Abstract
Background B-type ephrins are membrane-bound proteins that maintain tissue function in several organs. We previously reported that ephrin-B1 is localized at the slit diaphragm of glomerular podocytes. However, the function of ephrin-B1 at this location is unclear.
Methods We analyzed the phenotype of podocyte-specific ephrin-B1 knockout mice and assessed the molecular association of ephrin-B1 and nephrin, a key molecule of the slit diaphragm, in HEK293 cells and rats with anti-nephrin antibody-induced nephropathy.
Results Compared with controls, ephrin-B1 conditional knockout mice displayed altered podocyte morphology, disarrangement of the slit diaphragm molecules, and proteinuria. Ephrin-B1 expressed in HEK293 cells immunoprecipitated with nephrin, which required the basal regions of the extracellular domains of both proteins. Treatment of cells with an anti-nephrin antibody promoted the phosphorylation of nephrin and ephrin-B1. However, phosphorylation of ephrin-B1 did not occur in cells expressing a mutant nephrin lacking the ephrin-B1 binding site or in cells treated with an Src kinase inhibitor. The phosphorylation of ephrin-B1 enhanced the phosphorylation of nephrin and promoted the phosphorylation of c-Jun N-terminal kinase (JNK), which was required for ephrin-B1–promoted cell motility in wound-healing assays. Notably, phosphorylated JNK was detected in the glomeruli of control mice but not ephrin-B1 conditional knockout mice. In rats, the phosphorylation of ephrin-B1, JNK, and nephrin occurred in the early phase (24 hours) of anti-nephrin antibody-induced nephropathy.
Conclusions Through interactions with nephrin, ephrin-B1 maintains the structure and barrier function of the slit diaphragm. Moreover, phosphorylation of ephrin-B1 and, consequently, JNK are involved in the development of podocyte injury.
Keywords: cell adhesion, Cell Signaling, glomerular filtration barrier, nephrin, podocyte, proteinuria
It is now understood that proteinuria in several kinds of kidney diseases results from the dysfunction of a slit diaphragm bridging the neighboring foot processes of the glomerular visceral epithelial cell (podocyte).1–3 The slit diaphragm is a unique cell–cell junction and is reported to be a variant of tight junction. In the past two decades, some molecules have been identified as critical components of the slit diaphragm. However, its precise molecular composition and the mechanism regulating the structure and function of the slit diaphragm are not well understood.
Ephrin and Eph are membrane-bound proteins that function as receptor-ligand pairs. Ephrins are divided into two subclasses.4 B-type ephrins have a transmembrane domain followed by a short cytoplasmic region containing four tyrosine residues and a PDZ domain-binding motif at the C-terminal end. B-type ephrins are expressed in several tissues, and ephrin-B plays a critical role in maintaining tissue function in several major organs.5–9 However, few studies analyzing the role of ephrin-B in the kidney have been reported. We previously reported that ephrin-B1 was expressed at the slit diaphragm and interacted with nephrin, a key molecule of the slit diaphragm.7 However, the role of ephrin-B1 at the slit diaphragm and the precise functional association with nephrin were unclear.
Here, we show that podocyte-specific ephrin-B1 conditional knockout (CKO) mice displayed alteration of the podocyte morphology, disarrangement of the slit diaphragm molecules, and proteinuria. Analyses with the HEK cell expression system revealed that nephrin-binding ephrin-B1 interacted with nephrin via the basal regions of extracellular domain. Nephrin-binding ephrin-B1 was phosphorylated by extracellular nephrin stimulation. The phosphorylation of ephrin-B1 was detected in rat glomeruli of the nephrotic model, induced by anti-nephrin antibody injection. Further, nephrin-binding ephrin-B1 regulated the phosphorylation of JNK in glomeruli independently of nephrin phosphorylation. Taken together, it is conceivable that ephrin-B1 in the podocyte is essential for maintaining the integrity of the glomerular filtration barrier and plays a critical role as a signal molecule controlling the podocyte functions.
Methods
Animal Experiments
All animal experiments conformed to the National Institutes of Health Guide for the care and Use of Laboratory Animals. All animal experiments were conducted in compliance with the protocol, which was reviewed by the Institutional Animal Care and Use Committee and approved by the President of Niigata University (permit no. 27, Niigata University Res.441–1). The method for the generation of the podocyte-specific ephrin-B1 CKO mice and the method for the induction of the rat nephrotic model are described in the Supplemental Material.
RT-PCR, Immunofluorescence, Western Blot Analysis, and Morphologic Analysis
Semiquantitative RT-PCR with isolated glomerular RNA was performed basically according to the method described previously.10–12 Tissues were homogenized, and then total RNA was extracted (n=3). The immunofluorescence studies with CKO mice and rats were performed basically according to the method previously reported.13–16 The antibodies used in this study are summarized in Supplemental Table 1. The stainings were evaluated in 30 glomeruli of each mouse, in a blinded manner. Western blot analysis with the transfected cells and isolated glomeruli of ephrin-B1 CKO mice was performed basically according to the method described previously.17 The width of foot process was evaluated by the method of Bechtel et al.18 Five randomly selected glomeruli of five mice each were evaluated.
Assays with HEK293 Cell Transfection and Immunoprecipitation Assay
HEK293 cells were transfected with the plasmid constructs by calcium phosphate method. The information of the plasmid constructs is provided in the Supplemental Material. Immunoprecipitation assays were performed basically according to the method described previously.19,20 In vitro phosphorylation assay was performed basically according to the method described previously.21 Transfected cells were stimulated with mouse anti-nephrin antibody22 or EphB2-Fc for 10 minutes. The phosphorylation was analyzed by immunoblotting. To analyze the pathways of the phosphorylation, the transfected cells were pretreated with PP2 or SP600125 before stimulation with mouse anti-nephrin antibody or EphB2-Fc. Wound-healing assays with HEK293 cells were performed basically according to the method of Cho et al.21 The gap area was quantified using Image J software. The percentage of wound closure was determined as (gap area at 0 hours−gap area at 24 hours)/gap area at 0 hours×100.
Cultured Podocytes
A conditionally immortalized mouse podocyte cell line was kindly donated by Dr. Peter Mundel (Albert Einstein College of Medicine, Bronx, NY). Cultivation of the cell was conducted as described previously.23
Human Kidney Specimens
The kidney specimens were obtained from a patient with minimal change nephrotic syndrome (MCNS) treated at the Department of Pediatrics, Niigata City General Hospital. After the routine studies had been performed, the samples were stored at −70°C until further use. As a control, we used the normal part of the kidney, obtained via nephrectomy. This study was approved by the Ethics Committee of the Niigata City General Hospital. Informed consent was obtained from the patient.
