Abstract
Background Laminin α5β2γ1 (LM-521) is a major component of the GBM. Mutations in LAMB2 that prevent LM-521 synthesis and/or secretion cause Pierson syndrome, a rare congenital nephrotic syndrome with diffuse mesangial sclerosis and ocular and neurologic defects. Because the GBM is uniquely accessible to plasma, which permeates endothelial cell fenestrae, we hypothesized that intravenous delivery of LM-521 could replace the missing LM-521 in the GBM of Lamb2 mutant mice and restore glomerular permselectivity.
Methods We injected human LM-521 (hLM-521), a macromolecule of approximately 800 kD, into the retro-orbital sinus of Lamb2−/− pups daily. Deposition of hLM-521 into the GBM was investigated by fluorescence microscopy. We assayed the effects of hLM-521 on glomerular permselectivity by urinalysis and the effects on podocytes by desmin immunostaining and ultrastructural analysis of podocyte architecture.
Results Injected hLM-521 rapidly and stably accumulated in the GBM of all glomeruli. Super-resolution imaging showed that hLM-521 accumulated in the correct orientation in the GBM, primarily on the endothelial aspect. Treatment with hLM-521 greatly reduced the expression of the podocyte injury marker desmin and attenuated the foot process effacement observed in untreated pups. Moreover, treatment with hLM-521 delayed the onset of proteinuria but did not prevent nephrotic syndrome, perhaps due to its absence from the podocyte aspect of the GBM.
Conclusions These studies show that GBM composition and function can be altered in vivo via vascular delivery of even very large proteins, which may advance therapeutic options for patients with abnormal GBM composition, whether genetic or acquired.
Keywords: glomerular filtration barrier, podocyte, laminin, glomerular basement membrane
The glomerular filtration barrier (GFB) between the vasculature and the urinary space consists of three layers: the fenestrated endothelium with its glycocalyx, the podocytes with their foot processes and slit diaphragms, and the intervening glomerular basement membrane (GBM).1 Proper cell-cell and cell-matrix interactions, as well as complex intermolecular interactions within the GBM, are crucial to establish and maintain glomerular permselectivity.2 Defects in any one of the three layers, whether genetic or acquired, may cause proteinuria and eventual ESRD.3,4
The best evidence for the importance of the GBM in conferring glomerular permselectivity is the fact that genetic defects in its collagen IV (collagen α3α4α5[IV]) or laminin (laminin α5β2γ1; LM-521) components can cause proteinuria and ESRD.1 Mutations that affect the type IV collagen α3, α4, or α5 chains cause Alport syndrome, which is characterized by kidney disease usually accompanied by deafness and lens and retinal defects.5–7 Alport syndrome first manifests as hematuria in young children and only later progresses to proteinuria and ESRD by adolescence or young adulthood, although new treatment regimens incorporating ACE inhibition slow progression to proteinuria and ESRD.8,9
In contrast, mutations in LAMB2 that affect the laminin β2 chain of LM-521 cause a congenital nephrotic syndrome that can present either as isolated congenital nephrotic syndrome or as a major manifestation of Pierson syndrome, which also includes severe eye and neurologic problems.10 This spectrum of phenotypes depends in part on the nature of the specific LAMB2 mutations present in the patient,11 with nonsense and splice site mutations usually causing the more severe Pierson syndrome.12 Studies of animal models of Pierson syndrome and its variants have revealed important details about its pathophysiology, especially regarding the mechanisms leading to nephrotic syndrome.13–20
GBM diseases can be viewed as attractive candidates for reparative protein therapy, because the GBM is directly accessible to protein delivery via the bloodstream. Unlike most other capillaries, the glomerular capillaries are lined by fenestrated endothelial cells, which allows the plasma to reach the GBM so that water and dissolved solutes (such as electrolytes, metabolic waste products, and proteins) can cross into the urinary space in a permselective fashion.4 On the other hand, the GBM components known to be affected by mutations are large macromolecules: collagen IV trimers are approximately 550 kD, and LM-521 trimers are approximately 800 kD. Because these are secreted from cells as preassembled trimers,21 it is highly unlikely that exogenous individual chains (such as laminin β2 or collagen α5[IV]) could integrate into the GBM and function properly, even if they could be delivered to it intravenously (iv). Therefore, logic dictates that laminin or collagen IV trimers be employed for protein therapy approaches.
Given the commercial availability of recombinant human LM-521 (hLM-521) and the high homology between the human and mouse proteins, we delivered hLM-521 iv to Lamb2−/− mice to determine whether it would reach and deposit into the GBM and improve glomerular permselectivity. Biochemical and cell biologic studies demonstrated that hLM-521 was in the form of a trimer, could bind its receptors, and could support cell adhesion and spreading. Although retroorbitally injected hLM-521 accumulated within the endothelial aspect of the GBM, slowed the onset of proteinuria, and attenuated podocyte injury and foot process effacement (FPE), it did not prevent nephrotic syndrome. Nonetheless, these studies demonstrate that very large proteins can be delivered to the GBM, can remain there for weeks, and can affect the GBM’s function as a component of the filtration barrier.
Methods
Characterization of hLM-521
Recombinant hLM-521 with an NH2-terminal FLAG tag on LAMA5 was purchased from BioLamina (Sundbyberg, Sweden). SDS-PAGE using 4%–15% Mini-Protean TGX gels (Bio-Rad, Hercules, CA) was performed under nonreducing and reducing (with β-mercaptoethanol) conditions by standard methods. Proteins were detected with the Pierce Silver Stain kit (Thermo Fisher Scientific, Rockford, IL). Sandwich ELISA was performed by standard methods using a goat antibody to FLAG (Abcam, Cambridge, MA) to coat the plate and a mouse antibody to human LAMC1 (clone 2E8; MilliporeSigma, Burlington, MA) to detect captured hLM-521 with an HRP-conjugated goat anti-mouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and colorimetric development in TMB substrate (Thermo Fisher Scientific). Cell adhesion and receptor inhibition assays using K562 cells were performed as previously described.22
Mice and Intravenous Injections
All animal procedures were performed according to protocols approved by the Washington University Institutional Animal Care and Use Committee. Lamb2−/− mice have been described previously.13,23 Lamb2−/− mice receiving injections also carried the Muscle Creatine Kinase–rat LAMB2 transgene to prevent lethal neuromuscular junction defects.14 Rag1 null mice24 were purchased from The Jackson Laboratory (Bar Harbor, ME). Native equine ferritin was purchased from Sigma (St. Louis, MO). Mice were injected iv with hLM-521 or ferritin via the retroorbital sinus beginning at postnatal day (P) 11, 12, or 13.25
Analysis of the Distribution of hLM-521 and Ferritin after Injection
For immunostaining of 7-µm frozen sections, mouse anti-human LAMA5 clone 4C7 (MilliporeSigma) was used with a mouse-on-mouse kit (Vector Laboratories, Burlingame, CA) and with rabbit anti-mouse agrin.26 Rabbit anti-equine ferritin antibody (Biomatik, Wilmington, DE) was used with rat anti-mouse nidogen (MilliporeSigma). Fluorochrome-conjugated secondary antibodies were purchased from Invitrogen (Carlsbad, CA). Images were acquired with a Nikon C1 confocal system and analyzed in Nikon Elements software (Nikon Instruments, Melville, NY).
