Abstract
Background With No Lysine kinase (WNK) signaling regulates mammalian renal epithelial ion transport to maintain electrolyte and BP homeostasis. Our previous studies showed a conserved role for WNK in the regulation of transepithelial ion transport in the Drosophila Malpighian tubule.
Methods Using in vitro assays and transgenic Drosophila lines, we examined two potential WNK regulators, chloride ion and the scaffold protein mouse protein 25 (Mo25), in the stimulation of transepithelial ion flux.
Results In vitro, autophosphorylation of purified Drosophila WNK decreased as chloride concentration increased. In conditions in which tubule intracellular chloride concentration decreased from 30 to 15 mM as measured using a transgenic sensor, Drosophila WNK activity acutely increased. Drosophila WNK activity in tubules also increased or decreased when bath potassium concentration decreased or increased, respectively. However, a mutation that reduces chloride sensitivity of Drosophila WNK failed to alter transepithelial ion transport in 30 mM chloride. We, therefore, examined a role for Mo25. In in vitro kinase assays, Drosophila Mo25 enhanced the activity of the Drosophila WNK downstream kinase Fray, the fly homolog of mammalian Ste20-related proline/alanine-rich kinase (SPAK), and oxidative stress-responsive 1 protein (OSR1). Knockdown of Drosophila Mo25 in the Malpighian tubule decreased transepithelial ion flux under stimulated but not basal conditions. Finally, whereas overexpression of wild-type Drosophila WNK, with or without Drosophila Mo25, did not affect transepithelial ion transport, Drosophila Mo25 overexpressed with chloride-insensitive Drosophila WNK increased ion flux.
Conclusions Cooperative interactions between chloride and Mo25 regulate WNK signaling in a transporting renal epithelium.
Keywords: kidney, hypertension, hypokalemia, Cab39, Malpighian tubule, NKCC
The regulation of epithelial ion transport in the mammalian nephron ensures homeostasis of the internal milieu, including electrolyte concentrations and effective circulating volume. With No Lysine kinases (WNKs) play important roles in this process. Gain-of-function mutations in WNK1 and WNK4 in humans cause familial hyperkalemic hypertension (also known as pseudohypoaldosteronism type 2 or Gordon syndrome), in which patients have hypertension and hyperkalemia.1 WNKs act through the intermediary Ste20 kinases Ste20-related proline/alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1), leading to the phosphorylation and activation of SLC12 family cation-chloride cotransporters, including the sodium-chloride cotransporter (NCC) and sodium-potassium 2 chloride cotransporters (NKCCs) NKCC1 and NKCC2.2–4 NCC and NKCC2 reabsorb sodium chloride in the distal convoluted tubule and thick ascending limb, respectively, of the mammalian nephron.
Earlier work showed that NKCC1 phosphorylation and activity increase with lowering of intracellular chloride, suggesting the existence of a chloride-sensitive kinase.5,6 Our prior studies showing that chloride binds directly to the active site of human WNK1, inhibiting autophosphorylation and thereby, WNK activation,7 definitively proved that WNKs are chloride-sensitive kinases. Chloride regulation of WNK3 and WNK4 was subsequently shown.8 However, direct proof that chloride regulates transepithelial ion flux through WNK regulation has been lacking.
Another potential modulator of WNK-SPAK/OSR1 signaling is the scaffold protein mouse protein 25 (Mo25; also called calcium binding protein 39). Mo25 acts synergistically with WNKs to activate SPAK/OSR1 in vitro,9 and it activates NKCC1 and NKCC2 activity in Xenopus oocytes together with WNK-SPAK/OSR1 pathway components.10,11 Mo25 is expressed in the thick ascending limb and distal convoluted tubule in the mammalian nephron,12 but a role for Mo25 in transepithelial ion transport has not been shown.
Drosophila melanogaster has four renal tubules (Malpighian tubules), which are in the abdominal cavity bathed by hemolymph (equivalent to plasma in mammals). The tubules are blind ended, and therefore, urine generation occurs through the secretion of a potassium chloride–rich fluid by the main segment of the tubules. Transepithelial cation secretion, primarily potassium, occurs via the principal cells, whereas chloride secretion occurs via the neighboring stellate cells (Figure 6D).13–17 We previously showed that approximately 30% of transepithelial potassium flux and fluid secretion is dependent on principal cell D. melanogaster With No Lysine kinase (DmWNK), the fly SPAK/OSR1 homolog Fray, and the secretory fly NKCC encoded by the Ncc69 gene.18,19 Bathing the tubules in hypotonic medium resulted in increased transepithelial potassium flux in a WNK-, Fray-, and NKCC-dependent manner,18 suggesting that WNK signaling may be activated in these conditions. Here, we show that, in hypotonic medium, intracellular chloride decreases in the renal epithelial cells, resulting in WNK activation at 30–60 minutes. Relief of chloride inhibition together with Mo25 is sufficient to stimulate WNK signaling and transepithelial ion transport. We thus directly link changes in intracellular chloride, WNK signaling, and ion flux in a transporting renal epithelium and show an important role for Mo25.
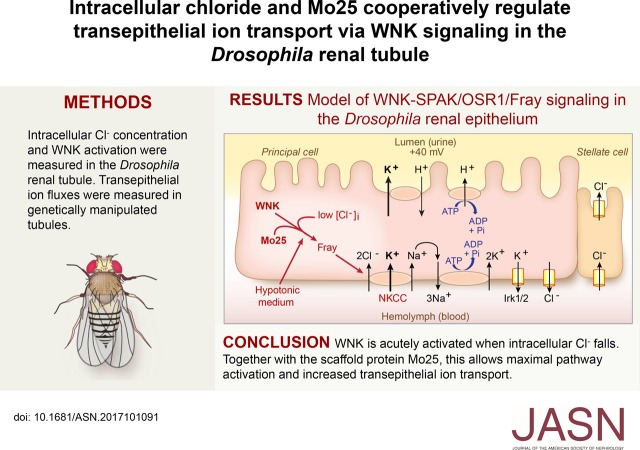
Figure 6.
Relief of chloride inhibition of With No Lysine kinase (WNK) and concomitant mouse protein 25 (Mo25) overexpression is sufficient to increase transepithelial ion flux in standard bathing medium. (A) Transepithelial potassium flux was measured using the Ramsay assay in standard bathing medium (isotonic conditions, approximately 30 mM intracellular chloride). Compared with control tubules, tubules in which FrayT206E was expressed during adulthood in principal cells with or without concomitant overexpression of D. melanogaster mouse protein 25 (DmMo25) had no difference in transepithelial potassium flux. Genotypes were control: w; tub-GAL80ts20/+; c42-GAL4/+ and experimental: w; tub-GAL80ts20 UAS-DmMo25/+; c42-GAL4/+, w; tub-GAL80ts20/UAS-FrayT206E; c42-GAL4/+, and w; tub-GAL80ts20 UAS-DmMo25/UAS-FrayT206E; c42-GAL4/+. Flies were reared at 18°C throughout development and shifted to 28°C for 2 days before testing to allow induction of the FrayT206E and DmMo25 transgenes; n=40–41 tubules per genotype. P=0.13, one-way ANOVA. (B) Compared with control tubules, tubules in which the wild type (WT) D. melanogaster With No Lysine kinase (DmWNK) was expressed during adulthood in principal cells with or without concomitant overexpression of DmMo25 had no difference in potassium flux. Genotypes were control: w; tub-GAL80ts20/+; c42-GAL4/+ and experimental: w; tub-GAL80ts20 UAS-DmWNKWT/+; c42-GAL4/+ and w; tub-GAL80ts20 UAS-DmWNKWT/UAS-DmMo25; c42-GAL4/+. Flies were reared at 18°C throughout development and shifted to 28°C for 2 days before testing to allow induction of the DmWNKWT and DmMo25 transgenes; n=24–29 tubules per genotype. P=0.16, one-way ANOVA. (C) Compared with control tubules, tubules overexpressing DmMo25 during adulthood had no difference in potassium flux, whereas tubules expressing both DmMo25 and chloride-insensitive DmWNKL421F had increased potassium flux. Genotypes were control: w; tub-GAL80ts20/+; c42-GAL4/+ and experimental: w; tub-GAL80ts20 UAS-DmMo25/+; c42-GAL4/+ and w; tub-GAL80ts20 UAS-DmMo25/UAS-DmWNKL421F; c42-GAL4/+. Flies were reared at 18°C throughout development and shifted to 28°C for 2 days before testing to allow induction of the DmWNKL421F and DmMo25 transgenes; n=24–25 tubules per genotype. P=0.002, one-way ANOVA. Adjusted P values (Tukey multiple comparisons test) were 0.62 for w; tub-GAL80ts20/+; c42-GAL4/+ versus w; tub-GAL80ts20 UAS-DmMo25/+; c42-GAL4/+, 0.03 for w; tub-GAL80ts20 UAS-DmMo25/+; c42-GAL4/+ versus w; tub-GAL80ts20 UAS-DmMo25/UAS-DmWNKL421F; c42-GAL4/+ and 0.002 for w; tub-GAL80ts20/+; c42-GAL4/+ versus w; tub-GAL80ts20 UAS-DmMo25/UAS-DmWNKL421F; c42-GAL4/+. *P=0.03; **P=0.002. (D) Model. The principal cell apical vacuolar H+-ATPase drives fluid secretion65 by extruding protons to generate a lumen-positive transepithelial potential difference.16 This is thought to drive exchange of protons for cations, primarily potassium in the Drosophila renal tubule. Transepithelial chloride secretion through the stellate cells is also driven by the lumen-positive charge.16,17 Both the sodium-potassium 2 chloride cotransporter (NKCC), encoded by Ncc69, and inwardly rectifying potassium channels, Irk1 and Irk2, are required for transepithelial potassium flux.19,21 Sodium entering the epithelial cells through NKCC is recycled through the Na+/K+-ATPase.19 There is also a basolateral chloride conductance with molecular identity that is unknown.48 WNK and Fray (the Drosophila Ste20-related proline/alanine-rich kinase [SPAK]/oxidative stress response 1 [OSR1] homolog) are positive regulators of potassium flux through NKCC, and hypotonicity stimulates transepithelial potassium flux in a WNK/Fray/NKCC-dependent manner.18 Here, we show that intracellular chloride concentrations in renal tubule epithelial cells fall in hypotonic conditions, with a concomitant increase in tubule WNK activity. Mo25 is also required for maximal ion transport. A mutation that abolishes chloride inhibition of WNK together with Mo25 overexpression is sufficient to stimulate transepithelial ion flux. These results implicate both chloride and Mo25 as important regulators of WNK signaling in a transporting renal epithelium.
