoral response require urgent research, including the detection of conserved antigens and characterization of their immunogenic potential. Also, specific isotype responses and their kinetics should be further studied, along with their effect on scrub typhus pathogenesis and diverse aspects of humoral and cellular responses. The development of accurate animal models may constitute a powerful tool for the characterization and modulation of these responses.
Contribution of T Cells and Cytotoxic Lymphocytes to Protection and Pathogenesis
According to reports based on human patients and experimental animal studies, CMI has a significant role in protective responses to Orientia tsutsugamushi, being more important in the development of cross-protective responses than humoral immunity (132, 226–233). One study found that mice that survived a first Gilliam strain challenge were able to survive a subsequent lethal i.p. Karp challenge, despite a lack of cross-reactive antibodies. Moreover, complete protection of naive mice was observed after the passive transfer of splenocytes from Gilliam-inoculated immune mice, a protective effect that was otherwise absent when transferred cells were T cell depleted. Interestingly, no protection against lethal Karp challenge was observed when only sera of Gilliam-immunized animals were transferred to naive mice, confirming that humoral responses had a marginal role in these protective responses (226). In another study, gamma-irradiated Karp strain bacteria, which are able to enter the cells but are unable to replicate, were employed to perform i.p. immunization of mice, resulting in a nonlethal outcome. These irradiated immunogens yielded complete cell-mediated protection against subsequent lethal challenges performed 24 days after immunization with the Karp and Kato strains, providing further support for the role of CMI in homologous and heterologous protection (227). When formalin-inactivated Orientia bacteria were employed instead, neither homologous nor heterologous protection was achieved, which may be related to a decrease in antigen immunogenicity (227). In accordance to those previous results, lymphocyte proliferation triggered by both homologous and heterologous antigens was observed in an in vitro mouse lymphocyte proliferation assay performed after immunization with the Karp, Kato, or Gilliam strain (229). It was later reported that proliferating T cells were able to secrete MIF and interferon (234). A previously reported study using an in vitro assay indicated that CD4+ T cells are activated after encountering infected moDCs, as revealed by IFN-γ secretion (117). Nevertheless, patients with acute scrub typhus present with peripheral CD4+ lymphopenia and regulatory T cell downregulation, along with massive T cell apoptosis, which may contribute to the poor generation of memory responses and to an unregulated cytotoxic T lymphocyte (CTL) response (235).
To date, several reports have yielded robust data indicating that protection against Orientia infection results after IFN-γ upregulation. High IFN-γ levels were seen in C3H/He mice previously s.c. immunized with the Gilliam strain, after i.p. challenge with viable Orientia bacteria. The correlation of these levels with rickettsemia strongly suggested that this cytokine has an important role in protective responses (230). A protective role was observed in another study, where an IFN-γ-producing immune T cell line previously exposed to specific antigens provided protection after being transferred to challenged naive mice. Interestingly, this cell line, which was suggested to be of a T helper phenotype, showed partial reactivity to Karp but not Kato strain antigens in a lymphocyte proliferation assay (236). In NHPs, one report indicated that T cells respond with IFN-γ secretion after Orientia infection (188), results which were recently supported by a study carried out in a rhesus macaque model where Orientia-specific cellular IFN-γ secretion was observed at between 7 and 28 dpi through an ex vivo IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assay (237). The detection of the production of this cytokine in peripheral blood mononuclear cells has been related to the improved control of bacteremia in NHPs vaccinated with a DNA vaccine (pKarp47 DNA) (238). In human scrub typhus patients, an early response of IFN-γ and the IFN-γ-inducing cytokines IL-15 and IL-18 occurs early during the acute phase of infection (144–146). Additionally, a proinflammatory-shaped response is evident, and T cell recruitment may be enhanced by the release of the chemokines CXCL9 and CXCL10. Furthermore, increased levels of granzymes A and B indicate the upregulation of cytotoxic activity (144–146, 232). These responses are likely due to the cytosolic sensing of Orientia, as discussed above, and support the importance of CMI and cytotoxicity in early responses against this infection.
Cytotoxicity is an important mechanism that is usually required to eliminate cytosolic pathogens. The major effectors of these response are CTLs, which are mainly CD8+ cells. These responses are less studied for Orientia than for the Rickettsia genus (239). Early reports observing a cytotoxicity effect showed that splenocytes derived from Gilliam strain-infected C3H/He mice are able to lyse L929 fibroblasts in vitro. Recently, using a BALB/c mice footpad inoculation model with the Karp strain, it was demonstrated that the spleen and lung are highly infiltrated by active CD8+ T cells at 21 dpi. The CD8+ response is required for the protection of mice, since its depletion results in elevated bacterial loads from 14 dpi onwards and in mouse mortality, while the adoptive transfer of these cells to i.p. challenged naive mice prevents lethality. Complementary roles were addressed in a C57BL/6 mice model, where an increased percentage of CD8+ T cells was present in the pulmonary lymphocyte compartment for several weeks, and their depletion at 84 dpi caused the reactivation of bacterial growth. Interestingly, the activity of CD8+ T cells was required to control infection and protect mice from lethality even when increased IFN-γ levels and activated macrophage responses were observed in lung and liver. Also, the cytotoxic activity of CD8+ T cells was responsible for liver and lung injury. Those results underscore the relevance of these cells in orchestrating a protective response that requires several mediators and in restricting the growth of Orientia in acute and persistent infection. Moreover, those results demonstrate the role of this cell subset in tissue injury observed in mouse models that resemble human pathology (231).
Finally, it is interesting to note that humoral immunity also requires T cells for proper development, as concluded in a study performed with athymic mice, where antibody responses were dependent on the transfer of T cells (233). Summarizing the data from recent studies, and considering the characteristics of the infective cycle and systemic dissemination of Orientia tsutsugamushi in scrub typhus, is clear that a protective response requires efficient cross talk between humoral and cellular immunity, with a tight control of effector cells, in order to successfully achieve pathogen clearance without compromising the host due to exacerbated inflammatory responses. The role of T cells and cytotoxicity against Orientia infection seems to be crucial for several aspects of adaptive immunity; however, functional studies unveiling the contribution of CTLs, apart from the ones mentioned above, as well as studies of other T cell subpopulations in O. tsutsugamushi infection are scarce, particularly in the context of infections that resemble human pathology. This branch of the immune response should be of high priority in future studies, since it may constitute an essential component of the response required to achieve the complete elimination of the pathogen from infected tissues, acting as a scaffold for humoral and cytotoxic responses and as a basis for the development of robust cross-protective immunological memory, which is a crucial goal to reach.
CONCLUDING REMARKS
Scrub typhus remains a neglected disease despite the substantial health burden that it generates annually. The development of an effective and cross-protective vaccine constitutes an imperative goal for the prevention and control of this severe infection, but current efforts have yielded ineffective results. This bacterium has a complex infectious cycle that involves several hosts and cell types, where it displays a plethora of molecular mechanisms required for successful infection and cycle consummation. In this context, great research progress has been achieved regarding details of the Orientia-host cell interplay, which have shown that this bacterium extensively modulates human innate immune responses. However, important aspects of innate response subversion are still not elucidated, including apoptosis modulation and phagosome/autophagosome escape mechanisms as well as enhanced cytosolic replication withstanding nitric oxide production. The availability of the complete genomes of two Orientia strains may help in an understanding of these processes and furthermore has given evidence of several potential pathogenic mechanisms. For example, the massive existence of secretion systems and effectors proteins, along with some recent discoveries of their roles as virulence factors, has raised an interesting hypothesis about the extent and mechanisms of the interaction network mediated by Orientia soluble molecules, making this a fertile field for future investigations. The incorporation of new methodologies may be useful for expanding current knowledge, as reported for other intracellular pathogens, where chromatin immunoprecipitation (ChIP) studies have shed light on the direct DNA-binding capacity of Ank proteins in Anaplasma phagocytophilum (240, 241) and Ehrlichia chaffeensis (242, 243). Similar improved methodologies for intracellular organisms were recently reported (244), and efforts from research groups will be required to expand the tools needed for dissecting Orientia modulation of host cells. Additionally, new releases of draft Orientia genomes (245) may also be employed to facilitate an understanding of the molecular complexity of different Orientia strains and the genetic bases of pathogenicity, as exemplified by recent work addressing strain differences in antibiotic resistance (246). Genomic information should also be exploited for the detection of conserved subdominant antigens, which may be crucial for the generation of vaccines that elicit cross-protective responses, as discussed above.
The bacterial ligands and host cell receptors that participate in the specific recognition of Orientia remain largely unknown, with some exceptions. However, it is well documented that infection and activation of phagocytic and nonphagocytic innate cells induce the upregulation of cytokines and chemokines that drive a proinflammatory state, which has been consistently studied by employing in vitro systems. It seems that these responses are insufficient to control infection and additionally may be involved in scrub typhus immunopathogenesis. It is interesting to analyze IL-10 secretion, which has been consistently observed in several in vitro and in vivo experiments, as well as studies carried out in scrub typhus patients (48, 130, 135, 141, 144, 146). Although not addressed by mechanistic studies, the role of this cytokine seems to be critical in shaping disease outcomes, possibly being involved in the modulation of the hypersecretion of inflammatory cytokines and bacterial persistence. Further research, integrating new molecular techniques and employing recently established mouse models, will have a fundamental role in determining the impact of key molecules and actors in the inflammatory response, including ligands and receptors involved in infection sensing.
Fluorescent chemical probes that allow the tracking and live imaging of cell compartments are promising tools that are expanding our knowledge of the basic biology of Orientia and revealing striking characteristics, such as the presence of peptidoglycan-like structures that may be involved in host cell recognition by unique patterns, which should be further characterized (11, 247). Moreover, studies based on TLR2-deficient mice have revealed interesting roles for this molecule (47), and it is expected that future mechanistic studies performed with models that closely resemble human infection will greatly improve our understanding of defense and pathogenic mechanisms. The establishment of these models constitutes a unique advancement, since in the past, several studies addressing immune responses were performed by using either i.p. or i.v. inoculation, displaying responses that do not reflect those observed in mite-borne natural human infection. Further diversification of inoculation routes is necessary to explore different aspects of the pathology and immune responses triggered by Orientia infection. Laboratory-reared chigger infection models may be the most appropriate approach to study several aspects of Orientia infection, without neglecting vector-associated factors. Although successful Orientia challenges employing this inoculation route in mice have been reported (126, 248), it has not been routinely employed in the latest studies. Furthermore, to better understand natural human Orientia infection, and to address gaps regarding human immune responses, this laboratory-reared chigger inoculation approach may be employed on humanized mice, which constitute an interesting tool that has been unexplored to date. These mice display a functional human immune system, since they carry a mutation in the IL-2 receptor γ chain locus that renders them severely immunodeficient, a condition that permits the subsequent engrafting of primary hematopoietic cells and tissues from humans (249, 250). This combined approach would allow the assessment of several factors that are frequently bypassed, such as the immunomodulatory effects that arthropod saliva might exert (251). It would also provide a unique platform to investigate the infective process of Orientia and human immune responses. We expect that efforts will be made to overcome technical difficulties related to the establishment of the laboratory-reared chigger inoculation model.
