Abstract
The picture of HDL cholesterol (HDL-C) as the “good” cholesterol has eroded. This is even more surprising because there exists strong evidence that HDL-C is associated with cardiovascular disease (CVD) in the general population as well as in patients with impairment of kidney function and/or progression of CKD. However, drugs that dramatically increase HDL-C have mostly failed to decrease CVD events. Furthermore, genetic studies took the same line, as genetic variants that have a pronounced influence on HDL-C concentrations did not show an association with cardiovascular risk. For many, this was not surprising, given that an HDL particle is highly complex and carries >80 proteins and several hundred lipid species. Simply measuring cholesterol might not reflect the variety of biologic effects of heterogeneous HDL particles. Therefore, functional studies and the involvement of HDL components in the reverse cholesterol transport, including the cholesterol efflux capacity, have become a further focus of study during recent years. As also observed for other aspects, CKD populations behave differently compared with non-CKD populations. Although clear disturbances have been observed for the “functionality” of HDL particles in patients with CKD, this did not necessarily translate into clear-cut associations with outcomes.
Keywords: lipids, cardiovascular disease, progression of chronic renal failure, reverse, cholesterol transport, high-density lipoprotein
This review focuses on HDL in the context of CKD. It considers the epidemiologic as well as the genetic evidence and discusses the heterogeneity of HDL particles, as well as their components and their involvement in cholesterol efflux and reverse cholesterol transport. It points to the discrepancies between different findings in non-CKD populations, which can often not be confirmed in CKD populations, a phenomenon also well known from interventional studies in fields of study beyond lipid metabolism.
Normally, HDL particles have potent anti-inflammatory, antioxidative, and antithrombotic properties caused by several components carrying these properties. These are enzymes, apolipoproteins, complements, and other components. As reviewed extensively in the past,1 certain conditions such as CKD with systemic oxidative stress and inflammation substantially reduce these capabilities of HDL particles and can transform them into the opposite pro-oxidant and proinflammatory direction.2 This has consequences for the interrelated pathways and, for example, the ability of HDL to prevent oxidation of LDL,3 reduced monocyte chemotactic activity,4 or disturbances in the reverse cholesterol transport.
Furthermore, it is emphasized that some of the inconsistencies between studies in CKD populations in the literature might be caused by the heterogeneity of populations. HDL-C concentrations, composition of HDL particles, disturbances in the functionality, and especially the reverse cholesterol transport might be different between various stages of kidney impairment, especially between patients with and without nephrotic syndrome, as well as between patients with ESRD treated with hemodialysis, peritoneal dialysis, or kidney transplantation.
A Brief Introduction to Lipoprotein Pathways
Lipids such as cholesterol and triglycerides are not soluble in water and are therefore transported in blood and other body fluids together with proteins assembled to lipoproteins. The number of these proteins is quite large and >80 in the case of HDL particles.5 They contribute not only to the structure of lipoproteins but also to the metabolic control by activation or inhibition of enzymes, and interaction with lipoprotein receptors.
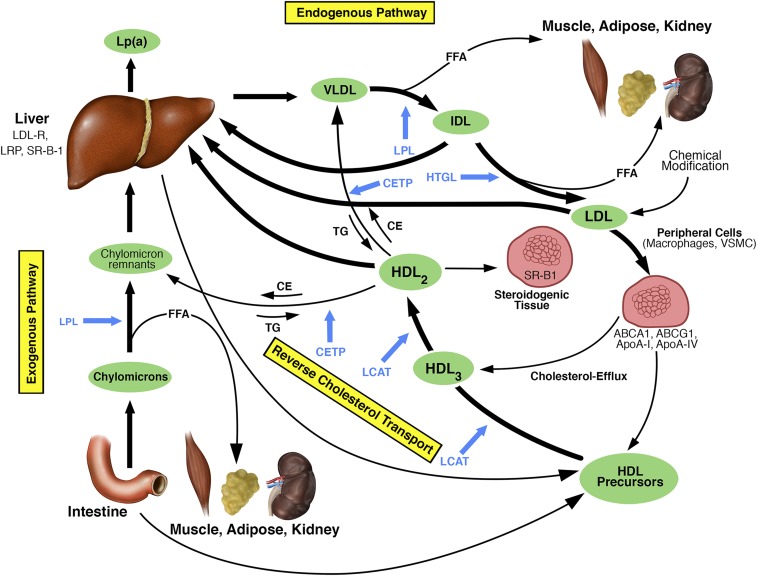
Figure 1 illustrates the two main lipoprotein pathways. The exogenous pathway transports dietary lipids from the intestine as triglyceride-rich chylomicrons. Chylomicrons are catabolized by lipoprotein lipase to chylomicron remnants, which are finally taken up by the liver. This process generates free fatty acids, which are absorbed mainly by liver, muscle, and adipose tissue for either energy production or storage. Other organs such as the kidney can also take them up. A cellular overload with free fatty acids results in an accumulation of toxic derivatives, promoting cellular dysfunction and damage, a metabolic disorder called lipotoxicity.6
Figure 1.
Schematic illustration of the major normal lipoprotein metabolic pathways. Blue arrows refer to points of action of the respective enzymes in blue. ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; ApoA-I, apolipoprotein A-I; ApoA-IV, apolipoprotein A-IV; CE, cholesterol ester; CETP, cholesteryl ester transfer protein; FFA, free fatty acid; HTGL, hepatic triglyceride lipase; IDL, intermediate-density lipoprotein; LCAT, lecithin–cholesterol acyltransferase; LDL-R, LDL receptor; LPL, lipoprotein lipase; LRP, LDL-R–related protein; SR-B1, scavenger receptor B1; TG, triglyceride; VLDL, very low-density lipoprotein; VSMC, vascular smooth muscle cells. Reprinted and adapted from reference 7, with permission.
The assembly and secretion of triglyceride-rich VLDL particles characterize the endogenous pathway. They transport triglycerides from the liver to peripheral tissues and are hydrolyzed by the lipoprotein lipase to intermediate-density lipoproteins, which either can be taken up by the liver or can be further hydrolyzed to LDL particles. This conversion results in a depletion of triglycerides and a relative increase in cholesterol content. LDL transports cholesterol primarily to hepatocytes but also to peripheral tissues. During circulation, LDL can become chemically modified, oxidized, and taken up by macrophages and vascular smooth muscle cells. Macrophages overloaded with cholesteryl esters can be transformed into foam cells, a crucial step in atherosclerosis development.
HDL is a key player in the reverse cholesterol transport, shuttling cholesterol from peripheral cells to the liver and to steroidogenic organs for steroid hormone production. This important step relieves peripheral cells and especially macrophages from cholesterol burden. HDL particles are characterized by a very high protein content (roughly 45%), cholesterol (25%), phospholipids (25%), and triglycerides (5%). However, this percentage varies depending on the HDL particle size. HDL precursor particles are secreted by the liver and intestine and can absorb free cholesterol from cell membranes. This process is mediated by ATP-binding cassette (ABC) transporter A1, ABCG1, scavenger receptor class B type 1 (SR-B1), apoA-I, apoA-IV, and others. ApoA-I as the major apolipoprotein of HDL activates the enzyme lecithin–cholesterol acyltransferase, which esterifies the accepted free cholesterol. HDL3 particles are transformed into larger HDL2 particles by acquisition of additional apolipoproteins, cholesteryl esters, and triglycerides.7 Large HDL particles bind to SR-B1 in liver and steroidogenic tissues. It is believed that the HDL particle is not internalized and degraded as a whole particle, but docks to this receptor and releases the cholesteryl ester cargo to the cell.8,9 Furthermore, cholesteryl esters can take an alternative route via transfer from HDL to triglyceride-rich lipoproteins such as chylomicron remnants or VLDL mediated by the cholesteryl ester transfer protein (CETP) in exchange for triglycerides.10
HDL-C and CVD in the General Population
A decade ago, everything was so much easier. There was bad cholesterol and good cholesterol, and even the general population was educated on this topic. The epidemiologic evidence was quite strong, with a meta-analysis of 68 prospective studies including >300,000 participants free of CVD at baseline and a follow-up time of almost 3 million person-years. This study described an adjusted 22% decrease in risk of coronary heart disease for each increase in HDL-C of 15 mg/dl.11
This observation was a clear mandate for the pharmaceutical industry to develop drugs that increase HDL-C. The first and very obvious target was the inhibition of CETP, which plays a major role in the reverse cholesterol transport, relieving the peripheral cells from a cholesterol overload.12 A handful of drugs with that target have been developed that have increased HDL-C by up to >100%. Phase 3 trials prematurely stopped three of the drugs because of either off-target effects with an increase in death rates, or lack of efficacy.13 Very recently, the results on anacetrapib have been reported as one of the last substances in a running phase 3 trial including 30,449 adults with atherosclerotic vascular disease who were already receiving an effective LDL cholesterol–lowering regimen at baseline. This trial has met its primary end point by significantly reducing major coronary events by 9% compared with placebo (hazard ratio, 0.91; 95% confidence interval, 0.85 to 0.97; P=0.004).14 Interestingly, this drug did not only increase HDL-C by 104% compared with placebo, but also decreased non–HDL-C values by 18%. From other interventional studies, this decrease in non–HDL-C alone has been expected to result in a 10% relative reduction in risk of coronary death and myocardial infarction. This was very similar to the observed changes.14 In addition, lipoprotein(a) [Lp(a)] was lowered by 25%. Furthermore, the increase in HDL-C by 46 mg/dl did not meet the expectations from associated effect sizes from observational studies by far. It is therefore likely that the observed effects on major coronary events might be caused by the effect on apoB-containing lipoproteins such as LDL cholesterol and Lp(a), rather than by effects on HDL-C.15
The enthusiasm toward the idea to increase HDL-C was dampened further by genetic studies that did not find an association for genetic variants with CVD, although these variants showed a pronounced association with HDL-C concentrations.16–18 For example, observational epidemiologic studies revealed that an increase of HDL-C by 1 SD is associated with a 38% lower risk for myocardial infarction. However, no increase in risk was observed when the same increase in HDL-C concentrations was caused by a group of genetic variants that are exclusively associated with these higher HDL-C concentrations.19 Even the opposite can be the case: a loss-of-function variant in the SR-B1 gene impairs posttranslational processing of SR-B1 and abrogates selective HDL-C uptake. Heterozygous carriers of this variant have about 70% higher HDL-C concentrations and, interestingly, 2.8-fold higher HDL2b concentrations. Contrary to expectations, carriers of the mutation have a 79% higher risk of CHD.20 This might be interpreted as a severe disturbance in HDL particle uptake and the measured HDL-C concentration might not reflect the functionality of the HDL particles.
