Abstract
The red blood cell (RBC) storage interval has been extended from less than a week to the current storage interval of 6–8 weeks. Regulatory criteria for extending storage rely upon a minimal degree of hemolysis and acceptable in vivo 24-h post transfusion recovery. Clinical studies of safety and efficacy have never been required. Concerns have arisen that RBC toward the end of storage develop a ‘storage lesion’ with previously unrecognized toxicity. Of the several mechanisms proposed, the bolus of iron delivered to macrophages as a result of hemolysis of stored RBC might pose a particular risk to patients with existing infections. We developed a canine model of pneumonia to compare the toxicity of stored RBC transfusion. We described increased mortality after transfusion of old RBC. We found that transfused older RBC increased mortality, in vivo hemolysis, circulating cell-free hemoglobin that scavenges nitric oxide, and elevations of non-transferrin bound and plasma labile iron. Disappearance of circulating iron correlated with increased mortality, worsening pulmonary function, and bacterial proliferation. Washing decreased the mortality associated with transfusing older RBC, but had the opposite effect on fresher blood. With low doses of bacteria, survival was unaffected by the age of blood, whereas high bacteria doses masked any effect of RBC age on mortality. Older RBC may have adverse effects, but the patient’s clinical status, the age, volume and method of preparation of the RBC may be critical variables. Several mechanisms may account for this toxicity, but in the presence of bacterial infection, availability of iron likely plays a major role.
Keywords: blood components, red cell components, transfusion medicine (in general)
Introduction
Blood preservative solutions were developed to eliminate reliance on vein-to-vein transfusion and to improve blood supply and logistics [1]. The storage interval of refrigerated blood has been extended progressively from less than a week when whole blood was collected into a citrate/glucose solution to as much as 2 months [2]. Regulations and practices vary internationally. Licensed preservative solutions permit a red blood cell (RBC) shelf life as long as 7 weeks. However, RBCs degrade progressively during the weeks of refrigerated storage and may become less effective as they age. Whereas no one expects RBCs to perform at the end of several weeks of refrigerated storage exactly as they do when they exit the donor’s vein, neither do clinicians anticipate that stored blood will harm their patients. This question of toxicity has recently been challenged [3, 4].
Stored blood does not age gracefully. Changes in percentage haemolysis, osmotic fragility and gross morphology were among the earliest observations that came to define the ‘storage lesion’ (see below). Detailed assessments of RBC metabolism and quality have been reviewed elsewhere [5]. The principal question is whether these changes have significant clinical consequences.
The criteria for extending RBC shelf life when new storage systems are proposed rely first upon a panel of in vitro studies, although without precise acceptance ranges. Most countries specify a minimal degree of haemolysis at outdate (1% in the USA and 0·8% in Europe). The ‘gold standard’ remains in vivo 24-h post-transfusion recovery of more than 75% of cells labelled with radiochromium at the component outdate [6, 7]. Clinical trials of safety and efficacy have never been required (or performed) for licensure. The first widely used anticoagulant preservative, acid citrate dextrose, permitted RBC storage for up to 21 days [8]. Most blood providers extended the RBC shelf life to 28 days when phosphate was added to the preservative, to 35 days with the addition of adenine, and to 42 days when ‘additive solutions’ became available [9–11]. The longest shelf life currently in clinical use is 49 days [12]. A new additive solution has been recently certified for 56-day storage in Europe, but licensed for only 42-day storage in the USA [11].
At present, blood prepared for most adult patients requiring transfusion still follows the first-in, first-out principle with the oldest compatible unit available given first, a strategy designed to manage blood inventories not to optimize patient care. However, many clinicians harbour the belief that ‘fresh’ blood is superior to stored RBC. Yet, no definition of ‘fresh’ has been widely accepted. Paediatricians often define ‘fresh’ as 7-day storage or less, whereas a vocal minority of practitioners request that fresh blood be ‘warm to the touch,’ an impracticality in the era of testing and good manufacturing practice (GMP). Until recently, evidence that fresher RBCs result in clinical benefit has been surprisingly scarce. During the last 15 years, an increasing number of retrospective and prospective studies have raised concerns that patients receiving older blood have an increased morbidity and mortality risk compared with patients receiving fresher units [13–23]. Only one of these dealt specifically with infection [16]. A smaller number of reports has failed to confirm such associations [24–27]. In response, some blood services have already elected to limit RBC shelf life to less than what licensure permits in the reasonable expectation that shortened storage improves RBC quality. (Table 1) Japan, for example, has elected to restrict the ‘42-day RBC’ to a 21-day shelf life since 1995; the shorter storage period reduced the risk of septic reactions from slow-growing bacteria and permitted universal irradiation of RBC to prevent transfusion-associated graft-versus-host disease (GvHD) [28]. The UK has chosen to limit RBC storage to 35 days. The Nether-lands has found that with 35-day storage, RBC discard rates remained <2%. The NIH Clinical Center in the USA has instituted on 1 February 2014, a policy of routinely quarantining RBCs once storage has reached the last licensed 7 days unless clinical indications, such as a rare blood type generates an authorized medical exception. All RBC units, which are licensed for 42-day storage using AS-3 additive solution (saline–adenine–glucose–citrate–phosphate), have been transfused since with a storage time of 35 days or less.
Table 1.
