Abstract
Background Thrombospondin type 1 domain–containing 7A (THSD7A) has been identified as a pathogenic autoantigen in membranous nephropathy (MN). However, the THSD7A epitopes targeted by patient autoantibodies are unknown.
Methods We performed an in silico analysis of the THSD7A multidomain structure, expressed the folded domains in HEK293 cells, and tested for domain reactivity with 31 serum samples from patients with THSD7A-associated MN using Western and native blotting. Immunogenicity of the antigen domains was further investigated by cDNA immunization of rabbits and mice.
Results We characterized the extracellular topology of THSD7A as a tandem string of 21 thrombospondin type 1 domains. Overall, 28 serum samples (90%) recognized multiple epitope domains along the molecule. Detailed epitope mapping revealed that the complex consisting of the first and second N-terminal domains (amino acids 48–192) was recognized by 27 of 31 patient serum samples (87%). Serum recognizing one or two epitope domains showed lower anti-THSD7A antibody levels than serum recognizing three or more epitope domains. During follow-up, a loss of epitope recognition was observed in seven of 16 patients, and it was accompanied by decreasing antibody levels and remission of proteinuria. In four of 16 patients, epitope recognition patterns changed during follow-up. Notably, immunization experiments in rabbits and mice revealed that induced antibodies, like patient autoantibodies, preferentially bound to the most N-terminal domains of THSD7A.
Conclusions Our data show that the immune response in THSD7A-associated MN is polyreactive and that autoantibodies predominantly target the most N-terminal part of THSD7A.
Keywords: glomerular disease, membranous nephropathy, clinical immunology
Primary membranous nephropathy (MN) is an autoimmune disease and a major cause of nephrotic syndrome in adult patients. The clinical outcome varies, with about 30% of patients experiencing spontaneous remission, whereas another 20%–30% develop ESRD within 10 years.1 Two podocyte-expressed autoantigens have been identified in primary MN so far: phospholipase A2 receptor 1 (PLA2R1) and thrombospondin type 1 domain–containing 7A (THSD7A).2,3 Anti-PLA2R1 antibody levels associate with clinical outcome of affected patients.4–6 Therefore, measurement of anti-PLA2R1 antibodies is useful for diagnosis, individual risk assessment, and monitoring of treatment in patients with MN, including the time after transplantation.7–10 The prevalence of THSD7A-associated MN is significantly lower compared with that of PLA2R1-associated MN, and the clinical usefulness of anti-THSD7A autoantibody measurement is currently under investigation.11 Noteworthy, 20% of white patients with THSD7A-associated MN have concurrent malignancies, suggesting that intensive screening for malignancies is advised in these patients.12,13
The identification of antigen epitopes in renal autoimmune diseases, such as anti-glomerular basement membrane disease and ANCA vasculitis, has contributed to the understanding of the disease mechanisms in these entities.14,15 Recently, the most N-terminal part of PLA2R1 was identified as the immunodominant epitope region in patients with PLA2R1-associated MN.16,17 PLA2R1 contains at least two more epitope regions involved in autoimmune processes in MN, and epitope spreading from the N-terminus toward the C-terminus might associate with a poor clinical outcome and a reduced response to immunosuppressive therapy.18,19
In this study, we identified the autoantibody binding sites in THSD7A. Furthermore, we characterized the association of individual epitope profiles and changes of epitope recognition patterns over time with the clinical presentation. Additionally, we experimentally investigated the immune response against THSD7A using animal models of active immunization.
Methods
Design and Generation of THSD7A Fragments
THSD7A was split into three parts: d1_d4 (Ala-48 to Ala-423), d5_d10 (Thr-424 to Gln-831), and d11_d21 (Ser-832 to His-1535). To define more precise epitope regions, we designed fragments of THSD7A containing three or two domains each: d1_d2 (Ala-48 to Gln-192), d2_d3 (Trp-117 to Cys-246), d3_d4 (Gln-193 to Ala-423), d5_d6 (Thr-424 to Tyr-574), d7_d8 (Asp-575 to Thr-695), d9_d10 (Val-696 to Gln-831), d11_d12 (Ser-832 to Asp-959), d13_d14 (Lys-960 to Asn-1095), d15_d16 (Gln-1096 to Tyr-1220), d17_d18 (His-1221 to Tyr-1341), and d19_d21 (Arg-1342 to His-1535). An additional d1_d3 (Ala-48 to Cys-246) construct was designed for purification of domain-specific antibodies. All variants were generated by PCR and cloned into the eukaryotic expression vector pCSE2.5 (provided by Thomas Schirrmann, Braunschweig, Germany). This vector has been optimized for secretory protein production in suspension cultures of HEK293–6E cells.20 The cDNA of a full-length, flag-tagged THSD7A variant served as the PCR template (Origene). All constructs were designed to be secreted to the cell culture medium. All constructs contained a C-terminal 6× his tag. Full sequencing validated the accuracy of all constructs.
Cell Culture, Cell Transfection, and Recombinant Protein Expression
HEK293 cells were kept in culture and transfected with the generated constructs. Cells were harvested and lyzed in 50 mM Tris (pH 7.4), 1 mM EDTA, 150 mM NaCl, and 1% Triton. All expressions were validated by Western blot and immunologic detection using an anti-his antibody (1:1000; Thermo Scientific, Cramlington, United Kingdom). Details on these procedures are presented in Supplemental Material.
Western Blot and Immunologic Detection
If reducing conditions were desired, samples were heated in 20% β-mercaptoethanol. Proteins were separated by electrophoresis and subsequently transferred to methanol-soaked polyvinylidene difluoride membranes. Membranes were then blocked for 2 hours at room temperature followed by incubation with the primary antibody. Sera (human, mouse, and rabbit) were diluted 1:100. All sera were tested using horseradish peroxidase–conjugated anti-human IgG4 and antitotal human IgG, and protein bands were visualized using a chemiluminescence substrate. Details on these procedures and the used materials are presented in Supplemental Material.
Native Blotting (Dot Blot Analyses) and Immunologic Detection
His-tagged thrombospondin type 1 (TSP-1) domain constructs (d1_d2 to d19_d21) were purified under native conditions using an Ni-NTA resin (His-Pur, 88221; Thermo Scientific) and applying the batch method according to the manufacturer’s instructions. Purified proteins were then dotted on nitrocellulose membranes and allowed to dry for 10 minutes at room temperature. Membranes were then blocked in 4% dry milk in PBS plus Tween 0.08% (PBS-T) for 3 hours followed by incubation with human serum (1:100) or anti-his antibody (1:1000) in PBS-T with 0.5% dry milk. Horseradish peroxidase–conjugated secondary anti-human or anti-mouse IgG was used as the secondary antibody. Details of these procedures and the used material are presented in Supplemental Material.