For super-resolution imaging by stochastic optical reconstruction microscopy (STORM), kidney fragments were prepared, cryosectioned at 200 nm, and immunostained as previously described27 with the following modifications. Before quenching fixative in glycine solution, sections were incubated in 10 mM Tris (pH=9), 1 mM EDTA, and 0.05% Tween-20 for 20 minutes at 65°C for antigen retrieval. Primary antibodies were as follows: clone 4C7 (human laminin α5 [hLAMA5] LG domain), clone 2E8 (hLAMC1 amino terminus), and rabbit anti-mouse agrin LG domain.26 Single-dye secondary antibodies (Jackson Immunoresearch, West Grove, PA) were made by conjugating to Cy3 (GE Healthcare, Pittsburgh, PA) or Alexa 647 (Invitrogen). Fifteen thousand images were acquired per color in three-frame cycles using Nikon N-STORM and analyzed with NIS-Elements AR software V4.50 (Nikon Instruments).
Analysis of the Effects of hLM-521 on the Glomerulus
Antibodies for immunostaining of 7-µm frozen sections were as follows: rabbit anti-WT1 (Santa Cruz Biotechnology), mouse anti-desmin (clone D33; Dako, Carpinteria, CA), rat anti-mouse platelet endothelial cell adhesion molecule (PECAM) (BD Biosciences, San Jose, CA), rabbit anti-mouse LM-111 (L9393; Sigma), and rabbit anti-mouse agrin.26 Fluorochrome-conjugated secondary antibodies were purchased from Invitrogen (Carlsbad). To quantify podocyte injury, at least 880 WT1-positive podocytes from at least 60 glomeruli from each mouse were examined for the presence of the injury marker desmin. Samples for electron microscopy (EM) were prepared as described previously.13 To assess the extent of podocyte FPE, foot processes along at least 500 µm of total GBM length were quantified using ImageJ (http://rsbweb.nih.gov/ij/) on electron micrographs taken from at least four glomeruli of each animal. Two-tailed, unpaired t tests were used to determine statistical significance in the quantification assays. Differences were considered significant with P<0.05.
Analysis of Urinary Albumin
Creatinine concentrations were quantified by QuantiChrom creatinine assay kit (Hayward, CA) and used to normalize the amounts of urine analyzed by SDS-PAGE and Coomassie blue staining. Albumin concentration was determined by densitometry and comparison to BSA standards loaded on the same gel. Statistical significance between treatment groups was assessed as above.
Results
Characterization of Recombinant hLM-521
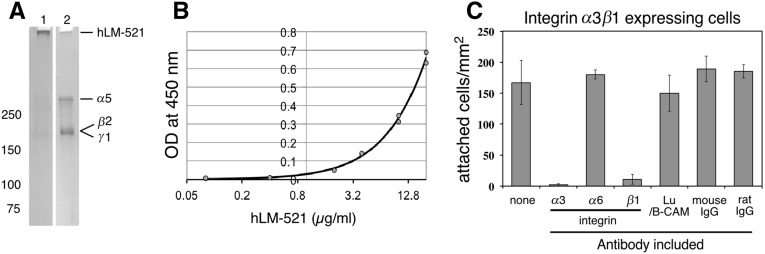
Recombinant hLM-521 purchased from BioLamina was characterized biochemically and cell biologically. SDS-PAGE analysis showed that the nonreduced material migrated at approximately 800 kD, the expected size for the intact disulfide-bonded heterotrimer. After reduction, the components of the trimer were resolved into bands that migrated at approximately 400 kD (α5 chain) and approximately 180–200 kD (β2 and γ1 chains) (Figure 1A). Sandwich ELISAs using antibodies to human laminin γ1 and to the FLAG tag attached to the laminin α5 LN domain confirmed that laminin α5 and γ1 were in a complex (Figure 1B), consistent with the trimer being intact.
Figure 1.
Recombinant hLM-521 is trimeric and adhesive in vitro. (A) Five nanograms of recombinant hLM-521 trimers were subjected to 4%–15% SDS-PAGE and detected by silver staining. The trimers (approximately 800 kD) were too large to migrate out of the well without reduction (lane 1) but separated into approximately 400-kD α5 chains and β2 and γ1 chains that comigrated at approximately 180–200 kD under reducing conditions (lane 2). (B) With a sandwich ELISA using anti-FLAG as a capturing antibody and anti-hLAMC1 as a detection antibody, hLM-521 showed a concentration-dependent (from 0.08 to 20 µg/ml) increase in OD at 450 nm. (C) K562 cells expressing integrin α3β1 were plated onto hLM-521–coated dishes and assayed for the number of cells adhered. Adhesion was specifically blocked by antibodies to integrin α3 and β1 but not by antibodies to integrin α6 or to Lu or by nonimmune mouse and rat IgG.
Next we tested the bioactivity of hLM-521 in cell adhesion assays using nonadherent parental K562 cells and K562 cells expressing either integrin α3β1, integrin α6β1, or the Lutheran cell adhesion molecule (Lu), all of which have been shown to bind the laminin α5 LG domain.22,28 Parental cells did not adhere to LM-521, but cells expressing integrin α3β1 (Figure 1C), α6β1, or Lu (data not shown) adhered well. For cells expressing integrin α3β1, adhesion was inhibited by antibodies to α3 or β1 but not by the other antibodies tested (Figure 1C). All together, these data suggest that the recombinant hLM-521 trimer is intact, the constituent chains are disulfide bonded, and the protein is bioactive.
