Abstract
Cardiovascular disease is the leading cause of mortality in patients receiving hemodialysis. Cardiovascular events in these patients demonstrate a day-of-week pattern; i.e., they occur more commonly during the last day of the long interdialytic interval and the first session of the week. The hemodialysis process causes acute decreases in cardiac chamber size and pulmonary circulation loading and acute diastolic dysfunction, possibly through myocardial stunning and other non–myocardial-related mechanisms; systolic function, in contrast, is largely unchanged. During interdialytic intervals volume overload, acid-base, and electrolyte shifts, as well as arterial and myocardial wall changes, result in dilatation of right cardiac chambers and pulmonary circulation overload. Recent studies suggest that these alterations are more extended during the long interdialytic interval or the first dialysis session of the week and are associated with excess volume overload or removal, respectively, thus adding a mechanism for the day-of-week pattern of mortality in patients receiving hemodialysis. This review summarizes the existing data from echocardiographic studies of cardiac morphology and function during the hemodialysis session, as well as during the interdialytic intervals.
Keywords: hemodialysis, interdialytic interval, echocardiography, diastolic dysfunction, myocardial stunning, pulmonary circulation overload
Cardiovascular morbidity and mortality in patients receiving hemodialysis is notoriously high, with the adjusted risk of death being about ten times higher than in the general population.1 Cardiovascular disease accounts for about 50% of deaths in hemodialysis both in European countries1 and the US.2,3 Among these patients, serious arrhythmias and sudden cardiac arrests, rather than acute myocardial infarction or stroke, are the most frequent causes of cardiovascular death.3
It is established that excessive mortality in ESRD can only partially be explained by traditional risk factors.4 Nontraditional, “uremia-related” risk factors, such as abnormal calcium-phosphate metabolism promoting vascular calcification, anemia, endothelial dysfunction, insulin resistance, and chronic inflammation, probably contribute to development of structural and functional cardiovascular abnormalities.5 Accelerated arteriosclerosis leads to arterial stiffness, left ventricular hypertrophy (LVH), and heart failure, which predispose patients to arrhythmia and cardiac arrest.6 Sudden death probably results from a combination of compromised myocardial substrate with an arrhythmogenic trigger.7 In these patients, specific macro- and microscopic remodeling changes, including intermyocardiocytic fibrosis, form an arrhythmogenic cardiac substrate8 and induce systolic and diastolic dysfunction.9 Furthermore, evidence gathered over the last decade indicates that the hemodialysis treatment per se may induce myocardial ischemia and left ventricular (LV) dysfunction.10 Although the pathogenesis of ventricular remodeling and dysfunction is complex, from a hemodynamic point of view, hypertension, arterial stiffness, valvular alterations, and increased preload, due to hypervolemia and high blood-flow arteriovenous fistulas (AVFs), are major risk factors.5
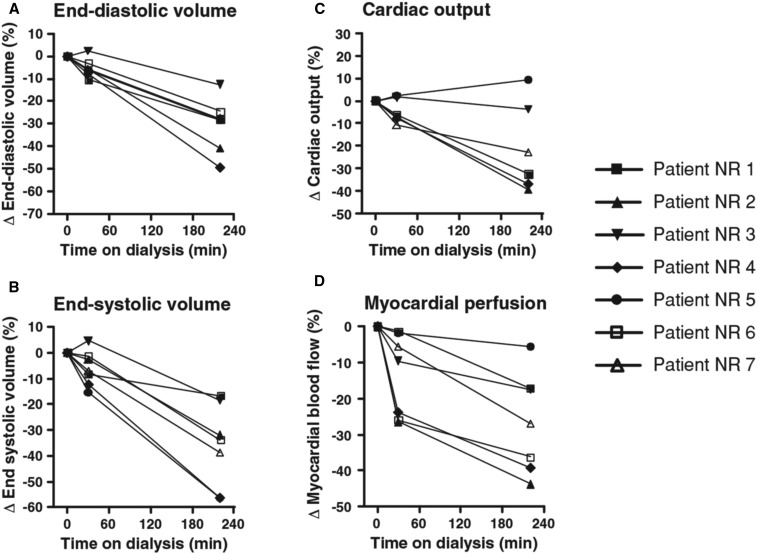
Among several observations in the field of changes in systolic function during dialysis, previous positron emission tomography (PET) studies provided intriguing results. A seminal study in four patients by McIntyre et al.11 in 2008 showed that global myocardial blood flow was acutely reduced during hemodialysis and that stress-induced segmental LV dysfunction correlated with the reduction in myocardial blood flow. An almost contemporary PET-based study12 documented that, as early as 30 minutes from the start of the hemodialysis session, in the absence of significant volume removal, myocardial blood flow was reduced by 13% of average, and that it was further reduced to −26% from baseline at the end of dialysis, after subtraction of 2.5 L on average (Figure 1). Importantly, no patient in this study experienced intradialytic hypotension and BP was the same before and after dialysis. The above implicate both volume- and non–volume-related mechanisms in systolic function changes during hemodialysis.
Figure 1.
PET imaging of the heart during hemodialysis. Relative change from baseline of the (A) LV end-diastolic volume, (B) LV end-systolic volume, (C) cardiac output, and (D) myocardial blood flow. Each line represents an individual patient. In each of these figures, the same symbols are used for individual patients. Reprinted from reference 12, with permission. NR, number.