Purification of Domain-Specific Antibodies from Patient Sera
The shortest protein fragment containing the epitope of interest was transferred to a polyvinylidene difluoride membrane in the highest possible quantity. The protein-containing part of the membrane was cut out and incubated in patient serum (1:100) in 0.5% skim milk in PBS-T overnight at 4°C with agitation. After washing the membrane three times with PBS-T, the bound antibodies were eluted from the membrane by three subsequent incubations with 200 μl IgG elution buffer for 5 minutes (Thermo Scientific) at room temperature. The eluted solution was immediately neutralized with 1 M Tris-HCl (pH 9). Before use as the primary antibody for immunologic detection, 0.5% skim milk in PBS-T was added.
Statistical Analyses
Remission of proteinuria was defined as proteinuria of <3.5 g/24 h and at least 50% reduction from the time of inclusion. Data are given as median and interquartile range (IQR). A Mann–Whitney U test was performed to assess for statistical significance. For analyses of categorical data, a Fisher exact test was performed. Statistical significance was defined as P<0.05.
Animal Care
Wild-type male BALB/c mice (8–12 weeks old) were bred in the animal facility of the University Medical Center Hamburg-Eppendorf. Animals had free access to water and standard animal chow.
Immunization of Rabbits and Mice with THSD7A cDNA
A mixture of expression constructs encoding full-length mouse and human THSD7A was conjugated to 1-μm gold particles (Bio-Rad Laboratories, Munich, Germany). These were ballistically injected into rabbits and mice at the antibody core unit of the University Medical Center Hamburg-Eppendorf. The rabbits received four immunizations in 3- to 6-week intervals, each with 12 shots of plasmid-conjugated gold particles (1 μg DNA per 1 mg gold per shot). The mice received four immunizations in 3- to 6-week intervals, each with four shots of plasmid-conjugated gold particles (1 μg DNA per 1 mg gold per shot). Serum was obtained 3 weeks after the last immunization. The specificity of the antiserum for native mouse and human THSD7A was verified using immunofluorescence staining of transfected Chinese hamster ovary cells as described previously.21 All animal experiments were performed according to national and institutional animal care and ethical guidelines, and they were approved by the veterinarian agency of Hamburg and the local animal care committee.
Epitope Domains in Mouse THSD7A
Eleven mouse TSP-1 fragments corresponding to the previously designed human TSP-1 constructs were designed: d1_d2 (Ala-37 to Gln-181), d2_d3 (Trp-106 to Glu-236), d3_d4 (Lys-182 to Ala-412), d5_d6 (Thr-413 to Tyr-563), d7_d8 (Asp-564 to Thr-684), d9_d10 (Val-685 to His-820), d11_d12 (Ser-821 to Asp-888), d13_d14 (Lys-889 to Asn-1084), d15_d16 (Gln-1085 to Tyr-1208), d17_d18 (His-1209 to Tyr-1329), and d19_d21 (Arg-1330 to Arg1523). All variants were generated by PCR, cloned into the eukaryotic expression vector pCSE2.5, and expressed in HEK293 cells as described above for human TSP-1 domains.
Immunohistochemical Analyses
Immunohistochemical analyses were performed essentially as described previously.21 Details are presented in Supplemental Material.
Results
Domain Analyses of THSD7A
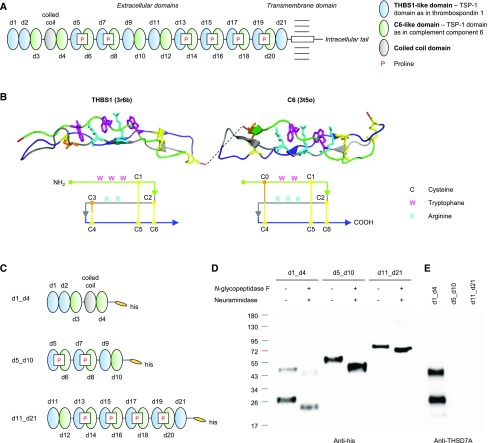
THSD7A is a glycosylated 250-kD type 1 transmembrane protein composed of a large extracellular N-terminal region, a single-pass transmembrane domain, and a short intracellular C-terminal tail.3 On the basis of automated basic local alignment search tool analyses, THSD7A was previously reported to contain 11 TSP-1 (Pfam domain PF00090) repeats.3 We now took into consideration the relative position of conserved cysteine, tryptophan, and arginine residues and performed position-sensitive iterative basic local alignment search tool searches and structure-based alignments of THSD7A with TSP-1 domains from the Protein Data Bank (pdb). This re-evaluation of the THSD7A architecture revealed a tandem string of 21 TSP-1 domains (d1–d21) (Figure 1A, Supplemental Figure 1), most of which are separated by short linkers of one to nine amino acid residues. These TSP-1 domains show high structural homology either to the TSP-1 domains of thrombospondin 1 (THBS1; pdb code 3r6b) or complement component 6 (C6; pdb code 3t5o; containing two TSP-1 domains) and F-spondin (pdb 1szl; containing one TSP-1 domain).22–24 THBS1 and C6 domains both consist of three antiparallel peptide strands (Figure 1B). The first strand contains two or three tryptophan residues that interlace with two or three arginine residues from the second strand. The three peptide strands are tightly connected by three disulfide bridges between cysteine residues. The first disulfide bridge connects the first strand and the third strand (C1–C5), and the second disulfide bridge connects the second strand and the third strand (C2–C6). However, THBS1 and C6 domains differ in the position of the third disulfide bridge. In THBS1, it connects the second and third strands (C3–C4), whereas in C6, it connects C4 to C0, a cysteine near the N-terminus of the first strand (Figure 1B).
Figure 1.
Structural analysis of thrombospondin type 1 domain–containing 7A (THSD7A) reveals 21 consecutive thrombospondin type 1 (TSP-1) domains. (A) Schematic view of the THSD7A structure. The extracellular part consists of 21 thrombospondin type 1 (TSP-1) domains (referred to as d1–d21) and one coiled coil domain. P, proline. (B) Predicted three-dimensional structure and schematic view of THBS1- and complement component 6 (C6)–like domains with three strand-connecting disulfide bonds each (yellow/orange). (C) Schematic view of the three his-tagged THSD7A fragments. (D) Western blot of the three THSD7A fragments before and after deglycosylation with N-glycopeptidase F and neuraminidase with an anti-his antibody under reducing conditions. (E) Western blot of THSD7A fragments with a coiled coil domain–directed commercial antibody.
