Abstract
Background Diabetic nephropathy (DN) is a leading cause of ESRD in the United States, but the molecular mechanisms mediating the early stages of DN are unclear.
Methods To assess global changes that occur in early diabetic kidneys and to identify proteins potentially involved in pathogenic pathways in DN progression, we performed proteomic analysis of diabetic and nondiabetic rat glomeruli. Protein S (PS) among the highly upregulated proteins in the diabetic glomeruli. PS exerts multiple biologic effects through the Tyro3, Axl, and Mer (TAM) receptors. Because increased activation of Axl by the PS homolog Gas6 has been implicated in DN progression, we further examined the role of PS in DN.
Results In human kidneys, glomerular PS expression was elevated in early DN but suppressed in advanced DN. However, plasma PS concentrations did not differ between patients with DN and healthy controls. A prominent increase of PS expression also colocalized with the expression of podocyte markers in early diabetic kidneys. In cultured podocytes, high-glucose treatment elevated PS expression, and PS knockdown further enhanced the high-glucose–induced apoptosis. Conversely, PS overexpression in cultured podocytes dampened the high-glucose– and TNF-α–induced expression of proinflammatory mediators. Tyro3 receptor was upregulated in response to high glucose and mediated the anti-inflammatory response of PS. Podocyte-specific PS loss resulted in accelerated DN in streptozotocin-induced diabetic mice, whereas the transient induction of PS expression in glomerular cells in vivo attenuated albuminuria and podocyte loss in diabetic OVE26 mice.
Conclusions Our results support a protective role of PS against glomerular injury in DN progression.
Keywords: diabetic nephropathy, podocytes, Protein S, proteome
Diabetic nephropathy (DN) is a leading cause of ESRD in the United States.1 Although the current management of tight glycemic control and renin angiotensin system blockade slows the progression of DN, many patients continue to progress toward ESRD.1 Thus, elucidating the mechanisms that mediate the early stages of diabetic kidney injury may help identify novel preventive and therapeutic measures for DN. Toward this goal, we performed proteomic analysis of glomeruli isolated from diabetic and nondiabetic control rats. We identified protein S (PS) to be among the highly upregulated proteins, which was reversed with insulin treatment. Interestingly, the increase in glomerular PS levels in diabetic rats did not correlate with the serum concentrations of PS, suggestive of kidney cell–specific upregulation of PS in the diabetic kidney.
PS is a plasma glycoprotein whose function is well established as an essential cofactor for activated protein C–dependent inhibition of coagulation factors FVa and FVIIIa.2 In diabetic kidneys, thrombomodulin-dependent activated protein C formation has been shown to mediate cytoprotection by inhibiting glomerular endothelial cell and podocyte apoptosis,3 suggesting that PS may have a renoprotective function. In addition to its anticoagulant function, PS also activates a family of protein tyrosine kinase receptors, Tyro-3, Axl, and Mer (TAM) receptors, that have multiple biologic functions.4 Although PS and its structural homolog GAS6 are both ligands of TAM receptors, due to their differential binding affinities for individual TAM receptors,5,6 PS and GAS6 have divergent functions.7 GAS6 binds predominantly to Axl, with some affinity to Tyro3, but little affinity to Mer. In contrast, PS binds predominantly to Tyro3 and Mer with little affinity to Axl.4,8 In the context of kidney injury, GAS6 has been shown to induce Axl-mediated mesangial cell proliferation and glomerular hypertrophy in early DN9,10 and to promote inflammation in GN.11,12 Although the role of PS in kidney cells has not been explored, in other cell types PS has been shown to be a negative regulator of immune and inflammatory responses, and involved in the clearance of apoptotic cells13,14 and in the inhibition of VEGF-A–mediated angiogenesis.15 Given these contrasting effects of PS in comparison to GAS6, we posited that the increased PS expression in early diabetic kidney injury might be renoprotective. Our results now show that PS is significantly increased in the glomerular cells including the podocytes during the early stages of DN, and that the loss of PS specifically in podocytes aggravated proteinuria, inflammation, and podocyte injury and loss in streptozotocin (STZ)-induced diabetic kidneys. Conversely, the induction of PS overexpression in glomerular cells attenuated albuminuria and podocyte loss in OVE26 diabetic mice, confirming the renoprotective role of PS in diabetic kidneys.
Methods
Study Approval
All animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee at Icahn School of Medicine at Mount Sinai.
Statistical Analyses
Data are expressed as mean±SEM. Unpaired t test was used to analyze data between two groups. ANOVA with Bonferroni post hoc test was used when more than two groups were present. All experiments were repeated at least three times, and representative experiments are shown. Statistical significance was achieved when P<0.05.
A complete, detailed description of methods can be found in the Supplemental Material.
Results
Identification of PS from Proteomic Analysis of Diabetic Rat Glomeruli
Proteomic analysis was performed on isolated glomeruli of rats injected with either low-dose STZ or with citrate buffer vehicle. Rats were euthanized at either 6 or 12 weeks after the onset of diabetes in the experimental group (n=5 for each time point). An additional group of diabetic rats were treated with insulin to maintain tight glycemic control between weeks 6 and 12 and euthanized at 12 weeks after the onset of diabetes (n=5). Sex- and age-matched nondiabetic control rats were used as controls (n=5 for each time point). Body weight, blood glucose, kidney-to–body weight ratio, and urine albumin-to-creatinine measurements are shown in Supplemental Table 1. The top ten of the proteins whose expression was significantly changed in diabetic rats, but was reversed by insulin treatment, are listed in Table 1. Among them, PS had nearly two- to three-fold increase in diabetic glomeruli at both 6 and 12 weeks of diabetes, but its level was significantly decreased in diabetic rats treated with insulin (Table 1). Because PS is a homolog of GAS6, which was shown to be involved in early diabetic glomerular hypertrophy and DN pathogenesis,10 we selected PS for further study.
Table 1.
Top ten identified proteins with most significant fold changes in expression levels at 12 wk after induction of diabetes
| Protein Name | Gene | Cont 6 wk | Cont 12 wk | STZ 6 wk | STZ 12 wk | STZ+Ins 12 wk | P Value | Adjusted Q Value |
|---|---|---|---|---|---|---|---|---|
| C4b-binding protein α chain | C4bpa | 1.0 | 1.0 | 2.1 | 2.4 | 1.6 | 1.055e−07 | 1.787e−05 |
| Vitamin K–dependent PS | Pros1 | 1.0 | 1.0 | 2.1 | 2.4 | 1.7 | 1.055e−07 | 8.933e−06 |
| Brain acid soluble protein 1 | Basp1 | 1.0 | 1.0 | 0.5 | 0.4 | 0.7 | 2.266e−07 | 1.278e−05 |
| DnaJ homolog subfamily C member 2 | Dnajc2 | 1.0 | 1.0 | 0.9 | 0.4 | 0.4 | 2.266e−07 | 9.586e−06 |
| Solute carrier family 6, member 18 | Slc6a18 | 1.0 | 1.0 | 0.5 | 0.4 | 0.6 | 2.266e−07 | 7.669e−06 |
| Apo A-I | Apoa1 | 1.0 | 1.0 | 1.5 | 2.3 | 1.3 | 4.028e−07 | 1.136e−05 |
| Complement C4 | C4 | 1.0 | 1.0 | 2.1 | 2.2 | 1.7 | 1.53e−06 | 3.699e−05 |
| C4b-binding protein β chain | C4bpb | 1.0 | 1.0 | 2.3 | 1.9 | 1.0 | 7.856e−05 | 0.0017 |
| Solute carrier family 43, member 3 | Slc43a3 | 1.0 | 1.0 | 1.4 | 1.9 | 1.1 | 7.856e−05 | 0.0015 |
| Alanyl aminopeptidase | Anpep | 1.0 | 1.0 | 0.6 | 0.5 | 0.7 | <0.001 | 0.0018 |
Protein expression levels at 6 and 12 wk of diabetes (STZ 6wk, STZ 12wk) are indicated as fold changes relative to age-matched nondiabetic vehicle-injected controls. We used a Z-test to test for significance of fold changes for individual proteins, and the Benjamini–Hochberg procedure to adjust for multiple hypothesis testing. Cont, control vehicle-injected; STZ, STZ-injected; Ins, treated with insulin.
