Abstract
The endocochlear potential (EP) provides part of the electrochemical drive for sound-driven currents through cochlear hair cells. Intense noise exposure (110 dB SPL, 2 h) differentially affects the EP in three inbred mouse strains (C57BL/6 [B6], CBA/J [CBA], BALB/cJ [BALB]) (Ohlemiller and Gagnon, 2007, Hearing Research 224:34–50; Ohlemiller et al., 2011, JARO 12:45–58). At least for mice older than 3 mos, B6 mice are unaffected, CBA mice show temporary EP reduction, and BALB mice may show temporary or permanent EP reduction. EP reduction was well correlated with histological metrics for injury to stria vascularis and spiral ligament, and little evidence was found for holes or tears in the reticular lamina that might ‘short out’ the EP. Thus we suggested that the genes and processes that underlie the strain EP differences primarily impact cochlear lateral wall, not the organ of Corti. Our previous work did not test the range of noise exposure conditions over which strain differences apply. It therefore remained possible that the relation between exposure severity and acute EP reduction simply has a higher exposure threshold in B6 mice compared to CBA and BALB. We also did not test for age dependence. It is well established that young adult animals are especially vulnerable to noise-induced permanent threshold shifts (NIPTS). It is unknown, however, whether heightened vulnerability of the lateral wall contributes to this condition. The present study extends our previous work to multiple noise exposure levels and durations, and explicitly compares young adult (6–7 wks) and older mice (>4 mos). We find that the exposure level-versus-acute EP relation is dramatically strain-dependent, such that B6 mice widely diverge from both CBA and BALB. For all three strains, however, acute EP reduction is greater in young mice. Above 110 dB SPL, all mice exhibited rapid and severe EP reduction that is likely related to tearing of the reticular lamina. By contrast, EP-versus-noise duration examined at 104 dB suggested that different processes contribute to EP reduction in young and older mice. The average EP falls to a constant level after ~7.5 min in older mice, but progressively decreases with further exposure in young mice. Confocal microscopy of organ of Corti surface preparations stained for phalloidin and zonula occludens-1 (ZO-1) indicated this corresponds to rapid loss of outer hair cells (OHCs) and formation of both holes and tears in the reticular lamina of young mice. In addition, when animals exposed at 119 dB were allowed to recover for 1 mo, only young B6 mice showed collapse of the EP to ≤5 mV. Confocal analysis suggested novel persistent loss of tight junctions in the lateral organ of Corti. This may allow paracellular leakage that permanently reduces the EP. From our other findings, we propose that noise-related lateral wall pathology in young CBA and BALB mice promotes hair cell loss and opening of the reticular lamina. The heightened vulnerability of young adult animals to noise exposure may in part reflect special sensitivity of the organ of Corti to acute lateral wall dysfunction at younger ages. This feature appears genetically modifiable.
Keywords: Stria vascularis, Noise-induced hearing loss, Tight junction, Cochlea, Holes, Tears, Mouse
1. Introduction
Mammalian cochlear injury targets of noise exposure include the organ of Corti and the stria vascularis. Changes in either can affect the endocochlear potential (EP), which provides much of the electrochemical driving force for sound-induced currents through hair cells (Wangemann, 2006). Depending on model and conditions, the EP may temporarily either increase or decrease. Acute EP increases have been attributed to changes in the organ of Corti such as prolonged hair cell depolarization or decreased conductance of transducer channels (Ohlemiller et al., 2016). Acute EP decreases appear attributable to injury to the stria or spiral ligament (Ohlemiller and Gagnon, 2007; Ohlemiller et al., 2011a) or to increased leakiness of the organ of Corti, which may ‘short out’ the EP (Ahmad et al., 2003). Within this framework, the EP may serve either as an indicator of events in the organ of Corti, or as the primary metric of interest, since lateral wall pathology that directly decreases the EP will elevate thresholds independent of any changes in the organ of Corti.