The first two (N-terminal) TSP-1 domains of THSD7A are tandem THBS1-like domains, and the third and fourth TSP-1 domains are C6-like domains separated by a unique and highly basic coiled coil domain (Figure 1A). Domains d5–d21 are arranged in an alternating fashion of THBS1-like and C6-like domains. Six linker regions contain a proline residue, which is often found in linker regions of TSP-1 domains in the pdb and likely stabilizes the angle between the adjacent THBS1- and C6-like domains due to limited flexibility. The overall domain architecture of THSD7A is highly conserved in vertebrate evolution. It is found in all extant teleost and cartilaginous fish, including the elephant shark (Callorhinchus milii), the slowest evolving of all known vertebrates.25
Expression of Three THSD7A Fragments
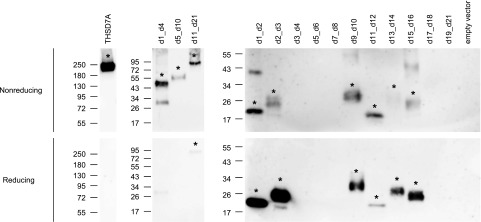
To define epitope regions targeted by anti-THSD7A autoantibodies, we designed three fragments of the antigen. The first fragment contained the first four TSP-1 domains, including the coiled coil domain interposed between the two C6-like domains (d1_d4) (Figure 1C). The middle fragment contained the three subsequent THBS1-C6 pairs (d5_d10), and the third fragment ranged from the 11th to the last TSP-1 domain shortly before the transmembrane region (d11_d21). Transfection of HEK293 cells with subsequent Western blotting under reducing and nonreducing conditions revealed efficient expression of all constructs (Figure 1D, Supplemental Figure 2). The fragments d5_d10 and d11_d21 showed single bands at 60 and 90 kD, respectively, whereas detection of d1_d4 revealed two distinct bands, the predicted band at 50 kD and an additional band at 30 kD, suggesting post-translational proteolytic cleavage.26 All three fragments were found to be glycosylated and showed a shift in size after enzymatic deglycosylation with N-glycopeptidase F and neuraminidase (Figure 1D). Immunologic detection with a commercially available anti-THSD7A antibody raised against the coiled coil domain exclusively recognized the d1_d4 construct (Figure 1E).
Clinical Characteristics and Serum Recognition of the Three THSD7A Fragments in the Study Cohort
We tested the reactivity of sera from 31 patients with biopsy-proven MN with the three THSD7A fragments by Western blotting under nonreducing conditions. Before study inclusion, all patients tested positive for anti-THSD7A antibodies in the serum by a recently developed indirect immunofluorescence test.11 Nineteen (61%) patients were men, and the median age was 67 years old (IQR, 53.5–74.5 years old). The median time between renal biopsy and first serum collection was 1 month (IQR, 0.0–1.0 months), median proteinuria at the time of first serum collection was 7.1 g/d (IQR, 4.8–9.9 g/d), and median serum creatinine was 1.3 mg/dl (IQR, 0.8–1.6 mg/dl). Nine of the patients were diagnosed with a malignant tumor before diagnosis of MN or during follow-up (median time, 3 months; IQR, 1–6 months).
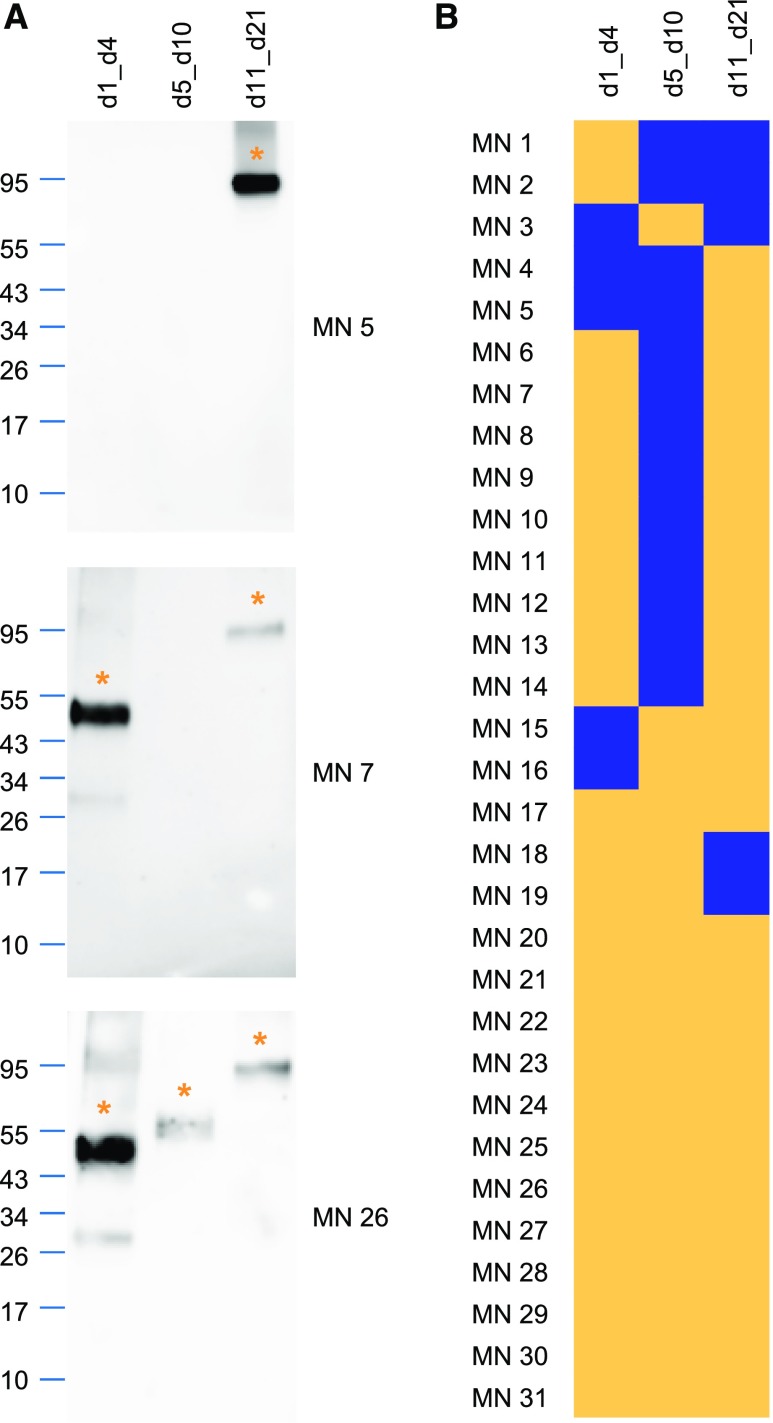
All sera reacted with at least one of the expressed constructs (Figure 2, Supplemental Figure 3). The d1_d4 and the d11_d21 constructs were each recognized by 84% of patient sera, whereas the d5_d10 construct was recognized by 58% of patient sera. More than one fragment was recognized by 84%, and all three fragments were recognized by 42% of the sera. Sera from healthy control individuals that were analyzed under identical conditions failed to react with any of the THSD7A fragments (Supplemental Figure 4A). Taken together, these results suggest the presence of multiple autoantibody binding sites in THSD7A, with most reactivity in the more N-terminal and the more C-terminal extracellular parts of the antigen.
