Abstract
Background The kidneys have a central role in the generation, turnover, transport, and excretion of metabolites, and these functions can be altered in CKD. Genetic studies of metabolite concentrations can identify proteins performing these functions.
Methods We conducted genome-wide association studies and aggregate rare variant tests of the concentrations of 139 serum metabolites and 41 urine metabolites, as well as their pairwise ratios and fractional excretions in up to 1168 patients with CKD.
Results After correction for multiple testing, genome-wide significant associations were detected for 25 serum metabolites, two urine metabolites, and 259 serum and 14 urinary metabolite ratios. These included associations already known from population-based studies. Additional findings included an association for the uremic toxin putrescine and variants upstream of an enzyme catalyzing the oxidative deamination of polyamines (AOC1, P-min=2.4×10−12), a relatively high carrier frequency (2%) for rare deleterious missense variants in ACADM that are collectively associated with serum ratios of medium-chain acylcarnitines (P-burden=6.6×10−16), and associations of a common variant in SLC7A9 with several ratios of lysine to neutral amino acids in urine, including the lysine/glutamine ratio (P=2.2×10−23). The associations of this SLC7A9 variant with ratios of lysine to specific neutral amino acids were much stronger than the association with lysine concentration alone. This finding is consistent with SLC7A9 functioning as an exchanger of urinary cationic amino acids against specific intracellular neutral amino acids at the apical membrane of proximal tubular cells.
Conclusions Metabolomic indices of specific kidney functions in genetic studies may provide insight into human renal physiology.
Keywords: chronic kidney disease, genome-wide association studies, metabolites, renal tubular epithelial cells
Metabolites are small molecules that represent intermediates or products of metabolic processes. They play important roles in energy generation, signaling, and the regulation of enzymatic reactions. The concentrations of these small molecules in cells and body fluids result from a balance of their intake and generation, their transport across compartments, and their breakdown and excretion. The kidneys play a central role in all of these processes, providing a rationale for metabolomics research in nephrology.1 Previous studies have linked blood metabolite concentrations to common genetic markers across the genome and found a strong genetic component to the measured concentrations of many metabolites.2–10 The implicated genes are often involved in balancing blood metabolite concentrations through metabolite generation (e.g., encoding the rate-limiting enzyme), turnover, or excretion (e.g., encoding metabolite transporters). In CKD, reduced GFR leads to elevated concentrations of many metabolites in blood. We therefore hypothesized that the presence of CKD represents a “challenge” model for metabolite handling. Under such a model, metabolites may be quantifiable that are usually below the limit of detection, and renal mechanisms that facilitate active metabolite reabsorption or excretion may be altered and could inform about tubular functions. Studying metabolite concentrations in patients with CKD may therefore allow for the detection of genetic loci influencing such processes.
Only a few previous studies have investigated genetic influences on metabolite concentrations in urine,11–13 which are of particular interest for the field of nephrology. Not only does the urine contain metabolites that are exclusively or predominantly generated in the kidneys and secreted into urine, but urinary metabolite concentrations also allow for the modeling of specific renal functions that may be affected in the presence of CKD. For example, genetic screens for the metabolite’s fractional excretion (FE) could identify tubular transport proteins for this metabolite, or genetic variants associated with the ratio of metabolites in the urine could identify substrates that are counter-transported across the apical tubular cell membrane.
We therefore set out to test the following hypotheses: first, genetic investigations of metabolite concentrations in serum and urine of patients with CKD can replicate findings from previous population-based studies and identify additional genetic loci that may reflect the metabolic challenge posed by CKD. Second, modeling of kidney-specific functions on the basis of metabolite concentrations can be used to gain insights into tubular transport mechanisms and metabolic reactions of importance in CKD or detectable in its presence. To test these hypotheses in a proof-of-principle study, we carried out genome-wide association studies (GWAS) of metabolite concentrations in serum and urine as well as their FEs and pairwise ratios in up to 1168 patients with CKD participating in the German Chronic Kidney Disease (GCKD) study.14
Methods
Study Design and Participants
The GCKD study is a prospective cohort study of patients with CKD treated by nephrologists. It was approved by the local ethics committees and registered in the national registry for clinical studies (DRKS 00003971). Between 2010 and 2012, 5217 eligible adult patients provided written consent and were enrolled into the study.14 Patients were included if they had an eGFR between 30 and 60 ml/min per 1.73 m2 or an eGFR>60 ml/min per 1.73 m2 and a urinary albumin-to-creatinine ratio (UACR) >300 mg/g, albuminuria >300 mg/d, a urinary protein-to-creatinine ratio >500 mg/g, or proteinuria >500 mg/d.15 Trained personnel obtained information on clinical data, socio-demographic factors, medical and family history, medications, and health-related quality of life. The leading cause of CKD was ascertained from the treating nephrologist. Moreover, biospecimens (plasma, serum, whole blood, spot urine) were collected in a standardized way at the enrollment visit, processed, and shipped frozen to a central laboratory for routine clinical chemistry and to a central biobank for future analyses following standard operating procedures.16 A complete description of study design and the recruited study population can be found elsewhere.14,15
For the current analysis, serum and urine specimens collected at baseline were selected for metabolite measurements from a subset of GCKD participants: all participants recruited from the Freiburg study center and additionally all GCKD patients from other study centers with autosomal-dominant polycystic kidney disease, focal segmental glomerulosclerosis, membranous nephropathy, membranoproliferative GN, rapid progressive GN, pauci-immune, and anti–glomerular basement membrane GN as the leading cause of CKD (Supplemental Material).
Genotyping and Imputation
Genomic DNA was extracted from whole blood using an automated magnetic bead–based technology, quantified and normalized on a pipetting robot platform, and available for 5123 GCKD participants. Genotyping was conducted for 2,612,357 markers at the Helmholtz Center Munich using Omni2.5Exome BeadChip arrays (Illumina, GenomeStudio, Genotyping Module Version 1.9.4). Data cleaning was carried out separately for the Omni2.5 content and the Exome Chip content of the array. For the Omni2.5 content, data QC and cleaning followed the protocol of Anderson et al.17 Per-individual QC steps included evaluation of call rate, sex check, heterozygosity, cryptic relatedness, and genetic ancestry. Altogether, 89 individuals were removed during the per-individual QC. In the per–single nucleotide polymorphism (SNP) QC steps, SNPs with <96% call rate, deviating from Hardy–Weinberg equilibrium (P value <1E−5), and those with duplicate positions were removed. Detailed steps of data cleaning are available in the Supplemental Material. The final dataset for the Omni2.5 genotypes contained 5034 individuals and 2,337,794 SNPs. The scripts from Anderson et al.17 which are on the basis of Plink v1.90 and R programming language were used in the genotyping data cleaning steps.
Cleaned Omni2.5 genotype data were then imputed using IMPUTE2 (v2.3.1)18 following the Best Practices for Imputation on the IMPUTE2 website (https://mathgen.stats.ox.ac.uk/impute/impute_v2.html#best_practices). SHAPEIT v219 was used for strand alignment as well as for prephasing. The 1000 Genomes Project ALL haplotypes – Phase 3 integrated variant set was used as the reference. Imputation was performed chunk-wise with 5-Mbp sequences in each chunk. Initial imputation yielded genotypes for 81,698,995 variants. Filtering for imputation quality (info) value of >0.8 and minor allele frequency (MAF) ≥1% led to 9,281,895 high-quality imputed markers that were subsequently used for GWAS.
Similarly, per-individual QC and per-SNP QC steps were performed for the Exome Chip content, with additional checks specific to exome chip data as in Guo et al.20 In the per-individual QC steps, 96 individuals were removed, and in the per-SNP QC, 3818 SNPs were removed on the basis of filtering for call rate >95% and Hardy–Weinberg equilibrium P value ≥1E−5. More detailed information of the Exome Chip QC is found in the Supplemental Material. The cleaned Exome Chip dataset contained genotypes of 226,233 variants from 5027 individuals. These cleaned data were postprocessed by zCall21 with a z-score threshold of six and then used in the Exome Chip association analyses.