Results
CKO Mice with Podocyte Ephrin-B1 Deleted after Birth Show Abnormal Proteinuria, Morphologic Abnormality, and Disarrangement of the Slit Diaphragm Molecules
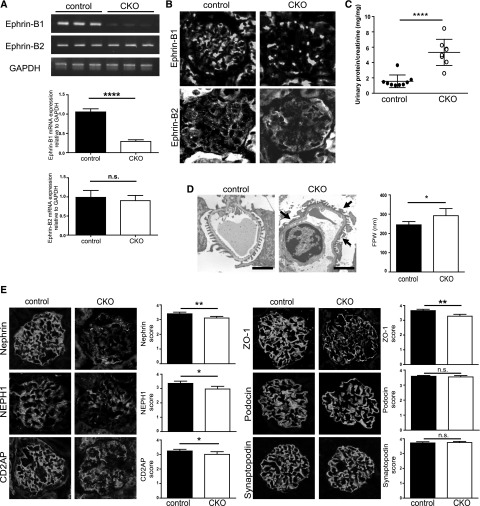
To investigate the function of ephrin-B1 in podocytes, we generated Nphs2-CreERT2; ephrin-B1loxP/loxP mice. To delete the expression of ephrin-B1, mice at 9–11 weeks of age were injected intraperitoneally with tamoxifen for 10 days, and the phenotype was analyzed on day 20 after the first injection. The efficiency of ephrin-B1 deletion was confirmed by RT-PCR and immunohistochemical analyses (Figure 1, A and B). Neither glomerular mRNA of ephrin-B2 nor immunostaining of ephrin-B2 in the glomeruli were increased in these mice (Figure 1, A and B). The CKO mice did not show body weight loss, abnormality of the kidney size, or abnormality of the size or number of glomeruli (Supplemental Table 2). The CKO mice showed proteinuria (Figure 1C). Foot process effacement was evident in the CKO mice (Figure 1D). The immunofluorescence study revealed that the stainings of nephrin, NEPH1, CD2AP, and ZO-1 in glomeruli were altered in ephrin-B1 CKO mice (Figure 1E). The quantitative analyses revealed that the stainings of nephrin, a key molecule for maintaining the barrier function of the slit diaphragm, and ZO-1, one of the critical cytoplasmic molecules at the slit diaphragm, were clearly altered (P<0.01). On the other hand, Western blotting revealed that the protein amounts of these molecules were not evidently changed (Supplemental Figure 1). These observations suggest that the localization of the slit diaphragm molecules was altered in the CKO mice, and that ephrin-B1 plays a role for maintaining the proper localization of the slit diaphragm molecules. It is conceivable that the altered localizations of these molecules contributed to the development of proteinuria in the ephrin-B1 CKO mice.
Figure 1.
Podocyte-specific ephrin-B1 CKO mice showed proteinuria. (A) RT-PCR analysis of the isolated glomeruli of ephrin-B1 CKO mice. Glomerular mRNA expression of ephrin-B1 was clearly decreased. Ephrin-B2 mRNA expression was not altered in ephrin-B1 CKO mice. (n=3 control mice, n=3 CKO mice; ****P<0.001, two-tailed t test). (B) Representative immunofluorescence findings of ephrin-B1 and ephrin-B2 in kidney sections. No ephrin-B1 staining in glomeruli was detected. The ephrin-B2 staining was unchanged. (C) Urinary protein-to-creatinine ratio. The CKO mice showed proteinuria. (n=9 control mice, n=8 CKO mice; ****P<0.001, two-tailed t test). (D) Electron microscopy and the semiquantitative data of the foot process width (FPW). The foot process effacement was evident in the CKO mice (arrows). Scale bars, 1 µm; * P<0.05, two-tailed t test. (E) Representative immunofluorescence findings and the data from the semiquantitative analysis of the stainings of the slit diaphragm–associated molecules. The stainings of nephrin and ZO-1 in glomeruli were clearly altered in the CKO mice. The alteration was also detected in the staining of NEPH1 and CD2AP. The stainings of podocin and synaptopodin were not altered in the CKO mice. (n=7 control mice, n=5 CKO mice; *P<0.05; **P<0.01, Mann–Whitney U test.).
Ephrin-B1 and Ephrin-B2 Appear in Presumptive Podocytes of the S-Shaped Body Stage, and the Expression of Ephrin-B2 Decreases with Maturation, Whereas Ephrin-B1 Expression Becomes Evident in the Matured Glomeruli
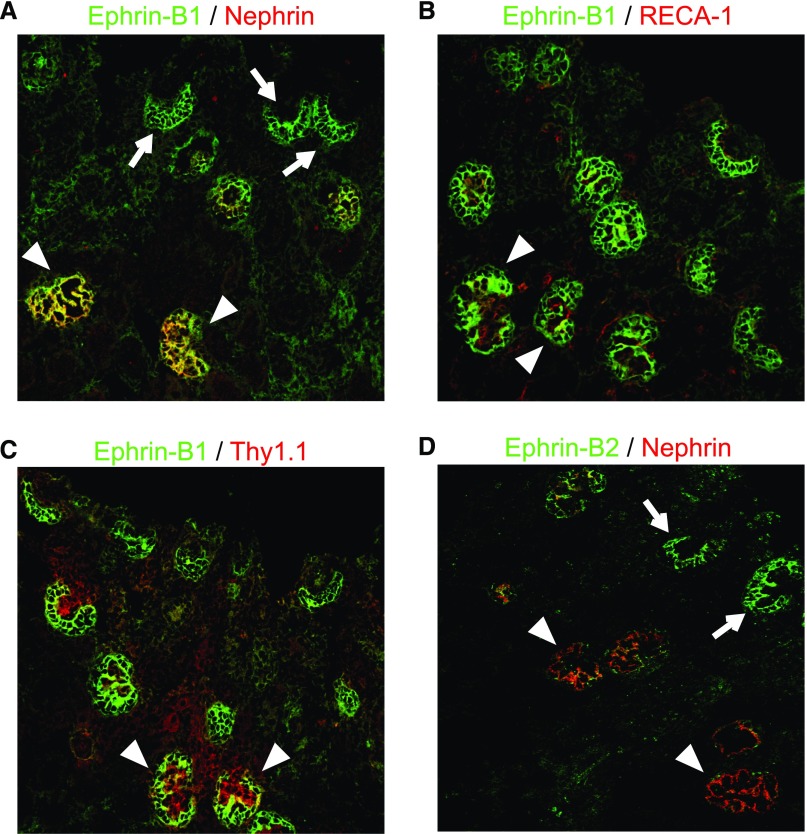
Next, we analyzed the developmental expression of ephrin-B1 with neonatal rat kidney sections. Ephrin-B1 appeared in presumptive podocytes of the S-shaped body stage when nephrin, a podocyte slit diaphragm component, was not yet detected (Figure 2A). The clear staining of ephrin-B1 was also detected in glomeruli of the capillary loop and the maturing stages. In these stages, the stainings of RECA-1, an endothelial cell marker, and Thy1.1, a mesangial cell marker, were clearly detected. The ephrin-B1 was colocalized with nephrin and was clearly separate from RECA-1 and Thy1.1 (Figure 2, B and C) in these stages. The expression of ephrin-B2 was also detected in presumptive podocytes of the S-shaped body stage (Figure 2D). However, in contrast to ephrin-B1, the ephrin-B2 expression became weak in the capillary loop and the maturing stages. The observations suggested that both ephrin-B1 and ephrin-B2 in podocytes play an important role in the early developing stage of glomerulogenesis, and ephrin-B1 plays a dominant role in the late maturing stage.
