Abstract
Background
The kidney is considered to be a structurally stable organ with limited baseline cellular turnover. Nevertheless, single cells must be constantly replaced to conserve the functional integrity of the organ. PDGF chain B (PDGF-BB) signaling through fibroblast PDGF receptor-β (PDGFRβ) contributes to interstitial-epithelial cell communication and facilitates regenerative functions in several organs. However, the potential role of interstitial cells in renal tubular regeneration has not been examined.
Methods
In mice with fluorescent protein expression in renal tubular cells and PDGFRβ-positive interstitial cells, we ablated single tubular cells by high laser exposure. We then used serial intravital multiphoton microscopy with subsequent three-dimensional reconstruction and ex vivo histology to evaluate the cellular and molecular processes involved in tubular regeneration.
Results
Single-tubular cell ablation caused the migration and division of dedifferentiated tubular epithelial cells that preceded tubular regeneration. Moreover, tubular cell ablation caused immediate calcium responses in adjacent PDGFRβ-positive interstitial cells and the rapid migration thereof toward the injury. These PDGFRβ-positive cells enclosed the injured epithelium before the onset of tubular cell dedifferentiation, and the later withdrawal of these PDGFRβ-positive cells correlated with signs of tubular cell redifferentiation. Intraperitoneal administration of trapidil to block PDGFRβ impeded PDGFRβ-positive cell migration to the tubular injury site and compromised the recovery of tubular function.
Conclusions
Ablated tubular cells are exclusively replaced by resident tubular cell proliferation in a process dependent on PDGFRβ-mediated communication between the renal interstitium and the tubular system.
Keywords: proliferation, renal stem cell, renal proximal tubule cell, tubular-interstitial crosstalk
The preservation of the structural integrity of an organ requires the continuous replacement of decayed cells. In some organs, such as the intestine, the liver, and the skin, a high turnover of cells is required to conserve the functional and structural integrity. Conversely, other organs, such as the kidney, have little cell turnover.1 Nevertheless, cells of all renal compartments decay during the lifespan of the organism and need to be replaced. If no adequate regeneration occurs, a progressive loss of the structural and functional integrity is inevitable. For the human kidney, approximately 70,000 cells of tubular origin are excreted in the urine per hour.2 Given an average of 1.5 million nephrons, this equals a loss of one tubular epithelial cell per nephron and day.
Tubular cells may be replaced by the proliferation and differentiation of neighboring cells of the tubular epithelium.3–5 These cells may be scattered resident stem/progenitor cells6–8 or common tubular cells.3,5,9–12 There is also evidence for a mesenchymal stem cell niche in the renal papilla, which may be the source of tubular progenitor cells.13 However, little is known about the role of the renal cortical interstitium in tubular cell turnover. Thus, in contrast to other organs, such as the skin, a proregenerative role of fibroblasts in epithelial regeneration in the kidney remains elusive. Finally, the immigration of extrarenal cells from the bone marrow may contribute to the healing of injured regions of the renal tubule.14
The investigation of tubular regeneration and cell turnover has been hampered by the lack of methods that would allow for following regenerative processes over time in an individual animal. In this study, we used serial imaging by intravital multiphoton microscopy (2-PM) of the same cortical regions in the kidney to determine the contribution of tubular and interstitial cells to tubular regeneration. To investigate the replacement of few tubular cells over time in the otherwise healthy organ, we ablated single tubular cells by focused laser exposure and analyzed the subsequent repair mechanisms. To examine the potential contribution of renal interstitial cells to tubular regeneration, we focused on PDGF receptor-β (PDGFRβ)–positive fibroblasts, which contribute to wound healing in other organs, such as the skin.
Our data suggest that (1) tubular cells are exclusively replaced by cells of tubular origin, (2) tubular cells migrate and proliferate to the site of cell loss and reconstitute the functional integrity of the tubule, and (3) the replacement of ablated tubular cells is supported by a targeted migration of interstitial PDGFRβ–positive cells to the location of tubular cell loss.
Methods
Animals
Male and female mice 6–12 weeks of age were randomly included. Pax8-iCre × Confetti and Pax8-iCre × GCaMP5 mice were generated by crossing Pax8-iCre mice [B6.Cg-Tg(Pax8-rtTA2S*M2)1Koes/J]15 with R26RConfetti [Gt(ROSA)26Sortm1(CAG-Brainbow2.1)Cle/J]16 and PC-G5-tdT [B6;129S6-Polr2atm1(CAG-GCaMP5g,-tdTomato)Tvrd/J]17 mice, respectively. After induction with doxycycline, Pax8-positive cells express either CFP, GFP, YFP, and RFP or the fluorescent calcium indicator protein variant GCaMP5G and td Tomato. PDGFRβ-iCre × mTmG and PDGFRβ-iCre × GCaMP5 were generated by crossing PDGFRβ-iCre mice [B6.Cg-Tg(Pdgfrb-cre/ERT2)6096Rha/J]18 with Rosa mTmG [Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J]19 and the above-mentioned PC-G5-tdT mice, respectively. After induction with tamoxifen, PDGFRβ-positive cells expressed either GFP (PDGFRβ-iCre × mTmG mice) or GCaMP5G and tdTomato (PDGFRβ-iCre × GCaMP5 mice). To detect renal proliferative cells in vivo, we used CyclinB1-GFP reporter mice [Tg(Pgk1-Ccnb1/EGFP)1Aklo/J].
Serial Intravital Multiphoton Imaging of Proximal Tubular and Interstitial Cells Using the Abdominal Imaging Window Technique
Intravital multiphoton imaging of the kidney was performed as described before.20–23 Repeated visualization of the kidney was achieved by the implantation of an abdominal imaging window.24 Single-tubular cell ablation was achieved by applying 30% laser excitation (860 nm) at maximal zoom for 2 seconds. This study focused on the regeneration of proximal tubular S1 segments, which were distinguished from S2 segments by differences in the autofluorescence as described before.25 As a second criterion to confirm S1 segment identity, we detected the internalization of injected FITC albumin.
Determination of Cell Migration and Proliferation
Cell migration was detected by comparing z stacks and three-dimensional reconstruction (Amira 5.3; Visage Imaging) data in a side by side fashion. Quantification of migration velocity was conducted in a simplified manner, including only migration in the x/y plane, using the ZEN2010 software (Carl Zeiss). Tubular cell proliferation was determined as the increase in the number of Hoechst 33324–labeled nuclei (12.5 μg/g body wt; Thermo Fischer) associated with monochromatic Confetti-positive areas in the regenerated epithelium of Pax8-iCre × Confetti mice.
Intracellular Calcium Measurement
Changes of intracellular calcium in tubular and interstitial cells were detected using Pax8-iCre × GCaMP5 and PDGFRβ-iCre × GCaMP5 mice, respectively, and expressed in a ratiometric fashion by normalizing GCaMP5G over tdTomato fluorescence.
Determination of Proximal Tubular Function
In vivo proximal tubular albumin uptake of the injured epithelium was assessed after FITC albumin injection (80 μg/g body wt; Sigma-Aldrich). Apical tubular FITC albumin fluorescence was measured before and 24, 48, and 164 hours after laser-induced cell ablation, and it was expressed in percentage of control values.
Immunohistochemistry and Ex Vivo Histology
Paraffin-embedded kidney sections were used to determine protein expression using commercially available antibodies. For ex vivo histology, the cortical renal area of fixed PDGFRβ-iCre × mTmG and Pax8-iCre × Confetti kidneys, which was attached to the abdominal imaging window, was separated and subsequently incubated with primary and secondary antibodies.
CLARITY
To further investigate interstitial PDGFRβ cell morphology, we performed CLARITY of PDGFRβ-iCre × mTmG kidneys as described before.26
Trapidil Treatment
Trapidil (Sigma-Aldrich) was dissolved in sterile saline at 10 mg/ml, and it was administered intraperitoneally (60 mg/kg three times per day).
