Abstract
Aryankalayil, M. J., Chopra, S., Levin, J., Eke, I., Makinde, A., Das, S., Shankavaram, U., Vanpouille-Box, C., Demaria, S. and Coleman, C. N. Radiation-Induced Long Noncoding RNAs in a Mouse Model after Whole-Body Irradiation. Radiat. Res. 189, 251–263 (2018).
Long noncoding RNAs (lncRNAs) are emerging as key molecules in regulating many biological processes and have been implicated in development and disease pathogenesis. Biomarkers of cancer and normal tissue response to treatment are of great interest in precision medicine, as well as in public health and medical management, such as for assessment of radiation injury after an accidental or intentional exposure. Circulating and functional RNAs, including microRNAs (miRNAs) and lncRNAs, in whole blood and other body fluids are potential valuable candidates as biomarkers. Early prediction of possible acute, intermediate and delayed effects of radiation exposure enables timely therapeutic interventions. To address whether long noncoding RNAs (lncRNAs) could serve as biomarkers for radiation biodosimetry we performed whole genome transcriptome analysis in a mouse model after whole-body irradiation. Differential lncRNA expression patterns were evaluated at 16, 24 and 48 h postirradiation in total RNA isolated from whole blood of mice exposed to 1, 2, 4,8 and 12 Gy of X rays. Sham-irradiated animals served as controls. Significant alterations in the expression patterns of lncRNAs were observed after different radiation doses at the various time points. We identified several radiation-induced lncRNAs known for DNA damage response as well as immune response. Long noncoding RNA targets of tumor protein 53 (P53), Trp53cor1, Dino, Pvt1 and Tug1 and an upstream regulator of p53, Meg3, were altered in response to radiation. Gm14005 (Morrbid) and Tmevpg1 were regulated by radiation across all time points and doses. These two lncRNAs have important potential as blood-based radiation biomarkers; Gm14005 (Morrbid) has recently been shown to play a key role in inflammatory response, while Tmevpg1 has been implicated in the regulation of interferon gamma. Precise molecular biomarkers, likely involving a diverse group of inducible molecules, will not only enable the development and effective use of medical countermeasures but may also be used to detect and circumvent or mitigate normal tissue injury in cancer radiotherapy.
INTRODUCTION
We are living in an increasingly discordant world in which we face a growing risk of terrorism and state-sponsored nuclear conflicts, as well as an increased dependence on nuclear energy. An accidental or intentional large-scale exposure to potentially lethal radiation doses is a recognized threat. In mass casualty scenarios, it would be imperative to differentiate people into categories ranging from those needing urgent and immediate medical aid to those with very low or no exposure who need assurance and possibly long-term follow-up but not immediate attention from a stressed medical system. A rapid and effective triage of the exposed population requires accurate radiation-sensitive biomarkers and easy-to-use and cost-effective assays. Timeliness of results is critical, since medical countermeasures (MCMs) may have a limited window of efficacy (1).
Currently, the criteria to assess exposure and tissue damage caused by radiation, in addition to a person’s location relative to the exposure, is based on 1. the time to onset of vomiting; 2. decline in absolute lymphocyte count; and 3. identification of chromosomal aberrations (2, 3). Onset of clinical symptoms such as fever, nausea, vomiting and a decrease in total blood count are important criteria for diagnosing acute radiation syndrome (ARS). Symptomatic changes are useful but can be nonspecific and are not sufficient for determining medical management (4, 5). Cytogenetic observations such as dicentric chromosomes and micronuclei in cultured peripheral blood lymphocytes are the current “gold standards” for assessing radiation biodosimetry (6). In addition to being labor and expertise intensive, use of cytogenetics is contingent on the presence of sufficient numbers of viable lymphocytes for the induction of mitosis (7). Cytogenetic assays require long preparation times (72 h), which are beyond the time needed for mitigation of the hematological ARS. Highly sensitive molecular signatures predicting accurate radiation dosimetry in a time-dependent manner need to be identified. Proteins like ataxia telangiectasia mutated (ATM), tumor protein 53 (TP53), phosphorylated histone 2AX (γ-H2AX) and cyclin dependent kinase inhibitor 1A (CDKN1A) are potential biomarkers for radiation exposure based on several cell line studies (8). Efforts are underway to identify genomic, proteomic, transcriptomic and metabolomic biomarkers (9–11).
With the advent of deep RNA sequencing techniques (12) and completion of the ENCyclopedia of DNA Elements (ENCODE) project (13), it has now become clear that 60–70% of the human genome is transcribed into RNA, of which only 2–2.5% is translated into proteins. The function and complexity of this copious amount of noncoding RNA is beginning to emerge. It is well known that various classes of noncoding RNA (ncRNA), e.g., tRNA, rRNA, snRNA, snoRNA, miRNA and piRNA, play crucial roles in protein synthesis, mRNA splicing, transcriptional regulation and gene silencing. A new class of ncRNAs identified in deep sequencing studies are the long noncoding RNAs (lncRNAs). They are arbitrarily defined as transcripts greater than 200 nt in length lacking a clear protein coding ability (14). Several lncRNAs, such as XIST, lincRNA-p21, HOTAIR and H19, to name a few, have been known to control gene transcription, gene silencing and/or chromatin modulation for decades (15). There are mechanistic differences among lncRNAs. They may fulfill their roles by acting as either signals, decoys, scaffolds, guides or by forming DNA-RNA triple helices (16, 17). LncRNA biomarkers are being investigated and identified in several disease conditions such as cancer and cardiovascular pathologies (18–23). Serum-circulating lncRNA signature has been proposed for assessing heart failure (24). Various published studies have proposed circulating lncRNA signatures as diagnostic and prognostic biomarkers in different types of cancers (25–29).
There is now ample evidence that lncRNA transcripts are modulated in response to radiation. Some lncRNAs have been found to confer radioresistance on different kinds of cancer cells (30–34) making them attractive targets in multimodal cancer therapies. Published studies of whole genome expression analysis have identified lncRNA transcripts changing in response to either ultraviolet (UV) or ionizing radiation in cultured peripheral blood mononuclear cells (PBMCs) (35), thymocytes (36), melanocytes (37) and human bronchial epithelial cells (38). High- and low-dose X rays were found to induce different subsets of lncRNAs in primary breast epithelial cells (39). To our knowledge, there have been no studies done previously to examine the changes in lncRNA expression profiles after whole-body irradiation in animal models. It is important to undertake such a study, since lncRNA biomarkers are emerging as potentially relevant for cancer care and in medical responses to accidental or intentional radiation exposure, based on the reasons discussed below.
Several lncRNAs are reported to play key roles in p53-dependent DNA damage response (38, 40–43) and in immune regulation (44–46). Tumor protein p53 pathway corepressor 1 (Trp53cor1) and damage-induced noncoding (Dino) are lncRNA targets of p53 and mediate p53-induced expression changes of target genes (40, 47). Other p53-associated lncRNAs, such as plasmacytoma variant translocation 1 (Pvt1), metastasis associated lung adenocarcinoma transcript 1 (MalatT), maternally expressed 3 (Meg3) and taurine upregulated gene 1 (Tug1), have relevance to different kinds of cancers (48–51). While Meg3 is speculated to act as a tumor suppressor by stabilizing P53 protein, Malat1, Pvt1 and Tug1 have oncogenic properties. The lncRNA Theiler’s murine encephalomyelitis virus persistence candidate gene 1 (Tmevpg1) has previously been shown to be expressed in natural killer cells, CD4+ and CD8+ T cells (52). The Morrbid-myeloid RNA regulator of Bim-induced death, Gm14005, has recently been shown to control the survival of myeloid cells and therefore impacts on the duration of an inflammatory response (53). Because radiation induces both DNA damage (54) and an associated systemic immune response (55), and lncRNAs are being identified to play key roles in these processes, it is logical to study lncRNA profile changes as potential radiation biodosimetry markers. Interestingly, we observed that all the above-mentioned lncRNAs change upon whole-body irradiation in our study.
The expression patterns of lncRNAs have greater organ and tissue specificity compared to protein coding RNAs (56). This characteristic makes them attractive targets in ascertaining organ-specific injury after partial-body exposures and clinical radiation therapy.
Comparisons of the most well-characterized lncRNAs point to the fact that as a class, lncRNAs make a highly complex and heterogeneous group. It would not be a surprise if several distinct sub-classes of lncRNAs were identified based on their structure, mechanisms of action and the level at which they operate. As functions of more and more lncRNAs are identified, it should eventually be possible to situate lncRNAs or the sub-classes at their proper place in the hierarchy of genome regulation. It is logical, therefore, that lncRNAs are included in any studies that address systems-level problems. Thus, in our studies to identify radiosensitive biomarkers, we are analyzing the lncRNA profiles along with miRNA and mRNA changes to investigate various aspects of transcriptional changes induced by radiation.
Here, we studied how lncRNA expression profiles change in the blood of female C57BL/6 mice after whole-body irradiation. A number of qualities make this study unique: 1. It is the first reported work assessing genome-wide lncRNA signatures from blood after whole-body irradiation; 2. It uses a wide range of radiation doses (1–8 Gy and 12 Gy, the latter being a lethal dose) in an animal model; and 3. It assesses lncRNA expression profiles at both early (16 h) and late (24 and 48 h) time points postirradiation.
MATERIALS AND METHODS
Animal Irradiations
Female C57BL/6 mice (6–8 weeks old) were whole-body X-ray irradiated using the Small Animal Radiation Research Platform (SARRP; Xstrahl Inc., Suwanee, GA.). Mice were placed in plastic containers and exposed to a single surface dose of 0–12 Gy at a dose rate of 1.05 Gy/min. Control mice (0 Gy) were placed in the same plastic container as the irradiated and sham-irradiated. Three animals per dose per time point have been included in all the studies. Separate sets of animals were used for the microarray analysis and the qPCR validations (RT2 lncRNA PCR Array mouse lncFinder and individual RT-PCR assays).