Longitudinal studies by echocardiography in patients with CKD stage 4 who progressed to ESRD reported a very high prevalence of LVH both in the prehemodialysis phase (85%) and after dialysis treatment initiation (79%).13 Severe LV systolic dysfunction (defined as ejection fraction ≤25%) has a 16% prevalence in incident hemodialysis patients.14 Less severe degrees of this alteration have a higher prevalence, particularly when LV systolic function is measured by midwall fractional shortening (prevalence 48%) which is a more sensitive indicator of LV function compared with ejection fraction (prevalence 22%).15 However, with a prevalence rate of 50%–75% LV diastolic dysfunction is not only more frequent than systolic dysfunction in this population, but also precedes the occurrence of the latter.16,17 Pulmonary hypertension is another frequent echocardiographic finding in patients receiving hemodialysis, with prevalence between 19% and 69%.18 An invasive study applying gold standard measurement of pulmonary pressure showed pulmonary hypertension at 78% of patients receiving dialysis with dyspnea unexplained from other causes.19
In recent years, large-scale population studies have shown that mortality and cardiovascular-related hospitalizations in hemodialysis are not evenly distributed throughout the days of the week, but are 25%–40% higher during the first hemodialysis day (Monday or Tuesday) than any other weekday; i.e., they commonly occur within the last hours of the long (3-day) interdialytic interval and the following dialysis session.20–22 The clustering of death and cardiovascular events in the first weekday suggests that extreme fluctuations in extracellular volume, accumulation of potentially toxic uremic solutes during the long interval, and the hemodynamic stress of the first hemodialysis session of the week may be implicated in myocardial disease and risk of death in these patients.23–27 Although this link between the long interval and worsened cardiovascular outcomes has attracted increasing attention, few studies have examined the underlying mechanisms. In this review, we summarize the current literature on the acute changes in left and right cardiac function during the hemodialysis session and during the short and the long interdialytic intervals.
Changes in Echocardiographic Indices during Hemodialysis Sessions
Echocardiography is a radiation-free, noninvasive, and widely available imaging technique for the diagnosis and management of patients with suspected or known heart diseases.16 This technique has been extensively applied to study heart morphology and function in patients receiving hemodialysis in resting conditions or during hemodialysis treatments. The echocardiographic parameters commonly measured are summarized in Table 1.
Table 1.
| Description | Parameters |
|---|---|
| Left ventricular size | LV end-diastolic diameter, LV end-diastolic volume index, LV end-systolic volume index |
| LVH | LVMi, interventricular septum diameter, posterior wall diameter |
| Left ventricular systolic function | LVEF, cardiac output, stroke volume |
| Left ventricular diastolic dysfunction and filling pressures | Peak early mitral diastolic velocity (E), peak late mitral diastolic velocity (A), ratio of early and late mitral diastolic velocities (E/A), deceleration time of E wave, ratio of peak early and early tissue-Doppler mitral diastolic velocities (E/Em) |
| Left atrial size | Left atrial volume index |
| Right ventricular size | Right ventricular end-diastolic diameter |
| Right ventricular systolic function | Peak tricuspid systolic velocity, myocardial performance index for RV (Tei index RV) |
| Right ventricular diastolic dysfunction and filling pressures | Ratio of peak early and early tissue-Doppler tricuspid diastolic velocities (E/Em RV), myocardial performance index for RV (Tei index RV) |
| Right ventricular and pulmonary circulation hemodynamics | Tricuspid regurgitation Vmax, tricuspid regurgitation peak gradient, RVSP, pulmonary vascular resistances |
| Right atrial size | Right atrial volume index |
| Right atrial pressure | Inferior vena cava diameter and collapsibility |
Increase in LV size is suggested by increase in LV end-diastolic diameter, LV end-diastolic volume index, and LV end-systolic volume index. LVH is suggested by increase in LVMi, interventricular septum diameter, and posterior wall diameter. Decrease in LV systolic function is suggested by reduced LVEF, cardiac output, stroke volume, stroke work (SW), and PVA. LV diastolic dysfunction is suggested by E, E/A, and deceleration time of E wave decrease as well as A increase. Increased LV filling pressures are suggested by E/Em increase. Increased left atrial size is suggested by an increase of left atrial volume index (LAVi). Increase of RV size is suggested by an increase of right ventricular end-diastolic diameter. Decrease in RV systolic function is suggested by reduced peak tricuspid systolic velocity and increased Tei index RV. RV diastolic dysfunction and increased RV filling pressures are suggested by increased E/Em RV and Tei index RV. Impaired RV and pulmonary circulation hemodynamics are suggested by increased Tricuspid regurgitation Vmax, tricuspid regurgitation peak gradient, RVSP, and pulmonary vascular resistances. Increased right atrial size is suggested by increased right atrial volume index. Increased right atrial pressure is suggested by increased inferior vena cava and reduced inferior vena cava collapsibility during respiration.
Effect of Hemodialysis on LV Diastolic Function
Diastolic filling is fundamental to maintain effective cardiac function. LV filling depends on the relationship between LV filling pressures, LV compliance, and the torsional recoil of LV (i.e., the untwisting of LV during diastole, which follows LV twisting during the preceding systole). Untwisting creates a suction effect which facilitates ventricular filling and produces the transmural gradient needed for myocardial perfusion. All of the structural myocardial changes discussed above reduce LV compliance and eventually raise intraventricular pressures. The study of diastolic function during hemodialysis is important both scientifically and clinically, but its assessment is complex, because echocardiographic measurements are affected by the changing LV loading conditions during hemodialysis. Table 2 presents a summary of echocardiographic studies evaluating association between diastolic function changes and volume status changes during hemodialysis.
Table 2.