Work in mice has shown that the extent of noise injury to the stria and spiral ligament and resulting EP reduction are genetically modifiable. Following a 2-hr broadband exposure at 110 dB SPL, three inbred mouse strains (C57BL/6J [B6], CBA/J [CBA], BALB/cJ [BALB]) show different phenotypes, such that B6 mice are unaffected, CBA mice show temporary EP reduction, and BALB mice show temporary or permanent EP reduction (Ohlemiller and Gagnon, 2007; Ohlemiller et al., 2010, 2011a). The EP reduction is correlated with histological injury metrics to stria vascularis, spiral ligament, and Reissner’s membrane. By contrast, little evidence was found for ‘holes’ left by rapid hair cell loss, or tears in the reticular lamina. Thus we suggested that the genes and processes that underlie these strain differences primarily impact cochlear lateral wall. One or more of the underlying genes lies on proximal chromosome 18 (Nirep QTL) (Ohlemiller et al., 2010, 2016).
Noise-related reticular lamina leaks that may reduce the EP appear to take two forms, both supported mostly by anecdotal evidence. At high noise levels (≥124 dB in guinea pigs and ≥116 dB in mice) mechanical trauma may tear the reticular lamina (Fredelius, 1988; Fredelius et al., 1990; Henderson et al., 1994; Hirose and Liberman, 2003; Hirose et al., 2005; Spongr et al., 1998; Thorne et al., 1984; Wang et al., 2002; Zheng and Hu, 2012). At much lower noise levels (82 dB), studies in chinchillas support the notion of rapid hair cell death that leaves holes in the reticular lamina (Harding and Bohne, 2004). Both tears and holes would promote mixing of endolymph and perilymph, ostensibly exposing hair cells to toxically high K+ levels, thereby magnifying hair cell and hearing loss. Such a process is supported by knockout models for tight junctional proteins such as vezatin, claudin-9, claudin-14, occludin, angulin-2 (Ildr1), and tricellulin (Bahloul et al., 2009; Ben-Yosef et al., 2003; Kitajiri et al., 2014; Morozko et al., 2014; Nakano et al., 2009; Nayak et al., 2013). Some of these models (claudin-9 and -14) show both reduced electrical resistance of tight junctions and hair cell loss, despite a normal EP. In mice, hair cell death that causes holes and EP reduction remains the exception to the rule, having only been reported in knockout models (Cohen-Salmon et al., 2002; Jin et al., 2016). On balance, observations of hair cell death from noise, ototoxins, or genetic causes support the predominance of mechanisms for quickly sealing off the reticular lamina so that electrical impedance and ionic integrity are maintained. The present work attempts to separate lateral wall and potential organ of Corti contributions to noise-related EP reduction.
Our previous work did not test the range of noise exposure conditions over which mouse strain differences apply. It thus remained possible that the relation between exposure level or duration and acute EP reduction simply has a higher exposure threshold in B6 mice compared to CBA and BALB. To address this, we measured EP changes in all three mouse strains in response to a wide range of 2-hr exposures (85–119 dB SPL). Our previous work also did not test for age dependence of results. It is well established that young adult animals are especially vulnerable to noise-induced permanent threshold shifts (NIPTS) (Ohlemiller et al., 2000, 2011b; Pujol, 1992; Saunders and Chen, 1982). It is unknown, however, whether heightened vulnerability of the lateral wall contributes to this early ‘vulnerable period’. Alternatively, the organ of Corti of young mice may be more affected by acute lateral wall dysfunction than in older animals. In the present work we separately examined mice whose age fell near the peak of the vulnerable period for mice (6–7 wks), and outside this period (>4 mos) (Henry, 1982). Because exaggerated loss of outer hair cells (OHCs) is a known aspect of the vulnerable period (Ohlemiller et al., 2000), for some exposure conditions we examined surface preparations of the organ of Corti using antibodies for hair cell cuticular plates and tight junctions. Our observations confirm striking genetic dependence of cochlear noise-related lateral wall injury and EP reduction, such that B6 mice are more resistant than CBA or BALB under most exposure conditions. They further suggest that noise injury to the lateral wall in young CBA and BALB mice exacerbates injury to the organ of Corti in the form of rapid OHC loss and formation of both holes and tears in the reticular lamina. While recovery of the EP indicates that holes and tears are typically repaired, the EP in young B6 mice showed persistent collapse to ≤5 mV after 2-hr exposure at 119 dB SPL. Surface preparations in these mice suggest novel, permanent, disruption of tight junctions in the reticular lamina.