Currently, interesting studies are unveiling the role of T cell subsets in defense against Orientia, particularly cytotoxic cells (231), but additional progress is required and expected to be addressed soon to understand the impact of each cell subset participating in CMI. The role of CD4+ cells requires urgent characterization due to their impact on immunological memory development, especially considering reports of CD4+ human lymphopenia (235). Finally, for achieving an accurate understanding of immune responses, it is necessary to conduct studies on the contribution of other less-studied cell subsets, including MAIT and regulatory T cells, which may be achieved via mechanistic studies thanks to the current availability of mouse models and diverse genetic backgrounds.
Several factors of this pathogen and the immune response elicited in human hosts turn the development of an effective cross-protective vaccine into a complex challenge for scientists. One of the greatest barriers is derived from the high genetic diversity and antigenic diversity among strains and their major surface proteins. However, other factors, such as (i) the natural response targeting nonconserved, immunodominant antigens; (ii) deficient naturally acquired immunity; (iii) the involvement of several immune cells in the replicative cycle of Orientia tsutsugamushi; (iv) the lack of established animal models for the realization of mechanistic studies; and (v) the limited knowledge on correlates of protective infection, also hinder the development of therapeutic modulation strategies and of a protective vaccine providing long-lasting and cross-protective responses. To date, it is well understood that effective immunity against Orientia requires the synergy of humoral responses and cellular components of both innate and adaptive responses, but a more realistic study approach will lead to a more precise understanding. We expect that future mechanistic studies based on animal models will allow researchers to dissect several branches of immunity against this pathogen, toward overcoming difficulties the underlie vaccine development. Moreover, other important goals to be achieved, such as the development of diagnostic and immunogenicity assays for field use as well as the evaluation of therapeutic agents and candidate vaccines, also require well-established animal models. Determining correlates of protection is therefore required, but this has not been achieved in humans. Interesting reports based on NHP models are providing a fertile field for future protection correlate studies and investigations into vaccine-induced immune responses (238). It is noteworthy that the recent establishment of a high-sensitivity IFN-γ-based ex vivo ELISPOT assay validated in both humans and a NHP model may provide a remarkable link between animal models and human studies and promises to improve field immunogenicity and candidate vaccine evaluation (237).
Finally, as stated above, this bacterium remains genetically intractable. Genetic manipulation of intracellular bacteria has been a challenging goal, mostly due to their mandatory intracellular lifestyle. It is noteworthy that during the last decade, genetic manipulation strategies have been successfully applied to Rickettsia, specially spotted fever group and typhus group rickettsiae (252, 253). Research groups should direct efforts toward overcoming this genetic intractability, since genetic manipulation tools, once protocolized, may notoriously improve the knowledge of Orientia pathogenesis and immunity.
ACKNOWLEDGMENTS
This work was funded by the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) (doctorate grant N-21170620 and FONDECYT grants 1070352 and 1170810), the Millennium Institute on Immunology and Immunotherapy (grant P09/016-F), Becton Dickinson, and FONDECYT grant 1150862.
Biographies

Fabián E. Díaz earned his doctor in veterinary medicine (D.V.M.) and master of veterinary science (M.V.Sc.) degrees at the Universidad de Chile in Santiago, Chile. He is currently in pursuit of a Ph.D. in Molecular Genetics and Microbiology at the School of Biological Sciences, Pontificia Universidad Católica de Chile (PUC), supported by a doctoral fellowship by CONICYT, Chile. His research interests have focused on the pathology, epidemiology, immunology, and vaccine development for diseases caused by vector-borne and intracellular pathogens (2014 to 2018).

Katia Abarca is a Full Professor at the School of Medicine, PUC. She earned her medical doctor (M.D.) degree at the University of Chile and her Pediatric Infectious Disease Specialist at the PUC. She obtained two master's degrees (M.Sc.), in Pediatrics at the PUC and in Molecular Biology Applied to Infectious Diseases at the London School of Hygiene and Tropical Medicine, London University. She is Head of the Postgraduate Program in Pediatrics at the School of Medicine, PUC, and Medical Director at the Vaccine Center, PUC. She has been awarded several important national and international research grants, published dozens of scientific articles, and integrated several scientific committees. Her research interests have been focused on pet-related zoonoses and pediatric vaccines. She is currently working as Principal Investigator in a research project related to the identification of Orientia tsutsugamushi in Chile, supported by a FONDECYT grant awarded from CONICYT, Chile.

Alexis M. Kalergis is a Full Professor at the School of Biological Sciences and Medicine at the Pontificia Universidad Católica de Chile. He obtained the Julius Marmur Award for his Ph.D. work on Microbiology and Immunology at the Albert Einstein College of Medicine in New York, NY. He performed postdoctoral training at AECOM and The Rockefeller University, supported by the Helen Hay Whitney Foundation. He is a member of the American Association of Immunologists; the American Society for Microbiology; and the Chilean Societies for Biology, Cell Biology, and Immunology. He was selected as the most outstanding young scientist by the Biology Society of Chile, as one of 50 Chilean young leaders, and awarded several important national and international research grants. He is the Director of the Millennium Institute on Immunology and Immunotherapy, an excellence research center recently appointed as an international center of excellence by the Federation of Clinical Immunology Societies (FOCIS). More than 200 young scientists have trained under his supervision. He has received several awards for his work, such as the Gold Medal by the World Intellectual Property Organization and the National Innovation Award, and published over 180 articles in leading journals. His group has recently developed a vaccine against human respiratory syncytial virus that is currently in clinical trials. His research focuses on the molecular interactions regulating the T cell-DC synapse and their contribution to pathogen immunity and self-tolerance during autoimmunity.
REFERENCES
- 1.Seong SY, Choi MS, Kim IS. 2001. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect 3:11–21. doi: 10.1016/S1286-4579(00)01352-6. [DOI] [PubMed] [Google Scholar]
- 2.Walker DH. 2016. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med 375:913–915. doi: 10.1056/NEJMp1608499. [DOI] [PubMed] [Google Scholar]
- 3.Chrispal A, Boorugu H, Gopinath KG, Prakash JA, Chandy S, Abraham OC, Abraham AM, Thomas K. 2010. Scrub typhus: an unrecognized threat in South India—clinical profile and predictors of mortality. Trop Doct 40:129–133. doi: 10.1258/td.2010.090452. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S, Song H, Liu Y, Li Q, Wang Y, Wu J, Wan J, Li G, Yu C, Li X, Yin W, Xu Z, Liu B, Zhang Q, Wan K, Li G, Fu X, Zhang J, He J, Hai R, Yu D, Walker DH, Xu J, Yu XJ. 2010. Scrub typhus in previously unrecognized areas of endemicity in China. J Clin Microbiol 48:1241–1244. doi: 10.1128/JCM.01784-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osuga K, Kimura M, Goto H, Shimada K, Suto T. 1991. A case of tsutsugamushi disease probably contracted in Africa. Eur J Clin Microbiol Infect Dis 10:95–96. doi: 10.1007/BF01964418. [DOI] [PubMed] [Google Scholar]
- 6.Weitzel T, Dittrich S, Lopez J, Phuklia W, Martinez-Valdebenito C, Velasquez K, Blacksell SD, Paris DH, Abarca K. 2016. Endemic scrub typhus in South America. N Engl J Med 375:954–961. doi: 10.1056/NEJMoa1603657. [DOI] [PubMed] [Google Scholar]
- 7.Kocher C, Jiang J, Morrison AC, Castillo R, Leguia M, Loyola S, Ampuero JS, Cespedes M, Halsey ES, Bausch DG, Richards AL. 2017. Serologic evidence of scrub typhus in the Peruvian Amazon. Emerg Infect Dis 23:1389–1391. doi: 10.3201/eid2308.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amano K, Tamura A, Ohashi N, Urakami H, Kaya S, Fukushi K. 1987. Deficiency of peptidoglycan and lipopolysaccharide components in Rickettsia tsutsugamushi. Infect Immun 55:2290–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho NH, Kim HR, Lee JH, Kim SY, Kim J, Cha S, Kim SY, Darby AC, Fuxelius HH, Yin J, Kim JH, Kim J, Lee SJ, Koh YS, Jang WJ, Park KH, Andersson SG, Choi MS, Kim IS. 2007. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc Natl Acad Sci U S A 104:7981–7986. doi: 10.1073/pnas.0611553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuxelius HH, Darby A, Min CK, Cho NH, Andersson SG. 2007. The genomic and metabolic diversity of Rickettsia. Res Microbiol 158:745–753. doi: 10.1016/j.resmic.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Atwal S, Giengkam S, Chaemchuen S, Dorling J, Kosaisawe N, VanNieuwenhze M, Sampattavanich S, Schumann P, Salje J. 19 June 2017. Evidence for a peptidoglycan-like structure in Orientia tsutsugamushi. Mol Microbiol doi: 10.1111/mmi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama K, Kurokawa K, Fukuhara M, Urakami H, Yamamoto S, Yamazaki K, Ogura Y, Ooka T, Hayashi T. 2010. Genome comparison and phylogenetic analysis of Orientia tsutsugamushi strains. DNA Res 17:281–291. doi: 10.1093/dnares/dsq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsay RW, Chang FY. 1998. Serious complications in scrub typhus. J Microbiol Immunol Infect 31:240–244. [PubMed] [Google Scholar]