Overview of studies evaluating changes in LV diastolic function echocardiographic indices during hemodialysis and association with intradialytic volume changes
| Author | Year | N | Time of Evaluation | Main Finding | Diastolic Function Change | Association between Changes in Indices and Volume | Volume Marker |
|---|---|---|---|---|---|---|---|
| Drighil et al.30 | 2008 | 17 | Before and after hemodialysis | LV E and E/A ratio, and RV E decrease | Deterioration | Yes (+) | Intradialytic weight loss |
| Sadler et al.31 | 1992 | 24 | Before, at 2 h, and after hemodialysis | LV and RV E and E/A ratio decrease | Deterioration | Yes (+) | Intradialytic weight loss |
| Dubin et al.32 | 2014 | 35 | Before and during the last hour of hemodialysis | LV E/Em ratio decrease | Deterioration | Yes (+) | NT-proBNP |
| Fijalkowski et al.33 | 2006 | 25 | Before and after hemodialysis | LV E and E/Em decrease | Deterioration | Yes (+) | Intradialytic weight loss |
| Graham et al.35 | 2003 | 17 | Before and after hemodialysis | LV E and E/A ratio decrease | Deterioration | No | Intradialytic weight loss |
| Assa et al.36 | 2013 | 109 | Before, at 60 and 180 intradialytic minutes, and after hemodialysis | LV E and Em decrease | Deterioration | No | BNP |
| Sarafidis et al.29 | 2016 | 41 | Before and after two separate hemodialysis sessions | LV and RV E decrease | Deterioration | Yes (+) | Intradialytic weight loss |
Studies are presented in chronologic order (“+” is used to present a positive correlation). E and Em, early non–tissue-Doppler and tissue-Doppler diastolic velocities (accordingly); A, late diastolic velocity; NT-proBNP, N-terminal pro b-type natriuretic peptide; BNP, brain natriuretic peptide.
As discussed before, structural myocardial alterations predisposing to LV dysfunction are frequent among patients receiving hemodialysis. Accordingly, before hemodialysis (i.e., at the zenith of volume expansion) LV pressure is increased, whereas transmitral flow velocity (Doppler) studies generally show reduced early mitral flow velocity (E). As a consequence, increased atrial filling (A) is prerequisite to maintain adequate LV filling and the E/A ratio is frequently reduced.28 It is important to note that the prehemodialysis volume expansion (high preload) would have been physiologically expected to raise mitral inflow (E). Therefore, volume expansion may lead to an underestimation of the true, underlying diastolic dysfunction.29 On the other hand, ultrafiltration during hemodialysis may lower blood volume to the point that LV volume and stroke volume decrease, thereby reducing LV filling pressure, E, and E/A ratio.30,31 Overall, the decrease in E posthemodialysis and the parallel changes in other diastolic function parameters32,33 are mainly seen as consequences of volume subtraction during dialysis.34 These changes in ventricular diastolic function are more prominent during the hemodialysis session that follows the 3-day interval, probably reflecting increased intradialytic volume removal.29 Two relevant studies35,36 concluded that there may also be a preload independent effect of hemodialysis on diastolic function. However, early mitral flow and the E/A declined across dialysis in both studies, and the lack of effect of preload on diastolic function parameters rested solely on the absence of a significant change in the propagation velocity of early diastolic filling by color myocardial-motion mode (M-mode) in the first study35 or the lack of relationship between changes in E or E/A and parameters of blood-volume status (relative blood volume and BNP).36
Effect of Hemodialysis on LV Systolic Function
In a study of 45 patients measuring tissue-Doppler systolic velocities and wave-intensity wall analysis by speckle-tracking echocardiography, parameters of LV systolic function improved after hemodialysis.37 These findings in part contradicted two previous studies,30,38 both showing a reduction in cardiac volumes after hemodialysis but no change in LV systolic function. In a subsequent study evaluating systolic function changes prehemodialysis, at 60 and at 180 minutes and posthemodialysis, 27% of patients developed regional LV systolic dysfunction during dialysis. Importantly, patients developing regional dysfunction had lower prehemodialysis LV ejection fraction (LVEF) (45.3±10.4 versus 51.8±9.5%, P<0.01) and higher LV mass index (LVMi) (89.9±24.6 versus 101.7±27.9 g/m2).39 In another study (41 patients) average LVEF and LV peak systolic myocardial velocity (LV S’) (by tissue Doppler imaging [TDI] echocardiography) increased during dialysis; these changes were accompanied by overall increased serum troponin I, myoglobin, and cardiac creatine kinase. Further analysis revealed that patients with greater rise in these biomarkers manifested a decline in LV S’ after dialysis, whereas no change in the same biomarkers was observed in patients with a rise in LV S’, suggesting that acute myocardial stress during hemodialysis may underlie LV systolic dysfunction.40 Of note, the reduction in the volume of cardiac chambers during dialysis may disturb proper assessment of systolic function by this treatment.41 Thus, differences in the magnitude of reduction in the volume of left cardiac chambers during hemodialysis may in part explain contrasting findings in the aforementioned studies. In this respect, we observed that ventricular systolic function remains stable, but myocardial performance and diastolic function are negatively affected from the start to the end of the dialysis session after the 3-day interval.29 Of note, a special population where systolic function is likely to be affected during dialysis are patients with frequent intradialytic hypotensive episodes; in these patients, previous echocardiographic studies showed greater reduction in LVEF from pre- to mid-dialysis compared with controls and suggested this inadequate compensation of LV systolic function as the main mechanism mediating intradialytic hypotension.42