Intravenously Administered hLM-521 Incorporates into the GBM of Lamb2−/− Mice
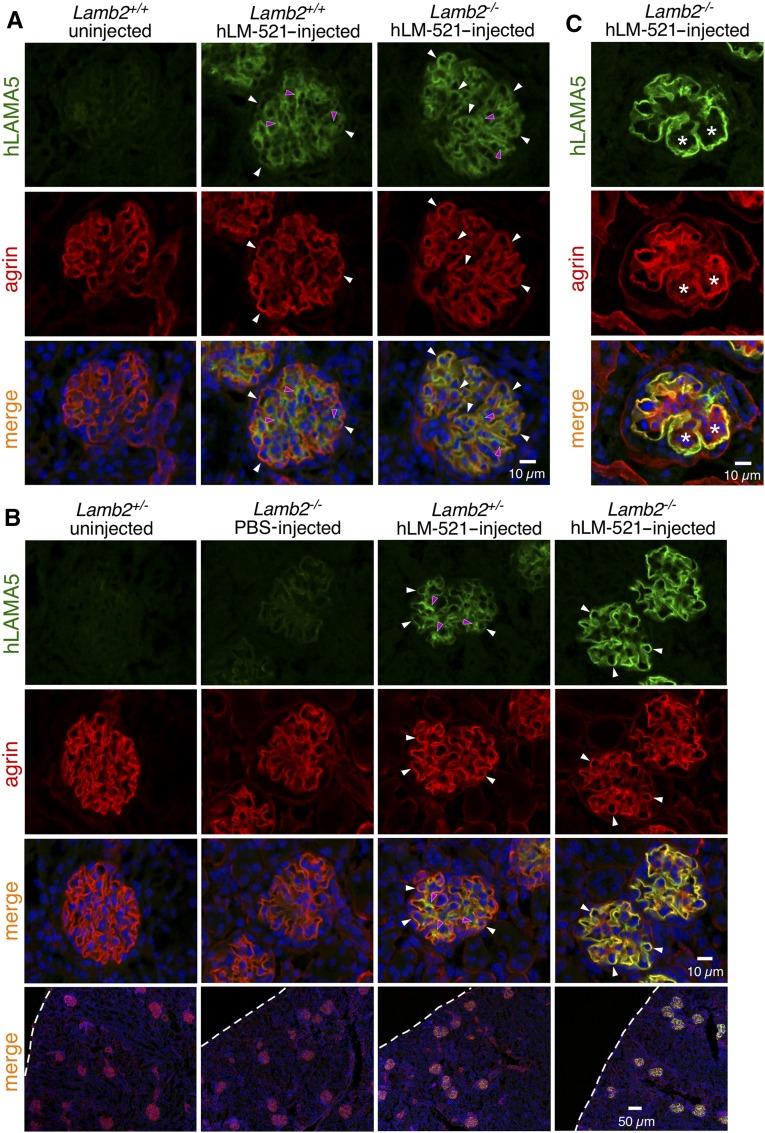
hLM-521 was injected iv into Lamb2−/− and control (Lamb2+/− or WT) pups beginning at approximately P11, 12, or 13 and on a daily basis for at least 5 days. The dose used was approximately 1.2 mg/kg. In a preliminary study, injected animals were euthanized either 15 or 45 minutes after the first injection, and the distribution of hLM-521 was examined with a mouse anti-hLAMA5–specific antibody. Even at the earliest time point, all glomeruli showed evidence of hLM-521 in the GBM, although there was more in the mutant GBM than in the control GBM; controls showed a mostly mesangial staining pattern (Figure 2A). Lamb2−/− mice injected once daily for 5 or more consecutive days showed more distinct accumulation of hLM-521 in the GBM of all glomeruli, compared with Lamb2+/− mice (Figure 2B). That the injected hLM-521 showed more robust deposition in the GBM of Lamb2−/− mice versus Lamb2+/− mice is likely due to the fact that (1) glomeruli receive a large volume of blood flow; (2) the glomerular endothelial cells are highly fenestrated, allowing even the large hLM-521 trimer to readily cross the endothelium; and (3) the reduced laminin level in the mutant GBM18 provides many sites for incorporation of the injected hLM-521. Chase studies showed that injected hLM-521 remained in the GBM of Lamb2−/− mice for at least 3 weeks (Figure 2C), providing initial evidence that it was stably incorporated into the GBM rather than transiently trapped within it.
Figure 2.
Injected hLM-521 is deposited into the GBM. Kidneys from (A) P13 mice either uninjected or at 15 minutes after one injection of hLM-521 (1.2 mg/kg), (B) P17 mice either uninjected or injected with PBS or hLM-521 once daily starting at P12 for 5 days, and (C) P37 mice injected with hLM-521 once daily starting at P12 for 6 days were subjected to confocal immunofluorescence assays. Antibodies to hLAMA5 (green) and agrin (red) were used with nuclear counterstaining (blue). (A) Lamb2+/+ mice showed mostly mesangial staining for hLAMA5 (magenta arrowheads in middle column) that did not overlap with the GBM marker agrin, and weak GBM staining of hLAMA5 (white arrowheads in middle column); Lamb2−/− mice showed hLAMA5 signals that were more distinct in the GBM than in the mesangial matrix (compare white arrowheads to magenta arrowheads in right column). (B) After five doses of hLM-521, Lamb2−/− mice displayed more robust accumulation of hLM-521 in the GBM compared with Lamb2+/− mice. Uninjected or PBS-injected mice showed no specific LAMA5 signal. Dashed lines demarcate the surface of the kidney. (C) After 20 days of chase, Lamb2−/− mice retained hLM-521 in the GBM, even when the mesangial matrix had expanded (asterisks).
In addition to kidney, injected hLM-521 was detected in endothelial basement membranes of adrenal glands of both Lamb2+/− and Lamb2−/− mice (Supplemental Figure 1A). However, unlike the retention of hLM-521 in the GBM for at least 3 weeks, the level of hLM-521 in adrenal glands was substantially reduced after 7 days of chase (Supplemental Figure 1A) and was barely detectable after 12 days (data not shown). We could not detect hLM-521 in the basement membranes of other tissues, including those that are highly vascularized, such as lung, liver, and skeletal muscle (data not shown), or that contain fenestrated endothelial cells, such as choroid plexus, pancreatic islets, and intestine (Supplemental Figure 1B). In addition to having fenestrated endothelial cells, adrenal glands, like the kidney, receive blood flow from the renal artery; this likely increases the chance of deposition of injected proteins.
Because the resolution of standard immunofluorescence microscopy is too low to pinpoint the location of proteins within the glomerular capillary wall, we used STORM, a form of super-resolution imaging, to localize the injected hLM-521. While attempting this analysis, a difficulty we encountered was the production of anti–hLM-521 antibodies by the mice receiving daily injections of hLM-521. Distinct, linear deposition of mouse IgG was detected in the GBM of injected Lamb2−/− mice, compared with weak, linear deposition of mouse IgG in untreated Lamb2−/− mice and undetectable mouse IgG in untreated Lamb2+/− control mice (Supplemental Figure 2A). The serum of hLM-521–injected mice contained antibodies that bound human glomerular and other renal basement membranes (Supplemental Figure 2C). Although the use of a mouse-on-mouse staining kit was sufficient to eliminate most of the signals from endogenous mouse antibodies in standard immunofluorescence assays (Figure 2), the extreme sensitivity of STORM required an alternative approach. To eliminate this problem for STORM studies, we mated the Lamb2 null allele onto the Rag1−/− genetic background to produce mice that lacked mature B and T cells and thus could not produce antibodies. The lack of antibody production upon hLM-521 injection was verified by the lack of accumulation of mouse IgG in the GBM (Supplemental Figure 2B).