Figure 2.
Patient autoantibodies recognize thrombospondin type 1 (TSP-1) domains along the whole extracellular part of thrombospondin type 1 domain-containing 7A (THSD7A). (A) Representative Western blots of the d1_d4, d5_d10, and d11_d21 fragments with three different sera under nonreducing conditions (serum dilution 1:100, anti-IgG4 as secondary antibody). Asterisks mark the recognized THSD7A fragments. (B) Heat map depicting the reactivity profiles of sera from 31 patients with THSD7A-associated membranous nephropathy (MN) with the three THSD7A fragments. Yellow indicates serum recognition of the construct, whereas blue indicates no reactivity. Patients were clustered according to their epitope recognition profiles.
Identification of Epitope Regions in THSD7A
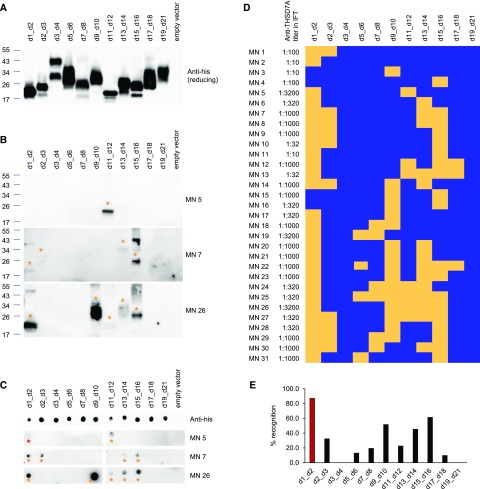
To more deeply map the epitopes for anti-THSD7A autoantibodies, we designed smaller fragments of the THSD7A antigen. These fragments contained two to three adjacent TSP-1 domains (d1_d2, d2_d3, d3_d4, d5_d6, d7_d8, d9_d10, d11_d12, d13_d14, d15_d16, d17_d18, and d19_d21). Transfection of HEK293 cells with subsequent Western blotting revealed efficient expression of all constructs (Figure 3A, Supplemental Figure 5). All constructs were found to be glycosylated, except d1_d2 and d11_d12 (Supplemental Figure 6).
Figure 3.
Patient autoantibodies recognize multiple epitopes in thrombospondin type 1 domain–containing 7A (THSD7A) with a predominance for the most N-terminal region. (A) Western blot of HEK293 cell–expressed soluble thrombospondin type 1 (TSP-1) domain constructs with an anti-his antibody under reducing conditions. (B) Representative Western blots of the TSP-1 domain constructs with three different sera under nonreducing conditions (serum dilution 1:100, anti-IgG4 as secondary antibody). Asterisks mark the recognized TSP-1 domain constructs. (C) Native blot (dot blot) analyses of the TSP-1 domain constructs with an anti-his antibody (row 1) and three representative sera (rows 2–4). Yellow asterisks mark the recognized TSP-1 domain constructs that were also recognized using Western blotting, whereas the red asterisk marks an additional TSP-1 domain recognition (MN 5). (D) Heat map depicting reactivity profiles of sera from all 31 patients with the small TSP-1 domain constructs as deduced from nonreduced Western blotting and native blotting. Yellow indicates serum recognition of the construct, whereas blue indicates no reactivity. IFT, indirect immunofluorescence test. (E) Recognition of the individual TSP-1 domains by patient sera in percentages.
We next tested the reactivity of the 31 sera with the TSP-1 domain constructs using Western blotting under nonreducing conditions. All but the d3_d4 and the d19_21 constructs were each recognized by at least three of the sera (Figure 3B, Supplemental Figure 7). Sera that reacted with d1_d4 in the first screening round also reacted with d1_d2 in the second screening round, whereas only nine (35%) of these sera additionally recognized d2_d3. Serum reactivity was independent of the glycosylation status of the protein (Supplemental Figure 8). The recognition of the TSP-1 domain constructs corresponded to the previously described recognition of the larger THSD7A fragments in all but one serum (MN 17). The one mismatched serum was positive for all three larger THSD7A fragments but failed to react with one of the smaller TSP-1 domain constructs in the d11_d21 area (Supplemental Figures 3 and 7). This suggests that this particular epitope was not covered with the chosen antigen fragmentation or that the sensitivity of detection was too low to show autoantibody binding in this case. Sera from healthy control individuals failed to react with any of the TSP-1 domain constructs (Supplemental Figure 4B).
To investigate the presence of epitopes that are sensitive to denaturation by SDS, we applied native blotting (dot blot analysis). All constructs were well recognized by an anti-his antibody under native conditions (Figure 3C). Subsequently, we tested 30 of the previously tested 31 patients with available serum. Twenty-one sera (70%) showed identical epitope profiles in native and Western blotting. However, three sera did not recognize a TSP-1 domain construct that was previously recognized using Western blotting (Supplemental Figure 9A), and two sera did not show any reactivity in native blotting (data not shown). Interestingly, four sera recognized additional TSP-1 domain constructs in native blotting, showing the presence of denaturation-sensitive epitopes in these patients (Figure 3C, Supplemental Figure 9B). Epitope recognition profiles as deduced from Western blotting and native blotting are shown in Figure 3D. Altogether, 28 of 31 (90%) serum samples recognized multiple domains, and d1_d2 (amino acids 48–192) was most frequently recognized (27 of 31 patients; 87%) followed by d15_d16, d9_d10, and d13_d14, which were recognized by 61%, 52%, and 45% of the sera, respectively (Figure 3E).
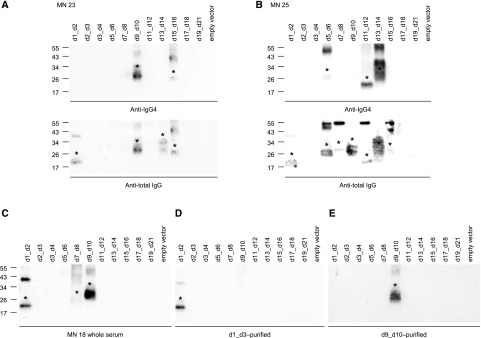
All Western blot experiments were performed using anti-human IgG4 and anti-total human IgG as secondary antibodies. Two sera recognized additional TSP-1 domain constructs when tested with anti-total IgG compared with anti-IgG4, showing the presence of domain-specific autoantibodies different from IgG4 in these patients (Figure 4, A and B). Both patients did not have an associated malignant disease. Taken together, these data show that autoantibodies from patients with THSD7A-associated MN preferentially bind to the most N-terminal region of THSD7A (amino acids 48–192) and that most sera recognize multiple epitope domains present in THSD7A.