Effect of Hemodialysis on Right Ventricular Function and Pulmonary Pressure
Right ventricular (RV) function and pulmonary pressure changes during hemodialysis were studied in small, uncontrolled studies. In 17 patients without overt heart disease, systolic pulmonary artery pressure decreased by 41% across a single session.30 In 27 patients with volume expansion, systolic, early, and late diastolic Doppler velocities of RV declined with fluid removal at 60 minutes during dialysis, but these changes were largely nonsignificant.43 In a pre-post hemodialysis study, systolic pulmonary artery pressure significantly decreased after the session, whereas tricuspid annulus plane systolic excursion (TAPSE) and RV fractional area change (RVFAC) showed a clear rise.44 We previously observed that right ventricular systolic pressure (RVSP, a parameter estimated by measuring the tricuspid regurgitation jet Vmax) significantly decreased after hemodialysis, whereas RV systolic function, evaluated with peak systolic RV velocity (RV S'), and pulmonary vascular resistance did not change.29 Likewise, RV diastolic function indices, such as RV E’, E’/A’ ratio, E/E’ ratio, and RV myocardial performance index (TEI RV), were significantly lower posthemodialysis. Importantly, in a study including ten patients with AVFs and ten with central venous catheters, patients with AVFs had higher posthemodialysis RV volume and lower TAPSE (a measure of diastolic distensibility), indicating that AVFs may further contribute in RV diastolic dysfunction and pulmonary circulation loading during hemodialysis.45
Changes in Echocardiographic Indices of Cardiac Function during the Interdialytic Intervals
Hemodialysis is an inherently unphysiologic therapy.46 Accumulation of uremic solutes and extracellular volume expansion peaks at the end of the interdialytic intervals, particularly the 3-day interval. Such cycles have per se detrimental effects in hemodialysis populations, as suggested by the cyclical early-week peaking in morbidity and mortality.21 Only a handful of studies have examined cardiac function changes during interdialytic intervals and just one compared changes in echocardiographic indices of LV and RV during the 3-day and the 2-day interdialytic interval.47 In a study of ten patients, parameters of diastolic function (peak early and late mitral velocities) recorded 1 hour after dialysis remained essentially similar with those measured after 24 hours.48 These findings suggest that cardiac function changes do not occur at 24 hours after the hemodialysis session, and may come later, closer to the upcoming session. In a study of five patients who had continuous central hemodynamic monitoring with an implantable monitor, LV filling pressure and RVSP increased gradually during the interdialytic periods and attained higher values after the 3-day than the 2-day interval. Pulmonary artery diastolic pressure was normal immediately after dialysis but elevated before the next session, attaining a level typically seen in symptomatic heart failure.49 A study over a single 3-day period in 80 Japanese patients examined the effect of a 2-minute handgrip exercise on LV function on the first, second, and third posthemodialysis day, showing an exercise-induced LV afterload mismatch at 72 hours. Indices of systolic function, such as resting stroke work (SW) (stroke volume multiplied by mean BP), were higher during the third day. However, E/E′ ratio and left atrial volume index (LAVi) were higher in the second and third day than directly posthemodialysis, indicating progressively impaired diastolic function.50 Arterial afterload assessed by effective arterial elastance and systemic vascular resistance were highest just after hemodialysis. During exercise, a significant increase in arterial elastance was observed during the third posthemodialysis day, which resulted in decreased SW, indicating that the myocardial oxygen consumption is gradually increasing, whereas systolic function during exercise is decreasing during the long interval.50
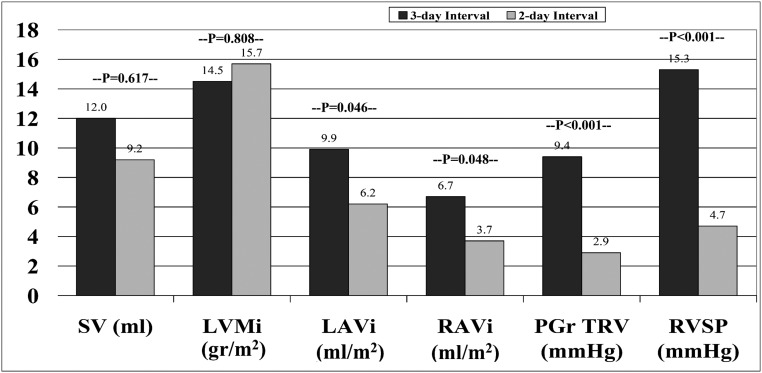
A limitation of all studies discussed above is their sequential, descriptive nature. In studies comparing changes occurring 1 and 2 days after hemodialysis, uncontrolled time-dependent factors extraneous to dialysis treatment may influence effects. To minimize this source of bias, we performed a crossover study in 41 patients, who were randomized to echocardiography recordings starting with either the 3-day or the 2-day interval.47 During both intervals, stroke volume (SV) and peak early diastolic velocities of LV showed a clear rise, whereas LVEF did not change. Changes in LV systolic and diastolic function indices were generally no different between the 2-day and 3-day interval. However, changes in left and right atrial volumes, RV systolic pressure, and tricuspid regurgitation peak gradient were more pronounced during the 3-day than the 2-day interval (Figure 2), suggesting increased pulmonary circulation and right ventricle loading over the 3-day period. On multivariate analysis, higher interdialytic weight gain, RV diastolic function (estimated by increased E/E’), and pulmonary vascular resistance were independently associated with higher RV systolic pressure. Overall, these findings suggested that atrial enlargement, an expression of diastolic dysfunction and volume overload, over the long interval imposes a hemodynamic burden to RV function and cardio-pulmonary circulation and a mechanistic factor for the heightened risk toward the end of the long interval.21
Figure 2.