Lamb2−/−; Rag1−/− and Lamb2+/−; Rag1−/− mice were injected iv daily for 6 days with hLM-521 and euthanized 1 day later; uninjected mice served as controls. The kidneys were processed and sectioned for standard immunofluorescence staining or for STORM. By standard immunofluorescence, Lamb2−/−; Rag1−/− mice showed a staining pattern for hLM-521 similar to that observed in Lamb2−/− mice, with distinct accumulation of hLM-521 in the GBM of all glomeruli (Supplemental Figure 3A). Chase studies revealed that the injected hLM-521 remained in the GBM for at least 11 days (Supplemental Figure 3B). This suggested that the robust deposition of hLM-521 in the GBM of Lamb2−/− mice (Figure 2) was not facilitated by the anti–hLM-521 antibodies in the GBM.
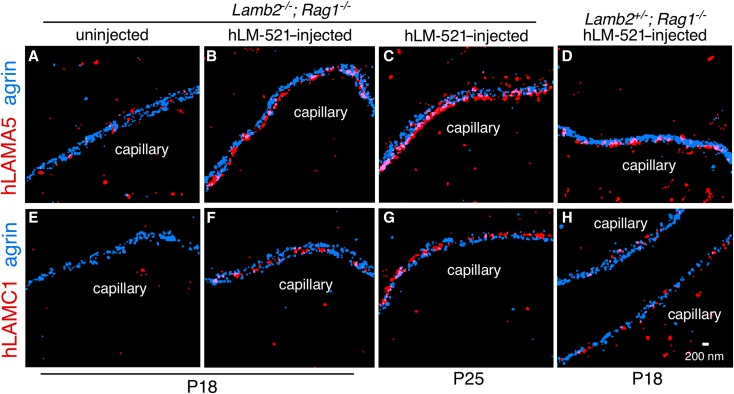
For STORM, kidney sections were stained with antibodies to the carboxyl terminus of hLAMA5 or to a domain near the amino terminus of human laminin γ1. They were costained with an antibody to mouse agrin that normally labels both outer aspects of the GBM, revealing two separate layers.27 Uninjected mice showed no specific hLM-521 signals, but mice injected with hLM-521 showed discrete linear hLAMA5 signals confined to the endothelial aspect and absent from the podocyte aspect of the GBM (Figure 3, A–C). Interestingly, hLM-521–injected Lamb2+/− mice showed interrupted linear staining for hLAMA5 at the endothelial aspect of the GBM (Figure 3D). In contrast, antibody to the human laminin γ1 amino terminus revealed linear staining of the GBM in hLM-521–injected mice that was present near the center of the GBM (Figure 3, E–H). All together, these data suggest that the injected hLM-521 readily crossed the glomerular endothelium and stably incorporated into the endothelial aspect of the GBM, with the carboxyl-terminal LG domain adjacent to the endothelial cells and the amino terminus at the center of the GBM; this orientation is consistent with our previous report of the orientation of endogenous LM-521.27 Despite these data supporting the stable and oriented incorporation of hLM-521 into the endothelial aspect of the GBM, further studies are required to determine how hLM-521 interacts with other GBM proteins and with cellular receptors.
Figure 3.
Injected hLM-521 is localized to the endothelial aspect of the GBM and properly oriented. Lamb2−/− and +/− mice also null for Rag1 either uninjected (A and E) or injected with hLM-521 once daily starting at P12 for 6 days and dissected at P18 or P25 were analyzed by STORM using antibodies to hLAMA5 (red) and agrin (blue). (B and C) hLM-521–injected Lamb2−/− mice showed discrete linear signals for human LAMA5 confined to the endothelial aspect of the GBM versus (D) the interrupted linear pattern in hLM-521–injected Lamb2+/− mice. (F and G) hLM-521–injected Lamb2−/− mice also showed linear staining of human laminin γ1 at the center of the GBM between the agrin layers compared with (H) the scattered staining in hLM-521–injected Lamb2+/− mice.
hLM-521 Slows the Onset of Proteinuria in Lamb2−/− Mice
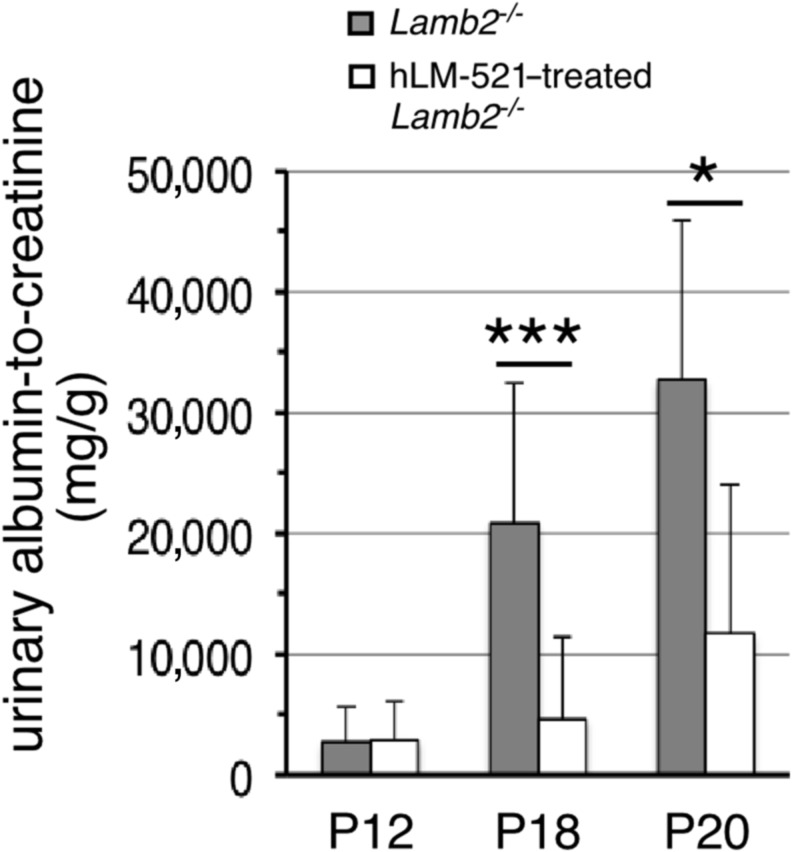
Having determined that the injected hLM-521 accumulates in the GBM, we investigated its effect on proteinuria. We began injecting mice iv at P11 or P12 and daily thereafter for 5–7 days or longer (see below). In Lamb2−/− mice with a urinary albumin-to-creatinine ratio (ACR) <9000 mg/g before injection, treatment with hLM-521 kept albuminuria low through P18, as compared with uninjected Lamb2−/− mice that showed the typical rise toward a nephrotic range level by P18 (Figure 4). At P20 the ACR for hLM-521–treated Lamb2−/− mice began to rise, but it was still significantly lower than that for untreated Lamb2−/− mice (Figure 4). The BUN level (mg/dl) of hLM-521–injected Lamb2−/− mice was not significantly different from that of uninjected Lamb2−/− mice (24.5±8.4, n=12 versus 19.3±4.9, n=9; P>0.05), suggesting that the reduction in albuminuria upon hLM-521 treatment was not due to a decreased GFR. By approximately P24, all hLM-521–treated Lamb2−/− mice had progressed to nephrotic range proteinuria (Supplemental Figure 4 and data not shown). The inability of hLM-521 treatment to sustain low proteinuria in mutant mice after P20 was not due to loss of hLM-521 from the GBM after P20, because the injected hLM-521 remained deposited in a linear pattern at the endothelial aspect of the GBM at P25, similar to that observed at P18 (Figure 3, compare B to C and F to G). This is consistent with the chase data obtained from standard immunofluorescence analyses (Figure 2C, Supplemental Figure 3B). Furthermore, the incomplete efficacy of hLM-521 treatment likely did not result from insufficient dosage of hLM-521, because daily injection of hLM-521 through at least P22 did not inhibit progression to nephrotic range proteinuria. For Lamb2−/− mice that showed ACR>9000 mg/g at P12, hLM-521 treatment did not significantly reduce the ACR compared with untreated Lamb2−/− mice (data not shown).