Figure 2.
Ephrin-B1 appeared in presumptive podocytes earlier than nephrin. Immunofluorescence findings with anti–ephrin-B1 (A–C) and anti–ephrin-B2 (D) antibodies (shown in green) costained with an anti-nephrin antibody, anti-RECA1 (endothelial cell marker) and anti-Thy1.1 (mesangial cell marker) (shown in red). (A) The staining of ephrin-B1 was first detected in presumptive podocytes of the early S-shaped body stage, when nephrin was not yet detected (arrows). The ephrin-B1 staining became evident in the capillary loop-stage glomeruli, when clear staining of nephrin was detected (arrowheads). (B and C) The stainings of RECA-1 and Thy1.1 were detected in glomeruli of the capillary loop and the maturing stages. The ephrin-B1 staining was clearly separate from RECA-1 and Thy1.1 (arrowheads). (D) The expression of ephrin-B2 was also detected in presumptive podocytes of the S-shaped body stage (arrows). The ephrin-B2 expression became to be weak in the capillary loop and the maturing stages (arrowheads).
Ephrin-B1 Interacted with Nephrin via the Basal Regions of the Extracellular Domain
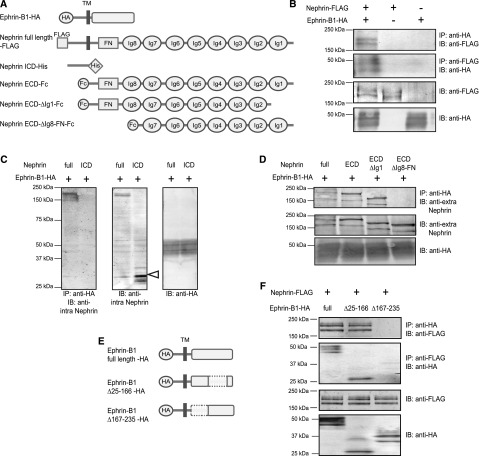
Next, we analyzed how ephrin-B1 interacted with nephrin, using the HEK293 cell transfection system. The immunoprecipitation assay revealed that ephrin-B1 did not interact with a truncated nephrin in which the extracellular site was deleted (Figure 3C). However, ephrin-B1 did interact with a truncated nephrin in which the intracellular site was deleted (Figure 3D). We found that ephrin-B1 did not interact with the truncated nephrin lacking the basal region of the extracellular domain containing a fibronectin-like site and the eighth Ig (Figure 3D), and that ephrin-B1 interacted with the truncated nephrin lacking the tip region from the N-end to the first Ig site. We also observed that nephrin did not interact with the truncated ephrin-B1 lacking the basal region of the extracellular domain (amino acids 167–235) (Figure 3F). These results show that ephrin-B1 interacted with nephrin via the basal regions of the extracellular domain.
Figure 3.
Ephrin-B1 interacts with nephrin via the basal regions of the extracellular domains. (A) Scheme of constructs of the full-length ephrin-B1, the full-length nephrin, and truncated nephrin. The full-length ephrin-B1 was tagged with the HA epitope. The full-length nephrin was tagged with the FLAG epitope, and the intracellular domain (ICD) of nephrin was tagged with the His epitope at the N-terminus. The extracellular domain (ECD) of nephrin, the ECD of nephrin lacking the Ig-like domain 1 (ECD-ΔIg1) of nephrin, and the ECD of nephrin lacking the Ig-like domain 8 and fibronectin type III domain (ECD-ΔIg8-FN) were coexpressed with IgG Fc at the C-terminus. (B) Ephrin-B1 interaction with nephrin. The nephrin band was detected in the precipitate with the anti-HA antibody from the lysate of HEK293 cells cotransfected with the full-length ephrin-B1-HA and the full-length nephrin-FLAG. The ephrin-B1 band was detected in the precipitate with anti-FLAG. (C) Ephrin-B1 does not interact with the ICD of nephrin. In this analysis, the antibody recognizing the ICD of nephrin was used. No bands were detected with anti-nephrin antibody in the anti-HA antibody precipitate from the lysate of the cells cotransfected with the full-length ephrin-B1 and the ICD of nephrin. The band of ICD of nephrin was detected in the cell lysates without immunoprecipitation (IP) (white arrowhead). (D) Ephrin-B1 interaction with the ECD containing the Ig-like domain 8 and fibronectin type III domain of nephrin. The antibody recognizing the center area of the ECD of nephrin was used. The nephrin band was detected in the anti-HA antibody precipitate from the lysates of the cells cotransfected with full-length ephrin-B1 and full-length nephrin. The nephrin bands were also detected in precipitates from the lysate of cells cotransfected with full-length ECD or ECD-ΔIg1. No nephrin bands were detected in precipitates from the lysate of cells cotransfected with ECD-ΔIg8-FN. (E) Scheme of constructs of the truncated ephrin-B1. The ephrin-B1 lacking the region of 25–166 amino acids, a EphB binding region (Δ25–166), and that lacking the region of 167–235 amino acids, a basal region of ECD (Δ167–235), were tagged with the HA epitope. (F) Nephrin interaction with the basal region of ephrin-B1. The nephrin band was detected in the precipitate with the anti-HA antibody from the lysate of HEK293 cells cotransfected with full-length nephrin-FLAG and ephrin-B1-Δ25–166-HA. The ephrin-B1 band was detected in the precipitate with anti-FLAG. No nephrin bands were detected in the precipitate with anti-HA from the lysate of cells cotransfected with full-length nephrin-FLAG and ephrin-B1-Δ167–235-HA. No ephrin-B1 bands were detected in the precipitate with anti-FLAG.