Changes in echocardiographic indices of LV and RV remodeling and function during the 3-day and 2-day interdialytic intervals. Interdialytic changes in stroke volume (SV) and LVMi were similar, but interdialytic changes in left atrial volume index (LAVi), right atrial volume index (RAVi), tricuspid regurgitation peak gradient (PGr TVR), and RVSP were greater during the 3-day compared with the 2-day interval, suggesting increased pulmonary circulation and right ventricle loading over the 3-day period. (Illustration based on results from Tsilonis et al.47)
Pathophysiologic Considerations for Intra- and Interdialytic Changes in Cardiac Function
Several mechanisms may be involved in echocardiographic changes in left and right cardiac function during hemodialysis sessions, in parallel with other cardiac effects of hemodialysis, such as electrocardiographic abnormalities and arrhythmias.11,51 Previous studies in patients receiving hemodialysis showed that chronic fluid overload is associated with impaired systolic and diastolic function,38 and correction of fluid overload with careful dry weight reduction results in improvement in these parameters.52,53 During hemodialysis, changes in intravascular volume represent a complex interaction between the degree of previous extracellular fluid overload, the speed of fluid removal from the intravascular department (ultrafiltration rate), and the speed of fluid movement from the interstitial to intravascular department. The reduction in vascular volume triggers hemodynamic changes, most commonly BP reduction, for which the heart should compensate with increased heart rate to maintain cardiac output.54 However, acute changes in plasma and tissue concentration in dialyzable substances, including electrolytes, major “uremic” metabolic products, and other, known or unknown, molecules may activate additional mechanisms that can compromise myocardial function, such as high-energy phosphate depletion, micro-vascular hypoperfusion, impaired sympathetic response, and reactive oxygen species generation.55,56 Such mechanisms induce calcium-dependent protease activity and result in troponin I proteolysis and intracellular calcium depletion.57,58 Consequently, a hemodialysis-induced relative “ischemia” occurs during sessions followed by delayed recovery of regional myocardial contractile function, a phenomenon known as “myocardial stunning,” exemplified with elegant studies with H215O PET scanning, as discussed above.10,11 Ventricular diastolic dysfunction is an established complication of transient myocardial ischemia, even after restoration of blood flow.59 Because coronary perfusion takes place during cardiac relaxation, ventricular diastolic dysfunction during hemodialysis may further induce a vicious cycle of cardiac ischemia and myocardial stunning.34 Non–myocardial-related mechanisms associated with ventricular dysfunction include intradialytic changes in electrolyte balance, such as serum calcium and phosphate levels, leading to myocardial fiber degeneration, interstitial calcium deposition, and interstitial fibrosis60; increased levels of fibroblast-growth-factor 23 (FGF-23) leading to LVH via activation of the calcineurin-NFAT signaling pathway through the FGF-23 receptor 4, an effect independent of Klotho61,62; and angiotensin II increase promoting myocardial stiffness through myocyte hypertrophy, fibroblast proliferation, and interstitial collagen accumulation.63
The exact pathophysiologic mechanisms underlying changes in cardiac function and sizing during interdialytic intervals are also obscure. Several factors could be involved, such as volume overload, acid-base, and electrolyte shifts, as well as arterial and myocardial wall changes.64 Interdialytic weight gain and cardiac chamber dilatation are associated in patients receiving hemodialysis, indicating that recurrent stretching of cardiac chambers between sessions results in long-term cardiac remodeling.65 Excess volume accumulation during the long interval was associated with greater left and right atrial enlargement and RVSP elevation compared with the short interval.47 Such findings are in agreement with the fact that patients with little or no residual urine excretion and increased interdialytic weight gain are more susceptible to adverse outcomes.66 During hemodialysis various factors affecting endothelial function are removed67; interdialytic accumulation of such factors may cause endothelial dysfunction resulting in abnormal elevation in afterload during exercise.50 Because central pressure increase may further contribute to development of ventricular dysfunction,68 another possible mechanism for interdialytic cardiac changes is the progressive increase in aortic systolic BP (SBP) and augmentation index, which are about 30% higher at the end of the 3-day than the 2-day interval.23,24,69 A rise in aortic SBP augments cardiac afterload and raises myocardial oxygen demand, thus increasing the likelihood for cardiac ischemia during diastole, when cardiac perfusion occurs.70
As discussed, large-scale observational studies support a specific day-of-week pattern of mortality in hemodialysis with an excess risk toward the end of the long interval and during the first session.21,71 This daily variation pattern is not present in patients receiving peritoneal dialysis or frequent hemodialysis.72,73 Ventricular dysfunction mostly occurs during the first session of the week29; although non–volume-related mechanisms may be present,36 this is also associated with intradialytic weight loss.29 High ultrafiltration volumes during sessions are related to subclinical myocardial stunning and microvascular ischemia.74 This agrees with the fact that aggressive ultrafiltration per se was also associated with increased risk of cardiovascular events.75 Progressive left atrial enlargement and dysfunction during interdialytic intervals induce lower LV filing capacity, decreased LV wall compliance, and greater pulmonary circulation loading and explain the more frequent occurrence of pulmonary edema toward the end of the 3-day interval.21 Moreover, right atrial dilatation may trigger serious arrhythmias and cardiac arrests76; i.e., the most common causes of death in hemodialysis.77 In the long-term, exposure of RV to elevated pulmonary pressure may result in compensatory RV hypertrophy, which deteriorates LV filling capacity via interventricular interaction, leading to further LV diastolic dysfunction.76 A high pulmonary capillary wedge pressure in patients receiving dialysis almost always results from a combination of volume overload and LV dysfunction,78 and pulmonary hypertension represents a strong and independent predictor of cardiovascular events and mortality in hemodialysis.79
Observations that frequent dialysis is associated with LVH reduction2,64 support the above notions. In the Frequent Hemodialysis Network (FHN) Daily Trial, during 12-months, six-weekly hemodialysis was associated with a 39% reduction in the risk of death or change in LVMi, as assessed by magnetic resonance imaging, compared with conventional dialysis.80 Although the twin FHN Nocturnal Trial did not show beneficial effects,81 a meta-analysis suggested that conversion from conventional (≤4 hours, three times weekly) to frequent (2–8 hours, >3 times weekly) or extended (>4 hours, three times weekly) hemodialysis was associated with improvements in LV morphology and systolic function, including LVMi and LVEF.73 Volume overload is the main determinant of adverse cardiac alterations during interdialytic intervals; in a post hoc analysis of the FHN trial, in patients with residual urine <100 ml/d, reduction in extracellular fluid volume was a significant determinant of left ventricular mass reduction.82
Conclusions
The acute effect of hemodialysis on echocardiographic indices of cardiac structure and function is evaluated in several studies. Their results are not uniform, but most of them conclude that from pre- to posthemodialysis cardiac chamber size and pulmonary circulation loading are gradually decreased and systolic function remains essentially unchanged, whereas diastolic function worsens. These changes in ventricular function may be attributed to global or regional myocardial hypoperfusion12,59 and factors such as myocardial interstitial calcium and collagen deposition and fibrosis9,60,63 resulting in myocardial stunning and wall movement abnormalities. On the other hand, studies on echocardiographic changes during interdialytic intervals are scarce. Current data indicate that preload and afterload increase during interdialytic intervals (mainly due to fluid accumulation) and result in LV and RV chamber increase, elevated filling pressures, and diastolic function deterioration, most markedly during the 3-day interval.47 Overall, the above data, along with the evidence of increased morbidity and mortality toward the end of the long interval, call for detailed heart imaging studies to examine whether these intradialytic and interdialytic alterations translate into long-term consequences in cardiac function and whether they mediate the day-of-week mortality pattern in conventional dialysis.