Metabolite Measurements
Samples were shipped frozen to BIOCRATES Life Sciences AG in Innsbruck (Austria) and stored at −80°C until the time of analysis in 2014/2015 when aliquots were thawed, centrifuged, and the supernatant was used for analysis. Following the manufacturer’s protocol of the commercially available AbsoluteIDQ p180 Kit, serum and urine samples were handled in a uniform and standard way that includes a precipitation/extraction step. Using that kit, >180 metabolites of different metabolite classes (amino acids, biogenic amines, acylcarnitines, [lyso-]phosphatidylcholines, sphingomyelins, and hexoses) were quantified from samples (input: 10 µl).
The quantitative assay was on the basis of PITC (phenylisothiocyanate)-derivatization in the presence of stable isotope-labeled internal standards, followed by flow injection analysis of acylcarnitines, lipids, and hexose and liquid chromatography of amino acids and biogenic amines, with detection accomplished by multiple reaction monitoring on a SCIEX 4000 QTrap mass spectrometer (SCIEX, Darmstadt, Germany). The experimental metabolomics measurement technique is described in detail in patents EP 1 897 014 B1 and EP 1 875 401 B1.22,23
Analytic performance was assessed by measuring replicates of human plasma samples spiked at three different concentrations as quality controls on each measurement plate along with the GCKD samples. Reference samples were within the predefined tolerances of the method. Accuracy of the measurements, determined by the accuracy of external calibrators, was in the normal range of the method (deviations from target ≤20%). Concentrations were normalized to the median of spiked reference samples to correct for possible batch effects.
Overall, serum measurements from 1200 individuals and urinary measurements from 1187 individuals could be obtained. All concentration values given are in micromolar (micromoles per liter). A detailed overview of the measured metabolites is provided in Supplemental Table 1. Measurements were further subjected to detailed quality control and data cleaning procedures (Supplemental Material).
Additional Variables
Serum creatinine was measured in the GCKD study in a central laboratory using an IDMS traceable enzymatic assay (Creatinine plus, Roche). eGFR values were calculated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration formula.24 The UACR was calculated from urinary albumin and creatinine measurements, where creatinine was measured using the same assay as in serum and albumin with the ALBU-XS assay (Roche/Hitachi Diagnostics GmbH, Mannheim, Germany).
GWAS and Exome Chip Analyses
Cleaned metabolite data were merged with genotype data yielding a dataset for 139 serum metabolites from 1168 individuals and 41 urinary metabolites from 1155 individuals for subsequent genetic analyses (Supplemental Material). The concentrations of the urinary metabolites were normalized using the probabilistic quotient25 to account for dilution. Within each matrix, pairwise ratios were calculated: Met A/Met B. For 37 of the metabolites, both serum and urinary concentrations were available, and the FE for each of these metabolites was calculated as (100×Metu×Creas)/(Mets×Creau) where Metu and Creau are the urinary concentrations of the metabolite and of creatinine, and Mets and Creas their serum concentrations. FEs and ratios were subjected to analyte level QC on the basis of evaluations of completeness, variability, and outliers, resulting in 34 FEs, 9591 serum metabolite ratios, and 820 urinary metabolite ratios for analyses. Before GWAS, all values (single metabolites, FEs, ratios) were transformed using rank-based inverse normal transformation.
An in-house GWAS analysis pipeline was set up using SNPTEST (v2.5.2; https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html) for association testing and GWAtoolbox (V2.2.4)26 for QC of the GWAS results. This pipeline was used for the GWAS of single serum and urinary metabolite concentrations and the FEs, using filtered imputed data of genotype dosages and assuming an additive genetic model. As in previous GWAS of metabolite concentrations,4,7 age and sex were included as covariates in all analyses; analyses of serum metabolite concentrations additionally included adjustment for log(eGFR). After correcting for multiple testing using a Bonferroni procedure, statistical significance was defined as P<3.6E−10 for 139 serum metabolites, P<1.2E−09 for 41 urinary metabolites, and P<1.5E−09 for 34 FEs; suggestive significance was defined as P value <5.0E−08. Regional association plots of loci with significant associations were generated by LocusZoom (v1.3).27
The proportion of variance in inverse-normal transformed metabolite trait (y) explained by an index SNP was calculated as R2=β2×var(SNP)/var(y), where β is the estimated effect of the SNP on y, var(SNP)=2×MAFSNP×(1−MAFSNP) according to the binomial distribution of the alleles.28
Because of the large number of evaluated ratios, a two-step analytic approach was used, with the first step consisting of GWAS of the 9591 serum and 820 urinary metabolite ratios using the software OmicABEL29 on the basis of cleaned, genotyped markers. Adjustment variables are the same as in the single variant analysis by SNPTEST. The OmicABEL results were filtered for associations with chi square–association statistics >30, which is equivalent to a P value <4.3E−8. Serum and urinary metabolite ratios with at least one SNP that passed this filter were then subjected in a second step to the SNPTEST-based GWAS pipeline using imputed genotype data, as for single metabolites and FEs. The Bonferroni-corrected significance threshold was set at 5E−8/9591=5.2E−12 for serum metabolite ratios to account for the conduct of 9591 GWAS, and 5E−8/820=6.1E−11 for urinary metabolite ratios. Additionally, the GWAS results of ratios were filtered on the “P-gain,” defined as the minimum of the two P values of the single metabolites composing the ratio divided by the P value of the ratio.30 Only associations with a P-gain>9591 in serum and P-gain>820 in urine were further considered.
For the analysis of the exome chip data, two kinds of gene-based tests of single metabolites, FEs, and metabolite ratios were carried out using the R package seqMeta (v1.6.5) and using the same phenotypes and adjustment variables as for the single variant associations: a burden test and the sequence kernel association test. For both tests, only variants with MAF<1% and likely to be functional (splicing, nonsynonymous, stopgain, and stoploss variants on the basis of the SNPInfo annotation file used in the CHARGE Consortium) were included.31 The test results were filtered and only genes with cumulative minor allele count ≥10 and with ≥2 variants per gene were retained. In total, 6924 genes met these filter criteria. To account for multiple testing, the overall significance threshold (0.05) was adjusted for the number of respective outcomes (per matrix), the number of genes (n=6924), and the number of performed tests (n=2). The significance thresholds were thus P<2.6E−08 for 139 serum metabolites, P<8.8E−08 for 41 urinary metabolites, P<3.8E−10 for 9591 serum metabolite ratios, P<4.4E−09 for 820 urinary metabolite ratios, and P<1.1E−07 for 34 FEs. Suggestive statistical significance was defined as P<1E−06.
Power Considerations
The power to detect associations between metabolites and genetic variants in a study of 1150 unrelated individuals such as ours is provided across a range of MAFs and effect sizes in Supplemental Table 10. For example, the power to detect an association at the genome-wide significance level of 5.0E−08 for genetic markers with MAF>0.1 and an absolute effect size >0.5 is >80%.
Results
Among the 1143 patients with CKD for whom both genetic information as well as serum and urinary metabolite measurements were available, the mean eGFR and the median UACR were 51.6 ml/min per 1.73 m2 and 160.8 mg/g, respectively (Table 1). The mean age was 56 years, and 63% of the patients were male. An overview of all metabolites studied, including their abbreviations, limits of detection, and coefficients of variation, is provided in Supplemental Table 1.
Table 1.