Nephrin and Nephrin-Binding Ephrin-B1 Are Phosphorylated by Extracellular Nephrin Stimulation
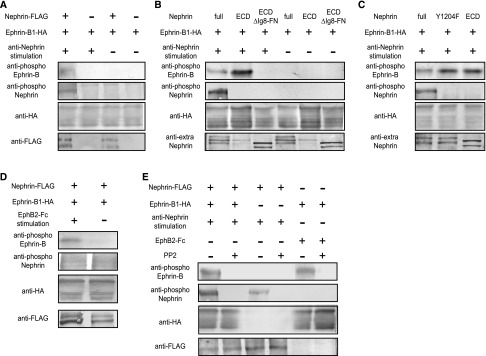
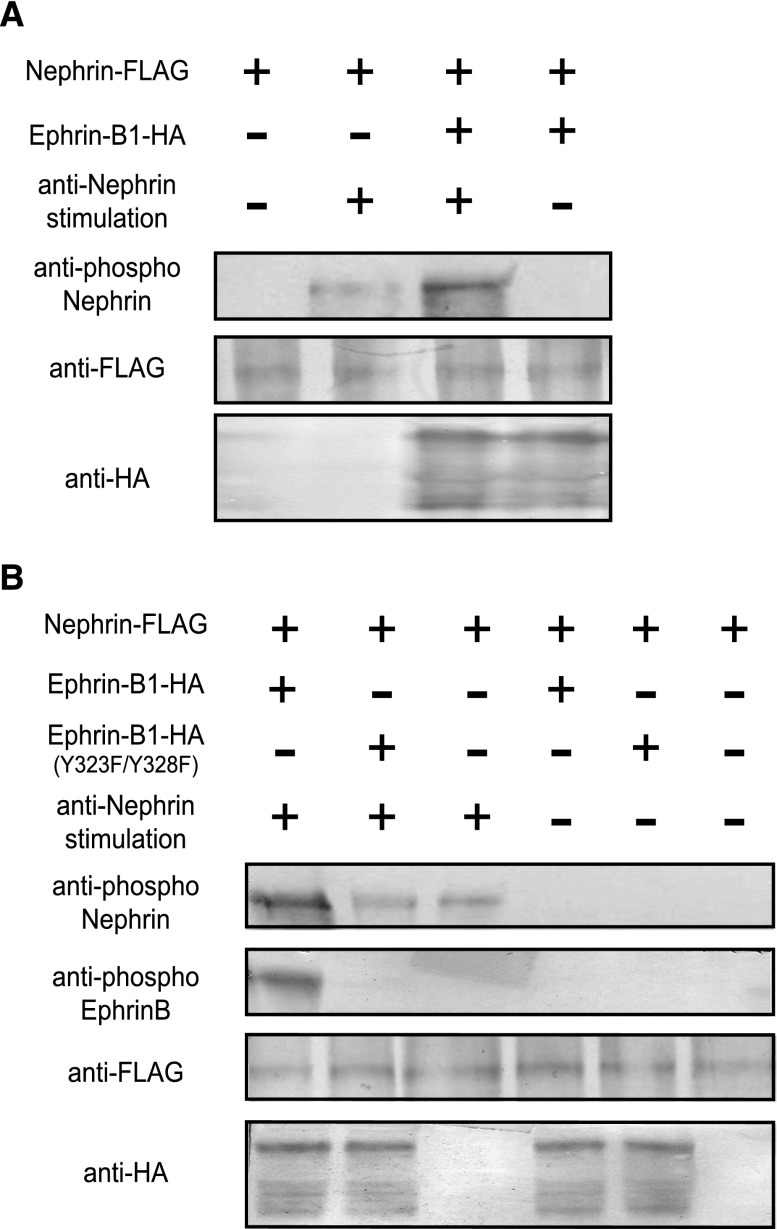
Next, the functional association of ephrin-B1 and nephrin was analyzed. The HEK cell assay revealed that the ephrin-B1 cotransfected with nephrin was phosphorylated by nephrin stimulation with anti-nephrin antibody (Figure 4A). By contrast, nephrin cotransfected with ephrin-B1 was not phosphorylated by ephrin-B1 stimulation with Eph (Figure 4D). It was observed that ephrin-B1 cotransfected with truncated nephrin lacking the ephrin-B1 binding site (Ig8-FN) was not phosphorylated by anti-nephrin antibody stimulation (Figure 4B), certifying that the binding of these molecules was required for induction of the phosphorylation of ephrin-B1 transmitted by the stimulated nephrin. The phosphorylation of nephrin-binding ephrin-B1 caused by nephrin stimulation was clearly inhibited by PP2, an Src kinase inhibitor (Figure 4E).
Figure 4.
Nephrin and nephrin-binding ephrin-B1 is phosphorylated by extracellular nephrin stimulation. (A) Ephrin-B1 coexpressed with nephrin on HEK293 cells was phosphorylated by stimulation with anti-nephrin antibody. (B) Ephrin-B1 coexpressed with the truncated nephrin lacking the ephrin-B1-binding site (Ig8-FN) was not phosphorylated by the anti-nephrin antibody. Ephrin-B1 coexpressed with the extracellular domain of nephrin (ECD) was evidently phosphorylated by stimulation with anti-nephrin antibody. (C) Ephrin-B1 cotransfected with the truncated nephrin in which the tyrosine residue (Y1204) was substituted by phenylalanine was phosphorylated more evidently by stimulation with the anti-nephrin antibody than ephrin-B1 cotransfected with wild-type nephrin. (D) Nephrin coexpressed with ephrin-B1 on HEK293 cells was not phosphorylated by ephrin-B1 stimulation with EphB2-Fc. (E) Phosphorylation of nephrin-binding ephrin induced by the anti-nephrin antibody was inhibited by PP2, indicating that the phosphorylation was Src kinase–dependent. The phosphorylation of nephrin caused by anti-nephrin and ephrin-B1 caused by EphB2-Fc were also Src kinase–dependent.
Ephrin-B1 cotransfected with the truncated nephrin in which the tyrosine residue (Y1204) was substituted by phenylalanine was phosphorylated more evidently by stimulation with anti-nephrin antibody than was the ephrin-B1 cotransfected with wild-type nephrin (Figure 4C). The results show that nephrin phosphorylation lowered the level of the phosphorylation of nephrin-binding ephrin-B1. We also found that the phosphorylation of nephrin induced by stimulation with anti-nephrin antibody was promoted if nephrin was bound to ephrin-B1 (Figure 5A). The promotion of nephrin phosphorylation was not detected if nephrin was bound to the ephrin-B1 in which the tyrosine residues (Y323/Y328) were substituted by phenylalanine. This shows that phosphorylated ephrin-B1 enhanced nephrin phosphorylation (Figure 5B).
Figure 5.
Nephrin phosphorylation is promoted by phosphorylated ephrin-B1. (A) Nephrin coexpressed with ephrin-B1 was phosphorylated by stimulation with anti-nephrin antibody more evidently than nephrin expressed alone without ephrin-B1. (B) The phosphorylation of nephrin was not promoted by the coexpression of the truncated ephrin-B1 when the tyrosine residues were substituted with phenylalanine (Y323F, Y328F).
Phosphorylation of Nephrin-Binding Ephrin-B1 Enhances JNK Phosphorylation Independently of Nephrin Signaling and Promotes Cell Motility
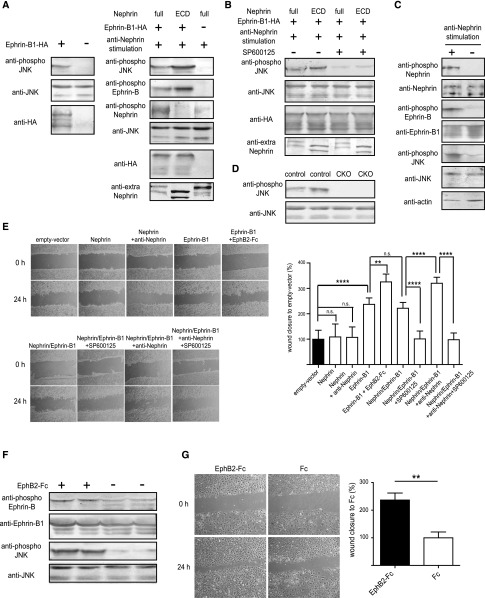
Next, we analyzed the role of the phosphorylated ephrin-B1 induced by the nephrin stimulation. We previously showed that ephrin-B1 phosphorylated JNK even if ephrin-B1 was not phosphorylated, and that the phosphorylated nephrin-binding ephrin-B1 induced by the anti-nephrin antibody promoted JNK phosphorylation (Figure 6A). By contrast, nephrin did not participate in the JNK phosphorylation even if it was phosphorylated (Figure 6A). We also observed that ephrin-B1 and nephrin were phosphorylated and JNK phosphorylation was promoted in the glomeruli, which were isolated from normal rats and were treated with anti-nephrin antibody in vitro (Figure 6C). In the Western blot analysis, no bands of the phosphorylated JNK were detected in the glomerular lysates of the ephrin-B1 CKO mice, although the band was detected in the glomerular lysates of the control mice (Figure 6D). This finding suggests that ephrin-B1 plays a major role in the phosphorylation of JNK in glomeruli.
