Abstract
Background As children progress to higher stages of AKI, the risk for adverse outcomes dramatically increases. No reliable methods exist to predict AKI progression in hospitalized children. To determine if biomarkers of inflammation and kidney injury can predict AKI progression, we conducted a three-center prospective cohort study of children undergoing cardiopulmonary bypass.
Methods On the first day of serum creatinine–defined AKI, we measured urine biomarkers (neutrophil gelatinase–associated lipocalin [NGAL], IL-18, kidney injury molecule 1, liver fatty acid binding protein [L-FABP], albumin, and cystatin C) and plasma biomarkers (IFN, IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, TNF-α, NGAL, and cystatin C). We defined AKI progression as a worsening of AKI stage or persisting stage 3 AKI (≥2 consecutive days).
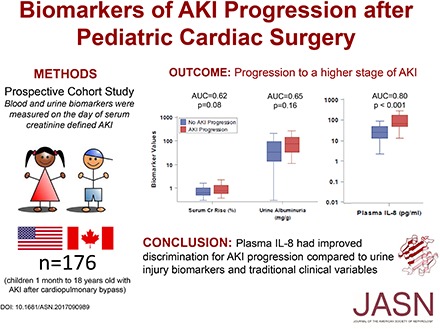
Results In all, 176 of 408 (43%) children developed postoperative AKI. Among the children with AKI, we diagnosed stages 1, 2, and 3 AKI in 145 (82.5%), 25 (14%), and six (3.5%) children, respectively, on the first day of AKI; 28 (7%) children had AKI progression. On the first day of AKI, nine of 17 biomarkers were significantly higher in patients with than without AKI progression. Urine L-FABP (among injury biomarkers) and plasma IL-8 (among inflammatory biomarkers) had the highest discrimination for AKI progression: optimism-corrected area under the curve, 0.70; 95% confidence interval, 0.58 to 0.81 and optimism-corrected area under the curve, 0.80; 95% confidence interval, 0.69 to 0.91, respectively.
Conclusions If validated in additional cohorts, plasma IL-8 could be used to improve clinical care and guide enrollment in therapeutic trials of AKI.
Keywords: acute renal failure, children, heart disease, pediatric nephrology, progression of renal failure, cytokines
AKI in children is associated with increased inpatient mortality, length of ventilation, and length of hospital stay.1–3 There is also evidence that the risk for adverse outcomes rises dramatically as the severity of AKI (or AKI stage) increases.4 Hospitalized children with AKI who progressed to stage 3 AKI (i.e., AKI worsened clinically from less severe AKI to the highest AKI stage) had a fivefold higher mortality risk than the children with stage 1 AKI.4 Additionally, in adult patients, the severity of AKI during hospitalization predicts the long-term development of hypertension, CKD, and ESRD.5–7 No reliable methods currently exist to predict progression of AKI to higher stages in hospitalized children. Developing novel biomarkers or clinical models that predict AKI progression could guide clinical care, resource allocation, and AKI clinical trial enrollment.8
Although elevated serum creatinine is useful in detecting changes in glomerular filtration, it often correlates poorly with the onset of AKI as well as AKI progression.9,10 Pediatric research has identified novel biomarkers that enhance prediction and diagnosis of AKI.11–18 Although these biomarkers detect intrinsic renal damage or the inflammatory process in AKI, they have not been evaluated for their ability to predict AKI progression in children. In this study, we investigated the prognostic utility of biomarkers to predict progression of AKI at the time of first diagnosis of clinical AKI.
Using the Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) prospective multicenter cohort of children undergoing cardiac surgery, we determined if blood and urine biomarkers of inflammation and kidney injury are associated with AKI progression and if these biomarkers provide adequate discrimination to predict AKI progression beyond traditional clinical variables.
Methods
Study Cohort
This is a prospective cohort study of children with congenital cardiac defects who were enrolled in two phases (from July 2007 to December 2009 and from January 2010 to December 2010) at Cincinnati Children’s Hospital, Montreal Children’s Hospital, and Yale New Haven Children’s Hospital. Children 1 month old to 18 years old who were scheduled to be placed on cardiopulmonary bypass (CPB) during cardiac surgery were enrolled in the TRIBE-AKI Study.3 Children were excluded if they had a history of renal transplantation or dialysis. Informed consent was obtained from all parents or legal guardians along with assent, when appropriate, from children. This study was approved by the institutional review board of each participating institution.
Sample Collection
Patients were recruited during preoperative evaluations within 1 month of surgery. Blood and urine specimens were collected before surgery or with anesthesia induction. Postoperatively, blood and urine were collected within 6 hours of intensive care unit arrival (day 1 sample) and then daily for up to 5 days. Biospecimens were centrifuged, and urine supernatant and plasma were aliquoted. Barcoded aliquots of urine and plasma were stored at −80°C in a continuously monitored biorepository until biomarker measurement. No additives or protease inhibitors were added.
