Abstract
Background
Trichinellosis is a serious zoonositc parasitosis worldwide. Because its clinical manifestations aren’t specific, the diagnosis of trichinellosis is not easy to be made. Trichinella spiralis muscle larva (ML) excretory–secretory (ES) antigens are the most widely applied diagnostic antigens for human trichinellosis, but the major drawback of the ES antigens for assaying anti-Trichinella antibodies is the false negative in the early Trichinella infection period. The aim of this study was to characterize the T. spiralis putative serine protease (TsSP) and to investigate its potential use for diagnosis of trichinellosis.
Methodology/Principal findings
The full-length TsSP sequence was cloned and expressed, and recombinant TsSP (rTsSP) was purified by Ni-NTA-Sefinose Column. On Western blotting analysis the rTsSP was recognized by T. spiralis-infected mouse serum, and the natural TsSP was identified in T. spiralis ML crude and ES antigens by using anti-rTsSP serum. Expression of TsSP was detected at various T. spiralis developmental stages (newborn larvae, muscle larvae, intestinal infective larvae and adult worms). Immunolocalization identified the TsSP principally in cuticles and stichosomes of the nematode. The sensitivity of rTsSP-ELISA and ES-ELISA was 98.11% (52/53) and 88.68% (47/53) respectively (P > 0.05) when the sera from trichinellosis patients were examined. However, while twenty-one serum samples of trichinellosis patients’ sera at 19 days post-infection (dpi) were tested, the sensitivity (95.24%) of rTsSP-ELISA was distinctly higher than 71.43% of ES-ELISA (P < 0.05). The specificity (99.53%) of rTsSP-ELISA was remarkably higher than 91.98% of ES-ELISA (P < 0.01). Only one out of 20 serum samples of cysticercosis patients cross-reacted with the rTsSP. Specific anti-Trichinella IgG in infected mice was first detected by rTsSP-ELISA as soon as 7 dpi and antibody positive rate reached 100% on 10 dpi, whereas the ES-ELISA did not permit detection of 100% of infected mice before 16 dpi.
Conclusions
The rTsSP is a potential early diagnostic antigen for human trichinellosis.
Author summary
Trichinellosis is an important parasitic zoonosis, and has a public health hazard and an economic impact on the safety of animal food. The diagnosis of trichinellosis is difficult and it is often misdiagnosed. There is an evident 2–3 week window stage between clinical manifestations and the anti-Trichinella IgG positive. Serine protease is a superfamily of proteolytic enzymes and exerts a major role in tissue invasion, larval development and survival of the parasites. A T. spiralis putative serine protease (TsSP) was characterized in ES proteins of T. spiralis intestinal infective larvae and adult worms by the immunoproteomics with early infection serum. In this study, the TsSP was expressed and purified. The results revealed that the TsSP was expressed at various T. spiralis stages (newborn larvae, muscle larvae, intestinal infective larvae and adult worms) and it was principally located in cuticle and stichosome of the nematode. The rTsSP was sensitive and specific for detection of anti-Trichinella IgG, and could be regarded as an early diagnostic marker of trichinellosis.
Introduction
Trichinellosis is an important food-borne parasitic disease worldwide. Trichinella infection occurs by ingesting raw or undercooked meat containing Trichinella muscle larvae [1]. T. spiralis is the main etiological agent of trichinellosis [2]. Outbreak of human trichinellosis was recorded in 55 countries around the world, and there were 65,818 cases and 42 deaths from trichinellosis reported from 41 countries during 1986–2009 [3]. Fifteen outbreaks of trichinellosis were documented in mainland China during 2004–2009 and pork is the dominating infection source [4,5]. A survey showed that the prevalence of porcine Trichinella infection in small pig farms in central China varied from 0.61% to 3.79% during 2010–2015, although the larval burdens in infected pigs was less than 2 larvae per gram of muscles [6,7]. Hence, trichinellosis has a public health hazard and an economic impact in meat food safety [8].
Since the symptoms and signs of trichinellosis aren’t specific, the diagnosis of trichinellosis isn’t easy to be established according to the clinical manifestations of this disease [9]. At present, the serological test widely applied for diagnosis of human trichinellosis is to detect anti-Trichinella IgG by ELISA and Western blotting with T. spiralis muscle larvae (ML) excretory/secretory (ES) antigens [10], but the principal drawback is the false negative in the early phase of this infection [11]. The occurrence of a 2–3 week window period of anti-Trichinella antibody negative is probable duo to the fact that the major ML ES antigen epitopes are the phase-specific for ML and not recognized by anti-Trichinella antibodies triggered by intestinal infective larvae (IIL) at 6 hours post infection (hpi) and adult worm (AW) at 3 dpi of the nematode in the early stage of Trichinella infection [12]. The ES antigens generated by the IIL and AW might firstly be exposed to host’s immune system and induced the generation of specific antibodies against the nematode. The recent investigation indicated that AW crude antigen positively reacted with swine and mouse infection sera at 7–8 dpi [13,14]. On Western blot analysis, the recombinant T. spiralis cystatin-like protein (rTsCLP) of IIL stage was probed by porcine infection sera at 15–20 dpi [15]. Anti-Trichinella IgG in serum samples of T. spiralis-infected mice was detected by ELISA using ES antigens of AW or IIL as soon as 8 dpi [16,17]. Therefore, it is likely that the diagnostic markers for early Trichinella infection will be exploited from the enteral worms of T. spiralis [18].
In our previous studies, immunoproteomics was used to investigate the early antigens for serodiagnosis of trichinellosis, and a putative serine protease was identified in the ES proteins from T. spiralis IIL and AW by mouse infection sera at 8–10 dpi and early trichinellosis patients’ sera at 19 dpi [12,19]. Additionally, the T. spiralis putative serine protease (TsSP) (GenBank accession no. ABY60762) was highly expressed in surface proteins of IIL stage compared with those of ML stage [20]. The aim of this study was to characteriz the TsSP and investigate the prospective diagnostic values of recombinant TsSP (rTsSP) for early trichinellosis.
Materials and methods
Ethics statement
The present study was performed in the light of National Guidelines for Experimental Animal Welfare (MOST of People’s Republic of China, 2006). All animal care and use in our research were reviewed and approved by the Life Science Ethics Committee of Zhengzhou University (No. SCXK 2015–0005). All the human serum samples were collected from adults, and the written informed consent was acquired from the adults before samples were used.
Parasites and experimental animals
T. spiralis isolate (ISS534) utilized in our study was acquired from a naturally infected domestic swine in Henan Province of central China. This isolate was passaged in BALB/c mice in our department. Six-week-old female BALB/c mice were provided by the Experimental Animal Center of Zhengzhou University (Zhengzhou, China). Mice were kept with specific pathogen-free conditions under suitable temperature and humidity.
Serum samples
Fifty-three serum samples from trichinellosis patients were obtained from two outbreaks of human trichinellosis in southwestern China [17]. The sera from patients with paragonimiasis (n = 20), schistosomiasis (n = 34), clonorchiasis (n = 7), cysticercosis (n = 20), echinococcosis (n = 20) and sparganosis (n = 7) were conserved in our laboratory. The diagnosis of these patients was established by fecal parasitological examination or serum specific antibody detection [11,21]. The sera from 104 presumably healthy persons, who came from non-endemic areas of trichinellosis and assayed negative for the before-mentioned helminthiases, were also examined in our study.
In order to observe the dynamics of anti-Trichinella IgG, nine mice were infected orally with 300 T. spiralis ML. About 100 μl of tail blood was collected from infected mice on alternate days during 2–30 dpi and serums were isolated. Serum samples from normal mice were obtained and utilized as the negative control.
Worm collection and antigen preparation
T. spiralis ML were obtained from experimentally infected mice at 35 dpi by using the artificial digestion method as described [22,23]. The IIL were recovered from intestines of the infected mice at 6 hpi [24], and the adult worms (AW) were separated from mouse duodenum and jejunum at 3 and 6 dpi, respectively [17]. The newborn larvae (NBL) were obtained from the adult females cultured in vitro in RPMI-1640 with 10% fetal bovine serum (FBS; Gibco) at 37°C in 5% CO2 for 24 h [25]. The crude soluble antigens of AW, NBL, ML and IIL, and the ML ES antigens were produced as described [26,27].