Terminal Blood Collection Using Cardiac Puncture
At 16, 24 and 48 h after whole-body irradiation (1, 2, 4, 8 and 12 Gy), anesthesia was induced with 2% isoflurane in oxygen for 5 min. A 25g needle attached to a 1-ml syringe was inserted into either notch of the mouse and directed towards the heart as determined by palpating for the heartbeat. Once the needle was inserted beneath the skin, gentle negative pressure was applied, by pulling backward on the plunger to collect approximately 500 pl of total blood. Blood (100 pl) was then transferred into five RNAprotect animal blood tubes (QIAGEN®, Valencia, CA) and gently inverted 8 to 10 times. After an incubation of 2 h at room temperature, the samples were stored at −20°C.
RNA Isolation
Total RNA was isolated using the RNeasy Protect Animal Blood Kit (QIAGEN) from the whole blood without separating serum or plasma. Briefly, all blood cells are lysed in the RNA Protect Animal Blood tubes (QIAGEN) and RNA (intracellular and extracellular) is precipitated along with cell debris. The pellet is washed once with RNase free water. RNA isolation is subsequently performed from the pellet using the manufacturer’s protocol. Total RNA obtained was further purified and concentrated using the RNA Clean-up and Concentration kit (Norgen Biotek Corp., Thorold, Canada). Quality and quantity of the RNA samples were assessed using an Agilent Bioanalyzer with the RNA6000 Nano Lab Chip (Agilent Technologies; Santa Clara, CA). The RNA integrity numbers were consistent for all the samples and ranged from 8–9.
Gene Expression Microarrays
Total RNA (50 ng) was reverse transcribed after priming with a DNA oligonucleotide containing the T7 RNA polymerase promoter 5′ to a d(T)24 sequence. After second-strand cDNA synthesis and purification of double-stranded cDNA, in vitro transcription was performed using T7 RNA polymerase. The quantity and quality of the cRNA was assayed by spectrophotometry and on the Agilent Bioanalyzer, as indicated, for total RNA analysis. cRNA was fragmented to uniform size and hybridized to SurePrint G3 mouse gene expression 8 × 60K, version 1 or 2 (design ID nos. 028005 and 074809, respectively; Agilent Technologies). Slides were washed and scanned on an Agilent SureScan Microarray Scanner. All arrays were processed with Agilent Feature Extraction software and data were analyzed with GeneSpring GX software (Agilent Technologies). To compare individual expression values across arrays, raw intensity data from each probe were normalized to the 75th percentile intensity of its array. Probes with intensity values above background in all samples within each group were used for further analysis. Differentially expressed probes at each time point (16, 24 and 48 h) for 0, 2, 4 and 8 Gy exposures were identified by analysis of variance (ANOVA) followed by Dunnett’s test for comparison of dose response against sham-irradiated controls. ANOVA results were further adjusted using the Benjamini-Hochberg method to control the false discovery rate (fdr). Statistical tests were considered significant after stringent selection of ≥ 2-fold change and a P value ≤0.01 between each treatment group and its control.
RT2 lncRNA PCR Array Mouse lncFinder
For lncRNA expression analysis we used RT2 lncRNA PCR lncFinder array (product no. 330721, cat. no. LAMM-0012A-24; QIAGEN) with predispensed primers against 84 known lncRNAs in a 96-well plate format. Some of the controls spotted on the plate are: housekeeping genes (Actb1, B2m, Rplp0, Rnsk), mouse genomic DNA contamination (MGDC) and several reverse transcription (RTC) controls. Three animals/dose point/time point were used for the study. This set of animals was different from the animals used in microarray analysis. Prior to reverse transcription, genomic DNA was enzymatically eliminated. RT2 PreAMP cDNA Synthesis Kit (cat. no. 330451, QIAGEN) was used for reverse transcribing 200 ng of total RNA and selectively preamplifying the 84 lncRNAs using RT2 lncRNA PreAMP Primer Mixes (LBM-001Z) in a PCR reaction going for 12 cycles. The preamplified PCR mix was dispensed onto the RT2 lncRNA PCR lncFinder array and qRT-PCR was performed using RT2 SYBR™ Green qPCR Master Mix (QIAGEN). PCR was performed in the Applied Biosystems’ thermal cycler (cat. no. 7500). PCR steps included the holding stage at 95°C for 10 min followed by 40 cycles of alternate denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. A melt curve analysis was also done to ensure the specificity of the corresponding RT-PCR reactions. For data analysis, the theshold cycle number (Ct) values were exported to an Excel® file (Microsoft® Corp., Redmond, WA) and uploaded into the RT2 PCR array data analysis web portal (https://www.qiagen.com/dataanalysiscenter). Relative expression was calculated with reference to the gene (Gt(ROSA)26Sor).
Statistical analyses were performed using the freely available software R. Comparison between the groups for each time point (16, 24 and 48 h) was performed using ANOVA. Comparison of exposed and sham-exposed control groups with multiple comparison correction was performed using Dunnett’s post hoc test. Differences were considered significant at P < 0.05. Adjusted P values (each exposed group against the sham-exposed control group) were computed by the functions provided in the package multcomp (http://CRAN.R-project.org/package=multcomp) (57).
Individual qRT-PCR reactions using RT2 qPCR primer assays along with RT2 First Strand Synthesis kit and RT2 SYBR Green qPCR Master Mix (all from Qiagen) were performed for the following: Cdkn1a (assay ID no. PPM02901B), Trp53cor1 (assay ID no. LPM12776A), Dino (40) (FP- GCAATGGTGTGCCTGACTAT; RP- ACTTCTGGCTTCCCAGAG) and Rplp0 (assay ID no. PPM03561B) in the 48-h mouse blood samples; Cdkn1a, Dino, Trp53cor1, Tmevpg1 (assay ID no. LPM06775A), Gm14005 (assay ID no. LPM08583A) and Gapdh (assay ID no. PPM02946E) in the mouse embryonic fibroblast samples. Relative expression was calculated as: 2−dCt where dCt = Ct [test gene] – Ct [Gt(ROSA)26Sor/Rplp0/Gapdh]. Fold change was calculated as 2−ddCt, where ddCt is calculated as: dCt = Ct (test gene) – Ct (Rplp0/Gapdh); ddCt = dCt (irradiated) – dCt (control)
Mouse Embryonic Fibroblast Culture Conditions and Treatment
Mouse embryonic fibroblasts (MEFs) were a kind gift from Dr. Andre Nussenzweig (NCI, Bethesda, MD). These studies were done to investigate LncRNA changes after irradiation at the cellular level. Cells were cultured in DMEM (Invitrogen™, Grand Island, NY) supplemented with 15% fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin as described elsewhere (58). At 24 h after plating, cells were X-ray irradiated at room temperature (320 kV, dose rate of 2.3 Gy/min; Precision X-ray Inc., North Branford, CT) with final doses of 2, 4 and 8 Gy and incubated for 24 h at 37°C and 5% CO2. Cells were trypsinized and total RNA was isolated using the miRNeasy® Mini Kit (QIAGEN) according to the manufacturer’s protocol. Experiments were performed in triplicates.
RESULTS
Whole Genome Microarray Analysis Revealed Extensive Changes in lncRNA Expression Profiles in Mouse Blood Samples after Whole-Body Irradiation
We performed a whole genome expression analysis on total RNA extracted from mouse blood samples after irradiation at different doses using whole genome mouse microarrays (Agilent). These arrays can capture the expression patterns of 4,578 lncRNA probes from the transcriptome of the mouse. The raw data were submitted to the Gene Expression Omnibus, NCBI repository (GSE104121). Total RNA was isolated from mouse blood after whole-body irradiation with either 2, 4 or 8 Gy at 16, 24 and 48 h postirradiation. Sham-irradiated animals served as controls (Fig. 1A). Intensity values were normalized to the 75th percentile intensity of above background probes on each array. lncRNA probes that passed a cutoff of twofold change and a P value cutoff of <0.01 (Welch t test) were selected for further analysis. One-way ANOVA was performed, and multiple comparison correction was performed using Dunnett’s test. lncRNA probes that passed this stringent statistical analysis were considered as differentially expressed. Supplementary Table S1 (http://dx.doi.org/10.1667/RR14891.1.S1) lists the number of lncRNA probes that passed the selection criteria at each step. The expression levels of 38 lncRNAs changed significantly at 16 h postirradiation irrespective of radiation dose (Fig. 1B and C). Similarly, 24 lncRNAs were regulated at 24 h, and expression of 168 lncRNAs was changed at 48 h, postirradiation, irrespective of the dose (Fig. 1B, D and E). There were larger numbers of lncRNAs that were differentially regulated at 48 h postirradiation compared to both 16 and 24 h.
FIG. 1.
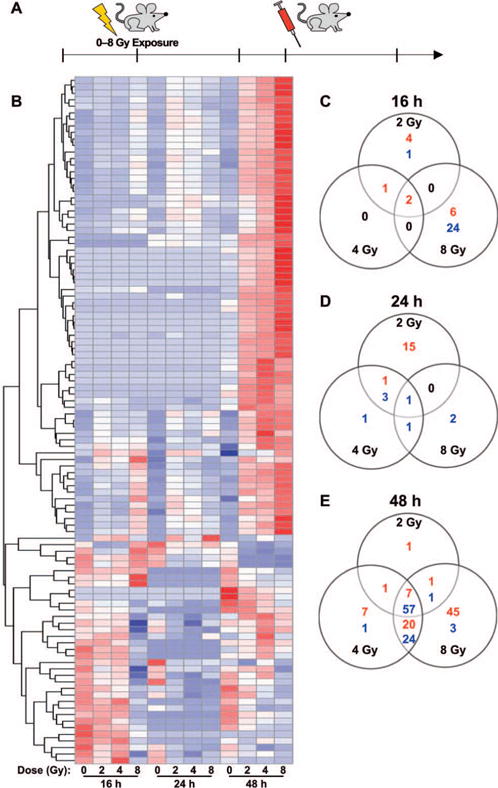
Whole genome expression analysis revealed lncRNA profiles changing in response to radiation. Panel A: Schematic showing experimental design (three mice/dose/time point were irradiated). Panel B: Heat map showing regulated lncRNA probes at 16, 24 and 48 h postirradiation. The lncRNA probes were clustered by hierarchical clustering using Euclidean distance metric. Panels C–E: Venn diagrams show the distribution of differentially regulated lncRNA probes across the dose points at 16, 24 and 48 h postirradiation, respectively. At each time point, ANOVA is followed by Dunnett’s t test to show the significant changes (FDR ≤ 0.01, intensity ratio ≥2 or ≤0.5) between irradiated (2, 4 and 8 Gy) and sham-irradiated controls. The number of up- and downregulated lncRNA probes are indicated in red and blue, respectively.