Disclosures
None.
Acknowledgments
This paper was not supported by any source and represents an original effort of the authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, et al. : Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302: 1782–1789, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Georgianos PI, Sarafidis PA: Pro: Should we move to more frequent haemodialysis schedules? Nephrol Dial Transplant 30: 18–22, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LYC, Ayanian J, et al. : US Renal Data System 2015 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 67: Svii, S1– 305, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoccali C: Cardiovascular risk in uraemic patients-is it fully explained by classical risk factors? Nephrol Dial Transplant 15: 454–457, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Zoccali C, Bolignano D, Mallamaci F: Left ventricular hypertrophy in chronic kidney disease. In: Oxford Textbook of Clinical Nephrology, 4th Ed., edited by Turner NN, Lameire N, Goldsmith DJ, Winearls CG, Himmelfarb J, Remuzzi G, et al, Oxford, Oxford University Press, 2015, pp 837–852 [Google Scholar]
- 6.Sarafidis PA, Bakris GL: Cardiovascular disease in CKD in 2014: New insights into cardiovascular risk factors and outcomes. Nat Rev Nephrol 11: 70–72, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Pun PH: The interplay between CKD, sudden cardiac death, and ventricular arrhythmias. Adv Chronic Kidney Dis 21: 480–488, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu DYY, Sinha S, Kalra PA, Green D: Sudden cardiac death in haemodialysis patients: Preventative options. Nephrology (Carlton) 19: 740–749, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Segall L, Nistor I, Covic A: Heart failure in patients with chronic kidney disease: A systematic integrative review. BioMed Res Int 2014: 937398, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntyre CW: Effects of hemodialysis on cardiac function. Kidney Int 76: 371–375, 2009 [DOI] [PubMed] [Google Scholar]
- 11.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CSR, et al. : Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasselaar JJ, Slart RHJA, Knip M, Pruim J, Tio RA, McIntyre CW, et al. : Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant 24: 604–610, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Bansal N, Keane M, Delafontaine P, Dries D, Foster E, Gadegbeku CA, et al. ; CRIC Study Investigators : A longitudinal study of left ventricular function and structure from CKD to ESRD: The CRIC study. Clin J Am Soc Nephrol 8: 355–362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE: Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11: 1277–1285, 1996 [PubMed] [Google Scholar]
- 15.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, et al. : Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J Am Soc Nephrol 15: 1029–1037, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Chiu DYY, Green D, Abidin N, Sinha S, Kalra PA: Echocardiography in hemodialysis patients: Uses and challenges. Am J Kidney Dis 64: 804–816, 2014 [DOI] [PubMed] [Google Scholar]
- 17.de Bie MK, Ajmone Marsan N, Gaasbeek A, Bax JJ, Groeneveld M, Gabreels BA, et al. : Left ventricular diastolic dysfunction in dialysis patients assessed by novel speckle tracking strain rate analysis: Prevalence and determinants. Int J Nephrol 2012: 963504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A, et al. : Pulmonary hypertension in CKD. Am J Kidney Dis 61: 612–622, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Pabst S, Hammerstingl C, Hundt F, Gerhardt T, Grohé C, Nickenig G, et al. : Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: Results of the PEPPER-study. PLoS One 7: e35310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleyer AJ, Russell GB, Satko SG: Sudden and cardiac death rates in hemodialysis patients. Kidney Int 55: 1553–1559, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Foley RN, Gilbertson DT, Murray T, Collins AJ: Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 365: 1099–1107, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Schaubel DE, Kalbfleisch JD, Bragg-Gresham JL, Robinson BM, Pisoni RL, et al. : Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule affects day-of-week mortality. Kidney Int 81: 1108–1115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgianos PI, Sarafidis PA, Haidich AB, Karpetas A, Stamatiadis D, Nikolaidis P, et al. : Diverse effects of interdialytic intervals on central wave augmentation in haemodialysis patients. Nephrol Dial Transplant 28: 2160–2169, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Koutroumbas G, Georgianos PI, Sarafidis PA, Protogerou A, Karpetas A, Vakianis P, et al. : Ambulatory aortic blood pressure, wave reflections and pulse wave velocity are elevated during the third in comparison to the second interdialytic day of the long interval in chronic haemodialysis patients. Nephrol Dial Transplant 30: 2046–2053, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuipers J, Usvyat LA, Oosterhuis JK, Dasselaar JJ, de Jong PE, Westerhuis R, et al. : Variability of predialytic, intradialytic, and postdialytic blood pressures in the course of a week: A study of Dutch and US maintenance hemodialysis patients. Am J Kidney Dis 62: 779–788, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP: Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int 79: 218–227, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Sigrist MK, Devlin L, Taal MW, Fluck RJ, McIntyre CW: Length of interdialytic interval influences serum calcium and phosphorus concentrations. Nephrol Dial Transplant 20: 1643–1646, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Mottram PM, Marwick TH: Assessment of diastolic function: What the general cardiologist needs to know. Heart 91: 681–695, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarafidis PA, Kamperidis V, Loutradis C, Tsilonis K, Mpoutsiouki F, Saratzis A, et al. : Haemodialysis acutely deteriorates left and right diastolic function and myocardial performance: An effect related to high ultrafiltration volumes? Nephrol Dial Transplant 32: 1402–1409, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Drighil A, Madias JE, Mathewson JW, El Mosalami H, El