HDL-C and CVD in CKD
The evidence that HDL-C concentrations are associated with prevalence and incidence of CVD events as well as mortality is quite contrasting.21–28 A recent study in a cohort of >33,000 patients on hemodialysis revealed a U-shaped association with an increased risk for total and CVD mortality in patients with HDL-C concentrations <30 mg/dl and >60 mg/dl.29 Many studies searched for alternative tools to predict CVD in these high-risk populations,2 such as various lipoprotein or apolipoprotein ratios.30,31 With more sophisticated techniques, many other HDL subfractions (up to ten) were identified and quantitated,32,33 again with more or less success concerning prediction of outcomes. We currently have an insufficient understanding of the physiologic and pathophysiologic role of these many subfractions. Therefore, the functionality of HDL particles came into the spotlight because of the increasing evidence that the proteome and lipidome of HDL particles is heavily disturbed not only in the uremic state, but also in very early stages of kidney impairment.34 This functionality is thought to be heavily related to cholesterol efflux with the related enzymes and apolipoproteins, or the modulation of inflammatory and immune cells.1,35 This could not only have an influence on CVD outcomes but also on CKD and CKD progression.
Epidemiologic Observations on HDL-C Concentrations in CKD
Several but not all epidemiologic studies pointed to an association between low HDL-C or a high triglyceride to HDL-C ratio and poor kidney function or progression of CKD.31,36–48 The very recent and largest study by Bowe et al.49 investigated the association between HDL-C levels and various CKD end points in a cohort of almost 2 million male veterans from the United States, with a median follow-up of 9 years. Individuals with HDL-C concentrations <30 mg/dl had a 10%–20% higher risk for CKD and/or progression of CKD compared with individuals with concentration ≥40 mg/dl, depending on the definition of the end point and how intensely they adjusted their results for confounders. The risk was still 8% higher for the most severe end point, defined by a composite outcome of ESRD, dialysis, or transplantation.49
Although these results were quite strong, it must not conceal the fact that epidemiologic studies cannot disentangle with certainty cause or consequence of an association of HDL-C with CKD outcomes. Even in prospective studies, HDL-C could already be influenced by very early and hard to detect stages of CKD long before CKD or progression of CKD became evident. As discussed recently,50 one possibility to see whether HDL-C influences kidney function would be to meta-analyze the data from trials that targeted HDL-C concentrations, and to investigate whether the intervention had a positive effect on eGFR and/or albumin-to-creatinine ratio during the observation period. This has been done in the most recent Randomized Evaluation of the Effects of Anacetrapib through Lipid Modification (REVEAL) trial and the results pointed in the opposite direction: the authors described a higher frequency of a decrease of eGFR <60 ml/min per 1.73 m2 in the anacetrapib group during the observation period compared with placebo (11.5% versus 10.6%; P=0.04), and a tendency toward a higher frequency of a 40% decline in eGFR (2.9% versus 2.5%; P=0.07).14 A meta-analysis of all available studies might be necessary to obtain a sufficient statistical and reliable power because the effect size of these drugs on kidney function might be low, and because the trials were primarily not designed to study the effect of the drugs on kidney function. It is interesting to note that in the Study of Heart and Renal Protection (SHARP) trial, the intervention with statins did not only show no effect on progression of kidney disease, but the baseline HDL-C concentration also did not modify this negative finding.51
Genetic Observations on HDL-C and CKD
During the last decade, a certain type of genetic studies, so-called Mendelian randomization studies, became quite popular to collect supportive evidence of a causal association between a biomarker and an outcome of interest.50,52 This principle relies on an instrumental variable in form of a genetic variant (e.g., single nucleotide polymorphisms [SNPs], copy number variants, etc.) that has a significant influence on a biomarker. If the biomarker is causally related to the outcome of interest, one would expect that the genetic variant is also associated with the outcome. In the case of low HDL-C concentrations and impaired kidney function, we would expect that a genetic variant that is associated with low HDL-C concentrations would also have an effect on eGFR if the association between HDL-C and kidney function was indeed causal.50
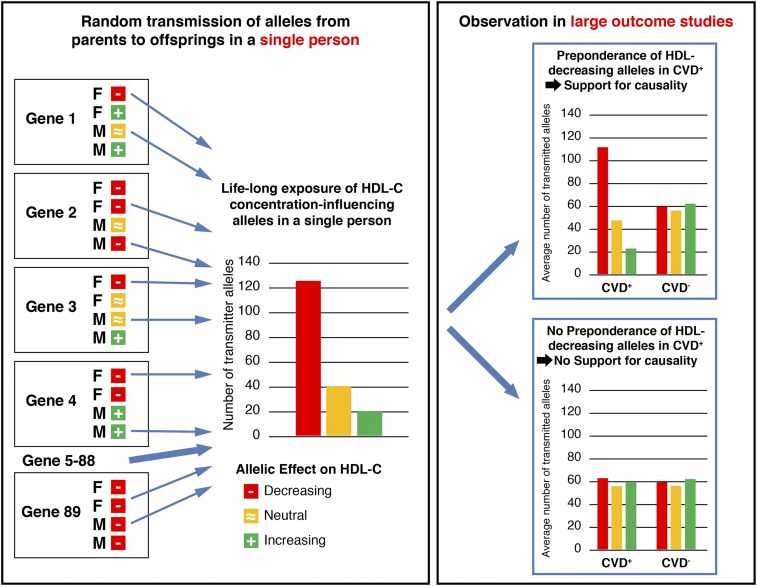
The name Mendelian randomization stems from the random assortment of alleles from parents to offspring during meiosis. If one of the parents carries an allele from a certain gene that causes lower HDL-C levels and one allele that causes normal HDL-C values, it is randomly determined which of the two alleles is transmitted to the offspring. This also holds true for the alleles from the other parent (Figure 2). It becomes even more complex because the effect of a single allele of a certain gene on HDL-C concentrations is usually quite low, with effect sizes < 1 mg/dl per risk allele. However, many genes are known to additively contribute to HDL-C concentrations and if the random assortment of alleles results in a preponderance of HDL-C–lowering alleles, an offspring might receive a large number of HDL-C–lowering alleles, to which they are exposed for their whole lives (Figure 2). It will thereby be determined in this early stage whether someone is exposed all his life to lower or higher HDL-C concentrations. A large number of HDL-C–lowering alleles would also mean a larger risk for CVD or an impairment of kidney function if HDL-C causally influences CVD and/or kidney function. On a population level, often a large number of investigated individuals is mandatory, depending on the effect sizes of the genetic variants on, for example, the HDL-C concentrations as the biomarker of interest and depending on the biologic effects of this marker on the outcome (Figure 2). Core assumptions and threats that could interfere with Mendelian randomizations were recently discussed in detail by Sekula et al.52
Figure 2.
Schematic illustration of a Mendelian randomization approach of HDL-C influencing genetic variants and outcomes of interest. Let us assume there exists a large number of genes that influence HDL-C concentrations (e.g., genes 1–89). For each gene, we know genetic variants that decrease and others that increase HDL-C, and others that are neutral. For each gene, it is determined randomly at the time of conception which of the two alleles from the father (F) and which of the two alleles from the mother (M) are transmitted to the offspring. Because many genes influence HDL-C concentrations, it is finally a question of whether an offspring has a preponderance of HDL-C–lowering alleles to which they are is exposed for their lifetime. If there is a causal effect of HDL-C concentrations on outcomes such as CVD or kidney function, one would expect that there will be also a preponderance of the HDL-C concentration-decreasing alleles in the patient groups with the outcome of interest compared with groups without that outcome. Usually, a large number of patients and controls have to be investigated to get reliable results.
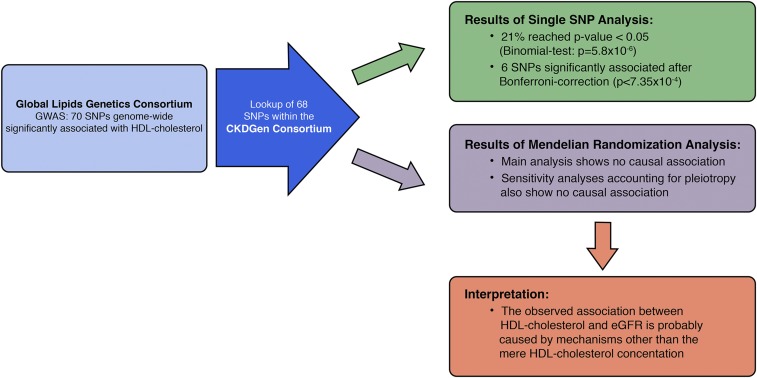
Following this idea, a recent study used 68 genetic SNP variants that were found to be associated with HDL-C in a genome-wide association study (GWAS) with >188,000 individuals, and tested their association with eGFR using the results from another GWAS on kidney function including >133,000 individuals (Figure 3).53 A larger number of HDL-C SNPs found was larger than could be accounted for by chance to be associated with eGFR (21% versus 5%). Most interestingly and in contrast to expectations, the genetic variants that showed the strongest associations with HDL-C levels (e.g., variants in the genes of CETP, LIPC, LIPG, or LPL) did not show any association with kidney function and vice versa. An extensive evaluation of pleiotropy indicated that the effects of some of the HDL-associated SNPs on eGFR cannot be mediated by HDL-C alone. That means for these SNPs, if there is indeed an association of HDL-C–associated SNPs with eGFR, then there must be additional mechanisms explaining this relationship than simply the cholesterol content of the HDL particle.53 A further publication using the same data sets but a slightly different approach revealed very similar HDL-C SNP effects on kidney function which, however, were finally significant: a difference of 1 SD of HDL-C (approximately 17 mg/dl) was associated with an increase of eGFR of only 0.8% (P=0.004).54 Even if the effect was significant, a causal effect of such a small magnitude would not explain the observed epidemiologic association of HDL-C on kidney function.49
Figure 3.