Methods
Fly Stocks
Fly rearing and crosses were done on standard cornmeal/yeast/molasses food (prepared in a central kitchen at the University of Texas Southwestern Medical Center or the University of Utah). Fly strains used are summarized in Table 1. The following transgenic lines were newly generated in this study and are described in more detail in Supplemental Material: w; UAS-ClopHensor c304, w; UAS-SPAKD219A, w; UAS-DmWNKWT, w; UAS-DmWNKL421F, w; UAS-DmMo25, and w; UAS-MmMo25. Except for w; UAS-ClopHensor c304, which has multiple inserts, all lines were outcrossed for at least five generations to the wBerlin genetic background at the laboratory of A.R.R. Flies expressing ClopHensor in the tubule were reared at 26°C. Other crosses were performed at 28°C, except in the case of experiments using tub-GAL80ts20, in which case crosses were performed at 18°C. Adult females were collected within 1–2 days of eclosion and placed on regular fly food for an additional 3–5 days before dissection of tubules. For experiments using tub-GAL80ts20, flies were shifted to 28°C for 2 days before experimentation.
Table 1.
Drosophila strains used
| Genotype | Description | Source | Ref. |
|---|---|---|---|
| w; c42-GAL4 | Principal cell GAL4 driver | Laboratory of Dow/Davies | Rosay et al.62 |
| w; tub-GAL80ts20 | Temperature-sensitive GAL4 repressor | BDSC 7019 | McGuire et al.66 |
| w; UAS-DmWNKRNAi | RNAi against Drosophila WNK | BDSC 42521 | |
| w; UAS-DmMo25RNAi | RNAi against Drosophila Mo25 | VDRC 34788 | Dietzl et al.67 |
| w; UAS-ClopHensor c304 | pH/chloride sensor | This study | |
| w; UAS-SPAKD219A | WNK substrate | This study | |
| w; UAS-DmWNKWT | Drosophila WNK | This study | |
| w; UAS-DmWNKL421F | Chloride-insensitive Drosophila WNK | This study | |
| w; UAS-DmMo25 | Drosophila Mo25 | This study | |
| w; UAS-MmMo25 | Mouse Mo25 | This study | |
| w;UAS-FrayT206E | Fray phosphomimicking mutation | Laboratory of A.R.R. | Wu et al.18 |
BDSC, Bloomington Drosophila Stock Center (Indiana University, Bloomington, IN); DmWNK, D. melanogaster With No Lysine kinase; RNAi, RNA interference; WNK, With No Lysine kinase; DmMo25, D. melanogaster mouse protein 25; Mo25, mouse protein 25; VDRC, Vienna Drosophila Resource Center (Vienna, Austria); WT, wild type.
WNK Autophosphorylation Assays
His-tagged DmWNK kinase domain, residues 261–534,18 was purified and allowed to autophosphorylate in vitro in 50 μl volume in buffers including 20 mM HEPES, pH 7.4, 20 mM Mg gluconate, 5 mM ATP, and NaCl to achieve a final chloride concentration of 50 or 150 mM. Reaction mixtures were prewarmed for 5 minutes at 30°C. Fifty micrograms purified WNK domain (5 mg/ml) was added to start the reaction and run over a 0-, 1-, 2-, or 4-hour time course at 30°C. Reactions were terminated by adding 17 μl 4× SDS sample buffer and heating the sample for 5 minutes. Autophosphorylation was monitored using mass spectrometry or Pro-Q Diamond phosphoprotein stain (ThermoFisher) after SDS-PAGE as detailed in Supplemental Material.
Measurement of Intracellular Chloride Concentration
Intracellular pH and chloride were measured in the renal tubule epithelium principal cell using the transgenic sensor, ClopHensor.20 pH and chloride calibrations were performed by bathing tubules expressing ClopHensor in varying pH/chloride baths in the presence of 10 μM tributyltinchloride (Sigma-Aldrich), 5 μM nigericin (Invitrogen), 5 μM carbonyl cyanide 3-chlorophenylhydrazone (Sigma-Aldrich), and 5 μM valinomycin (Sigma-Aldrich). Tubules were imaged using a Zeiss LSM510 confocal microscope, with excitation at 488 nm (green emission), 458 nm (cyan emission), and 543 nm (red emission). Pixel intensity in individual tubule epithelial cells was measured using ImageJ without image manipulation. The ratios of green to cyan versus pH and cyan to red versus chloride were entered into GraphPad Prism, and sigmoidal curves were interpolated as detailed in Supplemental Material. Curves allowed for subsequent calculation and paired comparison of pH and chloride from tubules bathed for 60 minutes in isotonic conditions in standard bathing medium and again after 60 minutes in hypotonic conditions. Standard bathing medium consists of a 1:1 mix of Drosophila saline and Schneider medium (Life Technologies). Drosophila saline consists of 117.5 mM NaCl, 20 mM KCl, 2 mM CaCl2, 8.5 mM MgCl2, 10.2 mM NaHCO3, 4.3 mM NaH2PO4, 15 mM HEPES, and 20 mM glucose, pH 7.0. The components of Schneider medium are listed in Supplemental Material. Hypotonic bath consists of standard bathing medium (measured osmolality of 335 mosm/kg18) diluted with water at a ratio of 90 μl water to 300 μl standard bathing medium (measured osmolality of 257 mosm/kg18).
Measurement of Tubule DmWNK Activity
Kinase-dead rat SPAK was transgenically expressed in the principal cell of the tubule as a substrate for endogenous DmWNK. Phosphorylation was monitored by measuring the ratio of phosphorylated Ste20-related proline/alanine-rich kinase (p-SPAK) to total Ste20-related proline/alanine-rich kinase (t-SPAK) as determined by Western blotting. Antibodies used were p-SPAK (Ser373)/phospho-OSR1 (Ser325; catalog no. 07–2273, lot nos. 2689632 and 2840398; Millipore), t-SPAK (2281, lot no. 4; Cell Signaling Technology; anti-STK39 [2E10], 117982; Abcam or anti-STK39 [2E10], catalog no. GTX83543, lot no. 821703924; GeneTex), and actin (JLA20-s; DSHB). Tubules were bathed in standard bathing medium, hypotonic bathing medium, or isotonic bathing media with varying potassium concentrations (Supplemental Tables 1 and 2).
Real-Time Quantitative RT-PCR
D. melanogaster mouse protein 25 (DmMo25) and DmWNK transcript abundances were quantified in knockdown tubules using quantitative RT-PCR. Quantitative PCR was performed using the CFX Connect Real-Time PCR detecting system (Bio-Rad, Hercules, CA) with the iTaq Universal Probes Supermix (Bio-Rad). The TaqMan primer/probe sets for DmMo25 (Dm01822943_s1), DmWNK (Dm01792325_g1), and endogenous control RpL32 (Dm02143724_m1) were ordered from Invitrogen. For DmWNK, DNA standards for absolute quantification were made by PCR. Primers were designed outside the sequence targeted by the TaqMan primers: forward primer 5′ AGAAGCAACTCTCCAAGCAGCC 3′ and reverse primer 5′ CCAGCGGAACATTTTGAATAGG 3′. Four standard DNAs were used from 105 to 102 with tenfold dilution.
Measurement of Transepithelial Fluid Secretion and Potassium Flux
Ramsay assay to measure transepithelial fluid secretion, with measurement of secreted fluid potassium concentration using ion-specific electrodes, was performed as previously described.18,19,21,22 Renal tubules were dissected from adult females 4–6 days after eclosion and transferred to standard or hypotonic bathing medium (described above) under mineral oil. Secreted fluid droplet volume and potassium concentration were measured approximately 2 hours after initiation of the assay. Transepithelial potassium flux was calculated by multiplying fluid secretion rate (fluid droplet volume per secretion time) by potassium concentration. Potassium ionophore I cocktail B (Sigma-Aldrich) was used in the ion-specific electrodes, which were calibrated in each experiment against standards with varying potassium concentrations.