2. Methods
2.1. Animals
Procedures were approved by the Washington University Institutional Animal Care and Use Committee. Physiological recordings were conducted using C57BL/6J (n = 143), CBA/J (n = 200), and BALB/cJ (BALB, n = 208), all derived from breeders purchased from The Jackson Laboratory (JAX). Mice were either 6–7 weeks (‘young’) or 4–16 mos of age (‘older’) at the time of evaluation. The age of older BALBs was capped at 13 mos as our published data indicated that BALBs may show age-associated EP decline thereafter (Ohlemiller et al., 2006). All samples were randomly composed by gender, and no gender effects were detected in any feature reported here.
2.2. EP recording
All animals underwent a single EP measurement, obtained from the cochlear lower basal turn of the left ear. For EP recording, animals were anesthetized (60 mg/kg sodium pentobarbital, IP) and positioned ventrally in a custom headholder. Core temperature was maintained at 37.5 ± 1.0 °C using a thermostatically-controlled heating pad in conjunction with a rectal probe (Yellow Springs Instruments Model 73A). An incision was made along the midline of the neck and soft tissues were blunt dissected and displaced laterally to expose the trachea and left bulla. A tracheostomy was then made and the musculature over the bulla was cut posteriorly to expose the bone overlying the round window. Using a fine drill, a hole was made in the left cochlear capsule directly over scala media of the lower basal turn. Glass capillary pipettes (40–80 MΩ) filled with 0.15 M KCl were mounted on a hydraulic microdrive (Frederick Haer) and advanced until a stable positive potential was observed that did not change with increased electrode depth. The signal from the recording electrode was led to an AM Systems Model 1600 intracellular amplifier. A silver/silver chloride ball inserted into the neck muscles served as ground.
2.3. Noise exposure
Noise exposures were performed in a foam-lined, single-walled soundproof room (IAC). Fully awake and unrestrained animals were placed singly or in pairs in modified cages (food, water, bedding removed) positioned up to two cages at once directly under an exponential horn. All noise was octave band (8–16 kHz), generated digitally using custom Labview routines running on a PC. The signal was output to a Tucker-Davis Technologies RZ6 signal processor, then to a Crown D-150A power amplifier that drove the speaker. Depending on the experiment, noise level varied from 85 to 119 dB SPL in 3 dB increments. To characterize EP temporal dynamics, at two noise intensities (104 and 119 dB) exposure duration was varied from 14 s to 2 h in 2× increments.
2.4. Nonlinear curve fitting
Acute EP-versus-noise duration data (Fig. 4) were fitted using a nonlinear least-squares optimization (lsqnonlin) in Matlab to a Boltzmann function of the form:
where EPinitial is the EP value at the start of the noise exposure; EPfinal is the asymptote EP corresponding to a constant final EP value; t0 is the time at the center or point of inflection, and dt is a time constant that varies the slope in the transition region between the two EP values (see Fig. 10). EPfinal is not asserted to represent a permanent post-noise steady state, but rather, a value that persists prior to repair whose time course we did not characterize. A Boltzmann or logistic function is commonly used to describe voltage-dependent ion channel conductances (e.g., Beluzzi et al., 1985; Ehrenstein et al., 1970). In the present framework, intense noise is taken to cause an abnormal increase in the conductance between endolymphatic and perilymphatic spaces, producing a time-dependent reduction in the EP. The Boltzmann function well describes problems of current flow and chemical diffusion, and is appropriate for considering the thermodynamics of atoms and molecules in various applications in physiology (Dubois et al., 2009).
Fig. 4.
Mean and scatter of acute EP versus noise duration for 104 dB SPL (left column) and 119 dB SPL (right column) on semi-log axes. Exposure duration varied from 14 s to 2 h. 2-h data are taken from Fig. 1. B6 mice were not included in experiments at 104 dB since they showed little EP reduction at this level. A. Older CBA/J and BALB exposed to 104 dB. B. Young CBA/J and BALB exposed to 104 dB. C. Means extracted from A and B for comparison by age. D. Older B6, CBA/J and BALB at 119 dB. E. Young B6, CBA/J and BALB at 119 dB. F. Means extracted from D and E for comparison.