Badaoui N, Ramdani B, et al. : Haemodialysis: Effects of acute decrease in preload on tissue Doppler imaging indices of systolic and diastolic function of the left and right ventricles. Eur J Echocardiogr 9: 530–535, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Sadler DB, Brown J, Nurse H, Roberts J: Impact of hemodialysis on left and right ventricular Doppler diastolic filling indices. Am J Med Sci 304: 83–90, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Dubin RF, Beatty AL, Teerlink JR, Schiller NB, Alokozai D, Johansen KL: Associations of tissue Doppler imaging with NT-proBNP and hs-TnT: A pilot study in end-stage renal disease. Echocardiography 31: 1205–1212, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fijalkowski M, Koprowski A, Gruchala M, Galaska R, Debska-Slizien A, Rogowski J, et al. : Effect of preload reduction by hemodialysis on myocardial ultrasonic characterization, left atrial volume, and Doppler tissue imaging in patients with end-stage renal disease. J Am Soc Echocardiogr 19: 1359–1364, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Selby NM, McIntyre CW: The vicious cycle of dialysis-induced cardiac injury--are dynamic changes in diastolic function involved? Am J Kidney Dis 62: 442–444, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Graham RJ, Gelman JS, Donelan L, Mottram PM, Peverill RE: Effect of preload reduction by haemodialysis on new indices of diastolic function. Clin Sci (Lond) 105: 499–506, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Assa S, Hummel YM, Voors AA, Kuipers J, Groen H, de Jong PE, et al. : Changes in left ventricular diastolic function during hemodialysis sessions. Am J Kidney Dis 62: 549–556, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Bjällmark A, Larsson M, Nowak J, Lind B, Hayashi SY, do Nascimento MM, et al. : Effects of hemodialysis on the cardiovascular system: Quantitative analysis using wave intensity wall analysis and tissue velocity imaging. Heart Vessels 26: 289–297, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, et al. : Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging 3: 586–594, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Assa S, Hummel YM, Voors AA, Kuipers J, Westerhuis R, de Jong PE, et al. : Hemodialysis-induced regional left ventricular systolic dysfunction: Prevalence, patient and dialysis treatment-related factors, and prognostic significance. Clin J Am Soc Nephrol 7: 1615–1623, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yildiz G, Kayataş M, Candan F, Yilmaz MB, Zorlu A, Sarikaya S: What is the meaning of increased myocardial injury enzymes during hemodialysis? A tissue Doppler imaging study. Cardiorenal Med 3: 136–153, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung KC, Huang HL, Chu CM, Chen CC, Hsieh IC, Chang ST, et al. : Evaluating preload dependence of a novel Doppler application in assessment of left ventricular diastolic function during hemodialysis. Am J Kidney Dis 43: 1040–1046, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Yang NI, Wang CH, Hung MJ, Chen YC, Wu IW, Lee CC, et al. : Real-time three-dimensional echocardiography provides advanced haemodynamic information associated with intra-dialytic hypotension in patients with autonomic dysfunction. Nephrol Dial Transplant 25: 249–254, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Arinc H, Gunduz H, Tamer A, Ozhan H, Akdemir R, Saglam H, et al. : Use of tissue Doppler to assess right ventricle function in hemodialysis patients. Am J Nephrol 25: 256–261, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Najafian J, Taheri S, Mahaki B, Molavi S, Alami S, Khalesi S, et al. : Comparing right ventricular function and pulmonary artery pressure before and shortly after hemodialysis in patients with end-stage renal disease. Adv Biomed Res 4: 197, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Lullo L, Floccari F, Polito P: Right ventricular diastolic function in dialysis patients could be affected by vascular access. Nephron Clin Pract 118: c257–c261, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Kjellstrand CM, Evans RL, Petersen RJ, Shideman JR, Von Hartitzsch B, Buselmeier TJ: The “unphysiology” of dialysis: A major cause of dialysis side effects? Hemodial Int 8: 24–29, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Tsilonis K, Sarafidis PA, Kamperidis V, Loutradis C, Georgianos PI, Imprialos K, et al. : Echocardiographic parameters during long and short interdialytic intervals in hemodialysis patients. Am J Kidney Dis 68: 772–781, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Ie EHY, Vletter WB, ten Cate FJ, Nette RW, Weimar W, Roelandt JRTC, et al. : Preload dependence of new Doppler techniques limits their utility for left ventricular diastolic function assessment in hemodialysis patients. J Am Soc Nephrol 14: 1858–1862, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Braunschweig F, Kjellström B, Söderhäll M, Clyne N, Linde C: Dynamic changes in right ventricular pressures during haemodialysis recorded with an implantable haemodynamic monitor. Nephrol Dial Transplant 21: 176–183, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Obokata M, Negishi K, Marwick TH, Kurosawa K, Ishida H, Ito K, et al. : Comparison of different interdialytic intervals among hemodialysis patients on their echocardiogram-based cardiovascular parameters. Am Heart J 169: 523–530.e2, 2015 [DOI] [PubMed] [Google Scholar]
- 51.Abe S, Yoshizawa M, Nakanishi N, Yazawa T, Yokota K, Honda M, et al. : Electrocardiographic abnormalities in patients receiving hemodialysis. Am Heart J 131: 1137–1144, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Hirayama S, Ando Y, Sudo Y, Asano Y: Improvement of cardiac function by dry weight optimization based on interdialysis inferior vena caval diameter. ASAIO J 48: 320–325, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Machek P, Jirka T, Moissl U, Chamney P, Wabel P: Guided optimization of fluid status in haemodialysis patients. Nephrol Dial Transplant 25: 538–544, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Georgianos PI, Sarafidis PA, Malindretos P, Nikolaidis P, Lasaridis AN: Hemodialysis reduces augmentation index but not aortic or brachial pulse wave velocity in dialysis-requiring patients. Am J Nephrol 34: 407–414, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Stegmayr B: Dialysis procedures alter metabolic conditions. Nutrients 9: 548–557, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fotbolcu H, Duman D, Ecder SA, Oduncu V, Cevik C, Tigen K, et al. : Attenuated cardiovascular response to sympathetic system activation during exercise in patients with dialysis-induced hypotension. Am J Nephrol 33: 491–498, 2011 [DOI] [PubMed] [Google Scholar]