Sequence analysis of TsSP gene
The complete TsSP cDNA sequence was acquired from the GenBank database with accession no. ABY60762. The Pepstats software was applied to predict molecular weight (MW), isoelectric point (pI) and transmembrane helices of the TsSP protein [28,29]. The putative N-glycosylation site was verified with the NetNGly1.0 server (http://www.cbs.dtu.dk/services/NetNGlyc/). The potential B and T cell epitope of the TsSP was calculated with the DNAStar software and the online server of BepiPred (http://www.cbs.dtu.dk/services/BepiPred/), respectively [30]. The tertiary structure of the TsSP protein was predicted on the Expasy website (http://web.expasy.org/). The identification of protein motifs and catalytic triad of the TsSP was from aligning the multiple protein sequences [31].
Cloning, expression, and purification of rTsSP
The total ML RNA was extracted with Trizol reagent (Invitrogen, USA). The full-length TsSP sequences were amplified via PCR with specific primers carrying enzyme BamHI and PstI sites (bold and italicized) (5'-GGGATCCATGATCCTTTTCAAGTGCTTATTTCT-3' and 5'-GCGCTGCAGTCAGCAAACTCAATTTATTTAGAT-3'). The TsSP gene coding regions without a 18 amino acid signal peptide were produced by PCR with oligonucleotide primers carrying enzyme BamHI and PstI sites (bold and italicized) (5'-TTCGGATCCAATTATGAA TGTGGCACCTTAC-3' and 5'-CCGCTGCAGTTAACGGAAAAAAGTGAATGAT-3'). PCR amplification reaction included 25μl premix (DNA polymerase, dNTPs and PCR buffer), 0.5 μl cDNA, 0.4μl DNA polymerase, 1.0 μl 10 μM of each primer, 22 μl ddH2O. The cycling procedure was as follows: 98°C for 5 min; 30 cycles of at 94°C for 3min, 94°C for 45 s,60°C for 45 s, 72°C for 90 s, and finally 5 min at 72°C. The final purified PCR product was digested and cloned into the pGEM-T vector (Promega, USA), then sub-cloned into the pQE-80L carrying the N-terminus His-tag (Novagen, USA). The recombinant pQE-80L/TsSP was transformed into Escherichia coli BL21 (DE3) (Novagen). The rTsSP expression was induced by using 0.5 mM IPTG for 4 h at 30°C. The rTsSP were purified with a Ni-NTA His-tag affinity kit (Novagen). The rTsSP protein were identified on SDS–PAGE analysis [32]. The concentration of the rTsSP protein was assayed as described [33].
Phylogenetic analysis of the TsSP
The sequences of serine protease homologues from other organisms were aligned using the default settings in the program Clustal X [34]. The phylogenetic relationship among TsSP and other homologues was assayed by using a phylogenetic tree constructed in the MEGA 5.0 under the maximum parsimony algorithm with 1 000 bootstrap replications [35].
Preparation of anti-rTsSP serum
Thirteen BALB/c mice were immunized with rTsSP. Each mouse was injected abdomen subcutaneously with 20 μg of rTsSP emulsified in Freund’s complete adjuvant, then the mice were boosted twice with the same amount of rTsSP emulsified with Freund’s incomplete adjuvant at an intervals of 10 days [36]. About 50 μl of blood sample from immunized mice were obtained at 10 days after final immunization and serum anti-rTsSP antibody titer was assayed by ELISA with 2 μg/ml rTsSP as coating antigen [37].
Western blotting analysis
Samples consisted of 5μg rTsSP, 15μg ML crude antigens and ML ES antigens per lane. The protein was separated on SDS-PAGE with 12% separation gel, subsequently transferred onto the membranes (Millipore, USA) at 18 V for 35 min in a semi-dry transfer cell (Bio-Rad, USA) [26]. The membrane was blocked by 5% skim milk in Tris–buffered saline with 0.05% Tween-20 (TBST) at room temperature for 2 h, and incubated with 1:100 dilutions of different sera (anti-rTsSP serum, serum of T. spiralis-infected mice collected at 42 dpi, immune serum from mice immunized with ML ES and crude antigens, and uninfected normal mouse serum) at 4°C overnight. Following being washed, the membrane was incubated with 1:10 000 dilutions of HRP-conjugated goat anti-mouse IgG at 37°C for 1 h. The membrane was colored by use of 3,3’-diaminobenzidine tetrahydrochloride (DAB; Sigma), and terminated by washing the membrane with deionized water.
To detect the relative TsSP expression level in T. spiralis different stages, 15 μg/lane of soluble proteins of ML, IIL, AW and NBL was separated with SDS-PAGE and identified by Western blotting with 1:100 dilutions of anti-rTsSP serum [38]. Rabbit anti-β-actin antibody diluted at 1:400 was utilized as a quantitative protein control to detect β-actin expression. After it was washed three times with TBST, the color development was performed by the enhanced chemiluminescence (ECL) kit (CWBIO, Beijing, China) [39]. The relative expression level of the TsSP protein at various T. spiralis phases was determined with Image J software.
RT-PCR assay of TsSP transcription
Total RNA was extracted respectively from diverse T. spiralis phases (ML, IIL, AW, and NBL) with Trizol reagent (Invitrogen). The RT-PCR was carried out according to the previous report [38]. By using as an internal control, T. spiralis glyceraldehyde-3-phosphate dehydrogenase (GAPDH, GenBank accession No. AF452239) was amplified as a housekeeping gene in our study. PBS was used as a negative control template in all PCR assays.
ELISA
The crude proteins from different T. spiralis phases (NBL, ML, IIL and AW) and ES proteins from AW, ML and IIL were prepared as described [27,40]. The above-mentioned antigens and rTsSP were diluted to a final concentration of 1.5 μg/ml. The ELISA procedure was performed as described previously [11]. Briefly, the microtiter plate was coated with the antigens at 4°C overnight. Following being washed with PBST, it was blocked with 5% skimmed milk in PBST at 37°C for 2 h. After washing again, the plate was incubated at 37°C for 1 h with 1:200 dilutions of trichinellosis patients’ serum or 1:100 dilutions of mouse serum, subsequently incubated with HRP-conjugated anti-human/mouse antibody IgG (1:10 000) at 37°C for 1h. After the last washing, the coloration was developed by incubation with o-phenylenediamine dihydrochloride (OPD; Sigma) plus 30% H2O2 for 30 min. The reaction was ceased by 2M H2SO4. The absorbance (optical density, OD) was measured at 490 nm, and all serum samples were assayed in duplicate. The ratio < 2.1 of assayed serum/negative serum OD values was taken as negative and the ratio ≥2.1 as positive [41]. The cut-off value of rTsSP-ELISA and ES-ELISA for detection of the patient’s serum was 0.35 and 0.45, respectively. The cut-off value of the above two ELISA for detection of experimentally infected mice was 0.20 and 0.21, respectively.
Immunofluorescent test (IFT)
To confirm whether the TsSP expressed on the surface of T. spiralis diverse stages, the whole worms were used in IFT [42]. Additionally, the tissue sections with 3 μm thickness of female adults at 3 dpi, ML and IIL were separately cut by a microtome. The intact nematodes and their sections were blocked in 5% normal goat serum diluted with PBS, and incubated using a 1:10 dilution of mouse immune serum, infection serum or negative control serum. FITC-labeled goat anti-mouse IgG diluted at 1:100 (Santa Cruz, USA) was utilized as the second antibody. After they were washed with PBST, the intact nematode and sections were examined under a fluorescent microscopy (Olympus, Japan).
Statistical analysis
The statistical analysis of data was carried out by using SPSS 17.0 software. All the data were shown as arithmetic means ± standard deviation (SD). The comparison of the TsSP expression level in T. spiralis various stages was performed with one-way ANOVA. Chi-square test was used to determine the difference between groups. The statistical test was regarded significant at P < 0.05.
Results
Bioinformatic analysis of TsSP sequences
Bioinformatics analysis revealed that the full-length cDNA sequence of the TsSP gene was 1372 bp (CDS: 2–1290 bp). The predicted MW and pI of TsSP were 47.55 kDa and 8.73, respectively. The signal peptides were located at 1–18 aa (MILFKCLFLLAYTTLAFA). The mature serine protease consisted of 411 amino acid residues of 45.2 kDa, and no transmembrane helix was detected, indicating that the TsSP is a secretory protein. Only one N-glycosylation site 78–81 (NGSQ) of the TsSP was identified. Secondary structures of the TsSP had 18 potential B cell epitopes. The SMART analysis results demonstrated that the TsSP had a domain (at 37-277aa) of trypsin-like serine protease carrying an active site of classic catalytic triad. In three-dimensional model, the motif of catalytic triad (Serine–Histidine–Aspartate) constituted a functional domain carrying substrate binding sites (Fig 1).