Characteristics of the study sample
| Characteristic | All Patients in the GCKD Study (n=5217) | GCKD Patients with Genetic and Metabolite Information (n=1143) |
|---|---|---|
| Male sex, % (n) | 60 (3132) | 62.9 (719) |
| Mean age (SD), yr | 60.1 (11.96) | 56 (13.1) |
| Mean BMI (SD), kg/m2a | 29.8 (5.97) | 28.6 (5.64) |
| Mean systolic BP (SD), mm Hga | 139.5 (20.36) | 138.5 (19.11) |
| Diabetes, % (n) | 35.5 (1853) | 23 (263) |
| Mean serum creatinine (SD), mg/dla | 1.5 (0.48) | 1.5 (0.52) |
| Mean eGFR (SD), ml/min per 1.73 m2a | 49.5 (18.22) | 51.6 (21.12) |
| Mean urinary creatinine (SD), mg/dla | 82.1 (55.58) | 79.4 (53.72) |
| Median urinary albumin-to-creatinine ratio (IQR), mg/ga | 50.9 (9.66–391.79) | 160.8 (24.35–872.27) |
| CKD cause: primary glomerular disease, % (n) | 18.7 (978) | 52.4 (599) |
The number of GCKD patients with genetic data and either serum measurements or urinary measurements is slightly higher: n=1168 and n=1155, respectively. BMI, body mass index; IQR, interquartile range.
These variables were not available for some patients (proportion<2%).
Genetic Association Studies of Metabolites in Patients with CKD: Model Selection
As a first step, we tested whether known genetic associations with metabolites could be replicated in a CKD population, and evaluated the potential influence of covariate selection and, specifically for urinary metabolites, the method used to account for dilution. We systematically selected variants with large genetic effects on metabolites from early publications of metabolite GWAS3,12 where both variant and metabolite were available in our data. These known genetic associations also achieved genome-wide significance among patients with CKD, as illustrated by associations between serum nonanoylcarnitine concentrations and an SNP in ACADL or serum butyrylcarnitine concentrations and an SNP in ACADS, as well as urinary lysine concentrations and an SNP in SLC7A9 (Table 2). Adjustment of serum metabolite associations for eGFR led to increased statistical significance, mainly due to smaller SEM. Thus, genetic screens with metabolite concentrations in serum were adjusted for eGFR in subsequent analyses. In urine, the association between rs7247977 in SLC7A9 and lysine concentrations was best detected when probabilistic quotient–normalization25 was used to account for urine dilution, rather than normalization by urinary creatinine concentrations. The association changed little upon further adjustment for UACR. Hence, subsequent GWAS for urinary metabolite concentrations were adjusted for age and sex.
Table 2.
Evaluation of known genetic associations in a CKD population, exemplified for nonanoylcarnitine (serum) and lysine (urine)
| Metabolitea | Normalizationb | Model | SNP | Chr | Position | Ref/Effect Allele | Freq Effect Allele | Effect | SEM | P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Serum | ||||||||||
| Nonanoylcarnitine, C9 | NA | adjustment for age, sex | rs2286963 | 2 | 211060050 | T/G | 0.34 | 0.46 | 0.041 | 7.0E−29 |
| NA | adjustment for age, sex, eGFR | 0.46 | 0.036 | 3.1E−35 | ||||||
| Butyrylcarnitine, C4 | NA | adjustment for age, sex | rs2014355 | 12 | 121175524 | T/C | 0.28 | 0.43 | 0.043 | 1.6E−22 |
| NA | adjustment for age, sex, eGFR | 0.41 | 0.038 | 1.4E−25 | ||||||
| Urine | ||||||||||
| Lysine | creatinine-normalized | adjustment for age, sex | rs7247977 | 19 | 33358355 | T/C | 0.39 | 0.22 | 0.042 | 2.6E−07 |
| creatinine-normalized | adjustment for age, sex, lnUACR | 0.23 | 0.041 | 1.5E−08 | ||||||
| pq-normalized | adjustment for age, sex | 0.29 | 0.042 | 7.3E−12 | ||||||
| pq-normalized | adjustment for age, sex, lnUACR | 0.29 | 0.042 | 9.7E−12 |
Chr, chromosome; Ref, reference; Freq, frequency; NA, not available; pq, probabilistic quotient.
Metabolite measurements were transformed before analysis using rank-based inverse normal transformation. Small differences in comparison to results from subsequent GWAS originate from differences in software and covariate adjustment.
We compared the state-of-the-art normalization with urinary creatinine and the probabilistic quotient normalization25 to evaluate correction of urinary metabolite concentrations for urine dilution.
GWAS and Burden Tests Identify Significant Associations with 25 Serum Metabolites
We carried out GWAS of common SNPs (MAF>0.01) with concentrations of 139 metabolites in serum. The genomic inflation factors ranged from 0.99 to 1.03, consistent with the absence of inflation from systematic errors. Multiple previously reported associations were identified at genome-wide significance (P<3.6E−10, see Methods), such as genetic variants in NAT8 and N-acetyl-ornithine (P=1.0E−62), CPS1 and glycine (P=2.4E−20), PRODH and proline (P=1.8E−17), ACADL and nonanoylcarnitine (P=1.3E−35), and FADS1 and phosphatidylcholines such as PC aa C36:5 (P=4.7E−14; Table 3). The 25 metabolites for which significant associations were found mapped into 13 loci.
Table 3.
Common variants (MAF≥0.05) associated with serum metabolites at genome-wide significance (P<3.6E−10)
| Metabolite | SNP | Chr | Position | NEA | EA | Effect | SEM | P Value | AF_EA | Proportion of Variance Explained | Genes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C10 | rs12134854 | 1 | 76137606 | T | C | −0.30 | 0.04 | 1.43E−12 | 0.31 | 0.04 | SLC44A5(dist=60807),ACADM(dist=52437) |
| Ac-Orn | rs375811360 | 2 | 73870324 | C | G | −0.84 | 0.05 | 1.04E−62 | 0.21 | 0.24 | NAT8 |
| C9 | rs3764913 | 2 | 211074909 | T | C | 0.46 | 0.04 | 1.34E−35 | 0.34 | 0.09 | ACADL |
| Gly | rs1047891 | 2 | 211540507 | C | A | 0.39 | 0.04 | 2.43E−20 | 0.31 | 0.07 | CPS1 |
| C18:2 | rs12194000 | 6 | 110776012 | A | G | −0.31 | 0.05 | 3.44E−11 | 0.24 | 0.03 | SLC22A16 |
| C4 | rs146203232 | 6 | 160521885 | C | T | −0.54 | 0.06 | 1.57E−18 | 0.09 | 0.05 | IGF2R |
| Putrescine | rs4725366 | 7 | 150522054 | A | G | −0.36 | 0.05 | 2.42E−12 | 0.81 | 0.04 | TMEM176A(dist=19846),AOC1(dist=27511) |