Schematic overview on the study design, main findings, and interpretation of the results derived from two genome-wide association studies on HDL-C and eGFR. For explanation, see Genetic Observations on HDL-C and CKD. Reprinted from reference 53, with permission.
This observation is in line with the idea that we actually do not know the guilty party in the HDL particle that is responsible for the outcome. That means that HDL-C represents a surrogate marker that does not reflect the responsible culprit or the real HDL functionality. Particularly, the functionality of HDL particles has been a major focus of investigations over the last two decades, with inspiring but also contrasting results.
Cholesterol Efflux Capacity and HDL Functionality
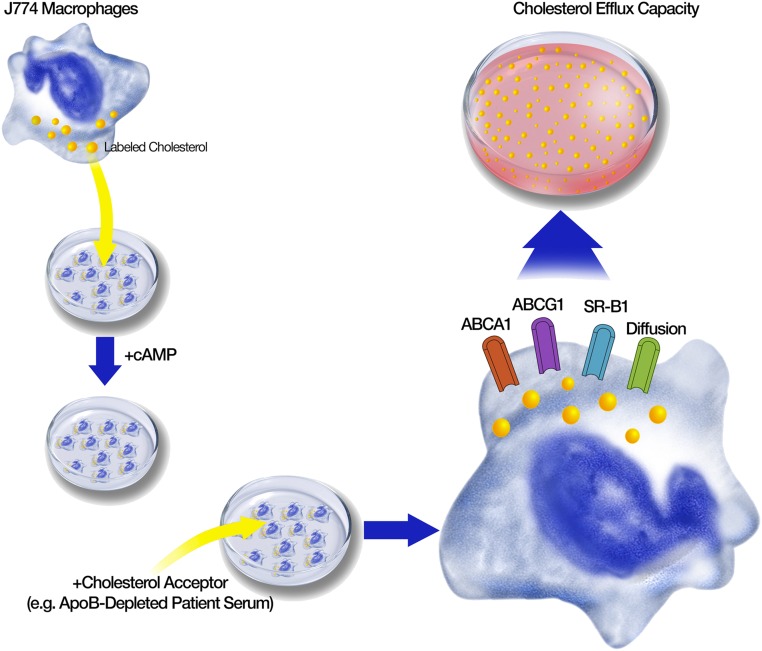
A key component in the development of atherosclerosis is the overloading of macrophages with cholesterol in the arterial wall, resulting in foam cells. The relief of macrophages from cholesterol is thus a crucial atheroprotective step. Therefore, functional in vitro assays have been developed that measure the cholesterol efflux capacity. Figure 4 illustrates the principle of these assays. Mouse macrophage cell lines such as J774 are cultivated and used as donor cells. They are enriched with cholesterol by exposure to radiolabeled or fluorescence-labeled cholesterol and stimulated with cAMP to induce cholesterol transporters. The cholesterol acceptor, usually whole serum or plasma, apoB-depleted serum, or isolated HDL from the patient under investigation, is then added to the medium. ApoB-depleted serum, obtained by precipitation, is often preferred because this fast and simple procedure removes LDL, intermediate-density lipoproteins, and VLDL as well as Lp(a) from the serum in a far more gentle manner compared with ultracentrifugation, which might detach some of the many functionally important proteins from the HDL particle. The apoB-depleted serum abolishes the transfer of labeled cholesterol from cells to these apoB-containing lipoproteins, and exclusively analyzes the cholesterol transport capacity to HDL particles. After incubation, the efflux of labeled cholesterol from the cells to the medium is quantified and reflects the cholesterol transport mediated by ABCA1, ABCG1, SR-B1, and aqueous diffusion. The principle of the assay can be modified in different ways and thereby influences which of the transporters is more or less in the focus of the investigation. Strongly standardized criteria have to be applied to ensure a reliable measurement of the efflux capacity. It is not clear whether differences in the assay design might have caused some of the discrepant results in the literature (reviewed by Anastasius et al.55).
Figure 4.
Principle of cholesterol efflux capacity assays. Macrophage cell lines are enriched with labeled cholesterol and stimulated with cAMP to induce cholesterol transporters. Then a cholesterol acceptor, such as apoB-depleted serum of the patient under investigation, is added to the medium. After incubation the efflux of labeled cholesterol from the cells to the medium is quantified and reflects the cholesterol transport mediated by ABCA1, ABCG1, SR-B1, and aqueous diffusion.
The situation in patients with CKD points to an impaired cholesterol efflux. Holzer et al.34 studied the proteome and lipid composition of HDL from patients on hemodialysis. They found pronounced changes in HDL with increased amounts of serum amyloid A1, albumin, lipoprotein-associated phospholipase A2, apoC-III, triglycerides, and lysophospholipids, as well as decreased amounts of phospholipids. These changes in uremic HDL were associated with a decreased cholesterol efflux from macrophages34 and a shift toward a proinflammatory phenotype. The anti-inflammatory and antiapoptotic functions were more severely suppressed in patients receiving hemodialysis rather than peritoneal dialysis.56 Yamamoto et al. made similar observations in patients on hemodialysis: besides a profound efflux impairment, they described a reduced chemotactic ability and an increased macrophage cytokine response with increased concentrations of TNF-α, IL-6, and IL-1-β. When patients on hemodialysis received statins, they presented with a reduced inflammatory response but impaired cholesterol acceptor function did not improve.57 In further studies, monocyte subsets were analyzed as predictors of CVD events in patients with CKD stages 2–4: higher CD14++CD16+ monocyte counts independently predicted CVD events. These monocytes showed a preferential lipid accumulation and high CD36, CD68, and low ABCA1 expression, and finally, a low cholesterol efflux.58 These findings are in line with a reduced ABCA1 mRNA in monocytes from patients with CKD of various etiologies with CKD stages 4–5 but not on dialysis when compared with control monocytes.59
Many studies from the general population and in patients with CVD showed a clear association between decreased cholesterol efflux capacity and cardiovascular outcomes.60–63 This association was usually independent of HDL-C concentrations.
Studies investigating the cholesterol efflux capacity in patients with CKD did not find evidence for an association with CVD. The CARE FOR HOMe study included 526 patients with CKD stages 2–4 at baseline. The cholesterol efflux capacity was not significantly different between patients with and without prevalent CVD at baseline. It also did not predict CVD events that occurred during the prospective observation period of an average of 4.6 years.64 A post hoc analysis of the German Diabetes Dialysis Study (4D-study) on 1147 patients with diabetes who were receiving hemodialysis, with a median follow-up of 4.1 years, also did not find an association between efflux capacity and a combined end point (including a composite of cardiac death, nonfatal myocardial infarction. and stroke), with cardiac events and all-cause mortality.65 A further study investigated a cohort of 495 kidney transplant recipients with a median follow-up of 7 years. Baseline cholesterol efflux capacity did not predict cardiovascular and total mortality. Interestingly, a high efflux capacity at baseline was associated with a lower risk for graft failure, independent of apoA-I, HDL-C, and creatinine clearance.66
The reason of the discrepant results between non-CKD and CKD populations is not clear. One explanation might be that the CVD in patients with CKD is not as much of atherosclerotic nature with atherothrombotic events such as myocardial infarctions, but is probably more connected to arteriosclerotic changes of the smaller vessels with thickening of the medial arterial layer resulting in cardiac fibrosis and hypertrophy, heart failure, and arrhythmia.67 Further, it is not clear why cholesterol efflux capacity was not associated with CVD in transplanted patients but with graft survival in the same cohort. It might reflect different vascular and inflammatory processes in the transplant vasculopathy with site-specific endothelial processes. It could also be that the cholesterol efflux from kidney cells such as mesangial cells or podocytes is of major importance for the homeostasis in the kidney allografts. Overall, we have to keep in mind that currently, only a few studies are available that were conducted on different stages of CKD. Clearly, larger and more extended studies with standardized experimental protocols are required.
HDL-Related Components
HDL particles are highly complex and carry >80 proteins and some hundred lipid species,5 as well as a dozen micro-RNAs.68 Therefore, recent studies (reviewed by Annema and von Eckardstein5,69) described dysfunctional HDL particles70 that lose their atheroprotective effect, probably because of compositional modifications.69 Table 1 gives an overview on the proteome of HDL particles but is far from being complete, and more details can be found at the HDL Proteome Watch (http://homepages.uc.edu/∼davidswm/HDLproteome.html). Only a few of them have been investigated in the context of CKD and especially of progression of CKD.
Table 1.