Examples for RBC storage times currently approved vs. applied
| Additive solution and shelf life days) | ||
|---|---|---|
|
|
||
| Approved | Applied in clinical service | Country |
| 49 days | 49 days | Switzerland, Germany (parts) |
| 49 days | 42 days | Germany (parts) |
| 42 days | 42 days | Argentina, Australia, Austria, Brazil, Canada, China, France, Germany, India, Italy, Malaysia, Switzerland, Saudi Arabia, Spain, Thailand, Tunisia, USA |
| 42 days | 35 days | China; Netherlands, United Kingdom (uniform for the countries); Germany (several large blood services) |
| 42 days | 21 days | Japan (All RBC irradiated) |
| 35 days | 35 days | China, Ethiopia, India, Malaysia, Oman, Pakistan, Tunisia |
| 21 days | 21 days | China, Saudi Arabia (few blood services) |
Clinical studies comparing safety and efficacy of fresh and old-stored RBC fall into four general categories: (1) the majority of publications as cited above involve observational studies of different patient populations that demonstrate some statistical association between prolonged storage of allogeneic blood and disease state. All of these studies have been criticized for limitations in size, design or methodology. Some studies examine mortality, others morbidity, while still others have reported on such surrogate measures as length of hospital or ICU stay, recurrence of cancer, changes in gastric intramucosal pH, serum lactate levels or decreased oxygen delivery to different organs [13–23]. Definitions of ‘fresh’ and ‘old’ differ as do the RBC preparations and transfusion practice. (2) Three studies involve autologous transfusion of healthy normal volunteers with their own fresh or stored RBC [29–31]. (3) Studies in animal models provide an opportunity to design trials that cannot be performed in patients or in normal volunteers. (4) Finally, several large prospective randomized controlled trials (RCTs) have either been completed recently or are in progress. None deals specifically with the issue of infection. A comprehensive review comparing fresh and old RBC for transfusion has recently been published [32].
RBC storage lesion
Red blood cell quality may actually improve during the first few days of storage. Bacterial contamination during the donation process may be reduced within the first few hours of storage of whole blood, before the leucocytes are removed. Storage at +4°C for several days reduces the risk of spirochaete transmission, some cell-associated viruses and parasites and the mononuclear cells responsible for GvHD [28].
Whereas no credible claim has been made that RBC quality improves during storage beyond the first few days, an abundance of in vitro evidence implicates refrigerated storage as increasingly detrimental to RBC quality [33, 34]. The original concern regarding RBC storage time and maximal acceptable shelf life in patient care evolved from observations of these RBC changes which are referred to collectively as the ‘storage lesion(s)’ [35–37]. The RBC undergoes metabolic changes and oxidative stress during the storage causing metabolites, proteins and particles, such as microvesicles, to be released into the supernatant. Numerous other changes, in cellular biochemistry, lipid concentration as well as membrane composition, carbohydrate alterations, oxidative injury to lipids and proteins, oxygen affinity and delivery, and adhesion of RBCs to endothelial cells, have been reported. Secondary risks include accumulation of potassium and plasticizer from the container. Many of these changes are summarized in Table 2. The damage affecting the membrane and cytoskeleton results in altered RBC deformability and shape and compromise of the ability of the cell to transit the microcirculation [38]. Some of the RBC undergo lysis in the storage container, while others lyse in the circulation upon transfusion or are rapidly removed by macrophages.
Table 2.
The RBC storage lesion: selected changes in haemoglobin, cytoplasm and membrane
| Changes occurring in the RBC | Oxidative stress | ||
| Echinocytes, reversible ↑ | CD47 ↓ | Protein oxidation ↑ | |
| Sphero-echinocytes, irreversible ↑↑ | Deformability ↓ | Lipid peroxidation ↑ | |
| Osmotic fragility ↑ | Oxygen delivery ↓ | Prostaglandin and isoprostanes ↑ | |
| Microvesicles (procoagulant) ↑ | Na-K-ATPase ↓ | ||
| Membrane rigidity ↑ | |||
| Phosphatidylserine (PS) exposure ↑ | |||
| Oxygen affinity of haemoglobin ↑ | |||
| Vascular endothelium adherence ↑ | |||
| Metabolic changes | Changes in the additive solution | ||
| Lactate ↑ | 2,3-DPG ↓ | K+ ↑ | pH ↓ |
| Phosphate ↓ | H+ ↑ | ||
| ATP, ADP, AMP ↓ | free haemoglobin (NO scavenger) ↑ | ||
| Glutathione ↓ | free haem and iron ↑ | ||
| S-nitroso haemoglobin ↓ | soluble lipids (platelet-activating factor) ↑ | ||
| Nitric oxide (NO) ↓ | phospholipid vesicles ↑ | ||
| cytokines (IL1, IL6, IL8, TNF) ↑ | |||
| histamine ↑ | |||
| complement ↑ | |||
| enzymes ↑ |
The dynamics of the process vary widely: Some effects occur within a few hours of blood donation, whereas other effects represent the accumulation of changes which occur over days and weeks. Further, donor-related biological variability (phenotype) should be expected for recovery, survival and storage. The temporal pattern linking clinical outcomes with the critical determinant of the RBC storage lesion may also vary among patients and their different clinical conditions.
Regulatory criteria for extending storage rely upon a set of in vitro studies that specify a minimal degree of haemolysis and acceptable in vivo 24-h post-transfusion recovery at the end of RBC shelf life. (see above). 51Cr-labelling, widely used to study RBC survival in vivo, has intrinsic variability in addition to the donor-to-donor biological variability [6]. Flow cytometry has shown a nearly identical long-term RBC survival in vivo for fresh and old RBCs once an early-removal fraction of the older RBCs is eliminated [7]. Haemoglobin increments at 48 h did not differ between fresh and old RBCs in a study involving 10 patients, whereas 2,3-DPG was significantly lower up to 48 h after transfusion of the old RBCs [39]. Data in mice, dogs and sheep support the hypotheses that NO depletion and free iron release, occurring as a consequence of the RBC storage lesion, contribute to the detrimental effects of old RBCs [40–42] A bolus of iron released by aging RBC may play a particular role in the setting of infection, both by producing a pro-inflammatory response to transfused red blood cells through the effects of reactive oxygen species on stress pathways and by supplying iron to pathogens through their high-affinity siderophores [40, 43]. However, given the extent of the changes in the RBC, other mechanisms may well be involved.