Figure 6.
Phosphorylation of nephrin-binding ephrin-B1 enhances JNK phosphorylation independently of nephrin signaling and promotes cell motility. (A) Transfection of ephrin-B1 induced JNK phosphorylation. Phosphorylation of nephrin-binding ephrin-B1 induced by the anti-nephrin antibody promoted the phosphorylation of JNK. The ephrin-B1 that bound to the truncated nephrin lacking the intracellular domain was phosphorylated more evidently than the ephrin-B1 bound to the full-length nephrin, and the phosphorylated ephrin-B1 induced the JNK phosphorylation more effectively. Nephrin without coexpression of ephrin-B1 was also phosphorylated by the anti-nephrin antibody, but the phosphorylated nephrin did not induce JNK phosphorylation. (B) The JNK phosphorylation detected by the anti–phospho-JNK antibody was clearly inhibited by SP600125, a JNK inhibitor. (C) Ephrin-B1 and nephrin were phosphorylated and JNK phosphorylation was promoted in the glomeruli, which were isolated from normal rats and were treated with anti-nephrin antibody in vitro. (D) No bands for the phosphorylated JNK were detected in glomerular lysates of ephrin-B1 CKO mice, although the clear band was detected in that of the control mice. (E) Wound-healing assay with HEK cells transfected with nephrin and/or ephrin-B1. Representative images of migrating cells obtained at 0 or 24 hours after wound formation are shown in the left panel. The migration was quantified by calculating the cell-covered area using Image J software (right panel). The cell motility was promoted in the HEK cells transfected with ephrin-B1 but not in those transfected with nephrin. The phosphorylated nephrin did not promote cell motility. The ephrin-B1 phosphorylated by the EphB2-Fc promoted cell motility more than the unphosphorylated ephrin-B1. Nephrin-binding ephrin-B1 phosphorylated by the anti-nephrin antibody also promoted cell motility. The cell motility of the HEK cells transfected with ephrin-B1 was suppressed by SP600125, a JNK inhibitor to basal level of the cells without any transfection. (F) Ephrin-B in cultured podocytes was phosphorylated by the stimulation with EphB2-Fc, and the phosphorylated ephrin-B1 promoted JNK phosphorylation. (G) Wound-healing assay with cultured podocytes. Ephrin-B1 phosphorylated by EphB2-Fc promoted cell motility. The assays were repeated at least four times. **P<0.01; ****P<0.001, two-tailed t test.
Wound-healing assays with HEK cells transfected with nephrin and/or ephrin-B1 showed that ephrin-B1 promoted the cell motility, whereas nephrin did not participate in the promotion of cell motility. We found that the phosphorylated nephrin-binding ephrin-B1 induced by the anti-nephrin antibody promoted cell motility (Figure 6E). The degree of motility of the HEK cells was associated with the degree of JNK phosphorylation. The cell motility of the HEK cells transfected with ephrin-B1 was suppressed to normal level by SP600125, a JNK inhibitor (Figure 6E). We also found that the phosphorylation of ephrin-B1 and the consequent JNK phosphorylation promoted cell motility with cultured podocytes (Figure 6, F and G). These results indicate that ephrin-B1 plays a pivotal role in the regulation of cell motility by regulating JNK phosphorylation.
Ephrin-B1, JNK, and Nephrin Are Phosphorylated in the Rat Nephrotic Model Induced by Anti-Nephrin Antibody Injection
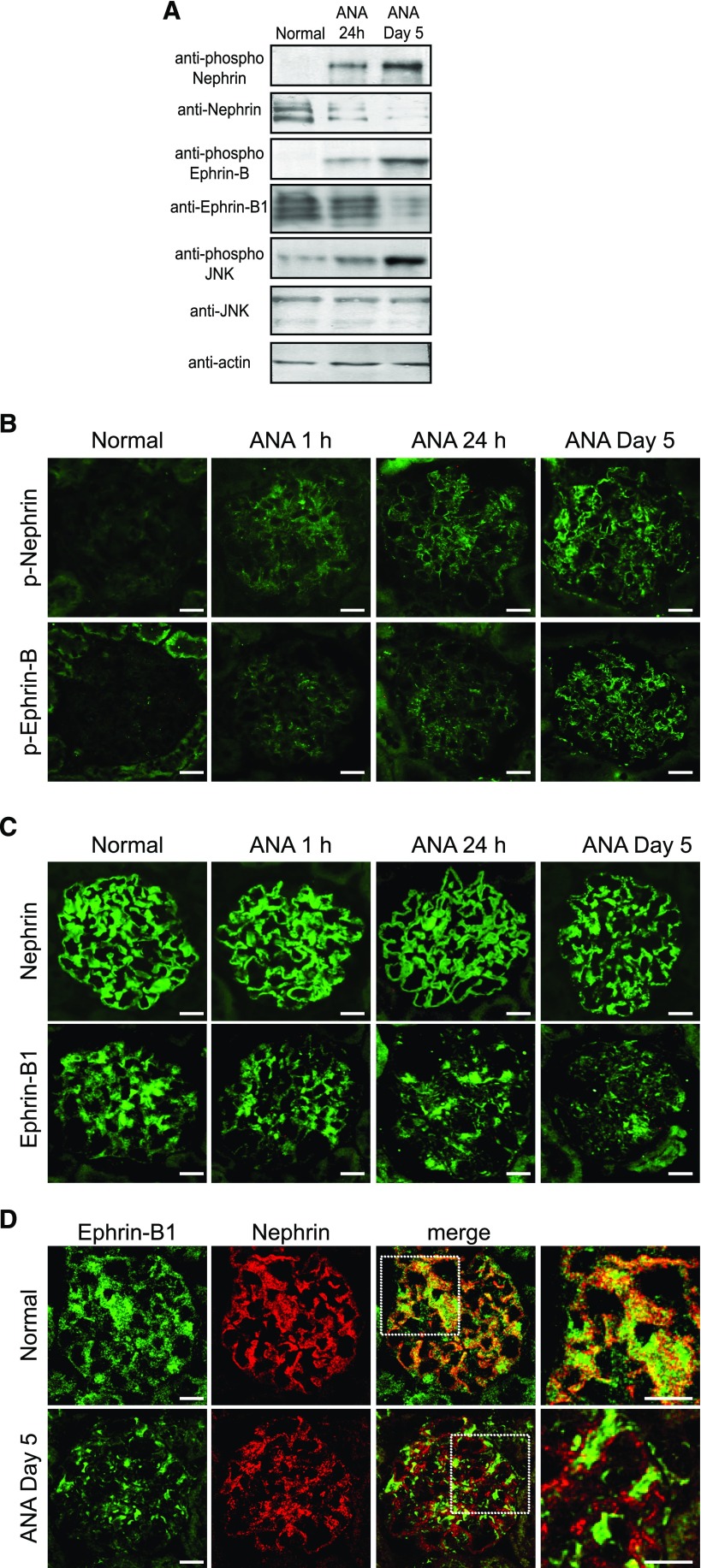
Next, we analyzed the role of ephrin-B1 in the development of the rat nephrotic model induced by injection of the anti-nephrin antibody. Western blot analysis with glomerular lysate showed that not only nephrin but also ephrin-B was phosphorylated, and that the JNK phosphorylation was promoted in the nephrotic model. The phosphorylation of these molecules was detected at 24 hours after the disease induction, and became evident on day 5 when severe proteinuria was detected (Figure 7A). The immunofluorescence study showed that ephrin-B and nephrin were already phosphorylated at 1 hour after disease induction, and that the phosphorylation of ephrin-B and nephrin became evident on day 5 (Figure 7B). The expression of ephrin-B1 was clearly decreased (Figure 7C) and the remaining ephrin-B1 was clearly dissociated from nephrin in the proteinuric state (Figure 7D). These observations suggested that the phosphorylation of ephrin-B1 and the JNK phosphorylation enhanced by phosphorylated ephrin-B1 are important initiation events, and that the consequent downregulation of ephrin-B1 and the disinteraction of epnrin-B1 and nephrin are involved in the development of this nephrotic model.