Fig. 10.
Disruption of ZO-1 labeling in medial organ of Corti is associated with near-loss of ZO-1 labeling in lateral organ of Corti. Images compare four mice exposed at 104 dB SPL (top) without disruption of ZO-1 in OHC region with four mice exposed at 119 dB SPL with ZO-1 disruption in the OHC region (bottom). Missing/altered ZO-1 label in the medial and lateral organ (demarcated by dashed line) coincide. Arrows indicate regions of missing/altered ZO-1 label. Scale bar: 6
2.5. Immunocytochemistry and confocal imaging
To identify changes in the reticular lamina that might explain EP trends, we performed confocal immunocytochemical analysis of organ of Corti surface preparations in acute noise-exposed mice and unexposed controls. These animals did not undergo EP recording. Analyses focused on both young and older mice of all three strains exposed for 2 h at either 104 or 119 dB SPL. In addition, both young and older B6 mice that were exposed at 119 dB SPL and then allowed to survive for 1–3 mos were examined as surface preparations. One cochlea from 3 to 12 animals was examined to discern qualitative trends. Examinations emphasized the basal turn, since all our EP recordings were from the basal turn. For all conditions tested, injury to the cochlear apical turn (acute hair cell loss and opening of the reticular lamina) appeared less severe than in the base (not shown).
Inner ears were fixed by intra-cardiac perfusion of 4% para-formaldehyde, then immersed in the same fixative for 15 min at room temperature. After rinsing with phosphate buffered saline (PBS), temporal bones were decalcified with 0.1 M EDTA for 48 h at 4 °C. Samples were incubated at room temperature for 2 h in blocking solution (5% normal horse serum in 0.2% Triton X-100 in PBS). Cochleae were incubated overnight at room temperature with combinations of following primary antibodies: Hair cells were labeled with antibody against Myosin 7a (catalogue #25-6790, Proteus Biosciences, 1:500) and tight junctions were labeled with antibody against ZO-1 (catalogue #339100, Life technologies, 1:100). The apical surfaces of the sensory epithelia were stained using Phalloidin (catalogue #A12379, Life technologies; 25 μl/ml). For ZO-1 labeling, temporal bones were fixed for 20 min on ice using fresh, cold 10% trichloro-acetic acid (TCA, Sigma Aldrich), followed by immediate transfer into 1X PBS. Tissue was then dissected and permeabilized and blocked using 5% normal horse serum in 0.2% Triton X-100 in PBS solution for 2 h at room temperature. Samples were probed overnight at 4 °C with mouse monoclonal anti-ZO-1 antibody. Because zonulae occludins-1 (ZO-1) appears to be a requisite component of normally functioning epithelial tight junctions (Rodgers et al., 2013; Tornavaca et al., 2015; Van Itallie et al., 2009), we used ZO-1 labeling to infer the presence or absence of tight junctions in the reticular lamina. Following incubation in primaries, specimens were rinsed 5X in PBS and treated for 2 h in corresponding secondary antibodies. The secondary antibody solutions also contained DAPI (catalogue #D9542, Sigma-Aldrich, 1 μg/ml), in order to label cell nuclei. Specimens were cover-slipped in glycerol:PBS (9:1), and fluorescence imaging was performed using a Zeiss LSM 700 confocal microscope. Z-series images were obtained with either 20× air or 63× oil objective (zoom of 1.6) beginning at apical surface of hair cells and extending through the basilar membrane. Image processing and 3D rendering was performed using Volocity 3D image analysis software (Ver 6.1.1, PerkinElmer).