| C0 | rs1171616 | 10 | 61468589 | G | T | 0.38 | 0.05 | 2.84E−15 | 0.77 | 0.05 | SLC16A9 |
| PC ae C38:4 | rs11320420 | 11 | 61542006 | GA | G | −0.28 | 0.04 | 2.35E−10 | 0.33 | 0.04 | MYRF |
| PC ae C38:5 | rs11320420 | 11 | 61542006 | GA | G | −0.33 | 0.04 | 2.79E−13 | 0.33 | 0.05 | MYRF |
| PC ae C36:5 | rs11320420 | 11 | 61542006 | GA | G | −0.34 | 0.04 | 1.47E−14 | 0.33 | 0.05 | MYRF |
| PC ae C38:5 | rs174537 | 11 | 61552680 | G | T | −0.32 | 0.04 | 9.45E−13 | 0.33 | 0.04 | MYRF |
| PC ae C36:5 | rs174537 | 11 | 61552680 | G | T | −0.34 | 0.04 | 2.47E−14 | 0.33 | 0.05 | MYRF |
| PC aa C38:5 | rs174537 | 11 | 61552680 | G | T | −0.36 | 0.04 | 1.37E−16 | 0.33 | 0.06 | MYRF |
| PC aa C38:4 | rs102275 | 11 | 61557803 | T | C | −0.40 | 0.04 | 9.76E−21 | 0.33 | 0.07 | TMEM258 |
| lysoPC a C20:4 | rs102275 | 11 | 61557803 | T | C | −0.50 | 0.04 | 2.63E−32 | 0.33 | 0.11 | TMEM258 |
| PC aa C36:4 | rs102274 | 11 | 61557826 | T | C | −0.41 | 0.04 | 8.07E−21 | 0.33 | 0.07 | TMEM258 |
| PC aa C36:3 | rs174560 | 11 | 61581764 | T | C | 0.28 | 0.04 | 2.52E−10 | 0.29 | 0.03 | FADS1 |
| PC aa C36:5 | rs174566 | 11 | 61592362 | A | G | −0.32 | 0.04 | 4.66E−14 | 0.33 | 0.05 | FADS1(dist=7833),FADS2(dist=3351) |
| C4 | rs2014355 | 12 | 121175524 | T | C | 0.42 | 0.04 | 3.90E−27 | 0.28 | 0.07 | ACADS |
| PC aa C28:1 | rs7160525 | 14 | 64232220 | G | A | 0.42 | 0.05 | 6.57E−15 | 0.16 | 0.05 | SGPP1(dist=37464),SYNE2(dist=87463) |
| SM (OH) C14:1 | rs112329286 | 14 | 64239877 | T | TTTTA | 0.36 | 0.05 | 5.44E−12 | 0.16 | 0.04 | SGPP1(dist=45121),SYNE2(dist=79806) |
| PC ae C32:1 | rs1077989 | 14 | 67975822 | A | C | −0.28 | 0.04 | 1.97E−12 | 0.48 | 0.04 | TMEM229B |
| Asn | rs2282377 | 14 | 104571820 | C | G | 0.43 | 0.06 | 1.23E−11 | 0.13 | 0.04 | ASPG |
| Pro | rs117935223 | 22 | 18911333 | C | A | 0.62 | 0.07 | 1.79E−17 | 0.09 | 0.06 | PRODH |
Sample size: n=1168. Chr, chromosome; NEA, noneffect allele; EA, effect allele; AF_EA, allele frequency of effect allele; Genes, gene the SNP maps into or genes neighboring the SNP and the corresponding distances in base pairs.
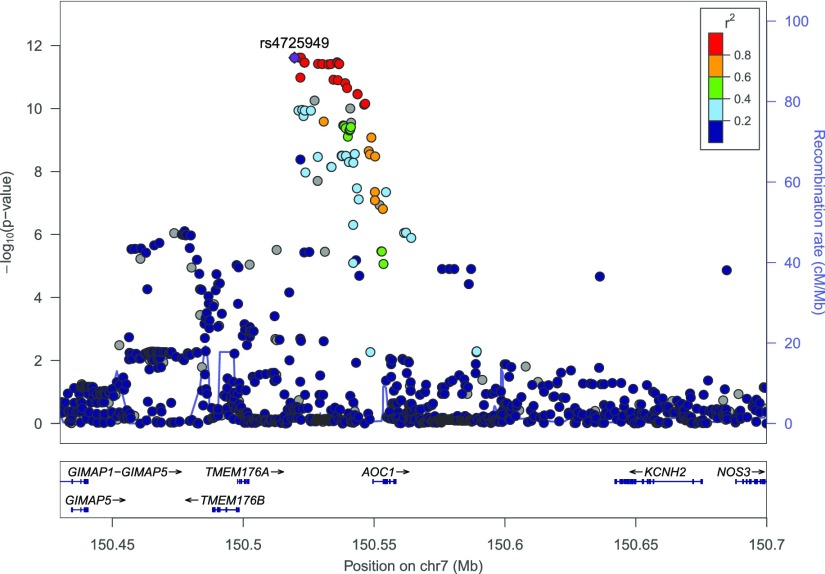
One locus, intergenic to TMEM176A and AOC1, showed a novel genome-wide significant association with concentration of serum putrescine (P=2.4E−12), a known uremic toxin (Figure 1).32 The effect of the G allele at the index SNP in this locus, rs4725366, on serum putrescine concentration was twice as large in individuals with eGFR<30 ml/min per 1.73 m2 compared with those with eGFR≥60 (−0.28 versus −0.60, Table 4). The relationship between the genetic effect size and eGFR, however, was not linear, and a statistical interaction term between SNP and eGFR category was not statistically significant (P=0.3). SNP rs4725366 is significantly associated with the expression of the neighboring AOC1 gene (P=1.5e−22 in testis in the GTEx resource33 that does not contain eQTL information from kidney tissue). AOC1 encodes amine oxidase copper-containing 1, an enzyme catalyzing the oxidative deamination of polyamines such as histamine, putrescine, and cadaverine.
Figure 1.
Regional association plot illustrates the presence of serum putrescine-associated SNPs upstream of AOC1 and downstream of TMEM176A on chromosome 7. y axis, negative log10(P value) from GWAS analysis; x axis, genomic position. Linkage disequilibrium with the index SNP rs4725949 (purple) in the 1000 Genomes Project EUR reference population is color-coded as detailed in the legend.
Table 4.
Effect of G allele of rs4725366 (AOC1 locus) on serum putrescine concentrations stratified by eGFR
| eGFR Category (ml/min per 1.73 m2) | n | Effect | SEM | P Value |
|---|---|---|---|---|
| ≥60 | 293 | −0.28 | 0.10 | 6.37E−03 |
| 45–59 | 343 | −0.41 | 0.09 | 4.16E−06 |
| 30–44 | 391 | −0.30 | 0.09 | 1.34E−03 |
| <30 | 117 | −0.60 | 0.15 | 1.12E−04 |
n, sample size.
A full table listing the index variant for metabolites with at least one suggestive association signal (P<5E−08, see Methods) is shown in Supplemental Table 2. These include loci not significantly associated with blood metabolite concentrations in previous studies, such as the PTPRN2 locus and concentrations of lysophosphatidylcholine with acyl residue C18:1 (P=1.7E−08). Because this locus also showed suggestive evidence of association with the blood concentrations of another lipid in a previous metabolite GWAS,7 such associations are excellent candidates to achieve genome-wide significance in future, larger association studies.
We additionally examined the aggregate effect of rare deleterious variants within the same gene on serum metabolite concentrations. No gene passed the significance threshold for multiple testing (P<2.6E−08, see Methods), but the burden of rare deleterious variants in four genes showed suggestive evidence for association (P<1E−06) with at least one metabolite (Supplemental Table 3). Among these was an association between serum concentrations of histidine and rare variants in the HAL gene (P-burden=7.1E−08), encoding histidine ammonia-lyase.34 Rare mutations in HAL can cause autosomal-recessive histidinemia (MIM #235800), and associations between rare genetic variants in HAL and blood histidine concentrations have previously been reported in a population-based study.9 In that study, 14 rare HAL variants were underlying the association signal with blood histidine concentrations, whereas in our study 17 such variants were identified (nine of which overlapped). These findings indicate that rare deleterious variants also influence metabolite concentrations in patients with CKD. Because additional and unique potentially deleterious variants are identified with each new study population examined, results in the suggestive significance range identified in our study likely contain other true positive findings that await discovery in future experimental or larger genetic association studies.