Figure 4.
Patient autoantibodies are IgG4-predominant and domain-specific. (A and B) Western blot under nonreducing conditions of TSP-1 domain constructs with sera from two patients with thrombospondin type 1 domain–containing 7A (THSD7A)–associated membranous nephropathy (MN) using anti-IgG4 (upper panels) and anti-total IgG (lower panels) as secondary antibodies. Asterisks mark the recognized TSP-1 domain constructs. (C) Western blot under nonreducing conditions of TSP-1 domain constructs with serum from one patient with THSD7A-associated MN. (D and E) Antibodies that were purified using recombinant (D) d1_d3 and (E) d9_d10 exclusively bound d1_d2 and d9_d10, respectively. Asterisks mark the recognized TSP-1 domain constructs.
Domain and Conformation Specificity of Identified Autoantibodies
We next investigated whether the identified autoantibodies were indeed distinct domain-specific antibodies or whether one clone of antibodies recognized several epitopes along the antigen due to sequence homology between the TSP-1 domains (Supplemental Figure 1A). We purified domain-specific IgG using recombinant d1_d3 and d9_d10 from a patient serum that recognized d1_d2, d7_d8, and d9_d10 (Figure 4C). The d1_d3-purified antibodies exclusively recognized d1_d2, whereas the d9_d10-purified antibodies showed exclusive reactivity with d9_d10 in Western blot analysis (Figure 4, D and E). The experiment was repeated with three more patient sera with consistent results (not shown). These results indicate that the variety of domain recognition in our patients is due to distinct autoantibodies against several epitopes present in THSD7A.
Autoantibodies from patients with THSD7A-associated MN have been reported to recognize THSD7A exclusively under nonreducing conditions in Western blot analysis, suggesting autoantibody binding to a conformation-dependent epitope within the antigen.3 We tested nine of the previously analyzed sera under both reducing and nonreducing conditions in a standardized side by side Western blot experiment. Sera were probed on full-length THSD7A; the d1_d4, d5_d10, and d11_d21 fragments; and the small TSP-1 domain constructs. Under reducing conditions, all investigated sera completely lost reactivity with full-length THSD7A; partially or completely lost reactivity with d1_d4, d5_d10, and d11_d21; and completely retained reactivity with the small TSP-1 domain constructs (Figure 5) (not shown). Sera from healthy control individuals failed to react with the TSP-1 domain constructs under reducing conditions (Supplemental Figure 4). These experiments suggest that the small TSP-1 fragments are capable of refolding after loss of the reducing agent during protein transfer.
Figure 5.
Patient autoantibodies recognize conformation-dependent epitopes in thrombospondin type 1 domain–containing 7A (THSD7A). Images depict Western blots of full-length THSD7A, the three THSD7A fragments, and the small thrombospondin type 1 (TSP-1) domain constructs with one representative serum (MN 27) under nonreducing (upper panels) and reducing conditions (lower panels).
Association of Epitope Recognition Profiles with Clinical Characteristics and Epitope Recognition during Follow-Up
We next analyzed the association of the THSD7A epitope profiles (Figure 3D) with clinical characteristics of the study cohort (Table 1). Patients whose sera recognized only one or two TSP-1 domain constructs had significantly lower anti-THSD7A antibody levels and statistically nonsignificant lower proteinuria compared with patients whose sera recognized at least three TSP-1 domain constructs (P=0.02 and P=0.07, respectively). Remission of proteinuria was achieved by 86% of patients whose sera recognized one or two TSP-1 domain constructs, but it was only achieved by 50% of patients whose sera recognized three or more TSP-1 domains (P=0.18) (Table 1).
Table 1.
Epitope recognition and clinical characteristics of the cohort
| Clinical Characteristics | Sera with Recognition of One or Two TSP-1 Domain Constructs | Sera with Recognition of More Than Two TSP-1 Domain Constructs | P Value |
|---|---|---|---|
| No. of patients (%) | 10 (32) | 21 (68) | N/A |
| No. of epitopes, median (IQR) | 2.0 (1.0–2.0) | 4.0 (4.0–5.0) | <0.001 |
| Age, yr, median (IQR) | 67.0 (52.0–76.5) | 65.0 (54.0–69.0) | 0.75 |
| Men (%) | 5 (50) | 14 (67) | 0.45 |
| Proteinuria, g/d, median (IQR) | 5.8 (3.4–7.4) | 7.7 (5.3–10.7) | 0.07 |
| Serum creatinine, mg/dl, median (IQR) | 1.2 (0.9–1.6) | 1.2 (0.8–1.8) | 0.69 |
| Anti-THSD7A antibody level, median (IQR) | 210 (32–320) | 1000 (320–1000) | 0.02 |
| Patients with malignancy (%) | 3 (30) | 6 (29) | >0.99 |
| Patients with partial or complete remission of proteinuria during follow-up (%) | 6 of 7 (86) | 8 of 16 (50) | 0.18 |
Follow-up data on proteinuria were available for 23 of the patients included in our cohort. TSP-1, thrombospondin type 1; N/A, not applicable; IQR, interquartile range; THSD7A, thrombospondin type 1 domain–containing 7A.
Follow-up sera were available from 16 of 31 patients in the study, and they were examined for changes in epitope recognition over time. In five patients, epitope profiles did not change during follow-up (Supplemental Figure 10). All of these patients had stable anti-THSD7A antibody levels as measured by immunofluorescence test and nephrotic-range proteinuria throughout the follow-up time. Sera from seven patients lost reactivity with one or more constructs (Supplemental Figure 11). The anti-THSD7A antibody levels decreased in six of these patients, and five patients had a remission of proteinuria. The remaining four patients had a change in their epitope profile during follow-up (Supplemental Figure 12). We found no specific patterns for the loss or gain of epitope recognition (i.e., preferential loss or gain of reactivity with one specific TSP-1 domain construct compared with another) (Supplemental Table 1).