- 14.Nakayama K, Yamashita A, Kurokawa K, Morimoto T, Ogawa M, Fukuhara M, Urakami H, Ohnishi M, Uchiyama I, Ogura Y, Ooka T, Oshima K, Tamura A, Hattori M, Hayashi T. 2008. The whole-genome sequencing of the obligate intracellular bacterium Orientia tsutsugamushi revealed massive gene amplification during reductive genome evolution. DNA Res 15:185–199. doi: 10.1093/dnares/dsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, Mathai M, Raoult D. 2003. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci 990:359–364. doi: 10.1111/j.1749-6632.2003.tb07391.x. [DOI] [PubMed] [Google Scholar]
- 16.Jeong YJ, Kim S, Wook YD, Lee JW, Kim KI, Lee SH. 2007. Scrub typhus: clinical, pathologic, and imaging findings. Radiographics 27:161–172. doi: 10.1148/rg.271065074. [DOI] [PubMed] [Google Scholar]
- 17.Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, Purty S. 2010. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India 58:24–28. [PubMed] [Google Scholar]
- 18.Varghese GM, Trowbridge P, Janardhanan J, Thomas K, Peter JV, Mathews P, Abraham OC, Kavitha ML. 2014. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis 23:39–43. doi: 10.1016/j.ijid.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Peter JV, Sudarsan TI, Prakash JA, Varghese GM. 2015. Severe scrub typhus infection: clinical features, diagnostic challenges and management. World J Crit Care Med 4:244–250. doi: 10.5492/wjccm.v4.i3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajapakse S, Weeratunga P, Sivayoganathan S, Fernando SD. 2017. Clinical manifestations of scrub typhus. Trans R Soc Trop Med Hyg 111:43–54. doi: 10.1093/trstmh/trx017. [DOI] [PubMed] [Google Scholar]
- 21.Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA, Strickman D. 1996. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet 348:86–89. doi: 10.1016/S0140-6736(96)02501-9. [DOI] [PubMed] [Google Scholar]
- 22.Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D. 1999. Azithromycin activities against Orientia tsutsugamushi strains isolated in cases of scrub typhus in Northern Thailand. Antimicrob Agents Chemother 43:2817–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tantibhedhyangkul W, Angelakis E, Tongyoo N, Newton PN, Moore CE, Phetsouvanh R, Raoult D, Rolain JM. 2010. Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. Int J Antimicrob Agents 35:338–341. doi: 10.1016/j.ijantimicag.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valbuena G, Walker DH. 2012. Approaches to vaccines against Orientia tsutsugamushi. Front Cell Infect Microbiol 2:170. doi: 10.3389/fcimb.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paris DH, Shelite TR, Day NP, Walker DH. 2013. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg 89:301–307. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuya Y, Yoshida Y, Katayama T, Kawamori F, Yamamoto S, Ohashi N, Tamura A, Kawamura A Jr. 1991. Specific amplification of Rickettsia tsutsugamushi DNA from clinical specimens by polymerase chain reaction. J Clin Microbiol 29:2628–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuya Y, Yoshida Y, Katayama T, Yamamoto S, Kawamura A Jr. 1993. Serotype-specific amplification of Rickettsia tsutsugamushi DNA by nested polymerase chain reaction. J Clin Microbiol 31:1637–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly DJ, Marana DP, Stover CK, Oaks EV, Carl M. 1990. Detection of Rickettsia tsutsugamushi by gene amplification using polymerase chain reaction techniques. Ann N Y Acad Sci 590:564–571. doi: 10.1111/j.1749-6632.1990.tb42267.x. [DOI] [PubMed] [Google Scholar]
- 29.Le Viet N, Laroche M, Thi Pham HL, Viet NL, Mediannikov O, Raoult D, Parola P. 2017. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. PLoS Negl Trop Dis 11:e0005397. doi: 10.1371/journal.pntd.0005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murai K, Tachibana N, Okayama A, Shishime E, Tsuda K, Oshikawa T. 1992. Sensitivity of polymerase chain reaction assay for Rickettsia tsutsugamushi in patients' blood samples. Microbiol Immunol 36:1145–1153. doi: 10.1111/j.1348-0421.1992.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 31.Paris DH, Aukkanit N, Jenjaroen K, Blacksell SD, Day NP. 2009. A highly sensitive quantitative real-time PCR assay based on the groEL gene of contemporary Thai strains of Orientia tsutsugamushi. Clin Microbiol Infect 15:488–495. doi: 10.1111/j.1469-0691.2008.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paris DH, Blacksell SD, Newton PN, Day NP. 2008. Simple, rapid and sensitive detection of Orientia tsutsugamushi by loop-isothermal DNA amplification. Trans R Soc Trop Med Hyg 102:1239–1246. doi: 10.1016/j.trstmh.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 33.Singhsilarak T, Leowattana W, Looareesuwan S, Wongchotigul V, Jiang J, Richards AL, Watt G. 2005. Short report: detection of Orientia tsutsugamushi in clinical samples by quantitative real-time polymerase chain reaction. Am J Trop Med Hyg 72:640–641. [PubMed] [Google Scholar]
- 34.Sonthayanon P, Chierakul W, Wuthiekanun V, Blacksell SD, Pimda K, Suputtamongkol Y, Pukrittayakamee S, White NJ, Day NP, Peacock SJ. 2006. Rapid diagnosis of scrub typhus in rural Thailand using polymerase chain reaction. Am J Trop Med Hyg 75:1099–1102. [PubMed] [Google Scholar]
- 35.Paris DH, Stephan F, Bulder I, Wouters D, van der Poll T, Newton PN, Day NPJ, Zeerleder S. 2015. Increased nucleosomes and neutrophil activation link to disease progression in patients with scrub typhus but not murine typhus in Laos. PLoS Negl Trop Dis 9:e0003990. doi: 10.1371/journal.pntd.0003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadosaka T, Kimura E. 2003. Electron microscopic observations of Orientia tsutsugamushi in salivary gland cells of naturally infected Leptotrombidium pallidum larvae during feeding. Microbiol Immunol 47:727–733. doi: 10.1111/j.1348-0421.2003.tb03442.x. [DOI] [PubMed] [Google Scholar]
- 37.Kitaoka M, Asanuma K, Otsuji J. 1974. Transmission of Rickettsia orientalis to man by Leptotrombidium akamushi at a scrub typhus endemic area in Akita Prefecture, Japan. Am J Trop Med Hyg 23:993–999. doi: 10.4269/ajtmh.1974.23.993. [DOI] [PubMed] [Google Scholar]
- 38.Traub R, Wisseman CL Jr, Jones MR, O'Keefe JJ. 1975. The acquisition of Rickettsia tsutsugamushi by chiggers (trombiculid mites) during the feeding process. Ann N Y Acad Sci 266:91–114. doi: 10.1111/j.1749-6632.1975.tb35091.x. [DOI] [PubMed] [Google Scholar]
- 39.Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, Suwancharoen D, Panlar P, Saisongkorh W, Rolain JM, Raoult D, Suputtamongkol Y. 2006. Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol 100:363–370. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- 40.Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, Richards AL, Burgess TH, Wierzba TF, Putnam SD. 2012. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am J Trop Med Hyg 86:246–253. doi: 10.4269/ajtmh.2012.11-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourgeois AL, Olson JG, Fang RC, Huang J, Wang CL, Chow L, Bechthold D, Dennis DT, Coolbaugh JC, Weiss E. 1982. Humoral and cellular responses in scrub typhus patients reflecting primary infection and reinfection with Rickettsia tsutsugamushi. Am J Trop Med Hyg 31:532–540. doi: 10.4269/ajtmh.1982.31.532. [DOI] [PubMed] [Google Scholar]
- 42.Chattopadhyay S, Richards AL. 2007. Scrub typhus vaccines: past history and recent developments. Hum Vaccin 3:73–80. doi: 10.4161/hv.3.3.4009. [DOI] [PubMed] [Google Scholar]
- 43.Mendell NL, Bouyer DH, Walker DH. 2017. Murine models of scrub typhus associated with host control of Orientia tsutsugamushi infection. PLoS Negl Trop Dis 11:e0005453. doi: 10.1371/journal.pntd.0005453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keller CA, Hauptmann M, Kolbaum J, Gharaibeh M, Neumann M, Glatzel M, Fleischer B. 2014. Dissemination of Orientia tsutsugamushi and inflammatory responses in a murine model of scrub typhus. PLoS Negl Trop Dis 8:e3064. doi: 10.1371/journal.pntd.0003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shelite TR, Saito TB, Mendell NL, Gong B, Xu G, Soong L, Valbuena G, Bouyer DH, Walker DH. 2014. Hematogenously disseminated Orientia tsutsugamsushi [sic]-infected murine model of scrub typhus. PLoS Negl Trop Dis 8:e2966. doi: 10.1371/journal.pntd.0002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soong L, Wang H, Shelite TR, Liang Y, Mendell NL, Sun J, Gong B, Valbuena GA, Bouyer DH, Walker DH. 2014. Strong type 1, but impaired type 2, immune responses contribute to Orientia tsutsugamushi-induced pathology in mice. PLoS Negl Trop Dis 8:e3191. doi: 10.1371/journal.pntd.0003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gharaibeh M, Hagedorn M, Lilla S, Hauptmann M, Heine H, Fleischer B, Keller C. 2016. Toll-like receptor 2 recognizes Orientia tsutsugamushi and increases susceptibility to murine experimental scrub typhus. Infect Immun 84:3379–3387. doi: 10.1128/IAI.00185-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soong L, Mendell NL, Olano JP, Rockx-Brouwer D, Xu G, Goez-Rivillas Y, Drom C, Shelite TR, Valbuena G, Walker DH, Bouyer DH. 2016. An intradermal inoculation mouse model for immunological investigations of acute scrub typhus and persistent infection. PLoS Negl Trop Dis 10:e0004884. doi: 10.1371/journal.pntd.0004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shelite TR, Liang Y, Wang H, Mendell NL, Trent BJ, Sun J, Gong B, Xu G, Hu H, Bouyer DH, Soong L. 2016. IL-33-dependent endothelial activation contributes to apoptosis and renal injury in Orientia tsutsugamushi-infected mice. PLoS Negl Trop Dis 10:e0004467. doi: 10.1371/journal.pntd.0004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, Jones M, Jenjaroen K, Vongsouvath M, Ferguson DP, Blacksell SD, Newton PN, Day NP, Turner GD. 2012. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis 6:e1466. doi: 10.1371/journal.pntd.0001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urakami H, Tsuruhara T, Tamura A. 1983. Penetration of Rickettsia tsutsugamushi into cultured mouse fibroblasts (L cells): an electron microscopic observation. Microbiol Immunol 27:251–263. doi: 10.1111/j.1348-0421.1983.tb03587.x. [DOI] [PubMed] [Google Scholar]