GWAS and Burden Tests of Urinary Metabolites Confirm Population-Based Findings in CKD
Next, we carried out GWAS of common SNPs and metabolite concentrations in urine. Among the 41 metabolites that passed quality control, significant associations were observed for two previously reported loci, SLC7A9 and urinary lysine concentrations (P=4.7E−12) and NAT8 and urinary N-acetylornithine concentrations (P=9.6E−12, Supplemental Table 4). These results were stable to additional adjustment for UACR. Suggestive associations were detected for several loci that were previously described as associated with the metabolite’s blood concentrations, such as SLC22A1 and butyrylcarnitine.10 The rare variant analyses did not lead to the identification of an aggregate effect of deleterious variants in any gene with P<1E−06. The number of loci associated with urinary metabolite concentrations among patients with CKD in this study needs to be interpreted in light of the assay used to quantify metabolites: the BIOCRATES AbsoluteIDQ p180 Kit is a targeted assay originally developed to quantify blood metabolite concentrations, only a subset of which are detected in urine (Supplemental Table 1).
GWAS and Burden Tests of Serum Metabolite Ratios Highlight Variants Central to Lipid and Acylcarnitine Metabolism
Metabolite concentrations can be used to model physiologic functions of the kidney. Specifically, ratios of metabolites can reflect not only the activity of enzymatic reactions, but also co- or counter-transport at the apical or basolateral membrane of tubular cells, resulting in synchronized changes of these metabolites in urine and blood, respectively. We therefore first evaluated the association between common genetic variants and metabolite ratios in serum. Genome-wide significant associations were identified between genetic variants and 259 metabolite ratios mapping into 11 loci (Supplemental Table 5), many of which implicated known genes central to lipid biosynthesis. These analyses confirmed FADS1 as a major player in the metabolism of phosphatidylcholines, ACADM and ACADS in metabolism of medium-chain acylcarnitines, SPTLC3 in the synthesis of sphingomyelins, as well as PDXDC1 in phospholipid metabolism.3,35
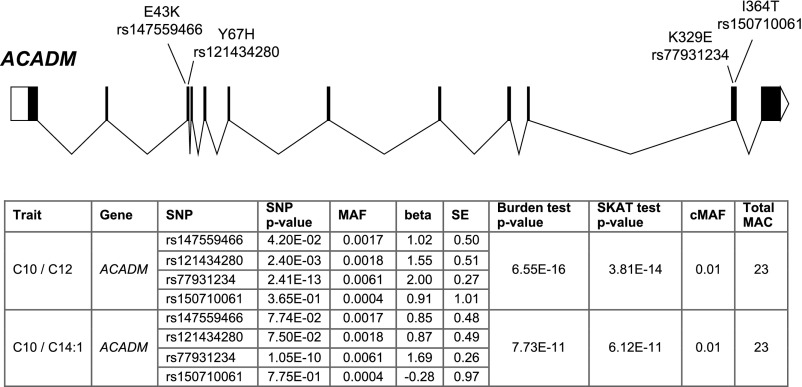
In the gene-based tests of rare variants on serum metabolite ratios, we identified an exome-wide significant association (P<2.4E−10) for one gene, ACADM. Significant associations were observed for several metabolite ratios, with the lowest P value observed for the decanoylcarnitine/dodecanoylcarnitine ratio from the burden test (P=6.6E−16, Figure 2). The low burden test P value, despite the modest sample size and low frequency of the evaluated deleterious variants (MAF<0.01), suggested large and concordant effects of the contributing variants affecting the metabolite concentrations. Indeed, the four variants in ACADM that contributed to the test were all associated with increases in the decanoylcarnitine/dodecanoylcarnitine ratio (Figure 2). ACADM encodes medium-chain acyl-CoA dehydrogenase, which catalyzes the initial reaction in the β-oxidation of C4 to C12 straight-chain acyl-CoAs. Mutations in the ACADM gene cause MCAD deficiency (MIM #201450), a condition that is part of newborn screening on the basis of acylcarnitine analysis in blood. Three of the four variants detected in our screen have been reported as cause of classic MCAD deficiency if present in the homozygous state (p.E43K, p.Y67H, p.K329E), confirming the functional and clinical relevance of these mutations. The mutation p.K329E gives rise to a severe disease phenotype, whereas the clinical picture is milder for the mutations p.E43K and p.Y67H, suggesting residual enzyme function. These observations from monogenic manifestations of the disease are consistent with the effect sizes observed for heterozygous carriers in our study, with p.K329E showing the largest effect on the decanoylcarnitine/dodecanoylcarnitine ratio. The last variant identified in our study, p.I364T, is yet of uncertain clinical significance (Figure 2). Our findings reveal a relatively high carrier frequency of potentially deleterious ACADM mutations with large effects on metabolite concentrations in an adult population of European ancestry (23 of 1157=2.0%; 1.9% without p.I364T). This relatively high frequency is also observed among European ancestry individuals in the gnomAD database, suggesting that this finding is more widely applicable. Full results of all serum metabolite ratios associated at suggestive significance with rare variants in one or more genes are listed in Supplemental Table 6.
Figure 2.
Rare coding variants in ACADM are cumulatively associated with the serum concentrations of acylcarnitine ratios. The upper panel contains a schematic representation of the ACADM gene and the mapping of the identified deleterious variants to exons. The table contains P values for the cumulative effect of the variants on the concentrations of the listed acylcarnitine ratios, as well as the association P values for the single variants that contribute to the gene-based tests (burden test and sequence kernel association test, see Methods). The allele frequencies of these variants in European individuals in gnomAD database are: 0.0032 for rs147559466, 0.00099 for rs121434280, 0.0061 for rs77931234, and 0.00049 for rs150710061. C10, decanoylcarnitine; C12, dodecanoylcarnitine; C14:1, Tetradecenoylcarnitine; cMAF, cumulative MAF; MAC, minor allele count; SKAT, Sequence Kernel Association Test.