In Vitro Kinase Assays
In vitro kinase assays using purified Fray or constitutively active FrayT206E with or without purified DmMo25 and the N terminus of Drosophila NKCC (Ncc69; amino acids 1–204) were performed as previously described.18 Approximately 2.5 μg glutathione S-transferase (GST)-Fray or GST-FrayT206E with or without 10 μg GST-Ncc69[1–204] and 10 μg GST-DmMo25 was incubated in 50 μl kinase buffer containing 10 mM HEPES, pH 7.4, 1 mM dithiothreitol, 5 mM MgCl2, 10 μM ATP, and 10 μCi γ-32P-ATP (3000 Ci/mmol; Perkin-Elmer, Waltham, MA). After incubation for 1 hour at 30°C, incorporation of phosphate was determined after electrophoresis of samples and autoradiography.
Statistical Methods
All statistical analyses were performed in GraphPad Prism, version 7. Means were compared by t test or one- or two-way ANOVA as specified in the figures. Linear regression and interpolation of sigmoidal curves were also performed in GraphPad Prism.
Additional details are in Supplemental Material.
Results
DmWNK Is a Chloride-Sensitive Kinase
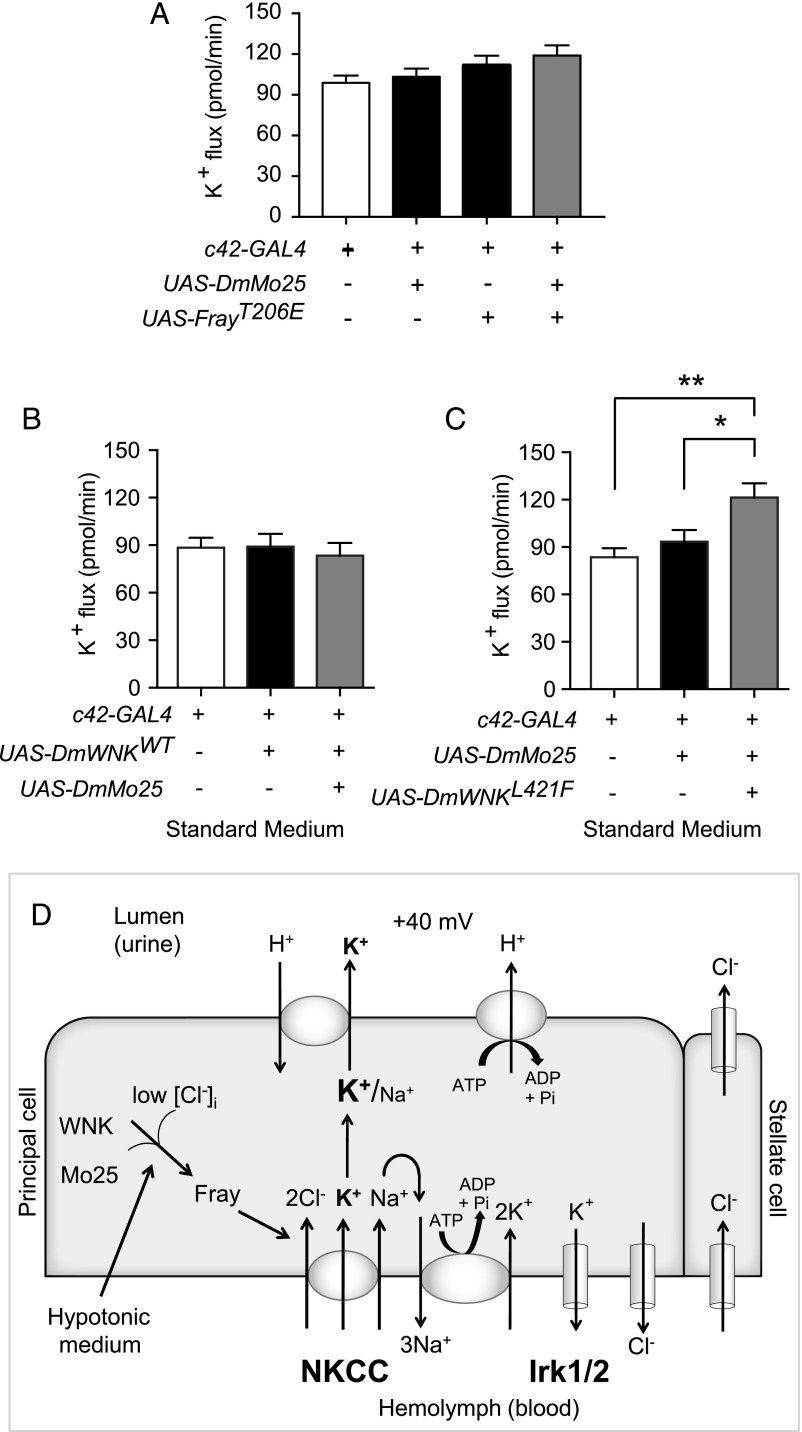
Because most cells undergo regulatory volume decrease after hypotonic swelling, with efflux of potassium and chloride,23 we hypothesized that intracellular chloride decreases in fly renal tubule epithelial cells bathed in hypotonic medium, resulting in WNK activation. The DmWNK kinase domain is 73% identical to human WNK1,24 and the chloride binding region, including amino acids that bind chloride in human WNK1,7 is perfectly conserved (Figure 1A). We, therefore, tested whether chloride inhibits DmWNK in vitro. We purified the DmWNK kinase domain (Supplemental Figure 1A) and allowed it to autophosphorylate in the presence of 50 or 150 mM chloride. We measured autophosphorylation on the activation loop Ser434 using mass spectrometry (Figure 1B, Supplemental Figure 1B) or the Pro-Q Diamond phosphoprotein stain (Figure 1C, Supplemental Figure 1C). In both cases, there was less autophosphorylation of DmWNK in high-chloride concentrations. Because autophosphorylation is required for kinase activation, this shows that chloride is a negative regulator of DmWNK.
Figure 1.
D. melanogaster With No Lysine kinase (DmWNK) autophosphorylation is inhibited by chloride in vitro. (A) Alignment of the chloride binding domain of human With No Lysine kinase 1 (HsWNK1) and DmWNK kinase domains shows conservation of this region. Chloride binding residues7 are highlighted. (B) DmWNK kinase domain (amino acids 261–534) was purified (Supplemental Figure 1A) and allowed to autophosphorylate in vitro in buffers containing 50 or 150 mM NaCl. Autophosphorylation at Ser 434 (Supplemental Figure 1B) was monitored at 0, 1, 2, and 4 hours using mass spectrometry. (C) DmWNK kinase domain autophosphorylation was performed as in B. The reaction was terminated, protein was electrophoresed using SDS-PAGE, and the Pro-Q Diamond phosphoprotein stain was used to determine kinase autophosphorylation followed by band densitometry for quantification. Three independent experiments were performed (Supplemental Figure 1C). Results were normalized to the 0-hour time point for each experiment. In this figure and subsequent figures, mean±SEM is shown; 50 versus 150 mM chloride values at each time point were compared using multiple t tests, with Holm–Sidak correction for multiple comparisons. Adjusted P values are shown. *P=0.03 at 1 hour; ***P=0.003 at 2 hours; ***P<0.001 at 4 hours.