Figure 4.
hLM-521 treatment delays albuminuria in Lamb2−/− mice. Mice were untreated or injected with hLM-521 once daily starting at P11 or P12 for 5–7 days. Urine was collected before injection and at various time points thereafter for assessing ACRs (milligrams per gram). In Lamb2−/− mice with albuminuria<9000 before injection, treatment with hLM-521 kept proteinuria low through P18, compared with uninjected Lamb2−/− mice that showed high proteinuria at P18 (4651±6770, n=12 versus 20,826±11,580, n=8; ***P<0.001). At P20 albuminuria in treated mice began to rise but was still significantly lower than that in untreated Lamb2−/− mice (11,696±12,406, n=5 versus 32,711±13,184, n=5; *P<0.05).
To examine whether the inability of hLM-521 to prevent nephrotic range proteinuria in Lamb2−/− mice that initially responded to treatment was caused by anti–hLM-521 antibodies, Lamb2−/−; Rag1−/− mice that were unable to produce antibodies were injected at P12 daily for 5–7 days and examined for albuminuria. Out of six mice tested, four started with ACR>11,000 mg/g at P12 and did not show reduced proteinuria upon hLM-521 treatment. In contrast, for the two mice with milder proteinuria at P12, hLM-521 treatment suppressed the rise of albuminuria through P18, but it did not prevent the progression to nephrotic range proteinuria after P20 in the one mouse that was followed to P25 (data not shown). Together, these results suggest that anti–hLM-521 antibodies cannot be responsible for progression to nephrotic range proteinuria.
To rule out the possibility that the delayed onset of proteinuria in hLM-521–treated Lamb2−/− mice was due to nonspecific macromolecule-mediated blockage of the GFB, we injected 2 mg/kg ferritin (MW=440 kD) iv into mice at P12 daily for 6 days (a molarity three times that of hLM-521) and collected tissues at P18 for immunofluorescence staining. Ferritin staining was not present in the GBM of injected Lamb2+/+ or Lamb2−/− mice, but it was detected in the cytoplasm of some tubular cells, with more distinct staining in Lamb2−/− versus Lamb2+/+ tubules (Supplemental Figure 5). Consistent with previous findings,15 these results suggest that Lamb2−/− mice have a leaky GFB that allowed ferritin to pass through without becoming trapped within the GBM, precluding it from being able to affect proteinuria. Together with the STORM results above that show properly oriented hLM-521 in the GBM, these data indicate that the delayed onset of proteinuria in Lamb2−/− mice treated with hLM-521 did not result from nonspecific clogging of the GFB.
hLM-521 Attenuates Podocyte Injury
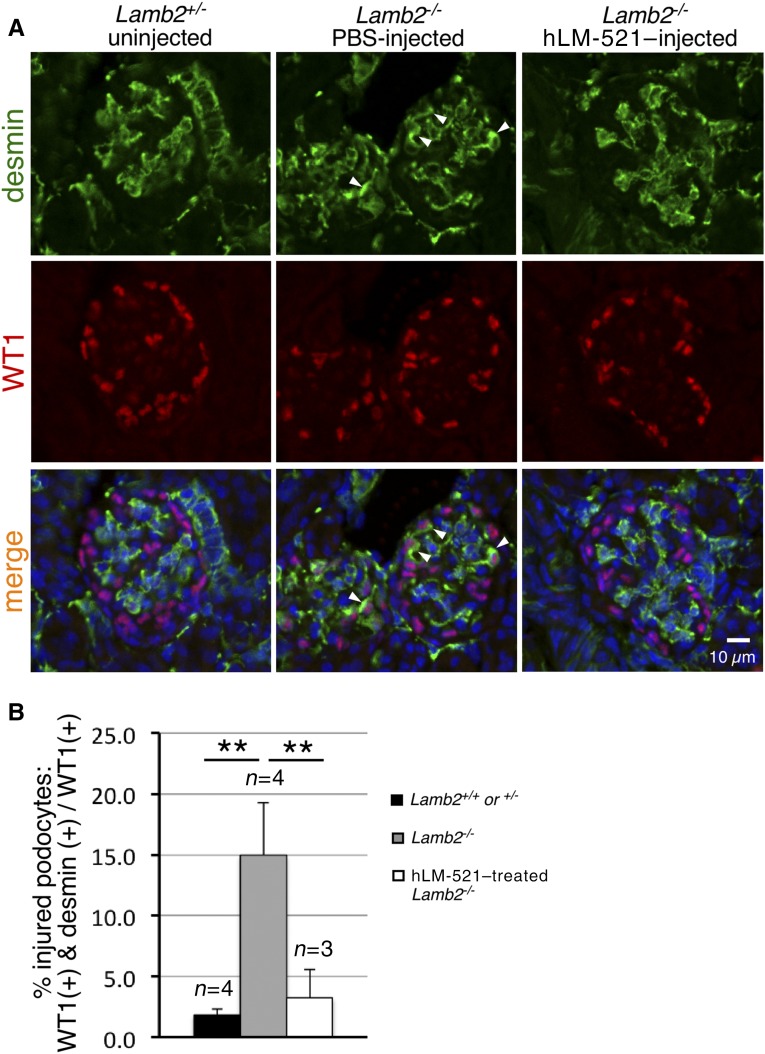
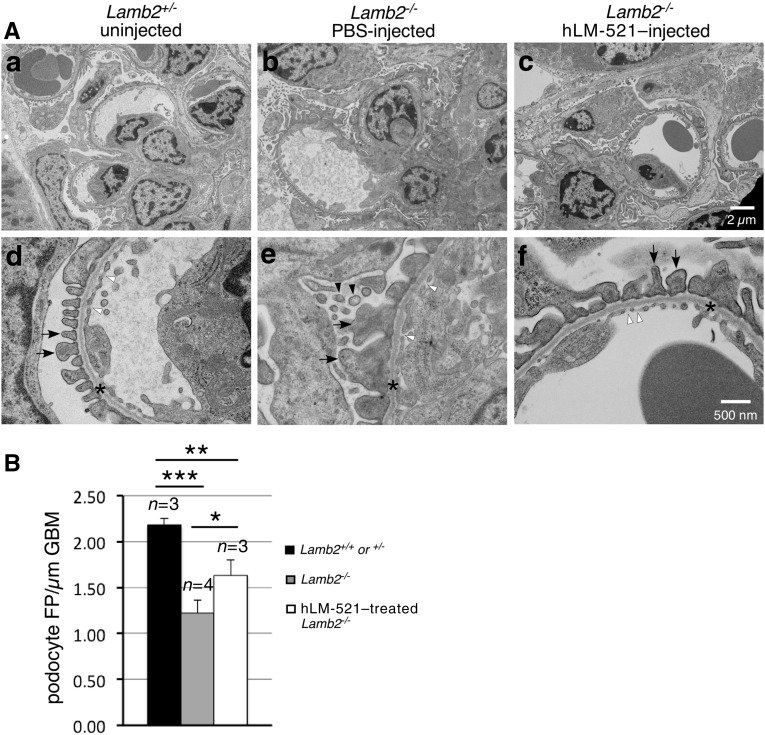
Having determined that the injected hLM-521 does not reach the podocyte aspect of the GBM and therefore cannot directly interact with podocytes, we investigated how the incorporation of hLM-521 into the endothelial aspect of the GBM might indirectly affect podocyte phenotype. We examined podocyte expression of desmin, an established marker of podocyte injury.18,29 Sections of P18 kidneys from Lamb2+/− and Lamb2−/− mice either untreated or injected with hLM-521 or PBS were costained for desmin and WT1, a podocyte nuclear protein (Figure 5A). The ratio of the number of WT1-desmin double-positive cells (injured podocytes) to the number of WT1-positive cells (total