Data Collection
The following detailed cardiac surgery admission information was available for all participants: age, sex, race, type of congenital heart disease, and CPB time. We used the risk adjustment for congenital heart surgery 1 (RACHS-1) consensus-based scoring system to categorize the complexity of surgery.19 Higher scores signify more complex surgery.
Outcome Definitions
AKI was defined as the development of at least stage 1 AKI defined by AKI Network (AKIN)20 as at least a ≥50% rise or a ≥0.3 mg/dl rise from baseline serum creatinine during hospitalization after cardiac surgery. Stage 2 AKI was defined as a doubling in serum creatinine from baseline. Stage 3 AKI was defined as a tripling in serum creatinine from baseline or receiving acute dialysis during the hospital stay. AKI progression was defined as a worsening of AKIN stage: from stage 1 to stage 2 or 3 or from stage 2 to 3. Patients diagnosed with progressive or persisting stage 3 AKI (stage 3 AKI for ≥2 consecutive days) were classified as having AKI progression. We determined the preoperative eGFR using the CKD in Children study equation.21 Age-adjusted percentiles for eGFR values were obtained on the basis of previously published data on normal pediatric renal function.22,23 Pre- and postoperative serum creatinine levels were measured in the same clinical laboratory for each patient at all sites.
AKI Biomarker Measurements
For this study, biomarkers were measured on the initial day that the serum creatinine first attained the stage 1 AKI threshold (initial clinical evidence of AKI). We categorized our biomarkers as “traditional” biomarkers (serum creatinine and urine albuminuria), cystatin C (urine and plasma), “kidney injury” biomarkers (urinary neutrophil gelatinase–associated lipocalin [NGAL], IL-18, kidney injury molecule 1 [KIM-1], liver fatty acid binding protein [L-FABP], and plasma NGAL), and “inflammatory” biomarkers (plasma interferon-gamma [IFN], IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, and tumor necrosis factor-alpha [TNF]). These biomarkers were selected on the basis of prior pediatric AKI research, on the basis of pilot studies within the TRIBE-AKI consortium, and with consideration for pathophysiologic mechanisms of AKI progression.11–18,24 Personnel measuring the biomarkers were blinded to clinical outcomes. If a patient had insufficient biospecimen to measure a specific biomarker, the patient was excluded from analyses for that biomarker. Details of the measurement of urine NGAL, IL-18, KIM-1, L-FABP, albumin, and cystatin C as well as blood NGAL and cystatin C have been previously described.3,11–13,23,25 These biomarkers were measured at a median of 2 years after sample collection. The plasma inflammatory biomarkers IFN, IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, and TNF were measured using the Meso Scale Discovery platform (Meso Scale Discovery, Gaithersburg, MD) at a median of 8 years after collection. The intra-assay coefficients of variation for IFN, IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, and TNF were 8.9%, 7.8%, 7.9%, 11.3%, 6.5%, 7.7%, 11.3%, 6.4%, and 10.9%, respectively.
Statistical Analyses
Continuous variables were compared with two-sample t tests or Wilcoxon rank sum tests, and dichotomous variables were compared with chi-squared tests or Fisher exact tests. Mixed effects models were used to determine the effect of storage time on biomarker levels (Supplemental Figure 1, Supplemental Table 1).26 We performed multivariate analyses to examine the association between the covariates of age, sex, race, preoperative GFR, RACHS-1, and CPB time with the outcome of AKI progression. We determined the adjusted odds ratio (using logistic regression) for biomarkers to predict AKI progression. We adjusted for baseline covariates of age, sex, race, RACHS-1, study site, and CPB time. We used area under the receiver operating curve (AUC) to determine the ability of the biomarkers and pairs of biomarkers to discriminate between patients with and without AKI progression. We determined the AUCs of pairs of plasma inflammatory biomarkers as well as a pair of kidney injury and inflammatory biomarkers with the highest individual AUC to predict AKI progression. The apparent AUC directly estimated from the model is optimistically biased by both resubstitution bias and model selection bias, and therefore, we calculated an optimism-corrected AUC.27,28 The optimism-corrected AUC was estimated using a bootstrap procedure with 1000 replicates. A bootstrap procedure is a statistical method where the original dataset is repeatedly sampled with replacement to create several random bootstrap datasets, each of the same size as the original dataset. The model fitted using the bootstrap dataset was applied to the original dataset as well as the bootstrap dataset. The average difference in predictive abilities is the optimism. The average optimism in AUCs was subtracted from the apparent AUC to get the optimism-adjusted AUC. SAS version 9.4 was used for analyses.