- 52.Moron CG, Popov VL, Feng HM, Wear D, Walker DH. 2001. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol 14:752–759. doi: 10.1038/modpathol.3880385. [DOI] [PubMed] [Google Scholar]
- 53.Urakami H, Tsuruhara T, Tamura A. 1982. Intranuclear Rickettsia tsutsugamushi in cultured mouse fibroblasts (L cells). Microbiol Immunol 26:445–447. doi: 10.1111/j.1348-0421.1982.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 54.Rikihisa Y, Ito S. 1982. Entry of Rickettsia tsutsugamushi into polymorphonuclear leukocytes. Infect Immun 38:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuhara M, Fukazawa M, Tamura A, Nakamura T, Urakami H. 2005. Survival of two Orientia tsutsugamushi bacterial strains that infect mouse macrophages with varying degrees of virulence. Microb Pathog 39:177–187. doi: 10.1016/j.micpath.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Cho BA, Cho NH, Seong SY, Choi MS, Kim IS. 2010. Intracellular invasion by Orientia tsutsugamushi is mediated by integrin signaling and actin cytoskeleton rearrangements. Infect Immun 78:1915–1923. doi: 10.1128/IAI.01316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ha NY, Cho NH, Kim YS, Choi MS, Kim IS. 2011. An autotransporter protein from Orientia tsutsugamushi mediates adherence to nonphagocytic host cells. Infect Immun 79:1718–1727. doi: 10.1128/IAI.01239-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JH, Cho NH, Kim SY, Bang SY, Chu H, Choi MS, Kim IS. 2008. Fibronectin facilitates the invasion of Orientia tsutsugamushi into host cells through interaction with a 56-kDa type-specific antigen. J Infect Dis 198:250–257. doi: 10.1086/589284. [DOI] [PubMed] [Google Scholar]
- 59.Ha NY, Sharma P, Kim G, Kim Y, Min CK, Choi MS, Kim IS, Cho NH. 2015. Immunization with an autotransporter protein of Orientia tsutsugamushi provides protective immunity against scrub typhus. PLoS Negl Trop Dis 9:e0003585. doi: 10.1371/journal.pntd.0003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ihn KS, Han SH, Kim HR, Huh MS, Seong SY, Kang JS, Han TH, Kim IS, Choi MS. 2000. Cellular invasion of Orientia tsutsugamushi requires initial interaction with cell surface heparan sulfate. Microb Pathog 28:227–233. doi: 10.1006/mpat.1999.0344. [DOI] [PubMed] [Google Scholar]
- 61.Longley RL, Woods A, Fleetwood A, Cowling GJ, Gallagher JT, Couchman JR. 1999. Control of morphology, cytoskeleton and migration by syndecan-4. J Cell Sci 112(Part 20):3421–3431. [DOI] [PubMed] [Google Scholar]
- 62.Kim HR, Choi MS, Kim IS. 2004. Role of syndecan-4 in the cellular invasion of Orientia tsutsugamushi. Microb Pathog 36:219–225. doi: 10.1016/j.micpath.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Ewing EP Jr, Takeuchi A, Shirai A, Osterman JV. 1978. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun 19:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim SW, Ihn KS, Han SH, Seong SY, Kim IS, Choi MS. 2001. Microtubule- and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center. Infect Immun 69:494–500. doi: 10.1128/IAI.69.1.494-500.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi JH, Cheong TC, Ha NY, Ko Y, Cho CH, Jeon JH, So I, Kim IK, Choi MS, Kim IS, Cho NH. 2013. Orientia tsutsugamushi subverts dendritic cell functions by escaping from autophagy and impairing their migration. PLoS Negl Trop Dis 7:e1981. doi: 10.1371/journal.pntd.0001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu H, Lee JH, Han SH, Kim SY, Cho NH, Kim IS, Choi MS. 2006. Exploitation of the endocytic pathway by Orientia tsutsugamushi in nonprofessional phagocytes. Infect Immun 74:4246–4253. doi: 10.1128/IAI.01620-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cho BA, Cho NH, Min CK, Kim SY, Yang JS, Lee JR, Jung JW, Lee WC, Kim K, Lee MK, Kim S, Kim KP, Seong SY, Choi MS, Kim IS. 2010. Global gene expression profile of Orientia tsutsugamushi. Proteomics 10:1699–1715. doi: 10.1002/pmic.200900633. [DOI] [PubMed] [Google Scholar]
- 68.Schnupf P, Portnoy DA. 2007. Listeriolysin O: a phagosome-specific lysin. Microbes Infect 9:1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Stachowiak R, Bielecki J. 2001. Contribution of hemolysin and phospholipase activity to cytolytic properties and viability of Listeria monocytogenes. Acta Microbiol Pol 50:243–250. [PubMed] [Google Scholar]
- 70.Ge Y, Rikihisa Y. 2011. Subversion of host cell signaling by Orientia tsutsugamushi. Microbes Infect 13:638–648. doi: 10.1016/j.micinf.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Ogawa M, Satoh M, Kataoka M, Ando S, Saijo M. 2017. Nitric oxide enhanced the growth of an obligated intracellular bacterium Orientia tsutsugamushi in murine macrophages. Microb Pathog 107:335–340. doi: 10.1016/j.micpath.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Urakami H, Tsuruhara T, Tamura A. 1984. Electron microscopic studies on intracellular multiplication of Rickettsia tsutsugamushi in L cells. Microbiol Immunol 28:1191–1201. doi: 10.1111/j.1348-0421.1984.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 73.Hanson B. 1987. Improved plaque assay for Rickettsia tsutsugamushi. Am J Trop Med Hyg 36:631–638. doi: 10.4269/ajtmh.1987.36.631. [DOI] [PubMed] [Google Scholar]
- 74.Moree MF, Hanson B. 1992. Growth characteristics and proteins of plaque-purified strains of Rickettsia tsutsugamushi. Infect Immun 60:3405–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim MJ, Kim MK, Kang JS. 2013. Involvement of lipid rafts in the budding-like exit of Orientia tsutsugamushi. Microb Pathog 63:37–43. doi: 10.1016/j.micpath.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 76.Gao LY, Kwaik YA. 2000. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol 8:306–313. doi: 10.1016/S0966-842X(00)01784-4. [DOI] [PubMed] [Google Scholar]
- 77.Sahni SK, Rydkina E. 2009. Host-cell interactions with pathogenic Rickettsia species. Future Microbiol 4:323–339. doi: 10.2217/fmb.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim MK, Seong SY, Seoh JY, Han TH, Song HJ, Lee JE, Shin JH, Lim BU, Kang JS. 2002. Orientia tsutsugamushi inhibits apoptosis of macrophages by retarding intracellular calcium release. Infect Immun 70:4692–4696. doi: 10.1128/IAI.70.8.4692-4696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joshi SG, Francis CW, Silverman DJ, Sahni SK. 2004. NF-kappaB activation suppresses host cell apoptosis during Rickettsia rickettsii infection via regulatory effects on intracellular localization or levels of apoptogenic and anti-apoptotic proteins. FEMS Microbiol Lett 234:333–341. doi: 10.1111/j.1574-6968.2004.tb09552.x. [DOI] [PubMed] [Google Scholar]
- 80.Kasuya S, Nagano I, Ikeda T, Goto C, Shimokawa K, Takahashi Y. 1996. Apoptosis of lymphocytes in mice induced by infection with Rickettsia tsutsugamushi. Infect Immun 64:3937–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kee SH, Cho KA, Kim MK, Lim BU, Chang WH, Kang JS. 1999. Disassembly of focal adhesions during apoptosis of endothelial cell line ECV304 infected with Orientia tsutsugamushi. Microb Pathog 27:265–271. doi: 10.1006/mpat.1999.0304. [DOI] [PubMed] [Google Scholar]
- 82.Kim MK, Kee SH, Cho KA, Chung MH, Lim BU, Chang WH, Kang JS. 1999. Apoptosis of endothelial cell line ECV304 persistently infected with Orientia tsutsugamushi. Microbiol Immunol 43:751–757. doi: 10.1111/j.1348-0421.1999.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 83.Tantibhedhyangkul W, Ben Amara A, Textoris J, Gorvel L, Ghigo E, Capo C, Mege JL. 2013. Orientia tsutsugamushi, the causative agent of scrub typhus, induces an inflammatory program in human macrophages. Microb Pathog 55:55–63. doi: 10.1016/j.micpath.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Tantibhedhyangkul W, Prachason T, Waywa D, El Filali A, Ghigo E, Thongnoppakhun W, Raoult D, Suputtamongkol Y, Capo C, Limwongse C, Mege JL. 2011. Orientia tsutsugamushi stimulates an original gene expression program in monocytes: relationship with gene expression in patients with scrub typhus. PLoS Negl Trop Dis 5:e1028. doi: 10.1371/journal.pntd.0001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deretic V. 2012. Autophagy: an emerging immunological paradigm. J Immunol 189:15–20. doi: 10.4049/jimmunol.1102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ko Y, Choi JH, Ha NY, Kim IS, Cho NH, Choi MS. 2013. Active escape of Orientia tsutsugamushi from cellular autophagy. Infect Immun 81:552–559. doi: 10.1128/IAI.00861-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rikihisa Y. 1984. Glycogen autophagosomes in polymorphonuclear leukocytes induced by rickettsiae. Anat Rec 208:319–327. doi: 10.1002/ar.1092080302. [DOI] [PubMed] [Google Scholar]
- 88.Deretic V. 2011. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol Rev 240:92–104. doi: 10.1111/j.1600-065X.2010.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ogata H, Renesto P, Audic S, Robert C, Blanc G, Fournier PE, Parinello H, Claverie JM, Raoult D. 2005. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol 3:e248. doi: 10.1371/journal.pbio.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ogata H, La Scola B, Audic S, Renesto P, Blanc G, Robert C, Fournier PE, Claverie JM, Raoult D. 2006. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genet 2:e76. doi: 10.1371/journal.pgen.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wallden K, Rivera-Calzada A, Waksman G. 2010. Type IV secretion systems: versatility and diversity in function. Cell Microbiol 12:1203–1212. doi: 10.1111/j.1462-5822.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christie PJ, Whitaker N, Gonzalez-Rivera C. 2014. Mechanism and structure of the bacterial type IV secretion systems. Biochim Biophys Acta 1843:1578–1591. doi: 10.1016/j.bbamcr.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cerveny L, Straskova A, Dankova V, Hartlova A, Ceckova M, Staud F, Stulik J. 2013. Tetratricopeptide repeat motifs in the world of bacterial pathogens: role in virulence mechanisms. Infect Immun 81:629–635. doi: 10.1128/IAI.01035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bang S, Min CK, Ha NY, Choi MS, Kim IS, Kim YS, Cho NH. 2016. Inhibition of eukaryotic translation by tetratricopeptide-repeat proteins of Orientia tsutsugamushi. J Microbiol 54:136–144. doi: 10.1007/s12275-016-5599-5. [DOI] [PubMed] [Google Scholar]