Interestingly, almost all the lncRNA expression changes at 16 h postirradiation were observed in the 8 Gy samples and not in the 2 and 4 Gy (Fig. 1C). We identified eight lncRNAs induced in response to low-dose radiation and early time point (2 Gy, 16 h) from the microarray data. However, we did not detect many lncRNAs changing at 24 h after 8 Gy irradiation. Sixty-four lncRNAs changed 48 h postirradiation across all radiation doses (Fig. 1E). In addition, compared to 44 lncRNAs that were commonly altered after 4 and 8 Gy irradiation, there were only two lncRNAs that were commonly altered at 48 h after 2 and 8 Gy irradiation (Fig. 1E). Supplementary Tables S2–S4 (http://dx.doi.org/10.1667/RR14891.LS2) list all the lncRNA probes that passed the statistical analysis. The genomic coordinates and the sequence (spotted on the array slides) of the lncRNAs differentially altered after irradiation were used to determine if any of these were annotated, using GENCODE version V15 and RefSeq databases. We found annotations for 15 lncRNAs, of which 11 had a sequence match in BLAST. The annotation information is included in Supplementary Tables S2–S4.
RT2 lncRNA PCR Array Mouse lncFinder Analysis Identified Several Disease-Associated lncRNAs Changing Because of Exposure to Radiation
Since most of the lncRNAs in the microarray were unannotated at the time we did these experiments, we also analyzed how known, experimentally verified lncRNAs might change upon whole-body irradiation. Towards this end, we utilized the RT2 lncRNA PCR Array mouse lncFinder assays, which profile the expression of 84 well-characterized lncRNAs. Correlation to involvement in disease manifestation such as cancer, gene expression, and biological processes have been suggested for each of these 84 lncRNAs. Assays were performed to span a wide range of doses (1, 2, 4, 8 and 12 Gy) across three different time points. A total of 17, 18 and 16 lncRNAs were respectively found to be significantly changing at 16, 24, and 48 h postirradiation regardless of the dose given. The raw Ct (threshold cycle) values observed in the lncFinder array qPCRs are listed in Supplementary Table S5 (http://dx.doi.org/10.1667/RR14891.1.S3). 1U0038B12Rik, Miat and Neat1 were exclusively regulated at 16 h postirradiation (Table 1). H19, Jpx, Kcnq1ot1 and Snhg1 were exclusively regulated at the 24 h postirradiation (Table 2). Firre and Meg3 were exclusively regulated at 48 h postirradiation (Table 3). 1700020I14Rik, Gm14005(Morrbid), Gas5 and Pvt1 were induced across all the time points in response to radiation (Tables 1–3). To better illustrate between the dose differences, lncRNAs (Pvt1, Gm14005, Gas5 and Snhg8) differentially expressed at all the time points in at least one of the doses have also been represented in graphical format. Among these, Pvt1, Gm14005 and Gas5 were upregulated in response to radiation where Snhg8 was downregulated (Fig. 2).
TABLE 1.
List of the lncRNAs Significantly Regulated at the 16 h Time Point across Different Doses Compared to Sham-Irradiated Controls
| lncRNA |
P value AoV |
0 Gy
|
1 Gy
|
2 Gy
|
4 Gy
|
8 Gy
|
12 Gy
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meana | SDb | Meana | SDb | P | Meana | SDb | P | Meana | SDb | P | Meana | SDb | P | Meana | SDb | P | ||
| 1110038B12Rik | 0.044 | 0.542 | 0.182 | 0.341 | 0.023 | 0.344 | 0.390 | 0.048 | 0.583 | 0.257 | 0.146 | 0.111 | 0.406 | 0.252 | 0.675 | 0.102 | 0.049 | 0.011 |
| 1700020I14Rik | 0.024 | 5.228 | 0.507 | 5.944 | 2.729 | 0.998 | 12.661 | 2.632 | 0.028 | 7.470 | 2.909 | 0.792 | 4.006 | 4.616 | 0.975 | 4.167 | 1.890 | 0.986 |
| 1810053B23Rik | 0.025 | 0.017 | 0.014 | 0.013 | 0.017 | 0.996 | 0.052 | 0.029 | 0.073 | 0.015 | 0.011 | 1.000 | 0.004 | 0.006 | 0.765 | 0.002 | 0.001 | 0.661 |
| AI504432 | 0.002 | 0.269 | 0.177 | 0.349 | 0.292 | 0.997 | 1.464 | 0.300 | 0.002 | 0.507 | 0.334 | 0.798 | 0.303 | 0.456 | 1.000 | 0.250 | 0.158 | 1.000 |
| C130071C03Rik | 0.004 | 0.001 | 0.000 | 0.001 | 0.000 | 0.726 | 0.001 | 0.000 | 0.054 | 0.002 | 0.000 | 0.001 | 0.001 | 0.000 | 0.034 | 0.001 | 0.000 | 0.125 |
| Crnde | 0.017 | 0.007 | 0.002 | 0.020 | 0.002 | 0.598 | 0.046 | 0.003 | 0.009 | 0.044 | 0.023 | 0.014 | 0.027 | 0.019 | 0.237 | 0.023 | 0.003 | 0.402 |
| Ftx | 0.001 | 0.007 | 0.007 | 0.005 | 0.008 | 0.993 | 0.031 | 0.010 | 0.002 | 0.004 | 0.003 | 0.950 | 0.002 | 0.001 | 0.660 | 0.001 | 0.000 | 0.635 |
| Gas5 | 0.005 | 2.284 | 1.337 | 4.794 | 0.752 | 0.198 | 4.755 | 1.774 | 0.208 | 7.515 | 1.078 | 0.004 | 6.251 | 1.410 | 0.025 | 8.062 | 2.094 | 0.002 |
| Gm14005 | 0.007 | 1.022 | 0.240 | 2.656 | 1.787 | 0.385 | 5.591 | 0.367 | 0.003 | 3.666 | 1.091 | 0.078 | 1.873 | 2.098 | 0.860 | 1.600 | 0.194 | 0.964 |
| Gm15832 | 0.004 | 0.126 | 0.026 | 0.239 | 0.103 | 0.815 | 0.396 | 0.233 | 0.157 | 0.263 | 0.088 | 0.694 | 0.576 | 0.202 | 0.012 | 0.701 | 0.136 | 0.002 |
| Malat1 | 0.03 | 40.931 | 12.166 | 32.029 | 11.536 | 0.998 | 39.792 | 9.247 | 1.000 | 30.557 | 14.837 | 0.996 | 133.120 | 83.912 | 0.032 | 38.508 | 10.196 | 1.000 |
| Miat | 0.004 | 0.001 | 0.000 | 0.001 | 0.000 | 0.730 | 0.001 | 0.000 | 0.040 | 0.002 | 0.000 | 0.001 | 0.001 | 0.000 | 0.035 | 0.001 | 0.000 | 0.128 |
| Neat1 | 0.027 | 1.612 | 0.170 | 1.705 | 0.880 | 1.000 | 2.201 | 0.708 | 1.000 | 1.608 | 0.686 | 1.000 | 14.078 | 10.704 | 0.018 | 2.506 | 0.680 | 0.999 |
| Pvt1 | 0.002 | 6.017 | 1.906 | 9.970 | 1.290 | 0.202 | 14.031 | 2.287 | 0.005 | 17.271 | 1.463 | 0.000 | 12.102 | 4.017 | 0.030 | 11.630 | 1.980 | 0.046 |
| Rab10os | 0.053 | 0.176 | 0.036 | 0.151 | 0.004 | 0.915 | 0.158 | 0.053 | 0.974 | 0.130 | 0.051 | 0.547 | 0.172 | 0.058 | 1.000 | 0.062 | 0.020 | 0.024 |
| Snhg8 | 0.015 | 0.213 | 0.061 | 0.152 | 0.037 | 0.462 | 0.131 | 0.062 | 0.226 | 0.084 | 0.066 | 0.031 | 0.081 | 0.039 | 0.028 | 0.041 | 0.002 | 0.005 |
| Tmevpg1 | 0.033 | 0.018 | 0.008 | 0.012 | 0.008 | 0.586 | 0.002 | 0.001 | 0.030 | 0.008 | 0.010 | 0.191 | 0.002 | 0.000 | 0.025 | 0.002 | 0.001 | 0.028 |
Notes. Mouse lncFinder arrays were utilized to profile the status of 84 characterized lncRNAs at different radiation doses (1, 2, 4, 8 and 12 Gy). RNA was isolated 16 h postirradiation, converted to cDNA, and PCR assays were performed according to the manufacturer’s protocol. Statistical significance was calculated using ANOVA along with Dunnett’s test to compute P values.
Mean = average relative expression compared to endogenous control [Gt(ROSA)26Sor].
SD = standard deviation in irradiated vs. sham-irradiated samples.
TABLE 2.