PS Expression Is Increased in Diabetic Glomeruli
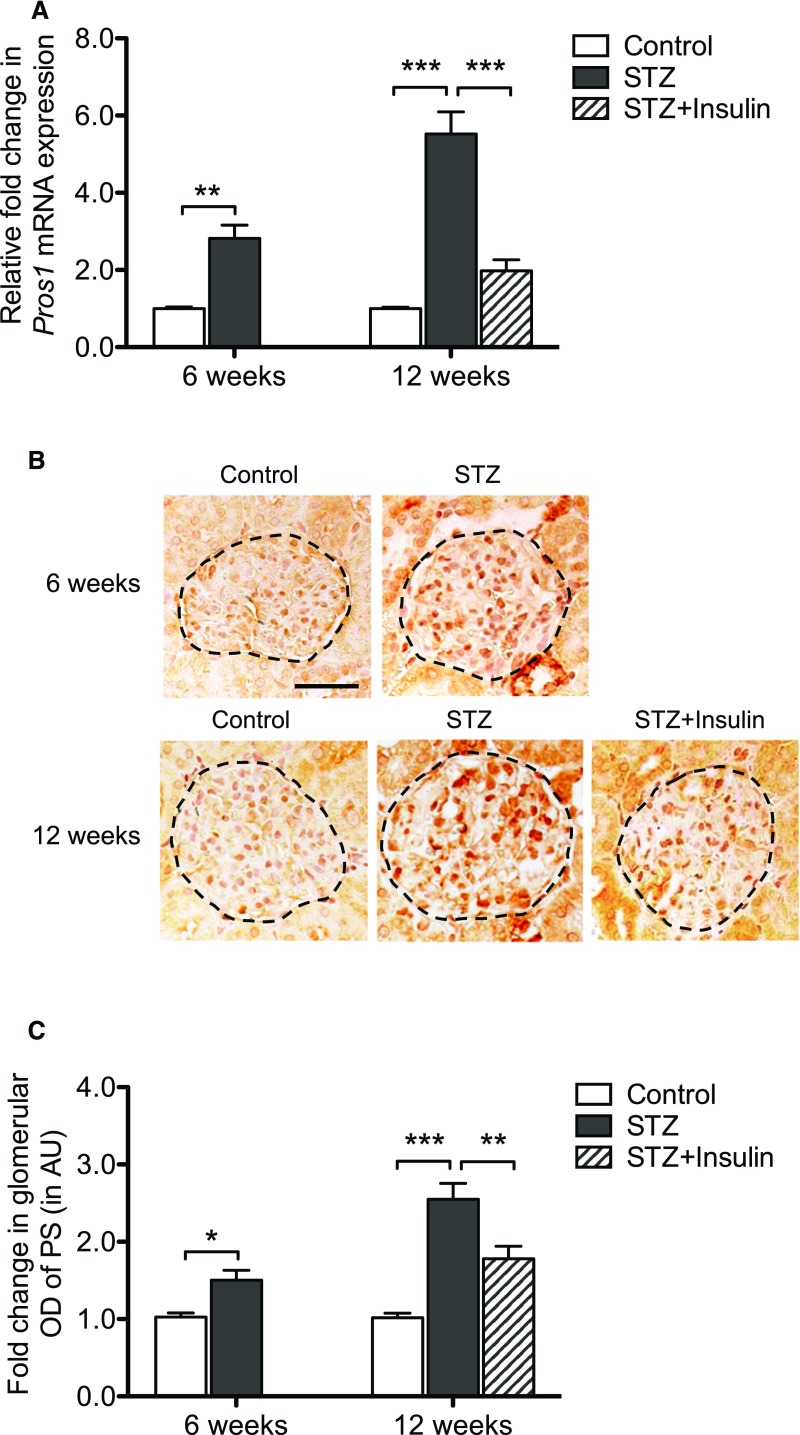
Real-time PCR analysis and immunostaining showed that both mRNA and protein levels of PS were increased in the diabetic rat glomeruli compared with nondiabetic glomeruli and that their levels were partially reversed by insulin treatment (Figure 1, A–C). We did not detect any significant changes in serum levels of PS in all groups (Supplemental Figure 1A), but urinary PS levels increased significantly in diabetic rats at both 6 and 12 weeks (Supplemental Figure 1B).
Figure 1.
Expression of PS is increased in diabetic rat glomeruli. (A) Real-time PCR was performed to measure Pros1 mRNA expression in glomeruli isolated from diabetic and control rats euthanized at either 6 or 12 weeks after the induction of diabetes (DM). Pros1 mRNA expression was also measured in diabetic rats treated with insulin between 6 and 12 weeks (DM+Insulin). (B) Representative images of immunostaining for PS are shown. Scale bar, 50 μm. (C) Glomerular region was selected, and OD was measured and quantified as a relative fold change to nondiabetic control mice (n=5; *P<0.05, **P<0.01, and ***P<0.001 when compared between indicated groups). AU, arbitrary units.
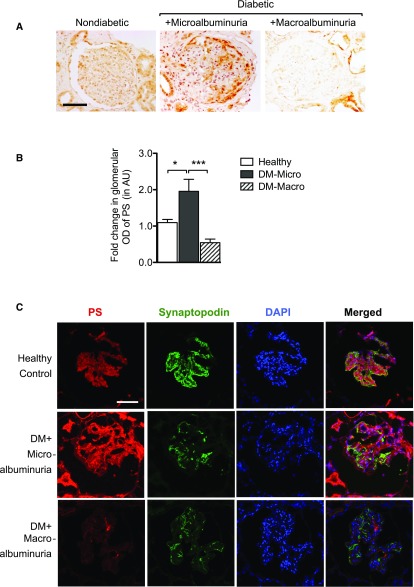
Phenotypes of most rodent models of DN, including the STZ-induced DN, are considered to be representative of the early stage of DN. Recent studies suggest disparate or even opposite patterns of renal gene expression profiles between diabetic animals and patients,16 which may be in part due to the differences in early (rodent models) versus late stages (human samples) of DN. Therefore, we next examined whether PS expression was altered in human diabetic kidneys with early and advanced DN (clinical information of the patients is summarized in Supplemental Table 2). Immunohistochemical analysis showed a marked increase of PS in glomerular cells of kidneys of patients with diabetes with microalbuminuria, but it was barely detectable in those with macro albuminuria (Figure 2, A and B). We further verified that the increased glomerular PS expression partially colocalized with podocyte marker synaptopodin (Figure 2C). Consistent with the above data in diabetic rats, there were no significant changes in plasma levels of free PS between control patients and patients with diabetes with early or late DN (Supplemental Figure 1, B and C). Our results suggest that there is a temporal increase of PS in kidneys of early DN, but not late DN, likely due to the local production of PS in glomerular cells.
Figure 2.