Types of RBC preparation and shelf life
Many RBC preparations and storage conditions designed to reduce RBC changes in the storage container have been studied in the belief that in vitro alterations will influence clinical outcomes [44] RBC preparation and storage solutions continue to be modified [45]. The general approach to RBC storage has become standardized under GMP. However, unlike small molecule drugs, the RBC source material is a biological product derived from human volunteer blood donors and cannot be rigorously standardized. Substantial donor-to-donor variability in RBC storage, haemolysis and survival has been recognized since the 1960s. The RBCs of approximately two-thirds of donors store better than the mean, and ‘super donors’ whose RBC store exceptionally well have been identified [6, 44]. Because of this variability, each RBC unit is considered a ‘lot’ or ‘batch’. Biomarkers to predict suboptimal storage and to detect ‘poor storers’ do not currently exist, but would be extremely valuable. One example, the RBC enzyme G6PD, has hundreds of recognized variants which may or may not affect RBC storage and quality [46].
Whole blood is typically collected into sterile plastic bags and anticoagulated with a defined concentration of citrate. Leucocyte removal during RBC preparation is commonplace, but not universal. The shelf life of RBC in standard anticoagulant-preservative solutions varies from 21 to 35 days. At the time of initial preparation, the packed RBC may be transferred into a second bag with an ‘additive solution’ and approved for a 42-day RBC shelf life. A variety of primary anticoagulant-preservative solutions and additive solutions are available around the world [11]. RBC for storage may also be irradiated at the time of preparation, or irradiated subsequently and returned to inventory for further refrigerated storage. Approval of storage containers, preservatives, additives and virtually any change in manufacturing depends on in vivo RBC recovery and survival as well as on measurement of certain analytes thought critical for RBC survival. The RBC analytes considered most important are adenosine 5′-triphosphate (ATP) concentration, a measure of cell viability, and 2,3-diphosphoglycerate (2,3-DPG) recovery rate, a marker of oxygen affinity and release [6]. RBCs are regulated by drug acts, biological medicine regulations, pharmaceutical affairs laws and national standards which vary by country [47, 48].
Meta-analysis of studies on mortality
We conducted a formal meta-analysis of studies comparing survival rates associated with the transfusion of fresh vs. older RBCs [4]. Seventeen to 387 130 patients were enrolled from 1991 to 2009 in three RCTs, six prospective observational and 12 retrospective studies. Across these 21 studies, the effects were similar and overall mortality was increased significantly for patients receiving older RBCs. Based on these predominantly retrospective data, published mortality rates and the odd ratios, one would have to transfuse between 97 and 69 428 patients with exclusively fresh RBCs to save one life.
Six studies enrolled trauma patients, six cardiac surgery patients and nine a mix of varied patient populations. The results of each of these three subgroups were consistent with an overall increase in mortality associated with older RBCs, as were results comparing small and large studies (<500 vs. >500 patients) and studies of patients receiving on average three RBCs or less vs. more than three RBCs per patient. Seven studies reported serious adverse events; three others showed an overall increase in multiple-organ dysfunction associated with transfusion of older blood, suggesting an increased risk of death from old RBCs. Although these studies were not designed to evaluate the role of blood storage in infection, three studies did report an overall significant increase in pneumonia. Furthermore, a meta-analysis is only as powerful as the individual studies that are analysed, and conclusions drawn from any meta-analysis should be considered only hypothesis generating and not definitive.
Studies with small and large animals
Animal studies can be designed to address specific physiologic questions and mechanisms unable to be studied in patients or volunteers. Such studies are particularly important for understanding the underlying mechanism, unlikely to be attained by RCTs, and eventually result in the formulation of improved RBC units. The results of these studies may contribute to the design of RCTs as much as they complement the results of RCTs. There are benefits and limitations for RBC transfusion in animal models. Animal studies may be the only way to test hypotheses regarding specific models of infection, trauma and exsanguinating haemorrhage and to compare the extremes of RBC storage. However, the RBC of different animals vary in size, number, haemoglobin content, structure, function, membrane composition, fragility, antigenicity and a number of other factors. The RBC of different animals may store differently, and the time course and nature of the storage lesion may not be comparable to human RBC. The crucial question is whether data and conclusions from animal models can be sufficiently applicable to change clinical transfusion practice.
Murine
Mouse and human RBCs show a progressive decline in survival depending on storage time, unlike the precipitous loss of viability reported for rat RBC [49, 50]. Non-trans-ferrin-bound iron concentration and acute tissue iron deposition were increase after transfusion of old murine RBCs, which results in an inflammatory response not seen after fresh mouse RBC infusion [40]. The increased non-transferrin-bound iron in murine serum was implicated in enhancing bacterial growth in vitro, an observation that was confirmed subsequently in a prospective human study [31]. Old mouse RBCs that were cleared rapidly post-transfusion induced a cytokine storm, a finding not replicated in human volunteer studies [31, 51]. Furthermore, mice pretreated with endotoxin derived from lipo-polysaccharide isolated from Escherichia coli 0111:B4 became moribund after infusion of stored, but not fresh RBC. While not a true infection model, these studies, together with the bacterial growth observations, suggest that iron release to macrophages from prolonged storage of RBC may mediate adverse effects through increased susceptibility to sepsis.