Statistical Analyses
Data were analyzed by ANOVA with Bonferroni post hoc test using Graph Pad Prism 5 (GraphPad Software). All data are given as mean±SEM. P<0.05 was considered significant.
Supplemental Material has a detailed description.
Results
Resident Proximal Tubular Cells Migrate and Proliferate in Response to Laser-Induced Tubular Cell Ablation
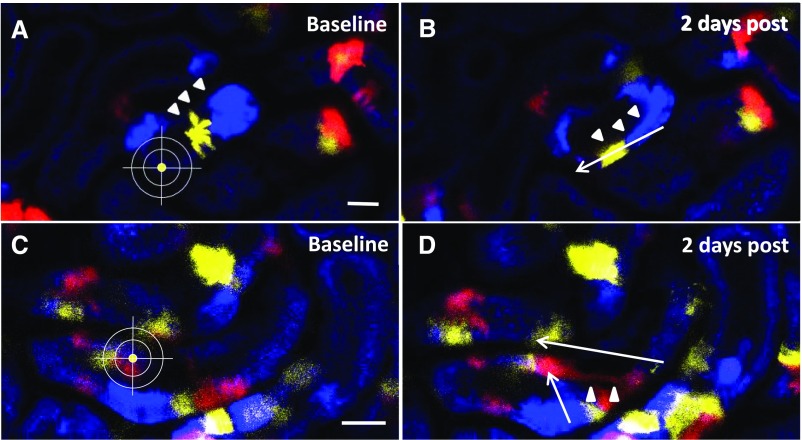
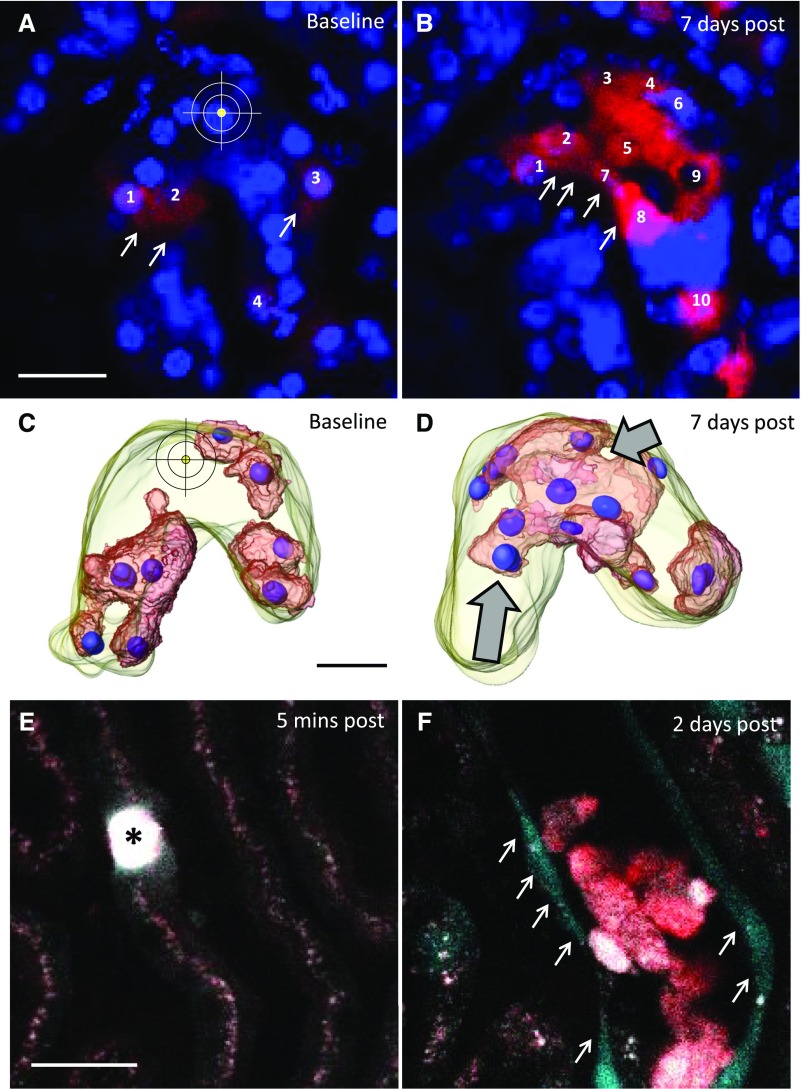
We first aimed to determine the role of resident proximal tubular cells in tubular regeneration. Single cells of the S1 segment of the proximal tubule were ablated by high-laser power exposure to mimic the loss of single cells in the healthy kidney. S1 proximal tubular segments were identified in vivo by the endocytosis of FITC albumin and the characteristic autofluorescence as described before.25 To track tubular regenerative processes, we performed lineage tracing of partially induced Pax8-iCre × Confetti mice. One to two days after local tubular injury, Confetti-positive tubular cells near the injury site changed from a cubical (Figure 1, A and C) to a flask-like shape (Figure 1, B and D), which was accompanied by the migration of these cells toward the site of injury (Figure 1). Seven days after the injury, large monochromatic cell clusters were found in and around the injury site (Figure 2, B and D), revealing a different distribution pattern of Confetti-positive tubule cells compared with baseline. Furthermore, the number of cell nuclei associated with monochromatic cell clusters was increased 7 days after injury compared with baseline conditions (208.3%±30.1% increase in nuclei in monochromatic areas; P<0.05; n=5), suggesting cell proliferation of resident proximal tubular cells. Intravital serial 2-PM imaging of CyclinB1-GFP reporter mice, in which replicating cells are identified by GFP expression, further confirmed these observations. Two days after laser-induced tubular cell ablation, we found a strong epithelial GFP signal within the affected tubular epithelium, indicating proliferating tubular cells adjacent to the site of injury (Figure 2F).
Figure 1.
Resident tubular cells undergo distinct morphologic changes and migrate in response to laser-induced tubular cell ablation. To investigate if and how resident proximal tubular cells respond to local tubular injury, we used partially induced Pax8-iCre × Confetti mice with single-tubular cell expression of CFP (blue), GFP (green), YFP (yellow), or RFP (red). Serial two-photon imaging (A and C) before and (B and D) 2 days after tubular cell ablation revealed characteristic morphologic changes of the adjacent tubular epithelium. Single Confetti-positive tubular cells turned from a cubical to a narrower and flask-like shape (arrowheads), which was accompanied by a distinct cell migration toward the injury site (arrows; average migration velocity: 1.87±0.14 μm/h; n=8).
Figure 2.
Resident tubular cells proliferate in response to laser-induced tubular cell ablation. To investigate whether tubular cells proliferate in response to laser-induced cell ablation, we performed (A and B) cell lineage tracing by serial imaging of Pax8-iCre × Confetti mice followed by (C and D) three-dimensional reconstruction. Injection of Hoechst 33342 allowed for the counting of tubular cell nuclei associated with monochromatic areas within the injured tubular epithelium. Note the different distribution pattern of the RFP-positive area (B and D, arrows) 7 days after tubular cell ablation (A and C) compared with baseline conditions. (B and D, arrows) Although originally located to more remote areas, 7 days after tubular cell ablation, monochromatic cell clusters were found within the injury site, suggesting tubular cell migration and clonal proliferation. (B) Quantification of tubular cell nuclei associated with the RFP-positive area before and 7 days after tubular cell ablation further suggested proliferation of resident tubular epithelial cells. Scale bars, 20 μm. (E and F) To further assess injury-induced tubular cell proliferation, we performed intravital serial imaging of CyclinB1-GFP reporter kidneys. In this genetic mouse model, replicating cells express GFP during the S, G2, and M phases of the cell cycle. (E) Five minutes after tubular cell ablation, there were no GFP-positive cells within or around the injury site (asterisk). (F) In contrast, 2 days after tubular cell ablation, a strong epithelial GFP signal (arrows) was detected in the affected tubular epithelium, indicating proliferation of resident tubular cells after local cell ablation. Furthermore, shed autofluorescent epithelial material of the injured tubular segment was found in the tubular lumen.