podocytes) was calculated to determine the percentage of injured podocytes (Figure 5B). Untreated and PBS-treated Lamb2−/− kidneys showed a seven-fold increase in podocyte injury versus Lamb2+/+ and Lamb2+/− controls. Treatment with hLM-521 significantly reduced podocyte injury in Lamb2−/− kidneys, to a level that was not significantly different from Lamb2+/+ and Lamb2+/− controls. Ultrastructural analysis of the GFB from P18 mice showed that treatment with hLM-521 reduced the extent of villous transformation that is typically observed in podocytes when proteinuria is significant (Figure 6A). The hLM-521 treatment also significantly attenuated podocyte FPE in Lamb2−/− mice as assessed by the density of foot processes along the GBM (Figure 6). However, the density of foot processes in hLM-521–treated Lamb2−/− mice was significantly lower than that in Lamb2+/+ and Lamb2+/− controls.
Figure 5.
hLM-521–treated Lamb2−/− mice show attenuated podocyte injury. (A) Kidneys collected from P18 mice either untreated or injected with PBS or hLM-521 once daily for 5 days were sectioned and immunostained for desmin (green) and WT1, a podocyte nucleus marker (red), and examined by confocal microscopy after nuclear counterstaining (blue). Desmin was normally detected in mesangial cells (Lamb2+/−; left column). In PBS-injected Lamb2−/− mice (middle column), there was occasional ectopic expression of desmin in WT1-positive podocytes (arrowheads). With hLM-521 treatment (right column), there was a dramatic reduction in desmin-positive podocytes. (B) Quantification of the ratio of desmin-WT1 double-positive cells to total WT1-positive cells shows the significant reduction in podocyte injury with hLM-521 treatment (3.2%±2.3%, n=3 versus 14.9%±4.3%, n=4; **P<0.01).
Figure 6.
Podocyte FPE is reduced in hLM-521–treated Lamb2−/− mice. Kidneys collected from P18 mice uninjected or injected with PBS or hLM-521 once daily for 5 days were analyzed by transmission electron microscopy. (A) The GBMs (asterisks in d–f) appeared normal, detected between the podocyte foot processes (arrows) and the endothelium (fenestrae marked with white arrowheads). Villous transformation in podocytes of Lamb2−/− mice (black arrowheads in e) was reduced (f) in Lamb2−/− mice treated with hLM-521. (B) When quantified by number of foot processes per micrometer of GBM, uninjected or PBS-injected Lamb2−/− mice showed significant reduction in the density of foot processes compared with Lamb2+/+ and Lamb2+/− controls (1.22±0.14, n=4 versus 2.18±0.07, n=3 glomeruli; ***P<0.001). hLM-521 treatment significantly increased the density of podocyte foot processes in Lamb2−/− mice (1.63±0.17, n=3; *P<0.05). FP, foot processes.
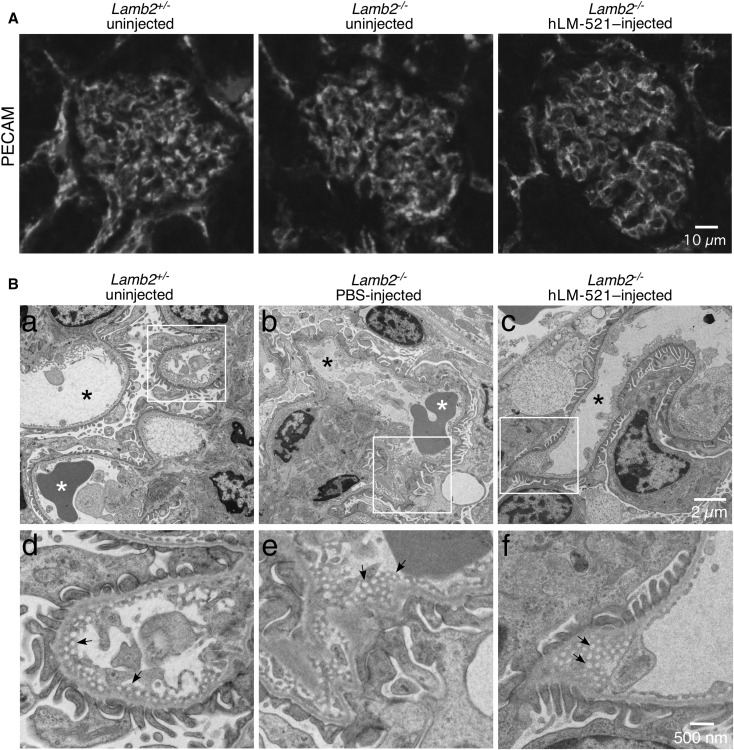
We also investigated whether accumulation of hLM-521 at the endothelial aspect of the GBM had any effect on the endothelium that might explain the reduction in proteinuria. By immunofluorescence staining, localization of PECAM in the glomerular capillary wall of both untreated and hLM-521–treated Lamb2−/− mice was similar to that of Lamb2+/− mice at P18 (Figure 7A). Ultrastructural analysis of the glomerular endothelial fenestrae of Lamb2−/− mice at P18 revealed a normal organization, similar to Lamb2+/− mice (Figure 7B). Treatment with hLM-521 did not change fenestral organization in Lamb2−/− mice (Figure 7B).