PS expression is altered in human DN. Immunostaining for PS was performed on healthy donor nephrectomy specimens and on kidney biopsy samples of patients with diabetes with microalbuminuria (UACR<300 mg/g) and with macroalbuminuria (UACR>300 mg/g). (A) Representative images of six subjects in each group are shown (400× original magnification; scale bar, 50 μm). (B) Glomerular area was selected, and OD was measured and quantified as a relative fold change to healthy donor specimens (n=6; *P<0.05 and ***P<0.001 when compared between indicated groups). (C) Representative images of diabetic human kidneys immunostained for both PS and synaptopodin (×400 original magnification). DAPI was used to visualize the nuclear staining. AU, arbitrary units; DAPI, 4′,6-diamidino-2-phenylindole; DM, diabetes mellitus; Macro, macroalbuminuria; Micro, microalbuminuria; PS, protein S; UACR, urinary albumin-to-creatinine ratio.
High Glucose Increases PS Expression in Podocytes
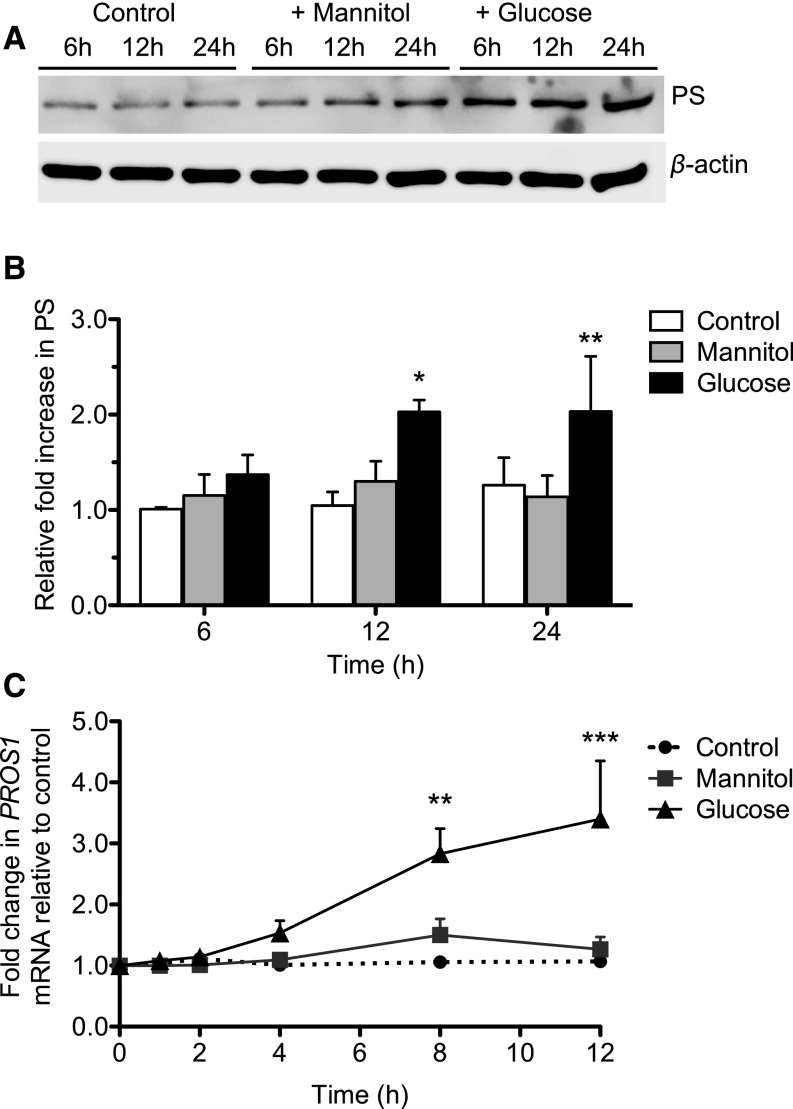
Because PS expression was localized more prominently in podocytes of diabetic kidneys, we sought to determine the regulation of PS expression in cultured human podocytes. Incubation of podocytes with high glucose induced both mRNA and protein levels of PS in comparison to cells incubated in control media with normal glucose or with high mannitol (Figure 3, A–C), suggesting that the induction of PS in hyperglycemic milieu may account in part for the observed increase of PS in early DN kidneys. To determine whether insulin had a direct effect on PS regulation, we treated the podocytes with either high glucose or insulin and found that insulin alone did not induce PS (Supplemental Figure 2). The mechanism of reduced PS expression in kidney with advanced DN remains to be determined.
Figure 3.
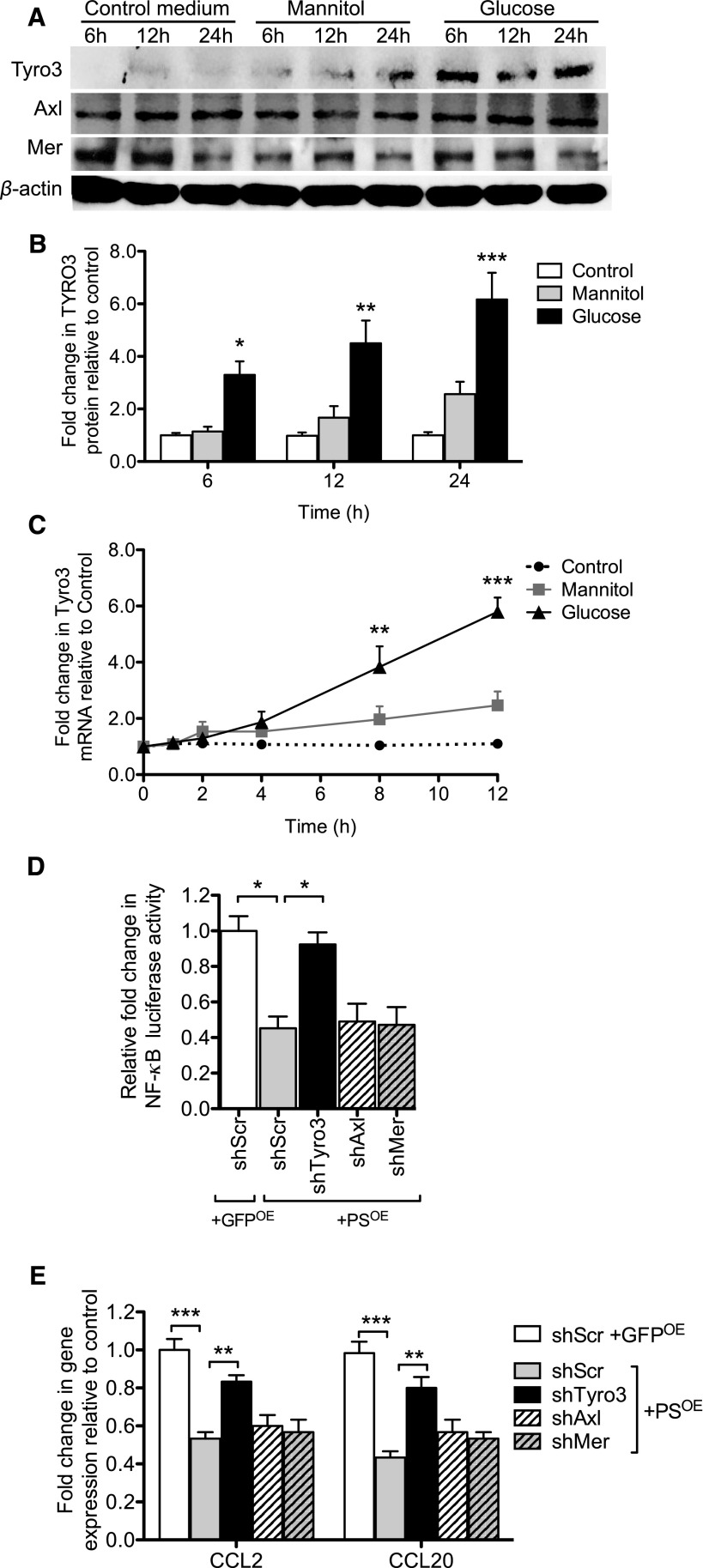
High glucose increases PS expression in cultured human podocytes. Human podocytes were incubated with normal glucose (5 mM), high mannitol (30 mM mannitol), or high glucose (30 mM) for 24 hours by adding the indicated concentrations of glucose and mannitol in the glucose-free medium. Western blotting and real-time PCR were performed to assess PS expression levels. (A) Representative western blot of three independent experiments is shown. (B) Densitometry analyses were performed for western blots. (C) PROS1 mRNA expression was measured by real-time PCR. (n=3; *P<0.05, **P<0.01, ***P<0.001 versus all other groups.)