Guinea pig
Transfusion of 28-day guinea pig RBCs led to intravascular haemolysis, hypertension, vascular injury and kidney dysfunction [52, 53] This plasma haemoglobin-driven toxicity could be attenuated by infusion of haptoglobin. The precise mechanism of injury is unknown but may involve the direct toxicity of cell-free haemoglobin, iron, scavenging of nitric oxide (NO) or a combination of such mechanisms. Renal injury associated with stored blood transfusion has not been a feature in other animal models.
Dogs
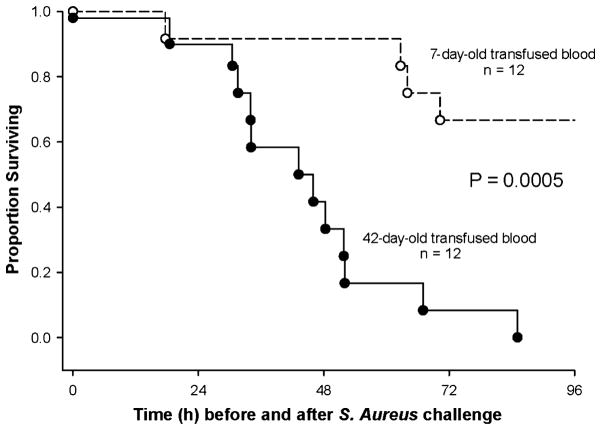
The most striking results of any animal study on prolonged storage of RBCs so far have been observed in canines (Fig. 1): 42-day-old dog RBCs dramatically increased mortality in dogs with experimental Staphylococcus aureus pneumonia [41]. This model was developed to test the extremes of blood storage in a clinical setting of severe infection and massive transfusion. The bacterial challenge dose was optimized in a validated blinded canine model to account for the effects of multiple transfusions on mortality. Bacterial dose was titrated so that animals were sufficiently ill to demonstrate a difference in toxicity from stored RBC if one existed, but not so ill as to mask the effect with high mortality [43]. Canines with pneumonia were treated like critically ill patients with bacterial pneumonia in an intensive care unit. RBCs were collected and prepared by an FDA-licensed canine blood bank mimicking procedures and technologies used for human RBCs [52, 53].
Fig. 1.
Survival curves in Staphylococcus aureus-challenged dogs comparing fresh and old blood. Kaplan–Meier plots over the 96-h course of 12 dogs exchange transfused with 42-day-old RBC (solid circle, solid line) and 12 dogs exchange transfused with 7-day-old RBC (open circle, dashed line). Reprinted from Solomon et al. [41]).
Increased systemic and pulmonary artery pressures indicated that old RBCs were more vasoactive than fresh RBCs. Pulmonary hypertension caused right ventricular dilatation and, by adversely affecting left ventricular filling, resulted in marked tachycardia to maintain cardiac output. Prolonged haemolysis of old RBCs resulted in increased cell-free haemoglobin (CFH) and decreased haptoglobin. Old RBCs caused a steady rise in NO consumption capability of plasma for days, indicating the presence of oxyhaemoglobin, the vasoactive form of haemoglobin known to scavenge NO. Prolonged exposure to oxyhaemoglobin resulted in ischaemic vascular damage at the site of tissue injury in the lung causing gas exchange abnormalities, pulmonary arterial hypertension and an increased risk of death.
Non-transferrin-bound and labile iron, the toxic iron moiety, were elevated only during transfusion, but not associated with survival [41] NO scavenging and in vivo haemolysis were augmented after transfusion of old RBCs, which appeared to result in an excess of non-transferrin-bound and labile iron, but worsened outcome only in the presence of an established infection [43]. The availability of iron, circulating 8–12 h after bacterial challenge, may have promoted bacterial growth and contributed to mortality.
In the canine model, transfusing 42-day-old stored blood washed with a commercial cell washer (Haemonetics Corp., Braintree, MA, USA) improved survival rates when compared with unwashed stored blood of the same age [54]. Transfusing washed 7-day-old stored blood had an unexpectedly different and opposite effect that worsened survival rates (Fig. 2). Transfusing 42-day-old washed stored blood lessened the degree of lung injury when compared with unwashed controls, decreased vasopressor requirements (reversed shock) and improved cardiac performance. In contrast, transfusing washed fresher blood had a different and opposite effect that made these same parameters worse when compared with unwashed fresh controls. Washing fresh red blood cells with saline solution introduces some membrane damage, making RBCs more susceptible to in vivo haemolysis than are fresh unwashed cells. The effect overall is the opposite of that observed with older blood: an increase in CFH levels with washing but much lower levels than seen with the unwashed blood that still contains very fragile but intact old RBCs.
Fig. 2.