List of major components of the HDL proteome
| Proteins | Function |
| Apolipoproteins | |
| A-I | Major apolipoprotein of HDL; activates LCAT |
| A-II | Enhances hepatic triglyceride lipase activity |
| A-IV | Participates in reverse cholesterol transport; probably involved in the activation of LCAT and LPL |
| C-I | Activates LCAT; modulates CETP activity; inhibits hepatic lipase |
| C-II | Activator of LPL |
| C-III | Inhibitor of LPL and hepatic lipase |
| C-IV | Involved in triglyceride metabolism |
| D | Associated with LCAT |
| E | Ligand for several members of the LDL receptor gene family (especially LDL-R and LRP) |
| F | Also known as lipid transfer inhibitor protein (LTIP); inhibits CETP |
| H | Also known as β-2-glycoprotein 1; regulates platelet aggregation |
| J | Also called clusterin; binds hydrophobic molecules, interacts with cell receptors |
| L-1 | Trypanolytic factor of human serum |
| M | Binding of small hydrophobic molecules |
| Enzymes | |
| LCAT | Esterification of cholesterol to cholesteryl esters |
| PON1 | Paraoxonase 1: hydrolysis of homocysteine thiolactone; hydrolyses a wide variety of substrates |
| PAF-AH (LpPLA2) | Lipoprotein-associated phospholipase A2: hydrolysis of short-chain oxidized phospholipids |
| GSPx-3 | Plasma glutathione selenoperoxidase 3: reduction of hydroperoxides by glutathione |
| Lipid transfer proteins | |
| PLTP | Phospholipid transfer protein: conversion of HDL into larger and smaller particles, transport of LPS; positive acute-phase reactant |
| CETP | Shuttles cholesteryl esters and triglycerides between HDL and apoB-containing lipoproteins. |
| Acute-phase proteins | |
| SAA | Serum amyloid A; acute-phase protein |
| LBP | LPS binding protein; binds phospholipids, thereby acting as a lipid exchange protein similar to CETP and PLTP |
| α-1 acid glycoprotein 2 | Also called orosomucoid-2; negative acute-phase reactant |
| α-2 HS glycoprotein | Also called fetuin-A; promotes endocytosis; possesses opsonic properties |
| Fibrinogen α-chain | Precursor of fibrin, cofactor in platelet aggregation |
| Complement components | |
| C3 | Complement activator through both classic and alternative activation pathways |
| C4 | Activation of the classic pathway of the complement system |
| C4b-binding protein | Controls the classic pathway. |
| C9 | Pore-forming subunit of the membrane attack complex |
| Vitronectin | Inhibitor of the membrane-damaging effect of the terminal cytolytic pathway |
| Proteinase inhibitors | |
| α-1 antitrypsin | Inhibitor of serine proteinases |
| α-2 antiplasmin | Inhibits plasmin and trypsin and inactivates chymotrypsin |
| Haptoglobin-related protein | Decoy substrate to prevent proteolysis |
| Other proteins | |
| Retinol-binding protein | Delivers retinol from liver to peripheral tissues |
| Transthyretin | Carries the thyroid hormone thyroxine (T4) and retinol-binding protein bound to retinol |
| Serotransferrin | Iron transport glycoprotein |
| Vitamin D-binding protein | Vitamin D binding and transport |
| α-1B glycoprotein | Function unknown |
| Hemopexin | Iron binding protein |
This list is incomplete because only the proteins detected and confirmed by other studies are provided, according to Kontush et al. and Shal et al.159,160 LCAT, lecithin–cholesterol acyltransferase; LPL, lipoprotein lipase; LDL-R, LDL-receptor; LRP, lipoprotein receptor-related protein. Modified from reference 159, with permission.
Usually HDL-binding peptides, proteins, and microRNAs (miRNAs) are small and the binding of these components to HDL components, especially under uremic conditions might be different compared with healthy controls. Unbound molecules are cleared by glomerular filtration and might therefore accumulate in plasma in the case of impaired GFR. An accumulation in plasma could therefore be an effect rather than a cause of GFR decline. However, when bound to HDL particles that are not filtered, these molecules are more attractive candidates as causative biomarkers.
ApoA-IV
ApoA-IV is the third most abundant HDL-associated protein and has similar properties as apoA-I. It is involved in the reverse cholesterol transport.71,72 This glycoprotein is mainly produced in the small intestine73 and is a structural protein of chylomicrons, VLDL, HDL, or is found free in plasma.74,75 It activates the lipoprotein lipase,76 lecithin–cholesterol acyltransferase,77 and is involved in the CETP-mediated transfer of cholesteryl esters from HDL to LDL.78 It has antiatherogenic and antioxidative properties.71,72,76,79,80 In line with that, mice overexpressing human or mouse apoA-IV showed less atherosclerotic lesions in the aorta.79,80 A mouse model injected with lipid-free apoA-IV revealed a more stable plaque phenotype and a reduced incidence of acute plaque disruptions.81 This is in line with epidemiologic data in humans that observed lower apoA-IV concentrations in patients with CVD.75,82–86 Low apoA-IV levels predicted an increased risk for all-cause mortality and sudden cardiac death in patients with CKD on hemodialysis.87
The glomerular filtration capacity is one of the main determinants of apoA-IV concentration. Until recently, most of the data on apoA-IV and kidney function came from patients with primary CKD: apoA-IV already starts to increase in early stages of eGFR impairment.86 However, the concentrations are modified by proteinuria and serum albumin levels in the sense that patients with a low eGFR have lower apoA-IV levels than expected when the serum albumin level is also low, compared with high serum albumin levels.85 Patients with tubular proteinuria had significantly higher amounts of apoA-IV in their urine than those with a purely glomerular type of proteinuria. This has generated the idea that apoA-IV is glomerular-filtrated and subsequently reabsorbed in the proximal tubular cells under physiologic circumstances.85 These observations are in line with a pronounced immunoreactivity mainly in the tubular system in histologic investigations in healthy human kidneys.88 The concentrations of apoA-IV are highest in ESRD independent of whether treatment is performed by hemodialysis or peritoneal dialysis.89
Besides several studies in patients with primary CKD,39,85–87,89–93 only one large study in the general population including roughly 6000 individuals has been performed.94 There was an inverse correlation between apoA-IV concentrations and eGFR: individuals in the third and fourth quartile of apoA-IV concentrations had 1.8 and 5.1 ml/min per 1.73 m2 lower eGFR values, respectively, compared with the first quartile of apoA-IV. The probability for the presence of an eGFR<60 ml/min per 1.73 m2 was 1.46-fold and 3.47-fold higher in the third and fourth quartile, respectively, versus the first quartile of apoA-IV. At least in the general population, apoA-IV was not significantly associated with the presence of a urinary albumin-to-creatinine ratio ≥30 mg/g. This supports the idea that apoA-IV concentrations are influenced by the glomerular filtration capacity rather than by albuminuria in the general population.94 It is currently not known, whether the higher apoA-IV concentrations in individuals with kidney impairment are also a response to the disease enhancing the reverse cholesterol transport to relieve mesangial cells from an overload of cholesterol.94 The increase of antioxidative apoA-IV could also be a reaction to the high oxidative stress in CKD.95
Genetic support for a causal association between kidney function and apoA-IV concentrations came from a recent GWAS.96 This study used 53 SNPs that have been identified to be associated with kidney function.97 It found that the more genetic eGFR-decreasing kidney function variants an individual carried, the higher their apoA-IV concentrations were.96 However, the opposite analysis of whether SNPs that have a significant influence on apoA-IV concentrations also have an effect on kidney function, remained negative and did not find any of the three apoA-IV SNPs recently identified96 as being associated with eGFR.98
Finally, a prospective study in patients with primary nondiabetic mild to moderate CKD has investigated whether apoA-IV levels predict progression of CKD, defined as doubling of baseline serum creatinine and/or terminal renal failure necessitating RRT.39 Each increase of apoA-IV by 10 mg/dl increased the risk for a progression by 62% independent of baseline GFR. Patients with apoA-IV levels above the median had a significantly faster progression compared with patients with concentrations below the median.39 A recent report confirmed apoA-IV to predict rapidly declining kidney function in patients with type 2 diabetes mellitus independent of conventional clinical variables.99
This finding of high apoA-IV concentrations and CKD progression was unexpected, considering the physiologic functions of apoA-IV. It has been speculated that apoA-IV is either not fully functionally active or that the increase of apoA-IV is an unsuccessful attempt to slow or to halt the progression. High apoA-IV levels could also reflect an aspect of renal impairment that is not parallel to GFR decline.39
Apo L1
ApoL1 is a minor component of HDL-C particles in the more dense fractions HDL3b/c.100 ApoL1 concentrations have been suspected to only be a surrogate of other antioxidative and anti-inflammatory proteins, such as paraoxonases.101
In 2010, the description of an association of certain APOL1 genetic variants (called G1 and G2) with the susceptibility to nondiabetic CKD significantly contributed to the understanding of the 3–4-fold higher incidence rates for ESRD in blacks compared with whites.102,103 About 13% of blacks carry high-risk genotypes. This frequency is drastically increased to around 70% in patients of this ethnicity with FSGS, HIV-associated nephropathy, and to 45% in patients with hypertension-associated kidney disease.102,103 These risk alleles are practically nonexistent in nonblack populations.104,105 Sequencing studies in that gene region did not reveal other independent risk variants than G1 and G2.106,107
ApoL1 is highly interesting from an evolutionary standpoint and is kind of a Trojan horse, causing human resistance against Trypanosoma brucei brucei. When HDL particles are taken up as nutrients by these parasites, the HDL-attached apoL1 is inserted in the membranes of the parasites and results in pore formation and lysis of the parasites. Interestingly, Trypanosoma brucei rhodesiense has developed a serum resistance-associated protein (SRA) that binds to apoL1 and thereby avoids pore formation. This finally results in sleeping sickness. However, the two genetic variants G1 and G2 of the APOL1 gene are located in the SRA-interacting domain of apoL1. Their presence no longer allows the binding of SRA to apoL1 resulting in resistance against T. brucei rhodesiense.104 On the other hand, carriers of these alleles have a markedly increased risk for nondiabetic CKD as well as kidney function decline.108–112 The mechanism for this is still a matter of debate recently reviewed by Dummer et al.,104 and might mainly be related to mechanisms such as lysosomal membrane permeabilization and autophagic cell death. Others observed that the risk variants increased cytoplasmic cell swelling mediated by an increased efflux of intracellular K+ and a subsequent influx of Na+ with Cl− and H2O.113