Immune Response against THSD7A after Active Immunization
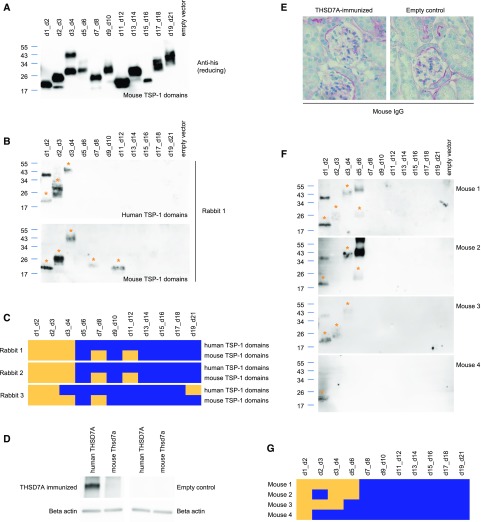
We next evaluated the epitope regions in human and mouse THSD7A that are targeted by antibodies raised against THSD7A in three rabbits by coimmunization with human and mouse THSD7A cDNA. We have previously shown that these antibodies induce MN with severe nephrotic syndrome when transferred into mice.21 A detailed sequence analysis of mouse THSD7A showed that it shares over 90% of amino acid sequence homology with human THSD7A and that it is also composed of 21 TSP-1 domains with the identical pattern of THBS1- and C6-like domains (Supplemental Figure 13). Consequently, we used the same cloning and expression techniques for the mouse TSP-1 domains as described for the human TSP-1 domains. All mouse TSP-1 domain constructs were well expressed in HEK293 cells and secreted to the culture medium (Figure 6A). We tested the three rabbit antisera for their recognition of human and mouse TSP-1 domains under nonreducing conditions. Strikingly, all three rabbit antisera recognized the most N-terminal region d1_d2 of both human and mouse THSD7A (Figure 6, B and C, Supplemental Figure 14), which was also the most frequently recognized epitope region in our patient cohort (Figure 3E). There is no lower homology between the rabbit, mouse, and human proteins in the d1_d2 region compared with the other TSP-1 domains (Supplemental Table 2). Therefore, a lack of homology in this region cannot be the reason for the preferred generation of autoantibodies against the most N-terminal region of THSD7A. Interestingly, the highly pathogenic rabbit sera additionally recognized epitope domains in the more C-terminal region of mouse THSD7A, also resembling the situation found in our patient cohort.
Figure 6.
Rabbits and mice generate antibodies against the most N-terminal region of thrombospondin type 1 domain–containing 7A (THSD7A) after coimmunization with human and mouse THSD7A cDNA. (A) Western blot of HEK293 cell–expressed soluble mouse thrombospondin type 1 (TSP-1) domain constructs with an anti-his antibody under reducing conditions. (B) Western blot under nonreducing conditions of human (upper panel) and mouse (lower panel) TSP-1 domain constructs with one representative rabbit antiserum (serum dilution 1:100). Asterisks mark the recognized TSP-1 domain constructs. (C) Heat map depicting the reactivity profiles of three rabbit antisera with human and mouse TSP-1 domain constructs in Western blot analysis. Yellow indicates serum recognition of the construct, whereas blue indicates no reactivity. (D) Representative Western blot under nonreducing conditions of recombinant human and mouse THSD7A with serum from one of four mice that were immunized with a combination of human and mouse THSD7A cDNA. Four mice were mock immunized (empty control). (E) Representative immunohistochemical staining for mouse IgG in one THSD7A-immunized mouse and one control mouse. (F) Western blot under nonreducing conditions and (G) heat map analysis of reactivity of four mouse antisera with the human TSP-1 domain constructs. Asterisks in F mark the recognized TSP-1 domain constructs. Yellow in G indicates serum recognition of the construct, whereas blue indicates no reactivity.
In a similar approach, we immunized four mice by ballistic cDNA immunization with a mixture of human and mouse THSD7A cDNA. As controls, four mice were mock immunized. THSD7A-immunized mice generated antibodies that recognized human but not mouse THSD7A (Figure 6D), showing that self-tolerance could not be overcome with this particular immunization technique. Accordingly, mice did not show glomerular binding of mouse IgG in immunohistochemical analysis (Figure 6E) and did not develop proteinuria (not shown). Notably, sera from all THSD7A-immunized mice also recognized d1_d2 (Figure 6, F and G). As for rabbit and human THSD7A, the amino acid sequence homology in the N-terminal region of mouse and human THSD7A is comparable (approximately 90% identity) with that of the other TSP-1 domains (Supplemental Table 2), indicating that differences in the amino acid sequence are not the predominant stimulus for the animals’ immune system to target this region. Taken together, these data show that induced rabbit and mouse anti-THSD7A antibodies, like patient autoantibodies, predominantly target the most N-terminal domains of THSD7A.
Discussion
This study identifies the epitope regions in THSD7A that are targeted by autoantibodies from patients with MN. Using comprehensive sequence alignments, we characterized the extracellular part of THSD7A as a series of 21 TSP-1 domains. On the basis of their amino acid sequence, these TSP-1 domains can be further classified as either THBS1-like or C6-like. THBS1 itself acts as an adhesive glycoprotein that can interact with components of the extracellular matrix,27–30 cell receptors,31–37 and proteases,38,39 possibly leading to activation of downstream signaling pathways, alterations of protein localization, proteolytic processing, and protein internalization.40 The homology of the THBS1-like domains in THSD7A with THBS1 may give a hint on the presently unknown biologic functions of THSD7A on podocytes and allow speculation on potential pathomechanisms in THSD7A-associated MN.
In an unbiased approach to identify the epitope regions in THSD7A, we designed three consecutive fragments of the antigen and tested for recognition by patient autoantibodies. Most patient sera reacted with several THSD7A fragments with a predominance for the more N-terminal and the more C-terminal part of the antigen. Additional epitope mapping revealed that the d1_d2 construct (amino acids 48–192) was recognized by 87% of the sera, indicating that the dominant epitope in THSD7A-associated MN is located within the most N-terminal part of the antigen. In contrast, the reactivity in the more C-terminal part distributed over several epitope domains. Interestingly, the preference of autoantibodies for the most N-terminal region of the antigen is a similarity of THSD7A- and PLA2R1-associated MN. However, whereas patient autoantibodies bind to the most N-terminal region of PLA2R1 in all investigated patients,17,18 this is not the case for THSD7A. Furthermore, most sera from patients with THSD7A-associated MN recognize more than one antigen domain, and epitope profiles vary between the different patients. This is in contrast to PLA2R1, for which only three epitope regions have been identified so far.18 It is known from other antibody-mediated autoimmune diseases, such as Grave disease, pemphigus vulgaris, and anti-brush border antibody disease, that autoantibodies target a great variety of epitopes that distribute over the whole extracellular regions of the respective autoantigens but with an N-terminal predominance.41–44
Compared with patients whose sera recognized only one or two TSP-1 domains, patients whose sera recognized more than two TSP-1 domains had higher anti-THSD7A antibody levels, tended to higher levels of proteinuria, and achieved a remission of proteinuria in the observation period less often. During follow-up, seven patients lost recognition of one or more epitopes, which paralleled a decrease in anti-THSD7A antibody levels and in most patients, a remission of proteinuria. From these data it is not clear whether it is the individual epitope profile, the number of recognized epitopes, the antibody titer alone, or a combination of these factors that drive disease. Further studies are needed to investigate these issues.