PS Regulates Glucose-Induced Apoptosis and NF-κB–Mediated Inflammation in Podocytes
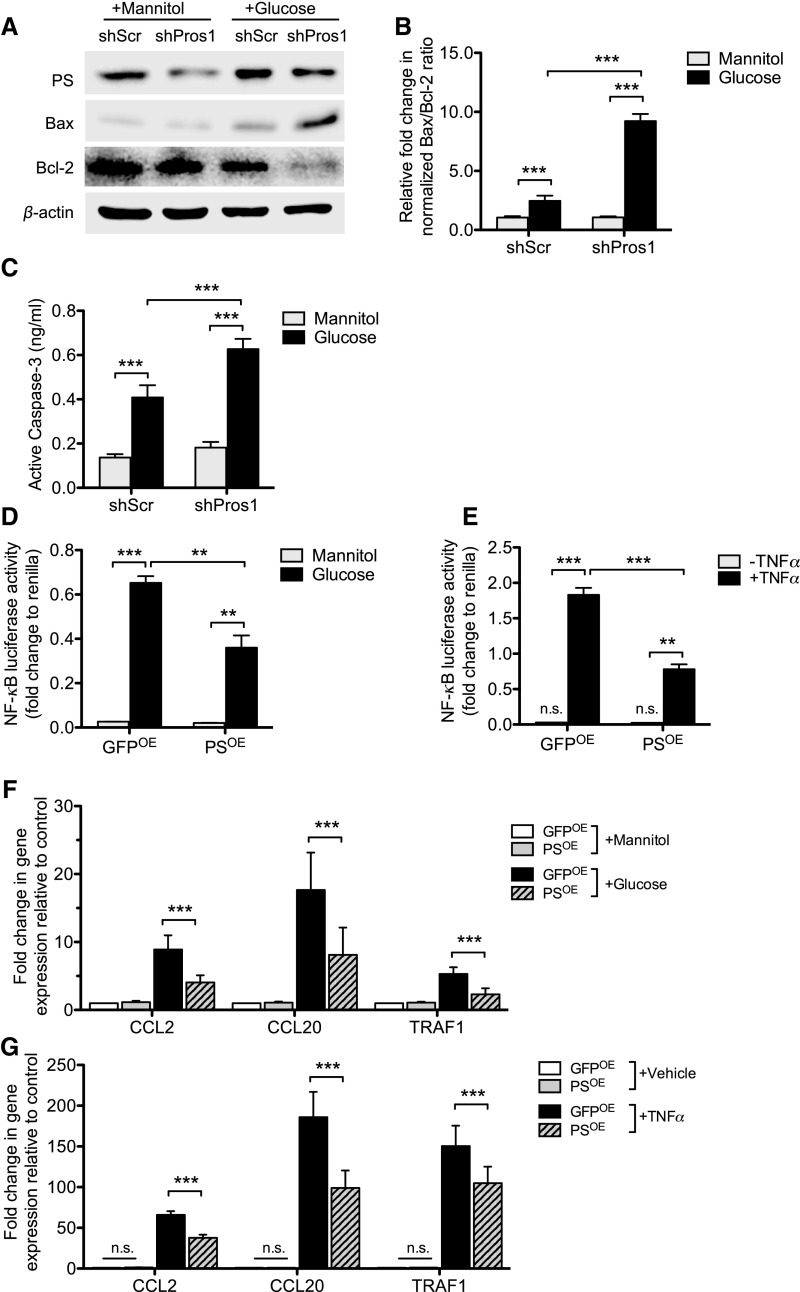
Hyperglycemia-induced podocyte loss is an early event in DN.17 Because PS is known to exert an antiapoptotic effect through the binding to and signaling through its cognate TAM receptors,18 we next examined whether the reduced expression of PS might affect podocyte survival under high-glucose conditions. shRNA-mediated silencing of PROS1 in podocytes incubated with high glucose led to a further increase in BAX expression and suppression of BCL-2 expression (Figure 4, A and B) and to increased Caspase-3 activity (Figure 4C), indicating that reduced PS expression exacerbated the high-glucose–induced podocyte apoptosis. These observations were further validated by using a second set of PS-specific shRNA to rule out any nonspecific effects (Supplemental Figure 3).
Figure 4.
PS prevents hyperglycemia-induced apoptosis and dampens TNF-α-induced inflammation. (A–C) Podocytes stably transduced with lentivirus expressing either scrambled shRNA (shScr) or Pros1 shRNA (shPros1) were incubated with high glucose (30 mM) or high mannitol (5 mM glucose±25 mM mannitol) for 24 hours. (A) Western blots were performed for PS, BAX, and BCL2. Representative blots of three independent experiments are shown. Densitometry analyses were performed for western blots of Bax and Bcl-2 after normalization with β-actin. (B) Active Caspase-3 concentration was measured by ELISA. (n=3; *P<0.05, **P<0.01, and ***P<0.001 when compared between indicated groups.) (D-G) Podocytes were transiently transfected with PROS1 overexpressing vector (PSOE) or control GFP vector (GFPOE). NF-κB luciferase reporter assay (D) with high mannitol (25 mM) or high glucose (30 mM) for 48 hours or (E) with TNF-α (10 ng) for 24 hours. NF-κB luciferase reporter activity is shown as fold change to renilla luciferase activity. (F-G) Real-time PCR analysis of NF-κB–targeted gene expression (F) in high glucose condition or (G) with TNF-α treatment is shown. (n=3; *P<0.05, **P<0.01, and ***P<0.001 when compared between indicated groups.)
Because PS is a negative regulator of inflammatory responses,19 and TNF-α–induced NF-κB signaling is a driver of DN pathogenesis,20,21 we examined whether the overexpression of PS can mitigate the high-glucose– or TNF-α-induced inflammatory responses. Podocytes were transfected with either PS overexpressing vector (PSOE) or control vector expressing green fluorescent protein (GFPOE) together with NF-κB luciferase reporter and renilla luciferase plasmids. Forty-eight hours post-transfection, cells were exposed to either high glucose or TNF-α (10 ng/ml) for an additional 24 hours. We found that the overexpression of PS suppressed both high-glucose– or TNF-α-induced NF-κB luciferase reporter activity (Figure 4, D and E), as well as the expression of several NF-κB–mediated proinflammatory genes, by real-time PCR (Figure 4, F and G).