Fig 1. The predicted three-dimensional model of T. spiralis TsSP protein.
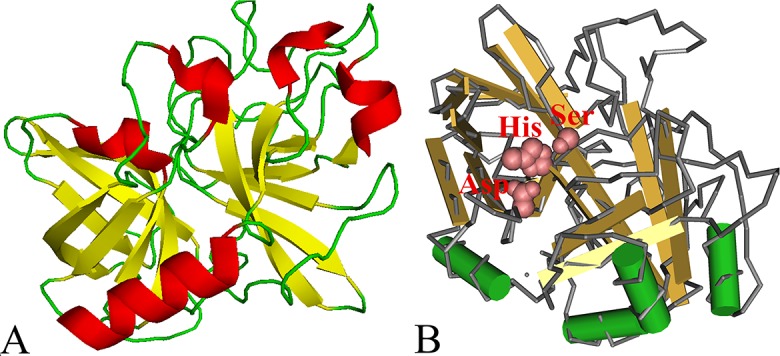
A: The predicted three-dimensional structure of TsSP protein, there are 4 α-helixes (in red), 14 β-strand (in yellow), and 19 irregular coils (in green); B: Catalytic residues Ser-His-Asp form a pocket-shaped functional domain. The active site of TsSP was highlighted with red color.
Sequence alignment and the comparison of TsSP
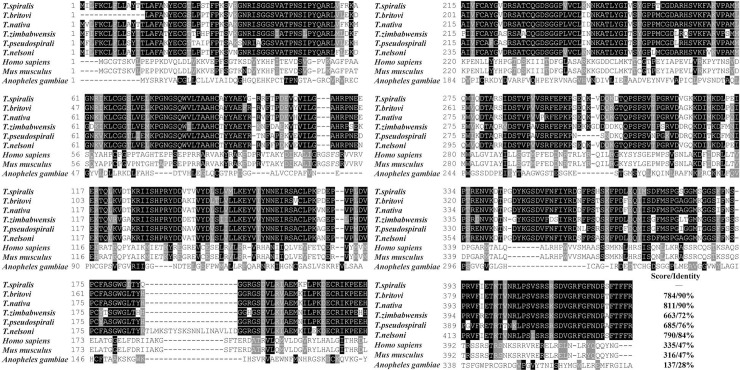
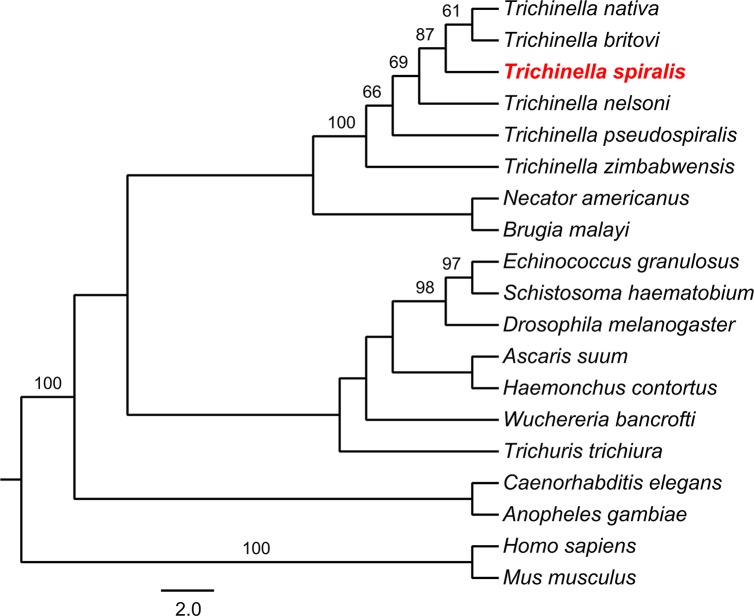
A homology comparison of TsSP and other serine protease orthologues in the genus Trichinella was determined (Fig 2), among these sequences, the highest homology was between T. spiralis and T. nativa (with 90% identity). As shown in the phylogenetic tree generated with TsSP and its orthologues (Fig 3), the Trichinella genus was displayed as a monophyletic group with bootstrap value of 87. Within the Trichinella, the close relationships among T. spiralis, T. nativa and T. britovi were supported with a high bootstrap value (95).
Fig 2. Sequence alignment of serine protease from Trichinella spiralis (ABY60762) with other species of the genus Trichinella and other organisms.
The serine proteases was from Trichinella spp.: T. britovi (KRY59723.1), T. nativa (KRZ48330.1), T. zimbabwensis (KRZ02345.1), T. pseudospirali (KRY01512.1), T. nelsoni (KRX27556.1), Homo sapiens (CAB91984.1), Mus musculus (EDL11329.1), Anopheles gambiae (CAB90819.1). The multiple sequences alignment was performed in the Clustal X and displayed using BOXSHADE. Black shade indicated that residues identical to TsSP, and conservative substitutions were shaded grey. The numbers at the end of each sequence represent the score and percent identity to TsSP.
Fig 3. Cladogram of analysis of TsSP.
The maximum parsimony tree of serine protease from 19 organisms calculated using MEGA. The GenBank accession numbers of each serine protease are as follows: Trichinella spiralis (ABY60762.1), Trichinella nativa (KRZ48330.1), Trichinella nelsoni (KRX27556.1), Trichinella britovi (KRY59723.1), Trichinella pseudospiralis (KRY01512.1), Trichinella zimbabwensis (KRZ02345.1), Homo sapiens (CAB91984.1), Mus musculus (EDL11329.1), Caenorhabditis elegans (CCD65324.1), Drosophila melanogaster (NP_001262565.1), Trichuris trichiura (CDW53654.1), Ascaris suum (ERG81033.1), Necator americanus (XP_013308358.1), Brugia malayi (XP_001900088.1), Wuchereria bancrofti (EJW79555.1), Haemonchus contortus (CDJ82043.1), Echinococcus granulosus (EUB62466.1), Anopheles gambiae (CAB90819.1), Schistosoma haematobium (KGB34910.1). Bootstrap values which are higher than 80 are indicated on branches.
Cloning and expression of recombinant TsSP
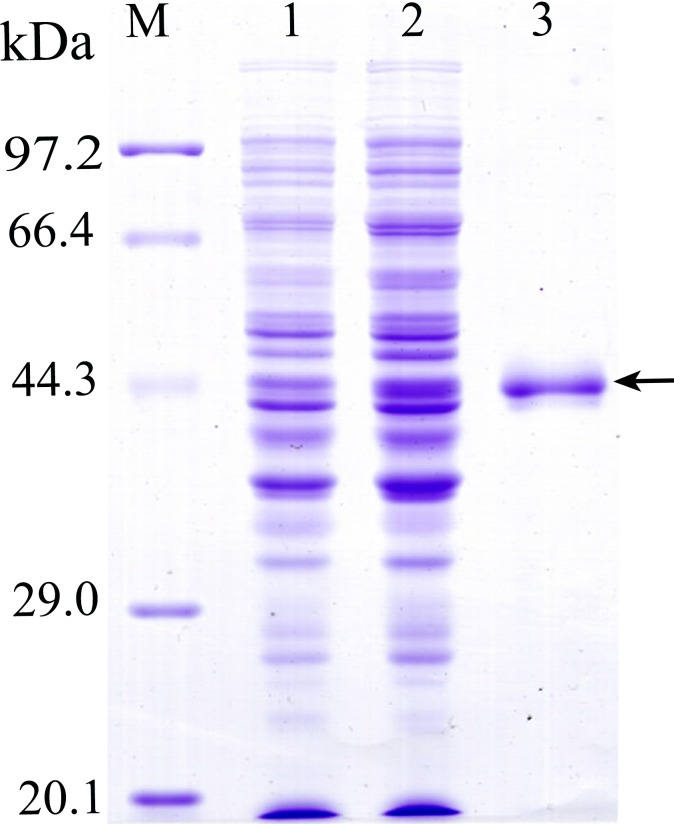
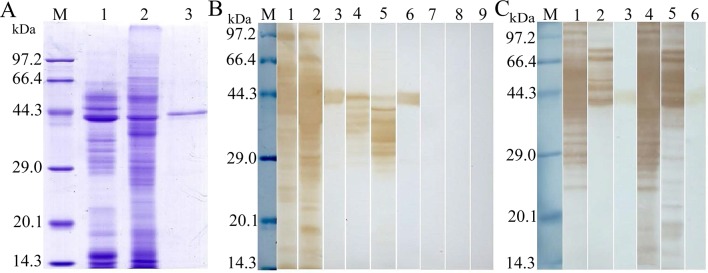
The complete TsSP cDNA sequences without signal peptide were 1236 bp. The open reading frame (ORF) of TsSP encoded a 45.2 kDa protein of 411 amino acids. The TsSP coding sequences were cloned into the pQE-80L. Following induction, SDS-PAGE analysis showed that the recombinant bacteria harboring pQE-80L/TsSP expressed a protein band with 45.2 kDa. After being purified, the rTsSP had a single distinct protein band (Fig 4). The molecular weight (45.2 kDa) of the rTsSP was consistent with its expected size.