- 57.McIntyre CW: Haemodialysis-induced myocardial stunning in chronic kidney disease - a new aspect of cardiovascular disease. Blood Purif 29: 105–110, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Zuidema MY, Dellsperger KC: Myocardial stunning with hemodialysis: Clinical challenges of the cardiorenal patient. Cardiorenal Med 2: 125–133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braunwald E, Kloner RA: The stunned myocardium: Prolonged, postischemic ventricular dysfunction. Circulation 66: 1146–1149, 1982 [DOI] [PubMed] [Google Scholar]
- 60.Galetta F, Cupisti A, Franzoni F, Femia FR, Rossi M, Barsotti G, et al. : Left ventricular function and calcium phosphate plasma levels in uraemic patients. J Intern Med 258: 378–384, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. : FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wyatt CM, Drüeke TB: Fibroblast growth factor receptor 4: The missing link between chronic kidney disease and FGF23-induced left ventricular hypertrophy? Kidney Int 89: 7–9, 2016 [DOI] [PubMed] [Google Scholar]
- 63.Raizada V, Hillerson D, Amaram JS, Skipper B: Angiotensin II-mediated left ventricular abnormalities in chronic kidney disease. J Investig Med 60: 785–791, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Georgianos PI, Sarafidis PA, Sinha AD, Agarwal R: Adverse effects of conventional thrice-weekly hemodialysis: Is it time to avoid 3-day interdialytic intervals? Am J Nephrol 41: 400–408, 2015 [DOI] [PubMed] [Google Scholar]
- 65.Ozdogan O, Kayikcioglu M, Asci G, Ozkahya M, Toz H, Sezis M, et al. : Left atrial volume predicts mortality in low-risk dialysis population on long-term low-salt diet. Am Heart J 159: 1089–1094, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, et al. ; Frequent Hemodialysis Network Trial Group : Effects of frequent hemodialysis on ventricular volumes and left ventricular remodeling. Clin J Am Soc Nephrol 8: 2106–2116, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hewitson CL, Whiting MJ, Barbara JA, Mangoni AA: Acute effects of haemodialysis on biochemical modulators of endothelial function. J Intern Med 262: 571–580, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Subherwal S, de las Fuentes L, Waggoner AD, Heuerman S, Spence KE, Davila-Roman VG: Central aortic pressure is independently associated with diastolic function. Am Heart J 159: 1081–1088, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karpetas A, Sarafidis PA, Georgianos PI, Protogerou A, Vakianis P, Koutroumpas G, et al. : Ambulatory recording of wave reflections and arterial stiffness during intra- and interdialytic periods in patients treated with dialysis. Clin J Am Soc Nephrol 10: 630–638, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Protogerou AD, Papaioannou TG, Blacher J, Papamichael CM, Lekakis JP, Safar ME: Central blood pressures: Do we need them in the management of cardiovascular disease? Is it a feasible therapeutic target? J Hypertens 25: 265–272, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Fotheringham J, Fogarty DG, El Nahas M, Campbell MJ, Farrington K: The mortality and hospitalization rates associated with the long interdialytic gap in thrice-weekly hemodialysis patients. Kidney Int 88: 569–575, 2015 [DOI] [PubMed] [Google Scholar]
- 72.Krishnasamy R, Badve SV, Hawley CM, McDonald SP, Boudville N, Brown FG, et al. : Daily variation in death in patients treated by long-term dialysis: Comparison of in-center hemodialysis to peritoneal and home hemodialysis. Am J Kidney Dis 61: 96–103, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Susantitaphong P, Koulouridis I, Balk EM, Madias NE, Jaber BL: Effect of frequent or extended hemodialysis on cardiovascular parameters: A meta-analysis. Am J Kidney Dis 59: 689–699, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flythe JE, Kimmel SE, Brunelli SM: Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 79: 250–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Majos E, Dąbrowski R, Szwed H: The right ventricle in patients with chronic heart failure and atrial fibrillation. Cardiol J 20: 220–226, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Saran R, Li Y, Robinson BE: Chapter 6: Mortality. Am J Kidney Dis 67: S219–S226, 2016 [Google Scholar]
- 78.Zoccali C, Puntorieri E, Mallamaci F: Lung congestion as a hidden threat in end-stage kidney disease: A call to action. Nephrol Dial Transplant 28: 2657–2660, 2013 [DOI] [PubMed] [Google Scholar]
- 79.Agarwal R: Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant 27: 3908–3914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. ; FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rocco MV, Lockridge RS Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, et al. ; Frequent Hemodialysis Network (FHN) Trial Group : The effects of frequent nocturnal home hemodialysis: The Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 80: 1080–1091, 2011. 21775973 [Google Scholar]
- 82.Raimann JG, Chan CT, Daugirdas JT, Depner T, Gotch FA, Greene T, et al. ; Frequent Hemodialysis Network (FHN) Trial Group : The effect of increased frequency of hemodialysis on volume-related outcomes: A secondary analysis of the Frequent Hemodialysis Network Trials. Blood Purif 41: 277–286, 2016 [DOI] [PubMed] [Google Scholar]
- 83.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. : Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23: 685–713, 2010 [DOI] [PubMed] [Google Scholar]
- 84.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. : Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16: 233–270, 2015 [DOI] [PubMed] [Google Scholar]
- 85.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. : Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10: 165–193, 2009 [DOI] [PubMed] [Google Scholar]