Because intracellular calcium is known to play a role in cell migration27 and proliferation,28 we used Pax8-iCre × GCaMP5 reporter mice to determine changes in tubular intracellular calcium [Ca2+]. Laser-induced ablation of one to two tubular cells caused a transient calcium wave along the affected tubular segment within the first 5–10 seconds after injury (Supplemental Figure 1A). Twelve hours after injury, there was a marked increase of [Ca2+] levels in single tubular cells, which were located within 200 μm of the injury site (Supplemental Figure 1B, 12 hours after injury). Within the first 24 hours after injury, these tubular cells migrated toward the injury site (Supplemental Figure 1B). Consistent with our observations in Pax8-iCre × Confetti mice, we detected a characteristic flattening of the tubular epithelium, which was also associated with elevated [Ca2+] levels (Supplemental Figure 1B, 24 hours after injury). These morphologic changes of the tubular epithelium occurred before cell proliferation (Supplemental Figure 1B, 48–72 hours after injury). Seven days after the cell ablation, proximal tubular GCaMP5G-to-tdTomato ratio had returned to baseline values (Supplemental Figure 1, C and D), and the regenerated tubular epithelium appeared morphologically normal (Supplemental Figure 1D). There were no significant tubular calcium alterations in control experiments (Supplemental Figure 2).
Proximal Tubular Cells Are Replaced by Cells of Tubular Origin
In vivo acquired z stacks of the regenerated tubular segment (7 days after tubular cell ablation) in fully induced Pax8-iCre × GCaMP5 mice revealed no tdTomato-negative cells in the tubular epithelium, suggesting that there was no integration of extratubular cells (Supplemental Figure 1D).
Proximal Tubular Cell Dedifferentiation Precedes Tubular Regeneration
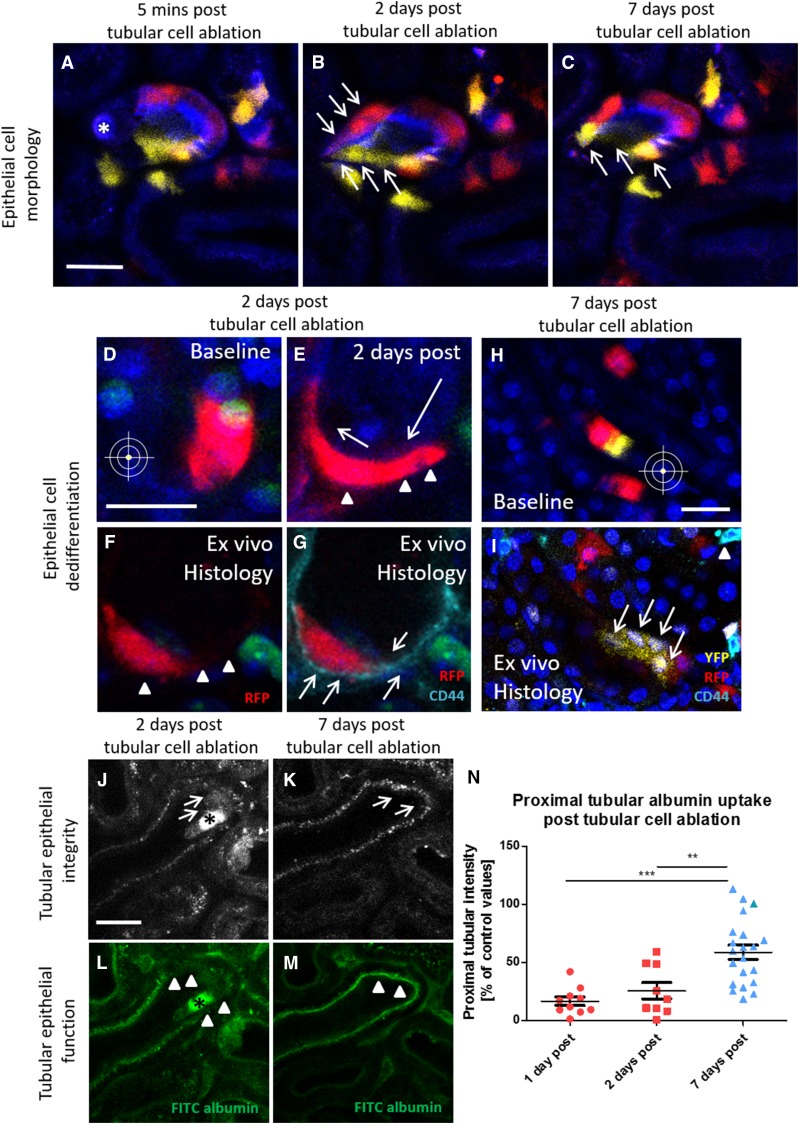
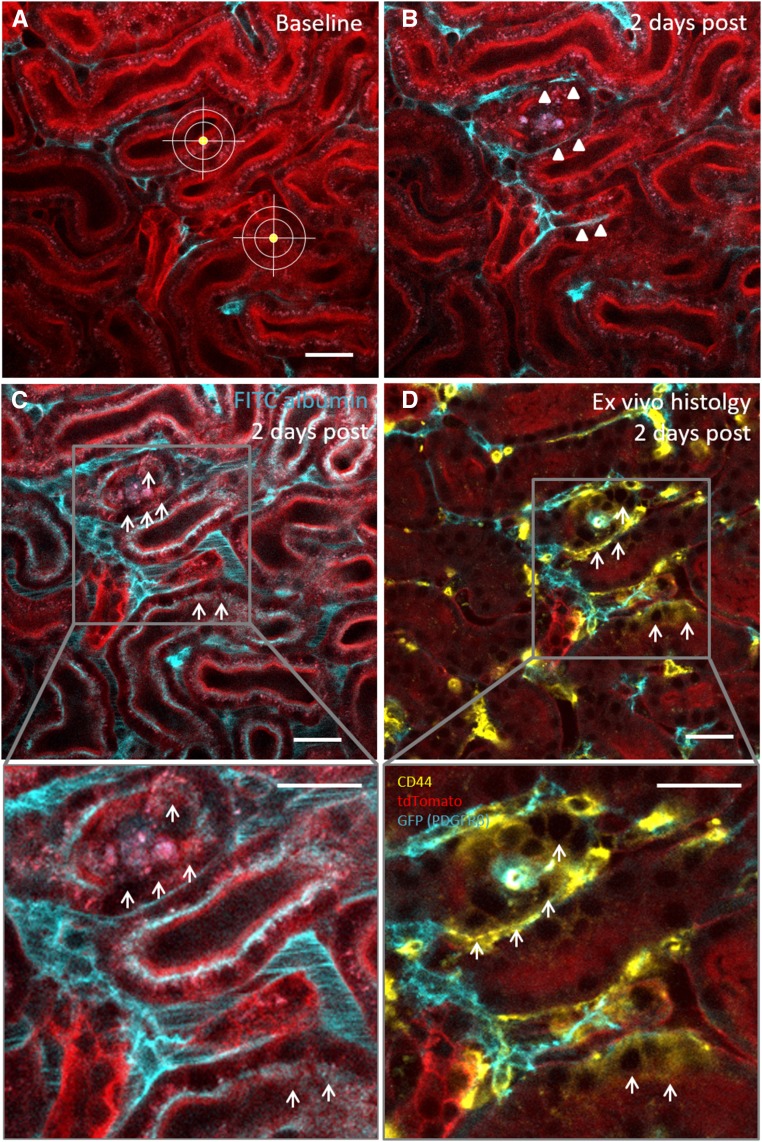
Our results suggest that proximal tubular regeneration is exclusively mediated by the migration and proliferation of resident tubule cells with a distinct morphologic phenotype. We hypothesized that the characteristic flattening of injury-responding tubule cells (Figure 3, B and E) was a sign of dedifferentiation,5 and we performed ex vivo histology for the dedifferentiation marker CD44. In control regions of Pax8-iCre × Confetti kidneys, there was no tubular CD44 expression (Supplemental Figure 3). However, 2 days after laser-induced tubular cell ablation, we reidentified migrating Confetti-positive tubule cells on the fixed tissue and detected a strong epithelial expression of CD44 (Figure 3, F and G). Two days after cell ablation, injury-adjacent tubular cells further showed strongly impaired albumin reuptake capacity (Figure 3, L and N), which was often accompanied by the shedding of tubular cell material into the tubular lumen (Figure 3J). Consistent with these findings, we found an inverse expression pattern for CD44 and the multiligand receptor megalin within the injured tubular epithelium. Thus, CD44-negative proximal tubular cells showed normal megalin expression and performed FITC albumin uptake as determined in vivo. In contrast, CD44-positive proximal tubule cells showed markedly reduced megalin expression and reduced FITC albumin internalization in vivo (Supplemental Figure 4). Seven days after tubular cell ablation, injury-responding tubule cells had regained a cubical cell morphology (Figure 3C) and showed virtually no epithelial CD44 staining (Figure 3I). The regenerated tubule segment further revealed an intact epithelial morphology (Figure 3K) and regained 58.6%±6.1% (n=21) of control tubule albumin reuptake capacity (Figure 3, M and N).