List of the lncRNAs Significantly Regulated at the 24 h Time Point across Different Doses Compared to Sham-Irradiated Controls
| lncRNA |
P value AoV |
0 Gy
|
1 Gy
|
2 Gy
|
4 Gy
|
8 Gy
|
12 Gy
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meana | SDb | Meana | SDb | P | Meana | SDb | P | Meana | SDb | P | Meana | SDb | P | Meana | SDb | P | ||
| 1700020I14Rik | 0.01 | 2.675 | 0.682 | 3.800 | 0.802 | 1.000 | 15.832 | 10.331 | 0.103 | 19.181 | 6.911 | 0.034 | 12.504 | 4.794 | 0.282 | 24.040 | 8.644 | 0.007 |
| 1810053B23Rik | 0.025 | 0.004 | 0.002 | 0.010 | 0.007 | 1.000 | 0.152 | 0.116 | 0.349 | 0.305 | 0.181 | 0.019 | 0.219 | 0.121 | 0.106 | 0.214 | 0.085 | 0.116 |
| AI504432 | 0.01 | 0.102 | 0.033 | 0.229 | 0.102 | 1.000 | 3.042 | 2.645 | 0.293 | 3.354 | 1.223 | 0.219 | 1.441 | 0.453 | 0.869 | 6.978 | 3.822 | 0.005 |
| Airn | 0.028 | 0.070 | 0.018 | 0.079 | 0.032 | 0.983 | 0.071 | 0.016 | 1.000 | 0.044 | 0.028 | 0.495 | 0.018 | 0.017 | 0.047 | 0.033 | 0.015 | 0.205 |
| Crnde | 0.025 | 0.007 | 0.003 | 0.014 | 0.006 | 0.985 | 0.031 | 0.020 | 0.368 | 0.052 | 0.021 | 0.029 | 0.053 | 0.010 | 0.025 | 0.043 | 0.027 | 0.091 |
| Ftx | 0.017 | 0.003 | 0.001 | 0.007 | 0.003 | 1.000 | 0.556 | 0.493 | 0.039 | 0.629 | 0.186 | 0.019 | 0.289 | 0.126 | 0.416 | 0.418 | 0.065 | 0.143 |
| Gas5 | 0.002 | 3.845 | 0.098 | 6.947 | 1.629 | 0.061 | 9.618 | 1.005 | 0.001 | 8.427 | 1.567 | 0.006 | 7.192 | 2.111 | 0.041 | 9.560 | 0.737 | 0.001 |
| Gm14005 | 0.003 | 0.366 | 0.072 | 1.409 | 0.041 | 0.998 | 11.350 | 8.595 | 0.041 | 15.744 | 3.770 | 0.005 | 9.354 | 3.249 | 0.106 | 15.536 | 4.554 | 0.005 |
| Gm15051 | 0 | 0.001 | 0.000 | 0.005 | 0.001 | 0.986 | 0.021 | 0.017 | 0.143 | 0.046 | 0.008 | 0.001 | 0.011 | 0.004 | 0.673 | 0.052 | 0.017 | 0.000 |
| H19 | 0.016 | 0.002 | 0.002 | 0.002 | 0.002 | 1.000 | 0.032 | 0.026 | 0.175 | 0.047 | 0.027 | 0.028 | 0.022 | 0.008 | 0.478 | 0.049 | 0.017 | 0.021 |
| Jpx | 0 | 0.058 | 0.003 | 0.050 | 0.028 | 0.891 | 0.019 | 0.007 | 0.007 | 0.007 | 0.002 | 0.001 | 0.010 | 0.003 | 0.001 | 0.002 | 0.003 | 0.000 |
| Kcnq1ot1 | 0 | 0.166 | 0.004 | 0.153 | 0.031 | 0.956 | 0.119 | 0.047 | 0.157 | 0.035 | 0.012 | 0.000 | 0.026 | 0.022 | 0.000 | 0.018 | 0.014 | 0.000 |
| Pvt1 | 0.003 | 3.982 | 0.596 | 7.793 | 1.632 | 0.690 | 13.114 | 3.533 | 0.069 | 16.004 | 4.856 | 0.015 | 21.608 | 7.787 | 0.001 | 13.951 | 1.466 | 0.045 |
| Rab10os | 0.054 | 0.152 | 0.027 | 0.186 | 0.027 | 0.346 | 0.179 | 0.019 | 0.540 | 0.187 | 0.015 | 0.318 | 0.125 | 0.022 | 0.565 | 0.153 | 0.033 | 1.000 |
| Snhg1 | 0.014 | 0.144 | 0.044 | 0.166 | 0.077 | 0.947 | 0.099 | 0.038 | 0.536 | 0.066 | 0.003 | 0.124 | 0.051 | 0.008 | 0.057 | 0.046 | 0.017 | 0.044 |
| Snhg8 | 0.003 | 0.196 | 0.056 | 0.235 | 0.079 | 0.769 | 0.153 | 0.029 | 0.694 | 0.074 | 0.037 | 0.031 | 0.065 | 0.021 | 0.020 | 0.089 | 0.034 | 0.060 |
| Tmevpg1 | 0.001 | 0.028 | 0.012 | 0.014 | 0.006 | 0.074 | 0.008 | 0.007 | 0.008 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
Notes. Mouse lncFinder arrays were utilized to profile the status of 84 characterized lncRNAs at different radiation doses (1, 2, 4, 8 and 12 Gy). RNA was isolated 24 h postirradiation, converted to cDNA, and PCR assays were performed according to the manufacturer’s protocol. Statistical significance was calculated using ANOVA along with Dunnett’s test to compute P values.
Mean = average relative expression compared to endogenous control [Gt(ROSA)26Sor].
SD = standard deviation in irradiated vs. sham-irradiated samples.
TABLE 3.
List of the lncRNAs Significantly Regulated at the 48 h Time Point across Different Doses Compared to Sham-Irradiated Controls
| lncRNA |
P value AoV |
0Gy
|
1 Gy
|
2 Gy
|
4 Gy
|
8 Gy
|
12 Gy
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meana | SDb | Meana | SDb | P | Meana | SDb | P | Meana | SDb | P | Meana | SDb | P | Meana | SDb | P | ||
| 1700020I14Rik | 0.007 | 10.976 | 4.295 | 10.462 | 2.859 | 1.000 | 29.318 | 3.624 | 0.021 | 30.273 | 3.121 | 0.015 | 27.433 | 11.723 | 0.039 | 19.843 | 8.714 | 0.381 |
| 1810053B23Rik | 0.041 | 0.156 | 0.094 | 0.108 | 0.045 | 0.614 | 0.168 | 0.028 | 0.998 | 0.104 | 0.008 | 0.550 | 0.069 | 0.027 | 0.143 | 0.040 | 0.026 | 0.039 |
| AI504432 | 0.007 | 0.891 | 0.261 | 2.095 | 0.643 | 0.990 | 8.338 | 1.033 | 0.078 | 11.980 | 2.114 | 0.008 | 10.920 | 3.340 | 0.015 | 9.919 | 7.349 | 0.029 |
| Airn | 0.022 | 0.161 | 0.033 | 0.237 | 0.166 | 0.998 | 0.177 | 0.027 | 1.000 | 0.194 | 0.007 | 1.000 | 0.228 | 0.188 | 0.999 | 1.049 | 0.686 | 0.014 |
| Firre | 0.055 | 0.319 | 0.104 | 0.277 | 0.328 | 1.000 | 0.099 | 0.063 | 0.700 | 0.185 | 0.072 | 0.933 | 0.230 | 0.173 | 0.987 | 0.783 | 0.438 | 0.128 |
| Gas5 | 0.005 | 2.913 | 0.995 | 3.332 | 0.556 | 0.998 | 7.351 | 1.142 | 0.026 | 9.076 | 2.705 | 0.003 | 6.009 | 2.296 | 0.144 | 5.902 | 1.172 | 0.164 |
| Gm14005 | 0.019 | 3.307 | 1.358 | 6.299 | 5.282 | 0.982 | 23.191 | 6.985 | 0.026 | 23.356 | 9.173 | 0.025 | 20.808 | 12.497 | 0.053 | 11.737 | 3.903 | 0.529 |
| Gm15051 | 0 | 0.011 | 0.001 | 0.016 | 0.012 | 0.986 | 0.044 | 0.005 | 0.060 | 0.067 | 0.008 | 0.002 | 0.069 | 0.011 | 0.001 | 0.088 | 0.030 | 0.000 |
| Gm15832 | 0.002 | 0.184 | 0.051 | 0.243 | 0.111 | 0.879 | 0.157 | 0.052 | 0.994 | 0.520 | 0.160 | 0.003 | 0.139 | 0.052 | 0.955 | 0.301 | 0.047 | 0.403 |
| Malat1 | 0.047 | 126.011 | 76.639 | 103.769 | 115.408 | 0.998 | 32.877 | 6.965 | 0.590 | 71.807 | 40.252 | 0.908 | 64.633 | 51.955 | 0.861 | 288.616 | 153.626 | 0.153 |
| Meg3 | 0.002 | 0.081 | 0.070 | 0.119 | 0.089 | 1.000 | 0.048 | 0.034 | 1.000 | 0.091 | 0.018 | 1.000 | 0.275 | 0.236 | 0.912 | 1.381 | 0.744 | 0.002 |
| Pvt1 | 0.05 | 3.802 | 1.556 | 5.026 | 1.518 | 0.648 | 7.188 | 0.778 | 0.024 | 4.543 | 0.097 | 0.917 | 3.758 | 1.583 | 1.000 | 4.346 | 1.246 | 0.974 |
| Snhg8 | 0.027 | 0.348 | 0.012 | 0.450 | 0.139 | 0.531 | 0.272 | 0.073 | 0.758 | 0.315 | 0.051 | 0.987 | 0.159 | 0.078 | 0.091 | 0.220 | 0.127 | 0.339 |
| Tmevpg1 | 0 | 0.031 | 0.016 | 0.068 | 0.018 | 0.005 | 0.012 | 0.006 | 0.170 | 0.012 | 0.007 | 0.196 | 0.002 | 0.001 | 0.025 | 0.003 | 0.001 | 0.035 |
| Tug1 | 0.026 | 3.939 | 2.013 | 3.614 | 1.706 | 0.998 | 4.774 | 0.649 | 0.882 | 6.634 | 1.021 | 0.084 | 4.357 | 1.040 | 0.992 | 7.056 | 0.423 | 0.042 |
Notes. Mouse lncFinder arrays were utilized to profile the status of 84 characterized lncRNAs at different radiation doses (1, 2, 4, 8 and 12 Gy). RNA was isolated 48 h postirradiation, converted to cDNA, and PCR assays were performed according to the manufacturer’s protocol. Statistical significance was calculated using ANOVA along with Dunnett’s test to compute P values.