Results
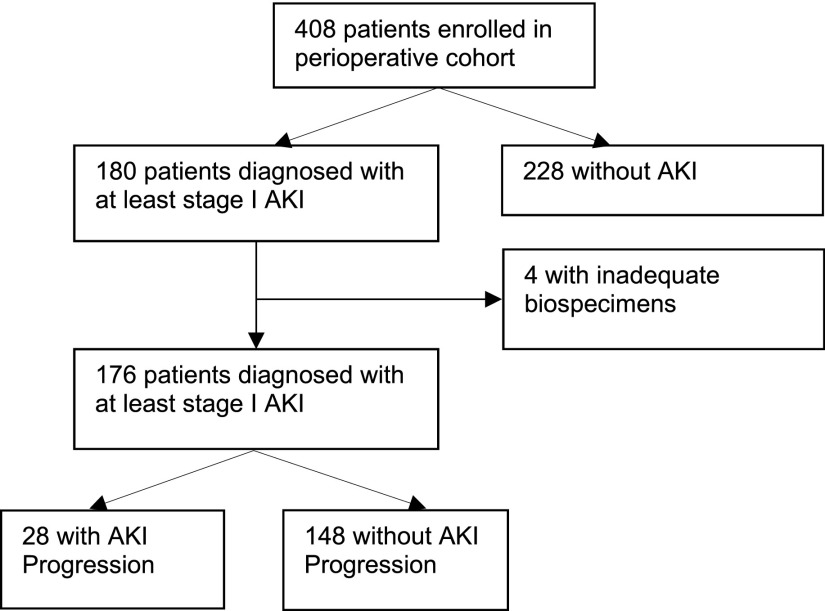
Of the 408 children who were enrolled in the cohort, 176 (43%) were diagnosed with at least stage 1 AKI after cardiac surgery (Figure 1). Among the 176 children with AKI, 28 (16%) children met the criteria for AKI progression. Table 1 shows the demographic and perioperative characteristics of participants with and without AKI progression. Children with AKI who progressed were less likely to have an elective surgery and had higher CPB time. Patient age and RACHS-1 category were not associated with AKI progression. Children with AKI progression had longer lengths of hospital stay, intensive care unit stay, and time of ventilation. Approximately two thirds of patients with AKI progression had an initial AKI severity of stage 1 (shown in Table 2). Three (11%) of 28 children with progressive AKI required acute dialysis.
Figure 1.
Flow diagram of patient selection into the study cohort.
Table 1.
Baseline and postoperative characteristics by AKI progression status
| Characteristic | AKI without Progression, n=148 | AKI with Progression, n=28 | P Valuea |
|---|---|---|---|
| Age, yr | 2.7 (4.0) | 1.9 (2.9) | 0.51 |
| Women | 67 (45%) | 12 (43%) | 0.81 |
| Nonwhite race | 25 (17%) | 7 (25%) | 0.31 |
| Preoperative eGFR | 98.3 (32.0) | 106.3 (32.5) | 0.19 |
| RACHS-1 category | |||
| 1 | 1 (1%) | 0.92 | |
| 2 | 76 (52%) | 13 (46%) | |
| 3 | 61 (41%) | 13 (46%) | |
| 4 | 9 (6%) | 2 (7%) | |
| CPB time, min | 116.0 (64.6) | 142.3 (54.8) | 0.003 |
| Crossclamp time, min | 48.0 (46.8) | 59.5 (42.5) | 0.09 |
| Type of surgery, urgent | 12 (8%) | 6 (21%) | 0.03 |
| Type of surgery | |||
| Septal defect repair | 47 (33%) | 7 (27%) | 0.50 |
| Inflow/outflow tract or valve procedure | 19 (13%) | 2 (8%) | |
| Combined procedure | 76 (54%) | 17 (65%) | |
| Index hospitalization | |||
| Dialysis | 0 (0%) | 3 (11%) | <0.001 |
| Length of stay in ICU, d | 4.4 (4.68) | 10.7 (10.5) | <0.001 |
| Length of stay in hospital, d | 9.6 (10.0) | 19.2 (18.5) | <0.001 |
| In-hospital death | 4 (3%) | 1 (4%) | 0.80 |
| No. of days on ventilator | 2.0 (1.6) | 3.5 (1.6) | <0.001 |
RACHS-1, risk adjustment of congenital heart surgery 1; CPB, cardiopulmonary bypass; ICU, intensive care unit.
P values were obtained by the Kruskal–Wallace test.
Table 2.