To determine whether the antiapoptotic and anti-inflammatory effects of PS observed above are mediated by the activation of TAM receptors, we first examined their expression in podocytes. All three TAM receptors were expressed in podocytes, but only Tyro3 expression was upregulated in both protein and mRNA levels under high-glucose conditions (Figure 5, A–C, Supplemental Figure 4). To ascertain the contribution of each receptor in PS-mediated anti-inflammatory effects, we next silenced the individual TAM receptors by using lentiviral vectors expressing specific shRNAs to each receptor (Supplemental Figure 5, A and B). Indeed, the silencing of Tyro3, but not of Mer or Axl, led to a significant mitigation of the anti-inflammatory effects of PS in podocytes (Figure 5, D and E, Supplemental Figure 5, C and D), suggesting that Tyro3 specifically mediates the cytoprotective effects of PS in podocytes.
Figure 5.
Tyro3 mediates the effects of PS in podocytes. (A–C) Podocytes were incubated with normal glucose, high mannitol, or high glucose for 24 hours. Western blots were performed for each TAM receptor. (A) Representative blots of three independent experiments are shown. Densitometry analysis of Tyro3 is shown (densitometric analysis of Axl and Mer are included in Supplemental Figure 2). (C) PROS1 mRNA expression was measured by real-time PCR (mRNA expression of Axl and Mer are included in Supplemental Figure 2). (n=3; *P<0.05, **P<0.01, and ***P<0.001 versus Control.) (D and E) Podocytes stably transduced with lentivirus expressing either scrambled shRNA (shScr), Tyro3 shRNA (shTyro3), Axl shRNA (shAxl), or Mer ShRNA (ShMer) were transfected with NF-κB luciferase reporter and renilla luciferase, together with either control GFP overexpression vector (GFPOE) or PS overexpression vector (PSOE). (D) NF-κB luciferase reporter activity is shown as fold change to renilla luciferase activity. (E) Real-time PCR analysis of selected NF-κB–targeted gene expression was performed in these cells. (n=3; *P<0.05, **P<0.01, and ***P<0.001 when compared between indicated groups.)
Diabetic Glomerulopathy Is Aggravated in Podocyte-Specific Pros1-Null Mice
Because global Pros1 knockout mice are not viable,13 and significant upregulation of PS was found in podocytes in early diabetic kidneys, we interrogated the effects of podocyte-specific loss of PS in DN. Pros1 floxed mice (from Dr. G. Lemke) were crossed with podocin-Cre (Pod-Cre) transgenic mice (Jackson Laboratory, ME). Resulting Pros1fl/fl;Pod-Cre (PS-KO) mice were viable, fertile, and indistinguishable from the Pros1fl/fl wildtype (WT) littermates. We confirmed the podocyte-specific ablation of Pros1 by western blot analysis of primary podocytes isolated from PS-KO and WT mice (Figure 6A). Immunostaining of PS with synaptopodin further confirmed the loss of PS specifically in podocytes (Figure 6B). At baseline, PS-KO mice did not develop proteinuria and kidney injury when they were euthanized at 6–12 months of age (data not shown).
Figure 6.
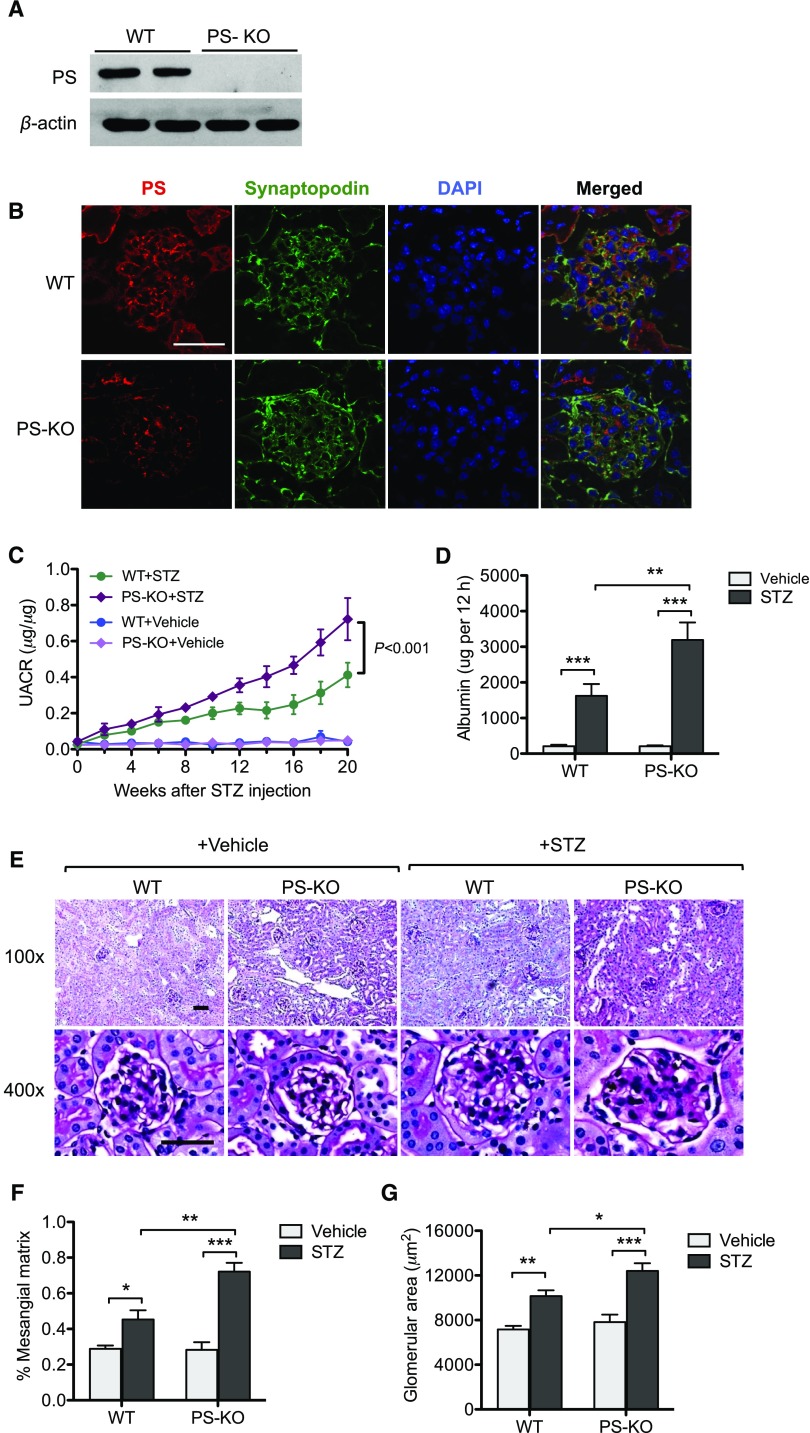
Diabetic glomerulopathy is aggravated in PS-KO mice. (A) Western blot analysis of PS in the lysates of primary podocytes isolated from PS-KO and control WT mice. (B) Immunostaining of PS and synaptopodin in the kidney shows loss of PS specifically in podocytes in PS-KO mice. (C) Urinary albumin-to-creatinine ratio is shown over the course of 20 weeks post–diabetes onset. (D) The 12-hour urinary albumin excretion rate was determined at 20 weeks post–diabetes onset. (E) Representative images of periodic acid–Schiff–stained kidneys of vehicle- or STZ-injected WT and PS-KO at both low (×200) and high (×400) magnification. (F and G) Quantification of (F) glomerular area and (G) percentage of mesangial matrix area are shown (60 glomeruli per group; n=6; *P<0.05, **P<0.01, and ***P<0.001 when compared between indicated groups). DAPI, 4′,6-diamidino-2-phenylindole; PS-KO+Vehicle, PS-KO injected vehicle control; WT+Vehicle, WT injected with vehicle control; PS-KO+STZ, PS-KO injected with STZ; WT+STZ, WT injected with STZ; UACR, urinary albumin-to-creatinine ratio.