Fig 4. SDS-PAGE analysis of the rTsSP.
M: protein molecular weight marker; 1: protein lysates from recombinant E. coli before being induced, 2: protein lysates of recombinant E. coli induced by IPTG; 3: rTsSP purified by Ni-NTA-Sefinose Column. Arrow indicates rTsSP.
Humoral immune responses elicited by immunization with rTsSP
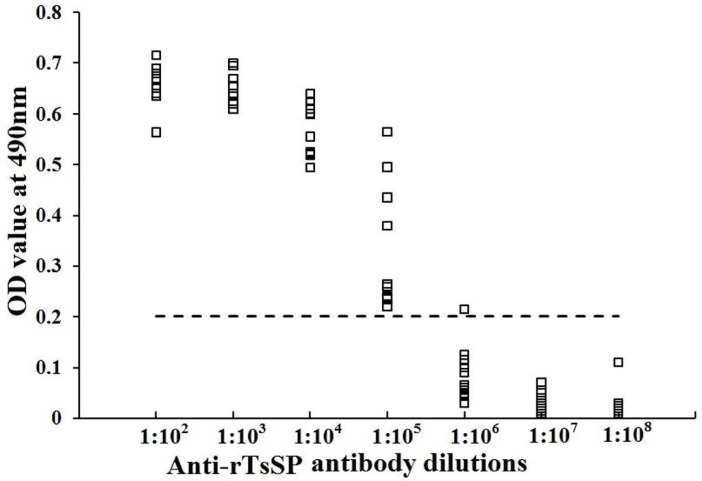
To determine humoral immune responses to rTsSP in immunized mice, serum specific anti-rTsSP IgG titers at days 10 after the final immunization were measured by ELISA. As shown in Fig 5, anti-rTsSP antibodies could be triggered by the immunization with rTsSP. The titer of serum anti-rTsSP IgG was 1:105 following the last immunization, indicating that the rTsSP has a high immunogenicity.
Fig 5. Serum anti-rTsSP IgG titers determined by ELISA.
Microtiter plates were coated with 2 μg rTsSP /ml and incubated overnight at 4°C. After being blocked with 5% nonfat milk, the plates were incubated at 37°C for 1 h with different dilutions of immune sera. Normal mouse sera (n = 20) diluted at 1:100 were assayed as negative controls. HRP-conjugated IgG was utilized as the second antibodies. The coloration was developed by incubation with the substrate OPD. The absorbance at 490 nm was assayed following adding 2 M H2SO4. The cut-off values (0.202) are expressed with a dotted line.
Western blot analysis of the rTsSP
The results of SDS-PAGE analysis showed that the ML crude antigens had 44 bands with MW of 14.7–97.2 kDa, ML ES antigens had 29 bands with 14.4–96.3 kDa, and the rTsSP had only one band with 45.2 kDa (Fig 6A). On Western blot analysis the rTsSP was probed with anti-rTsSP serum and infection serum. The native TsSP proteins with 25–47 kDa in T. spiralis ML crude and ES proteins were recognized with anti-rTsSP serum (Fig 6B). Furthermore, the rTsSP was also recognized by immune serum from mice immunized with ML ES or crude antigens (Fig 6C). The results indicated that TsSP is one protein component from somatic and ES products of T. spiralis ML.
Fig 6. Western blot analysis of rTsSP antigenicity.
(A) SDS-PAGE analysis of ML ES antigens from T. spiralis (lane 1), ML crude antigen (lane 2), and rTsSP (lane 3). (B) Western blotting of the rTsSP. T. spiralis ML ES antigens (lane 1) and ML crude antigens (lane 2) and rTsSP (lane 3) were probed with mouse infection sera. The natural TsSP protein in ML ES (lane 4), ML crude antigens (lane 5) and rTsSP (lane 6) were identified by using anti-rTsSP serum. Normal mouse sera did not probe the ES (lane7) and crude antigens (lane 8), and rTsSP (lane 9). (C) Western blotting of ML ES (lane 1 and 4) and crude antigens (lane 2 and 5), and rTsSP (lane 3 and 6) probed with immune sera from mice vaccinated with ES and crude antigens.
RT-PCR assay of TsSP transcription at different stages
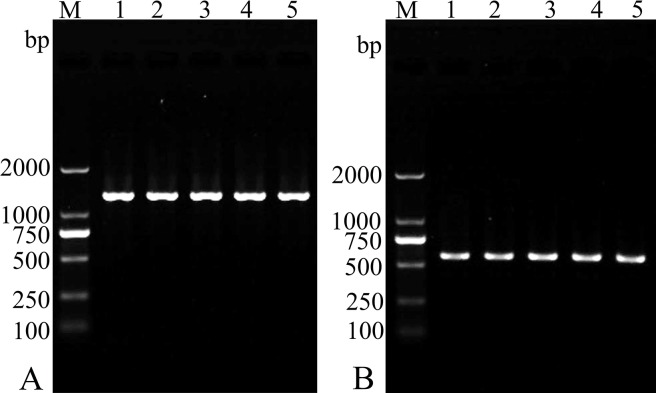
The TsSP transcription at different T. spiralis stages was assayed by RT-PCR assay and the transcription of GAPDH gene was used as an internal control. The TsSP mRNA transcript (1236 bp) was observed at all T. spiralis lifecycle stages (NBL, ML, IIL and AW). Moreover, the primers for the housekeeping gene (GAPDH) also produced the expected band (570 bp) in different developmental stages (Fig 7).
Fig 7. RT-PCR assay of TsSP transcription at different T. spiralis phases.
RT-PCR assay of the mRNA transcription of TsSP (A) and GAPDH (B) at various T. spiralis stages. M: DNA marker; Lane 1: ML; Lane 2: IIL; Lane 3: AW at 3 dpi; Lane 4: AW at 6 dpi; Lane 4: NBL.
ELISA and Western blot analysis of TsSP expression at various stages
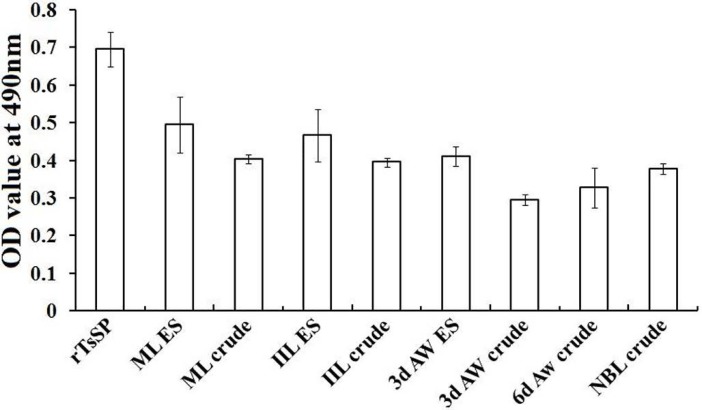
The results of ELISA revealed that the rTsSP and the native TsSP in crude and ES products of different stages (NBL, ML, IIL and AW) were identified by using anti-rTsSP serum (Fig 8). The results of Western blot analysis demonstrated that the native TsSP of 45.2 kDa in crude antigens of various stages were also probed with anti-rTsSP serum (Fig 9). These results further indicated that the TsSP was expressed at various developmental phases, and existed in both the somatic and ES proteins of the nematode. The TsSP expression level in IIL and NBL were obviously higher than those in the other three stages (ML, AW at 3 and 6 dpi) (P < 0.05).