Tubule WNK Activity Increases When Intracellular Chloride Decreases
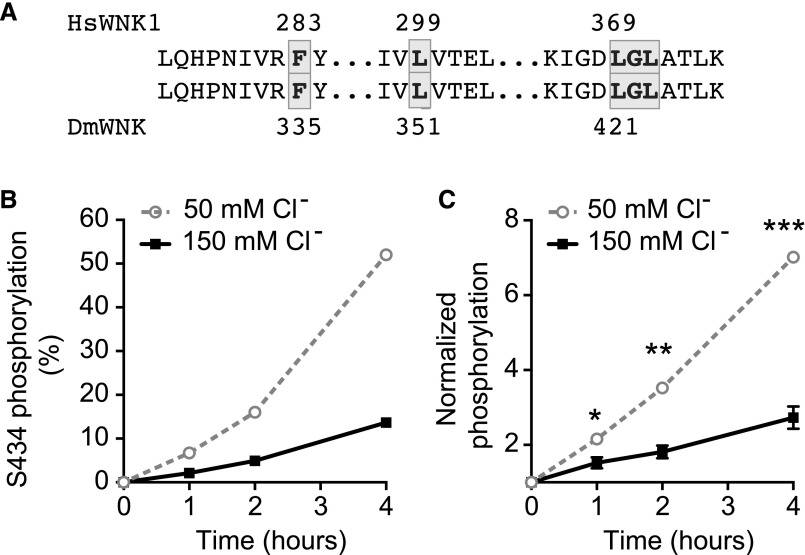
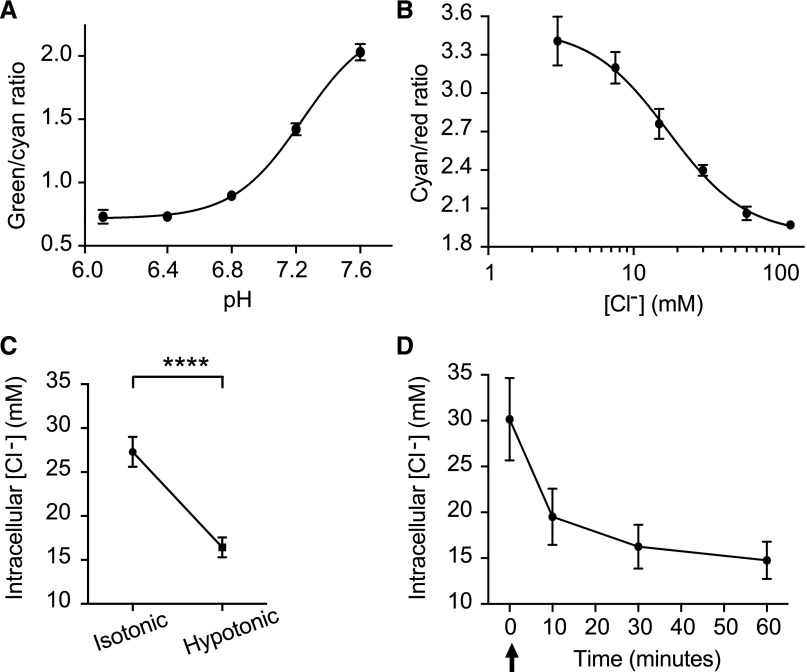
We used the transgenic sensor, ClopHensor,20,25 to measure intracellular chloride in renal tubule epithelial cells. ClopHensor simultaneously measures pH and chloride. The green-to-cyan ratio provides a readout of pH, whereas the cyan-to-red ratio provides a readout of chloride concentration. Sigmoidal calibration curves were obtained by equilibrating intracellular and extracellular pH or chloride using a cocktail of nigericin, valinomycin, carbonyl cyanide 3-chlorophenylhydrazone, and tributyltinchloride (Figure 2, A and B). We then used ClopHensor to measure intracellular pH and chloride concentration in tubules bathed for 1 hour in isotonic medium and then changed to hypotonic medium for 1 hour. Intracellular pH was 7.25±0.08 (mean±SEM) after 1 hour in isotonic medium and 7.26±0.07 after 1 hour in hypotonic medium, a nonsignificant difference (P=0.59, paired t test). Intracellular chloride concentration decreased from 27±1.7 mM in isotonic conditions to 16±1.1 mM in hypotonic conditions (P<0.001, paired t test) (Figure 2C). In a separate set of tubules, we measured intracellular pH and chloride concentrations after 1 hour in isotonic medium and then, 10, 30, and 60 minutes after changing to hypotonic medium. pH values were 7.40±0.01 in isotonic medium and 7.39±0.02, 7.39±0.02, and 7.42±0.02 at 10, 30, and 60 minutes in hypotonic medium, respectively (linear regression, y=0.0004397x+7.389; slope not significantly different from zero; P=0.24). Chloride concentration decreased from 30±4.5 mM in isotonic conditions to 20±3.1, 16±2.4, and 15±2.0 mM at 10, 30, and 60 minutes in hypotonic medium, respectively (Figure 2D). There was a greater decrease in intracellular chloride concentration when the tubules were bathed in hypotonic medium containing lower potassium and chloride concentrations, consistent with potassium and chloride efflux in hypotonic conditions (Supplemental Figure 2).
Figure 2.
Intracellular chloride concentration decreases in hypotonic bathing medium. (A) pH calibration curve for Drosophila renal tubules expressing the transgenic pH and chloride sensor, ClopHensor, in the principal cells of the main segment. The green-to-cyan ratio, which is pH sensitive, was measured at varying intracellular pH by equilibrating the tubule in solutions containing varying pH in the presence of nigericin, tributyltinchloride, carbonyl cyanide 3-chlorophenylhydrazone, and valinomycin. Genotype was w; c42-GAL4/UAS-ClopHensor c304. The c42-GAL4 driver is expressed in the principal cells of the tubule main segment62; n=9 cells were analyzed at each pH. (B) Chloride calibration curve obtained as in A except with variation of chloride concentration; n=9 cells were analyzed at each chloride concentration. (C) Intracellular chloride decreases in renal tubule principal cells bathed in hypotonic medium. Tubules from flies expressing ClopHensor in the tubule principal cells (w; c42-GAL4/UAS-ClopHensor c304) were bathed in standard bathing medium (isotonic) for 60 minutes followed by standard bathing medium diluted with water (hypotonic) for 60 minutes. The same cells were imaged in isotonic and hypotonic conditions, and the green-to-cyan and cyan-to-red ratios were measured to determine intracellular pH and chloride concentrations; n=21 cells from eight tubules. ****P<0.001, paired t test. (D) Tubules from ClopHensor-expressing flies were bathed in standard bathing medium for 60 minutes. At time 0 (arrow), tubule bathing solution was exchanged to the hypotonic bath, and the same tubules were reimaged at 10, 30, and 60 minutes of exposure to the hypotonic bathing solution. Green-to-cyan and cyan-to-red ratios were measured from the same cells in the tubule over time; n=9 cells from three tubules.
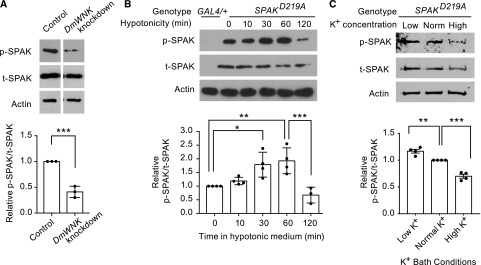
To examine whether endogenous tubule DmWNK is activated in low-chloride conditions, we transgenically expressed kinase-dead rat SPAK (SPAKD219A) in the principal cells of the tubule using the GAL4/UAS system.26 We reasoned that SPAK would serve as a phosphorylation substrate for DmWNK given the similarity of SPAK to Fray,27 and we used a kinase-dead SPAK to abolish SPAK autophosphorylation. We used antibodies to p-SPAK and t-SPAK substrate and used the p-SPAK-to-t-SPAK ratio as a readout of DmWNK activity. In tubules in which DmWNK was knocked down in the principal cells (Supplemental Figure 3), the p-SPAK-to-t-SPAK ratio decreased, indicating that SPAK indeed serves as a substrate for tubule DmWNK (Figure 3A, Supplemental Figure 4A). Normalized to control, transcript levels of DmWNK in the knockdown tubules were 0.40 (Supplemental Figure 3) as measured with real-time quantitative RT-PCR, correlating well with the p-SPAK-to-t-SPAK ratio in control versus knockdown tubules (0.41).
Figure 3.
With No Lysine kinase (WNK) activity in the tubule increases when intracellular chloride concentration decreases, and it is modulated by extracellular potassium. (A) Phosphorylation of rat Ste20-related proline/alanine-rich kinase (SPAK) reflects tubule D. melanogaster With No Lysine kinase (DmWNK) kinase activity. Kinase-dead (autophosphorylation-incompetent) rat SPAK was transgenically expressed in the Drosophila renal tubule principal cells as a substrate for endogenous DmWNK. Genotype was w; UAS-SPAKD219A/+; c42-GAL4/+. Phosphorylated Ste20-related proline/alanine-rich kinase (p-SPAK) and total Ste20-related proline/alanine-rich kinase (t-SPAK) were detected using specific antibodies, and the ratio of p-SPAK to t-SPAK was used as a measure of WNK activity. In the right column, SPAK phosphorylation was measured in tubules, in which endogenous Drosophila WNK was knocked down using RNA interference, resulting in decreased SPAK phosphorylation. Genotype was w; UAS-SPAKD219A UAS-WNKRNA interference/+; c42-GAL4/+. Three independent experiments were performed with 15 tubules per genotype pooled in each experiment (Supplemental Figure 4A), and the ratio from DmWNK knockdown tubules was normalized to the ratio from control tubules. ***P<0.001, unpaired t test. (B) Tubule DmWNK activity increases at 30 and 60 minutes in hypotonic bathing medium, and then, it is downregulated at 120 minutes. Tubules expressing kinase-dead rat SPAK (w; UAS-SPAKD219A/+; c42-GAL4/+) were bathed in standard bathing medium for 60 minutes and then transferred to hypotonic medium for the times shown. The first lane contains tubules expressing only c42-GAL4 without rat SPAK expression (w; c42-GAL4/+). Four independent experiments were performed (Supplemental Figure 4B). Results were analyzed using one-way ANOVA with multiple comparisons with Sidak correction. *P<0.05; **P<0.01; ***P<0.001. (C) Tubule DmWNK activity is increased in tubules bathed in low-potassium bathing medium (12 mM potassium) compared with normal potassium bathing medium (22 mM), and it is decreased in tubules bathed in high-potassium bathing medium (32 mM) (Supplemental Tables 1 and 2 show complete bath composition). Hemolymph potassium concentration in adult D. melanogaster is approximately 26 mM.63,64 The results were normalized to the normal potassium bath conditions. Measured osmolality of the three baths was the same (337, 337, and 338 mosm/kg, respectively), and it is equivalent to isotonic standard bathing medium (335 mosm/kg as measured in our previous study18). SPAK phosphorylation was measured in tubules expressing kinase-dead rat SPAK in the principal cells (w; UAS-SPAKD219A/+; c42-GAL4/+). Four independent experiments were performed (Supplemental Figure 4C). Results were analyzed using one-way ANOVA with multiple comparisons with Sidak correction. **P<0.01; ***P<0.001.
We next measured tubule DmWNK activity again using SPAKD219A phosphorylation as a readout after 1 hour in isotonic conditions and over a 1-hour time course in hypotonic conditions. In the absence of SPAKD219A expression, p-SPAK and t-SPAK bands were not observed, indicating antibody specificity. The p-SPAK-to-t-SPAK ratio was increased at 30 and 60 minutes, and then, it decreased at 120 minutes (Figure 3B, Supplemental Figure 4B). This indicates DmWNK activation in hypotonic conditions at 30 and 60 minutes, correlating with the nadir of intracellular chloride concentration (Figure 2D), with subsequent downregulation.