Mean = average relative expression compared to endogenous control [Gt(ROSA)26Sor].
SD = standard deviation in irradiated vs. sham-irradiated samples.
FIG. 2.
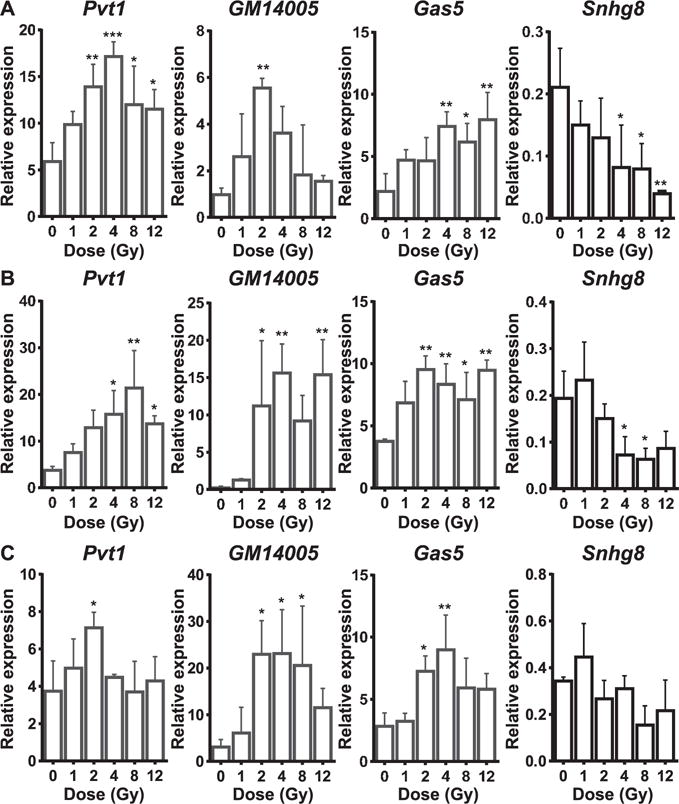
RT2 lncRNA PCR Array mouse lncFinder profiling of several characterized lncRNAs as radiation targets. Panels A–C: Bar graphs show dose responsiveness of Pvtl, Gm14005 and Gas5 and Snhg8 at 16, 24 and 48 h postirradiation, respectively. Relative expressions (2−dCt) are plotted across doses. dCt was calculated against endogenous control gene, Gt(ROSA)26Sor. All graphs show mean ± SD from three animals/dose/time point. ANOVA followed by a Dunnett’s post hoc test (treatments compared to control) was performed to compute statistical significance. *P < 0.05; **P < 0.01 and ***P < 0.001.
Microarray and lncFinder Data Comparison Revealed 2 lncRNAs Commonly Regulated in Both Datasets
To find overlap between the microarray and the lncFinder data sets, we compared the list of 15 annotated lncRNAs from the microarray data to the lncFinder data. We found only two lncRNAs regulated in both the microarray data and the lncFinder data. 1110038B12Rik was found to be downregulated at 16 h postirradiation in both the microarray and the lncFinder qPCR assay (Fig. 3A and B). Tug1 was another lncRNA that was found to be induced in both the microarray and the lncFinder data 48 h postirradiation (Fig. 3C and D). This comparison also succeeded in validating the microarray data at the qRT-PCR level.
FIG. 3.
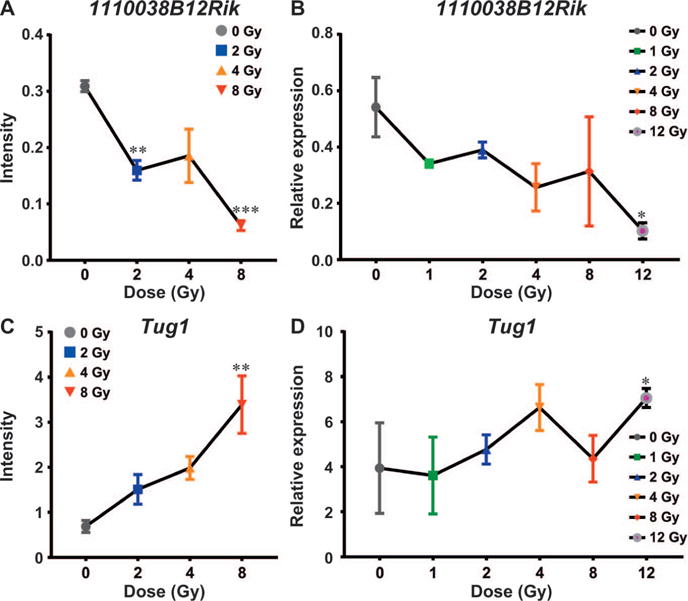
Comparison of 1110038B12Rik and Tug1 between the microarray and the lncFinder data. Panels A and B: Line graphs showing downregulation of 1110038B12Rik in response to increasing radiation doses at the 16 h time point in the microarray and lncFinder data, respectively. Panels C and D: Line graphs showing upregulation of Tug1 in response to increasing radiation doses at the 48 h time point in the microarray and lncFinder data. *P < 0.05; **P < 0.01 and ***P < 0.001.
Concomitant Induction of Cdkn1a, Trp53cor1 and Dino in Response to Radiation
RT-PCR analysis revealed induction of the canonical p53 targets, Cdkn1a and the Trp53cor1 lncRNA upon irradiation (Fig. 4A and B). Expression of a novel p53 target, lncRNA Dino was also induced upon irradiation in a dose-dependent manner. We could not detect any expression of Dino in the sham-irradiated controls (Fig. 4C). Therefore, the dose-dependent fold induction was calculated with respect to 1 Gy (Fig. 4D). Interestingly, Dino is transcribed from the same strand as Cdkn1a partially overlapping Cdkn1a locus, while Trp53cor1 is transcribed from the opposite strand upstream from the Cdkn1a locus on mouse chromosome 17 (Fig. 4E). Cdkn1a, Trp53cor1 and Dino were also found to be regulated as a response to radiation in MEFs (Fig. 5A). Gm14005 (Morrbid), myeloid cell specific, was not altered upon irradiation in the MEFs (Fig. 5B).
FIG. 4.
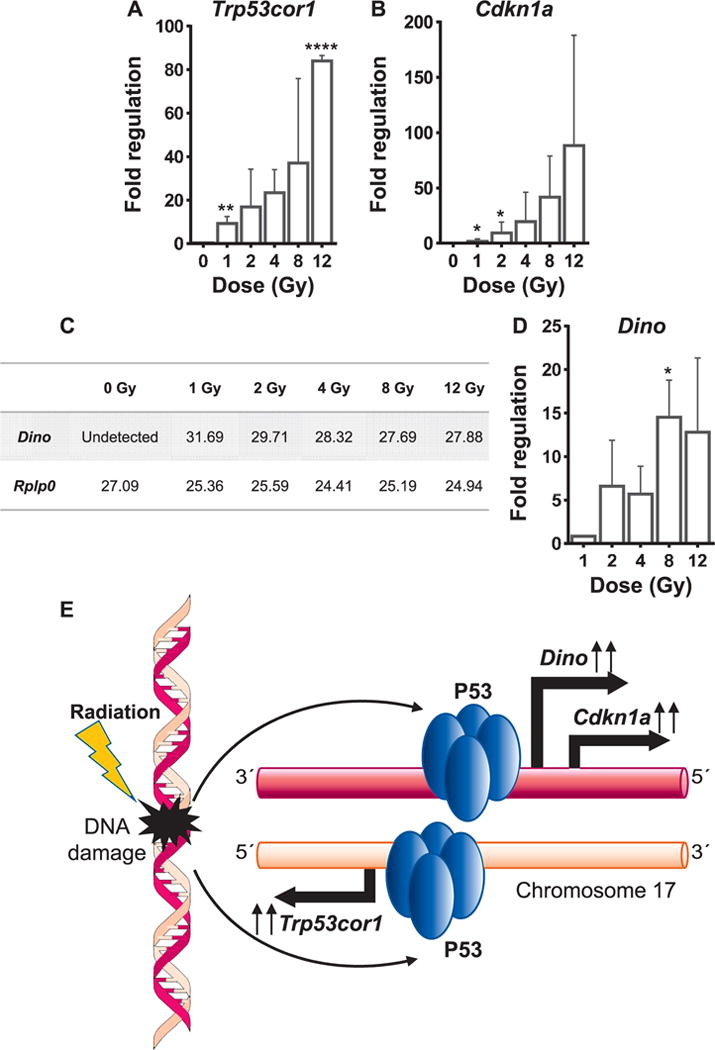
Concomitant induction of Trp53cor1, Cdknla and Dino. Panels A and B: qRT-PCR analysis showing fold change in the expression of Trp53cor1 and Cdknla, respectively, at different doses compared to 0 Gy at 48 h postirradiation. Panel C: Table showing mean Ct (threshold cycle number) values of Dino and Rplp0 observed in the qRT-PCR assays performed on the 48 h time point samples. Panel D: qRT-PCR analysis showing fold change values of Dino at various doses compared to 1 Gy at the 48-h time point. Rplp0 was used as the normalizing control. All graphs show mean ± SD from three animals/dose/time point. Statistical significance was calculated using one-way ANOVA, and Dunnett’s test was performed to calculate pair-wise P values of treatment sets compared to the control set. Panel E: Schematic showing concomitant induction of Cdknla, Trp53cor1 and Dino from the mouse chromosome 17 by P53. *P < 0.05, **P < 0.01 and ****P < 0.0001
FIG. 5.