Fig 8. Anti-rTsSP IgG level of immunized mice determined by ELISA using antigens from various stages.
OD values shown for each group (n = 13) were the arithmetic mean ± standard deviation (SD) of serum anti-rTsSP IgG levels.
Fig 9. Western blot analyses of the TsSP expression levels at diverse T. spiralis phases.
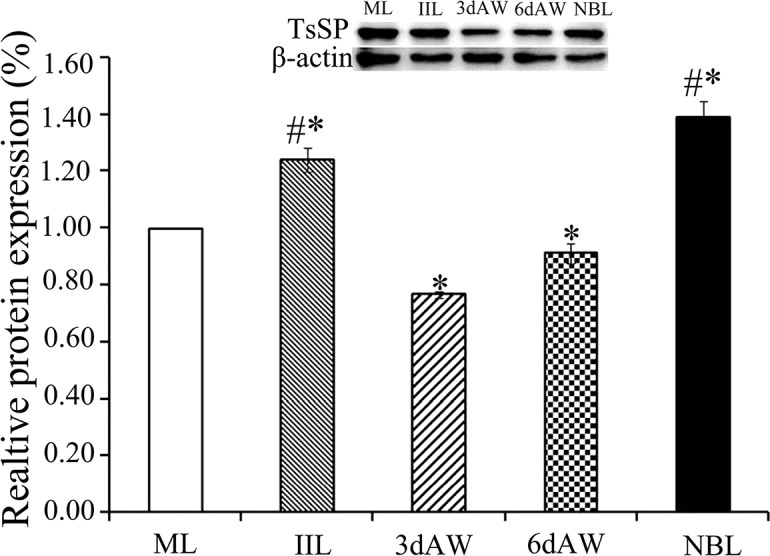
Expression levels of TsSP protein with 45.2kDa in crude antigens of diverse T. spiralis phase (AW, NBL, ML and IIL) were determined by Western blot with 1:100 dilutions of anti-rTsSP serum. The graph reveals the relative protein expression assayed with densitometry in 3 independent experiments. Asterisk (*) shows statistical differences (P < 0.05) relative to the ML. Pound sign (#) indicates remarkably statistical differences (P <0.01) relative the ML.
Immunolocalization of TsSP by IFT
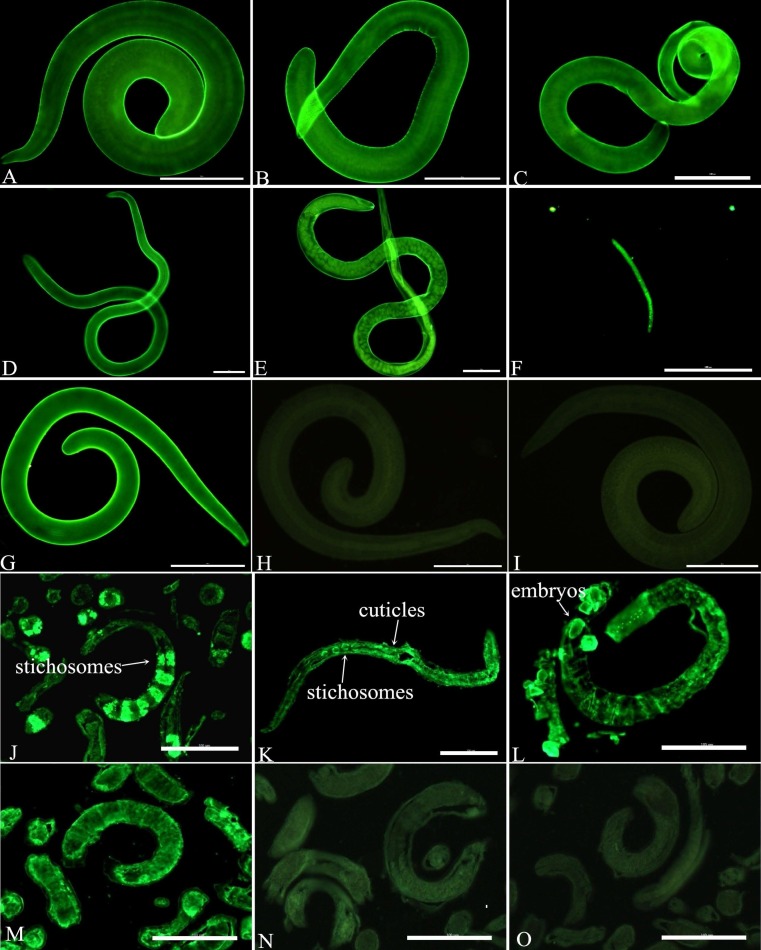
The IFT using intact parasite revealed that the immunostaining was found on cuticles of different stages (AW, NBL, ML and IIL) by using anti-rTsSP serum (Fig 10). While tissue sections of the nematode were incubated by anti-rTsSP serum, the staining was detected in cuticles and stichosomes of ML, IIL, AW and the embryos within uterus of female adult at 3 dpi.
Fig 10. Immunolocalization of TsSP at different T. spiralis phases.
A-I: IFT with intact worm of different T. spiralis phases probed by anti-rTsSP serum. The apparent immunostaining is observed on cuticles of ML (A), 6 hpi IIL (B), 24 hpi IIL (C), 3 dpi female adult (D), 6 dpi female adult (E), and NBL (F). The ML probed by infection serum (G) was utilized as a positive serum control; the ML incubated with normal mouse serum (H) and PBS (I) were applied to negative controls. When worm sections were incubated by anti-rTsSP serum, positive staining is seen in cuticles and stichosomes of ML (J), IIL (K) and female adult at 3 dpi (L). The ML probed by infection serum (M) was employed as a positive serum control; ML reveals no staining with normal mouse serum (N) and PBS (O) as a negative control. Scale-bars: 100 μm.
Detection of anti-Trichinella IgG in patients
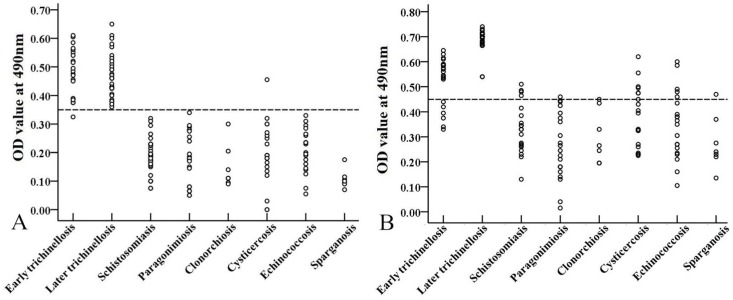
The sensitivity of rTsSP-ELISA and ES-ELISA for detection of anti-Trichinella IgG in serum samples from trichinellosis patients was 98.11% (52/53) and 88.68% (47/53), respectively (χ2 = 2.910, P = 0.088). As the patients’ serum samples at 35 dpi were tested, the sensitivity of two antigens reached 100% (32/32). Nevertheless, while the patients’ samples at 19 dpi were examined, the sensitivity of the rTsSP was 95.24% (20/21), which was obviously higher than 71.43% (15/21) of ES antigens (χ2 = 4.286, P = 0.038) (Table 1). The specificity of the rTsSP and ES antigens was 99.53% (211/212) and 91.98% (195/212) (χ2 = 14.853, P = 0), when they were applied for detecting anti-Trichinella IgG in sera of patients with other parasitosis and healthy individuals. The cross-reaction of rTsSP with sera of patients with other parasitic diseases was not observed except for one serum sample from patients with cysticercosis (Fig 11).