Previous studies have shown that changes in plasma potassium result in changes in NCC phosphorylation in the distal convoluted tubule, with low potassium stimulating NCC phosphorylation and high potassium having the opposite effect.28–33 NCC phosphorylation in response to low potassium is dependent on an intact WNK-SPAK/OSR1 signaling pathway and has been suggested to occur by changing intracellular chloride concentration.32,34,35 We, therefore, examined the effect of low- and high-potassium bathing medium (Supplemental Tables 1 and 2) without changes in osmolality or other ions on tubule DmWNK activity. DmWNK activity was increased in the low-potassium bath and decreased in the high-potassium bath (Figure 3C, Supplemental Figure 4C).
Relief of WNK Chloride Inhibition Is Not Sufficient to Increase Transepithelial Ion Transport
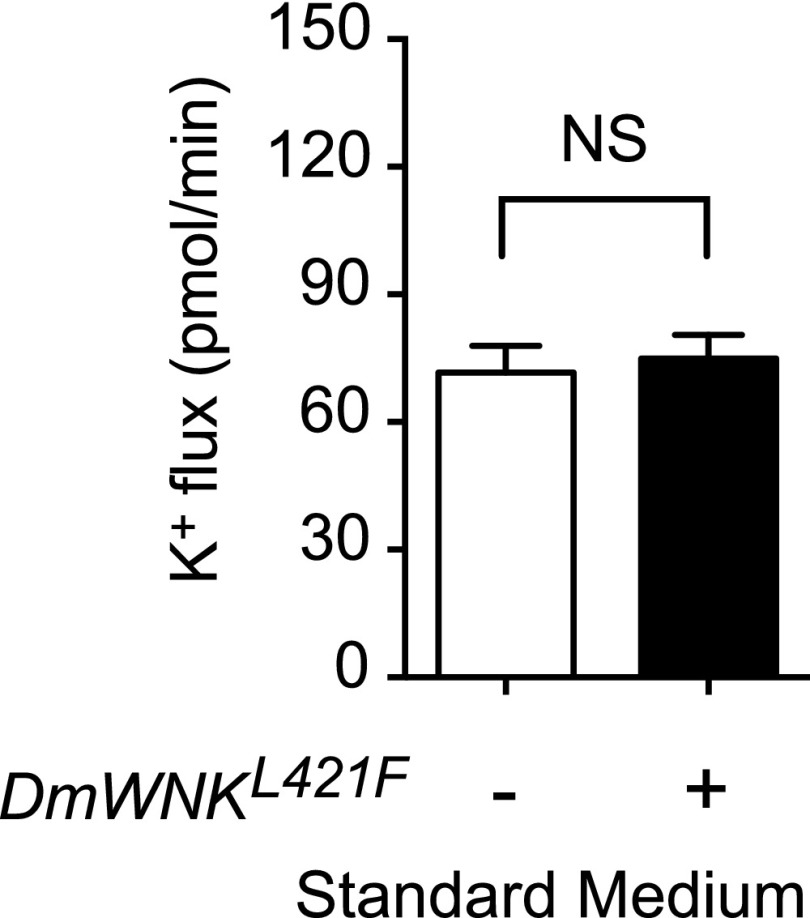
We previously synthesized chloride-insensitive WNK mutants on the basis of the structure of the chloride binding site.7 We could, therefore, determine whether relief of WNK chloride inhibition is sufficient to increase transepithelial potassium flux. Mutation of the chloride binding Leu369 to Phe in human WNK1 decreases chloride sensitivity in vitro.7 We made the homologous L421F mutation (Figure 1A) in DmWNK and expressed the mutant kinase in the principal cell during adulthood. No change in transepithelial potassium flux was seen in isotonic conditions, in which intracellular chloride concentrations are approximately 30 mM (Figure 4, Table 2). We, therefore, considered additional factors that could regulate WNK pathway signaling.
Figure 4.
Relieving chloride inhibition of With No Lysine kinase (WNK) is not sufficient to increase transepithelial ion transport. Human WNK1 with a leucine to phenylalanine mutation in the chloride binding amino acid, Leu 369, is resistant to chloride inhibition.7 The homologous mutation, L421F, was introduced into D. melanogaster With No Lysine kinase (DmWNK) and transgenically expressed in adult tubule principal cells. Tubules were bathed in isotonic standard bathing medium, and fluid secretion and transepithelial potassium flux were measured using the Ramsay assay and ion-specific electrodes (Table 2). Genotypes were control: w; tub-GAL80ts20/+; c42-GAL4/+ and experimental: w; tub-GAL80ts20/UAS-DmWNKL421F; c42-GAL4/+. Flies were reared at 18°C throughout development and shifted to 28°C for 2 days before testing to allow induction of the DmWNKL421F transgene in adult tubule principal cells; n=17–20 tubules per genotype. P=0.69, unpaired t test.
Table 2.
Secretion rate, [K+], and potassium flux
| Genotype | Secretion Rate, nl/min | [K+], mM | K+ Flux, pmol/min per Tubule | n | Slope |
|---|---|---|---|---|---|
| Chloride-insensitive WNK expression in adult principal cells, standard bathing medium | |||||
| w; tub-GAL80ts20/+; c42-GAL4/+ | 0.49±0.03 | 143±9.0 | 72±6.2 | 17 | 56.1 |
| w; tub-GAL80ts20/UAS-DmWNKL421F; c42-GAL4/+ | 0.54±0.03 | 137±5.8 | 75±5.6 | 20 | |
| Principal cell Mo25 knockdown | |||||
| w; c42-GAL4/+, standard bathing medium | 0.48±0.05 | 134±6.9 | 68±8.1 | 23 | 56.6 |
| w; c42-GAL4/+, hypotonic | 1.09±0.08 | 108±5.9 | 125±11.9 | 21 | |
| w; UAS-DmMo25RNAi/+; c42-GAL4/+, standard bathing medium | 0.41±0.04 | 138±4.5 | 58±6.5 | 22 | |
| w; UAS-DmMo25RNAi/+; c42-GAL4/+, hypotonic | 0.64±0.07 | 103±3.1 | 65±7.0 | 24 | |
| Principal cell Mo25 rescue, hypotonic | |||||
| w; c42-GAL4/+ | 0.75±0.04 | 137±3.7 | 104±5.8 | 39 | 51.7 |
| w; UAS-DmMo25RNAi/+; c42-GAL4/+ | 0.49±0.03 | 125±4.5 | 64±5.4 | 40 | |
| w; UAS-DmMo25RNAi/+; c42-GAL4/UAS-MmMo25 | 0.93±0.05 | 136±3.8 | 127±8.2 | 31 | |
| Principal cell overexpression of FrayT206E with Mo25, standard bathing medium | |||||
| w; tub-GAL80ts20/+; c42-GAL4/+ | 0.65±0.03 | 150±4 | 99±5.4 | 40 | 52.2 |
| w; tub-GAL80ts20 UAS-DmMo25/+; c42-GAL4/+ | 0.67±0.04 | 153±3 | 103±6.0 | 41 | |
| w; tub-GAL80ts20/UAS-FrayT206E; c42-GAL4/+ | 0.72±0.03 | 152±4 | 112±6.9 | 41 | |
| w; tub-GAL80ts20 UAS-DmMo25/UAS-FrayT206E; c42-GAL4/+ | 0.77±0.04 | 154±3 | 119±7.5 | 41 | |
| Principal cell overexpression of wild-type WNK ± Mo25, standard bathing medium | |||||
| w; tub-GAL80ts20/+; c42-GAL4/+ | 0.64±0.04 | 137±5.4 | 88±6.2 | 29 | 50.7 |
| w; tub-GAL80ts20 UAS-DmWNKWT/+; c42-GAL4/+ | 0.68±0.05 | 131±5.6 | 89±8.0 | 26 | |
| w; tub-GAL80ts20 UAS-DmWNKWT/UAS-DmMo25; c42-GAL4/+ | 0.66±0.05 | 126±8.2 | 83±8.0 | 24 | |
| Principal cell overexpression of chloride-insensitive WNK with Mo25, standard bathing medium | |||||
| w; tub-GAL80ts20/+; c42-GAL4/+ | 0.62±0.04 | 136±3.9 | 84±5.7 | 24 | 58.7 |
| w; tub-GAL80ts20 UAS-DmMo25/+; c42-GAL4/+ | 0.67±0.05 | 138±5.1 | 93±7.4 | 25 | |
| w; tub-GAL80ts20 UAS-DmMo25/UAS-DmWNKL421F; c42-GAL4/+ | 0.84±0.07 | 145±1.9 | 121±9.0 | 24 |
All values shown are mean±SEM. Slope is the mean slope per tenfold difference in [K+] of the electrodes used for each experiment. Note that potassium flux was calculated separately for each tubule analyzed, and therefore, the value of mean secretion rate × mean [K+] may differ slightly from measured potassium flux due to rounding. K+, potassium; DmWNK, D. melanogaster with no lysine kinase; DmMo25, D. melanogaster mouse protein 25; MmMo25, M. musculus mouse protein 25; RNAi, RNA interference; WT, wild type.