First stage of AKI diagnosis and peak AKI stage
| First AKI stage | Peak AKI Stage | |||
|---|---|---|---|---|
| Stage 1 AKI | Stage 2 AKI | Stage 3 AKI | Total | |
| Stage 1 AKI | 125 | 10a | 10a | 145 |
| Stage 2 AKI | 0 | 23 | 2a | 25 |
| Stage 3 AKI | 0 | 0 | 6a | 6 |
| Total | 125 | 33 | 18 | 176 |
Patients with AKI progression.
Traditional Biomarkers
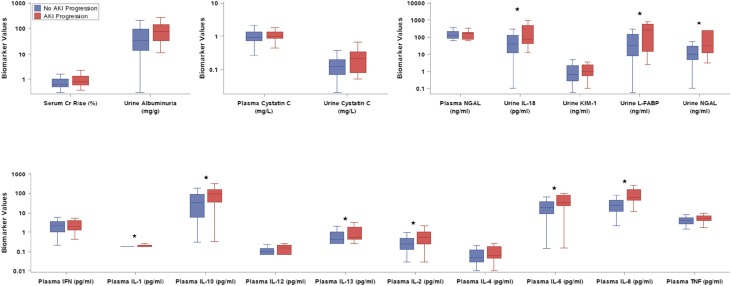
Median serum creatinine percent rise and urine albuminuria were higher on the day of diagnosis in AKI progressors (Figure 2, Supplemental Table 2). CPB time and preoperative eGFR percentile were the two significant clinical predictors of AKI progression. The clinical prediction model devised of CPB time and preoperative eGFR percentile yielded an optimism-corrected AUC of 0.67 (95% confidence interval [95% CI], 0.55 to 0.78). There was no statistically significant interaction between age (<2 or ≥2 years old) and biomarker levels.
Figure 2.
Nine of the 17 biomarkers measured on the first day of AKI were higher in children with AKI progression. Urine and plasma biomarkers were measured on the first day of serum creatinine–defined AKI. The y axis is displayed on a logarithmic scale. Boxes represent the median and interquartile range (25th and 75th percentiles), and whiskers represent the range of values. The asterisks represent a significant difference (P<0.05) in comparison of AKI and non-AKI progression. Cr, creatinine; KIM-1, kidney injury molecule 1; L-FABP, liver fatty acid binding protein; NGAL, neutrophil gelatinase–associated lipocalin.
Kidney Injury Biomarkers
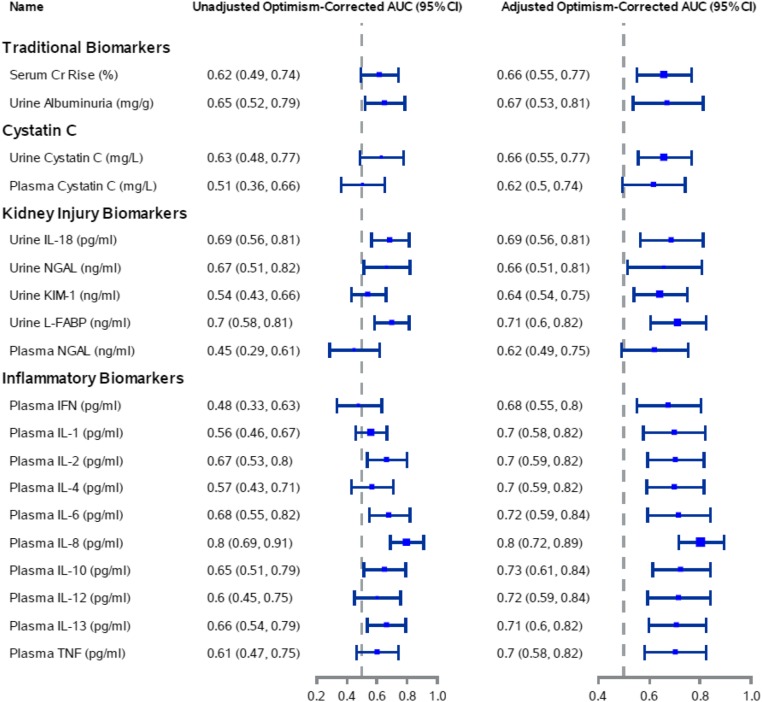
Figure 3 displays the unadjusted and adjusted optimism-corrected AUCs of the kidney injury biomarkers (IL-18, NGAL, KIM-1, and L-FABP) to predict AKI progression. The apparent AUCs were also calculated (Supplemental Table 3). IL-18 and L-FABP had the highest individual optimism-corrected AUCs of 0.69 and 0.70, respectively. We also determined the discriminative performance of urine and blood biomarkers to distinguish which patients with stage 1 AKI (Supplemental Table 4) or stage 1 and stage 2 AKI (Supplemental Table 5) will progress to a higher AKI stage.