Table 1. Detection of anti-Trichinella IgG antibodies in serum samples of patients with trichinellosis and other parasitic diseases by rTsSP-ELISA.
| Sera of patients with | No. of serum samples | ELISA with rTsSP antigens | ELISA with ML ES antigens | ||
|---|---|---|---|---|---|
| OD value ( ± S) | No. of positive serum samples (%) | OD value ( ± S) | No. of positive serum samples (%) | ||
| Trichinellosis | 53 | 0.47±0.09 | 52 (98.11) | 0.62±0.11 | 47 (88.68) |
| Early trichinellosis* | 21 | 0.48±0.08 | 20 (95.24) | 0.52±0.10 | 15 (71.43) |
| Later trichinellosis# | 32 | 0.47±0.08 | 32 (100) | 0.69±0.04 | 32 (100) |
| Schistosomiasis | 34 | 0.19±0.08 | 0 (0) | 0.33±0.10 | 4 (11.76) |
| Paragonimiasis | 20 | 0.18±0.09 | 0 (0) | 0.26±0.14 | 1 (5.00) |
| Clonorchiasis | 7 | 0.16±0.10 | 0 (0) | 0.30±0.11 | 1 (14.29) |
| Cysticercosis | 20 | 0.20±0.11 | 1 (5.00) | 0.38±0.12 | 5 (25.00) |
| Echinococcosis | 20 | 0.22±0.09 | 0 (0.00) | 0.35±0.14 | 5 (25.00) |
| Sparganosis | 7 | 0.11±0.03 | 0 (0) | 0.27±0.10 | 1 (14.29) |
| Healthy persons | 104 | 0.17±0.05 | 0 (0) | 0.21±0.08 | 0 (0) |
*Early trichinellosis: The sera of early patients with trichinellosis were collected at 19 days post infection.
#Later trichinellosis: The sera of later patients with trichinellosis were collected at 35 days post infection.
Fig 11.
Scatter plot of optical density values of rTsSP-ELISA (A) and ES-ELISA (B) for detection of anti-Trichinella IgG in serum samples of patients with trichinellosis and other parasitic diseases. The cut-off value is represented by the dotted line.
Dynamics of serum anti-Trichinella IgG in experimentally infected mice
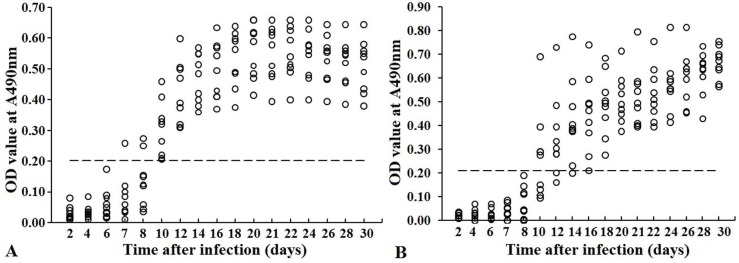
Serum anti-Trichinella IgG levels in infected mice at different time intervals post infection were measured by rTsSP-ELISA and ES-ELISA, respectively. Specific anti-Trichinella IgG was first detected by rTsSP-ELISA on 7 dpi and antibody positive rate reached 100% on 10 dpi (Fig 12A); when ES-ELISA was used, the specific antibody was first detected on 10 dpi and antibody detection reached 100% on 16 dpi (Fig 12B).
Fig 12. Kinetics of serum anti-Trichinella IgG in mice experimentally infected with 300 muscle larvae.
Anti-Trichinella IgG was detected by rTsSP-ELISA (A) and ES-ELISA (B). The cut-off value is represented by the dotted line.
Discussion
Previous studies showed there is an evident 2–3 week window of anti-Trichinella IgG negative after Trichinella infection, the antibody detection rate could not attain 100% till 1–3 months following Trichinella infection in humans [43,44]. The conventional ELISA with ML ES antigens lacks perfect sensitivity at the beginning of Trichinella infection, so improvements of diagnostic antigens would be of clinical value. In theory, detection of circulating antigens or DNA from T. spiralis live worms seems an ideal early diagnostic method for trichinellosis. But the levels of Trichinella circulating antigens in serum samples are usually lower and its detection rate in patients with clinical trichinellosis was usually only 30–50% [45,46]. Moreover, the persistence of Trichinella DNA is transient in blood circulation and the feces of infected hosts [23,47]. Therefore, determination of Trichinella circulating antigens or DNA has not been used for diagnosis of human trichinellosis. Up to now, determination of anti-Trichinella IgG is the most widely applied diagnostic method of trichinellosis, which is recommended by WHO and the International Commission on Trichinellosis (ICT) [10,48]. Therefore, it would be beneficial to identify antigens better able to diagnose recent Trichinella infection.
Serine protease (or serine proteinase) is a superfamily of widespread proteolytic enzymes in parasites, they exert an important part in physiological and pathological proceses during parasite infection [49]. The protease is related with the larval invasion, molting, digestion and fibrinolysis in parasitic nematodes [50,51]. Previous studies indicated that some secreted serine proteases were found in ES products from T. spiralis ML and AW, including serine protease TspSP-1 and trypsin-like 45 kDa antigen [52,53], and the enzymic activity of the native serine proteases in T. spiralis ML and AW ES proteins was also detected by biochemistry assay [54,55]. Our previous studies demonstrated that while the ML were activated into IIL and cocultivated with intestinal epithelial cells (IEC), the serine protease expression level in IIL stage was evidently increased as compared with ML stage [56,57], suggeting that the serine proteases might be involved in the larval invasion of host’s enteral mucosa. These serine proteases might be the target molecular antigens of the early host’s immune response, and they are possiblly used as the new diagnostic antigens for early trichinellosis [58].
The complete TsSP cDNA sequence was cloned and expressed in this study. The TsSP is attributed to the trypsin-like serine protease superfamily and has 90% identity with T. nativa which is another encapsulated Trichinella species [59]. After being purified, the rTsSP was strongly immunogenic and used for generating anti-rTsSP antibodies. Immunization of mice with the rTsSP elicited specific humoral immune response against rTsSP. The ELISA results revealed that the titer of specific anti-rTsSP IgG in immune serum was 1:105. On Western blotting, the rTsSP protein was recognized with anti-rTsSP serum and mouse infection serum. As shown in Fig 6B, by using anti-rTsSP serum several native TsSP proteins was identified in T. spiralis ML crude and ES antigens. The TsSP might have different isoforms, or the protein was possibly processed by means of post-translational modifications/alternative splicing [11,60,61]. The process might be involved in the phosphorylation, methylation or acetylation of the TsSP after being translated, and they are possible important for the biological functions of the TsSP [38,62,63]. Additionally, it is also possible because the TsSP is a member of serine protease family, and they have the same functional domains.
The TsSP mRNA transcription was detected by RT-PCR at all T. spiralis developmental stage (AW, NBL, ML, IIL) (Fig 7). The TsSP expression was found by ELISA at various stage, but as shown in Fig 9, the TsSP expression level in IIL and NBL were obviously higher than those in the other three stages (ML, AW at 3–6 dpi) on Western blot anlysis. The IFT results demonstrated immunostaining was principally located in cuticle and stichosome of the nematode (Fig 10). Our results indicated that the TsSP was expressed at various T. spiralis phases and the TsSP was likely from the worm’s ES products. Previous studies showed another serine proteases (TspSP-1.2) was also expressed in T. spiralis different stages [38]. The results suggested that the TsSP is an essential protein and act a pivotal part in T. spiralis larval invasion and development. The the enzymatic activity and biological funtions of the rTsSP need to be studied in further experiments.
To investigate the potential use of rTsSP for serodiagnosis of human trichinellosis, rTsSP-ELISA method was establised and applied to assay anti-Trichinella IgG in trichinellosis patients’ serum samples, and the sensitivity was compared with those of ES-ELISA. The results revealed that the sensitivity of rTsSP-ELISA and ES-ELISA was 98.11% (52/53) and 88.68% (47/53), respectively (P > 0.05). Nevertheless, while the trichinellosis patients’ serum samples at 19 dpi were examined, the sensitivity (95.24%) of rTsSP was significantly higher than 71.43% (15/21) of ES antigens (P < 0.05), demostrating that the rTsSP protein was useful for the early diagosis of human trichinellosis. The specificity (99.53%) of the rTsSP was also superior to 91.98% of the ES antigens (P < 0.01). The cross-reaction of the rTsSP was seen only with one out of 20 serum samples of cysticercosis patients. The sensitivity and specificity of rTsSP are similar with that of recombinant T. spiralis 31 kDa protein [11]. The sensitivity of rTsSP for diagnosing early trichinellosis is comparative to those of ELISA using IIL or AW ES antigens, but the specificity of rTsSP-ELISA has an evident advantage over those of IIL and AW ES antigens [16,17]. Importantly, the anti-Trichinella IgG in 100% of the mice infected with 300 muscle larvae was detected by rTsSP-ELISA as soon as 10 dpi, but the ES-ELISA did not permit detection of 100% of infected mice before 16 dpi. The results suggested that the TsSP protein might be secreted by the nematode into the host’s peripheral blood circulation at the early infection stage and elicited an early specific anti-Trichinella antibody response continuing to the muscle stage [16]. Furthermore, our previous study has showed that the rTsSP could be recognized by early mouse infection sera at 8–10 dpi on Western blotting analysis [58]. Consequently, the rTsSP could be of value as potential novel antigen for the early diagnosis of T. spiralis infection in humans.