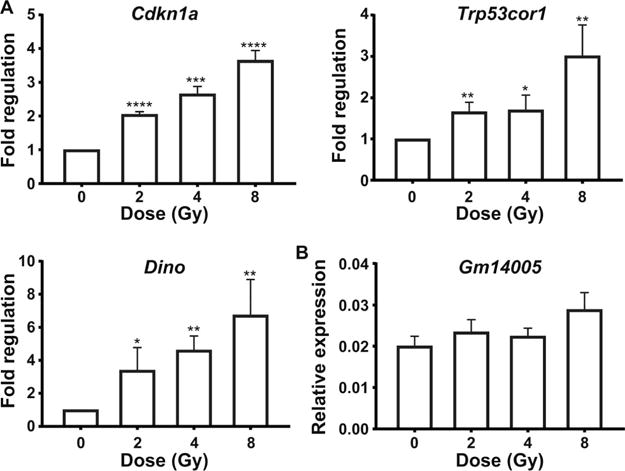
Status of p53 LncRNA targets and Gm14005 in mouse embryonic fibroblast cells. Panel A: qRT-PCR analysis for Cdkn1a, Trp53cor1 and Dino in MEFs. Panel B: qRT-PCR analysis for Gm14005 in MEFs. Each data point depicts mean ± SD relative expression from three independent experiments across different doses compared to 0 Gy. Gapdh was used as the endogenous normalizing control to calculate fold changes. Statistical significance was calculated using unpaired t test. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Identification of Tmevpgl in this Mouse Model as a Potential Radiation-Altered Blood-Based Biomarker
The expression of Tmevpg1 decreases in a dose-dependent manner at 16 and 24 h postirradiation (Fig. 6A and B). The expression of Tmevpg1 is seen to increase transiently in the 1 Gy irradiated sample compared to the control at the 48-h time point. All the higher doses repressed the expression of Tmevpg1 at 48 h postirradiation (Fig. 6C). We could not detect any expression or induction of Tmevpg1 in MEFs (data not shown). Interestingly, the expression levels of Tmevpg1 correlated well with the total RNA recovered from equal volumes of blood from the control and 2–12 Gy irradiated samples (Fig. 6D). Tmevpg1 might be considered as a potential radiation-responsive blood-based biomarker.
FIG. 6.
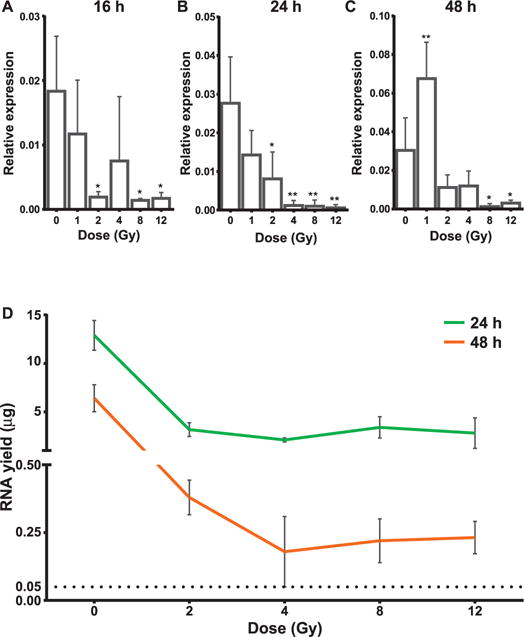
Dose-dependent downregulation of Tmevpg1. Panels A–C: Bar graphs showing the dose-responsive downregulation of Tmevpg1 at 16, 24 and 48 h postirradiation, respectively. Relative expressions (2−dCt) are plotted across doses. dCt was calculated against endogenous control gene, Gt(ROSA)26Sor. All graphs show mean ± SD from three animals/dose/time point. ANOVA followed by a Dunnett’s post hoc test (treatments compared to control) was performed to compute statistical significance. *P < 0.05 and **P < 0.01. Panel D: Concomitant reduction in the total RNA yield in a dose-dependent manner at all time points. Graph shows mean ± SD. N = 3 for all the samples.
DISCUSSION
The last decade has seen a significant increase in the description and understanding of lncRNAs once considered to be part of “junk DNA”. LncRNA genes are being shown to play important roles in development and diseases. They can regulate protein coding genes either in cis or trans, making them important chromatin modifiers. Expression of the lncRNA Xist leads to inactivation of the X chromosome by recruiting the polycomb repressive complex2 (PRC2) (59). Likewise, lncRNA HOTAIR recruits PRC2 to silence the HOXD locus located on a different chromosome (60). P53 exerts its actions of global gene repression through induction of lincRNA-p21 (Trp53cor1) (47). There is clear evidence now that lncRNAs play important roles in nuclear compartmentalization (61). Numerous such examples show that lncRNAs are emerging as critical players in transcriptional regulation, chromatin modulation and assembling protein complexes. Given the crucial functions lncRNAs play in normal physiology, it is no surprise that changes in their expression are reported in disease conditions such as cancer (62), cardiovascular pathology (18), metabolic disorders (63) and nervous system conditions (64, 65). lncRNA transcripts also exhibit high temporal and tissue-specific expression, are resistant to plasma RNase activity, and are therefore relatively stable in plasma for longer periods of time (15). All these characteristics, combined with the knowledge that lncRNA expression is modulated in response to radiation, make them attractive candidate biomarkers for radiation biodosimetry studies.
Very few published studies have delved into identifying lncRNA signatures in response to radiation. Beer et al. have shown that radiation regulates several lncRNAs in a time-dependent manner in PBMCs (35). The caveat in their study is that the radiation dose chosen (60 Gy) was very high and might not be encountered even in accidental situations. In another published study, the response of bronchial epithelial cells to varied doses of radiation (2, 4 and 8 Gy) was investigated (38). The effects of both low- and high-dose radiation on lncRNA expression have been studied in mouse thymocytes (36). Tang et al. have shown that lncRNAs may coordinate the response of mammary tissue to low-dose ionizing radiation by modulating the expression of protein coding genes (66). To our knowledge, there are no published studies on changes in the lncRNA expression patterns after whole-body irradiation in animal models. Therefore, it was of interest to explore the potential of lncRNAs as biomarkers for radiation biodosimetry and as potential indicators of organ-specific injury. We examined the lncRNA expression changes in the blood samples of whole-body irradiated mice compared to sham-irradiated mice. We performed the study using three different doses (2, 4 and 8 Gy) and collected RNA at three different time points (16, 24 and 48 h) postirradiation. We have identified 41 lncRNAs as early time point (16 h) markers for the 8 Gy dose. A substantial number of lncRNA expression changes (304) were observed 48 h postirradiation, of which most changed after 8 Gy irradiation. At only 48 h after low-dose (2 Gy) irradiation, we observed a considerable number of lncRNA expression changes. Most of these were also observed at the higher dose of 8 Gy, although there were 20 lncRNAs whose expression changed only after 2 Gy irradiation 48 h later. At the 24-h time point, we found a greater number of lncRNAs changing after 2 and 4 Gy compared to 8 Gy irradiation.
Given that lncRNA biology is both new and complex, there are limited data on their function and mechanisms of action. We found annotations for 15 of the lncRNAs spotted on the arrays. Since we did not find several well-known lncRNAs even after the annotation, we performed the RT2 lncRNA PCR Array mouse lncFinder assays to profile the status of 84 lncRNAs, which have been reported to have some disease correlation in the literature. We did not find any lncRNAs differentially expressed in response to a low dose of 1 Gy. Gas5 and Pvt1 were prominent lncRNAs that changed in response to intermediate- to high-dose radiations at the 16- and 24-h time points. Snhg8 was also changed in response to intermediate- to high-dose radiation at the 16-h time point. The growth arrest-specific transcript 5 (GAS5) has been shown to have tumor suppressor properties in several cancers. Radiation leads to DNA damage, activating the p53-dependent DNA repair pathways. Several of the p53-dependent lncRNA targets (Pvt1, Trp53cor1, Tug1, Dino) changed in response to radiation. Tug1 has previously been shown to be induced in response to radiation in several normal and cancer cell lines (35, 67). Tug1 induction in response to high-dose radiation was observed in both the microarray and the lncFinder analysis at the late time point of 48 h. We observed concomitant induction of Cdkn1a, Trp53cor1 and Dino. All three genes are important p53 targets and play significant roles in downstream p53 responses. It appears that their adjacent location on mouse chromosome 17 leads to their concomitant induction. This signature was detected in the mouse blood, and validated in MEFs. Interestingly Cdkn1a, Trp53cor1 and Dino genes appear in close proximity in humans on chromosome 6 (40, 47).
An important finding from this study is the identification of Tmevpg1 as an early downregulated lncRNA even at a dose of 2 Gy. Downregulation of Tmevpg1 was also observed from 2 Gy onwards at 24 and 48 postirradiation. Tmevpg1 gene locus is in a cluster of genes along with IFN-gamma and homologs of interleukin-10 in both mice and humans. It was found to be expressed in PBMCs and downregulated upon immune stimulation (52). It has been proposed that Tmevpg1 might be involved in the control of the Ifng expression. Previously, our group has reported induction of immune regulatory genes (IFN-related signature in particular) in response to radiation (68). Radiation-induced alteration of Tmevpg1, even at lower doses and early time points, corroborates well with these earlier observations. It can be proposed that Tmevpg1 acts as a radiation-responsive lncRNA at higher doses in this mouse model, just like another lymphocyte microRNA biomarker miR-150 as reported previously by others.