Because not all carriers of the risk genotypes develop CKD, a “second hit” theory has been developed that involves the innate immune system and the coagulation system.114 This is in line with a significant interaction between the APOL1 high-risk genotype and factor VIIIc and protein C for the development of ESRD.115 Recent data showed that soluble urokinase plasminogen activator receptor (suPAR), a marker of immune activation and systemic inflammation, modifies the association between APOL1 risk variants and decline in kidney function: the risk was attenuated in patients with lower suPAR, and enforced in those with higher suPAR levels. The authors identified high-affinity interactions between apoL1 and suPAR, as well as between apoL1 and αvβ3 integrin. They showed that the G1 and G2 risk variants synergized with suPAR in the activation of αvβ3 integrin on podocytes, which leads to the formation of autophagosomes, dysregulation of the actin cytoskeleton, and cell detachment.116
Recent studies also investigated the association of these high-risk variants G1 and G2 with CVD outcomes and the results were quite heterogeneous in the general population.117–120 A cohort of black patients with hypertension-attributed CKD followed for up to 12 years found APOL1 risk variants not to be associated with an overall risk for CVD, although some signals for cardiovascular mortality were observed.121 Furthermore, these risk variants were associated with prevalent CVD in a cohort of patients with SLE.122
Serum Amyloid A
A shotgun proteomics approach identified 49 HDL-associated proteins in a uremia-specific pattern. One of the most interesting ones was serum amyloid A (SAA) because it promoted inflammatory cytokine production and inversely correlated with the anti-inflammatory potency of HDL.123 A follow-up study in transplanted patients revealed that after restoration of kidney function, many of these proteins do not change and the functional alterations of HDL in these patients might be contributing to the remaining high cardiovascular risk after transplantation.124 Interestingly, one of the proteins that modified the effect of HDL on CVD is SAA. As long as concentrations of this acute phase protein are below the 80th percentile, HDL-C was inversely and independently associated with all-cause and cardiovascular mortality as seen in non-CKD populations. However, when SAA was above the 80th percentile, HDL-C lost its protective function and high HDL-C concentrations were even associated with an increased all-cause and cardiovascular mortality. This might be explained by the observation that excessive SAA can displace antiatherogenic apoA-I from the HDL surface as an inflammatory response, and in extreme circumstances, SAA can account for up to 80% of the HDL proteins.5 This risk-modifying effect was not only shown in the general population but also in a CVD group,125 as well as in patients on hemodialysis with diabetes mellitus.126 Cell culture studies revealed that HDL supplemented with SAA significantly inhibited the nitric oxide (NO) production and significantly increased the endothelial production of reactive oxygen species.125
Asymmetric and Symmetric Dimethylarginine
Asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) are structurally related to L-arginine and may interfere with L-arginine–related signaling. However, they differ in their functional effects on NO synthesis. ADMA is an established competitive inhibitor of the NO synthase, which is less the case for SDMA.127 Both ADMA and SDMA are associated with CVD as well as total mortality.128,129 Several studies described ADMA and SDMA to be connected to kidney impairment,130 as well as to the progression of CKD.131–140 Recent data identified SDMA within HDL particles of CKD patients but not in healthy controls, and SDMA was considered to be the culprit that modifies HDL and reverts the endothelial-protective properties. This modified HDL induces endothelial dysfunction, impairs endothelial repair, increases BP, and activates endothelial TLR-2 signaling, resulting in enhanced reactive oxygen species production and inhibition of endothelial NO bioavailability.2 SDMA itself is considered as functionally inactive, and it was therefore hypothesized that SDMA may form a complex with apoA-I, the major apo of HDL particles: apoA-I inhibited endothelial NO production after supplementation with SDMA, whereas apoA-I without SDMA did not significantly affect endothelial NO production.2 Interestingly, recent data found a pronounced correlation between serum SDMA and SDMA in HDL. A higher serum SDMA was independently associated with a lower HDL-associated cholesterol efflux capacity and an accumulation of SDMA in HDL abolished the anti-inflammatory and regenerative properties of HDL.141 Because this accumulation of SDMA in HDL seems to be quite specific for CKD and is already present in very early phases of kidney impairment, it might be an important contributor to the disturbances in cholesterol efflux in patients with CKD. These data are in line with the finding that HDL from children with CKD compared with those without CKD inhibited NO production, promoted superoxide production, increased the expression of vascular cell adhesion molecule 1 in human aortic endothelial cells, and reduced cholesterol efflux from macrophages.21 Interestingly, two recent meta-analyses with slightly different study inclusion criteria found a significant association of ADMA but not of SDMA with CVD outcomes in patients with kidney diseases.128,129
Advanced Oxidation Protein Products
Advanced oxidation protein products (AOPPs) are markers of oxidative stress and are carried by oxidized plasma proteins such as oxidized albumin. They accumulate in renal disease as well as CVD.142,143 AOPP-albumin binds with high affinity to SR-B1 and thereby blocks the binding of HDL to SR-B1 and the cholesterol ester uptake. Albumin isolated from patients on hemodialysis but not from healthy controls markedly inhibited SR-B1–mediated HDL-cholesteryl ester transfer in vitro, dependent on the AOPP content of albumin. It has been suggested that this results in an abnormal composition of HDL in CKD.144 Studies in patients with IgA nephropathy described renal AOPPs as a predictor for progression of the disease.145,146 Experimental studies demonstrated that AOPPs have an influence on mesangial cell perturbations as well as on podocyte apoptosis.147,148
miRNAs
miRNAs are small, noncoding RNAs that have an influence on gene expression of their targeted genes by posttranscriptional regulation of mRNA stability and translation.149 They are not only found in the intracellular but also in the extracellular space, where they participate in intercellular communication. To avoid neutralization by ribonucleases, they are protected by an association with protein and lipid carriers.150 One of the important carriers are HDL lipoproteins.151 There is growing evidence that miRNAs control many genes involved in HDL metabolism, thereby regulating HDL biogenesis, cellular cholesterol efflux, HDL-C uptake in the liver, and many steps in reverse cholesterol transport.152 A number of miRNAs are already known to control key elements in this cascade, such as ABCA1, ABCG1, and SR-B1 (for review, see Canfrán-Duque et al.152). A pronounced network of regulators at different sites result in a granular regulation of the key elements in HDL metabolism and reverse cholesterol transport. For example, miR-33 not only influences HDL biogenesis by controlling ABCA1, but also cholesterol efflux from peripheral cells by controlling ABCA1 and ABCG1, bile acid synthesis by regulating CYP7A1, as well as bile acid secretion by influencing ABCB11 or ATP8B1, and thereby bile excretion.153,154 In addition to miR-33, ABCA1 and ABCG1 at the peripheral cells are regulated by miR26, miR-27, and miR-758. The uptake of cholesterol via SR-B1 is regulated by miR-185, miR-96, miR-223, and miR-125a.152 We are only just beginning to understand this extremely flexible regulation system. Epidemiologic studies of these miRNAs and their influence on metabolic or cardiovascular outcomes are starting to appear, but their results are contrasting (for review see Willeit et al.155). Many methodological problems need to be solved before the reliability of miRNAs as potential biomarkers can be investigated in depth.
miRNAs seem to play an important role in renal physiology and pathophysiology.156,157 It is suggested that miRNA processing in renal cells and especially podocytes is of utmost importance for renal morphology and function. They are involved in water homeostasis, osmoregulation, renin production, calcium sensing, sodium and potassium handling, and renal senescence.158 Because of the wide range of involvement of miRNAs in pivotal processes of cell cycle, stem cell differentiation, and apoptosis, a further layer in the understanding of the regulation of lipoprotein metabolism and development of atherosclerosis has started to be introduced.156 However, we are currently missing large studies that investigate the influence of this regulatory layer on outcomes in patients with CKD.
Conclusions
The field of HDL-C is full of discrepancies. Epidemiologic studies are not in line with genetic studies and intervention studies that increase HDL-C. The latter two are not in favor of HDL-C as a causal factor for CVD and/or CKD. However, the content of cholesterol in the HDL particle might not be the responsible culprit, considering that HDL particles contain a very large number of proteins and lipids. Therefore, during recent years, there has been a major shift of focus toward the functionality of HDL particles and the role of its components in the reverse cholesterol transport and especially on cholesterol efflux capacity. Many disturbances have been seen in patients with CKD and we are only at the beginning of this journey, for which further studies are required. The results have been heterogeneous, which might on the one hand be explained by the heterogeneity of kidney disease and the different stages of the disease itself, and on the other hand by the insufficient standardization and accuracy of the various assays. The associations between the observed disturbances and end points were often much stronger in non-CKD than in CKD populations. There is still much to learn before this knowledge can be effectively translated into potential diagnostic and therapeutic strategies.
Disclosures
The author declares no competing interests. The Medical University of Innsbruck holds patents on apoA-IV as a marker for kidney impairment and progression of CKD.