In summary, this study demonstrated that the TsSP was expressed at various T. spiralis developmental stages, it was likely from the worm’s ES products, and mainly located in cuticle and stichosome of this nematode. The rTsSP was strongly immunogenic. Sensitivity and specificity of rTsSP for detecting anti-Trichinella IgG antibodies are superior to the conventional ML ES antigens which are widely used at present. The rTsSP had the potential valuable as a new diagnositic antigen for early trichinellosis. But more serum samples from patients with trichinellosis and other nematode infection (ascariasis, trichuriasis, hookworm infection, filariasis, etc.) should be tested to further evaluate its sensitivity and specificity.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by grants of the National Natural Science Foundation of China (nos. 81672043, 81471981 and U1704284). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rostami A, Gamble HR, Dupouy-Camet J, Khazan H, Bruschi F (2017) Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol 64: 65–71. doi: 10.1016/j.fm.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 2.Wang ZQ, Li LZ, Jiang P, Liu LN, Cui J (2012) Molecular identification and phylogenetic analysis of Trichinella isolates from different provinces in mainland China. Parasitol Res 110: 753–757. doi: 10.1007/s00436-011-2549-3 [DOI] [PubMed] [Google Scholar]
- 3.Murrell KD, Pozio E (2011) Worldwide occurrence and impact of human trichinellosis 1986–2009. Emerg Infect Dis 17: 2194–2202. doi: 10.3201/eid1712.110896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui J, Wang ZQ, Xu BL (2011) The epidemiology of human trichinellosis in China during 2004–2009. Acta Trop 118: 1–5. doi: 10.1016/j.actatropica.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 5.Cui J, Wang ZQ (2011) An epidemiological overview of swine trichinellosis in China. Vet J 190: 324–328. [DOI] [PubMed] [Google Scholar]
- 6.Cui J, Jiang P, Liu LN, Wang ZQ (2013) Survey of Trichinella infections in domestic pigs from northern and eastern Henan, China. Vet Parasitol 194: 133–135. doi: 10.1016/j.vetpar.2013.01.038 [DOI] [PubMed] [Google Scholar]
- 7.Jiang P, Zhang X, Wang LA, Han LH, Yang M, et al. (2016) Survey of Trichinella infection from domestic pigs in the historical endemic areas of Henan province, central China. Parasitol Res 115: 4707–4709. doi: 10.1007/s00436-016-5240-x [DOI] [PubMed] [Google Scholar]
- 8.Bruschi F (2012) Trichinellosis in developing countries: is it neglected? J Infect Dev Ctries 6: 216–222. [DOI] [PubMed] [Google Scholar]
- 9.Dupouy-Camet J, Kociecka W, Bruschi F, Bolas-Fernandez F, Pozio E (2002) Opinion on the diagnosis and treatment of human trichinellosis. Expert Opin Pharmacother 3: 1117–1130. doi: 10.1517/14656566.3.8.1117 [DOI] [PubMed] [Google Scholar]
- 10.Gamble HR, Pozio E, Bruschi F, Nockler K, Kapel CMO, et al. (2004) International commission on trichinellosis: Recommendations on the use of serological tests for the detection of Trichinella infection in animals and man. Parasite 11: 3–13. doi: 10.1051/parasite/20041113 [DOI] [PubMed] [Google Scholar]
- 11.Cui J, Wang L, Sun GG, Liu LN, Zhang SB, et al. (2015) Characterization of a Trichinella spiralis 31 kDa protein and its potential application for the serodiagnosis of trichinellosis. Acta Trop 142: 57–63. doi: 10.1016/j.actatropica.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 12.Liu JY, Zhang NZ, Li WH, Li L, Yan HB, et al. (2016) Proteomic analysis of differentially expressed proteins in the three developmental stages of Trichinella spiralis. Vet Parasitol 231: 32–38. doi: 10.1016/j.vetpar.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Pan W, Sun X, Zhao X, Yuan G, et al. (2015) Immunoproteomic profile of Trichinella spiralis adult worm proteins recognized by early infection sera. Parasit Vectors 8: 20 doi: 10.1186/s13071-015-0641-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu DM, Wen H, Wang LA, Hu CX, Qi X, et al. (2017) Identification of early diagnostic antigens in soluble proteins of Trichinella spiralis adult worms by Western blot. Trop Biomed 34: 191–198. [PubMed] [Google Scholar]
- 15.Tang B, Liu MY, Wang LB, Yu SY, Shi HN, et al. (2015) Characterisation of a high-frequency gene encoding a strongly antigenic cystatin-like protein from Trichinella spiralis at its early invasion stage. Parasite Vector 8: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun GG, Liu RD, Wang ZQ, Jiang P, Wang L, et al. (2015) New diagnostic antigens for early trichinellosis: the excretory-secretory antigens of Trichinella spiralis intestinal infective larvae. Parasitol Res 114: 4637–4644. doi: 10.1007/s00436-015-4709-3 [DOI] [PubMed] [Google Scholar]
- 17.Sun GG, Wang ZQ, Liu CY, Jiang P, Liu RD, et al. (2015) Early serodiagnosis of trichinellosis by ELISA using excretory-secretory antigens of Trichinella spiralis adult worms. Parasite Vector 8: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang ZQ, Shi YL, Liu RD, Jiang P, Guan YY, et al. (2017) New insights on serodiagnosis of trichinellosis during window period: early diagnostic antigens from Trichinella spiralis intestinal worms. Infect Dis Poverty 6: 41 doi: 10.1186/s40249-017-0252-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu RD, Jiang P, Wen H, Duan JY, Wang LA, et al. (2016) Screening and characterization of early diagnostic antigens in excretory-secretory proteins from Trichinella spiralis intestinal infective larvae by immunoproteomics. Parasitol Res 115: 615–622. doi: 10.1007/s00436-015-4779-2 [DOI] [PubMed] [Google Scholar]
- 20.Liu RD, Cui J, Liu XL, Jiang P, Sun GG, et al. (2015) Comparative proteomic analysis of surface proteins of Trichinella spiralis muscle larvae and intestinal infective larvae. Acta Trop 150: 79–86. doi: 10.1016/j.actatropica.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 21.Cui J, Li N, Wang ZQ, Jiang P, Lin XM (2011) Serodiagnosis of experimental sparganum infections of mice and human sparganosis by ELISA using ES antigens of Spirometra mansoni spargana. Parasitol Res 108: 1551–1556. doi: 10.1007/s00436-010-2206-2 [DOI] [PubMed] [Google Scholar]
- 22.Gamble HR, Bessonov AS, Cuperlovic K, Gajadhar AA, van Knapen F, et al. (2000) International Commission on Trichinellosis: recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet Parasitol 93: 393–408. [DOI] [PubMed] [Google Scholar]
- 23.Li F Wang ZQ, Cui J. (2010) Early detection by polymerase chain reaction of migratory Trichinella spiralis larvae in blood of experimental infected mice. Foodborne Pathog Dis 7: 887–892. doi: 10.1089/fpd.2009.0472 [DOI] [PubMed] [Google Scholar]
- 24.Liu RD, Cui J, Liu XL, Jiang P, Sun GG, et al. (2015) Comparative proteomic analysis of surface proteins of Trichinella spiralis muscle larvae and intestinal infective larvae. Acta Trop 150: 79–86. doi: 10.1016/j.actatropica.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 25.Wu ZL, Nagano I, Takahashi Y, Maekawa Y (2016) Practical methods for collecting Trichinella parasites and their excretory-secretory products. Parasitol Int 65: 591–595. doi: 10.1016/j.parint.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Wang ZQ, Cui J (2013) Protein changes in Trichinella spiralis muscle larvae in vitro induced by bovine bile. Vet Parasitol 194: 164–167. doi: 10.1016/j.vetpar.2013.01.046 [DOI] [PubMed] [Google Scholar]
- 27.Yang W, Li LG, Liu RD, Sun GG, Liu CY, et al. (2015) Molecular identification and characterization of Trichinella spiralis proteasome subunit beta type-7. Parasite Vector 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8: 785–786. doi: 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 29.Rice P, Longden I, Bleasby A (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16: 276–277. [DOI] [PubMed] [Google Scholar]
- 30.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S (1999) SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50: 213–219. [DOI] [PubMed] [Google Scholar]