Figure 7.
The Lamb2−/− glomerular endothelium appears normal with or without hLM-521 treatment. Kidneys collected from P18 Lamb2−/− mice, either uninjected or injected with PBS or hLM-521 once daily starting at P12 for 5 days, and from uninjected Lamb2+/− mice were analyzed by (A) immunofluorescence staining for PECAM and (B) transmission electron microscopy. (A) Localization of PECAM in the glomerular capillary wall of both untreated and hLM-521–treated Lamb2−/− mice was similar to that of Lamb2+/− mice. (B) In all mice the glomerular endothelium was thin and well fenestrated (arrows in d–f), and the capillary loops were patent (black asterisks in a–c). The boxed regions in (a–c) are magnified in (d–f), respectively. White asterisks, red blood cells.
Discussion
Protein therapy has been proven effective in mouse models of basement membrane protein–associated diseases. For example, LM-111 delivered intraperitoneally weekly starting at P10 improves muscle strength and life span of Lama2 mutant mice that model congenital muscular dystrophy.30 Delivery of one dose of collagen VII iv at birth improves the dermal-epidermal adherence and life span of Col7a1 mutant mice that model recessive dystrophic epidermolysis bullosa.31 Here we tested whether hLM-521 delivered iv could reach the GBM and improve glomerular filtration defects in the Lamb2−/− mouse model of Pierson syndrome.
Our results demonstrate that after injection into the retroorbital sinus of Lamb2−/− mice, hLM-521 specifically deposited in the kidneys and adrenal glands but not in other tissues. hLM-521 was detected in nearly all GBM segments, and it remained for at least 3 weeks; this suggests stable incorporation into the GBM rather than nonspecific trapping, a conclusion also supported by super-resolution imaging showing a uniform and proper orientation of hLM-521 within the GBM. Data from Lamb2−/−; Rag1−/− mice indicate that the retention of hLM-521 in the GBM was not enhanced by anti–hLM-521 antibodies. In contrast to the GBM deposition in Lamb2−/− mice, control mice displayed a primarily mesangial pattern of hLM-521 deposition. This differential deposition of hLM-521 may relate to the increased porosity of the abnormally “fluid” Lamb2−/− GBM and increased availability of laminin binding sites therein. It could also involve an abnormal endothelial glycocalyx.
The fenestrations in the glomerular endothelial cells, which were found to be ultrastructurally normal, should favor efficient passage of hLM-521 into the GBM. Although the endothelial cells of the choroid plexus, pancreatic islets, and intestine are also fenestrated, and their basement membranes normally contain LM-521, injected hLM-521 was not detected in these tissues. The much greater volume of blood flow to the kidney as compared with these tissues, coupled with the high rate of fluid flow across the GFB, may account for this. The transient accumulation of hLM-521 in the adrenals, which receive some blood supply from the renal artery, may also stem from greater blood flow.
Super-resolution STORM imaging showed that iv injected hLM-521 incorporated into the endothelial aspect of the GBM in an orientation consistent with what we previously reported for LM-521 in mice.27 This proper orientation argues against nonspecific trapping of the protein. However, injected LM-521 did not reach the podocyte side of the GBM. This is also consistent with our previous results showing that hLAMA5 expressed from a transgene in either endothelial cells or podocytes accumulates only within the aspect of the GBM nearest the synthesizing cells; laminin does not cross over to the opposite side of the GBM.27 This suggests that the dense layer of matrix proteins at the center of the GBM, which includes collagen α3α4α5(IV)27 and likely also other proteins,32 serves as an efficient barrier to large macromolecules.
hLM-521 treatment of Lamb2−/− mice slowed the onset of proteinuria until P18 but was unable to suppress the progression to nephrotic range proteinuria after P20, even with treatments beyond P20. We could find no evidence that the incomplete rescuing effect of hLM-521 was associated with injury to glomerular endothelial cells. Our best explanation for the partial rescuing effect of hLM-521 is that the injected hLM-521 only deposited in the endothelial aspect of the GBM but could not cross over to the podocyte aspect and restore a complete GFB for the long term. In support of this, podocyte-specific knockout of Lama5 causes proteinuria despite the persistence of LAMA5 on the endothelial aspect of the GBM.33 As accomplished with transgene approaches in mouse models of muscular dystrophy,34–37 it may be possible to design smaller matrix proteins that can cross to the podocyte aspect of the GBM, incorporate, and improve barrier function across the entire width of the GBM.
Even without reaching the podocytes, hLM-521 slowed the onset of proteinuria and dramatically reduced accumulation of the podocyte injury marker desmin in Lamb2−/− mice through P18. This suggests that the podocyte injury normally observed in Lamb2−/− mice is not due to lack of LM-521 and its signaling to podocytes but is perhaps due to proteinuria and the increased exposure to albumin. Our data also showed that hLM-521 treatment was associated with moderate attenuation of FPE, but the mechanism for this is currently unclear. That the hLM-521 treatment had more effect on slowing podocyte injury (on the basis of desmin staining) than on reducing FPE is consistent with accumulation of desmin in podocytes being a later event than FPE in mice with proteinuria and podocyte injury.
In conclusion, our studies demonstrate that the approximately 800-kD hLM-521 trimeric protein can be delivered to the GBM, remains there for several weeks to modify GBM composition, improves podocyte homeostasis, and delays the increase in proteinuria. Although hLM-521 treatment alone did not prevent the onset of nephrotic syndrome, its administration together with a regimen of drugs to reduce proteinuria could improve the outcome of patients with Pierson syndrome or with milder forms of LM-521 deficiency or dysfunction.
Disclosures
K.T. is a cofounder and shareholder in BioLamina AB. B.L.H. is the founder and a shareholder of Prothelia Inc.
Supplementary Material
Acknowledgments
We thank Jennifer Richardson for genotyping the mice and preparing electron microscopy samples, Gloriosa Go for measuring urinary creatinine levels, the Mouse Genetics Core for mouse husbandry and urine collections, the Kidney Translational Research Core for normal human kidney sections, and the Washington University Center for Cellular Imaging (supported by the Diabetes Research Center, National Institutes of Health [NIH] P30DK020579). We also thank Takako Sasaki for the agrin antibody.