Figure 7.
Ephrin-B1 and nephrin are phosphorylated in the glomeruli of rats with the anti-nephrin antibody (ANA)–induced nephropathy. (A) Representative Western blot findings of glomerular lysate of rats with ANA-induced nephropathy. The clear bands for the phosphorylated nephrin, ephrin-B1, and JNK were detected at 24 hours after disease induction, and they became evident on day 5 when proteinuria peaked. (B and C) Representative immunofluorescence images of nephrin and ephrin-B1 in the kidney sections of ANA–induced nephropathy. (B) Stainings of the anti–phospho-nephrin and the anti–phospho-ephrin-B antibodies. The phosphorylation of nephrin and ephrin-B1 was already detected at 1 hour after ANA injection. The stainings with phospho-nephrin and phospho-ephrin-B antibodies became extensive on day 5. (C) Stainings of the anti-nephrin and anti–ephrin-B1 antibodies. Nephrin staining was not clearly altered in the early phase (1 hour), and nephrin staining was clearly altered on day 5. The staining of ephrin-B1 shifted to a discontinuous pattern and was clearly downregulated already at 1 hour, and the alteration became more evident on day 5. (D) Dual labeling of immunofluorescence images of ephrin-B1 (green) and nephrin (red). Ephrin-B1 was colocalized with nephrin in normal rat glomeruli. Ephrin-B1 and nephrin were clearly dissociated on day 5. Scale bars, 20 μm.
Ephrin-B1 Staining in Podocytes Is Clearly Decreased in a Human Patient with Active Nephrotic Syndrome
Positive staining of ephrin-B1 was detected in glomeruli of normal human kidney sections. Ephrin-B1 staining was broadly detected in glomeruli but was dominant in the podocyte. Stainings of nephrin, a slit diaphragm component, and podocalyxin, a transmembrane protein restrictedly localized at the apical surface of podocyte, were also detected. The expression of ephrin-B1 as well as nephrin was clearly reduced in glomeruli of a patient with relapsing nephrotic syndrome, whereas podocalyxin staining was reserved (Supplemental Figure 2).
Discussion
We have previously reported that ephrin-B1 was expressed at the slit diaphragm, and the expression was clearly decreased in a rat nephrotic model.7 In this study, first we analyzed the phenotype of podocyte-specific ephrin-B1 CKO mice. It was reported that global ephrin-B1 knockout mice resulted in embryonic lethality because of the defects in the skeletal patterning.5,6 To analyze the role of ephrin-B1 in the maintenance of slit diaphragm, the ephrin-B1 podocyte gene was inactivated after birth. This study is the first report analyzing the role of ephrin-B1 after birth with the inducible ephrin-B1 CKO mice. We demonstrated that ephrin-B1 CKO mice displayed disarrangement of the slit diaphragm molecules, effacement of foot processes, and proteinuria. Our findings show that ephrin-B1 participates in the maintenance of the proper molecular arrangement of the slit diaphragm molecules and the barrier function of the slit diaphragm.
Nephrin, a key molecule of the slit diaphragm, is identified as a molecule coded by NPHS1, a gene of a Finnish congenital nephrotic syndrome, and is a 1241-amino-acid residue transmembrane protein of the Ig super family with a single transmembrane domain.8 Nephrin has a long extracellular domain containing eight Ig-like modules and a single fibronectin type III module. An earlier study with electron microscopy showed that the slit diaphragm exhibited a zipper-like substructure with alternating, periodic cross bridges extending from the opposite podocyte plasma membranes (Karnovski model).9 Ruotsalainen et al.24 proposed that nephrin molecules extending from two adjacent foot processes are likely to interact with each other in the slit through homophilic interactions, as has been shown for other Ig cell adhesion molecules such as N-CAM,25 C-CAM,26 and L1.27 Ephrin-B1 is a transmembrane protein with 346 amino acid residues with a single transmembrane site.28,29 The extracellular domain of ephrin-B1 is much shorter than that of nephrin. Our study shows that ephrin-B1 binds nephrin not via the cytoplasmic site, but via the basal regions of the extracellular domains (Figure 3). It is plausible that ephrin-B1 does not bind nephrin extending from the opposite foot process, as in trans form, but binds nephrin from the same foot process as in cis form. We hypothesize that ephrin-B1 serves as a “prop” for nephrin to maintain the structure and the barrier function of the slit diaphragm.
In addition to its role as a structural protein of the slit diaphragm, nephrin serves as a signaling molecule to transmit signals from the slit diaphragm into the cells.30–36 However, the precise signaling pathways from nephrin are not yet fully elucidated. In this study, we found that not only nephrin but also nephrin-binding ephrin-B1 was phosphorylated by extracellular nephrin stimulation (Figure 4). This shows that ephrin-B1 at the slit diaphragm serves as a signaling molecule to transmit the signals detected by nephrin.