Gm14005 (Morrbid) is another important lncRNA identified in this study as a radiation-responsive lncRNA at all investigated time points. Higher levels of Morrbid are observed in hyper-eosinophilic patients. It is expressed in the cells of the myeloid lineage (neutrophils, eosinophils and monocytes) and protects these cells against apoptosis (53). Radiation causes a dramatic reduction in the peripheral blood lymphocyte count and a transient rise in granulocyte count, leading to an increase in the relative percentage of the neutrophils and other cells in the whole blood. We speculate that the observed upregulation of Morrbid is due to an increase in the relative percentage of the cells of the myeloid origin in the irradiated samples compared to the controls. To the best of our knowledge, this is the first study reporting Dino, Gm14005 and Tmevpg1 as radiation-responsive lncRNAs in a mouse model after whole-body irradiation.
Based on the current findings, we propose that lncRNAs can be developed as biomarkers to assess radiation biodosimetry. It is likely that a family of RNAs will be useful to not only estimate whole-body dose but also organ-specific damage, which is a concept we are pursuing. We are currently working with minipig and non-human primate samples to validate the lncRNA signatures in higher order mammals. Owing to the tissue specificity observed in lncRNA expression patterns, these might help to evaluate normal tissue toxicity and injury during clinical radiation therapy.
Supplementary Material
Table S1. Total number of lncRNA probes on the array (4,578), details of the selection criteria that were followed and number of lncRNA probes passing the selection criteria at each step, for any of the dose points (2, 4 and 8 Gy) for the time points studied.
Tables S2–S4. One-way ANOVA was performed, and comparisons were made among 2, 4 and 8 Gy irradiated versus nonirradiated control samples for respective time points, as explained in Materials and Methods. Tables depict Agilent’s systematic identification and accession information. Group-specific P values ≤ 0.01 are highlighted in green; intensity ratios ≥ 2 are highlighted in red; intensity ratios ≤ 0.5 are highlighted in blue. Annotation information in the form of RefSeq ID and lncRNA name has been included for some of the lncRNAs (“-” in the Blast matched column indicates no BLAST match observed; “blast” in the Blast matched column indicates BLAST match was observed).
Table S5. Raw Ct value (threshold cycle number) observed in the RT2 MouseFinder lncRNA arrays at the 16 h time point from triplicate experiments across all the samples.
Acknowledgments
This study was supported by the NIH Intramural Research Program, National Cancer Institute, Center for Cancer Research and National Institute of Allergy and Infectious Diseases (IAA no. NRC-13028). We thank Patricia Rivera-Solis and Katherine Wilsdon for their excellent technical assistance. We also thank Dr. Konrad Huppi (NCI, NIH) for his expert advice.
Footnotes
Editor’s note. The online version of this article (DOI: 10.1667/RR14891.1) contains supplementary information that is available to all authorized users.
References
- 1.Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, et al. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep. 2011;5:202–12. doi: 10.1001/dmp.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong KF, Siu LLP, Ainsbury E, Moquet J. Cytogenetic biodosimetry: What it is and how we do it. Hong Kong Med J. 2013;19:168–73. [PubMed] [Google Scholar]
- 3.Romm H, Oestreicher U, Kulka U. Cytogenetic damage analysed by the dicentric assay. Ann Ist Super Sanita. 2009;45:251–9. [PubMed] [Google Scholar]
- 4.Dörr AH, Abend M, Blakely WF, Bolduc DL, Boozer D, Costeira T, et al. Using Clinical Signs and Symptoms for Medical Management of Radiation Casualties – 2015 NATO Exercise. Radiat Res. 2017;187:273–86. doi: 10.1667/RR14619.1. [DOI] [PubMed] [Google Scholar]
- 5.Demidenko E, Williams BB, Swartz HM, Demidenko E, Williams BB, Swartz HM. Radiation dose prediction using data on time to emesis in the case of nuclear terrorism radiation. Radiat Res. 2009;171:310–9. doi: 10.1667/RR1552.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Lemos Pinto MMP, Santos NFG, Amaral A. Current status of biodosimetry based on standard cytogenetic methods. Radiat Environ Biophys. 2010;49:567–81. doi: 10.1007/s00411-010-0311-3. [DOI] [PubMed] [Google Scholar]
- 7.Chao NJ. Accidental or intentional exposure to ionizing radiation: Biodosimetry and treatment options. Exp Hematol. 2007;35:24–7. doi: 10.1016/j.exphem.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Marchetti F, Coleman MA, Jones IM, Wyrobek AJ. Candidate protein biodosimeters of human exposure to ionizing radiation. Int J Radiat Biol. 2006;82:605–39. doi: 10.1080/09553000600930103. [DOI] [PubMed] [Google Scholar]
- 9.Sproull M, Kramp T, Tandle A, Shankavaram U, Camphausen K. Multivariate analysis of radiation responsive proteins to predict radiation exposure in total-body irradiation and partial-body irradiation models. Radiat Res. 2017;187:251–8. doi: 10.1667/RR14558.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sproull M, Camphausen K. State-of-the-art advances in radiation biodosimetry for mass casualty events involving radiation exposure. Radiat Res. 2016;186:423–35. doi: 10.1667/RR14452.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sproull M, Kramp T, Tandle A, Shankavaram U, Camphausen K. Serum amyloid A as a biomarker for radiation exposure. Radiat Res. 2015;184:14–23. doi: 10.1667/RR13927.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veneziano D, Di Bella S, Nigita G, Laganà A, Ferro A, Croce CM. Noncoding RNA: Current deep sequencing data analysis approaches and challenges. Hum Mutat. 2016;37:1283–98. doi: 10.1002/humu.23066. [DOI] [PubMed] [Google Scholar]
- 13.Pennisi E. ENCODE Project writes eulogy for junk DNA. Science. 2012;337:1159–61. doi: 10.1126/science.337.6099.1159. [DOI] [PubMed] [Google Scholar]
- 14.Evan JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Invest. 2016;126:2775–82. doi: 10.1172/JCI84421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–81. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–14. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Syed J, Sugiyama H. RNA-DNA Triplex formation by long noncoding RNAs. Cell Chem Biol. 2016;23:1325–33. doi: 10.1016/j.chembiol.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Archer K, Broskova Z, Bayoumi AS, Teoh JP, Davila A, Tang Y, et al. Long non-coding RNAs as master regulators in cardiovascular diseases. Int J Mol Sci. 2015;16:23651–67. doi: 10.3390/ijms161023651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bar C, Chatterjee S, Thum T. Long noncoding RNAs in cardiovascular pathology, diagnosis, and therapy. Circulation. 2016;134:1484–99. doi: 10.1161/CIRCULATIONAHA.116.023686. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Xue W, Lv J, Han P, Liu Y, Cui B. Identification of potential long non-coding RNA biomarkers associated with the progression of colon cancer. Oncotarget. 2017;8:75834–43. doi: 10.18632/oncotarget.17924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Li H, Zhang L, Zhang C, Yan W. Identification of novel long non-coding RNA biomarkers for prognosis prediction of papillary thyroid cancer. Oncotarget. 2017;8:46136–44. doi: 10.18632/oncotarget.17556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou M, Zhang Z, Zhao H, Bao S, Cheng L, Sun J. An immunerelated six-lncRNA signature to improve prognosis prediction of glioblastoma multiforme. Mol Neurobiol. 2017:1–14. doi: 10.1007/s12035-017-0572-9. [DOI] [PubMed] [Google Scholar]
- 23.Tu Z, He D, Deng X, Xiong M, Huang X, Li X, et al. An eight-long non-coding RNA signature as a candidate prognostic biomarker for lung cancer. Oncol Rep. 2016;36:215–22. doi: 10.3892/or.2016.4817. [DOI] [PubMed] [Google Scholar]
- 24.Xuan L, Sun L, Zhang Y, Huang Y, Hou Y, Li Q, et al. Circulating long non-coding RNAs NRON and MHRT as novel predictive biomarkers of heart failure. J Cell Mol Med. 2017;9:1803–14. doi: 10.1111/jcmm.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Wang Y-Q, Weng W-W, Zhang Q-Y, Yang X-Q, Gan H-L, et al. A serum-circulating long noncoding RNA signature can discriminate between patients with clear cell renal cell carcinoma and healthy controls. Oncogenesis. 2016;5:1–7. doi: 10.1038/oncsis.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He B, Zeng J, Chao W, Chen X, Huang Y. Serum long non-coding RNAs MALAT1, AFAP1-AS1 and AL359062 as diagnostic and prognostic biomarkers for nasopharyngeal carcinoma. Oncotarget. 2017;8:41166–77. doi: 10.18632/oncotarget.17083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhang K, Luo Z, Liu L, Wu L, Liu J. Circulating long non-coding HOX transcript antisense intergenic ribonucleic acid in plasma as a potential biomarker for diagnosis of breast cancer. Thorac Cancer. 2016;7:627–32. doi: 10.1111/1759-7714.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ke D, Li H, Zhang Y, An Y, Fu H, Fang X. The combination of circulating long noncoding RNAs AK001058, INHBA-AS1, MIR4435-2HG, and CEBPA-AS1 fragments in plasma serve as diagnostic markers for gastric cancer. Oncotarget. 2017;8:21516–25. doi: 10.18632/oncotarget.15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu ZL, Shen CT, Sun ZK, Wei WJ, Zhang XY, Song HJ, et al. Circulating long non-coding RNAs act as biomarkers for predicting 131I uptake and mortality in papillary thyroid cancer patients with lung metastases. Cell Physiol Biochem. 2016;40:1377–90. doi: 10.1159/000453190. [DOI] [PubMed] [Google Scholar]