- 95.Menard R, Sansonetti P, Parsot C. 1994. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J 13:5293–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watson AA, Alm RA, Mattick JS. 1996. Identification of a gene, pilF, required for type 4 fimbrial biogenesis and twitching motility in Pseudomonas aeruginosa. Gene 180:49–56. doi: 10.1016/S0378-1119(96)00403-9. [DOI] [PubMed] [Google Scholar]
- 97.Mavris M, Page AL, Tournebize R, Demers B, Sansonetti P, Parsot C. 2002. Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol Microbiol 43:1543–1553. doi: 10.1046/j.1365-2958.2002.02836.x. [DOI] [PubMed] [Google Scholar]
- 98.Walburger A, Koul A, Ferrari G, Nguyen L, Prescianotto-Baschong C, Huygen K, Klebl B, Thompson C, Bacher G, Pieters J. 2004. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science 304:1800–1804. doi: 10.1126/science.1099384. [DOI] [PubMed] [Google Scholar]
- 99.Broms JE, Forslund AL, Forsberg A, Francis MS. 2003. PcrH of Pseudomonas aeruginosa is essential for secretion and assembly of the type III translocon. J Infect Dis 188:1909–1921. doi: 10.1086/379898. [DOI] [PubMed] [Google Scholar]
- 100.Broms JE, Edqvist PJ, Forsberg A, Francis MS. 2006. Tetratricopeptide repeats are essential for PcrH chaperone function in Pseudomonas aeruginosa type III secretion. FEMS Microbiol Lett 256:57–66. doi: 10.1111/j.1574-6968.2005.00099.x. [DOI] [PubMed] [Google Scholar]
- 101.Edqvist PJ, Aili M, Liu J, Francis MS. 2007. Minimal YopB and YopD translocator secretion by Yersinia is sufficient for Yop-effector delivery into target cells. Microbes Infect 9:224–233. doi: 10.1016/j.micinf.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 102.Qin A, Mann BJ. 2006. Identification of transposon insertion mutants of Francisella tularensis tularensis strain Schu S4 deficient in intracellular replication in the hepatic cell line HepG2. BMC Microbiol 6:69. doi: 10.1186/1471-2180-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dieppedale J, Sobral D, Dupuis M, Dubail I, Klimentova J, Stulik J, Postic G, Frapy E, Meibom KL, Barel M, Charbit A. 2011. Identification of a putative chaperone involved in stress resistance and virulence in Francisella tularensis. Infect Immun 79:1428–1439. doi: 10.1128/IAI.01012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rocak S, Linder P. 2004. DEAD-box proteins: the driving forces behind RNA metabolism. Nat Rev Mol Cell Biol 5:232–241. doi: 10.1038/nrm1335. [DOI] [PubMed] [Google Scholar]
- 105.Cordin O, Banroques J, Tanner NK, Linder P. 2006. The DEAD-box protein family of RNA helicases. Gene 367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 106.Rikihisa Y, Lin M. 2010. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Curr Opin Microbiol 13:59–66. doi: 10.1016/j.mib.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Khodor S, Price CT, Habyarimana F, Kalia A, Abu Kwaik Y. 2008. A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol Microbiol 70:908–923. doi: 10.1111/j.1365-2958.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Voth DE. 2011. ThANKs for the repeat: intracellular pathogens exploit a common eukaryotic domain. Cell Logist 1:128–132. doi: 10.4161/cl.1.4.18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Min CK, Kwon YJ, Ha NY, Cho BA, Kim JM, Kwon EK, Kim YS, Choi MS, Kim IS, Cho NH. 2014. Multiple Orientia tsutsugamushi ankyrin repeat proteins interact with SCF1 ubiquitin ligase complex and eukaryotic elongation factor 1 alpha. PLoS One 9:e105652. doi: 10.1371/journal.pone.0105652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.VieBrock L, Evans SM, Beyer AR, Larson CL, Beare PA, Ge H, Singh S, Rodino KG, Heinzen RA, Richards AL, Carlyon JA. 2014. Orientia tsutsugamushi ankyrin repeat-containing protein family members are type 1 secretion system substrates that traffic to the host cell endoplasmic reticulum. Front Cell Infect Microbiol 4:186. doi: 10.3389/fcimb.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beyer AR, VieBrock L, Rodino KG, Miller DP, Tegels BK, Marconi RT, Carlyon JA. 2015. Orientia tsutsugamushi strain Ikeda ankyrin repeat-containing proteins recruit SCF1 ubiquitin ligase machinery via poxvirus-like F-box motifs. J Bacteriol 197:3097–3109. doi: 10.1128/JB.00276-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beyer AR, Rodino KG, VieBrock L, Green RS, Tegels BK, Oliver LD Jr, Marconi RT, Carlyon JA. 3 February 2017. Orientia tsutsugamushi Ank9 is a multifunctional effector that utilizes a novel GRIP-like Golgi localization domain for Golgi-to-endoplasmic reticulum trafficking and interacts with host COPB2. Cell Microbiol doi: 10.1111/cmi.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abbas W, Kumar A, Herbein G. 2015. The eEF1A proteins: at the crossroads of oncogenesis, apoptosis, and viral infections. Front Oncol 5:75. doi: 10.3389/fonc.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mateyak MK, Kinzy TG. 2010. eEF1A: thinking outside the ribosome. J Biol Chem 285:21209–21213. doi: 10.1074/jbc.R110.113795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palucka K, Banchereau J. 2002. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol 14:420–431. doi: 10.1016/S0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 116.Reddick LE, Alto NM. 2014. Bacteria fighting back: how pathogens target and subvert the host innate immune system. Mol Cell 54:321–328. doi: 10.1016/j.molcel.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chu H, Park SM, Cheon IS, Park MY, Shim BS, Gil BC, Jeung WH, Hwang KJ, Song KD, Hong KJ, Song M, Jeong HJ, Han SH, Yun CH. 2013. Orientia tsutsugamushi infection induces CD4+ T cell activation via human dendritic cell activity. J Microbiol Biotechnol 23:1159–1166. doi: 10.4014/jmb.1303.03019. [DOI] [PubMed] [Google Scholar]
- 118.Gorvel L, Textoris J, Banchereau R, Ben Amara A, Tantibhedhyangkul W, von Bargen K, Ka MB, Capo C, Ghigo E, Gorvel JP, Mege JL. 2014. Intracellular bacteria interfere with dendritic cell functions: role of the type I interferon pathway. PLoS One 9:e99420. doi: 10.1371/journal.pone.0099420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bar-Haim E, Gat O, Markel G, Cohen H, Shafferman A, Velan B. 2008. Interrelationship between dendritic cell trafficking and Francisella tularensis dissemination following airway infection. PLoS Pathog 4:e1000211. doi: 10.1371/journal.ppat.1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Edelson BT. 2012. Dendritic cells in Listeria monocytogenes infection. Adv Immunol 113:33–49. doi: 10.1016/B978-0-12-394590-7.00006-3. [DOI] [PubMed] [Google Scholar]
- 121.Tam MA, Wick MJ. 2004. Dendritic cells and immunity to Listeria: TipDCs are a new recruit. Trends Immunol 25:335–339. doi: 10.1016/j.it.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 122.Randolph GJ, Angeli V, Swartz MA. 2005. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 123.Martín-Fontecha A, Lanzavecchia A, Sallusto F. 2009. Dendritic cell migration to peripheral lymph nodes, p 31–49. In Lombardi G, Riffo-Vasquez Y (ed), Dendritic cells. Springer, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 124.Forster R, Davalos-Misslitz AC, Rot A. 2008. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 125.Sunyakumthorn P, Paris DH, Chan TC, Jones M, Luce-Fedrow A, Chattopadhyay S, Jiang J, Anantatat T, Turner GD, Day NP, Richards AL. 2013. An intradermal inoculation model of scrub typhus in Swiss CD-1 mice demonstrates more rapid dissemination of virulent strains of Orientia tsutsugamushi. PLoS One 8:e54570. doi: 10.1371/journal.pone.0054570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lurchachaiwong W, Monkanna T, Leepitakrat S, Ponlawat A, Sattabongkot J, Schuster AL, McCardle PW, Richards AL. 2012. Variable clinical responses of a scrub typhus outbred mouse model to feeding by Orientia tsutsugamushi infected mites. Exp Appl Acarol 58:23–34. doi: 10.1007/s10493-012-9563-8. [DOI] [PubMed] [Google Scholar]
- 127.Shirai A, Saunders JP, Dohany AL, Huxsoll DL, Groves MG. 1982. Transmission of scrub typhus to human volunteers by laboratory-reared chiggers. Jpn J Med Sci Biol 35:9–16. doi: 10.7883/yoken1952.35.9. [DOI] [PubMed] [Google Scholar]
- 128.Cho NH, Seong SY, Huh MS, Han TH, Koh YS, Choi MS, Kim IS. 2000. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect Immun 68:594–602. doi: 10.1128/IAI.68.2.594-602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koo JE, Yun JH, Lee KH, Hyun JW, Kang HK, Jang WJ, Park KH, Koh YS. 2009. Activation of mitogen-activated protein kinases is involved in the induction of interferon beta gene in macrophages infected with Orientia tsutsugamushi. Microbiol Immunol 53:123–129. doi: 10.1111/j.1348-0421.2008.00098.x. [DOI] [PubMed] [Google Scholar]
- 130.Yun JH, Koh YS, Lee KH, Hyun JW, Choi YJ, Jang WJ, Park KH, Cho NH, Seong SY, Choi MS, Kim IS. 2005. Chemokine and cytokine production in susceptible C3H/HeN mice and resistant BALB/c mice during Orientia tsutsugamushi infection. Microbiol Immunol 49:551–557. doi: 10.1111/j.1348-0421.2005.tb03761.x. [DOI] [PubMed] [Google Scholar]
- 131.Yun JH, Koo JE, Koh YS. 2009. Mitogen-activated protein kinases are involved in tumor necrosis factor alpha production in macrophages infected with Orientia tsutsugamushi. Microbiol Immunol 53:349–355. doi: 10.1111/j.1348-0421.2009.00127.x. [DOI] [PubMed] [Google Scholar]