This work was funded by NIH grant R01DK078314 and a “Seeding Collaborations for Translational Research” supplement from the National Institute of Diabetes and Digestive and Kidney Diseases to J.H.M., as well as by the Knut and Alice Wallenberg Foundation and the Swedish Research Council.
M.-H.L., B.L.H., K.T., and J.H.M. designed the study; M.-H.L., J.B.M., Y.K., H.Y.S., and B.L.H. carried out experiments; M.-H.L., Y.K., H.Y.S., B.L.H., and J.H.M. analyzed the data; M.-H.L., Y.K., and B.L.H. made the figures; M.-H.L., K.T., and J.H.M. drafted and revised the paper; all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Repairing the GBM Step by Step,” on pages 1346–1347.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017060690/-/DCSupplemental.
References
- 1.Miner JH: Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol 26: 1413–1417, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miner JH: The glomerular basement membrane. Exp Cell Res 318: 973–978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh JH, Miner JH: The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol 9: 470–477, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott RP, Quaggin SE: Review series: The cell biology of renal filtration. J Cell Biol 209: 199–210, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, et al.: Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248: 1224–1227, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Hudson BG, Reeders ST, Tryggvason K: Type IV collagen: Structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 268: 26033–26036, 1993 [PubMed] [Google Scholar]
- 7.Lemmink HH, Mochizuki T, van den Heuvel LP, Schröder CH, Barrientos A, Monnens LAH, et al.: Mutations in the type IV collagen alpha 3 (COL4A3) gene in autosomal recessive Alport syndrome. Hum Mol Genet 3: 1269–1273, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Miner JH, Baigent C, Flinter F, Gross O, Judge P, Kashtan CE, et al.: The 2014 international workshop on Alport syndrome. Kidney Int 86: 679–684, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F: Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol 24: 364–375, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, et al.: Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat 31: 992–1002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, et al.: Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int 70: 1008–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, et al.: Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625–2632, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP: The renal glomerulus of mice lacking s-laminin/laminin beta 2: Nephrosis despite molecular compensation by laminin beta 1. Nat Genet 10: 400–406, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Miner JH, Go G, Cunningham J, Patton BL, Jarad G: Transgenic isolation of skeletal muscle and kidney defects in laminin beta2 mutant mice: Implications for Pierson syndrome. Development 133: 967–975, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarad G, Cunningham J, Shaw AS, Miner JH: Proteinuria precedes podocyte abnormalities inLamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272–2279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YM, Kikkawa Y, Miner JH: A missense LAMB2 mutation causes congenital nephrotic syndrome by impairing laminin secretion. J Am Soc Nephrol 22: 849–858, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YM, Zhou Y, Go G, Marmerstein JT, Kikkawa Y, Miner JH: Laminin β2 gene missense mutation produces endoplasmic reticulum stress in podocytes. J Am Soc Nephrol 24: 1223–1233, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh JH, Jarad G, VanDeVoorde RG, Miner JH: Forced expression of laminin beta1 in podocytes prevents nephrotic syndrome in mice lacking laminin beta2, a model for Pierson syndrome. Proc Natl Acad Sci USA 108: 15348–15353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bull KR, Mason T, Rimmer AJ, Crockford TL, Silver KL, Bouriez-Jones T, et al.: Next-generation sequencing to dissect hereditary nephrotic syndrome in mice identifies a hypomorphic mutation in Lamb2 and models Pierson’s syndrome. J Pathol 233: 18–26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funk SD, Bayer RH, Malone AF, McKee KK, Yurchenco PD, Miner JH: Pathogenicity of a human laminin β2 mutation revealed in models of Alport syndrome. J Am Soc Nephrol 29: XXX–XXX, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yurchenco PD, Quan Y, Colognato H, Mathus T, Harrison D, Yamada Y, et al.: The alpha chain of laminin-1 is independently secreted and drives secretion of its beta- and gamma-chain partners. Proc Natl Acad Sci USA 94: 10189–10194, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikkawa Y, Sasaki T, Nguyen MT, Nomizu M, Mitaka T, Miner JH: The LG1-3 tandem of laminin alpha5 harbors the binding sites of Lutheran/basal cell adhesion molecule and alpha3beta1/alpha6beta1 integrins. J Biol Chem 282: 14853–14860, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP: Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature 374: 258–262, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE: RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68: 869–877, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S: Retro-orbital injections in mice. Lab Anim (NY) 40: 155–160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, et al.: Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol 171: 139–152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suleiman H, Zhang L, Roth R, Heuser JE, Miner JH, Shaw AS, et al.: Nanoscale protein architecture of the kidney glomerular basement membrane. eLife 2: e01149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikkawa Y, Sudo R, Kon J, Mizuguchi T, Nomizu M, Hirata K, et al.: Laminin alpha 5 mediates ectopic adhesion of hepatocellular carcinoma through integrins and/or Lutheran/basal cell adhesion molecule. Exp Cell Res 314: 2579–2590, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Yaoita E, Kawasaki K, Yamamoto T, Kihara I: Variable expression of desmin in rat glomerular epithelial cells. Am J Pathol 136: 899–908, 1990 [PMC free article] [PubMed] [Google Scholar]
- 30.Rooney JE, Knapp JR, Hodges BL, Wuebbles RD, Burkin DJ: Laminin-111 protein therapy reduces muscle pathology and improves viability of a mouse model of merosin-deficient congenital muscular dystrophy. Am J Pathol 180: 1593–1602, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou Y, Guey LT, Wu T, Gao R, Cogan J, Wang X, et al.: Intravenously administered recombinant human type VII collagen derived from Chinese hamster ovary cells reverses the disease phenotype in recessive dystrophic epidermolysis bullosa mice. J Invest Dermatol 135: 3060–3067, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Lennon R, Byron A, Humphries JD, Randles MJ, Carisey A, Murphy S, et al.: Global analysis reveals the complexity of the human glomerular extracellular matrix. J Am Soc Nephrol 25: 939–951, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg S, Adair-Kirk TL, Senior RM, Miner JH: Maintenance of glomerular filtration barrier integrity requires laminin alpha5. J Am Soc Nephrol 21: 579–586, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKee KK, Crosson SC, Meinen S, Reinhard JR, Rüegg MA, Yurchenco PD: Chimeric protein repair of laminin polymerization ameliorates muscular dystrophy phenotype. J Clin Invest 127: 1075–1089, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinhard JR, Lin S, McKee KK, Meinen S, Crosson SC, Sury M, et al.: Linker proteins restore basement membrane and correct LAMA2-related muscular dystrophy in mice. Sci Transl Med 9: eaal4649, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentzinger CF, Barzaghi P, Lin S, Ruegg MA: Overexpression of mini-agrin in skeletal muscle increases muscle integrity and regenerative capacity in laminin-alpha2-deficient mice. FASEB J 19: 934–942, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Moll J, Barzaghi P, Lin S, Bezakova G, Lochmüller H, Engvall E, et al.: An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature 413: 302–307, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.