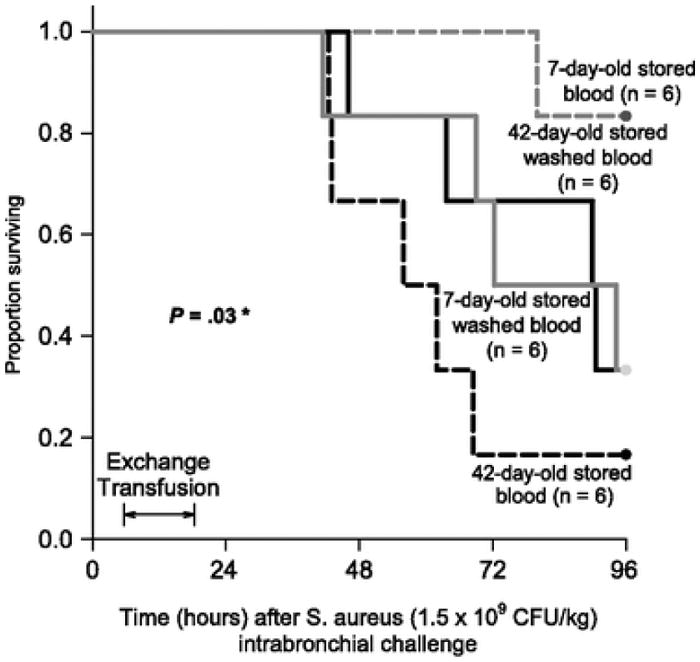
Survival curves in Staphylococcus aureus-challenged [Reprinted from Cortés-Puch et al. (54)] dogs comparing unwashed and washed blood. Kaplan–Meier plots throughout the 96-h study comparing the four types of transfused blood. The different types of blood are represented by different line patterns as follows: dashed black line (42-day-old unwashed blood), solid black line (42-day-old washed blood), solid grey line (7-day-old washed blood) and dashed grey line (7-day-old unwashed blood). There is a qualitative interaction between washing and the age of stored blood (P = 0·03). Reprinted from Cortés-Puch et al. [54]. *Qualitative interaction: there was a significantly different and opposite effect of washing on survival times depending on the age of blood, i.e., washing improves survival rates of 42-day-old and worsens survival rates of 7-day-old stored blood.
The dog model should be useful to determine whether the stored blood effect is specific to pneumonia and to haemolytic S. aureus or whether other pathogens, other sites of infection or models of sepsis, and settings of non-infectious severe illness lead to increased morbidity and mortality. Comparing infusions of licensed iron preparations to blood transfusion might help determine the mechanism(s). The model should also be useful for studying potential therapeutic interventions.
Sheep
Similar to the TRALI2 study in humans, which examined transfusion-related acute lung injury (TRALI) and was expanded to study RBC storage effects, a TRALI study prompted the development of a sheep model [42, 55]. Transfusion of 40-day-old autologous RBCs increased pulmonary vascular resistance and pressure, which was worsened by an NO synthase inhibitor and ameliorated by NO inhalation [40]. These observations were compatible with the NO and free iron findings in dogs. As TRALI is thought to be caused predominantly by plasma infusion and not by cellular components, initially the effect of the supernatant from stored human RBCs was tested in sheep [59]. Such human supernatants decreased arterial pressure and cardiac output in LPS-primed sheep more than did supernatants from stored human platelet components. Ovine RBCs stored for 35–42 days induced pulmonary arterial hypertension but not TRALI [56].
It is not clear to what extent rodent, canine, sheep, or for that matter any non-primate models, can mimic the variety of human clinical circumstances and whether canine or other RBCs are equivalent to similarly stored human RBCs. If transferable, the current canine study results may apply only to severely ill patients with infections and RBCs near the end of the shelf life permitted by the additive solutions used.
Preclinical studies with volunteers
The effect of fresh vs. old RBCs on physiological parameters can be assessed reliably and safely by transfusing autologous RBCs to healthy volunteers (Table 3). Such studies, unlike clinical trials, permit maximal separation of the age of stored RBCs. No differences were found for cognitive function, pulmonary function or hyperaemia in three different volunteer studies. The informative study by Hod et al. documented highly significant increases in serum iron and transferrin saturation at 4 h after transfusion of older RBCs. Ferritin concentrations increased from baseline only after transfusion of older RBCs [31]. While non-transferrin-bound iron concentration was not significantly increased after fresh RBCs, after transfusion of older RBCs, it progressively increased for 4 h. The ongoing haemolysis during RBC storage may explain the significantly increased serum total bilirubin peaking at 4 h, which correlated with a peak of unconjugated bilirubin in the minority of volunteers and a small, but significant, rise in serum-conjugated bilirubin. Statistically significant differences were observed in bacterial growth in vitro in serum samples from all volunteers surrounding the transfusions. Whereas the results in volunteer studies raise few concerns regarding the toxicity of stored RBC, the volumes transfused are small and the recipients healthy. The effects may be quite different in critically ill infected patients.
Table 3.
Assessment of the effects of RBC storage time on various physiological parameters in healthy volunteers
| Reference | Autologous RBC in crossover design | RBC characteristics | Outcome parameters | Summary of results | Country | Trial registration | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Volunteers (n) | RBC (n) | RBC storage time | Leukoreduction | |||||
| Weiskopf et al. [29] | 9 | 2 | 3·4 h vs. 23 days (median) | No | Anaemia-induced cognitive dysfunction | No difference in reversal of dysfunction | USA | |
| Hod et al. [31] | 14 | 2 | 3–7 days vs. 40–42 days | Yes | Iron, extravascular haemolysis, metabolism parameters, inflammation, bacterial growth in vitro | Significant changes in iron and extravascular haemolysis parameters only | USA | NCT01319552 |
| Berra et al. [64] | 9 | 1 | 3 days vs. 40 days | Yes | Reactive hyperemia index | No difference in hyperemia index | USA | |
| Weiskopf et al. [30] | 35 | 2 | 1·7 h vs. 24·5 days | Yes | Pulmonary function (gas exchange variables) | Equivalent slight declines in variables | USA | |
Randomized clinical trials (RCTs)
There is a dearth of reliable prospective controlled clinical data comparing the safety and effectiveness of stored RBC. Eleven RCTs evaluated RBC storage times with a median of 35 patients (range 10–237), and only two published RCTs addressed clinical outcomes. (Table 4) Physiological parameters have been the primary outcomes in most of the completed RCTs. None of these RCTs detected any effect of RBC storage time; however, none focused on patients with infections.