- 132.Jerrells TR, Osterman JV. 1982. Role of macrophages in innate and acquired host resistance to experimental scrub typhus infection of inbred mice. Infect Immun 37:1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nacy CA, Osterman JV. 1979. Host defenses in experimental scrub typhus: role of normal and activated macrophages. Infect Immun 26:744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Otterdal K, Janardhanan J, Astrup E, Ueland T, Prakash JA, Lekva T, Abraham OC, Thomas K, Damas JK, Mathews P, Mathai D, Aukrust P, Varghese GM. 2014. Increased endothelial and macrophage markers are associated with disease severity and mortality in scrub typhus. J Infect 69:462–469. doi: 10.1016/j.jinf.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 135.Kim MJ, Kim MK, Kang JS. 2006. Orientia tsutsugamushi inhibits tumor necrosis factor alpha production by inducing interleukin 10 secretion in murine macrophages. Microb Pathog 40:1–7. doi: 10.1016/j.micpath.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 136.Penaloza HF, Schultz BM, Nieto PA, Salazar GA, Suazo I, Gonzalez PA, Riedel CA, Alvarez-Lobos MM, Kalergis AM, Bueno SM. 2016. Opposing roles of IL-10 in acute bacterial infection. Cytokine Growth Factor Rev 32:17–30. doi: 10.1016/j.cytogfr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 137.Redpath S, Ghazal P, Gascoigne NR. 2001. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol 9:86–92. doi: 10.1016/S0966-842X(00)01919-3. [DOI] [PubMed] [Google Scholar]
- 138.Tufariello JM, Chan J, Flynn JL. 2003. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis 3:578–590. doi: 10.1016/S1473-3099(03)00741-2. [DOI] [PubMed] [Google Scholar]
- 139.Meghari S, Bechah Y, Capo C, Lepidi H, Raoult D, Murray PJ, Mege JL. 2008. Persistent Coxiella burnetii infection in mice overexpressing IL-10: an efficient model for chronic Q fever pathogenesis. PLoS Pathog 4:e23. doi: 10.1371/journal.ppat.0040023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sing A, Roggenkamp A, Geiger AM, Heesemann J. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J Immunol 168:1315–1321. doi: 10.4049/jimmunol.168.3.1315. [DOI] [PubMed] [Google Scholar]
- 141.Tsai MH, Chang CH, Tsai RK, Hong YR, Chuang TH, Fan KT, Peng CW, Wu CY, Hsu WL, Wang LS, Chen LK, Yu HS. 2016. Cross-regulation of proinflammatory cytokines by interleukin-10 and miR-155 in Orientia tsutsugamushi-infected human macrophages prevents cytokine storm. J Investig Dermatol 136:1398–1407. doi: 10.1016/j.jid.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 142.Cho KA, Jun YH, Suh JW, Kang JS, Choi HJ, Woo SY. 2010. Orientia tsutsugamushi induced endothelial cell activation via the NOD1-IL-32 pathway. Microb Pathog 49:95–104. doi: 10.1016/j.micpath.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 143.Koo JE, Hong HJ, Dearth A, Kobayashi KS, Koh YS. 2012. Intracellular invasion of Orientia tsutsugamushi activates inflammasome in ASC-dependent manner. PLoS One 7:e39042. doi: 10.1371/journal.pone.0039042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chung DR, Lee YS, Lee SS. 2008. Kinetics of inflammatory cytokines in patients with scrub typhus receiving doxycycline treatment. J Infect 56:44–50. doi: 10.1016/j.jinf.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 145.Iwasaki H, Mizoguchi J, Takada N, Tai K, Ikegaya S, Ueda T. 2010. Correlation between the concentrations of tumor necrosis factor-alpha and the severity of disease in patients infected with Orientia tsutsugamushi. Int J Infect Dis 14:e328–e333. doi: 10.1016/j.ijid.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 146.Kramme S, An LV, Khoa ND, Trin LV, Tannich E, Rybniker J, Fleischer B, Drosten C, Panning M. 2009. Orientia tsutsugamushi bacteremia and cytokine levels in Vietnamese scrub typhus patients. J Clin Microbiol 47:586–589. doi: 10.1128/JCM.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martinon F, Burns K, Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 148.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. 2010. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog 6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. 2010. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Latz E, Xiao TS, Stutz A. 2013. Activation and regulation of the inflammasomes. Nat Rev Immunol 13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hanson B. 1991. Comparative susceptibility to mouse interferons of Rickettsia tsutsugamushi strains with different virulence in mice and of Rickettsia rickettsii. Infect Immun 59:4134–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abdullah Z, Knolle PA. 2014. Scaling of immune responses against intracellular bacterial infection. EMBO J 33:2283–2294. doi: 10.15252/embj.201489055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Monroe KM, McWhirter SM, Vance RE. 2010. Induction of type I interferons by bacteria. Cell Microbiol 12:881–890. doi: 10.1111/j.1462-5822.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stifter SA, Feng CG. 2015. Interfering with immunity: detrimental role of type I IFNs during infection. J Immunol 194:2455–2465. doi: 10.4049/jimmunol.1402794. [DOI] [PubMed] [Google Scholar]
- 155.Page AV, Liles WC. 2013. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence 4:507–516. doi: 10.4161/viru.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chousterman BG, Swirski FK, Weber GF. 29 May 2017. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 157.Page AV, Tarr PI, Watkins SL, Rajwans N, Petruzziello-Pellegrini TN, Marsden PA, Kain KC, Liles WC. 2013. Dysregulation of angiopoietin 1 and 2 in Escherichia coli O157:H7 infection and the hemolytic-uremic syndrome. J Infect Dis 208:929–933. doi: 10.1093/infdis/jit268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, Krudsood S, Looareesuwan S, John CC, Liles WC, Kain KC. 2009. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 4:e4912. doi: 10.1371/journal.pone.0004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kumpers P, Lukasz A, David S, Horn R, Hafer C, Faulhaber-Walter R, Fliser D, Haller H, Kielstein JT. 2008. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care 12:R147. doi: 10.1186/cc7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Page AV, Kotb M, McGeer A, Low DE, Kain KC, Liles WC. 2011. Systemic dysregulation of angiopoietin-1/2 in streptococcal toxic shock syndrome. Clin Infect Dis 52:e157–e161. doi: 10.1093/cid/cir125. [DOI] [PubMed] [Google Scholar]
- 161.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 162.Liew FY, Pitman NI, McInnes IB. 2010. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 163.Chen WY, Li LC, Yang JL. 2017. Emerging roles of IL-33/ST2 axis in renal diseases. Int J Mol Sci 18:E783. doi: 10.3390/ijms18040783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Khawar MB, Abbasi MH, Sheikh N. 2016. IL-32: a novel pluripotent inflammatory interleukin, towards gastric inflammation, gastric cancer, and chronic rhino sinusitis. Mediators Inflamm 2016:8413768. doi: 10.1155/2016/8413768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ribeiro-Dias F, Saar Gomes R, de Lima Silva LL, Dos Santos JC, Joosten LA. 2017. Interleukin 32: a novel player in the control of infectious diseases. J Leukoc Biol 101:39–52. doi: 10.1189/jlb.4RU0416-175RR. [DOI] [PubMed] [Google Scholar]
- 166.Lanzavecchia A, Fruhwirth A, Perez L, Corti D. 2016. Antibody-guided vaccine design: identification of protective epitopes. Curr Opin Immunol 41:62–67. doi: 10.1016/j.coi.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 167.Oyarzun P, Kobe B. 2016. Recombinant and epitope-based vaccines on the road to the market and implications for vaccine design and production. Hum Vaccin Immunother 12:763–767. doi: 10.1080/21645515.2015.1094595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Coffman RL, Sher A, Seder RA. 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vasou A, Sultanoglu N, Goodbourn S, Randall RE, Kostrikis LG. 2017. Targeting pattern recognition receptors (PRR) for vaccine adjuvantation: from synthetic PRR agonists to the potential of defective interfering particles of viruses. Viruses 9:E186. doi: 10.3390/v9070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kumar H, Kawai T, Akira S. 2011. Pathogen recognition by the innate immune system. Int Rev Immunol 30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 171.Kawai T, Akira S. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 172.Min CK, Yang JS, Kim S, Choi MS, Kim IS, Cho NH. 2008. Genome-based construction of the metabolic pathways of Orientia tsutsugamushi and comparative analysis within the Rickettsiales order. Comp Funct Genomics 2008:623145. doi: 10.1155/2008/623145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Karlström Å, Heston SM, Boyd KL, Tuomanen EI, McCullers JA. 2011. Toll-like receptor 2 mediates fatal immunopathology in mice during treatment of secondary pneumococcal pneumonia following influenza. J Infect Dis 204:1358–1366. doi: 10.1093/infdis/jir522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wiersinga WJ, Wieland CW, Dessing MC, Chantratita N, Cheng AC, Limmathurotsakul D, Chierakul W, Leendertse M, Florquin S, de Vos AF, White N, Dondorp AM, Day NP, Peacock SJ, van der Poll T. 2007. Toll-like receptor 2 impairs host defense in Gram-negative sepsis caused by Burkholderia pseudomallei (melioidosis). PLoS Med 4:e248. doi: 10.1371/journal.pmed.0040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Reantragoon R, Boonpattanaporn N, Corbett AJ, McCluskey J. 2016. Mucosal-associated invariant T cells in clinical diseases. Asian Pac J Allergy Immunol 34:3–10. [PubMed] [Google Scholar]
- 176.Ussher JE, Klenerman P, Willberg CB. 2014. Mucosal-associated invariant T-cells: new players in anti-bacterial immunity. Front Immunol 5:450. doi: 10.3389/fimmu.2014.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moreira ML, Tsuji M, Corbett AJ, Araujo MSS, Teixeira-Carvalho A, Martins-Filho OA, Peruhype-Magalhaes V, Coelho-Dos-Reis JG. 2017. MAIT-cells: a tailor-made mate in the ancient battle against infectious diseases? Immunol Lett 187:53–60. doi: 10.1016/j.imlet.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 178.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua W-J, Yu YYL, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM. 2010. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jiang J, Wang X, An H, Yang B, Cao Z, Liu Y, Su J, Zhai F, Wang R, Zhang G, Cheng X. 2014. Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. Am J Respir Crit Care Med 190:329–339. doi: 10.1164/rccm.201401-0106OC. [DOI] [PubMed] [Google Scholar]