Next, we analyzed the downstream pathways of phosphorylation of nephrin-binding ephrin-B1. It has been reported that ephrin-B1 activates the phosphorylation of JNK even in an unphosphorylated state.21 Here, we show that JNK is phosphorylated even if the ephrin-B1 is not phosphorylated, and that the phosphorylation of nephrin-binding ephrin-B1 caused by nephrin stimulation promotes JNK phosphorylation, by analyses with HEK cells and isolated glomeruli (Figure 6, A and C). We also observed that nephrin does not have any effect on the phosphorylation of JNK even in its phosphorylated state. Our results imply that ephrin-B1 phosphorylates JNK independently of nephrin signaling. JNK is one of the key modulators of many cell events, such as apoptosis, cell migration, and the maintenance of the cell–cell junction.37–40 Here, we found that the motility of the HEK cells was promoted by transfection with ephrin-B1, and if the ephrin-B1 was phosphorylated, the motility was further promoted (Figure 6E). We also found that the degree of cell motility was associated with the degree of JNK phosphorylation, and that the promoted cell motility by ephrin-B1 was suppressed to normal level by a JNK inhibitor. Further, we found that the cell motility of cultured podocytes was increased, if ephrin-B1 and JNK were phosphorylated (Figure 6, F and G). It is understood that the strict regulation of podocyte motility is important to maintain the stability of podocytes,41,42 and that the increase in motility is involved in the pathogenesis of podocyte injury.43 Another important finding of this study is that no bands for the phosphorylated JNK were detected in the glomerular lysate of the podocyte-specific ephrin-B1 CKO mice, although the specific band was detected in that of wild-type mice. This implies that podocyte ephrin-B1 plays an important role in regulating the JNK signaling-mediated podocyte functions.
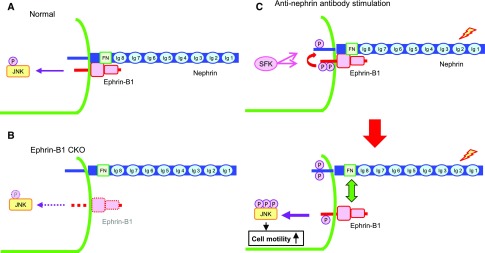
Next, we analyzed the role of ephrin-B1 in the development of the rat nephrotic model induced by anti-nephrin antibody injection (Figure 7). We found that ephrin-B1, as well as nephrin, was already phosphorylated at the very early phase in this nephrotic model, when proteinuria was not yet detected, and the phosphorylation became evident at the peak of proteinuria. We also observed that the expressions of ephrin-B1 and nephrin clearly decreased when proteinuria peaked and that the remaining ephrin-B1 and nephrin, of which major parts were phosphorylated, were dissociated. We also observed that the phosphorylation of JNK in glomeruli was promoted from the early phase of this nephrotic model. We have previously reported that proteinuria in this model results from the disarrangement of the slit diaphragm molecules, independent of any inflammatory factors.22,44,45 In this study, we also found that if the glomeruli isolated from normal rats were treated with anti-nephrin antibody in vitro, ephrin-B1 and nephrin were phosphorylated and JNK phosphorylation was promoted (Figure 6C). This implies that the phosphorylation of these molecules was induced without the participation of any circulating factors. It is reported that the JNK phosphorylation induces the downregulation and disinteraction of the tight junction proteins.38,39 Together with the observations in this study, it is conceivable that the phosphorylation of ephrin-B1 and the consequent JNK phosphorylation promoted by phosphorylated ephrin-B1 are important initiation events, and that the downregulation and disinteraction of the slit diaphragm components induced by the phosphorylated JNK are involved in the development of this nephrotic model. The scheme of the pathogenic model we propose is summarized in Figure 8.
Figure 8.
Ephrin-B1 bound to nephrin via extracellular domains plays a pivotal role in the regulation of cell motility by regulating JNK phosphorylation. (A) Ephrin-B1 at the slit diaphragm interacts with nephrin via the basal regions of the extracellular domains. In the physiologic state, nephrin and nephrin-binding ephrin-B1 are not phosphorylated. Ephrin-B1 is capable of phosphorylating JNK even in an unphosphorylated state. (B) If the expression of ephrin-B1 at the slit diaphragm is deleted, the localization of nephrin is altered. JNK in glomeruli is not phosphorylated. (C) If the extracellular domain of nephrin is stimulated, not only nephrin but also nephrin-binding ephrin-B1 is phosphorylated immediately after the stimulation, and the phosphorylated ephrin-B1 enhances the phosphorylation of nephrin. The phosphorylation of ephrin-B1 and nephrin are Src family kinase dependent (upper panel). The phosphorylated ephrin-B1 enhances JNK phosphorylation. The phosphorylated JNK promotes cell motility. The phosphorylated ephrin-B1 and nephrin are dissociated (lower panel).
Finally, we analyzed the expression of ephrin-B1 in human nephrotic syndrome. We confirmed that ephrin-B1 was expressed in podocytes in normal human kidney materials. We observed that the expression of the ephrin-B1 in glomeruli was clearly decreased in a patient with active MCNS (Supplemental Figure 2). Although further studies with more patients are necessary, this finding suggests that the decrease in the expression of ephrin-B1 at the slit diaphragm participates in the development of proteinuria in MCNS.
In conclusion, we have demonstrated that ephrin-B1 binds to nephrin via their extracellular domains and that the ephrin-B1–nephrin complex plays an essential role in maintaining the structure and barrier function of the slit diaphragm. Nephrin-binding ephrin-B1 was phosphorylated by extracellular nephrin stimulation and transferred the signals detected by nephrin downstream, via another route of nephrin signaling. Ephrin-B1 controls podocyte function through the JNK pathway (Figure 8). This study provides new insights to better understand podocyte biology and the pathogenesis of podocyte injury. The regulation of JNK phosphorylation could be a candidate for novel therapies of nephrotic syndrome.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors wish to thank Ms. Mutsumi Kayaba and Ms. Yukina Kitazawa for their excellent technical assistance, and Masaaki Nameta for taking electron microscopy images. The authors also thank Dr. Md. Murad Hossain for his help in performing immunoprecipitation assay.
Y.F. and H.K. designed the experiments and analyzed the data; Y.F. performed major parts of in vitro and in vivo experiments and wrote the manuscript; Y.Z. performed the developmental analysis; R.Y. performed genomic PCR; K.O. produced the transgenic mice; K.M. and T.W. performed the analysis with human samples; and H.K. conceived and directed the project and wrote the manuscript.
This work was supported by Grant-Aids for Scientific Research (B: 23390224 to H.K.) and Grant-Aid for Young Sciences (B: 24790839 and 16K197479 to Y.F., and 16K19483 to Y.Z.) from Ministry of Education, Culture, Sports, Science and Technology of Japan, a research aid from the Takeda Science Foundation to Y.F., and research support from Astellas Pharma Inc. to H.K.
A portion of this study was presented at the annual meeting of the American Society of Nephrology held in Chicago, IL, on November 17, 2016, and was published in abstract form.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017090993/-/DCSupplemental.