- 30.Yang XD, Xu HT, Xu XH, Ru G, Liu W, Zhu JJ, et al. Knockdown of long non-coding RNA HOTAIR inhibits proliferation and invasiveness and improves radiosensitivity in colorectal cancer. Oncol Rep. 2016;35:479–87. doi: 10.3892/or.2015.4397. [DOI] [PubMed] [Google Scholar]
- 31.Lu H, He Y, Lin L, Qi Z, Ma L, Li L, et al. Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumor Biol. 2016;37:1683–91. doi: 10.1007/s13277-015-3946-5. [DOI] [PubMed] [Google Scholar]
- 32.Jin C, Yan B, Lu Q, Lin Y, Ma L. The role of MALAT1/miR-1/slug axis on radioresistance in nasopharyngeal carcinoma. Tumor Biol. 2016;37:4025–33. doi: 10.1007/s13277-015-4227-z. [DOI] [PubMed] [Google Scholar]
- 33.Brodie S, Lee HK, Jiang W, Cazacu S, Xiang C, Poisson M, et al. The novel long non-coding RNA TALNEC2, regulates tumor cell growth and the stemness and radiation response of glioma stem cells. Oncotarget. 2017;8:31785–801. doi: 10.18632/oncotarget.15991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fotouhi Ghiam A, Taeb S, Huang X, Huang V, Ray J, Scarcello S, et al. Long non-coding RNA urothelial carcinoma associated 1 (UCA1) mediates radiation response in prostate cancer. Oncotarget. 2016;8:4668–89. doi: 10.18632/oncotarget.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beer L, Nemec L, Wagner T, Ristl R, Altenburger LM, Ankersmit HJ, et al. Ionizing radiation regulates long non-coding RNAs in human peripheral blood mononuclear cells. J Radiat Res. 2017;58:201–9. doi: 10.1093/jrr/rrw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao H, Dong Z, Wei W, Shao L, Jin L, Lv Y, et al. Integrative analysis for the role of long non-coding RNAs in radiation-induced mouse thymocytes responses. Acta Biochim Biophys Sin. 2017;49:51–61. doi: 10.1093/abbs/gmw114. [DOI] [PubMed] [Google Scholar]
- 37.Zeng Q, Wang Q, Chen X, Xia K, Tang J, Zhou X, et al. Analysis of lncRNAs expression in UVB-induced stress responses of melanocytes. J Dermatol Sci. 2016;81:53–60. doi: 10.1016/j.jdermsci.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Nie J, Peng C, Pei W, Zhu W, Zhang S, Cao H, et al. A novel role of long non-coding RNAs in response to X-ray irradiation. Toxicol Vitr. 2015;30:536–44. doi: 10.1016/j.tiv.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Tetradas M, Martín M, Repulles J, Huarte M, Genescà A, Tetradas M, et al. Distinct sets of lncRNAs are differentially modulated after exposure to high and low doses of X rays. Radiat Res. 2016;186:549–58. doi: 10.1667/RR14377.1. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt AM, Garcia JT, Hung T, Flynn RA, Shen Y, Qu K, et al. An inducible long noncoding RNA amplifies DNA damage signaling. Nat Genet. 2016;48:1370–6. doi: 10.1038/ng.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Audas TE, Lee S. Stressing out over long noncoding RNA Biochim. Biophys Acta. 2016;1859:184–91. doi: 10.1016/j.bbagrm.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossi E, Sanchez Y, Huarte M. Expanding the p53 regulatory network: LncRNAs take up the challenge. Biochim Biophys Acta. 2016;1859:200–8. doi: 10.1016/j.bbagrm.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Hall JR, Messenger ZJ, Tam HW, Phillips SL, Recio L, Smart RC. Long noncoding RNA lincRNA-p21 is the major mediator of UVB-induced and p53-dependent apoptosis in keratinocytes. Cell Death Dis. 2015;6:e1700. doi: 10.1038/cddis.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouyang J, Hu J, Chen JL. lncRNAs regulate the innate immune response to viral infection. Wiley Interdiscip Rev RNA. 2016;7:129–43. doi: 10.1002/wrna.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Zhong H, Xie X, Chen CY, Huang D, Shen L, et al. Long noncoding RNA derived from CD244 signaling epigenetically controls CD8 + T-cell immune responses in tuberculosis infection. Proc Natl Acad Sci. 2015;112:E3883–92. doi: 10.1073/pnas.1501662112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marques-Rocha JL, Samblas M, Milagro FI, Bressan J, Martinez JA, Marti A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015;29:3595–611. doi: 10.1096/fj.14-260323. [DOI] [PubMed] [Google Scholar]
- 47.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–19. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Gao G, Liu S, Yu L, Yan D, Yao X, et al. Long Noncoding RNA PVT1 as a novel diagnostic biomarker and therapeutic target for melanoma. Biomed Res Int. 2017;2017:7038579. doi: 10.1155/2017/7038579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan J, Qiu K, Li M, Liang Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015;589:3175–81. doi: 10.1016/j.febslet.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimoto R, Mayeda A, Yoshida M, Nakagawa S. MALAT1 long non-coding RNA in cancer. Biochim Biophys Acta. 2016;1859:192–9. doi: 10.1016/j.bbagrm.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Kanduri C. Long noncoding RNAs: Lessons from genomic imprinting. Biochim Biophys Acta. 2016;1859:102–11. doi: 10.1016/j.bbagrm.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Vigneau S, Rohrlich P, Brahic M. Tmevpg1, a candidate gene for the control of Theiler’s virus persistence, could be implicated in the regulation of gamma interferon. J Virol. 2003;77:5632–8. doi: 10.1128/JVI.77.10.5632-5638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotzin JJ, Spencer SP, McCright SJ, Kumar DBU, Collet MA, Mowel WK, et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature. 2016;537:239–43. doi: 10.1038/nature19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pateras IS, Havaki S, Nikitopoulou X, Vougas K, Townsend PA, Panayiotidis MI, et al. The DNA damage response and immune signaling alliance: Is it good or bad? Nature decides when and where. Pharmacol Ther. 2015;154:36–56. doi: 10.1016/j.pharmthera.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Candeias SM, Testard I. The many interactions between the innate immune system and the response to radiation. Cancer Lett. 2015;368:173–8. doi: 10.1016/j.canlet.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Gloss BS, Dinger ME. The specificity of long noncoding RNA expression. Biochim Biophys Acta. 2016;1859:16–22. doi: 10.1016/j.bbagrm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Hothom T, Bretz F, Westfall P. Simultaneous inference for general linear hypotheses. R package version. 2011;1:2–6. ( http://CRAN.R-project.org/package=multcomp) [Google Scholar]
- 58.Eke I, Koch U, Hehlgans S, Sandfort V, Stanchi F, Zips D, et al. PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1a. J Clin Invest. 2010;120:2516–27. doi: 10.1172/JCI41078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohlmaier A, Savarese F, Lachner M, Martens J, Jenuwein T, Wutz A. A chromosomal memory triggered by Xist regulates histone methylation in X inactivation. PLoS Biol. 2004;2:E171. doi: 10.1371/journal.pbio.0020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shevtsov SP, Dundr M. Nucleation of nuclear bodies by RNA. Nat Cell Biol. 2011;13:167–73. doi: 10.1038/ncb2157. [DOI] [PubMed] [Google Scholar]
- 62.Shi T, Gao G, Cao Y. Long noncoding RNAs as novel biomarkers have a promising future in cancer diagnostics. Dis Markers. 2016;2016:9085195. doi: 10.1155/2016/9085195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knoll M, Lodish HF, Sun L. Long non-coding RNAs as regulators of the endocrine system. Nat Rev Endocrinol. 2015;11:151–60. doi: 10.1038/nrendo.2014.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou X, Xu J. Identification of Alzheimer’s disease-associated long noncoding RNAs. Neurobiol Aging. 2015;36:2925–31. doi: 10.1016/j.neurobiolaging.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, Yuan L, Zhang X, Hamblin MH, Zhu T, Meng F, et al. Altered long non-coding RNA transcriptomic profiles in brain microvascular endothelium after cerebral ischemia. Exp Neurol. 2016;277:162–70. doi: 10.1016/j.expneurol.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang J, Huang Y, Nguyen DH, Costes SV, Snijders AM, Mao JH. Genetic background modulates lncRNA-coordinated tissue response to low dose ionizing radiation. Int J Genomics. 2015;2015:461038. doi: 10.1155/2015/461038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang H, Hu X, Zhang H, Li W. Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression. Radiat Oncol. 2017;12:65. doi: 10.1186/s13014-017-0802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.John-Aryankalayil M, Palayoor ST, Cerna D, Simone CB, II, Falduto MT, Magnuson SR, et al. Fractionated radiation therapy can induce a molecular profile for therapeutic targeting. Radiat Res. 2010;174:446–58. doi: 10.1667/RR2105.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Total number of lncRNA probes on the array (4,578), details of the selection criteria that were followed and number of lncRNA probes passing the selection criteria at each step, for any of the dose points (2, 4 and 8 Gy) for the time points studied.
Tables S2–S4. One-way ANOVA was performed, and comparisons were made among 2, 4 and 8 Gy irradiated versus nonirradiated control samples for respective time points, as explained in Materials and Methods. Tables depict Agilent’s systematic identification and accession information. Group-specific P values ≤ 0.01 are highlighted in green; intensity ratios ≥ 2 are highlighted in red; intensity ratios ≤ 0.5 are highlighted in blue. Annotation information in the form of RefSeq ID and lncRNA name has been included for some of the lncRNAs (“-” in the Blast matched column indicates no BLAST match observed; “blast” in the Blast matched column indicates BLAST match was observed).
Table S5. Raw Ct value (threshold cycle number) observed in the RT2 MouseFinder lncRNA arrays at the 16 h time point from triplicate experiments across all the samples.