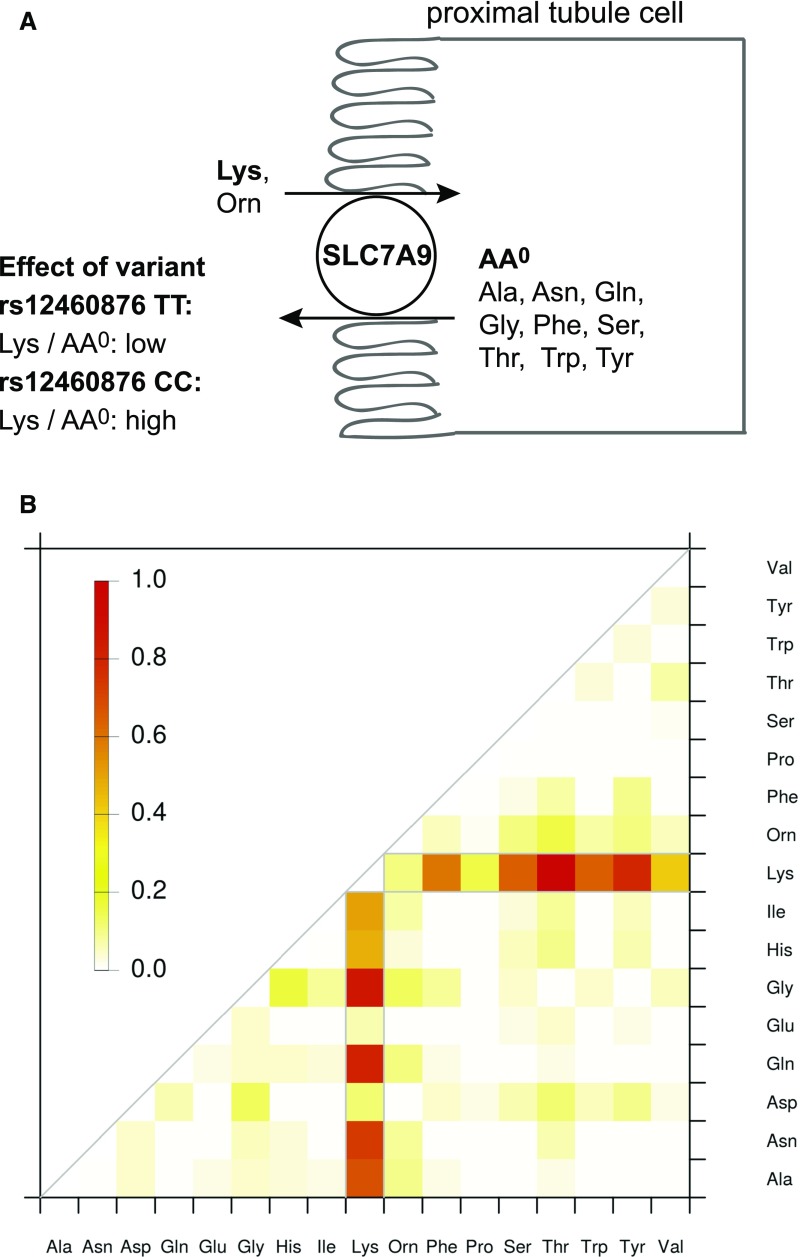
GWAS of Urine Metabolite Ratios Highlight Amino Acids Counter-Transported by SLC7A9
There were several genetic loci with significant SNP associations with urinary metabolite ratios (Supplemental Table 7). Interestingly, these associations delivered valuable insights into renal physiology: genetic variants in SLC7A9 showed significant associations with lysine-containing ratios that were many orders of magnitude stronger than the association of the respective SNP with urinary lysine concentrations alone (Figure 3). This is exemplified by the lysine/glutamine ratio (P=2.2E−23, P-gain=2.1E+11; see Methods), the lysine/tyrosine ratio (P=3.1E−22, P-gain=1.5E+10), and the lysine/threonine ratio (P=5.8E−22, P-gain=1.5E+10). Other significant lysine-containing ratios contained alanine, glycine, serine, asparagine, phenylalanine, and tryptophan. Of note, all of these amino acids carry no net electric charge at physiologic pH, consistent with the known role of SCL7A9 as an exchanger of cationic amino acids (lysine, arginine, and ornithine) and cystine in the renal tubular lumen against intracellular neutral amino acids (AA0) described in in vitro studies.36,37 This finding illustrates the potential of modeling metabolite concentrations to uncover specific physiologic functions of the kidney. The concept is illustrated in Figure 3A: individuals homozygous for the minor C allele at the intronic index SNP rs12460876 show higher urinary lysine/AA0 ratios than those homozygous for the major T allele, indicating less efficient function of the encoded SLC7A9 transport protein. Modeling of the association between rs12460876 and all pairwise amino acid ratios in urine revealed specific lysine/AA0 ratios with strong associations (Figure 3B), representing a readout of human SLC7A9 function in vivo. Other amino acid ratios showed little (mostly ornithine-containing ratios) or no association, such as lysine/AA− ratios (aspartate, glutamate).
Figure 3.
Counter-transport of amino acids by SLC7A9 in the renal proximal tubule cell is revealed by the analysis of urinary metabolite ratios. The upper part (A) contains a schematic representation of SLC7A9 function in the proximal tubule, reabsorbing dibasic urinary amino acids such as lysine in exchange for intracellular neutral amino acids. Individuals homozygous for the minor C allele at the index SNP rs12460876 show higher lysine/neutral amino acid (AA0) ratios in the urine, indicating less efficient lysine reuptake at the brush border. Listed amino acids are restricted to the ones measured in this study. The lower panel (B) represents a heatmap of the strength of the association between genotype at intronic rs12460876 (scaled chi square statistics) and the evaluated pairwise metabolite ratios in urine. It highlights the AA0 counter-transported against lysine in vivo. For comparison, lysine by itself has a scaled chi square statistic of 0.39, indicated by light orange color. ala, alanine; asn, asparagine; asp, aspartate; gln, glutamine; glu, glutamate; gly, glycine; his, histidine; ile, isoleucine; lys, lysine; orn, ornithine; phe, phenylalanine; pro, proline; ser, serine; thr, threonine; trp, tryptophan; tyr, tyrosine; val, valine.
The evaluation of the aggregate effect of rare variants on urinary metabolite ratios did not yield findings that achieved exome-wide significance (P<4.4E−09). There were, however, biologically plausible findings of suggestive significance (P<1E−06, Supplemental Table 8). For example, rare variants in SLC36A2 were associated with the urinary glycine-to-serine ratio (P=4.0E−07). This matches the known transport capacity of the encoded transporter for glycine and its localization to the S1 segment of the proximal tubule.13,38 Transport capacity for serine has only been described for murine SLC36A2 so far39 and could now be tested experimentally for human SLC36A2. Another example is the association between rare variants in PAH and the phenylalanine/tyrosine ratio (P=5.9E−07), which matches the function of the encoded phenylalanine hydroxylase that catalyzes the rate-limiting step in phenylalanine catabolism, the hydroxylation of phenylalanine to tyrosine. Although an earlier study had reported the association between rare variants in PAH and blood phenylalanine concentrations,9 our findings highlight that modeling of enzyme function as the ratio of the enzyme’s substrate and product adds additional information. In addition, the examination of urine adds information, because the gene is exclusively expressed in liver and kidney, and urinary concentrations integrate the information of reactions occurring in the kidney. More generally, these findings suggest that the study of metabolite ratios in urine of patients with CKD in a larger study sample is a promising avenue for future research.
GWAS and Burden Tests of Metabolite FEs
Lastly, GWAS of common SNPs with the FEs of 34 metabolites yielded one finding of genome-wide significance, the association between variants in the MLEC gene at the ACADS locus and the FE of butyrylcarnitine (C4, P=2.8E−14; Supplemental Table 9). However, these associations were not as significant as the ones with serum butyrylcarnitine concentration alone (P=3.9E−27, Table 3). No significant or suggestive associations (P<1E−06) were identified for the aggregate effect of rare deleterious variants in any gene and the FEs of the 34 evaluated metabolites. Thus, investigation of the FEs of metabolites did not provide additional insights.
Discussion
Our study has several main findings: first, genetic influences on metabolite concentrations in serum and urine from previous population-based studies2–4,7–9,11,40,41 translate to patients with CKD. Second, the study of patients with CKD revealed a novel association between concentrations of a uremic toxin, putrescine,42 and variation near the gene encoding amine oxidase copper-containing 1. Third, metabolite ratios are useful composite indices that reflect underlying physiologic mechanisms such as enzymatic reactions and counter-transport mechanisms of importance in nephrology, as exemplified by variants in SLC7A9 and the urinary ratios of lysine and several neutral amino acids. Our study generates multiple new hypotheses about the role of identified genes in human metabolism that can now be tested experimentally.
The great majority of genomic loci identified in our study have previously been detected in equally sized or larger GWAS of metabolite concentrations in population-based studies. Our study reveals that these genetic determinants of metabolite concentrations are also important in the setting of CKD. Thus, patients with both CKD and metabolite-increasing genotypes may represent a particularly high-risk group for adverse events related to such metabolites. The different techniques and assays used for metabolite quantification as well as the different transformations and covariate adjustments across these studies unfortunately preclude a systematic comparison of genetic effect sizes between population-based individuals and patients with CKD to answer the question of whether CKD as a metabolic challenge is reflected in different genetic effect sizes. A systematic comparison of genetic effect sizes between population-based individuals and patients with CKD should therefore be the focus of future joint studies that harmonize the methods for metabolite quantification and data analysis across studies a priori.