References
- 1.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Holthöfer H: Molecular architecture of the glomerular slit diaphragm: Lessons learnt for a better understanding of disease pathogenesis. Nephrol Dial Transplant 22: 2124–2128, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kawachi H, Shimizu F: Molecular composition and function of the slit diaphragm: Nephrin, the molecule responsible for proteinuria. Clin Exp Nephrol 4: 161–172, 2000 [Google Scholar]
- 4.Arvanitis D, Davy A: Eph/ephrin signaling: Networks. Genes Dev 22: 416–429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davy A, Aubin J, Soriano P: Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev 18: 572–583, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compagni A, Logan M, Klein R, Adams RH: Control of skeletal patterning by ephrinB1-EphB interactions. Dev Cell 5: 217–230, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto T, Karasawa T, Saito A, Miyauchi N, Han GD, Hayasaka K, et al.: Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte. Kidney Int 72: 954–964, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Tryggvason K: Unraveling the mechanisms of glomerular ultrafiltration: Nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 10: 2440–2445, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Rodewald R, Karnovsky MJ: Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 60: 423–433, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, et al.: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki K, Han GD, Miyauchi N, Hashimoto T, Nakatsue T, Fujioka Y, et al.: Angiotensin II type 1 and type 2 receptors play opposite roles in regulating the barrier function of kidney glomerular capillary wall. Am J Pathol 170: 1841–1853, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi A, Fukusumi Y, Yamazaki M, Kayaba M, Kitazawa Y, Tomita M, et al.: Angiotensin II type 1 receptor blockade ameliorates proteinuria in puromycin aminonucleoside nephropathy by inhibiting the reduction of NEPH1 and nephrin. J Nephrol 27: 627–634, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, et al.: Cloning of rat nephrin: Expression in developing glomeruli and in proteinuric states. Kidney Int 57: 1949–1961, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kawachi H, Koike H, Kurihara H, Sakai T, Shimizu F: Cloning of rat homologue of podocin: Expression in proteinuric states and in developing glomeruli. J Am Soc Nephrol 14: 46–56, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Fukusumi Y, Wakamatsu A, Takashima N, Hasegawa E, Miyauchi N, Tomita M, et al.: SV2B is essential for the integrity of the glomerular filtration barrier. Lab Invest 95: 534–545, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Nakatsue T, Koike H, Han GD, Suzuki K, Miyauchi N, Yuan H, et al.: Nephrin and podocin dissociate at the onset of proteinuria in experimental membranous nephropathy. Kidney Int 67: 2239–2253, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Otaki Y, Miyauchi N, Higa M, Takada A, Kuroda T, Gejyo F, et al.: Dissociation of NEPH1 from nephrin is involved in development of a rat model of focal segmental glomerulosclerosis. Am J Physiol Renal Physiol 295: F1376–F1387, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Bechtel W, Helmstädter M, Balica J, Hartleben B, Kiefer B, Hrnjic F, et al.: Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J Am Soc Nephrol 24: 727–743, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito A, Miyauchi N, Hashimoto T, Karasawa T, Han GD, Kayaba M, et al.: Neurexin-1, a presynaptic adhesion molecule, localizes at the slit diaphragm of the glomerular podocytes in kidneys. Am J Physiol Regul Integr Comp Physiol 300: R340–R348, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Wakamatsu A, Fukusumi Y, Hasegawa E, Tomita M, Watanabe T, Narita I, et al.: Role of calcineurin (CN) in kidney glomerular podocyte: CN inhibitor ameliorated proteinuria by inhibiting the redistribution of CN at the slit diaphragm. Physiol Rep 4: e12679, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho HJ, Hwang YS, Mood K, Ji YJ, Lim J, Morrison DK, et al.: EphrinB1 interacts with CNK1 and promotes cell migration through c-Jun N-terminal kinase (JNK) activation. J Biol Chem 289: 18556–18568, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orikasa M, Matsui K, Oite T, Shimizu F: Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J Immunol 141: 807–814, 1988 [PubMed] [Google Scholar]
- 23.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, et al.: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, et al.: Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A 96: 7962–7967, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiselyov VV, Berezin V, Maar TE, Soroka V, Edvardsen K, Schousboe A, et al.: The first immunoglobulin-like neural cell adhesion molecule (NCAM) domain is involved in double-reciprocal interaction with the second immunoglobulin-like NCAM domain and in heparin binding. J Biol Chem 272: 10125–10134, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Obrink B: CEA adhesion molecules: Multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol 9: 616–626, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonderegger P, Rathjen FG: Regulation of axonal growth in the vertebrate nervous system by interactions between glycoproteins belonging to two subgroups of the immunoglobulin superfamily. J Cell Biol 119: 1387–1394, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin D, Gish GD, Songyang Z, Pawson T: The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J Biol Chem 274: 3726–3733, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Kullander K, Klein R: Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol 3: 475–486, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Benzing T: Signaling at the slit diaphragm. J Am Soc Nephrol 15: 1382–1391, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, et al.: Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 23: 4917–4928, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, et al.: Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int 73: 556–566, 2008 [DOI] [PubMed] [Google Scholar]
- 33.George B, Verma R, Soofi AA, Garg P, Zhang J, Park TJ, et al.: Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. J Clin Invest 122: 674–692, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.New LA, Keyvani Chahi A, Jones N: Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins. J Biol Chem 288: 1500–1510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T: SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol 15: 3006–3015, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C, Jacobson K, Schaller MD: A role for JNK-paxillin signaling in cell migration. Cell Cycle 3: 4–6, 2004 [PubMed] [Google Scholar]
- 38.Samak G, Chaudhry KK, Gangwar R, Narayanan D, Jaggar JH, Rao R: Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem J 465: 503–515, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen D, Wei XT, Guan JH, Yuan JW, Peng YT, Song L, et al.: Inhibition of c-Jun N-terminal kinase prevents blood-brain barrier disruption and normalizes the expression of tight junction proteins clautin-5 and ZO-1 in a rat model of subarachnoid hemorrhage. Acta Neurochir (Wien) 154: 1469–1476, discussion 1476, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Dhanasekaran DN, Reddy EP: JNK signaling in apoptosis. Oncogene 27: 6245–6251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding WY, Saleem MA: Current concepts of the podocyte in nephrotic syndrome. Kidney Res Clin Pract 31: 87–93, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gee HY, Sadowski CE, Aggarwal PK, Porath JD, Yakulov TA, Schueler M, et al.: FAT1 mutations cause a glomerulotubular nephropathy. Nat Commun 7: 10822, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivanova EA, Arcolino FO, Elmonem MA, Rastaldi MP, Giardino L, Cornelissen EM, et al.: Cystinosin deficiency causes podocyte damage and loss associated with increased cell motility. Kidney Int 89: 1037–1048, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Kawachi H, Kurihara H, Topham PS, Brown D, Shia MA, Orikasa M, et al.: Slit diaphragm-reactive nephritogenic MAb 5-1-6 alters expression of ZO-1 in rat podocytes. Am J Physiol 273: F984–F993, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Kawachi H, Han GD, Miyauchi N, Hashimoto T, Suzuki K, Shimizu F: Therapeutic targets in the podocyte: Findings in anti-slit diaphragm antibody-induced nephropathy. J Nephrol 22: 450–456, 2009 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.