Table 4.
Completed randomized controlled trails (RCTs) without an effect of RBC storage time on outcome in different clinical settings
| Reference | Patients | Primary outcome parameters | Trial registration and acronym | ||
|---|---|---|---|---|---|
|
| |||||
| Treatment arm (n) | Control arm (n) | Clinical setting | |||
| Wasser et al. [24] | 118 | 119 | Cardiac surgery | Postoperative bleeding | |
| Schulman et al. [27] | 8 | 9 | Trauma | Mortality, infectious complications, respiratory failure | |
| Fernandes et al. [58] | 10 | 5 | Intensive care unit (ICU) with sepsis | Oxygen consumption, gastric mucosal pH change | |
| Walsh et al. [59] | 10 | 12 | ICU with mechanical ventilation | Gastric mucosal pH change, gastric to arterial PaCO2 gap | |
| Hebert et al. [25] | 26 | 31 | ICU | Mortality, infections, thrombotic events, ischaemic stroke | |
| Fernandes da Cunha et al. [60] | 26 | 26 | Donor exposure, (not powered for) mortality | ||
| 2005–2007, unpublished | 30 | 30 | Traumatic brain injury | Cerebral oxygen extraction ratio | NCT00141674 |
| Aubron et al. [61] | 25 | 26 | ICU | Feasibility study, (not powered for) mortality | |
| Kor et al. [26] | 50 | 50 | ICU with mechanical ventilation | Pulmonary function, (not powered for) mortality | TRALI2 trial NCT00751322 |
| Heddle et al. [62] | 309 | 601 | Acute care inpatients | Feasibility study, (not powered for) mortality | INFORM-P trial |
| Fergusson et al. [57] | 188 | 189 | Premature infants <1250 g | Mortality, necrotizing enterocolitis, retinopathy, bronchopulmonary dysplasia, intraventricular haemorrhage | ARIPI trial NCT00326924 ISRCTN65939658 |
| Yuruk et al. [63] | 10 | 10 | Haematology, outpatients | Sublingual microcirculation | |
The first of the larger RCTs addressing mortality as the primary outcome was recently completed [57]. The use of fresh RBCs compared with standard blood bank practice did not improve major neonatal morbidities, such as necrotizing enterocolitis, retinopathy of prematurity, bronchopulmonary dysplasia, intraventricular haemorrhage or death, in premature, very low-birthweight infants requiring transfusion. However, the average duration of RBC storage in the standard of care group (14·6 days) does not reflect the average RBC storage in the USA (18 days) and in many countries worldwide and certainly is not the practice of some centres that routinely store RBCs 21 days or longer. The five large ongoing RCTs (Table 5) define mortality or multiple-organ dysfunction as primary outcomes. The patient cohorts studied will be important for determining the applicability of the results and include acute care inpatients, critically ill patients in adult intensive care units (ICUs) and patients undergoing complex cardiac surgery. None of the trials specifically targets patients with severe infections, transplantation or trauma. The results of these large RCTs are not expected in the near future, although both the ABLE and RECESS studies have recently concluded recruitment.
Table 5.
Ongoing randomized controlled trails (RCTs) assessing the effect of RBC storage time on clinical outcomes
| Official title or Acronym | Patients | Primary outcome | Country | Years | |
|---|---|---|---|---|---|
|
| |||||
| Accrual goal (n) | Setting | ||||
| ABLE | 2510 | ICU | All cause mortality at day 90 | Canada 23 locations | 2008–2013 completed |
| Red cell storage Duration and outcomes in cardiac surgery | 2800 | All cardiopulmonary bypass patients | Mortality at day 30 post surgery | USA single centre | 2007–2014 recruiting |
| RECESS | 1696 | Scheduled complex cardiac surgery | Multiple-organ dysfunction score (MODS) | USA 26 locations | 2010–2013 recruiting |
| INFORM | 24 400 | Acute care inpatients | In-hospital mortality | Canada, USA, Australia | 2012–2014 recruiting |
| TRANSFUSE | 5000 | ICU excluding cardiac surgery | Mortality at day 90 | Australia, New Zealand, Finland | 2012–2016 recruiting |
Conclusion
Retrospective and prospective observational studies have suggested that fresher RBCs may benefit defined patient groups or all patients, whereas the only completed RCT failed to show improved outcomes in premature, very low-birthweight infants transfused with fresher cells [57]. By one calculation, between 97 and 69 428 patients needed to be treated with exclusively fresh RBC to save one life [4]. Studies in several animal models report evidence of organ toxicity and increased mortality when older blood is transfused, and suggest that patients with severe infections may represent one particularly vulnerable population. The ongoing RCTs should allow narrowing the range of the actual risk, which would determine the need for changes in transfusion practice. The effect of optimal RBCs storage may well vary according to different clinical settings.
Footnotes
Disclosures
The authors are full-time US government employees and have no conflicts of interest.