Figure 3.
Functional proximal tubule recovery involves epithelial cell dedifferentiation. Serial intravital imaging of a low dose–induced Pax8-iCre × Confetti kidney (A) 5 minutes and (B) 2 days after single-cell ablation revealed characteristic morphologic changes of migrating tubular cells. Compared with baseline conditions, injury-adjacent RFP- and YFP-positive tubular cells (B, arrows) turned from a cubical into a flat cell shape and migrated toward the injury site (A, asterisk). (C) Seven days after tubular cell ablation, the injury-responding tubular cells were localized at the site of injury and had regained a normal cubical cell morphology (arrows). Scale bar, 20 μm. Serial intravital imaging of Pax8-iCre × Confetti kidneys (D) before and (E) 2 days after tubular cell ablation again showed the (E, arrowheads) flattening and (E, arrows) migration of injury-adjacent RFP-positive tubular cells (2 days after tubular cell ablation). (F) Two days after cell ablation, the same RFP-positive cell was reidentified ex vivo in the fixed kidney tissue (arrowheads), and (G) it showed the expression of the dedifferentiation marker CD44 (cyan and arrows). Scale bar, 20 μm. (I) Seven days after tubular cell ablation, reidentification and CD44 staining (cyan) of the regenerated tubule (arrows) on fixed kidney tissue labeled only few interstitial cells (arrowhead) and revealed no epithelial expression of CD44. Different distribution pattern of Confetti-positive cells (H) at baseline and (I) 7 days after cell ablation further suggested migration. Note the clonal cell division of a single YFP-positive cell (I, arrows) at the site of injury (nuclei are labeled with Hoechst). Scale bar, 20 μm. (J and L) Two and (K and M) 7 days after laser-induced cell ablation, tubular epithelium integrity and function were assessed using serial intravital 2-PM. (J and K) The tubular epithelium was visualized by collecting autofluorescence (gray). (L and M) Subsequently, FITC albumin was injected in vivo (green), and proximal tubular albumin reuptake was determined. (J) Two days after cell ablation (asterisk), cell material was shed from the adjacent tubular epithelium (arrows). (L) Compromised tubular epithelial integrity was associated with strong functional impairment as indicated by the severe reduction of in vivo–injected FITC albumin uptake (green) within and adjacent to the injury site (arrowheads). Seven days after injury, the affected tubular epithelium had (K) morphologically (arrows) and (M) functionally (green FITC albumin uptake at the injury site) recovered. (N) Quantification of proximal tubular albumin uptake capacity at the injury site 1, 2, and 7 days after laser-induced cell ablation. Values are expressed as percentage of control proximal tubular albumin uptake. **P<0.01; ***P<0.001.
Local Tubular Injury Leads to Interstitial PDGFRβ Cell Activation and Recruitment toward the Injury Site
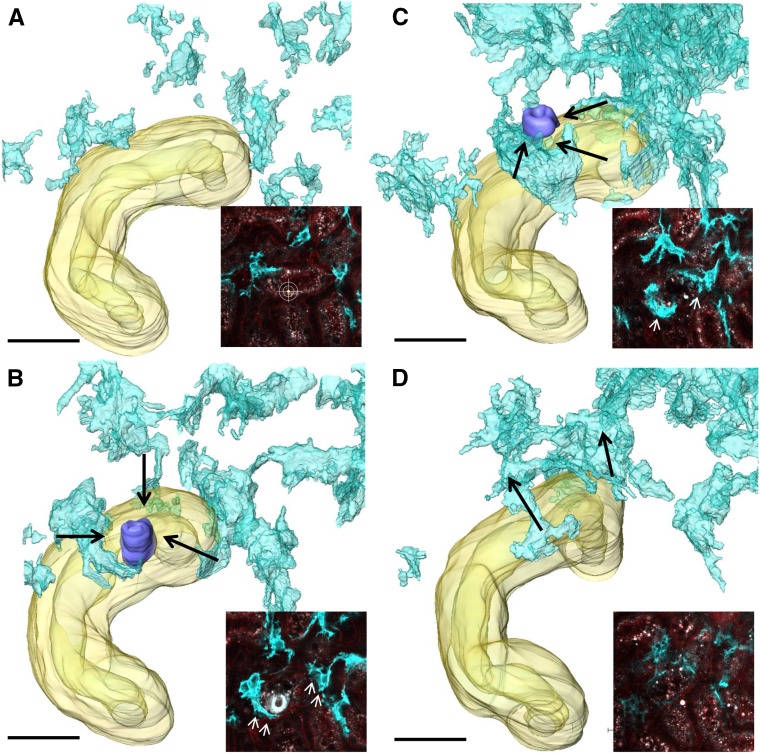
To further investigate a potential contribution of renal interstitial cells to tubular regeneration, we focused on PDGFRβ-positive fibroblasts, which contribute to wound healing in other organs, such as the skin.29,30 In vivo imaging and CLARITY of PDGFRβ-iCre × mTmG kidneys revealed two interstitial PDGFRβ cell types, which we distinguished as pericytes and fibroblasts by characteristic morphologic features. Fibroblasts had a dendritic-like cell shape with multiple long cell extensions connecting the cell bodies to the tubular epithelium (Supplemental Figure 5A, Supplemental Movie 1) and expressed the fibroblast marker vimentin (Supplemental Figure 5, C–E). In contrast, pericytes were identified by their characteristic interaction with glomerular capillaries (Supplemental Figure 5B) and coexpressed the pericyte marker neural/glial antigen 2 (Supplemental Figure 5, F–H). In vivo serial 2-PM imaging of PDGFRβ-iCre × mTmG mice before; 1, 3, 6, 12, 24, 48, and 72 hours after; and 7 days after laser-induced tubular cell ablation showed the targeted migration of PDGFRβ cells toward the tubular injury site. The average velocity of PDGFRβ fibroblasts migration was 1.6±0.2 μm/h (n=45) within the first 24 hours. Within 24–48 hours after injury, PDGFRβ cell recruitment led to the entire enclosure of the injured epithelium (Figure 4, Supplemental Figure 6) without integration of interstitial cells into the epithelium. Finally, 7 days after cell ablation, the recruited PDGFRβ cells had largely withdrawn from the affected tubular epithelium (Figure 4D, Supplemental Figure 6). We detected no injury-induced motility of PDGFRβ-positive pericytes.