- 180.Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. 2011. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol 32:212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 181.Georgel P, Radosavljevic M, Macquin C, Bahram S. 2011. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol 48:769–775. doi: 10.1016/j.molimm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 182.Loh L, Wang Z, Sant S, Koutsakos M, Jegaskanda S, Corbett AJ, Liu L, Fairlie DP, Crowe J, Rossjohn J, Xu J, Doherty PC, McCluskey J, Kedzierska K. 2016. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc Natl Acad Sci U S A 113:10133–10138. doi: 10.1073/pnas.1610750113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kang SJ, Jin HM, Won EJ, Cho YN, Jung HJ, Kwon YS, Kee HJ, Ju JK, Kim JC, Kim UJ, Jang HC, Jung SI, Kee SJ, Park YW. 2016. Activation, impaired tumor necrosis factor-alpha production, and deficiency of circulating mucosal-associated invariant T cells in patients with scrub typhus. PLoS Negl Trop Dis 10:e0004832. doi: 10.1371/journal.pntd.0004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rahimpour A, Koay HF, Enders A, Clanchy R, Eckle SBG, Meehan B, Chen Z, Whittle B, Liu L, Fairlie DP, Goodnow CC, McCluskey J, Rossjohn J, Uldrich AP, Pellicci DG, Godfrey DI. 2015. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med 212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sharma S, Thomas PG. 2014. The two faces of heterologous immunity: protection or immunopathology. J Leukoc Biol 95:405–416. doi: 10.1189/jlb.0713386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smadel JE, Ley HL Jr, Diercks FH, Traub R. 1950. Immunity in scrub typhus: resistance to induced reinfection. AMA Arch Pathol 50:847–861. [PubMed] [Google Scholar]
- 187.Smadel JE, Ley HL Jr, Diercks FH, Paterson PY, Wisseman CL Jr, Traub R. 1952. Immunization against scrub typhus: duration of immunity in volunteers following combined living vaccine and chemoprophylaxis. Am J Trop Med Hyg 1:87–99. doi: 10.4269/ajtmh.1952.1.87. [DOI] [PubMed] [Google Scholar]
- 188.MacMillan JG, Rice RM, Jerrells TR. 1985. Development of antigen-specific cell-mediated immune responses after infection of cynomolgus monkeys (Macaca fascicularis) with Rickettsia tsutsugamushi. J Infect Dis 152:739–749. doi: 10.1093/infdis/152.4.739. [DOI] [PubMed] [Google Scholar]
- 189.Bailey CA, Diercks FH, Proffitt JE. 1948. Preparation of a serological antigen and a vaccine for experimental tsutsugamushi disease. J Immunol 60:431–441. [PubMed] [Google Scholar]
- 190.Shishido A, Ohtawara M, Hikita M, Kitaoka M. 1959. The nature of immunity against scrub typhus in mice. II. The cross-protection test with mice for identification and differentiation of several strains of Rickettsia orientalis newly isolated in Japan. Jpn J Med Sci Biol 12:391–404. doi: 10.7883/yoken1952.12.391. [DOI] [PubMed] [Google Scholar]
- 191.Chen H-W, Zhang Z, Huber E, Mutumanje E, Chao C-C, Ching W-M. 2011. Kinetics and magnitude of antibody responses against the conserved 47-kilodalton antigen and the variable 56-kilodalton antigen in scrub typhus patients. Clin Vaccine Immunol 18:1021–1027. doi: 10.1128/CVI.00017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hanson B. 1985. Identification and partial characterization of Rickettsia tsutsugamushi major protein immunogens. Infect Immun 50:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Oaks EV, Rice RM, Kelly DJ, Stover CK. 1989. Antigenic and genetic relatedness of eight Rickettsia tsutsugamushi antigens. Infect Immun 57:3116–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ohashi N, Tamura A, Suto T. 1988. Immunoblotting analysis of anti-rickettsial antibodies produced in patients of Tsutsugamushi disease. Microbiol Immunol 32:1085–1092. doi: 10.1111/j.1348-0421.1988.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 195.Tamura A, Ohashi N, Urakami H, Takahashi K, Oyanagi M. 1985. Analysis of polypeptide composition and antigenic components of Rickettsia tsutsugamushi by polyacrylamide gel electrophoresis and immunoblotting. Infect Immun 48:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Oaks SC Jr, Hetrick FM, Osterman JV. 1980. A plaque reduction assay for studying antigenic relationships among strains of Rickettsia tsutsugamushi. Am J Trop Med Hyg 29:998–1006. doi: 10.4269/ajtmh.1980.29.998. [DOI] [PubMed] [Google Scholar]
- 197.Jiang J, Paris DH, Blacksell SD, Aukkanit N, Newton PN, Phetsouvanh R, Izzard L, Stenos J, Graves SR, Day NPJ, Richards AL. 2013. Diversity of the 47-kD HtrA nucleic acid and translated amino acid sequences from 17 recent human isolates of Orientia. Vector Borne Zoonotic Dis 13:367–375. doi: 10.1089/vbz.2012.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ohashi N, Fukuhara M, Shimada M, Tamura A. 1995. Phylogenetic position of Rickettsia tsutsugamushi and the relationship among its antigenic variants by analyses of 16S rRNA gene sequences. FEMS Microbiol Lett 125:299–304. doi: 10.1111/j.1574-6968.1995.tb07372.x. [DOI] [PubMed] [Google Scholar]
- 199.Ohashi N, Koyama Y, Urakami H, Fukuhara M, Tamura A, Kawamori F, Yamamoto S, Kasuya S, Yoshimura K. 1996. Demonstration of antigenic and genotypic variation in Orientia tsutsugamushi which were isolated in Japan, and their classification into type and subtype. Microbiol Immunol 40:627–638. doi: 10.1111/j.1348-0421.1996.tb01120.x. [DOI] [PubMed] [Google Scholar]
- 200.Eisemann CS, Osterman JV. 1981. Antigens of scrub typhus rickettsiae: separation by polyacrylamide gel electrophoresis and identification by enzyme-linked immunosorbent assay. Infect Immun 32:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Park CS, Kim IC, Lee JB, Choi MS, Choi SB, Chang WH, Kim I. 1993. Analysis of antigenic characteristics of Rickettsia tsutsugamushi Boryong strain and antigenic heterogeneity of Rickettsia tsutsugamushi using monoclonal antibodies. J Korean Med Sci 8:319–324. doi: 10.3346/jkms.1993.8.5.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang M, Zhao ZT, Yang HL, Zhang AH, Xu XQ, Meng XP, Zhang HY, Wang XJ, Li Z, Ding SJ, Yang L, Zhang LY. 2013. Molecular epidemiology of Orientia tsutsugamushi in chiggers and ticks from domestic rodents in Shandong, northern China. Parasit Vectors 6:312. doi: 10.1186/1756-3305-6-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lin CC, Chou CH, Lin TC, Yang MC, Cho CL, Chang CH, Yu HS, Lai CH, Chen LK, Hong YR. 2012. Molecular characterization of three major outer membrane proteins, TSA56, TSA47 and TSA22, in Orientia tsutsugamushi. Int J Mol Med 30:75–84. doi: 10.3892/ijmm.2012.1154. [DOI] [PubMed] [Google Scholar]
- 204.Kelly DJ, Fuerst PA, Ching W-M, Richards AL. 2009. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis 48:S203–S230. doi: 10.1086/596576. [DOI] [PubMed] [Google Scholar]
- 205.Ruang-Areerate T, Jeamwattanalert P, Rodkvamtook W, Richards AL, Sunyakumthorn P, Gaywee J. 2011. Genotype diversity and distribution of Orientia tsutsugamushi causing scrub typhus in Thailand. J Clin Microbiol 49:2584–2589. doi: 10.1128/JCM.00355-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Phetsouvanh R, Sonthayanon P, Pukrittayakamee S, Paris DH, Newton PN, Feil EJ, Day NPJ. 2015. The diversity and geographical structure of Orientia tsutsugamushi strains from scrub typhus patients in Laos. PLoS Negl Trop Dis 9:e0004024. doi: 10.1371/journal.pntd.0004024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Parola P, Blacksell SD, Phetsouvanh R, Phongmany S, Rolain J-M, Day NPJ, Newton PM, Raoult D. 2008. Genotyping of Orientia tsutsugamushi from humans with scrub typhus, Laos. Emerg Infect Dis 14:1483–1485. doi: 10.3201/eid1409.071259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.James SL, Blacksell SD, Nawtaisong P, Tanganuchitcharnchai A, Smith DJ, Day NP, Paris DH. 2016. Antigenic relationships among human pathogenic Orientia tsutsugamushi isolates from Thailand. PLoS Negl Trop Dis 10:e0004723. doi: 10.1371/journal.pntd.0004723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Varghese GM, Janardhanan J, Trowbridge P, Peter JV, Prakash JA, Sathyendra S, Thomas K, David TS, Kavitha ML, Abraham OC, Mathai D. 2013. Scrub typhus in South India: clinical and laboratory manifestations, genetic variability, and outcome. Int J Infect Dis 17:e981–e987. doi: 10.1016/j.ijid.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 210.Blacksell SD, Luksameetanasan R, Kalambaheti T, Aukkanit N, Paris DH, McGready R, Nosten F, Peacock SJ, Day NP. 2008. Genetic typing of the 56-kDa type-specific antigen gene of contemporary Orientia tsutsugamushi isolates causing human scrub typhus at two sites in north-eastern and western Thailand. FEMS Immunol Med Microbiol 52:335–342. doi: 10.1111/j.1574-695X.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- 211.Takhampunya R, Tippayachai B, Promsathaporn S, Leepitakrat S, Monkanna T, Schuster AL, Melendrez MC, Paris DH, Richards AL, Richardson JH. 2014. Characterization based on the 56-Kda type-specific antigen gene of Orientia tsutsugamushi genotypes isolated from Leptotrombidium mites and the rodent host post-infection. Am J Trop Med Hyg 90:139–146. doi: 10.4269/ajtmh.13-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Premaratna R, Blanton LS, Samaraweera DN, de Silva GNN, Chandrasena N, Walker DH, de Silva HJ. 2017. Genotypic characterization of Orientia tsutsugamushi from patients in two geographical locations in Sri Lanka. BMC Infect Dis 17:67. doi: 10.1186/s12879-016-2165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang HH, Huang IT, Lin CH, Chen TY, Chen LK. 2012. New genotypes of Orientia tsutsugamushi isolated from humans in Eastern Taiwan. PLoS One 7:e46997. doi: 10.1371/journal.pone.0046997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kim DM, Lee YM, Back JH, Yang TY, Lee JH, Song HJ, Shim SK, Hwang KJ, Park MY. 2010. A serosurvey of Orientia tsutsugamushi from patients with scrub typhus. Clin Microbiol Infect 16:447–451. doi: 10.1111/j.1469-0691.2009.02865.x. [DOI] [PubMed] [Google Scholar]