Mo25 Is Required for Maximal Transepithelial Ion Transport
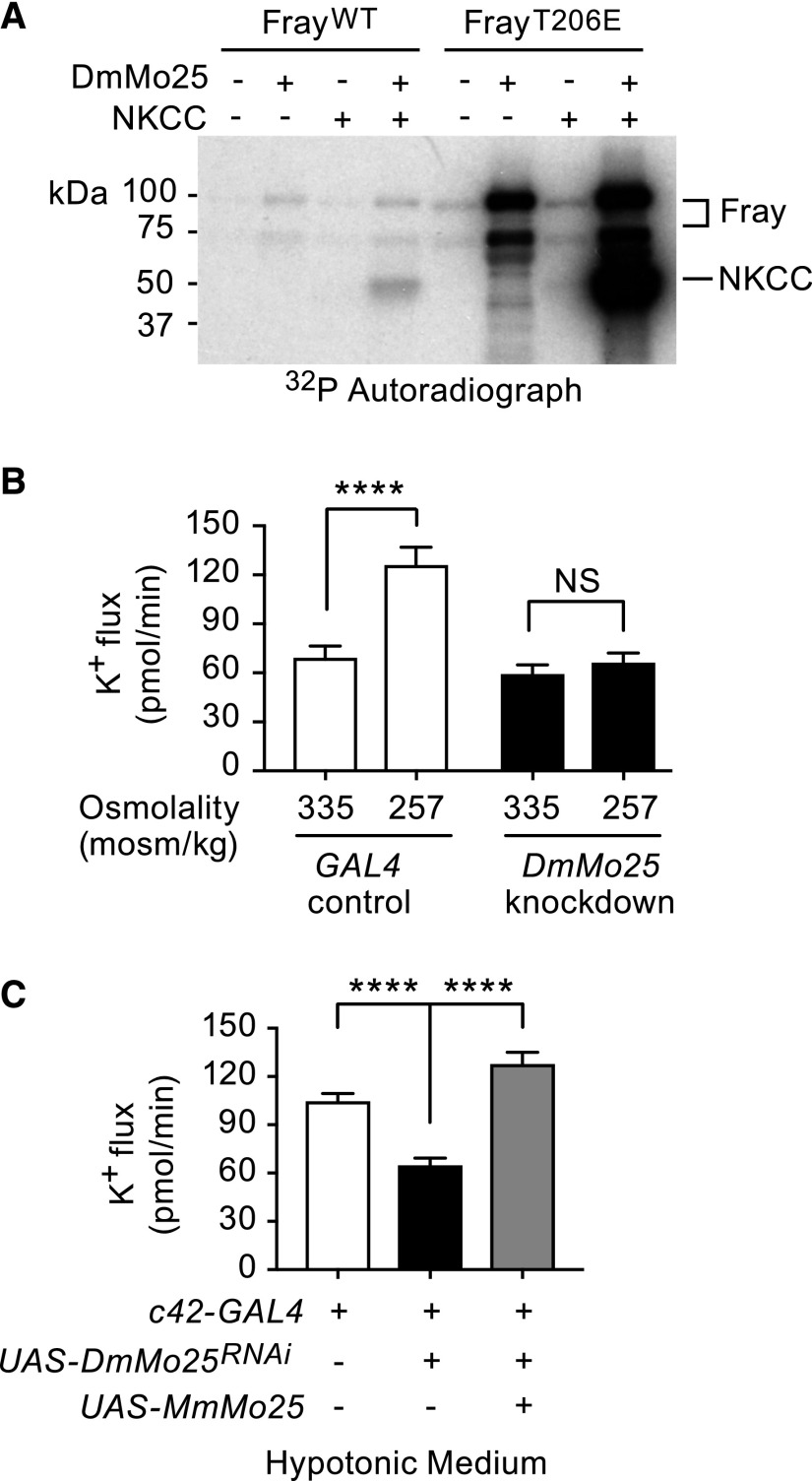
The scaffold protein Mo25/calcium binding protein 39 stimulates SPAK/OSR1 activity in vitro and seems to work synergistically with WNKs.9 Amino acids important for SPAK/OSR1 interactions with Mo25, including sites A-D Glu, Val, Tyr, and Trp/Glu/Trp in SPAK/OSR1 and Met260 in Mo25, are conserved in Fray and DmMo25,9,36 and Fray and DmMo25 interact in Drosophila developmental processes.37 We examined whether DmMo25 stimulates Fray activity by performing in vitro kinase assays using purified Drosophila proteins, DmMo25, Fray, and the N terminus of NKCC (encoded by Ncc69). DmMo25 stimulated autophosphorylation of wild-type Fray and had a more dramatic effect on FrayT206E, in which the T-loop threonine phosphorylated by WNK is mutated to a phosphomimicking glutamic acid. We have previously shown that FrayT206E phosphorylates the N terminus of fly NKCC in the absence of DmWNK.18 In the absence of WNK, wild-type Fray did not phosphorylate NKCC, whereas some phosphorylation of NKCC was seen when Mo25 was added to the reaction. Finally, Mo25 strongly stimulated FrayT206E phosphorylation of NKCC, suggesting synergistic effects of WNK and Mo25 in the activation of Fray (Figure 5A, Supplemental Figure 5).
Figure 5.
Mouse protein 25 (Mo25) stimulates Fray activity in vitro, and it is required for tubule transepithelial transport in stimulated (hypotonic) conditions. (A) Mo25 stimulates autophosphorylation of Fray (Drosophila Ste20-related proline/alanine-rich kinase [SPAK]/oxidative stress response 1 [OSR1] homolog; upper two bands) as well as sodium-potassium 2 chloride cotransporter (NKCC) phosphorylation (lower band) in vitro. The effect is more pronounced with FrayT206E, in which the T-loop Thr that is the target of With No Lysine kinase (WNK) phosphorylation is mutated to a phosphomimicking Glu. In vitro kinase assays were performed with purified Drosophila Fray, D. melanogaster mouse protein 25 (DmMo25), and the N terminus of Drosophila NKCC, Ncc69 (amino acids 1–204). A representative gel is shown of three independent experiments (Supplemental Figure 5). WT, wild type. (B) DmMo25 is required in the tubule principal cell for hypotonicity-stimulated transepithelial potassium flux. Transepithelial potassium flux was measured in control tubules (w; c42-GAL4/+) and DmMo25 knockdown tubules (w; UAS-DmMo25RNA interference (RNAi)/+; c42-GAL4/+) bathed in isotonic standard bathing medium or hypotonic medium (standard bathing medium diluted with water). Two-way ANOVA revealed significant effects of genotype (P<0.001), condition (P<0.001), and interaction (P<0.01). In a Sidak multiple comparisons test, hypotonic conditions resulted in a significant increase in potassium flux in control tubules but no change in DmMo25 knockdown tubules (P=0.82). There was no significant difference between control and DmMo25 knockdown tubules in isotonic conditions (P=0.67), whereas a significant difference was observed in hypotonic conditions (P<0.001). ****Adjusted P value <0.001. (C) Expression of mammalian Mo25 rescues the decreased potassium flux phenotype of DmMo25 knockdown tubules. Transepithelial potassium flux was measured in hypotonic conditions in control tubules (w; c42-GAL4/+), DmMo25 principal cell knockdown tubules (w; UAS-DmMo25RNAi/+; c42-GAL4/+), and DmMo25 knockdown tubules expressing mouse Mo25 (w; UAS-DmMo25RNAi/+; UAS-MmMo25/c42-GAL4). One-way ANOVA (P<0.001). In a Tukey multiple comparison test comparing all genotypes with one another, adjusted P values were <0.001 for the difference between w; c42-GAL4/+ and w; UAS-DmMo25RNAi/+; c42-GAL4/+ and also for the difference between w; UAS-DmMo25RNAi/+; c42-GAL4/+ and w; UAS-DmMo25RNAi/+; UAS-MmMo25/c42. Adjusted P value was 0.04 for the difference between w; c42-GAL4/+ and w; UAS-DmMo25RNAi/+; UAS-MmMo25/c42-GAL4. ****Adjusted P value <0.001.
To examine a role for Mo25 in transepithelial ion transport, we examined fluid secretion and transepithelial potassium flux in control and DmMo25 knockdown tubules in isotonic and hypotonic conditions. DmMo25 null flies are not viable,37 necessitating the use of RNA interference (RNAi) knockdown. Normalized to control, transcript levels of DmMo25 in principal cell knockdown tubules were 0.34 as measured with real-time quantitative RT-PCR (Supplemental Figure 3). DmMo25 knockdown had no effect on transepithelial potassium flux in isotonic conditions, but it abolished the hypotonic stimulation of potassium flux (Figure 5B, Table 2). The decrease in ion flux was rescued by expression of mouse MmMo25 (which is resistant to the DmMo25 RNAi, due to sequence differences), indicating that the DmMo25 RNAi phenotype was not due to off-target effects (Figure 5C, Table 2).
Relief of WNK Chloride Inhibition Together with Mo25 Is Sufficient to Increase Transepithelial Ion Transport
We next asked whether an increase in Mo25 is sufficient to increase transepithelial ion flux. Overexpression of Mo25 in the adult tubule principal cell did not increase ion flux in isotonic standard bathing medium (Figure 6, A and C, Table 2). We have previously shown that FrayT206E rescues ion flux in DmWNK knockdown tubules in standard medium18 and as shown above, that Mo25 stimulates the kinase activity of FrayT206E in vitro. However, overexpression of FrayT206E with or without Mo25 did not stimulate ion flux in standard medium (Figure 6A, Table 2). Wild-type DmWNK expression with or without Mo25 also did not stimulate ion flux (Figure 6B, Table 2). However, coexpression of Mo25 with chloride-insensitive DmWNKL421F increased transepithelial potassium flux in isotonic conditions (approximately 30 mM chloride) (Figure 6C, Table 2), recapitulating the increased fluid secretion and potassium flux seen in hypotonic conditions.