References
- 1.Robertson OH. Transfusion with preserved red blood cells. Br Med J. 1818;1:691–695. doi: 10.1136/bmj.1.2999.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein HG, Anstee DJ. Mollison’s Blood Transfusion in Clinical Medicine. 12. Oxford: Blackwell Publishing; 2014. pp. 366–379. [Google Scholar]
- 3.Koch CG, Li L, Sessler DI. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Sun J, Solomon SB, et al. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavenski K, Saidenberg E, Lavoie M, et al. Red blood cell storage lesions and related transfusion issues: a Canadian Blood Services research and development symposium. Trans-fus Med Rev. 2012;26:68–84. doi: 10.1016/j.tmrv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 7.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, et al. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–1485. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 8.Beutler E. Back to the future in RBC preservation. Transfusion. 2000;40:893–895. doi: 10.1046/j.1537-2995.2000.40080893.x. [DOI] [PubMed] [Google Scholar]
- 9.Högman CF, Hedlund K, Zetterstrom H. Clinical usefulness of red cells preserved in protein-poor mediums. N Engl J Med. 1978;299:1377–1382. doi: 10.1056/NEJM197812212992502. [DOI] [PubMed] [Google Scholar]
- 10.Moroff G, Holme S, Keegan T, et al. Storage of ADSOL-preserved red cells at 2.5 and 5.5 degrees C. comparable retention of in vitro properties. Vox Sang. 1990;59:136–139. doi: 10.1111/j.1423-0410.1990.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 11.Hess JR. An update on solutions for red cell storage. Vox Sang. 2006;91:13–19. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 12.Zehnder L, Schulzki T, Goede JS, et al. Erythrocyte storage in hypertonic (SAGM) or isotonic (PAGGSM) conservation medium: influence on cell properties. Vox Sang. 2008;95:280–287. doi: 10.1111/j.1423-0410.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 13.Purdy ER, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in ICU patients. Can J Anaesth. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 14.Zallen G, Offner PJ, Moore EE. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 15.Mynster T, Nielsen HJ. Storage time of transfused blood and disease recurrence after colorectal cancer surgery. Dis Colon Rectum. 2001;44:955–964. doi: 10.1007/BF02235483. [DOI] [PubMed] [Google Scholar]
- 16.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–716. doi: 10.1001/archsurg.137.6.711. [DOI] [PubMed] [Google Scholar]
- 17.Leal-Noval SR, Jara-López I, García-Garmendia JL, et al. Influence of red blood cell concentrates (RBCs) storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology. 2003;98:815–822. doi: 10.1097/00000542-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Basran S, Frumento RJ, Cohen A. The association between duration of storage of transfused red blood cells and morbidity and mortality after reoperative cardiac surgery. Anesth Analg. 2006;103:15–20. doi: 10.1213/01.ane.0000221167.58135.3d. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg JA, McGwin G, Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–282. doi: 10.1097/TA.0b013e31817c9687. [DOI] [PubMed] [Google Scholar]
- 20.Dean M, Samson D, Rooks M, et al. Aged packed red blood cells significantly reduce monocyte and dendritic cell inflammatory responses and cell proliferation in a human whole blood transfusion model [abstract] Vox Sang. 2011;101:29–30. [Google Scholar]
- 21.Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–3030. [PubMed] [Google Scholar]
- 22.Yap CH, Lau L, Krishnaswamy M, et al. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86:554–559. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 23.Frenzel T, Sibrowski W, Westphal M. Red-cell storage and complications of cardiac surgery. N Engl J Med. 2008;358:2841–2842. [PubMed] [Google Scholar]
- 24.Wasser MN, Houbiers JG, D’Amaro J, et al. The effect of fresh versus stored blood on post-operative bleeding after coronary bypass surgery: a prospective randomized study. Br J Haematol. 1989;72:81–84. doi: 10.1111/j.1365-2141.1989.tb07656.x. [DOI] [PubMed] [Google Scholar]
- 25.Hebert PC, Chin-Yee I, Fergusson D, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–1438. doi: 10.1213/01.ANE.0000148690.48803.27. [DOI] [PubMed] [Google Scholar]
- 26.Kor DJ, Kashyap R, Weiskopf RB, et al. Fresh red blood cell transfusion and short-term pulmonary, immunologic, and coagulation status: a randomized clinical trial. Am J Respir Crit Care Med. 2012;185:842–850. doi: 10.1164/rccm.201107-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulman CI, Nathe K, Brown M, et al. Impact of age of transfused blood in the trauma patient. J Trauma. 2002;52:1224–1225. doi: 10.1097/00005373-200206000-00036. [DOI] [PubMed] [Google Scholar]
- 28.Anderson K. Broadening the spectrum of patient groups at risk for transfusion-associated GVHD: implications for universal irradiation of cellular blood components. Transfusion. 2003;43:1652–1654. doi: 10.1111/j.0041-1132.2003.00631.x. [DOI] [PubMed] [Google Scholar]
- 29.Weiskopf RB, Feiner J, Hopf H, et al. Fresh blood and aged stored blood are equally efficacious in immediately reversing anaemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104:911–920. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Weiskopf RB, Feiner J, Toy P, et al. Fresh and stored red blood cell transfusion equivalently induce subclinical pulmonary gas exchange deficit in normal humans. Anesth Analg. 2012;114:511–519. doi: 10.1213/ANE.0b013e318241fcd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;25:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flegel WA, Natanson C, Klein HG. Does prolonged storage of red blood cells cause harm? Br J Haematol. 2014;165:3–16. doi: 10.1111/bjh.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin-Yee I, Arya N, d’Almeida MS. The red cell storage lesion and its implication for transfusion. Transfus Sci. 1997;18:447–458. doi: 10.1016/S0955-3886(97)00043-X. [DOI] [PubMed] [Google Scholar]