Figure 3.
Plasma IL-8 had the highest optimism-corrected area under the receiver operating curve (AUC) for the prediction of AKI progression. The adjusted model included the biomarker, cardiopulmonary bypass time, and preoperative eGFR percentile. The AUC with only the clinical variables is 0.67 (95% confidence interval [95% CI], 0.55 to 0.78). The optimism-corrected AUC was estimated using a bootstrap procedure with 1000 replicates. The model fitted using the bootstrap dataset was applied to the original dataset as well as the bootstrap dataset. The average difference in predictive abilities is the optimism. The average optimism in AUCs was subtracted from the apparent AUC to get the optimism-adjusted AUC. Cr, creatinine; KIM-1, kidney injury molecule 1; L-FABP, liver fatty acid binding protein; NGAL, neutrophil gelatinase–associated lipocalin.
Inflammatory Biomarkers
Figure 3 displays the performance of inflammatory biomarkers (IFN, IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, and TNF) to predict AKI progression. Plasma IL-8 had the strongest association with AKI progression. Median plasma IL-8 levels at the time of creatinine-based diagnosis of AKI were significantly higher in patients with AKI progression compared with those without AKI progression (63.81; interquartile range [IQR], 44.03–159.9 pg/ml versus 22.68; IQR, 12.11–43.23 pg/ml; P<0.001). Plasma IL-8, without and with the clinical model, provided similar optimism-corrected AUCs of 0.80 (95% CI, 0.69 to 0.91) and 0.80 (95% CI, 0.72 to 0.89), respectively.
Biomarker Combinations
We examined the combination of our most predictive kidney injury and inflammatory biomarkers, specifically urine L-FABP and plasma IL-8, to predict AKI progression. The combination of urine L-FABP and plasma IL-8 resulted in no improvement of discrimination for AKI progression, with unadjusted and adjusted optimism-corrected AUCs of 0.81 (95% CI, 0.70 to 0.92) and 0.80 (95% CI, 0.70 to 0.90), respectively. Among the pairs of plasma inflammatory biomarkers, no pair significantly improved discrimination for AKI progression compared with plasma IL-8 alone.
Discussion
Identifying children whose kidney function will continue to deteriorate after AKI is diagnosed is essential, because they are at the highest risk of adverse outcomes, including dialysis requirement and mortality.4 Pediatricians currently have established guidelines to diagnose AKI and document progression of AKI to higher stages, but there are no predictive biomarkers or risk models for AKI progression.29
Among the biomarkers, plasma IL-8 had the highest discrimination for predicting AKI progression. Plasma IL-8 had an AUC of 0.80 to predict AKI progression, substantially higher than serum creatinine rise, microalbuminuria, and the clinical model alone, which had AUCs of 0.62, 0.65, and 0.67, respectively. Additionally, there was no improvement in discrimination performance with the addition of clinical variables to IL-8. Multiple biomarkers, including urine NGAL and IL-18 as well as plasma IL-1, IL-2, IL-6, IL-10, and IL-13, were also significantly higher in patients with AKI progression. All of these biomarkers have multifaceted roles in inflammatory pathways and may be mediators or byproducts of progressive AKI.17,18,30–34 The effect of collinearity among inflammatory cytokines must be considered, although there is biologic plausibility for their role with AKI progression. Specifically, IL-6 and IL-10, prototypical pro- and anti-inflammatory cytokines, respectively, help modulate the inflammatory response and may determine the overall course of AKI and subsequent repair.14,30–32,35
IL-8 is a proinflammatory cytokine produced by monocyte, neutrophil, endothelial, and epithelial cells, and it acts as a potent neutrophil activator and chemoattractant.36 After ischemia-reperfusion injury, IL-8 is released by stem cells and endothelial progenitor cells into the systemic circulation, thereby increasing inflammation.37 Several studies have previously reported that plasma IL-8 is strongly associated with the development of AKI and other adverse outcomes after pediatric cardiac surgery.38–41 In a study on 817 critically ill adults receiving RRT for AKI, adding plasma IL-8 to a clinical model had predictive value for RRT requirement at 60 days after enrollment.42 IL-8 has also been reported to predict mortality in patients with AKI, but its ability to predict AKI progression has not been assessed.43
Inflammatory cytokines are promising biomarkers of AKI progression, because systemic and intrarenal inflammation plays an integral role in the development and progression of AKI.14,30,44,45 Furthermore, systemic inflammation is heightened after cardiac surgery, which raises blood cytokine levels and puts patients at increased risk for AKI.46 This proinflammatory state is primarily due to the patients’ blood making contact with the extracorporeal circuit, leading to neutrophil and complement system activation as well as cytokine production and endothelial dysfunction.47 Inflammatory cells and cytokines are known to directly damage cells and cause organ dysfunction, particularly in the kidneys.48 Our results suggest that inflammation is an integral component of AKI progression and that inflammatory biomarkers may be distinguishing between prerenal azotemia and an inflammatory tubular injury, which is more likely to progress. Additionally, in patients with AKI due to CPB-associated renal hypoperfusion, a proinflammatory milieu may be an important risk factor for AKI progression.