Diabetes was induced in 8-week-old WT and PS-KO mice with low-dose STZ injections, and all mice were euthanized 20 weeks postinjection. Blood glucose levels were monitored every 2 weeks, which were comparable between STZ-injected WT and PS-KO mice (Supplemental Figure 6A). Both WT and PS-KO diabetic mice displayed similar extents of weight loss (Supplemental Figure 6B) and kidney hypertrophy, as measured by kidney-to–body weight ratio (Supplemental Figure 6C), when compared with nondiabetic controls. However, we observed a significant increase in albuminuria in diabetic PS-KO mice in comparison to diabetic WT controls when examined by spot urine collection (Figure 6C) and by 12-hour urine collection at 20 weeks (Figure 6D). Histologic analysis of mouse kidneys also showed marked increase in mesangial matrix and in glomerular volume in diabetic PS-KO mice as compared with the diabetic WT controls (Figure 6, E–G).
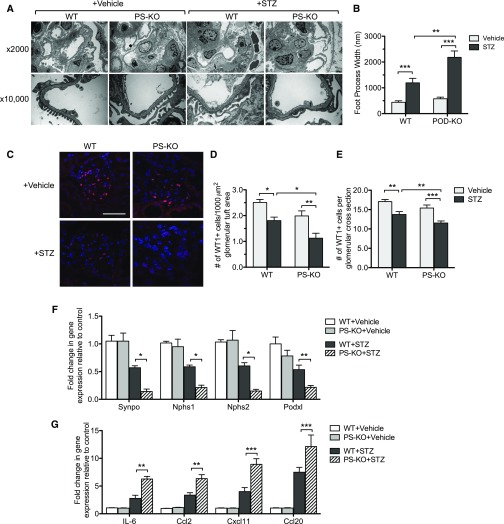
Consistent with worsened diabetic glomerulopathy in diabetic PS-KO mice, greater podocyte injury and loss were also observed in diabetic PS-KO mice (Figure 7). Electron microscopy revealed exacerbated podocyte foot process effacement in the diabetic PS-KO mice as compared with diabetic WT mice (Figure 7, A and B). In addition, there was a greater podocyte loss in diabetic PS-KO kidneys, as quantified by Wilms’ tumor-1 (WT-1)–positive cells (Figure 7, C–E). Real-time PCR analysis confirmed the decreased expression of podocyte markers (synaptopodin, nephrin, synaptopodin, and podocin) in diabetic PS-KO glomeruli as compared with diabetic WT glomeruli (Figure 7F), consistent with increased podocyte injury and loss. Furthermore, increased cell death in diabetic PS-KO glomeruli was confirmed by TUNEL staining (Supplemental Figure 7). Moreover, real-time PCR analysis showed increased expression of inflammatory mediators in diabetic PS-KO glomeruli in comparison to diabetic WT glomeruli (Figure 7G). Taken together, our data demonstrate a protective effect of PS against diabetic podocyte injury and loss in vivo.
Figure 7.
Podocyte injury and loss are worsened in diabetic PS-KO mice. (A) Representative transmission electron microscopy images of vehicle- or STZ-injected WT and PS-KO kidneys are shown at low (×2000) and high (×10,000) magnifications. (B) Quantification of foot process effacement is shown. (C) Representative images of WT-1 immunostained glomeruli are shown. (D and E) Quantification of WT-1+ cells are shown as (D) WT-1+ cell number per glomerular cross section and as (E) WT-1+ cell number per 1000 µm2 glomerular tuft area. (F) mRNA levels of WT-1, nephrin, synaptopodin, and podocin are shown as a relative fold change to vehicle control. (G) mRNA levels of inflammatory markers from isolated glomeruli are shown as a relative fold change to vehicle control. (60 glomeruli per group; n=6; *P<0.05, **P<0.01, and ***P<0.001 when compared between indicated groups.)
Induction of PS Expression in Diabetic Kidney Attenuated Albuminuria and Podocyte Injury
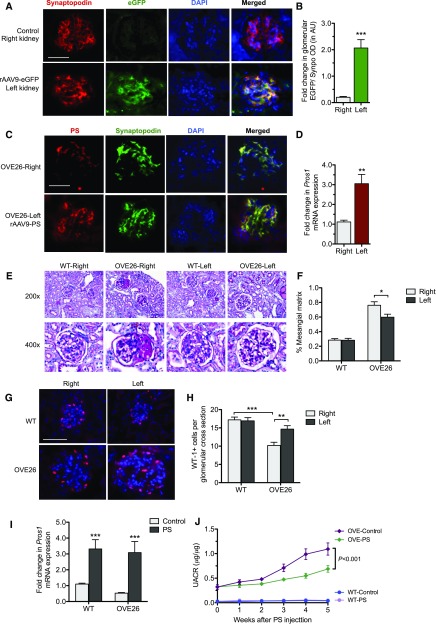
We next determined whether increased PS expression was sufficient to attenuate diabetic kidney injury in vivo. Because STZ-induced diabetes results in mild kidney disease, to detect the improvement of DN injury, we employed a diabetic mouse model with more severe DN, namely OVE26 mice in the FVB/N background.22,23 We induced a transient overexpression of PS in the kidney of OVE26 mice through intrarenal arterial injection of recombinant adeno-associated virus (rAAV9) expressing PS, as described in the Methods. We first confirmed the efficacy of the method by injection of enhanced green fluorescent protein–expressing rAAV9 (rAAV9-eGFP) into the renal artery of the left kidney. Examination of the kidneys 3 weeks postinjection showed a robust expression of eGFP in the glomeruli of the left kidney, but not in those of the right kidney (Figure 8, A and B). Glomerular expression of eGFP was confirmed by real-time PCR analysis of eGFP mRNA levels in the isolated glomeruli and in kidney cortices (Supplemental Figure 8A). Importantly, we observed that significant eGFP expression colocalized with synaptopodin expression, as shown by the quantification of eGFP and synaptopodin double-positive fluorescence areas in the glomeruli (Figure 8, A and B), suggesting a high infection rate of podocytes by rAAV9. We next injected the Pros1-expressing rAAV9 (rAAV9-PS) into the left kidneys of OVE26 mice at age 10 weeks when they developed a significant amount of proteinuria.22,23 Notably, 10-week-old OVE26 mice showed decreased PS expression compared with age-matched nondiabetic littermates (Supplemental Figure 8, B and C), consistent with the above observation that the severity of DN is associated with decreased PS expression. There was a significant increase in PS expression at 5 weeks postinjection in the rAAV9-injected (left) kidneys as compared with the contra-lateral (right) kidneys, which also showed a significant overlap with podocyte marker synaptopodin (Figure 8C), which was further confirmed by real-time PCR of isolated glomeruli (Figure 8D). Importantly, PS overexpression led to a significant reduction in mesangial expansion (Figure 8, E and F) and in podocyte loss (Figure 8, G and H) in the rAAv9-PS–injected left kidneys as compared with the uninjected right kidneys of OVE26 mice. To ascertain the effects on renal function by this transient overexpression of PS, we injected the rAAV9-PS or rAAV9-eGFP into both kidneys of diabetic OVE26 and nondiabetic control (WT) mice at 10 weeks of age, and the mice were similarly euthanized at 5 weeks postinjection. Real-time PCR analysis of isolated glomeruli confirmed a significant expression of PS in WT and OVE26 mice that received rAAV9-PS in comparison to those that received rAAV9-EGFP injection (Figure 8I). Urinary albumin-to-creatinine ratio showed a marked reduction of proteinuria in rAAV9-PS–injected OVE26 mice as compared with rAAV9-eGFP–injected OVE26 mice (Figure 8J). These data strongly suggest that PS could be a potential therapeutic target for DN.