3. Results
3.1. The EP-versus-noise level relation is highly divergent by mouse strain
Acute EP varied considerably as a function of exposure intensity and mouse strain (Fig. 1). In agreement with our earlier results, older B6 mice were highly resistant to EP reduction for noise levels up to 110 dB SPL (Fig. 1A). At 110 dB, EP values were widely scattered, while higher exposure levels reliably led to a precipitous drop to <40 mV. For B6 mice, 110 dB appeared to represent a transition from essentially normal EP values to drastically reduced values. By contrast with B6, EPs in older CBA and BALB suggested a decline from normal values for exposures as low as 98 dB (Fig. 1B and C). Although there was a great deal of scatter, mean EP values in CBA and BALB both showed an average ~60 mV plateau extending from 98 to 107 dB (Fig. 2B). Similar to B6, EPs in CBA and BALB showed a transition at 110–113 dB to values below 30 mV (Fig. 1B and C; Fig. 2B). Overall, the EP-vs.-noise level relation was substantially similar in CBA and BALB mice, suggestive of three plateaus with two intervening transitions. The left-most ‘normal’ range differs between CBA and BALB because BALBs are known to possess a lower EP throughout life than either CBA or B6 (Ohlemiller et al., 2006). The relation for B6 mice was more consistent with two plateaus with a single steep transition. As Fig. 2B makes clear, no amount of shift on the X-axis would bring the relation for B6 mice into register with those for CBA or BALB. The relation in B6 is qualitatively and quantitatively different from the other strains. However, all three strains share a substantial drop to values below ~40 mV for exposures of 110 dB and higher.
Fig. 1.
Mean and scatter of acute EP versus noise exposure level by strain and age on semi-log axes. A. C57BL/6J. B. CBA/J. C. BALB/cJ.
Fig. 2.
Replot of means from Fig. 1 for young mice (A) and older mice (B). Vertical dashed lines indicate noise levels chosen for variation of noise duration in subsequent experiments (Fig. 4).
The B6 EP data for 110 dB exposures are somewhat at odds with our previous results, which indicated completely normal EP for older B6 at this noise level (Ohlemiller and Gagnon, 2007). This difference may reflect the use of a narrower band of noise in the present study versus broadband noise used previously. A more restricted band of noise (more spatially focused energy) might favor tearing of the reticular lamina (see below). Because of the large number of B6 animals tested at 110 dB (Fig. 1A), we used this condition to examine the effect of broad age variation in our older sample (Fig. 3). We thought it particularly useful to examine older B6 mice, as these should have extensive basal hair cell loss due to the influence of the Cdh23753A (Ahl) allele (Johnson et al., 1997, 2000). Such large scale hair cell loss might cause structural weakening of the organ of Corti. Although Fig. 3 shows increased scatter in the EP for animals aged 14–16 mos, no significant correlation was found between acute EP and age. Thus neither advanced age nor associated hair cell loss appear to promote noise injury to the cochlear lateral wall or structural weakening of the organ so that the EP is reduced.
Fig. 3.

Scatter and linear regression for acute EP versus age in B6 mice exposed to 110 dB SPL.
3.2. The EP is more affected by noise in young mice
Each of the strains tested showed pronounced age effects, such that young mice were more affected. CBA and BALB mice were again similar to each other, yet very different from B6. On average, acute EPs in young CBA and BALB fell to a constant ~20 mV for exposures 101 dB SPL and higher (Fig. 1B and C; Fig. 2A). EPs in this plateau were similar to those seen for older animals for exposures above 110 dB. By contrast, while EPs in young B6 mice decreased from normal values for exposures above ~95 dB, reductions were generally modest for exposure levels up to 110 dB, where values abruptly declined in a manner similar to the older B6 mice (Fig. 1A; Fig. 2A).
3.3. Temporal dynamics of EP reduction differ for 104 and 119 dB exposures
The average functions in Figs. 1 and 2 are suggestive of distinct injury states over particular exposure ranges. These states vary by strain and age. One way to explore the underlying processes is to select exposure intensities that may correspond to different injury states and vary the exposure duration. We selected 104 dB and 119 dB as representative exposure intensities for the ‘intermediate’ and ‘low’ EP plateaus (see dashed vertical lines in Fig. 2). Fig. 4A,D compare results for older mice at 104 dB and 119 dB for exposure durations ranging 14 seconds-2 hrs. For experiments at 104 dB, B6 mice were not included, since they showed little EP reduction at this level. Although EPs were widely scattered at 104 dB, values for CBA and BALB appeared to occupy similar ranges for most exposure durations, and so were averaged together. At 119 dB, EPs for all three strains were similarly distributed, and were also averaged together. At 104 dB SPL, the mean EP fell from normal values for exposures ≥7.5 min, to an average 70–80 mV, but showed no further decline for durations up to 2 h. At 119 dB, EPs in older mice showed a rapid decline for durations greater than 1.88 min, then progressive reduction to typically <20 mV by 2 h.