- 215.Saunders JP, Brown GW, Shirai A, Huxsoll DL. 1980. The longevity of antibody to Rickettsia tsutsugamushi in patients with confirmed scrub typhus. Trans R Soc Trop Med Hyg 74:253–257. doi: 10.1016/0035-9203(80)90254-0. [DOI] [PubMed] [Google Scholar]
- 216.Bengtson IA. 1945. Apparent serological heterogeneity among strains of Tsutsugamushi disease (scrub typhus). Public Health Rep 60:1483–1488. doi: 10.2307/4585496. [DOI] [PubMed] [Google Scholar]
- 217.Topping NH. 1945. Tsutsugamushi disease (scrub typhus); the effects of an immune rabbit serum in experimentally infected mice. Public Health Rep 60:1215–1220. doi: 10.2307/4585418. [DOI] [PubMed] [Google Scholar]
- 218.Robinson DM, Huxsoll DL. 1975. Protection against scrub typhus infection engendered by the passive transfer of immune sera. Southeast Asian J Trop Med Public Health 6:477–482. [PubMed] [Google Scholar]
- 219.Bell EJ, Bennett BL, Whitman L. 1946. Antigenic differences between strains of scrub typhus as demonstrated by cross-neutralization tests. Proc Soc Exp Biol Med 62:134–137. doi: 10.3181/00379727-62-15398. [DOI] [PubMed] [Google Scholar]
- 220.Kim IS, Seong SY, Woo SG, Choi MS, Chang WH. 1993. High-level expression of a 56-kilodalton protein gene (bor56) of Rickettsia tsutsugamushi Boryong and its application to enzyme-linked immunosorbent assays. J Clin Microbiol 31:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kim IS, Seong SY, Woo SG, Choi MS, Kang JS, Chang WH. 1993. Rapid diagnosis of scrub typhus by a passive hemagglutination assay using recombinant 56-kilodalton polypeptides. J Clin Microbiol 31:2057–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Seong SY, Park SG, Huh MS, Jang WJ, Kim HR, Han TH, Choi MS, Chang WH, Kim IS. 1997. Mapping of antigenic determinant regions of the Bor56 protein of Orientia tsutsugamushi. Infect Immun 65:5250–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Choi MS, Seong SY, Kang JS, Kim YW, Huh MS, Kim IS. 1999. Homotypic and heterotypic antibody responses to a 56-kilodalton protein of Orientia tsutsugamushi. Infect Immun 67:6194–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Seong SY, Huh MS, Jang WJ, Park SG, Kim JG, Woo SG, Choi MS, Kim IS, Chang WH. 1997. Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect Immun 65:1541–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Seong SY, Kim MK, Lee SM, Odgerel Z, Choi MS, Han TH, Kim IS, Kang JS, Lim BU. 2000. Neutralization epitopes on the antigenic domain II of the Orientia tsutsugamushi 56-kDa protein revealed by monoclonal antibodies. Vaccine 19:2–9. doi: 10.1016/S0264-410X(00)00167-5. [DOI] [PubMed] [Google Scholar]
- 226.Shirai A, Catanzaro PJ, Phillips SM, Osterman JV. 1976. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun 14:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Eisenberg GH Jr, Osterman JV. 1977. Experimental scrub typhus immunogens: gamma-irradiated and formalinized rickettsiae. Infect Immun 15:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Eisenberg GH Jr, Osterman JV, Stephenson EH. 1980. Gamma-irradiated scrub typhus immunogens: analysis for residual, replicating rickettsiae. Infect Immun 28:295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jerrells TR, Osterman JV. 1983. Development of specific and cross-reactive lymphocyte proliferative responses during chronic immunizing infections with Rickettsia tsutsugamushi. Infect Immun 40:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Palmer BA, Hetrick FM, Jerrells TR. 1984. Gamma interferon production in response to homologous and heterologous strain antigens in mice chronically infected with Rickettsia tsutsugamushi. Infect Immun 46:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hauptmann M, Kolbaum J, Lilla S, Wozniak D, Gharaibeh M, Fleischer B, Keller CA. 2016. Protective and pathogenic roles of CD8+ T lymphocytes in murine Orientia tsutsugamushi infection. PLoS Negl Trop Dis 10:e0004991. doi: 10.1371/journal.pntd.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.de Fost M, Chierakul W, Pimda K, Dondorp AM, White NJ, Van der Poll T. 2005. Activation of cytotoxic lymphocytes in patients with scrub typhus. Am J Trop Med Hyg 72:465–467. [PubMed] [Google Scholar]
- 233.Kobayashi Y, Kawamura S, Oyama T. 1985. Immunological studies of experimental tsutsugamushi disease in congenitally athymic (nude) mice. Am J Trop Med Hyg 34:568–577. doi: 10.4269/ajtmh.1985.34.568. [DOI] [PubMed] [Google Scholar]
- 234.Jerrells TR, Palmer BA, Osterman JV. 1983. Gamma-irradiated scrub typhus immunogens: development of cell-mediated immunity after vaccination of inbred mice. Infect Immun 39:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cho B-A, Ko Y, Kim Y-S, Kim S, Choi M-S, Kim I-S, Kim H-R, Cho N-H. 2012. Phenotypic characterization of peripheral T cells and their dynamics in scrub typhus patients. PLoS Negl Trop Dis 6:e1789. doi: 10.1371/journal.pntd.0001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kodama K, Kawamura S, Yasukawa M, Kobayashi Y. 1987. Establishment and characterization of a T-cell line specific for Rickettsia tsutsugamushi. Infect Immun 55:2490–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sumonwiriya M, Paris DH, Sunyakumthorn P, Anantatat T, Jenjaroen K, Chumseng S, Im-erbsin R, Tanganuchitcharnchai A, Jintaworn S, Blacksell SD, Chowdhury FR, Kronsteiner B, Teparrukkul P, Burke RL, Lombardini ED, Richards AL, Mason CJ, Jones JW, Day NPJ, Dunachie SJ. 2017. Strong interferon-gamma mediated cellular immunity to scrub typhus demonstrated using a novel whole cell antigen ELISpot assay in rhesus macaques and humans. PLoS Negl Trop Dis 11:e0005846. doi: 10.1371/journal.pntd.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Paris DH, Chattopadhyay S, Jiang J, Nawtaisong P, Lee JS, Tan E, Dela Cruz E, Burgos J, Abalos R, Blacksell SD, Lombardini E, Turner GD, Day NP, Richards AL. 2015. A nonhuman primate scrub typhus model: protective immune responses induced by pKarp47 DNA vaccination in cynomolgus macaques. J Immunol 194:1702–1716. doi: 10.4049/jimmunol.1402244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Walker DH, Dumler JS. 2015. The role of CD8 T lymphocytes in rickettsial infections. Semin Immunopathol 37:289–299. doi: 10.1007/s00281-015-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Garcia-Garcia JC, Rennoll-Bankert KE, Pelly S, Milstone AM, Dumler JS. 2009. Silencing of host cell CYBB gene expression by the nuclear effector AnkA of the intracellular pathogen Anaplasma phagocytophilum. Infect Immun 77:2385–2391. doi: 10.1128/IAI.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sinclair SH, Rennoll-Bankert KE, Dumler JS. 2014. Effector bottleneck: microbial reprogramming of parasitized host cell transcription by epigenetic remodeling of chromatin structure. Front Genet 5:274. doi: 10.3389/fgene.2014.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhu B, Nethery KA, Kuriakose JA, Wakeel A, Zhang X, McBride JW. 2009. Nuclear translocated Ehrlichia chaffeensis ankyrin protein interacts with a specific adenine-rich motif of host promoter and intronic Alu elements. Infect Immun 77:4243–4255. doi: 10.1128/IAI.00376-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhu B, Kuriakose JA, Luo T, Ballesteros E, Gupta S, Fofanov Y, McBride JW. 2011. Ehrlichia chaffeensis TRP120 binds a G+C-rich motif in host cell DNA and exhibits eukaryotic transcriptional activator function. Infect Immun 79:4370–4381. doi: 10.1128/IAI.05422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hanson BR, Tan M. 2016. Intra-ChIP: studying gene regulation in an intracellular pathogen. Curr Genet 62:547–551. doi: 10.1007/s00294-016-0580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Liao HM, Chao CC, Lei H, Li B, Tsai S, Hung GC, Ching WM, Lo SC. 2016. Genomic sequencing of Orientia tsutsugamushi strain Karp, an assembly comparable to the genome size of the strain Ikeda. Genome Announc 4:e00702-16. doi: 10.1128/genomeA.00702-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liao HM, Chao CC, Lei H, Li B, Tsai S, Hung GC, Ching WM, Lo SC. 2017. Intraspecies comparative genomics of three strains of Orientia tsutsugamushi with different antibiotic sensitivity. Genom Data 12:84–88. doi: 10.1016/j.gdata.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Atwal S, Giengkam S, VanNieuwenhze M, Salje J. 2016. Live imaging of the genetically intractable obligate intracellular bacteria Orientia tsutsugamushi using a panel of fluorescent dyes. J Microbiol Methods 130:169–176. doi: 10.1016/j.mimet.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shirai A, Huxsoll DL, Dohany AL, Montrey RD, Werner RM, Gan E. 1982. Characterization of Rickettsia tsutsugamushi strains in two species of naturally infected, laboratory-reared chiggers. Am J Trop Med Hyg 31:395–402. doi: 10.4269/ajtmh.1982.31.395. [DOI] [PubMed] [Google Scholar]
- 249.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. 2012. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, Shultz LD. 2017. Humanized mouse models of clinical disease. Annu Rev Pathol 12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gillespie RD, Mbow ML, Titus RG. 2000. The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite Immunol 22:319–331. doi: 10.1046/j.1365-3024.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- 252.McClure EE, Chavez ASO, Shaw DK, Carlyon JA, Ganta RR, Noh SM, Wood DO, Bavoil PM, Brayton KA, Martinez JJ, McBride JW, Valdivia RH, Munderloh UG, Pedra JHF. 19 June 2017. Engineering of obligate intracellular bacteria: progress, challenges and paradigms. Nat Rev Microbiol doi: 10.1038/nrmicro.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Beare PA, Sandoz KM, Omsland A, Rockey DD, Heinzen RA. 2011. Advances in genetic manipulation of obligate intracellular bacterial pathogens. Front Microbiol 2:97. doi: 10.3389/fmicb.2011.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]