Discussion
We have previously shown that, as in the mammalian nephron, WNK-SPAK/OSR1 signaling regulates transepithelial ion transport in the Drosophila renal epithelium. We further showed that transepithelial ion flux increases when the tubules are bathed in hypotonic medium and that this stimulation of ion flux is dependent on DmWNK, the SPAK/OSR1 homolog Fray, and the tubule NKCC.18 Here, we examined the mechanism by which stimulation occurs. We show that intracellular chloride falls acutely in tubules exposed to hypotonic medium, decreasing to approximately 15 mM over the course of 1 hour. DmWNK, which we show is chloride sensitive in vitro, is simultaneously activated at 30 and 60 minutes. However, experiments with a chloride-insensitive DmWNK mutant suggested that relief of chloride inhibition was not sufficient to activate the pathway in this context, prompting us to examine another regulator of WNK-SPAK/OSR1 signaling, Mo25. We show a requirement for Mo25 in conditions of stimulated transepithelial ion transport and show that relief of chloride inhibition together with increased Mo25 can stimulate ion flux (Figure 6D).
Recent studies have suggested an important role for NCC in potassium homeostasis. NCC is dephosphorylated in animals consuming a high-potassium diet and phosphorylated in animals (and humans) consuming a low-potassium diet.28–32,38,39 Similarly, maneuvers that alter serum potassium through changes in aldosterone signaling or epithelial sodium channel activity also result in altered NCC phosphorylation, and these effects can be reversed by normalizing serum potassium through dietary manipulation.40–44 NCC activity influences downstream delivery of sodium to the aldosterone-sensitive distal nephron, where sodium-dependent potassium secretion occurs, and it also has effects on nephron remodeling.45
Increased NCC phosphorylation in conditions of low dietary potassium is dependent on WNK-SPAK/OSR1 signaling.32,34,35,46 Ellison and colleagues32 proposed that, as serum potassium concentration decreases, the basolateral membrane potential of the distal convoluted tubule epithelial cell hyperpolarizes, chloride exits through basolateral channels, and intracellular chloride concentration falls. This is expected to relieve chloride inhibition of WNK signaling, increasing NCC phosphorylation.32 This model is supported by studies in cultured cells, mathematic modeling,32 and studies in isolated distal convoluted tubules.33 Our results are supportive of this model. We show for the first time that a change in bathing medium acutely lowers intracellular chloride and that it simultaneously activates WNK signaling and increases transepithelial ion transport.18 Interestingly, in the mammalian distal convoluted tubule, intracellular chloride has been measured at approximately 10 mM,47 and it has been modeled to vary between approximately 10 and 20 mM under varying potassium conditions,32 similar to the intracellular chloride concentration that we observe in the fly renal tubule 30 and 60 minutes after bathing in hypotonic medium when WNK is activated. We also observed increased WNK activity in tubules bathed in low potassium in isotonic conditions and conversely, a decrease in WNK activity in tubules bathed in high-potassium isotonic conditions. Given the known potassium and chloride conductance of the basolateral membrane of the Drosophila tubule,48 these maneuvers are expected to lower and raise intracellular chloride, respectively, similar to what may occur in the distal convoluted tubule.
Chloride may not be the only physiologically relevant regulator of the pathway, however, as also suggested by other studies.33 Mo25 is expressed in mammalian nephron segments, in which WNKu-SPAK/OSR1 signaling regulates transepithelial ion transport, including the thick ascending limb and the distal convoluted tubule.12 This is the first demonstration of a role for Mo25 in transepithelial ion transport. Unlike DmWNK and Fray, which are required in the fly renal tubule for normal transepithelial ion transport in both isotonic and hypotonic conditions,18 DmMo25 knockdown does not affect ion flux in isotonic conditions, but it is only required in conditions of stimulated ion transport. This is consistent with in vitro biochemical data, showing that SPAK and OSR1 are able to phosphorylate downstream substrates, such as NCC, NKCC1, and NKCC2, after activation by WNK phosphorylation49–54 but that this activity is greatly stimulated by Mo25.9 Our in vitro data confirm that this is also the case with the Drosophila proteins. Work by Delpire and colleagues11 suggests that the mechanism by which Mo25 stimulates SPAK/OSR1 activity is through transautophosphorylation of SPAK/OSR1 dimers. Our data also suggest that the cooperative interaction of WNK and Mo25 is required for full pathway activation, because the gain-of-function phenotype seen in tubules expressing chloride-insensitive WNKL421F and Mo25 is not recapitulated by coexpression of phosphomimetic FrayT206E and Mo25. Indeed, the crystal structure of SPAKT243D, which presumably mimics that of WNK-phosphorylated SPAK, shows a partially activated conformation.55 One possibility is that both WNK and Mo25 have scaffolding functions that allow for more complete SPAK/OSR1 activation. Alternatively, WNK4 and Mo25 have been shown to activate NKCC1 and NKCC2 independently of SPAK/OSR110 and could also have additional downstream targets.
There may be differential requirements for Mo25 in different contexts of WNK-SPAK/OSR1 signaling depending on the degree of pathway activation required. Indeed, our finding that DmMo25 knockdown decreased transepithelial ion flux in stimulated hypotonic conditions but not in baseline isotonic conditions suggests that this is the case. In contrast, in HEK293 cells, knockdown of Mo25α decreased NKCC1 activity in both baseline and hypotonic low-chloride conditions.9 In mammals, the effect of Mo25 may partially depend on which WNK paralog is most active in a given cell type or tissue. Mammalian WNK1, -3, and -4 are differentially sensitive to chloride concentration in vitro8 and in heterologous cells,8,56 and it is possible that the relative importance of Mo25 will also depend on intracellular chloride concentrations as well as other yet to be discovered WNK regulators. Future studies will explore this issue. How Mo25 itself is regulated is also unknown. Furthermore, in some conditions, WNK4 in the presence of Mo25 directly activates NKCC1 and NKCC2,10 and our own data (Figure 5) and the data of others9 indicate that SPAK/OSR1/Fray can phosphorylate NKCC independently of WNK in the presence of Mo25. Additional studies are needed to define requirements for the various components of this signaling cassette, which may afford greater flexibility in varying physiologic conditions.
Our study has limitations. We did not measure cell volume, although inspection of ClopHensor-expressing tubule epithelial cells did not reveal obvious changes in cell size after shifting the tubules to hypotonic conditions, suggesting regulatory volume mechanisms are operative. Work by Miyazaki et al.57 in Xenopus A6 renal epithelial cells, in which cell volume and intracellular chloride were simultaneously measured with exposure to hypotonic medium, showed that there is an initial dilutional decrease in intracellular chloride concentration followed by a more gradual decrease due to regulatory volume decrease. Our time course of intracellular chloride concentration after hypotonic exposure qualitatively resembles their data, suggesting that similar mechanisms are at play. However, we have not identified the channels or transporters that may be responsible for the decrease in chloride concentration.
In summary, our study shows roles for intracellular chloride and the scaffold protein Mo25 in the regulation of WNK-SPAK/OSR1–dependent ion flux in a transporting renal epithelium. This has implications for pathway activation in the face of low dietary potassium, which is common in the United States58 and worldwide59 and contributes to the global epidemic of hypertension, cardiovascular morbidity, and mortality.59–61
Disclosures
None.
Supplementary Material
Acknowledgments
The authors wish to thank Julian Dow and Shireen Davies for the generous gift of fly lines as well as the Bloomington Drosophila Stock Center at Indiana University (supported by National Institutes of Health grant P40OD018537) and the Vienna Drosophila Resource Center for fly stocks. We thank Chou-Long Huang, Johannes Bischof, Konrad Basler, and the Drosophila Genomics Resource Center (supported by National Institutes of Health grant 2P40OD010949) for plasmids. We thank Diana Lim for assistance with figure preparation.
This project was supported by the National Institutes of Health: training grant 5T32DA007290 (to D.S.), grant EY10199 (to H.K.), grant DK110358 (to A.R.R. and E.J.G.), grant DK091316 (to A.R.R.), and grant DK106350 (to A.R.R.). In addition, this project was supported by the Welch Foundation, grant I1128 (to E.J.G.) and the American Heart Association, grant 16CSA28530002 (to E.J.G. and A.R.R.).
Parts of this manuscript were submitted in abstract form at the American Society of Nephrology Kidney Week (Sun et al., J Am Soc Nephrol 26: 189A, 2015) and Experimental Biology (Rodan et al., FASEB J 31: 856.13, 2017).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “WNKs on the Fly,” on pages 1347–1349.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017101091/-/DCSupplemental.