Figure 8.
Induction of PS expression in glomeruli attenuates podocyte injury and albuminuria in OVE26 mice. Four mice received injection of GFP-expressing rAAV9 into the left kidney for 3 weeks while the right kidneys were used as controls. (A) Representative images of immunofluorescence costaining between eGFP and synaptopodin are shown to compare the eGFP expression and localization between right kidney (control) and left kidney (rAAV9-eGFP injected). (B) Quantification of fluorescence intensity of eGFP/synaptopodin ratio between right and left kidneys from these mice (n=4; ***P<0.001 compared with the right kidneys). (C) Diabetic OVE26 mice at age 10 weeks were injected with the rAAV9-PS in the left kidneys and were euthanized 5 weeks postinjection. Representative image of immunostaining for PS and synaptopodin shows extensive overlap. (D) Pros1 mRNA levels were compared between left kidney (rAAV9-PS) and right kidney (control) by real-time PCR (n=4; **P<0.01 compared with right kidneys). (E) Representative images of PAS-stained kidneys. (F) Quantification of mesangial fraction (n=4; *P<0.05 compared with right kidneys). (G and H) Representative image of (G) WT-1 immunostaining and (H) quantification are shown (n=4; **P<0.01 and ***P<0.001 when compared between indicated groups). (I and J) In another set of experiments, OVE26 mice at age 10 weeks received either rAAV9-PS or rAAV9-eGFP in both kidneys. (I) Real-time PCR analysis confirmed an increase of PROS1 expression in the kidneys of mice injected with rAAV9-PS as compared with those injected with rAAV9-eGFP. (J) Urinary albumin-to-creatinine ratio (UACR) was determined weekly in these mice for 5 weeks (n=4; ***P<0.001 compared with between indicated groups). AU, arbitrary units; DAPI, 4′,6-diamidino-2-phenylindole; eGFP, enhanced green fluorescent protein; OVE-Control, OVE26 injected with rAAV9-eGFP; OVE-PS, OVE26 mice injected with rAAV-PS; WT-Control, wildtype injected with rAAV-eGFP; WT-PS, wildtype injected with rAAV9-PS.
Discussion
We performed a proteomic analysis of diabetic rat glomeruli in order to identify proteins that may be involved in disease pathogenesis in early DN. We identified several proteins with significant changes in their expression in diabetic glomeruli at both 6 and 12 weeks post STZ injection and whose expression was reversed when treated with insulin, suggesting that their expressions are likely to be regulated by hyperglycemia and/or insulin. Among the proteins identified, we chose to further interrogate the role of PS in early DN, because PS has previously been shown to have anti-inflammatory and anti-apoptotic effects through binding to TAM receptors,18,24 and apoptosis and inflammation of kidney cells are key components of DN.
Although PS is present abundantly in the plasma and is made mostly in the liver, the plasma levels of PS did not change between diabetic and nondiabetic rats and in human patients with diabetes with or without DN, demonstrating that the observed changes in our proteomic analysis of isolated glomeruli are likely due to changes to local production of PS by glomerular cells, which was also confirmed by immunostaining. In support of these observations, it has been shown that PS is also synthesized in cells and tissues other than the liver, including endothelial cells and smooth muscle cells.13 In contrast to our findings, the early studies suggest that the plasma PS levels increase in patients with diabetes.25,26 However, a more recent study shows that the plasma PS level did not change between normal controls, patients with diabetes with or without DN.27 The discrepancy among these studies could be due to the use of different assays and/or the heterogeneity of patient populations. Further studies with more standardized protocols and larger numbers of patient populations are required to reconcile the discrepant findings.
The regulation of PS expression is not well understood, because only a few studies have explored its mechanism. It was shown that miR-155 and miR-494 mediate the regulation of PROS128,29 and that PROS1 3′-UTR sequences contain three putative miR-494 binding sites. Interestingly, an increased expression of miR-155 was observed in the patients with DN and in the experimental DN animal models, which contributes to inflammation-mediated glomerular cell injury,30,31 and elevated urinary miR-494 predicts the progression of kidney disease.32 These studies suggest a potential role of miR-155 and miR-494 in the regulation of PROS1 in DN. IL-6 has also been shown to induce PS expression through the activation of Stat3 pathway,33 and Stat3 is known to be activated in diabetic kidneys.34 In addition, AMP kinase has been shown to mediate flow-induced PS expression in endothelial cells.35 We found that PS is expressed highly in podocytes and that high glucose, but not insulin, upregulates PS expression in cultured podocytes, suggesting that the effect of insulin in reducing PS expression in vivo is likely through the reduction in blood glucose. In human diabetic kidneys, we observed that PS expression is upregulated during the early stages, but not during the late stages of DN, suggesting that the upregulation of some factors induced by high glucose at early DN may be protective against the disease progression. We speculate that this protective mechanism is lost in patients with progressive DN and may contribute to its pathogenesis. Future studies are required to determine how PROS1 is regulated in diabetic kidneys during the early and late stages of DN.
In this study, we chose to focus on the role of PS in podocytes because we observed its prominent expression in podocytes of human diabetic kidneys. Podocyte injury is considered to be an important early event in DN pathogenesis,36 such that the reduction in podocyte density is the strongest predictor of progressive DN.37,38 We found that PS protects against podocyte loss in vivo and reduces high-glucose– and TNF-α-induced inflammatory response in cultured podocytes. Nevertheless, we cannot rule out the expression of PS and its potential contribution against DN injury in other glomerular cells, such as glomerular endothelial cells. Given that the mice with global endothelial cell–specific knockout of PS show development of thrombosis,13 the model may not be suitable for studying the effect of PS in DN; and glomerular endothelial cell–specific Cre mice are not currently available. Future studies are required to dissect the role of PS in other glomerular cells in DN.
Both ligands of TAM receptors, PS and Gas6, are constitutively γ-carboxylated on glutamic acid residues in their N-terminal domains by a vitamin K–dependent carboxylase during secretion from cells,39 and γ-carboxylation of PS and Gas6 appears to be required for full activation of specific cognate TAM receptors.6,40 It is not clear at present whether the upregulated PS specifically in glomerular cells including podocytes during diabetic injury is fully γ-carboxylated. Further studies are required to assess this important aspect of PS signal transduction. Nevertheless, our data strongly suggest that the effects of PS in podocytes are mediated through Tyro3, consistent with previous reports of preferential binding of PS to Tyro 3.6,8 Moreover, our data further indicate that PS/Tyro3 signaling pathway confers protection against high-glucose–mediated cellular injury in cultured podocytes, and that the podocyte-specific loss of PS aggravates early diabetic kidney injury in vivo. We speculate that the loss of PS/Tyro3 upregulation removes this protection and contributes to progressive DN. Future studies are required to confirm whether the loss of Tyro3 specifically in podocytes similarly aggravates DN. Importantly, to assess whether the increased PS expression would confer therapeutic benefit, we examined the in vivo effect of transient induction of PS expression in OVE26 mice, a type 1 diabetic mouse model with severe DN.22,23,41 We induced the PS expression in mice that had already developed significant proteinuria in order to detect a therapeutic effect. Indeed, the induction of PS expression in the glomerular cells by injection of a Pros1-overexpressing rAAV9, which showed a high prominent expression in podocytes, led to a significant improvement of DN, indicating that PS could be a potential therapeutic target for DN.