Acknowledgments
The author's work on apolipoprotein A-IV as well as reverse cholesterol transport has been supported by grants from the ‘Standortagentur Tirol’ and the Austrian Research Fund (FWF, W-1253 DK HOROS).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Vaziri ND, Navab M, Fogelman AM: HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol 6: 287–296, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Kränkel N, et al. : Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38: 754–768, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Morena M, Cristol JP, Dantoine T, Carbonneau MA, Descomps B, Canaud B: Protective effects of high-density lipoprotein against oxidative stress are impaired in haemodialysis patients. Nephrol Dial Transplant 15: 389–395, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M: In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int 76: 437–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annema W, von Eckardstein A: Dysfunctional high-density lipoproteins in coronary heart disease: Implications for diagnostics and therapy. Transl Res 173: 30–57, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Izquierdo-Lahuerta A, Martínez-García C, Medina-Gómez G: Lipotoxicity as a trigger factor of renal disease. J Nephrol 29: 603–610, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Kwan BCH, Kronenberg F, Beddhu S, Cheung AK: Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol 18: 1246–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Krieger M: Charting the fate of the “good cholesterol”: Identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem 68: 523–558, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Plochberger B, Röhrl C, Preiner J, Rankl C, Brameshuber M, Madl J, et al. : HDL particles incorporate into lipid bilayers - a combined AFM and single molecule fluorescence microscopy study. Sci Rep 7: 15886, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce C, Chouinard RA Jr, Tall AR: Plasma lipid transfer proteins, high-density lipoproteins, and reverse cholesterol transport. Annu Rev Nutr 18: 297–330, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. ; Emerging Risk Factors Collaboration: Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302: 1993–2000, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray KK, Vallejo-Vaz AJ: The evolving role of CETP inhibition: Beyond HDL cholesterol. Lancet 386: 412–414, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Barter PJ, Rye KA: Cholesteryl ester transfer protein inhibition is not yet dead--pro. Arterioscler Thromb Vasc Biol 36: 439–441, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, et al. ; HPS3/TIMI55–REVEAL Collaborative Group: Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 377: 1217–1227, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Hegele RA: CETP inhibitors - a new inning? N Engl J Med 377: 1284–1285, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. : Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 45: 1345–1352, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kronenberg F: Genetic variation in HDL-related genes and the association with cardiovascular disease: HDL particles as chameleons of lipoprotein metabolism. J Intern Med 270: 128–131, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Boes E, Coassin S, Kollerits B, Heid IM, Kronenberg F: Genetic-epidemiological evidence on genes associated with HDL cholesterol levels: A systematic in-depth review. Exp Gerontol 44: 136–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. : Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 380: 572–580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, et al. ; CHD Exome+ Consortium; CARDIoGRAM Exome Consortium; Global Lipids Genetics Consortium: Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 351: 1166–1171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shroff R, Speer T, Colin S, Charakida M, Zewinger S, Staels B, et al. : HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J Am Soc Nephrol 25: 2658–2668, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, et al. : HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol 25: 1073–1082, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch M, Kutkuhn B, Trenkwalder E, Bach D, Grabensee B, Dieplinger H, et al. : Apolipoprotein B, fibrinogen, HDL cholesterol, and apolipoprotein(a) phenotypes predict coronary artery disease in hemodialysis patients. J Am Soc Nephrol 8: 1889–1898, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Silbernagel G, Genser B, Drechsler C, Scharnagl H, Grammer TB, Stojakovic T, et al. : HDL cholesterol, apolipoproteins, and cardiovascular risk in hemodialysis patients. J Am Soc Nephrol 26: 484–492, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kronenberg F, Neyer U, Lhotta K, Trenkwalder E, Auinger M, Pribasnig A, et al. : The low molecular weight apo(a) phenotype is an independent predictor for coronary artery disease in hemodialysis patients: A prospective follow-up. J Am Soc Nephrol 10: 1027–1036, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C: Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 55: 648–658, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Stack AG, Bloembergen WE: Prevalence and clinical correlates of coronary artery disease among new dialysis patients in the United States: A cross-sectional study. J Am Soc Nephrol 12: 1516–1523, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar-Zadeh K: Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol 18: 293–303, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar-Zadeh K: Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant 29: 1554–1562, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang TI, Streja E, Soohoo M, Kim TW, Rhee CM, Kovesdy CP, et al. : Association of serum triglyceride to HDL cholesterol ratio with all-cause and cardiovascular mortality in incident hemodialysis patients. Clin J Am Soc Nephrol 12: 591–602, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamprea-Montealegre JA, Sharrett AR, Matsushita K, Selvin E, Szklo M, Astor BC: Chronic kidney disease, lipids and apolipoproteins, and coronary heart disease: The ARIC study. Atherosclerosis 234: 42–46, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Petersen AK, Stark K, Musameh MD, Nelson CP, Römisch-Margl W, Kremer W, et al. : Genetic associations with lipoprotein subfractions provide information on their biological nature. Hum Mol Genet 21: 1433–1443, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Gluba-Brzózka A, Franczyk B, Banach M, Rysz-Górzyńska M: Do HDL and LDL subfractions play a role in atherosclerosis in end-stage renal disease (ESRD) patients? Int Urol Nephrol 49: 155–164, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, et al. : Uremia alters HDL composition and function. J Am Soc Nephrol 22: 1631–1641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H, Fogo AB, Kon V: Kidneys: Key modulators of high-density lipoprotein levels and function. Curr Opin Nephrol Hypertens 25: 174–179, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cases A, Coll E: Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl 99: S87–S93, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Mänttäri M, Tiula E, Alikoski T, Manninen V: Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension 26: 670–675, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ: Plasma lipids and risk of developing renal dysfunction: The atherosclerosis risk in communities study. Kidney Int 58: 293–301, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Boes E, Fliser D, Ritz E, König P, Lhotta K, Mann JF, et al. : Apolipoprotein A-IV predicts progression of chronic kidney disease: The mild to moderate kidney disease study. J Am Soc Nephrol 17: 528–536, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Schaeffner ES, Kurth T, Curhan GC, Glynn RJ, Rexrode KM, Baigent C, et al. : Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol 14: 2084–2091, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Morton J, Zoungas S, Li Q, Patel AA, Chalmers J, Woodward M, et al. ; ADVANCE Collaborative Group: Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: Results of the ADVANCE study. Diabetes Care 35: 2201–2206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman M, Yang W, Akkina S, Alper A, Anderson AH, Appel LJ, et al. ; CRIC Study Investigators: Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clin J Am Soc Nephrol 9: 1190–1198, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashi K, Takayama M, Abe T, Kanda T, Hirose H, Shimizu-Hirota R, et al. : Investigation of metabolic factors associated with eGFR decline over 1 year in a Japanese population without CKD. J Atheroscler Thromb 24: 863–875, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Kang HT, Kim JK, Kim JY, Linton JA, Yoon JH, Koh SB: Independent association of TG/HDL-C with urinary albumin excretion in normotensive subjects in a rural Korean population. Clin Chim Acta 413: 319–324, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Tsuruya K, Yoshida H, Nagata M, Kitazono T, Hirakata H, Iseki K, et al. : Association of the triglycerides to high-density lipoprotein cholesterol ratio with the risk of chronic kidney disease: Analysis in a large Japanese population. Atherosclerosis 233: 260–267, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Tsuruya K, Yoshida H, Nagata M, Kitazono T, Iseki K, Iseki C, et al. : Impact of the triglycerides to high-density lipoprotein cholesterol ratio on the incidence and progression of CKD: A longitudinal study in a large Japanese population. Am J Kidney Dis 66: 972–983, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Bae JC, Han JM, Kwon S, Jee JH, Yu TY, Lee MK, et al. : LDL-C/apoB and HDL-C/apoA-1 ratios predict incident chronic kidney disease in a large apparently healthy cohort. Atherosclerosis 251: 170–176, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Bowe B, Xie Y, Xian H, Balasubramanian S, Al-Aly Z: Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int 89: 886–896, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Kronenberg F: High-density lipoprotein cholesterol on a roller coaster: Where will the ride end? Kidney Int 89: 747–749, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Haynes R, Lewis D, Emberson J, Reith C, Agodoa L, Cass A, et al. ; SHARP Collaborative Group; SHARP Collaborative Group: Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol 25: 1825–1833, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekula P, Del Greco M F, Pattaro C, Köttgen A: Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 27: 3253–3265, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coassin S, Friedel S, Köttgen A, Lamina C, Kronenberg F: Is high-density lipoprotein cholesterol causally related to kidney function? Evidence from genetic epidemiological studies. Arterioscler Thromb Vasc Biol 36: 2252–2258, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lanktree MB, Thériault S, Walsh M, Paré G: HDL cholesterol, LDL cholesterol, and triglycerides as risk factors for CKD: A Mendelian randomization study [published online ahead of print July 26, 2017]. Am J Kidney Dis doi:10.1053/j.ajkd.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 55.Anastasius M, Kockx M, Jessup W, Sullivan D, Rye KA, Kritharides L: Cholesterol efflux capacity: An introduction for clinicians. Am Heart J 180: 54–63, 2016 [DOI] [PubMed] [Google Scholar]