References
- 1.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, et al.: Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Zhang J, Khanna A, Hochdörfer T, Shang Y, Kahle KT: The WNK-SPAK/OSR1 pathway: Master regulator of cation-chloride cotransporters. Sci Signal 7: re3, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Shekarabi M, Zhang J, Khanna AR, Ellison DH, Delpire E, Kahle KT: WNK kinase signaling in ion homeostasis and human disease. Cell Metab 25: 285–299, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Hadchouel J, Ellison DH, Gamba G: Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol 78: 367–389, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Haas M, Forbush B 3rd: The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol 62: 515–534, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Russell JM: Sodium-potassium-chloride cotransport. Physiol Rev 80: 211–276, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ: Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7: ra41, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang C-L, Ellison DH: Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filippi BM, de los Heros P, Mehellou Y, Navratilova I, Gourlay R, Deak M, et al.: MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. EMBO J 30: 1730–1741, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponce-Coria J, Markadieu N, Austin TM, Flammang L, Rios K, Welling PA, et al.: A novel Ste20-related proline/alanine-rich kinase (SPAK)-independent pathway involving calcium-binding protein 39 (Cab39) and serine threonine kinase with no lysine member 4 (WNK4) in the activation of Na-K-Cl cotransporters. J Biol Chem 289: 17680–17688, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponce-Coria J, Gagnon KB, Delpire E: Calcium-binding protein 39 facilitates molecular interaction between Ste20p proline alanine-rich kinase and oxidative stress response 1 monomers. Am J Physiol Cell Physiol 303: C1198–C1205, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimm PR, Taneja TK, Liu J, Coleman R, Chen Y-Y, Delpire E, et al.: SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SH, et al.: Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol 274: R1039–R1049, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Linton SM, O’Donnell MJ: Contributions of K+:Cl- cotransport and Na+/K+-ATPase to basolateral ion transport in malpighian tubules of Drosophila melanogaster. J Exp Biol 202: 1561–1570, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Rheault MR, O’Donnell MJ: Analysis of epithelial K(+) transport in Malpighian tubules of Drosophila melanogaster: Evidence for spatial and temporal heterogeneity. J Exp Biol 204: 2289–2299, 2001 [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH: Separate control of anion and cation transport in malpighian tubules of Drosophila Melanogaster. J Exp Biol 199: 1163–1175, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Cabrero P, Terhzaz S, Romero MF, Davies SA, Blumenthal EM, Dow JAT: Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci U S A 111: 14301–14306, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Schellinger JN, Huang C-L, Rodan AR: Hypotonicity stimulates potassium flux through the WNK-SPAK/OSR1 kinase cascade and the Ncc69 sodium-potassium-2-chloride cotransporter in the Drosophila renal tubule. J Biol Chem 289: 26131–26142, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodan AR, Baum M, Huang C-L: The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am J Physiol Cell Physiol 303: C883–C894, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arosio D, Ricci F, Marchetti L, Gualdani R, Albertazzi L, Beltram F: Simultaneous intracellular chloride and pH measurements using a GFP-based sensor. Nat Methods 7: 516–518, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Baum M, Huang C-L, Rodan AR: Two inwardly rectifying potassium channels, Irk1 and Irk2, play redundant roles in Drosophila renal tubule function. Am J Physiol Regul Integr Comp Physiol 309: R747–R756, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schellinger JN, Rodan AR: Use of the Ramsay assay to measure fluid secretion and Ion flux rates in the Drosophila melanogaster Malpighian tubule [published online ahead of print November 15, 2015]. J Vis Exp doi:10.3791/53144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann EK, Lambert IH, Pedersen SF: Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193–277, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Rodan AR, Jenny A: WNK kinases in development and disease. Curr Top Dev Biol 123: 1–47, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhtarov M, Liguori L, Waseem T, Rocca F, Buldakova S, Arosio D, et al.: Calibration and functional analysis of three genetically encoded Cl(-)/pH sensors. Front Mol Neurosci 6: 9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brand AH, Perrimon N: Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Leiserson WM, Harkins EW, Keshishian H: Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron 28: 793–806, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S: Expression and phosphorylation of the Na+-Cl- cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, et al.: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA: Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitzthum H, Seniuk A, Schulte LH, Müller ML, Hetz H, Ehmke H: Functional coupling of renal K+ and Na+ handling causes high blood pressure in Na+ replete mice. J Physiol 592: 1139–1157, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, et al.: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penton D, Czogalla J, Wengi A, Himmerkus N, Loffing-Cueni D, Carrel M, et al.: Extracellular K+rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl--dependent and independent mechanisms. J Physiol 594: 6319–6331, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wade JB, Liu J, Coleman R, Grimm PR, Delpire E, Welling PA: SPAK-mediated NCC regulation in response to low-K+ diet. Am J Physiol Renal Physiol 308: F923–F931, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferdaus MZ, Barber KW, López-Cayuqueo KI, Terker AS, Argaiz ER, Gassaway BM, et al.: SPAK and OSR1 play essential roles in potassium homeostasis through actions on the distal convoluted tubule. J Physiol 594: 4945–4966, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Feng M, Shi Z, Hao Q, Song X, Wang W, et al.: Structural and biochemical insights into the activation mechanisms of germinal center kinase OSR1. J Biol Chem 289: 35969–35978, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Izumi Y, Matsuzaki F: The GC kinase Fray and Mo25 regulate Drosophila asymmetric divisions. Biochem Biophys Res Commun 366: 212–218, 2008 [DOI] [PubMed] [Google Scholar]
- 38.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AHJ, et al.: K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl- cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Frindt G, Houde V, Palmer LG: Conservation of Na+ vs. K+ by the rat cortical collecting duct. Am J Physiol Renal Physiol 301: F14–F20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, et al.: Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrier R, Boscardin E, Malsure S, Sergi C, Maillard MP, Loffing J, et al.: Severe salt-losing syndrome and hyperkalemia induced by adult nephron-specific knockout of the epithelial sodium channel α-subunit. J Am Soc Nephrol 27: 2309–2318, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canonica J, Sergi C, Maillard M, Klusonova P, Odermatt A, Koesters R, et al.: Adult nephron-specific MR-deficient mice develop a severe renal PHA-1 phenotype. Pflugers Arch 468: 895–908, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, et al.: Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol 26: 425–438, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boscardin E, Perrier R, Sergi C, Maillard M, Loffing J, Loffing-Cueni D, et al.: Severe hyperkalemia is rescued by low-potassium diet in renal βENaC-deficient mice. Pflugers Arch 469: 1387–1399, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Grimm PR, Coleman R, Delpire E, Welling PA: Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2597–2606, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castañeda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, Arroyo-Garza I, Vázquez N, Moreno E, et al.: Modulation of NCC activity by low and high K(+) intake: Insights into the signaling pathways involved. Am J Physiol Renal Physiol 306: F1507–F1519, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beck FX, Dörge A, Rick R, Schramm M, Thurau K: Effect of potassium adaptation on the distribution of potassium, sodium and chloride across the apical membrane of renal tubular cells. Pflugers Arch 409: 477–485, 1987 [DOI] [PubMed] [Google Scholar]
- 48.Ianowski JP, O’Donnell MJ: Basolateral ion transport mechanisms during fluid secretion by Drosophila Malpighian tubules: Na+ recycling, Na+:K+:2Cl- cotransport and Cl- conductance. J Exp Biol 207: 2599–2609, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Richardson C, Rafiqi FH, Karlsson HKR, Moleleki N, Vandewalle A, Campbell DG, et al.: Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Dowd BFX, Forbush B: PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J Biol Chem 278: 27347–27353, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, et al.: WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685–42693, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Anselmo AN, Earnest S, Chen W, Juang Y-C, Kim SC, Zhao Y, et al.: WNK1 and OSR1 regulate the Na+, K+, 2Cl- cotransporter in HeLa cells. Proc Natl Acad Sci U S A 103: 10883–10888, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HKR, et al.: Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J 397: 223–231, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson C, Sakamoto K, de los Heros P, Deak M, Campbell DG, Prescott AR, et al.: Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci 124: 789–800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor CA 4th, Juang Y-C, Earnest S, Sengupta S, Goldsmith EJ, Cobb MH: Domain-swapping switch point in Ste20 protein kinase SPAK. Biochemistry 54: 5063–5071, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bazúa-Valenti S, Chávez-Canales M, Rojas-Vega L, González-Rodríguez X, Vázquez N, Rodríguez-Gama A, et al.: The effect of WNK4 on the Na+-Cl- cotransporter is modulated by intracellular chloride. J Am Soc Nephrol 26: 1781–1786, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyazaki H, Shiozaki A, Niisato N, Marunaka Y: Physiological significance of hypotonicity-induced regulatory volume decrease: Reduction in intracellular Cl- concentration acting as an intracellular signaling. Am J Physiol Renal Physiol 292: F1411–F1417, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Cogswell ME, Zhang Z, Carriquiry AL, Gunn JP, Kuklina EV, Saydah SH, et al.: Sodium and potassium intakes among US adults: NHANES 2003-2008. Am J Clin Nutr 96: 647–657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, et al.; PURE Investigators : Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 371: 601–611, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP: Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ 346: f1378, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al.; PURE Investigators : Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 371: 612–623, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Rosay P, Davies SA, Yu Y, Sözen MA, Kaiser K, Dow JA: Cell-type specific calcium signalling in a Drosophila epithelium. J Cell Sci 110: 1683–1692, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Naikkhwah W, O’Donnell MJ: Salt stress alters fluid and ion transport by Malpighian tubules of Drosophila melanogaster: Evidence for phenotypic plasticity. J Exp Biol 214: 3443–3454, 2011 [DOI] [PubMed] [Google Scholar]
- 64.MacMillan HA, Hughson BN: A high-throughput method of hemolymph extraction from adult Drosophila without anesthesia. J Insect Physiol 63: 27–31, 2014 [DOI] [PubMed] [Google Scholar]
- 65.Dow JA, Maddrell SH, Görtz A, Skaer NJ, Brogan S, Kaiser K: The malpighian tubules of Drosophila melanogaster: A novel phenotype for studies of fluid secretion and its control. J Exp Biol 197: 421–428, 1994 [DOI] [PubMed] [Google Scholar]
- 66.McGuire SE, Mao Z, Davis RL: Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE 2004: pl6, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M, et al.: A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.