Figure 4.
Interstitial PDGFRβ cells are motile and recruited to the tubular injury site. To investigate the response of interstitial PDGFRβ cells to laser-induced tubular cell ablation, we performed in vivo serial imaging (insets) followed by three-dimensional reconstruction of PDGFRβ-iCre × mTmG kidneys at (A) baseline, (B) 1 day, (C) 2 days, and (D) 7 days after laser-induced injury. Interstitial PDGFRβ cells (cyan) were quickly recruited toward the injury site (purple) and enclosed the affected tubular epithelium within 1–2 days after laser-induced tubular cell ablation. (D) Seven days after injury, PDGFRβ cells had redispersed into the renal interstitium. Scale bars, 30 μm.
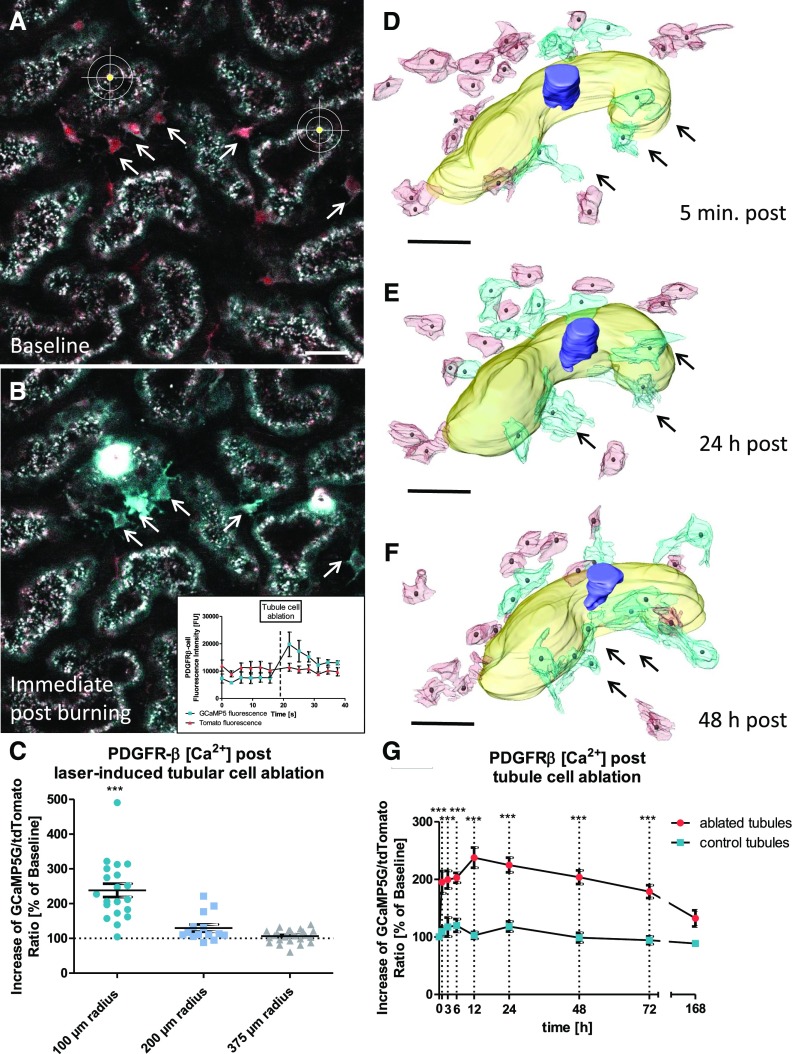
To further assess tubular injury–induced PDGFRβ activation and recruitment, we next measured PDGFRβ cell [Ca2+] levels using PDGFRβ-iCre × GCaMP5 mice. After laser-induced tubular cell ablation, PDGFRβ cell [Ca2+] levels increased immediately, with the most pronounced changes in close vicinity of the site of injury (Figure 5, A–C). Three-dimensional reconstructions of PDGFRβ-iCre × GCaMP5 kidneys revealed that PDGFRβ cells with rises in [Ca2+] levels in most cases showed cell-cell contacts with the affected tubular epithelium (Figure 5D). During the subsequent migration of these cells, the [Ca2+] levels of injury-responding PDGFRβ cells remained elevated (Figure 5, E–G). After 7 days, PDGFRβ [Ca2+] levels had returned to baseline levels (Figure 5G).
Figure 5.
PDGFRβ cell activation occurs predominantly in close proximity of the tubular injury site. Fully induced PDGFRβ-iCre × GCaMP5 mice, which coexpress tdTomato (red) and the fluorescent [Ca2+] indicator protein GCaMP5G (cyan) in PDGFRβ-positive cells (arrows), were investigated (A) before and (B) after laser-induced tubular cell ablation. Proximal tubules were visualized by collecting autofluorescence. (B) Note the strong increase of GCaMP5G fluorescence intensity (arrows and inset) immediately after tubular injury, whereas tdTomato fluorescent intensity remained unchanged (inset), indicating a strong increase of PDGFRβ [Ca2+] values. Scale bar, 20 μm. (C) Changes of PDGFRβ [Ca2+] levels in response to tubular cell ablation were expressed as the GCaMP5G-to-tdTomato ratio in percentage of baseline values. ***P<0.001 for 100-μm radius versus 200- and 375-μm radii. (D–F) To further investigate PDGFRβ [Ca2+] levels during interstitial cell recruitment, we performed serial intravital 2-PM imaging followed by three-dimensional reconstruction of PDGFRβ-iCre × GCaMP5 mice. (D) Three-dimensional reconstruction 5 minutes after tubular cell ablation revealed that initial PDGFRβ cell activation (high [Ca2+] levels; cyan and arrows) occurred predominantly in PDGFRβ cells with cell-cell contact to the injured tubular epithelium. (E) Within the first 24 hours, these early injury-responding PDGFRβ cells migrated toward the injury site (arrows). (E and F) Twenty-four to 48 hours after injury, additional PDGFRβ cells had been activated and recruited toward the tubular injury site (cyan cells). Scale bars, 30 μm. (G) [Ca2+] levels of PDGFRβ cells in close proximity of the injury site given as the GCaMP5G-to-tdTomato ratio in percentage of baseline values (n=25 each). There were no changes in PDGFRβ cell [Ca2+] in control areas. ***P<0.001 for ablated versus control tubules.