The phenomenon of epitope spreading has been described in human antibody-mediated autoimmune diseases45–49 and different autoimmune animal models.50–53 In the experimental MN model of Heymann nephritis, proteinuria increased with epitope spreading.54 This is in accordance with PLA2R1-associated MN, where epitope spreading was reported to relate with disease outcome.18,19 In our study, we did not find clear evidence for epitope spreading over time due to the small number of patients with THSD7A-associated MN and serologic follow-up. In the future, larger cohorts of patients will be needed to further dissect the relationship of epitope profiles at diagnosis and the possible phenomenon of epitope spreading with disease activity and long-term clinical outcome.
Our study has several limitations. First, we did not use a quantitative method, such as ELISA, to determine domain-specific autoantibody titers. Therefore, we cannot compare domain-specific antibody levels between patients and correlate domain-specific titers with disease activity and outcome. Second, Western blot experiments were standardized regarding protein loading (on the basis of anti-his reactivity under reducing conditions) and serum dilution (1:100) due to a limited amount of patient serum. It seems likely that a change in these parameters, such as higher or lower dilution of the used serum, may have led to slightly different epitope profiles for some patients. Third, antibody binding sites were mapped to peptides of 100–120 amino acids. Additional work is needed to define the genuine epitopes on the level of ten to 15 amino acids.
To investigate antibody generation against THSD7A, we analyzed rabbits and mice that were immunized with a combination of human and mouse THSD7A cDNA. Remarkably, all animals raised an antibody response against the first two N-terminal domains d1_d2 of both mouse and/or human THSD7A, and most animals recognized several other domains, resembling the situation in our patient cohort. Thereby, the N-terminus of THSD7A does not predispose to antibody formation due to a particularly low homology in this area between the three species orthologs. This indicates that other features of the N-terminal region, such as epitope accessibility, might be responsible for the predominant antibody response. However, autoantibodies were induced against foreign proteins in these experiments, and importantly, the pathogenicity of antibodies targeting specific domains within the antigen remains to be investigated in future studies.
Identification of the epitopes that are targeted by autoantibodies from patients with THSD7A-associated MN is of high interest for future investigations for the following reasons. (1) Autoimmunity may emerge as a result of molecular mimicry between microbial or tumor antigens and host proteins.55,56 Defining the precise antibody binding site(s) would allow alignments with microbial proteins to uncover yet unknown links between infections or tumors and primary MN. (2) The TSP-1 domains in THSD7A might differentially interact with other molecules and/or induce intracellular signaling.40 Interference of autoantibodies with these functions could represent a pathomechanism in the mediation of podocyte damage in MN. (3) Epitope blocking/competition therapy or systemic autoantibody extraction using the antibody binding fragment could constitute innovative therapies for patients with antibody-mediated autoimmune diseases.57 Also, expression of epitope-containing domains on T cells in combination with intracellular signaling domains can direct T cells to eliminate autoantibody-producing B cells through specific binding to the B cell receptor,58 representing another potential therapeutic intervention for patients with autoimmune primary MN that requires knowledge of the antibody binding domains.
In conclusion, our study shows that an autoimmune process with polyreactive IgG is part of the pathogenesis of THSD7A-associated MN. Additionally, we provide clinical and experimental evidence that the principal immune response targets the most N-terminal part of the protein, potentially enabling epitope-specific therapies in the future.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Eugen Kinzler and Daniela Bergleiter (III. Medizinische Klinik, University Medical Center Hamburg-Eppendorf, Hamburg, Germany) for technical assistance and Gudrun Dubberke, Fabienne Seyfried, and Sarah Hewald (Insitute of Immunology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany) for technical assistance with cDNA immunization.
This study was supported by grants from the Deutsche Forschungsgemeinschaft as part of the Sonderforschungsbereich 1192 (project B1 to E.H. and R.A.K.S.; project B2 to G.Z., R.A.K.S., and N.M.T.; and project B5 to F.K.-N.). E.H. is supported by the Else Kröner-Fresenius Stiftung.
The following colleagues participated in the recruitment of the patients for this study (in alphabetical order): Bahte S, Beckmann S, Bokemeyer D, Born B, Boser M, Budde K, Dellanna F, Ferber J, Fielitz JG, Floege J, Gerth J, Groll J, Grosser S, Hegner B, Hetzel GR, Hollenbeck M, Hoyer J, Isbell LK, Jabs W, Jacobson J, Kidder D, Koch C, Köhler S, Kortus-Götze B, Köstler F, Kresse S, Langer T, Leidig B, Messtorff K, Möller J, Peitzmeier C, Rosenburg C, Rump C, Sass C, Schmidtmann K, Schnegelsberg O, Tacuri-Strasser D, Thiele I, Tiedeken P, Treiber W, Vitu J, Vosskühler A, Walz G, Weiner S, and Worch P.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017070805/-/DCSupplemental.
References
- 1.Ruggenenti P, Fervenza FC, Remuzzi G: Treatment of membranous nephropathy: Time for a paradigm shift. Nat Rev Nephrol 13: 563–579, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al.: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al.: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofstra JM, Beck LH Jr, Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA: PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS One 9: e110681, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA: M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin J Am Soc Nephrol 9: 1883–1890, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck LH Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, et al.: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, et al.: Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1735–1743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl R, Hoxha E, Fechner K: PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N Engl J Med 363: 496–498, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Gupta G, Fattah H, Ayalon R, Kidd J, Gehr T, Quintana LF, et al.: Pre-transplant phospholipase A2 receptor autoantibody concentration is associated with clinically significant recurrence of membranous nephropathy post-kidney transplantation. Clin Transplant 30: 461–469, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Hoxha E, Beck LH Jr, Wiech T, Tomas NM, Probst C, Mindorf S, et al.: An indirect Immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J Am Soc Nephrol 28: 520–531, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoxha E, Wiech T, Stahl PR, Zahner G, Tomas NM, Meyer-Schwesinger C, et al.: A mechanism for cancer-associated membranous nephropathy. N Engl J Med 374: 1995–1996, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Cui Z, Lu J, Probst C, Zhang YM, Wang X, et al.: Circulating antibodies against thrombospondin type-I domain-containing 7A in Chinese patients with idiopathic membranous nephropathy. Clin J Am Soc Nephrol 12: 1642–1651, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, et al.: Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 363: 343–354, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth AJ, Ooi JD, Hess JJ, van Timmeren MM, Berg EA, Poulton CE, et al.: Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 123: 1773–1783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao L, Lam V, Waldman M, Glassock RJ, Zhu Q: Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J Am Soc Nephrol 26: 291–301, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fresquet M, Jowitt TA, Gummadova J, Collins R, O’Cualain R, McKenzie EA, et al.: Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol 26: 302–313, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seitz-Polski B, Dolla G, Payré C, Girard CA, Polidori J, Zorzi K, et al.: Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol 27: 1517–1533, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seitz-Polski