- 34.Solheim BG, Flesland O, Seghatchian J, et al. Clinical implications of red blood cell and platelet storage lesions: an overview. Transfus Apher Sci. 2004;31:185–189. doi: 10.1016/j.transci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Schrier SL, Hardy B, Bensch K, et al. Red blood cell membrane storage lesion. Transfusion. 1979;19:158–165. doi: 10.1046/j.1537-2995.1979.19279160285.x. [DOI] [PubMed] [Google Scholar]
- 36.Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107:1–9. doi: 10.1111/vox.12130. [DOI] [PubMed] [Google Scholar]
- 37.Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for “omics” analyses. Blood Transfus. 2012;10(Suppl 2):s7–s11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg JA, MacLennan PA, Vandromme-Cusick MJ, et al. The deleterious effect of red blood cell storage on microvascular response to transfusion. J Trauma Acute Care Surg. 2013;75:807–812. doi: 10.1097/TA.0b013e3182a74a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallis JP, Wells AW, Babb RG, et al. Effect of storage age of transfused blood on 48 hour Hb increment and recovery of 2,3 DPG in haematology patients. (Abstr) Br J Haematol. 2005;129:1. [Google Scholar]
- 40.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–1672. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonova G, Tung JP, Fraser JF, et al. A comprehensive ovine model of blood transfusion. Vox Sang. 2014;106:153–160. doi: 10.1111/vox.12076. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, Cortés-Puch I, Sun J, et al. Transfusion of older stored blood worsens outcomes in canines depending on the presence and severity of pneumonia. Transfusion. 2014;54:1712–1724. doi: 10.1111/trf.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hess JR. Scientific problems in the regulation of red blood cell products. Transfusion. 2012;52:1827–1835. doi: 10.1111/j.1537-2995.2011.03511.x. [DOI] [PubMed] [Google Scholar]
- 45.Greening DW, Glenister KM, Sparrow RL, et al. International blood collection and storage: clinical use of blood products. J Proteomics. 2010;73:386–395. doi: 10.1016/j.jprot.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 46.Francis RO, Jhang JS, Pham HP, et al. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang. 2013;105:271–282. doi: 10.1111/vox.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.UK Legislation. The Blood Safety and Quality Regulations 2005. 2005;2005(50) HEALTH AND SAFETY. Ref Type: Bill/Resolution. [Google Scholar]
- 48.Bundesärztekammer & Paul-Ehrlich-Institut: Richtlinien zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten (Hämotherapie) gemäß §§ 12 und 18 des Transfusionsgesetzes (TFG) Bundesanzeiger. 2010;62:3–36. [Google Scholar]
- 49.Gilson CR, Kraus TS, Hod EA, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.d’Almeida MS, Jagger J, Duggan M, et al. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: implications for animal models of transfusion. Transfus Med. 2000;10:291–303. doi: 10.1046/j.1365-3148.2000.00267.x. [DOI] [PubMed] [Google Scholar]
- 51.Hod EA, Spitalnik SL. Stored red blood cell transfusions: iron, inflammation, immunity, and infection. Transfus Clin Biol. 2012;19:84–89. doi: 10.1016/j.tracli.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baek JH, D’Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wardrop KJ. Selection of anticoagulant-preservatives for canine and feline blood storage. Vet Clin North Am Small Anim Pract. 1995;25:1263–1276. doi: 10.1016/s0195-5616(95)50153-6. [DOI] [PubMed] [Google Scholar]
- 54.Cortés-Puch I, Wang D, Sun J, et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood. 2014;123:1403–1411. doi: 10.1182/blood-2013-11-539353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tung JP, Fung YL, Nataatmadja M, et al. A novel in vivo ovine model of transfusion-related acute lung injury (TRALI) Vox Sang. 2011;100:219–230. doi: 10.1111/j.1423-0410.2010.01381.x. [DOI] [PubMed] [Google Scholar]
- 56.Baron DM, Yu B, Lei C, et al. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012;116:637–647. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fergusson DA, Hébert P, Hogan D, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 58.Fernandes CJ, Jr, Akamine N, De Marco FV, et al. Red blood cell transfusion does not increase oxygen consumption in critically ill septic patients. Crit Care. 2001;5:362–367. doi: 10.1186/cc1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walsh TS, McArdle F, McLellan SA, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32:364–371. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 60.Fernandes da Cunha DH, Nunes Dos Santos AM, Kopelman BI, et al. Transfusions of CPDA-1 red blood cells stored for up to 28 days decrease donor exposures in very low-birth-weight premature infants. Transfus Med. 2005;15:467–473. doi: 10.1111/j.1365-3148.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 61.Aubron C, Syres G, Nichol A, et al. A pilot feasibility trial of allocation of freshest available RBC cells versus standard care in critically ill patients. Transfusion. 2012;52:1196–1202. doi: 10.1111/j.1537-2995.2011.03437.x. [DOI] [PubMed] [Google Scholar]
- 62.Heddle N, Cook R, Arnold D, et al. The effect of blood storage duration on in-hospital mortality: a randomized controlled pilot feasibility trial. Transfusion. 2012;52:1203–1212. doi: 10.1111/j.1537-2995.2011.03521.x. [DOI] [PubMed] [Google Scholar]
- 63.Yürük K, Milstein DM, Bezemer R, et al. Transfusion of banked red blood cells and the effects on hemorrheology and microvascular hemodynamics in anemic hematology outpatients. Transfusion. 2013;53:1346–1352. doi: 10.1111/j.1537-2995.2012.03905.x. [DOI] [PubMed] [Google Scholar]
- 64.Berra L, Coppadoro A, Yu B, et al. Transfusion of stored autologous blood does not alter reactive hyperemia index in healthy volunteers. Anesthesiology. 2012;117:56–63. doi: 10.1097/ALN.0b013e31825575e6. [DOI] [PMC free article] [PubMed] [Google Scholar]