PDGFRβ Cell Motility Is Accompanied by the Functional Recovery of the Proximal Tubule
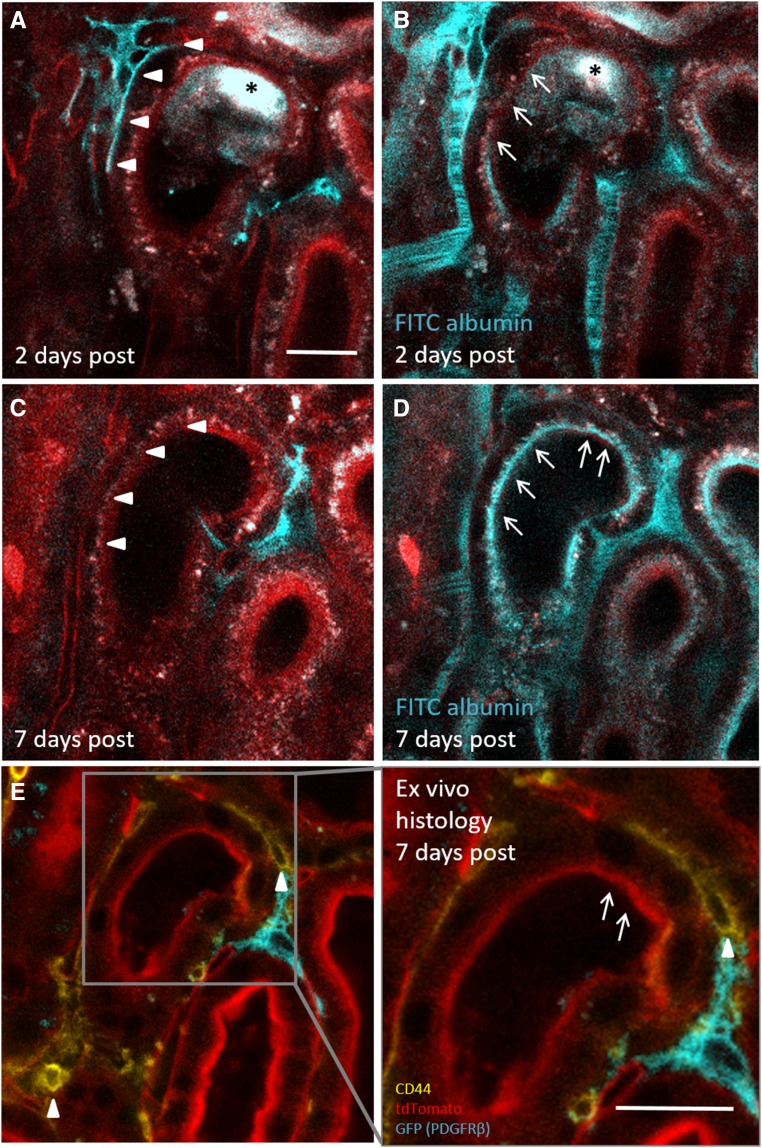
To better understand the role of PDGFRβ cell recruitment in tubular regeneration, we next investigated PDGFRβ cell motility in the due course of proximal tubular cell de- and redifferentiation. Two days after tubular cell ablation, we detected markedly decreased albumin reuptake capacity in the affected tubule segments, which were enclosed by recruited PDGFRβ cells (25.6%±7.1% of control tubules; n=9; P<0.001) (Figures 3N, 6C, and 7B). Ex vivo immunohistochemistry of the same dysfunctional tubular area revealed the de novo expression of the dedifferentiation marker CD44 (Figure 6D), which colocalized with the malfunctional area identified in vivo. Seven days after the injury, recruited PDGFRβ cells had withdrawn from the injury site (Figure 7C). This was associated with partially restored albumin uptake capacity (Figure 7D) and virtually no epithelial CD44 staining in the regenerated tubular epithelium (Figure 7E, inset), indicating redifferentiation of tubular cells on PDGFRβ cell withdrawal.
Figure 6.
PDGFRβ cell recruitment is accompanied by resident proximal tubular cell dedifferentiation. Serial 2-PM intravital imaging of PDGFRβ-iCre × mTmG mice (A) before and (B) 2 days after laser-induced tubular cell ablation confirmed recruitment of interstitial PDGFRβ cells (cyan) to the injury site (arrowheads). (C) In vivo injection of FITC-conjugated albumin (cyan) revealed functional impairment of the PDGFRβ cell–enclosed tubular epithelium (arrows in the inset). Scale bars, 20 μm. (D) To determine if reduced tubular albumin uptake capacity was a sign of resident tubular epithelial cell dedifferentiation, the same cortical area was reidentified ex vivo on fixed tissue and stained for the dedifferentiation marker CD44. Ex vivo histology showed epithelial CD44 expression (yellow and arrows) within the PDGFRβ cell–enclosed tubular epithelium, which colocalized with the malfunctional area observed in vivo (arrows). PDGFRβ cells are shown in cyan, and tdTomato is shown in red. Scale bars, 20 μm.
Figure 7.
PDGFRβ cell withdrawal from the site of injury is accompanied by resident proximal tubular cell redifferentiation. To investigate the timeline of proximal tubular injury and repair in relation to PDGFRβ cell dynamics, we performed serial intravital 2-PM imaging of PDGFRβ-iCre × mTmG mice before (not shown) and (A and B) 2 and (C and D) 7 days after laser-induced tubular cell ablation. (A and B) Two days after injury, PDGFRβ cell recruitment (arrowheads) to the injury site (asterisks) was accompanied by reduced in vivo FITC albumin uptake (arrows). (C and D) However, 7 days after injury, PDGFRβ cells had completely withdrawn from the injury site (arrowheads), and proximal tubular function had recovered (arrows). (E) Ex vivo histology of the regenerated tubule on fixed tissue revealed little to no epithelial CD44 staining (yellow) within the regenerated tubular segment (arrows in the inset), suggesting resident tubular cell redifferentiation. Few interstitial CD44-positive cells (arrowheads) confirmed the successful staining. Scale bars, 20 μm.
The Role of Platelet-Derived Growth Factor Chain B and PDGFRβ Signaling in PDGFRβ Cell Recruitment and Tubular Regeneration
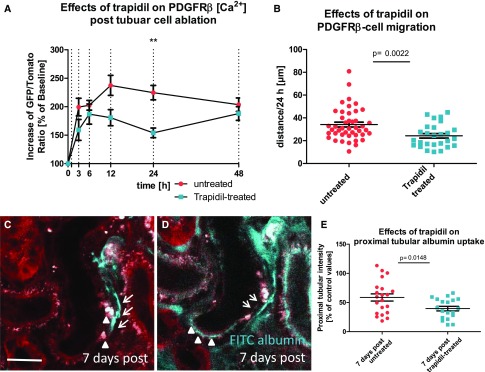
Because PDGF chain B (PDGF-BB) and fibroblast PDGFRβ signaling are known to support wound healing in other organs,31 we next evaluated the role of this pathway for tubular regeneration. Because we found an increased epithelial expression of PDGF-BB in injured tubules compared with unaffected tubules (Supplemental Figure 7), we blocked PDGFRβ signaling using systemic application of trapidil (60 mg/kg three times per day). Twenty-four hours after injury, when tubular PDGF-BB contents were high, PDGFRβ [Ca2+] levels were reduced by trapidil treatment compared with untreated mice (224.7%±12.7% versus 150.2%±7.7% of baseline for untreated versus trapidil treated; n=30 each; P<0.001) (Figure 8A). Furthermore, trapidil treatment decreased PDGFRβ cell migration velocity within the first 24 hours from 34.2±2.1 μm/24 h (n=44) in controls to 24.3±2 μm/24 h (n=27; P=0.002) in trapidil-treated animals (Figure 8B). Seven days after injury, PDGFRβ cells in trapidil-treated mice had not withdrawn from the injured tubular epithelium, and tubular cells often accumulated large vacuoles at the injury site (Figure 8C). Finally, the restoration of in vivo albumin uptake capacity after laser-induced tubular cell ablation was markedly reduced during trapidil application, averaging 39.5%±4.0% and 58.6%±6.1% of control tubules (n=21 each; P=0.02) for trapidil-treated and untreated animals, respectively (Figure 8, D and E).
Figure 8.
PDGFRβ-blockade compromises interstitial cell recruitment and recovery of proximal tubular function. (A) Effects of trapidil, a competitive PDGFRβ inhibitor, on PDGFRβ cell [Ca2+] level 3, 6, 12, 24, and 48 hours after laser-induced proximal tubular injury (n=25 each). **P<0.01 for trapidil-treated versus untreated tubules. (B) Trapidil treatment reduced PDGFRβ cell migration velocity compared with untreated experiments. Proximal tubule of a trapidil-treated animal 7 days after laser-induced tubular cell ablation (C) before and (D) after in vivo FITC albumin injection (cyan). (C) Unlike in untreated animals, PDGFRβ cells (cyan) had not redispersed after 7 days into the renal interstitium, but they still enclosed the injury site (arrows). In addition, the tubular epithelium contained large vacuoles at the injury site (arrowheads). (D) Seven days after injury, FITC albumin injection revealed scattered areas of functional (cyan FITC albumin uptake; arrowheads) and malfunctional albumin uptake (reduced or absent cyan FITC albumin uptake; arrows). Scale bar, 20 μm. (E) Trapidil treatment reduced the recovery of proximal tubular albumin reuptake capacity 7 days after injury.