There was one novel association between serum putrescine concentrations and genetic variants at the AOC1 locus, encoding an enzyme catalyzing the oxidative deamination of polyamines such as putrescine.43 The doubling in genetic effect size in patients with CKD with eGFR<30 compared with those with eGFR≥60 ml/min per 1.73 m2 may hint at the presence of dose-response effects with worsening kidney function and illustrate the value of studying “challenged” populations (such as disease cohorts) in general. Again, however, differences across studies complicate comparisons: the lack of reported associations with putrescine in previous population-based studies may be because it was not included on targeted assays or removed during quality control, because it was concentrated too lowly for quantification, because it was measured but not identified as putrescine, or because it showed no genetic associations and was therefore not reported. Although transcriptional activation of AOC1 has recently been linked to kidney morphogenesis in mice by affecting polyamine breakdown,44 it cannot be inferred from our study whether elevated putrescine concentrations occur as a consequence of CKD or contribute causally to CKD etiology and/or progression.
In terms of biologic insights, our study offers snapshots of renal physiology in humans and generates novel hypotheses for experimental confirmation. This former is exemplified by the identification of the neutral amino acids transported in exchange for urinary lysine in humans in vivo, and the latter by the potential transport capacity of human SLC36A2 for serine. Several previous studies reported associations between the same index or a proxy variant at the SLC7A9 locus with urinary lysine concentrations6,11–13 or some lysine-containing ratios,11 but without biologic interpretations in light of tubular counter-transport mechanisms. Interestingly, GWAS of eGFR in the general population could show that the rs12460876 C allele is associated not only with higher urinary lysine concentrations but also with higher eGFR and lower CKD prevalence and incidence,6,13,45 consistent with the association between higher urinary lysine concentrations and decreased risk for CKD.13 The molecular mechanisms connecting reduced SLC7A9-mediated transport, resulting in higher urinary lysine and cystine and higher intracellular AA0 concentrations, to better eGFR/lower CKD risk are presently unclear.
Studies of genetic influences on metabolite concentrations in patients with CKD have the potential to ultimately deliver insights of clinical importance; for example, by identifying genetic predispositions to unfavorable profiles of CKD risk factors or to CKD comorbidities. This may be especially relevant for rare variants with large effects on CKD risk factors, the presence and prevalence of which is illustrated by the discovery of rare coding ACADM variants in our study. Illuminating pathways that underlie processes such as hyperlipidemia or stone formation may eventually help to better treat or prevent these common CKD-related conditions. In addition, genetic discoveries related to metabolites that are considered uremic toxins such as putrescine can be used to test their causal involvement in disease progression once large-scale genetic studies of CKD progression become available.
Some limitations of our study warrant mention: the use of the BIOCRATES p180 assay for metabolite quantification results in a limited number of metabolites to study. Although nontargeted approaches to quantify metabolites result in a much greater number of metabolites for study and cover classic uremic toxins that are not part of the BIOCRATES assay, the latter was chosen in this study because it includes isotope-labeled standards for many metabolites that allow for comparison and integration of their concentrations across urine and blood. We could not comprehensively compare genetic effect sizes on metabolite concentrations in patients with CKD to those from previous population-based studies because of differences in methods to quantify metabolites and statistical data analysis. Our study was limited to individuals of European ancestry, and can therefore not necessarily be generalized. Twenty-four-hour urine collections were not available in the GCKD study, which may have led to increased variation of urinary metabolite concentrations because of differences in circadian rhythms resulting in reduced power to detect associations. Urate is one of few metabolites whose FE has been assessed from different urine collection methods in studies of healthy individuals. In these studies, the FEs for urate quantified from spot urine were similar to those from 24-hour urines,46–48 but this may not pertain to all metabolites.
Our study represents the first study of genetic influences on metabolite profiles in a large population of patients with CKD. We were able to examine up to 1168 patients with CKD and benefitted from the standardized data collection, sample preprocessing and storage procedures, DNA extraction, and genotyping in the GCKD study. Our study shows that genetic influences on metabolite profiles in the general population translate to patients with CKD and that novel genetic associations can be identified in this study population. Modeling of renal metabolite handling such as counter-transport mechanisms delivered novel insights into human renal (patho-)physiology and generated new hypotheses for experimental confirmation. Future extensions of sample size and metabolite coverage hold great promise to generate additional insights into human renal physiology in vivo.
Disclosures
J.W. is employed by BIOCRATES Life Sciences Aktiengesellschaft (AG) (Innsbruck, Austria).
Supplementary Material
Acknowledgments
We are grateful for the willingness of the patients to participate in the German Chronic Kidney Disease (GCKD) study. The enormous effort of the study personnel of the various regional centers is highly appreciated. We thank the large number of nephrologists who provide routine care for the patients and collaborate with the GCKD study. The GCKD Investigators are listed in the Supplementary Information.
The work of P.S., Y.L., and A.K. and metabolite quantification was supported by grants KO 3598/2-1 and KO 3598/4-1 of the German Research Foundation (DFG). The work of M.W., U.T.S., and A.K. was supported by DFG CRC 1140. P. Schlosser was supported by DFG CRC 992. A.K. was additionally supported by a DFG Heisenberg Professorship (KO 3598/3-1); U.T.S. was additionally supported by the Else Kroener Fresenius Kolleg Nierenfunktionsstörungen als Komplikation von Systemerkrankungen (NAKSYS). Genotyping was supported by Bayer Pharma Aktiengesellschaft (AG). Metabolite quantification was additionally supported by the Else Kroener Fresenius Foundation and the German Ministry of Education and Research (BMBF) Gerontosys II NephAge Project, 031 5896A. The GCKD study was/is funded by grants from the BMBF (grant number 01ER0804) and the Kuratorium fuer Heimdialyse (KfH) Foundation for Preventive Medicine.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Insights into CKD from Metabolite GWAS,” on pages 1349–1351.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017101099/-/DCSupplemental.