- 56.Holzer M, Schilcher G, Curcic S, Trieb M, Ljubojevic S, Stojakovic T, et al. : Dialysis modalities and HDL composition and function. J Am Soc Nephrol 26: 2267–2276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, et al. : Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol 60: 2372–2379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogacev KS, Zawada AM, Emrich I, Seiler S, Böhm M, Fliser D, et al. : Lower Apo A-I and lower HDL-C levels are associated with higher intermediate CD14++CD16+ monocyte counts that predict cardiovascular events in chronic kidney disease. Arterioscler Thromb Vasc Biol 34: 2120–2127, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Ganda A, Yvan-Charvet L, Zhang Y, Lai EJ, Regunathan-Shenk R, Hussain FN, et al. : Plasma metabolite profiles, cellular cholesterol efflux, and non-traditional cardiovascular risk in patients with CKD. J Mol Cell Cardiol 112: 114–122, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. : Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. : HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 371: 2383–2393, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritsch A, Scharnagl H, März W: HDL cholesterol efflux capacity and cardiovascular events. N Engl J Med 372: 1870–1871, 2015 [DOI] [PubMed] [Google Scholar]
- 63.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, et al. : Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol 3: 507–513, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bauer L, Kern S, Rogacev KS, Emrich IE, Zawada A, Fliser D, et al. : HDL cholesterol efflux capacity and cardiovascular events in patients with chronic kidney disease. J Am Coll Cardiol 69: 246–247, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Kopecky C, Ebtehaj S, Genser B, Drechsler C, Krane V, Antlanger M, et al. : HDL cholesterol efflux does not predict cardiovascular risk in hemodialysis patients. J Am Soc Nephrol 28: 769–775, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Annema W, Dikkers A, de Boer JF, Dullaart RP, Sanders JS, Bakker SJ, et al. : HDL cholesterol efflux predicts graft failure in renal transplant recipients. J Am Soc Nephrol 27: 595–603, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moody WE, Edwards NC, Chue CD, Ferro CJ, Townend JN: Arterial disease in chronic kidney disease. Heart 99: 365–372, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT: MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13: 423–433, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Annema W, von Eckardstein A: High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ J 77: 2432–2448, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, et al. : Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Steps 2 and 3. J Lipid Res 41: 1495–1508, 2000 [PubMed] [Google Scholar]
- 71.Steinmetz A, Barbaras R, Ghalim N, Clavey V, Fruchart J-C, Ailhaud G: Human apolipoprotein A-IV binds to apolipoprotein A-I/A-II receptor sites and promotes cholesterol efflux from adipose cells. J Biol Chem 265: 7859–7863, 1990 [PubMed] [Google Scholar]
- 72.Stein O, Stein Y, Lefevre M, Roheim PS: The role of apolipoprotein A-IV in reverse cholesterol transport studied with cultured cells and liposomes derived from an ether analog of phosphatidylcholine. Biochim Biophys Acta 878: 7–13, 1986 [DOI] [PubMed] [Google Scholar]
- 73.Utermann G, Beisiegel U: Apolipoprotein A-IV: A protein occurring in human mesenteric lymph chylomicrons and free in plasma. Isolation and quantification. Eur J Biochem 99: 333–343, 1979 [DOI] [PubMed] [Google Scholar]
- 74.Green PHR, Glickman RM, Riley JW, Quinet E: Human apolipoprotein A-IV. Intestinal origin and distribution in plasma. J Clin Invest 65: 911–919, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ezeh B, Haiman M, Alber HF, Kunz B, Paulweber B, Lingenhel A, et al. : Plasma distribution of apoA-IV in patients with coronary artery disease and healthy controls. J Lipid Res 44: 1523–1529, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Goldberg IJ, Scheraldi CA, Yacoub LK, Saxena U, Bisgaier CL: Lipoprotein ApoC-II activation of lipoprotein lipase. Modulation by apolipoprotein A-IV. J Biol Chem 265: 4266–4272, 1990 [PubMed] [Google Scholar]
- 77.Steinmetz A, Utermann G: Activation of lecithin: Cholesterol acyltransferase by human apolipoprotein A-IV. J Biol Chem 260: 2258–2264, 1985 [PubMed] [Google Scholar]
- 78.Guyard-Dangremont V, Lagrost L, Gambert P: Comparative effects of purified apolipoproteins A-I, A-II, and A-IV on cholesteryl ester transfer protein activity. J Lipid Res 35: 982–992, 1994 [PubMed] [Google Scholar]
- 79.Duverger N, Tremp G, Caillaud JM, Emmanuel F, Castro G, Fruchart JC, et al. : Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science 273: 966–968, 1996 [DOI] [PubMed] [Google Scholar]
- 80.Cohen RD, Castellani LW, Qiao JH, Van Lenten BJ, Lusis AJ, Reue K: Reduced aortic lesions and elevated high density lipoprotein levels in transgenic mice overexpressing mouse apolipoprotein A-IV. J Clin Invest 99: 1906–1916, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geronimo FRB, Barter PJ, Rye KA, Heather AK, Shearston KD, Rodgers KJ: Plaque stabilizing effects of apolipoprotein A-IV. Atherosclerosis 251: 39–46, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Kronenberg F, Stühlinger M, Trenkwalder E, Geethanjali FS, Pachinger O, von Eckardstein A, et al. : Low apolipoprotein A-IV plasma concentrations in men with coronary artery disease. J Am Coll Cardiol 36: 751–757, 2000 [DOI] [PubMed] [Google Scholar]
- 83.Manpuya MW, Guo J, Zhao Y: The relationship between plasma apolipoprotein A-IV levels and coronary heart disease. Chin Med J (Engl) 114: 275–279, 2001 [PubMed] [Google Scholar]
- 84.Omori M, Watanabe M, Matsumoto K, Honda H, Hattori H, Akizawa T: Impact of serum apolipoprotein A-IV as a marker of cardiovascular disease in maintenance hemodialysis patients. Ther Apher Dial 14: 341–348, 2010 [DOI] [PubMed] [Google Scholar]
- 85.Lingenhel A, Lhotta K, Neyer U, Heid IM, Rantner B, Kronenberg MF, et al. : Role of the kidney in the metabolism of apolipoprotein A-IV: Influence of the type of proteinuria. J Lipid Res 47: 2071–2079, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Kronenberg F, Kuen E, Ritz E, König P, Kraatz G, Lhotta K, et al. : Apolipoprotein A-IV serum concentrations are elevated in patients with mild and moderate renal failure. J Am Soc Nephrol 13: 461–469, 2002 [DOI] [PubMed] [Google Scholar]
- 87.Kollerits B, Krane V, Drechsler C, Lamina C, März W, Ritz E, et al. ; German Diabetes and Dialysis Study Investigators: Apolipoprotein A-IV concentrations and clinical outcomes in haemodialysis patients with type 2 diabetes mellitus--a post hoc analysis of the 4D Study. J Intern Med 272: 592–600, 2012 [DOI] [PubMed] [Google Scholar]
- 88.Haiman M, Salvenmoser W, Scheiber K, Lingenhel A, Rudolph C, Schmitz G, et al. : Immunohistochemical localization of apolipoprotein A-IV in human kidney tissue. Kidney Int 68: 1130–1136, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Kronenberg F, König P, Neyer U, Auinger M, Pribasnig A, Lang U, et al. : Multicenter study of lipoprotein(a) and apolipoprotein(a) phenotypes in patients with end-stage renal disease treated by hemodialysis or continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 6: 110–120, 1995 [DOI] [PubMed] [Google Scholar]
- 90.Dieplinger H, Lobentanz E-M, König P, Graf H, Sandholzer C, Matthys E, et al. : Plasma apolipoprotein A-IV metabolism in patients with chronic renal disease. Eur J Clin Invest 22: 166–174, 1992 [DOI] [PubMed] [Google Scholar]
- 91.Seishima M, Muto Y: An increased apo A-IV serum concentration of patients with chronic renal failure on hemodialysis. Clin Chim Acta 167: 303–311, 1987 [DOI] [PubMed] [Google Scholar]
- 92.Massy ZA, Kandoussi AM, Mamzer-Bruneel MF, Kreis H, Drüeke T, Lacour B: High serum apolipoprotein AIV levels in renal transplant recipients. Clin Nephrol 55: 156–158, 2001 [PubMed] [Google Scholar]
- 93.Kronenberg F, Lingenhel A, Neyer U, Lhotta K, König P, Auinger M, et al. : Prevalence of dyslipidemic risk factors in hemodialysis and CAPD patients. Kidney Int Suppl 84: S113–S116, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Stangl S, Kollerits B, Lamina C, Meisinger C, Huth C, Stöckl A, et al. : Association between apolipoprotein A-IV concentrations and chronic kidney disease in two large population-based cohorts: Results from the KORA studies. J Intern Med 278: 410–423, 2015 [DOI] [PubMed] [Google Scholar]
- 95.Qin X, Swertfeger DK, Zheng S, Hui DY, Tso P: Apolipoprotein AIV: A potent endogenous inhibitor of lipid oxidation. Am J Physiol 274: H1836–H1840, 1998 [DOI] [PubMed] [Google Scholar]
- 96.Lamina C, Friedel S, Coassin S, Rueedi R, Yousri NA, Seppälä I, et al. ; KORA Study Group: A genome-wide association meta-analysis on apolipoprotein A-IV concentrations. Hum Mol Genet 25: 3635–3646, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pattaro C, Teumer A, Gorski M, Chu AY, Li M, Mijatovic V, et al. ; ICBP Consortium; AGEN Consortium; CARDIOGRAM; CHARGe-Heart Failure Group; ECHOGen Consortium: Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun 7: 10023, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mack S, Coassin S, Vaucher J, Kronenberg F, Lamina C; ApoA-IV-GWAS Consortium: Evaluating the causal relation of apoA-IV with disease-related traits - A bidirectional two-sample Mendelian Randomization study. Sci Rep 7: 8734, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peters KE, Davis WA, Ito J, Winfield K, Stoll T, Bringans SD, et al. : Identification of novel circulating biomarkers predicting rapid decline in renal function in type 2 diabetes: The Fremantle Diabetes study phase II. Diabetes Care 40: 1548–1555, 2017 [DOI] [PubMed] [Google Scholar]
- 100.Kronenberg F: Apolipoprotein L1 and apolipoprotein A-IV and their association with kidney function. Curr Opin Lipidol 28: 39–45, 2017 [DOI] [PubMed] [Google Scholar]
- 101.Litvinov D, Mahini H, Garelnabi M: Antioxidant and anti-inflammatory role of paraoxonase 1: Implication in arteriosclerosis diseases. N Am J Med Sci 4: 523–532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. : Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, et al. : Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dummer PD, Limou S, Rosenberg AZ, Heymann J, Nelson G, Winkler CA, et al. : APOL1 kidney disease risk variants: An evolving landscape. Semin Nephrol 35: 222–236, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anderson BR, Howell DN, Soldano K, Garrett ME, Katsanis N, Telen MJ, et al. : In vivo modeling implicates APOL1 in nephropathy: Evidence for dominant negative effects and epistasis under anemic stress. PLoS Genet 11: e1005349, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Limou S, Nelson GW, Lecordier L, An P, O’hUigin CS, David VA, et al. : Sequencing rare and common APOL1 coding variants to determine kidney disease risk. Kidney Int 88: 754–763, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hawkins GA, Friedman DJ, Lu L, McWilliams DR, Chou JW, Sajuthi S, et al. : Re-sequencing of the APOL1-APOL4 and MYH9 gene regions in African Americans does not identify additional risks for CKD progression. Am J Nephrol 42: 99–106, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al. ; AASK Study Investigators; CRIC Study Investigators: APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen TK, Tin A, Peralta CA, Appel LJ, Choi MJ, Lipkowitz MS, et al. : APOL1 risk variants, incident proteinuria, and subsequent eGFR decline in blacks with hypertension-attributed CKD. Clin J Am Soc Nephrol 12: 1771–1777, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tin A, Grams ME, Estrella M, Lipkowitz M, Greene TH, Kao WH, et al. : Patterns of kidney function decline associated with APOL1 genotypes: Results from AASK. Clin J Am Soc Nephrol 11: 1353–1359, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, et