Although biomarkers of kidney injury enhanced a clinical model of AKI progression, they did not perform as well as individual inflammatory biomarkers. This may be partially due to the concept that kidney injury markers were from the urine and only able to detect local injury. Blood biomarkers that are circulating systemically may better predict AKI progression, because they more accurately detect multiorgan dysfunction and systemic inflammation. Of the individual kidney injury biomarkers, urinary L-FABP had the best individual biomarker performance. This may be due, in part, to the role of L-FABP in modulating the tubulointerstitial inflammatory response to injury.49,50
Prior research on novel biomarkers of AKI progression in children has focused on the performance of urine NGAL. Zappitelli et al.51 studied urinary NGAL in a cohort of 140 critically ill children. They determined that urine NGAL was a poor predictor of AKI progression (AUC, 0.63; 95% CI, 0.44 to 0.82). In another study, Trachtman et al.52 studied the role of urinary NGAL in 34 children with hemolytic uremic syndrome. They showed that higher NGAL levels measured during the first 5 days of hospitalization predicted future dialysis requirement.
This study has limitations. We lacked sufficient sample volume to include all of the patients from our prospective study. A larger sample size would have improved our power to assess the performance of these biomarkers as well as biomarker combinations to predict AKI progression. This study only reported on biomarkers on the first day of AKI. Serial measurement of biomarkers in real time and the change in biomarker concentration over time may provide a more accurate prediction of AKI progression. A combination of biomarkers that represent the multifactorial pathophysiology of AKI progression, including tubular injury, inflammation, repair, and renal hemodynamics, may have allowed us to better quantify the spectrum of injury.53 The number of AKI progression events was also a limitation, because AKI progression only occurs in a fraction of patients with AKI (16% of patients with AKI progressed to higher stages in this study). With a limited number of events per biomarker and in an effort to minimize resubstitution bias, we determined the optimism-corrected AUC.27 The optimism-corrected AUC was used as a method of internal validation to avoid overestimating biomarker performance. Reassuringly, the optimism-corrected AUCs were very similar to the apparent AUCs, which supports the validity of our findings. However, an independent dataset would be a higher standard for validating biomarker performance.54 Consideration should also be given to the multiple testing performed in our study and the risk of spurious results. Our sample size also limited our ability to compare biomarker performance in children younger than and older than 2 years of age, which should be a topic of future research given that performance of kidney biomarkers in children younger than 2 years is poorer.25,53,55 Nonetheless, our study is the largest prospective pediatric cardiac surgery cohort to study AKI, and as such, we were able to evaluate AKI progression in children. The strengths of our study include the prospective multicenter design and the known cause and time of AKI with robust assays for biomarker measurements.
We have shown that plasma IL-8 predicts AKI progression after cardiac bypass surgery in children. IL-8 improved prediction of AKI progression compared with albuminuria or percentage of serum creatinine rise. Next, we need validation studies in additional AKI cohorts to confirm our findings and establish cutoffs for clinical use. After validation, plasma IL-8 can be used to substantially improve clinical care and therapeutic trials of AKI progression. New clinical trials are essential, because there are no treatments to prevent AKI progression. IL-8 levels may be used in the design of clinical trials as a prognostic biomarker by identifying patients who will develop AKI progression and targeting them for enrollment. This would reduce trial costs substantially, because it would minimize enrollment of patients with AKI who do not progress and thus, do not contribute to the trial. For example, to perform a therapeutic trial of AKI progression, the highest quintile of plasma IL-8 levels can be used to enrich enrollment of children more likely to develop AKI progression. Without using a biomarker as a screening tool, the required sample size to detect a 30% reduction in the rate of AKI progression is 1599 when the event rate of AKI progression is 16%.56 If the highest quintile of IL-8 levels is used for selective enrollment, the sample size can be reduced by 73%: from 1599 to 435 children. Additionally, children whose AKI is most likely to progress should be targeted for more intensive care and nephrotoxin avoidance. Lastly, IL-8 inhibition may have therapeutic value to prevent AKI progression. Therefore, using plasma IL-8 to predict AKI progression has the potential to affect clinical care and improve the efficiency of AKI trials.