B, Debiec H, Rousseau A, Dahan K, Zaghrini C, Payre C, et al.: Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J Am Soc Nephrol 29: 401–408, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jäger V, Büssow K, Wagner A, Weber S, Hust M, Frenzel A, et al.: High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol 13: 52, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomas NM, Meyer-Schwesinger C, von Spiegel H, Kotb AM, Zahner G, Hoxha E, et al.: A heterologous model of thrombospondin type 1 domain-containing 7A-associated membranous nephropathy. J Am Soc Nephrol 28: 3262–3277, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klenotic PA, Page RC, Misra S, Silverstein RL: Expression, purification and structural characterization of functionally replete thrombospondin-1 type 1 repeats in a bacterial expression system. Protein Expr Purif 80: 253–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aleshin AE, Schraufstatter IU, Stec B, Bankston LA, Liddington RC, DiScipio RG: Structure of complement C6 suggests a mechanism for initiation and unidirectional, sequential assembly of membrane attack complex (MAC). J Biol Chem 287: 10210–10222, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pääkkönen K, Tossavainen H, Permi P, Rakkolainen H, Rauvala H, Raulo E, et al.: Solution structures of the first and fourth TSR domains of F-spondin. Proteins 64: 665–672, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, et al.: Elephant shark genome provides unique insights into gnathostome evolution. Nature 505: 174–179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo MW, Wang CH, Wu HC, Chang SJ, Chuang YJ: Soluble THSD7A is an N-glycoprotein that promotes endothelial cell migration and tube formation in angiogenesis. PLoS One 6: e29000, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvin NJ, Vance PM, Dixit VM, Fink B, Frazier WA: Interaction of human thrombospondin with types I-V collagen: Direct binding and electron microscopy. J Cell Biol 104: 1413–1422, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sercu S, Lambeir AM, Steenackers E, El Ghalbzouri A, Geentjens K, Sasaki T, et al.: ECM1 interacts with fibulin-3 and the beta 3 chain of laminin 332 through its serum albumin subdomain-like 2 domain. Matrix Biol 28: 160–169, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Dardik R, Lahav J: Multiple domains are involved in the interaction of endothelial cell thrombospondin with fibronectin. Eur J Biochem 185: 581–588, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Herndon ME, Stipp CS, Lander AD: Interactions of neural glycosaminoglycans and proteoglycans with protein ligands: Assessment of selectivity, heterogeneity and the participation of core proteins in binding. Glycobiology 9: 143–155, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Asch AS, Silbiger S, Heimer E, Nachman RL: Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem Biophys Res Commun 182: 1208–1217, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA: Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J Cell Biol 135: 533–544, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA: Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem 271: 21–24, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, et al.: Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 284: 1116–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calzada MJ, Sipes JM, Krutzsch HC, Yurchenco PD, Annis DS, Mosher DF, et al.: Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by alpha6beta1 integrin. J Biol Chem 278: 40679–40687, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Calzada MJ, Annis DS, Zeng B, Marcinkiewicz C, Banas B, Lawler J, et al.: Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J Biol Chem 279: 41734–41743, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Lawler J, Hynes RO: An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood 74: 2022–2027, 1989 [PubMed] [Google Scholar]
- 38.Bein K, Simons M: Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem 275: 32167–32173, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Strickland DK, Bornstein P: Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem 276: 8403–8408, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Resovi A, Pinessi D, Chiorino G, Taraboletti G: Current understanding of the thrombospondin-1 interactome. Matrix Biol 37: 83–91, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Nagayama Y, Wadsworth HL, Russo D, Chazenbalk GD, Rapoport B: Binding domains of stimulatory and inhibitory thyrotropin (TSH) receptor autoantibodies determined with chimeric TSH-lutropin/chorionic gonadotropin receptors. J Clin Invest 88: 336–340, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Futei Y, Amagai M, Sekiguchi M, Nishifuji K, Fujii Y, Nishikawa T: Use of domain-swapped molecules for conformational epitope mapping of desmoglein 3 in pemphigus vulgaris. J Invest Dermatol 115: 829–834, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Sekiguchi M, Futei Y, Fujii Y, Iwasaki T, Nishikawa T, Amagai M: Dominant autoimmune epitopes recognized by pemphigus antibodies map to the N-terminal adhesive region of desmogleins. J Immunol 167: 5439–5448, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Larsen CP, Trivin-Avillach C, Coles P, Collins AB, Merchant M, Ma H, et al.: LDL receptor-related protein 2 (megalin) as a target antigen in human kidney anti-brush border antibody disease. J Am Soc Nephrol 29: 644–653, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Zenzo G, Thoma-Uszynski S, Calabresi V, Fontao L, Hofmann SC, Lacour JP, et al.: Demonstration of epitope-spreading phenomena in bullous pemphigoid: Results of a prospective multicenter study. J Invest Dermatol 131: 2271–2280, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto T, Tsuruta D, Dainichi T, Hamada T, Furumura M, Ishii N: Demonstration of epitope spreading in bullous pemphigoid: Results of a prospective multicenter study. J Invest Dermatol 131: 2175–2177, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Chen JL, Hu SY, Jia XY, Zhao J, Yang R, Cui Z, et al.: Association of epitope spreading of antiglomerular basement membrane antibodies and kidney injury. Clin J Am Soc Nephrol 8: 51–58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD: Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med 182: 75–85, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goebels N, Hofstetter H, Schmidt S, Brunner C, Wekerle H, Hohlfeld R: Repertoire dynamics of autoreactive T cells in multiple sclerosis patients and healthy subjects: Epitope spreading versus clonal persistence. Brain 123: 508–518, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Thrasyvoulides A, Lymberi P: Evidence for intramolecular B-cell epitope spreading during experimental immunization with an immunogenic thyroglobulin peptide. Clin Exp Immunol 132: 401–407, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pöllinger B, Krishnamoorthy G, Berer K, Lassmann H, Bösl MR, Dunn R, et al.: Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med 206: 1303–1316, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vlase H, Nakashima M, Graves PN, Tomer Y, Morris JC, Davies TF: Defining the major antibody epitopes on the human thyrotropin receptor in immunized mice: Evidence for intramolecular epitope spreading. Endocrinology 136: 4415–4423, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Schwarz-Lauer L, Pichurin PN, Chen CR, Nagayama Y, Paras C, Morris JC, et al.: The cysteine-rich amino terminus of the thyrotropin receptor is the immunodominant linear antibody epitope in mice immunized using naked deoxyribonucleic acid or adenovirus vectors. Endocrinology 144: 1718–1725, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Shah P, Tramontano A, Makker SP: Intramolecular epitope spreading in Heymann nephritis. J Am Soc Nephrol 18: 3060–3066, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Wucherpfennig KW: Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest 108: 1097–1104, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, et al.: Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med 14: 1088–1096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ronco P, Debiec H: Pathophysiological advances in membranous nephropathy: Time for a shift in patient’s care. Lancet 385: 1983–1992, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, et al.: Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353: 179–184, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.