Discussion
In this study, we used serial intravital imaging followed by three-dimensional reconstruction and ex vivo histology to address tubular cell turnover and regeneration in the healthy kidney.
First, we found that proximal tubular cells are exclusively replaced by resident cells of tubular origin. Second, tubular cell replacement involves the migration and proliferation of dedifferentiated cells within the injured tubular epithelium. Third, interstitial PDGFRβ cells respond to tubular injury by marked increases in [Ca2+]. Fourth, interstitial PDGFRβ cells are motile and migrate toward the site of tubular cell loss. Fifth, interstitial cell recruitment spatially and temporarily coincides with tubular cell dedifferentiation, and the inhibition of PDGFRβ signaling compromises interstitial cell migration and tubular regeneration, suggesting a supportive role of renal PDGFRβ cells in tubular regeneration.
Despite the limited turnover rate of renal cells under physiologic conditions, the adult kidney has some ability to cope with injuries and restore its function.1 Indeed, human kidneys replace approximately one tubular cell per nephron per day while maintaining normal renal function.2 However, the mechanisms underlying tubular regeneration are still not fully evaluated.4 Although accumulating evidence indicates that the tubular epithelium is regenerated by cells of tubular origin,3,5,9–12 there are also data suggesting the contribution of a renal stem cell niche, which expresses mesenchymal markers and incorporates into the tubular epithelium on injury,13 and alternatively, the participation of bone marrow–derived cells.14,32,33 To mimic the physiologic loss of single tubular cells in the healthy kidney, we used the ablation of single tubular cells by high laser exposure in vivo.34,35 Serial imaging of Pax8-iCre × GCaMP5 mice revealed no tdTomato-negative cells in the tubular epithelium. This observation excludes the integration of Pax8-negative cells and suggests that tubular cell loss is replaced by cells of tubular origin.3,5,9–12 Consistent with this, our results strongly indicate that proximal tubular regeneration is exclusively performed by migrating and proliferating resident tubular cells.
The replacement of ablated tubular cells by remote cells requires their targeted migration to the site of injury. Thus, tubular cell migration was discussed to be involved in tubular regeneration.36,37 Here, we first provide in vivo evidence for tubular cell migration as an early response to proximal tubular cell loss. Furthermore, we detected pronounced structural changes of migrating proximal tubular cells, resembling the phenotype of dedifferentiated tubular epithelium cells.38,39 Other than a marked flattening of the cell bodies, dedifferentiated tubular cells are further characterized by the absence of an apical brush border38 and a distinct protein expression pattern, such as reduced megalin expression,40 and the de novo expression of specific stem cell markers, such as CD44,5 vimentin,36,40 and CD106.8 Functionally, we observed a strong reduction in the tubular albumin reuptake capacity, which was paralleled by the de novo expression of CD44, suggesting resident tubular cell dedifferentiation. This observation was not restricted to the site of injury but also occurred in the injury-adjacent segment of the same tubular epithelium. The concomitant flattening of the injury-adjacent epithelium was further associated with the shedding of apical cell material into the tubular lumen, suggesting the loss of the apical brush border, and it was paralleled by strongly reduced megalin expression in CD44-positive, dedifferentiated tubule cells. Thus, single-cell ablation within the intact tubular epithelium led to a pronounced remodeling of the affected tubular epithelium by adjacent resident tubular epithelial cells, which responded to the local injury by dedifferentiation, migration, and proliferation. Future studies need to address the mechanisms of resident tubular cell activation before dedifferentiation. However, our experiments in Pax8-iCre × GCaMP5 mice detected an immediate, injury-induced cell to cell propagation of a tubular calcium wave, which originated from the laser-induced cell ablation and then spread along the tubular epithelium. This data first showed a calcium-mediated cell to cell communication between proximal tubular cells, which may initiate tubular cell activation and dedifferentiation. Consistently, this early tubular calcium response was followed by the detection of single migrating tubular cells with high intracellular calcium levels. Changes of intracellular calcium are known to play a role during cell migration27 and proliferation.28 Because intracellular calcium levels are also strongly affected by apoptosis or necrosis,41 cell death of the injury-adjacent tubular epithelium could, in principle, account for the observed elevations of tubular calcium. However, large elevations of intracellular calcium were mainly found in single migrating tubule cells. Using the Pax8-iCre × Confetti model, we were able to track those migrating cells over several days. On the contrary to cell death and degradation, migrating tubule cells were found to regenerate the injured tubule segment by proliferation and subsequently redifferentiated into functional tubule cells. These observations strongly suggest that calcium elevations in the tubular epithelium are associated with tubular regeneration through migrating and proliferating resident tubule cells.
Tubular regeneration may be derived from specialized so-called scattered tubular epithelial cells. These cells may serve as resident stem cell/progenitors for tubular regeneration.8,42,43 In addition, a specific subset of progenitor cells in the Bowman’s capsule was suggested to participate in tubular regeneration.6,7 Alternatively, there is also evidence to suggest that every tubular cell may dedifferentiate and subsequently proliferate.3–5,12 Our observation that only single tubular epithelium cells migrate within the tubular epithelium may suggest a specialized subset of resident tubule cells, which gives rise to proliferation. However, we clearly detected a reversible phenotype shift of injury-adjacent tubular cells from a differentiated state to a dedifferentiated state, which was associated with de novo expression of CD44 and the lack of megalin expression. Our local injury model further revealed that the dedifferentiation marker CD44 was exclusively and only temporarily expressed in injury-adjacent tubule cells and that it was not expressed in healthy control areas of the same animal. Taken together, our results indicate that proximal tubular regeneration is facilitated by differentiated resident tubular epithelial cells, which undergo dedifferentiation and subsequent proliferation.
In contrast to other organs, little is known about proregenerative effects of renal fibroblasts in the kidney. PDGFRβ-positive fibroblasts in vivo showed a dendritic-like morphology, with cell extensions connecting them to the tubular epithelium. These results are in agreement with ultrastructural studies of CD73-positive fibroblasts that showed the spine-like processes connecting interstitial fibroblasts to the basal membrane of tubules, capillaries, and other fibroblasts.44,45 These properties suggest a morphologic basis for a potential crosstalk between renal epithelial cells and fibroblasts. Thus, the tubular epithelium and renal fibroblasts express connexine 43,46–49 which may mediate the propagation of calcium signaling between the tubular and interstitial compartments.
According to our observations, interstitial PDGFRβ cells are motile. Thus, these cells are activated by tubular injury and migrate toward the site of tubular cell loss. Most importantly, tubular cell dedifferentiation coincided with PDGFRβ cell recruitment, and CD44-positive tubular cells were usually surrounded by PDGFRβ-positive interstitial cells. These observations suggest a supportive role of PDGFRβ cells during tubular regeneration. The enclosure of the injured tubular epithelium by recruited renal fibroblasts may act as a mechanical stabilization of the denuded basement membrane during tubular cell migration and proliferation, and it may also support tubular regeneration. Thus, PDGFRβ blockade using trapidil markedly reduced interstitial PDGFRβ cell calcium levels and migration, which were accompanied by impaired tubular regeneration. Consistent with this observation, a recent study in rats showed that trapidil treatment after ischemia-reperfusion compromised tubular regeneration.50 In our study, trapidil treatment reduced the recovery of proximal tubular albumin reuptake capacity after tubular cell ablation. Mechanistically, the authors of this previous study suggested that trapidil treatment may disrupt the regenerative capacity of a tubular autocrine PDGF-BB/PDGFRβ axis.50 However, we and others51 found no epithelial expression of PDGFRβ. Considering that trapidil treatment also reduced interstitial PDGFRβ cell migration to the injury site, it seems likely that the compromised tubular regeneration during trapidil treatment is caused by an altered PDGF-BB/PDGFRβ–mediated paracrine crosstalk between tubular and interstitial cells.