al. ; Lupus Nephritis–End‐Stage Renal Disease Consortium: End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol 66: 390–396, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, et al. : Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol 27: 2842–2850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olabisi OA, Zhang JY, VerPlank L, Zahler N, DiBartolo S 3rd, Heneghan JF, et al. : APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci U S A 113: 830–837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Freedman BI, Skorecki K: Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol 9: 2006–2013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tin A, Grams ME, Maruthur NM, Astor BC, Couper D, Mosley TH, et al. : Hemostatic factors, APOL1 risk variants, and the risk of ESRD in the atherosclerosis risk in communities study. Clin J Am Soc Nephrol 10: 784–790, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hayek SS, Koh KH, Grams ME, Wei C, Ko YA, Li J, et al. : A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat Med 23: 945–953, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ito K, Bick AG, Flannick J, Friedman DJ, Genovese G, Parfenov MG, et al. : Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res 114: 845–850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mukamal KJ, Tremaglio J, Friedman DJ, Ix JH, Kuller LH, Tracy RP, et al. : APOL1 genotype, kidney and cardiovascular disease, and death in older adults. Arterioscler Thromb Vasc Biol 36: 398–403, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Langefeld CD, Divers J, Pajewski NM, Hawfield AT, Reboussin DM, Bild DE, et al. ; Systolic Blood Pressure Intervention Trial (SPRINT): Apolipoprotein L1 gene variants associate with prevalent kidney but not prevalent cardiovascular disease in the Systolic Blood Pressure Intervention Trial. Kidney Int 87: 169–175, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freedman BI, Rocco MV, Bates JT, Chonchol M, Hawfield AT, Lash JP, et al. ; SPRINT Research Group: APOL1 renal-risk variants do not associate with incident cardiovascular disease or mortality in the Systolic Blood Pressure Intervention Trial. Kidney Int Rep 2: 713–720, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen TK, Appel LJ, Grams ME, Tin A, Choi MJ, Lipkowitz MS, et al. : APOL1 risk variants and cardiovascular disease: Results from the AASK (African American Study of Kidney Disease and Hypertension). Arterioscler Thromb Vasc Biol 37: 1765–1769, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blazer A, Wang B, Simpson D, Kirchhoff T, Heffron S, Clancy RM, et al. : Apolipoprotein L1 risk variants associate with prevalent atherosclerotic disease in African American systemic lupus erythematosus patients. PLoS One 12: e0182483, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Weichhart T, Kopecky C, Kubicek M, Haidinger M, Döller D, Katholnig K, et al. : Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol 23: 934–947, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kopecky C, Haidinger M, Birner-Grünberger R, Darnhofer B, Kaltenecker CC, Marsche G, et al. : Restoration of renal function does not correct impairment of uremic HDL properties. J Am Soc Nephrol 26: 565–575, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zewinger S, Drechsler C, Kleber ME, Dressel A, Riffel J, Triem S, et al. : Serum amyloid A: High-density lipoproteins interaction and cardiovascular risk. Eur Heart J 36: 3007–3016, 2015 [DOI] [PubMed] [Google Scholar]
- 126.Kopecky C, Genser B, Drechsler C, Krane V, Kaltenecker CC, Hengstschläger M, et al. : Quantification of HDL proteins, cardiac events, and mortality in patients with type 2 diabetes on hemodialysis. Clin J Am Soc Nephrol 10: 224–231, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kittel A, Maas R: Pharmacology and clinical pharmacology of methylarginines used as inhibitors of nitric oxide synthases. Curr Pharm Des 20: 3530–3547, 2014 [DOI] [PubMed] [Google Scholar]
- 128.Willeit P, Freitag DF, Laukkanen JA, Chowdhury S, Gobin R, Mayr M, et al. : Asymmetric dimethylarginine and cardiovascular risk: Systematic review and meta-analysis of 22 prospective studies. J Am Heart Assoc 4: e001833, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schlesinger S, Sonntag SR, Lieb W, Maas R: Asymmetric and symmetric dimethylarginine as risk markers for total mortality and cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. PLoS One 11: e0165811, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vallance P, Leone A, Calver A, Collier J, Moncada S: Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339: 572–575, 1992 [DOI] [PubMed] [Google Scholar]
- 131.Fliser D, Kronenberg F, Kielstein JT, Morath C, Bode-Böger SM, Haller H, et al. : Asymmetric dimethylarginine and progression of chronic kidney disease: The mild to moderate kidney disease study. J Am Soc Nephrol 16: 2456–2461, 2005 [DOI] [PubMed] [Google Scholar]
- 132.Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C: Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: A competing risks modeling approach. J Am Soc Nephrol 16: 2449–2455, 2005 [DOI] [PubMed] [Google Scholar]
- 133.Hanai K, Babazono T, Nyumura I, Toya K, Tanaka N, Tanaka M, et al. : Asymmetric dimethylarginine is closely associated with the development and progression of nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant 24: 1884–1888, 2009 [DOI] [PubMed] [Google Scholar]
- 134.Tripepi G, Kollerits B, Leonardis D, Yilmaz MI, Postorino M, Fliser D, et al. : Competitive interaction between fibroblast growth factor 23 and asymmetric dimethylarginine in patients with CKD. J Am Soc Nephrol 26: 935–944, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eiselt J, Rajdl D, Racek J, Vostrý M, Rulcová K, Wirth J: Asymmetric dimethylarginine and progression of chronic kidney disease: A one-year follow-up study. Kidney Blood Press Res 39: 50–57, 2014 [DOI] [PubMed] [Google Scholar]
- 136.Looker HC, Colombo M, Hess S, Brosnan MJ, Farran B, Dalton RN, et al. ; SUMMIT Investigators: Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int 88: 888–896, 2015 [DOI] [PubMed] [Google Scholar]
- 137.Testa A, Mallamaci F, Leonardis D, Spoto B, Pisano A, Sanguedolce MC, et al. ; MAURO Study Investigators: Synergism between asymmetric dimethylarginine (ADMA) and a genetic marker of uric acid in CKD progression. Nutr Metab Cardiovasc Dis 25: 167–172, 2015 [DOI] [PubMed] [Google Scholar]
- 138.Abedini S, Meinitzer A, Holme I, März W, Weihrauch G, Fellstrøm B, et al. : Asymmetrical dimethylarginine is associated with renal and cardiovascular outcomes and all-cause mortality in renal transplant recipients. Kidney Int 77: 44–50, 2010 [DOI] [PubMed] [Google Scholar]
- 139.Heunisch F, Chaykovska L, von Einem G, Alter M, Dschietzig T, Kretschmer A, et al. : ADMA predicts major adverse renal events in patients with mild renal impairment and/or diabetes mellitus undergoing coronary angiography. Medicine (Baltimore) 96: e6065, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Emrich IE, Zawada AM, Martens-Lobenhoffer J, Fliser D, Wagenpfeil S, Heine GH, et al. : Symmetric dimethylarginine (SDMA) outperforms asymmetric dimethylarginine (ADMA) and other methylarginines as predictor of renal and cardiovascular outcome in non-dialysis chronic kidney disease [published online ahead of print November 3, 2017]. Clin Res Cardiol doi:10.1007/s00392-017-1172-4 [DOI] [PubMed] [Google Scholar]
- 141.Zewinger S, Kleber ME, Rohrer L, Lehmann M, Triem S, Jennings RT, et al. : Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur Heart J 38: 1597–1607, 2017 [DOI] [PubMed] [Google Scholar]
- 142.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. : Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49: 1304–1313, 1996 [DOI] [PubMed] [Google Scholar]
- 143.Descamps-Latscha B, Witko-Sarsat V, Nguyen-Khoa T, Nguyen AT, Gausson V, Mothu N, et al. : Advanced oxidation protein products as risk factors for atherosclerotic cardiovascular events in nondiabetic predialysis patients. Am J Kidney Dis 45: 39–47, 2005 [DOI] [PubMed] [Google Scholar]
- 144.Marsche G, Frank S, Hrzenjak A, Holzer M, Dirnberger S, Wadsack C, et al. : Plasma-advanced oxidation protein products are potent high-density lipoprotein receptor antagonists in vivo. Circ Res 104: 750–757, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Descamps-Latscha B, Witko-Sarsat V, Nguyen-Khoa T, Nguyen AT, Gausson V, Mothu N, et al. : Early prediction of IgA nephropathy progression: Proteinuria and AOPP are strong prognostic markers. Kidney Int 66: 1606–1612, 2004 [DOI] [PubMed] [Google Scholar]
- 146.Wang J, Liang M, Xu J, Cao W, Wang GB, Zhou ZM, et al. : Renal expression of advanced oxidative protein products predicts progression of renal fibrosis in patients with IgA nephropathy. Lab Invest 94: 966–977, 2014 [DOI] [PubMed] [Google Scholar]
- 147.Zhou LL, Hou FF, Wang GB, Yang F, Xie D, Wang YP, et al. : Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int 76: 1148–1160, 2009 [DOI] [PubMed] [Google Scholar]
- 148.Wei XF, Zhou QG, Hou FF, Liu BY, Liang M: Advanced oxidation protein products induce mesangial cell perturbation through PKC-dependent activation of NADPH oxidase. Am J Physiol Renal Physiol 296: F427–F437, 2009 [DOI] [PubMed] [Google Scholar]
- 149.Bartel DP: MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004 [DOI] [PubMed] [Google Scholar]
- 150.Vickers KC, Remaley AT: Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol 23: 91–97, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Michell DL, Vickers KC: Lipoprotein carriers of microRNAs. Biochim Biophys Acta 1861[12 Pt B]: 2069–2074, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Canfrán-Duque A, Ramírez CM, Goedeke L, Lin CS, Fernández-Hernando C: microRNAs and HDL life cycle. Cardiovasc Res 103: 414–422, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Allen RM, Marquart TJ, Albert CJ, Suchy FJ, Wang DQ, Ananthanarayanan M, et al. : miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol Med 4: 882–895, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li T, Francl JM, Boehme S, Chiang JY: Regulation of cholesterol and bile acid homeostasis by the cholesterol 7α-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology 58: 1111–1121, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Willeit P, Skroblin P, Kiechl S, Fernández-Hernando C, Mayr M: Liver microRNAs: Potential mediators and biomarkers for metabolic and cardiovascular disease? Eur Heart J 37: 3260–3266, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nassirpour R, Raj D, Townsend R, Argyropoulos C: MicroRNA biomarkers in clinical renal disease: From diabetic nephropathy renal transplantation and beyond. Food Chem Toxicol 98[Pt A]: 73–88, 2016 [DOI] [PubMed] [Google Scholar]
- 157.Denby L, Baker AH: Targeting non-coding RNA for the therapy of renal disease. Curr Opin Pharmacol 27: 70–77, 2016 [DOI] [PubMed] [Google Scholar]
- 158.Bai XY, Ma Y, Ding R, Fu B, Shi S, Chen XM: miR-335 and miR-34a promote renal senescence by suppressing mitochondrial antioxidative enzymes. J Am Soc Nephrol 22: 1252–1261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kontush A, Lindahl M, Lhomme M, Calabresi L, Chapman MJ, Davidson WS: Structure of HDL: Particle subclasses and molecular components. In: High Density Lipoproteins: From Biological Understanding to Clinical Exploitation, edited by Von Eckardstein A, Kardassis D, Cham, Springer International Publishing, 2015, pp 3–51 [Google Scholar]
- 160.Shah AS, Tan L, Long JL, Davidson WS: Proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J Lipid Res 54: 2575–2585, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]