- 31.Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. (2010. ) A new bioinformatics analysis tools framework at EMBLEBI. Nucleic Acids Res 695–699. doi: 10.1093/nar/gkp1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang SW, Wang ZQ, Cui J (2011) Protein change of intestinal epithelial cells induced in vitro by Trichinella spiralis infective larvae. Parasitol Res 108: 593–599. doi: 10.1007/s00436-010-2102-9 [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 7: 248–254. [DOI] [PubMed] [Google Scholar]
- 34.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and clustal X version 2.0. Bioinformatics 23: 2947–2948. doi: 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 35.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28: 2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui J, Ren HJ, Liu RD, Wang L, Zhang ZF, Wang ZQ. (2013. ) Phage-displayed specific polypeptide antigens induce significant protective immunity against Trichinella spiralis infection in BALB/c mice. Vaccine 31: 1171–1177. doi: 10.1016/j.vaccine.2012.12.070 [DOI] [PubMed] [Google Scholar]
- 37.Liu CY, Song YY, Ren HN, Sun GG, Liu RD, et al. (2017) Cloning and expression of a Trichinella spiralis putative glutathione S-transferase and its elicited protective immunity against challenge infections. Parasit Vectors 10: 448 doi: 10.1186/s13071-017-2384-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang B, Wang ZQ, Jin J, Ren HJ, Liu LN, et al. (2013) Cloning, expression and characterization of a Trichinella spiralis serine protease gene encoding a 35.5 kDa protein. Exp Parasitol 134: 148–154. doi: 10.1016/j.exppara.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 39.Wang ZQ, Zhang SB, Jiang P, Liu RD, Long SR, et al. (2015) The siRNA-mediated silencing of Trichinella spiralis nudix hydrolase results in reduction of larval infectivity. Parasitol Res 114: 3551–3557. doi: 10.1007/s00436-015-4650-5 [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Wang ZQ, Cui J (2013) Protein changes in Trichinella spiralis muscle larvae in vitro induced by bovine bile. Vet Parasitol 194: 164–167. doi: 10.1016/j.vetpar.2013.01.046 [DOI] [PubMed] [Google Scholar]
- 41.Liu LN, Wang ZQ, Zhang X, Jiang P, Qi X, et al. (2015) Characterization of Spirometra erinaceieuropaei Plerocercoid Cysteine Protease and Potential Application for Serodiagnosis of Sparganosis. PloS Neglect Trop Dis 9: e0003807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang YL, Wang ZQ, Li LG, Cui J (2013) Molecular characterization of Trichinella spiralis aminopeptidase and its potential as a novel vaccine candidate antigen against trichinellosis in BALB/c mice. Parasite Vector 6: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruschi F, Moretti A, Wassom D, Fioretti DP (2001) The use of a synthetic antigen for the serological diagnosis of human trichinellosis. Parasite 8: S141–S143. doi: 10.1051/parasite/200108s2141 [DOI] [PubMed] [Google Scholar]
- 44.Kociecka W, Bruschi F, Marini C, Mrozewicz B, Pielok L (2001) Clinical appraisal of patients and detection of serum antibodies by ELISA and CIA tests in late periods of Trichinella sp. invasion. Parasite 8:S147–S151. doi: 10.1051/parasite/200108s2147 [DOI] [PubMed] [Google Scholar]
- 45.Nishiyama T, Araki T, Mizuno N, Wada T, Ide T, et al. (1992) Detection of circulating antigens in human trichinellosis. Trans R Soc Trop Med Hyg 86: 292–293. [DOI] [PubMed] [Google Scholar]
- 46.Liu LN, Jing FJ, Cui J, Fu GY, Wang ZQ (2013) Detection of circulating antigen in serum of mice infected with Trichinella spiralis by an IgY-IgM mAb sandwich ELISA. Exp Parasitol 133: 150–155. doi: 10.1016/j.exppara.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 47.Liu XL, Ren HN, Shi YL, Hu CX, Song YY, et al. (2017) Early detection of Trichinella spiralis DNA in the feces of experimentally infected mice by using PCR. Acta Trop 166: 351–355. doi: 10.1016/j.actatropica.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 48.Dupouy-Camet J, Bruschi F. (2007) Management and diagnosis of human trichinel-losis In: Dupouy-Camet J., Murrell D. (Eds.), FAO/WHO/OIE Guidelines for the Surveillance, Management, Prevention and Control of Trichinellosis. OIE: 37–39. [Google Scholar]
- 49.Hedstrom L (2002) Serine protease mechanism and specificity. Chem Rev 102: 4501–4524. [DOI] [PubMed] [Google Scholar]
- 50.Nagano I, Wu Z, Nakada T, Boonmars T, Takahashi Y (2003) Molecular cloning and characterization of a serine proteinase gene of Trichinella spiralis. J Parasitol 89: 92–98. doi: 10.1645/0022-3395(2003)089[0092:MCACOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Wen Y, Cai YN, Vallee I, Boireau P, et al. (2015) Serine proteases of parasitic helminths. Korean J Parasitol 53: 1–11. doi: 10.3347/kjp.2015.53.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romaris F, North SJ, Gagliardo LF, Butcher BA, Ghosh K, et al. (2002) A putative serine protease among the excretory-secretory glycoproteins of L1 Trichinella spiralis. Mol Biochem Parasitol 122: 149–160. [DOI] [PubMed] [Google Scholar]
- 53.Robinson MW, Connolly B (2005) Proteomic analysis of the excretory-secretory proteins of the Trichinella spiralis L1 larva, a nematode parasite of skeletal muscle. Proteomics 5: 4525–4532. doi: 10.1002/pmic.200402057 [DOI] [PubMed] [Google Scholar]
- 54.Criado-Fornelio A, de Armas-Serra C, Gimenez-Pardo C, Casado-Escribano N, Jimenez-Gonzalez A, et al. (1992) Proteolytic enzymes from Trichinella spiralis larvae. Vet Parasitol 45: 133–140. [DOI] [PubMed] [Google Scholar]
- 55.Todorova VK, Knox DP, Kennedy MW (1995) Proteinases in the excretory/secretory products (ES) of adult Trichinella spiralis. Parasitology 111 (2): 201–208. [DOI] [PubMed] [Google Scholar]
- 56.Ren HJ, Cui J, Yang W, Liu RD, Wang ZQ (2013) Identification of differentially expressed genes of Trichinella spiralis larvae after exposure to host intestine milieu. PLoS One 8: e67570 doi: 10.1371/journal.pone.0067570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Wang ZQ, Cui J (2013) Proteomic analysis of the changed proteins of Trichinella spiralis infective larvae after co-culture in vitro with intestinal epithelial cells. Vet Parasitol 194: 160–163. doi: 10.1016/j.vetpar.2013.01.045 [DOI] [PubMed] [Google Scholar]
- 58.Wang ZQ, Liu RD, Sun GG, Song YY, Jiang P, et al. (2017) Proteomic Analysis of Trichinella spiralis Adult Worm Excretory-Secretory Proteins Recognized by Sera of Patients with Early Trichinellosis. Front Microbiol 8: 986 doi: 10.3389/fmicb.2017.00986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pozio E (2007) World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol 149: 3–21. doi: 10.1016/j.vetpar.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 60.Robinson MW, Greig R, Beattie KA, Lamont DJ, Connolly B (2007) Comparative analysis of the excretory-secretory proteome of the muscle larva of Trichinella pseudospiralis and Trichinella spiralis. Int J Parasitol 37: 139–148. doi: 10.1016/j.ijpara.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 61.Bien J, Cabaj W, Moskwa B (2015) Proteomic analysis of potential immunoreactive proteins from muscle larvae and adult worms of Trichinella spiralis in experimentally infected pigs. Folia Parasitol (Praha) 62. [DOI] [PubMed] [Google Scholar]
- 62.Gao F, Liu X, Wu XP, Wang XL, Gong D, et al. (2012) Differential DNA methylation in discrete developmental stages of the parasitic nematode Trichinella spiralis. Genome Biol 13: R100 doi: 10.1186/gb-2012-13-10-r100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y, Tong M, Bai X, Liu X, Cai X, et al. (2018) Comprehensive proteomic analysis of lysine acetylation in the goodborne pathogen Trichinella spiralis. Front Microbiol 8: 2674 doi: 10.3389/fmicb.2017.02674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.