In summary, our study suggests that renal interstitial cells are crucially involved in the baseline cellular turnover and the regeneration of the tubular epithelium in the healthy kidney. The interaction of interstitial and tubular renal cells may provide new therapeutic targets to support endogenous regenerative processes in kidney disease.
Disclosures
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB699/B7).
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Present addresses: Dr. Ina Maria Schiessl, Institute of Physiology and Biophysics, Zilkha Neurogenetic Institute, University of Southern California, Los Angeles, California. Dr. Alexandra Grill, Center of Thrombosis and Hemostasis University Medical Center, Mainz, Germany.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017101069/-/DCSupplemental.
References
- 1.Meyer-Schwesinger C: The role of renal progenitors in renal regeneration. Nephron 132: 101–109, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Prescott LF: The nephrotoxicity of analgesics. J Pharm Pharmacol 18: 331–353, 1966 [DOI] [PubMed] [Google Scholar]
- 3.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD: Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A 111: 1527–1532, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramann R, Kusaba T, Humphreys BD: Who regenerates the kidney tubule? Nephrol Dial Transplant 30: 903–910, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger K, Bangen JM, Hammerich L, Liedtke C, Floege J, Smeets B, et al.: Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci U S A 111: 1533–1538, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, et al.: Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Romagnani P, Kalluri R: Possible mechanisms of kidney repair. Fibrogenesis Tissue Repair 2: 3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, et al.: Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 30: 1714–1725, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, et al.: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al.: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M: Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol 294: C22–C28, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Kang HM, Huang S, Reidy K, Han SH, Chinga F, Susztak K: Sox9-positive progenitor cells play a key role in renal tubule epithelial regeneration in mice. Cell Reports 14: 861–871, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q: The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114: 795–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG: Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, et al.: An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, et al.: Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Gee JM, Smith NA, Fernandez FR, Economo MN, Brunert D, Rothermel M, et al.: Imaging activity in neurons and glia with a Polr2a-based and cre-dependent GCaMP5G-IRES-tdTomato reporter mouse. Neuron 83: 1058–1072, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, et al.: Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124: 161–173, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Schießl IM, Kattler V, Castrop H: In vivo visualization of the antialbuminuric effects of the angiotensin-converting enzyme inhibitor enalapril. J Pharmacol Exp Ther 353: 299–306, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Schießl IM, Hammer A, Kattler V, Gess B, Theilig F, Witzgall R, et al.: Intravital imaging reveals angiotensin II-induced transcytosis of albumin by podocytes. J Am Soc Nephrol 27: 731–744, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schießl IM, Castrop H: Angiotensin II AT2 receptor activation attenuates AT1 receptor-induced increases in the glomerular filtration of albumin: A multiphoton microscopy study. Am J Physiol Renal Physiol 305: F1189–F1200, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Schießl IM, Bardehle S, Castrop H: Superficial nephrons in BALB/c and C57BL/6 mice facilitate in vivo multiphoton microscopy of the kidney. PLoS One 8: e52499, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritsma L, Steller EJ, Ellenbroek SI, Kranenburg O, Borel Rinkes IH, van Rheenen J: Surgical implantation of an abdominal imaging window for intravital microscopy. Nat Protoc 8: 583–594, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Hato T, Winfree S, Dagher PC: Intravital imaging of the kidney. Methods 128: 33–39, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomer R, Ye L, Hsueh B, Deisseroth K: Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat Protoc 9: 1682–1697, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei C, Wang X, Zheng M, Cheng H: Calcium gradients underlying cell migration. Curr Opin Cell Biol 24: 254–261, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Munaron L, Antoniotti S, Lovisolo D: Intracellular calcium signals and control of cell proliferation: How many mechanisms? J Cell Mol Med 8: 161–168, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robson MC, Phillips LG, Thomason A, Robson LE, Pierce GF: Platelet-derived growth factor BB for the treatment of chronic pressure ulcers. Lancet 339: 23–25, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Pierce GF, Tarpley JE, Allman RM, Goode PS, Serdar CM, Morris B, et al.: Tissue repair processes in healing chronic pressure ulcers treated with recombinant platelet-derived growth factor BB. Am J Pathol 145: 1399–1410, 1994 [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Z, Sasaoka T, Fujimori T, Oya T, Ishii Y, Sabit H, et al.: Deletion of the PDGFR-beta gene affects key fibroblast functions important for wound healing. J Biol Chem 280: 9375–9389, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, et al.: Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195: 229–235, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, et al.: Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 15: 1794–1804, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Burford JL, Villanueva K, Lam L, Riquier-Brison A, Hackl MJ, Pippin J, et al.: Intravital imaging of podocyte calcium in glomerular injury and disease. J Clin Invest 124: 2050–2058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peti-Peterdi J, Sipos A: A high-powered view of the filtration barrier. J Am Soc Nephrol 21: 1835–1841, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witzgall R, Brown D, Schwarz C, Bonventre JV: Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffield JS, Bonventre JV: Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int 68: 1956–1961, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger K, Moeller MJ: Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin Nephrol 34: 394–403, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, et al.: Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol 229: 645–659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansson J, Hultenby K, Cramnert C, Pontén F, Jansson H, Lindgren D, et al.: Evidence for a morphologically distinct and functionally robust cell type in the proximal tubules of human kidney. Hum Pathol 45: 382–393, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Bhosale G, Sharpe JA, Sundier SY, Duchen MR: Calcium signaling as a mediator of cell energy demand and a trigger to cell death. Ann N Y Acad Sci 1350: 107–116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindgren D, Boström AK, Nilsson K, Hansson J, Sjölund J, Möller C, et al.: Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol 178: 828–837, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romagnani P: Family portrait: Renal progenitor of Bowman’s capsule and its tubular brothers. Am J Pathol 178: 490–493, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaissling B, Le Hir M: The renal cortical interstitium: Morphological and functional aspects. Histochem Cell Biol 130: 247–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaissling B, Hegyi I, Loffing J, Le Hir M: Morphology of interstitial cells in the healthy kidney. Anat Embryol (Berl) 193: 303–318, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Hillis GS, Duthie LA, Mlynski R, McKay NG, Mistry S, MacLeod AM, et al.: The expression of connexin 43 in human kidney and cultured renal cells. Nephron 75: 458–463, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Hossain MZ, Ao P, Boynton AL: Platelet-derived growth factor-induced disruption of gap junctional communication and phosphorylation of connexin43 involves protein kinase C and mitogen-activated protein kinase. J Cell Physiol 176: 332–341, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Ko K, Arora P, Lee W, McCulloch C: Biochemical and functional characterization of intercellular adhesion and gap junctions in fibroblasts. Am J Physiol Cell Physiol 279: C147–C157, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Moyer KE, Ehrlich HP: Modulation of human fibroblast gap junction intercellular communication by hyaluronan. J Cell Physiol 196: 165–170, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa T, Sasahara M, Haneda M, Kataoka H, Nakagawa H, Yagi M, et al.: Role of PDGF B-chain and PDGF receptors in rat tubular regeneration after acute injury. Am J Pathol 155: 1689–1699, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seifert RA, Alpers CE, Bowen-Pope DF: Expression of platelet-derived growth factor and its receptors in the developing and adult mouse kidney. Kidney Int 54: 731–746, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.