References
- 1.Kalim S, Rhee EP: An overview of renal metabolomics. Kidney Int 91: 61–69, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gieger C, Geistlinger L, Altmaier E, Hrabé de Angelis M, Kronenberg F, Meitinger T, et al.: Genetics meets metabolomics: A genome-wide association study of metabolite profiles in human serum. PLoS Genet 4: e1000282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Illig T, Gieger C, Zhai G, Römisch-Margl W, Wang-Sattler R, Prehn C, et al.: A genome-wide perspective of genetic variation in human metabolism. Nat Genet 42: 137–141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wägele B, et al. ; CARDIoGRAM : Human metabolic individuality in biomedical and pharmaceutical research. Nature 477: 54–60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, Lyytikäinen LP, et al.: Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet 44: 269–276, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rueedi R, Ledda M, Nicholls AW, Salek RM, Marques-Vidal P, Morya E, et al.: Genome-wide association study of metabolic traits reveals novel gene-metabolite-disease links. PLoS Genet 10: e1004132, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al.; Multiple Tissue Human Expression Resource (MuTHER) Consortium : An atlas of genetic influences on human blood metabolites. Nat Genet 46: 543–550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draisma HHM, Pool R, Kobl M, Jansen R, Petersen AK, Vaarhorst AAM, et al.: Genome-wide association study identifies novel genetic variants contributing to variation in blood metabolite levels. Nat Commun 6: 7208, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee EP, Yang Q, Yu B, Liu X, Cheng S, Deik A, et al.: An exome array study of the plasma metabolome. Nat Commun 7: 12360, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long T, Hicks M, Yu HC, Biggs WH, Kirkness EF, Menni C, et al.: Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet 49: 568–578, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Suhre K, Wallaschofski H, Raffler J, Friedrich N, Haring R, Michael K, et al.: A genome-wide association study of metabolic traits in human urine. Nat Genet 43: 565–569, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Raffler J, Friedrich N, Arnold M, Kacprowski T, Rueedi R, Altmaier E, et al.: Genome-wide association Study with targeted and non-targeted NMR metabolomics identifies 15 novel loci of urinary human metabolic individuality. PLoS Genet 11: e1005487, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon GM, Hwang SJ, Clish CB, Tin A, Yang Q, Larson MG, et al.; CKDGen Consortium : Urinary metabolites along with common and rare genetic variations are associated with incident chronic kidney disease. Kidney Int 91: 1426–1435, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Eckardt KU, Bärthlein B, Baid-Agrawal S, Beck A, Busch M, Eitner F, et al.: The German Chronic Kidney Disease (GCKD) study: Design and methods. Nephrol Dial Transplant 27: 1454–1460, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Titze S, Schmid M, Köttgen A, Busch M, Floege J, Wanner C, et al.; GCKD study investigators : Disease burden and risk profile in referred patients with moderate chronic kidney disease: Composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant 30: 441–451, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Prokosch HU, Mate S, Christoph J, Beck A, Köpcke F, Stephan S, et al.: Designing and implementing a biobanking IT framework for multiple research scenarios. Stud Health Technol Inform 180: 559–563, 2012 [PubMed] [Google Scholar]
- 17.Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT: Data quality control in genetic case-control association studies. Nat Protoc 5: 1564–1573, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howie BN, Donnelly P, Marchini J: A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5: e1000529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delaneau O, Zagury JF, Marchini J: Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods 10: 5–6, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, He J, Zhao S, Wu H, Zhong X, Sheng Q, et al.: Illumina human exome genotyping array clustering and quality control. Nat Protoc 9: 2643–2662, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein JI, Crenshaw A, Carey J, Grant GB, Maguire J, Fromer M, et al.; Swedish Schizophrenia Consortium; ARRA Autism Sequencing Consortium : zCall: A rare variant caller for array-based genotyping: Genetics and population analysis. Bioinformatics 28: 2543–2545, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsay, S.L.B.D. (Heidelberg 3084, AU), Guggenbichler, W. (Austrasse 26A, A-6063 Rum, AT), Weinberger, K.M. (Weidach 82, A-6414 Mieming, AT), Graber, A. (Fishnalerstrasse 14/Top3, A-6020 Innsbruck, AT), Stöggl, W.M. (Hoher Weg 9/7, A-6020 Innsbruck, AT); Device for quantitative analysis of a metabolite profile. (BIOCRATES Life Sciences AG (Innrain 66, 6020 Innsbruck, AT), 2014)
- 23.Ramsay, S.L.B.D. (Heidelberg 3084, AT), Stöggl, W.M. (Hoher Weg 9/7, A-6020 Innsbruck, AT), Weinberger, K.M. (Weidach 82, A-6414 Mieming, AT), Graber, A. (Fischnalerstrasse Innsbruck 14/Top 3, A-6020 Innsbruck, AT), Guggenbichler, W. (Austrasse 26A, A-6063 Rum, AT); Apparatus for analyzing a metabolite profile. (BIOCRATES Life Sciences AG (Innrain 66, 6020 Innsbruck, AT), 2014)
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dieterle F, Ross A, Schlotterbeck G, Senn H: Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem 78: 4281–4290, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Fuchsberger C, Taliun D, Pramstaller PP, Pattaro C; CKDGen consortium : GWAtoolbox: An R package for fast quality control and handling of genome-wide association studies meta-analysis data. Bioinformatics 28: 444–445, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al.: LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 26: 2336–2337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pattaro C, Teumer A, Gorski M, Chu AY, Li M, Mijatovic V, et al.; ICBP Consortium; AGEN Consortium; CARDIOGRAM; CHARGe-Heart Failure Group; ECHOGen Consortium : Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun 7: 10023, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabregat-Traver D, Sharapov SZ, Hayward C, Rudan I, Campbell H, Aulchenko Y, et al.: High-performance mixed models based genome-wide association analysis with omicABEL software. F1000 Res 3: 200, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen AK, Krumsiek J, Wägele B, Theis FJ, Wichmann HE, Gieger C, et al.: On the hypothesis-free testing of metabolite ratios in genome-wide and metabolome-wide association studies. BMC Bioinformatics 13: 120, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grove ML, Yu B, Cochran BJ, Haritunians T, Bis JC, Taylor KD, et al.: Best practices and joint calling of the HumanExome BeadChip: The CHARGE consortium. PLoS One 8: e68095, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, et al.; European Uremic Toxin Work Group : Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Consortium GT; GTEx Consortium : Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 348: 648–660, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suchi M, Harada N, Wada Y, Takagi Y: Molecular cloning of a cDNA encoding human histidase. Biochim Biophys Acta 1216: 293–295, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Demirkan A, van Duijn CM, Ugocsai P, Isaacs A, Pramstaller PP, Liebisch G, et al.; DIAGRAM Consortium; CARDIoGRAM Consortium; CHARGE Consortium; EUROSPAN consortium : Genome-wide association study identifies novel loci associated with circulating phospho- and sphingolipid concentrations. PLoS Genet 8: e1002490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munck BG: Lysine transport across the small intestine. Stimulating and inhibitory effects of neutral amino acids. J Membr Biol 53: 45–53, 1980 [DOI] [PubMed] [Google Scholar]
- 37.Munck BG: Transport of neutral and cationic amino acids across the brush-border membrane of the rabbit ileum. J Membr Biol 83: 1–13, 1985 [DOI] [PubMed] [Google Scholar]
- 38.Bröer S, Bailey CG, Kowalczuk S, Ng C, Vanslambrouck JM, Rodgers H, et al.: Iminoglycinuria and hyperglycinuria are discrete human phenotypes resulting from complex mutations in proline and glycine transporters. J Clin Invest 118: 3881–3892, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy DJ, Gatfield KM, Winpenny JP, Ganapathy V, Thwaites DT: Substrate specificity and functional characterisation of the H+/amino acid transporter rat PAT2 (Slc36a2). Br J Pharmacol 144: 28–41, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhee EP, Ho JE, Chen MH, Shen D, Cheng S, Larson MG, et al.: A genome-wide association study of the human metabolome in a community-based cohort. Cell Metab 18: 130–143, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burkhardt R, Kirsten H, Beutner F, Holdt LM, Gross A, Teren A, et al.: Integration of genome-wide SNP data and gene-expression profiles reveals six novel loci and regulatory mechanisms for amino acids and acylcarnitines in whole blood. PLoS Genet 11: e1005510, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Igarashi K, Ueda S, Yoshida K, Kashiwagi K: Polyamines in renal failure. Amino Acids 31: 477–483, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Chassande O, Renard S, Barbry P, Lazdunski M: The human gene for diamine oxidase, an amiloride binding protein. Molecular cloning, sequencing, and characterization of the promoter. J Biol Chem 269: 14484–14489, 1994 [PubMed] [Google Scholar]
- 44.Kirschner KM, Braun JF, Jacobi CL, Rudigier LJ, Persson AB, Scholz H: Amine oxidase copper-containing 1 (AOC1) is a downstream target gene of the Wilms tumor protein, WT1, during kidney development. J Biol Chem 289: 24452–24462, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Köttgen A, Pattaro C, Böger CA, Fuchsberger C, Olden M, Glazer NL, et al.: New loci associated with kidney function and chronic kidney disease. Nat Genet 42: 376–384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quaratino CP, Di Sciascio N, Rucci C, Ciaglia P, Giacomello A: The normal range of serum urate levels and of fractional urate excretion. Adv Exp Med Biol 370: 91–93, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Giacomello A, Di Sciascio N, Quaratino CP: Relation between serum triglyceride level, serum urate concentration, and fractional urate excretion. Metabolism 46: 1085–1089, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Perez-Ruiz F, Calabozo M, Erauskin GG, Ruibal A, Herrero-Beites AM: Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum 47: 610–613, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.