In conclusion, our data demonstrate a hyperglycemia-regulated renoprotective pathway mediated by PS in early DN. Future studies are needed to determine whether the therapeutic approaches to modulate this protective pathway specifically in the kidneys can prevent or halt the progression of DN without affecting the global coagulation activity.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank G. Lemke for Pros1 floxed mice.
J.C.H. is supported by Veterans Affairs Merit Award IBX000345C, National Institutes of Health (NIH) 1R01DK078897, NIH 1R01DK088541, NIH P01DK56492, and Chinese 973 fund 2012CB517601. E.U.A. was supported by an American Society of Nephrology Career Development Grant. The mass spectrometer used in this study was purchased with NIH grant, NS046593.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030234/-/DCSupplemental.
References
- 1.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, et al. : US renal data system 2013 annual data report. Am J Kidney Dis 63: A7, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Suleiman L, Negrier C, Boukerche H, Protein S: A multifunctional anticoagulant vitamin K-dependent protein at the crossroads of coagulation, inflammation, angiogenesis, and cancer. Crit Rev Oncol Hematol 88: 637–654, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, et al. : Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13: 1349–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 4.van der Meer JH, van der Poll T, van ’t Veer C: TAM receptors, Gas6, and protein S: Roles in inflammation and hemostasis. Blood 123: 2460–2469, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Hafizi S, Dahlback B: Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J 273: 5231–5244, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Tsou WI, Nguyen KQ, Calarese DA, Garforth SJ, Antes AL, Smirnov SV, et al. : Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J Biol Chem 289: 25750–25763, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studer RA, Opperdoes FR, Nicolaes GA, Mulder AB, Mulder R: Understanding the functional difference between growth arrest-specific protein 6 and protein S: An evolutionary approach. Open Biol 4: 140121, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lew ED, Oh J, Burrola PG, Lax I, Zagorska A, Traves PG, et al. : Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. eLife 3: e03385, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagai K, Arai H, Yanagita M, Matsubara T, Kanamori H, Nakano T, et al. : Growth arrest-specific gene 6 is involved in glomerular hypertrophy in the early stage of diabetic nephropathy. J Biol Chem 278: 18229–18234, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Nagai K, Matsubara T, Mima A, Sumi E, Kanamori H, Iehara N, et al. : Gas6 induces Akt/mTOR-mediated mesangial hypertrophy in diabetic nephropathy. Kidney Int 68: 552–561, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Yanagita M: The role of the vitamin K-dependent growth factor Gas6 in glomerular pathophysiology. Curr Opin Nephrol Hypertens 13: 465–470, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Fiebeler A, Park JK, Muller DN, Lindschau C, Mengel M, Merkel S, et al. : Growth arrest specific protein 6/Axl signaling in human inflammatory renal diseases. Am J Kidney Dis 43: 286–295, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Burstyn-Cohen T, Heeb MJ, Lemke G: Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J Clin Invest 119: 2942–2953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saller F, Brisset AC, Tchaikovski SN, Azevedo M, Chrast R, Fernandez JA, et al. : Generation and phenotypic analysis of protein S-deficient mice. Blood 114: 2307–2314, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraineau S, Monvoisin A, Clarhaut J, Talbot J, Simonneau C, Kanthou C, et al. : The vitamin K-dependent anticoagulant factor, protein S, inhibits multiple VEGF-A-induced angiogenesis events in a Mer- and SHP2-dependent manner. Blood 120: 5073–5083, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Hodgin JB, Nair V, Zhang H, Randolph A, Harris RC, Nelson RG, et al. : Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes 62: 299–308, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Susztak K, Raff AC, Schiffer M, Bottinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 18.Rezende SM, Simmonds RE, Lane DA: Coagulation, inflammation, and apoptosis: Different roles for protein S and the protein S-C4b binding protein complex. Blood 103: 1192–1201, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Cabezon R, Carrera-Silva EA, Florez-Grau G, Errasti AE, Calderon-Gomez E, Lozano JJ, et al. : MERTK as negative regulator of human T cell activation. J Leukoc Biol 97: 751–760, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedigo CE, Ducasa GM, Leclercq F, Sloan A, Mitrofanova A, Hashmi T, et al. : Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J Clin Invest 126: 3336–3350, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmid H, Boucherot A, Yasuda Y, Henger A, Brunner B, Eichinger F, et al. : European Renal c, DNABC: Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes 55: 2993–3003, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Huang Y, Li F, Zheng S, Epstein PN: FVB mouse genotype confers susceptibility to OVE26 diabetic albuminuria. Am J Physiol Renal Physiol 299: F487–F494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, et al. : Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes 53: 3248–3257, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S: TAM receptor signaling in immune homeostasis. Annu Rev Immunol 33: 355–391, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi H, Tatewaki W, Wada K, Shibata A: Plasma protein S in disseminated intravascular coagulation, liver disease, collagen disease, diabetes mellitus, and under oral anticoagulant therapy. Clin Chim Acta 182: 195–208, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Saito M, Kumabashiri I, Jokaji H, Asakura H, Uotani C, Otsuka M, et al. : The levels of protein C and protein S in plasma in patients with type II diabetes mellitus. Thromb Res 52: 479–486, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Ochodnicky P, Lattenist L, Ahdi M, Kers J, Uil M, Claessen N, et al. : Increased circulating and urinary levels of soluble TAM receptors in diabetic nephropathy. Am J Pathol 187: 1971–1983, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Ng CT, Dheen ST, Yip WC, Ong CN, Bay BH, Lanry Yung LY: The induction of epigenetic regulation of PROS1 gene in lung fibroblasts by gold nanoparticles and implications for potential lung injury. Biomaterials 32: 7609–7615, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Tay JW, Romeo G, Hughes QW, Baker RI: Micro-ribonucleic Acid 494 regulation of protein S expression. J Thromb Haemost 11: 1547–1555, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Liu Y, Li L, Su B, Yang L, Fan W, et al. : Involvement of inflammation-related miR-155 and miR-146a in diabetic nephropathy: Implications for glomerular endothelial injury. BMC Nephrol 15: 142, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khamaneh AM, Alipour MR, Sheikhzadeh Hesari F, Ghadiri Soufi F: A signature of microRNA-155 in the pathogenesis of diabetic complications. J Physiol Biochem 71: 301–309, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Szeto CC: Urine miRNA in nephrotic syndrome. Clin Chim Acta 436: 308–313, 2014 [DOI] [PubMed] [Google Scholar]
- 33.de Wolf CJ, Cupers RM, Bertina RM, Vos HL: Interleukin-6 induction of protein s is regulated through signal transducer and activator of transcription 3. Arterioscler Thromb Vasc Biol 26: 2168–2174, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Brosius FC 3rd, He JC: JAK inhibition and progressive kidney disease. Curr Opin Nephrol Hypertens 24: 88–95, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young A, Wu W, Sun W, Benjamin Larman H, Wang N, Li YS, et al. : Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler Thromb Vasc Biol 29: 1902–1908, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf G, Chen S, Ziyadeh FN: From the periphery of the glomerular capillary wall toward the center of disease: Podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Steffes MW, Schmidt D, McCrery R, Basgen JM: Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 59: 2104–2113, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Berkner KL: Vitamin K-dependent carboxylation. Vitam Horm 78: 131–156, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Geng K, Kumar S, Kimani SG, Kholodovych V, Kasikara C, Mizuno K, et al. : Requirement of gamma-carboxyglutamic acid modification and phosphatidylserine binding for the activation of Tyro3, Axl, and Mertk receptors by growth arrest-specific 6. Front Immunol 8: 1521, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alpers CE, Hudkins KL: Mouse models of diabetic nephropathy. Curr Opin Nephrol Hypertens 20: 278–284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.