Disclosures
P.D. reports being a coinventor on the neutrophil gelatinase–associated lipocalin patent. J.H.G., M.Z., Y.J., H.R.T.-P., C.A.d.F., F.P.W., S.C., and C.R.P. declare no financial interest.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health (NIH) grant R01HL085757 (to C.R.P.) to fund the Translational Research Investigating Biomarker Endpoints in AKI Consortium to study novel biomarkers of AKI in cardiac surgery. J.H.G. is supported by NIH career development grant K08DK110536. P.D. and C.R.P. are members of the NIH-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in AKI Consortium supported by NIH grant U01DK082185. P.D. is also supported by NIH grant P50DK096418. C.R.P. is supported by NIH grant K24DK090203 and NIH O'Brien Center grant P30 DK079310-07.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017090989/-/DCSupplemental.
References
- 1.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators : Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376: 11–20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, et al.: AKI in hospitalized children: Epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8: 1661–1669, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, et al.; TRIBE-AKI Consortium : Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: A prospective multicenter study. Crit Care Med 39: 1493–1499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, et al.: AKI in hospitalized children: Comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 10: 554–561, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE: The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu CY, Hsu RK, Yang J, Ordonez JD, Zheng S, Go AS: Elevated BP after AKI. J Am Soc Nephrol 27: 914–923, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu RK, Chawla LS, Wheeler DS, Goldstein SL: Renal angina: An emerging paradigm to identify children at risk for acute kidney injury. Pediatr Nephrol 27: 1067–1078, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Ismaili Z, Palijan A, Zappitelli M: Biomarkers of acute kidney injury in children: Discovery, evaluation, and clinical application. Pediatr Nephrol 26: 29–40, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, et al.; TRIBE-AKI Consortium : Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23: 905–914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, et al.; TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, et al.; TRIBE-AKI Consortium : Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol 8: 1079–1088, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zappitelli M, Greenberg JH, Coca SG, Krawczeski CD, Li S, Thiessen-Philbrook HR, et al.; Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) Consortium : Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr 169: 583–591, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg JH, Whitlock R, Zhang WR, Thiessen-Philbrook HR, Zappitelli M, Devarajan P, et al.; TRIBE-AKI Consortium : Interleukin-6 and interleukin-10 as acute kidney injury biomarkers in pediatric cardiac surgery. Pediatr Nephrol 30: 1519–1527, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, et al.: Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One 9: e93460, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucholz EM, Whitlock RP, Zappitelli M, Devarajan P, Eikelboom J, Garg AX, et al.; TRIBE-AKI Consortium : Cardiac biomarkers and acute kidney injury after cardiac surgery. Pediatrics 135: e945–e956, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al.: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 18.de Fontnouvelle CA, Greenberg JH, Thiessen-Philbrook HR, Zappitelli M, Roth J, Kerr KF, et al.; TRIBE-AKI Consortium : Interleukin-8 and tumor necrosis factor predict acute kidney injury after pediatric cardiac surgery. Ann Thorac Surg 104: 2072–2079, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI: Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 123: 110–118, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al.; Acute Kidney Injury Network : Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al.: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piepsz A, Tondeur M, Ham H: Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging 33: 1477–1482, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Zappitelli M, Krawczeski CD, Devarajan P, Wang Z, Sint K, Thiessen-Philbrook H, et al.; TRIBE-AKI consortium : Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int 80: 655–662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong L, Ma Q, Bennett M, Devarajan P: Urinary biomarkers of cell cycle arrest are delayed predictors of acute kidney injury after pediatric cardiopulmonary bypass. Pediatr Nephrol 32: 2351–2360, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zappitelli M, Coca SG, Garg AX, Krawczeski CD, Thiessen Heather P, Sint K, et al.; TRIBE-AKI Consortium : The association of albumin/creatinine ratio with postoperative AKI in children undergoing cardiac surgery. Clin J Am Soc Nephrol 7: 1761–1769, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu KD, Siew ED, Reeves WB, Himmelfarb J, Go AS, Hsu CY, et al.; ASSESS-AKI Study Investigators : Storage time and urine biomarker levels in the ASSESS-AKI study. PLoS One 11: e0164832, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr KF, Meisner A, Thiessen-Philbrook H, Coca SG, Parikh CR: RiGoR: Reporting guidelines to address common sources of bias in risk model development. Biomark Res 3: 2, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meisner A, Kerr KF, Thiessen-Philbrook H, Coca SG, Parikh CR: Methodological issues in current practice may lead to bias in the development of biomarker combinations for predicting acute kidney injury. Kidney Int 89: 429–438, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group : Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care 17: 204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang WR, Garg AX, Coca SG, Devereaux PJ, Eikelboom J, Kavsak P, et al.; TRIBE-AKI Consortium : Plasma IL-6 and IL-10 concentrations predict AKI and long-term mortality in adults after cardiac surgery. J Am Soc Nephrol 26: 3123–3132, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chawla LS, Seneff MG, Nelson DR, Williams M, Levy H, Kimmel PL, et al.: Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol 2: 22–30, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, et al.: Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 16: 3315–3325, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Sirota JC, Walcher A, Faubel S, Jani A, McFann K, Devarajan P, et al.: Urine IL-18, NGAL, IL-8 and serum IL-8 are biomarkers of acute kidney injury following liver transplantation. BMC Nephrol 14: 17, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL: Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 16: 3046–3052, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Sinuani I, Beberashvili I, Averbukh Z, Sandbank J: Role of IL-10 in the progression of kidney disease. World J Transplant 3: 91–98, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al.: Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258: 1798–1801, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Kuo MC, Patschan D, Patschan S, Cohen-Gould L, Park HC, Ni J, et al.: Ischemia-induced exocytosis of Weibel-Palade bodies mobilizes stem cells. J Am Soc Nephrol 19: 2321–2330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, et al.: Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: A case-control study. Crit Care 13: R104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell TC, Powell SL, Allen BK, Griffin RL, Warnock DG, Wang HE: Association of inflammatory and endothelial cell activation biomarkers with acute kidney injury after sepsis. Springerplus 3: 207, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kambhampati G, Ejaz NI, Asmar A, Aiyer RK, Arif AA, Pourafshar N, et al.: Fluid balance and conventional and novel biomarkers of acute kidney injury in cardiovascular surgery. J Cardiovasc Surg (Torino) 54: 639–646, 2013 [PubMed] [Google Scholar]
- 41.Oncel MY, Canpolat FE, Arayici S, Alyamac Dizdar E, Uras N, Oguz SS: Urinary markers of acute kidney injury in newborns with perinatal asphyxia (.). Ren Fail 38: 882–888, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Pike F, Murugan R, Keener C, Palevsky PM, Vijayan A, Unruh M, et al.; Biological Markers for Recovery of Kidney (BioMaRK) Study Investigators : Biomarker enhanced risk prediction for adverse outcomes in critically ill patients receiving RRT. Clin J Am Soc Nephrol 10: 1332–1339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, et al.; PICARD Study Group : Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 65: 1357–1365, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, et al.; Acute Dialysis Quality Initiative Consensus XIII Work Group : Inflammation in AKI: Current understanding, key questions, and knowledge gaps. J Am Soc Nephrol 27: 371–379, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS: Messengers without borders: Mediators of systemic inflammatory response in AKI. J Am Soc Nephrol 24: 529–536, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Brix-Christensen V, Petersen TK, Ravn HB, Hjortdal VE, Andersen NT, Tønnesen E: Cardiopulmonary bypass elicits a pro- and anti-inflammatory cytokine response and impaired neutrophil chemotaxis in neonatal pigs. Acta Anaesthesiol Scand 45: 407–413, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Brix-Christensen V: The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaesthesiol Scand 45: 671–679, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Seghaye MC, Duchateau J, Grabitz RG, Faymonville ML, Messmer BJ, Buro-Rathsmann K, et al.: Complement activation during cardiopulmonary bypass in infants and children. Relation to postoperative multiple system organ failure. J Thorac Cardiovasc Surg 106: 978–987, 1993 [PubMed] [Google Scholar]
- 49.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, et al.; TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 22: 1737–1747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanaguchi Y, Suzuki Y, Osaki K, Sugaya T, Horikoshi S, Tomino Y: Protective effects of L-type fatty acid-binding protein (L-FABP) in proximal tubular cells against glomerular injury in anti-GBM antibody-mediated glomerulonephritis. Nephrol Dial Transplant 26: 3465–3473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al.: Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: A prospective cohort study. Crit Care 11: R84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, et al.; Investigators of the HUS-SYNSORB Pk Multicenter Clinical Trial : Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: A novel marker of renal injury. Pediatr Nephrol 21: 989–994, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Srisawat N, Murugan R, Kellum JA: Repair or progression after AKI: A role for biomarkers? Nephron Clin Pract 127: 185–189, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Haukoos JS, Lewis RJ: Advanced statistics: Bootstrapping confidence intervals for statistics with “difficult” distributions. Acad Emerg Med 12: 360–365, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Greenberg JH, Parikh CR: Biomarkers for diagnosis and prognosis of AKI in children: One size does not fit all. Clin J Am Soc Nephrol 12: 1551–1557, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerr KF, Roth J, Zhu K, Thiessen-Philbrook H, Meisner A, Wilson FP, et al.: Evaluating biomarkers for prognostic enrichment of clinical trials. Clin Trials 14: 629–638, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.