3.4. Temporal dynamics of EP reduction are age-dependent
EP temporal dynamics for young mice are examined in Fig. 4B,E. At 104 dB SPL, rather than falling to a plateau as seen for older mice, the mean EP in the young mice continued to fall with increasing noise duration in a nearly log-linear manner. At 119 dB, mean EP also fell progressively, although more steeply, and no strain effects were apparent. Fig. 4C,F compare young versus older averages at 104 dB and 119 dB. Mean trends are qualitatively different for young and older mice at 104 dB. By contrast, young and older mice appear similar at 119 dB, independent of strain.
3.5. Exposure to 104 dB in young mice promotes rapid hair cell loss and openings in the reticular lamina
Severe reduction in the EP of young CBA and BALB mice might reflect openings in the reticular lamina to which these mice could be more prone. Fig. 5 shows typical acute surface views of the medial organ of Corti from young B6, BALB, and CBA mice exposed for 2 h at 104 dB SPL. As judged by staining for outer hair cell nuclei (DAPI), cuticular plates (phalloidin), and tight junctions between adjacent cells (ZO-1), young BALB and CBA mice undergo rapid OHC loss during the exposure, leaving holes in the reticular lamina. Young B6 mice appear much less prone to OHC loss at this exposure level, and show little evidence of holes. When the view is rotated and expanded to include the lateral organ of Corti (Fig. 6), the impression of gaps in the lamina at locations of hair cell loss is supported. This view, however, also reveals a surprising association between holes in the OHC region and tears between the third row of Deiters’ cells and Hensen’s cells (Fig. 6C, arrow). Fig. 6D–F show three more examples of tears laterally in other animals (arrows). Thus both holes and tears in the lamina likely contribute to acute EP reduction in young CBA and BALB mice. Based on Fig. 1, this kind of injury may occur for exposure levels down to 100 dB in these strains. Young B6 mice showed neither holes nor tears (Fig. 6G–I), in keeping with the notion that these are part of the process that reduces the EP in young CBA and BALB mice.
Fig. 5.
Exposure to 104 dB SPL for 2 h promotes rapid hair cell loss and openings in the reticular lamina in young mice. Images show whole mounts from the basal turn of the cochlea of C57BL/6J, BALB/cJ and CBA/J mouse immediately after noise exposure immunolabeled for DAPI (A,D,G), Phalloidin (B,E,H) or ZO-1 (C,F,I). Arrows indicate some regions of missing/pyknotic OHCs or lamina disruption. ZO-1 Images are from different animals. Scale bar: 13 μm (A,G,B,E,H) and 6 μm (C,F,I).
Fig. 6.
Representative images of cochlear sensory epithelium immunolabeled for myosin 7a, Phalloidin 488 and DAPI shows holes (A, 3D surface view and B, 3D-radial view, white arrow head) and tears (C, DIC, white arrow) in the reticular lamina of CBA/J mouse 2 h post noise exposure. D,E,F, additional DIC examples of tears from three more animals. G,H,I, Representative images showing intact reticular lamina of C57BL/6 mouse 2 h post noise exposure. Scale: 20 μm. DIC, Differential interference contrast.
Analysis of surface preparations from older mice exposed at 104 dB (Fig. 7) indicated that OHC loss or other disruption of the reticular lamina during noise exposure was minimal, irrespective of strain. Although the EP was not measured in the mice shown, previous and present EP data (Figs. 1, 2 and 4) indicate that the BALB and CBA mice shown would have exhibited moderate EP reduction, while the EP in the B6 mice would have been normal. This suggests that any EP reduction in older CBA and BALB mice was not due to openings in the reticular lamina, and is in keeping with our previous light microscope analyses at a higher sound level (110 dB SPL) (Ohlemiller and Gagnon, 2007; Ohlemiller et al., 2011a), which showed an intact lamina and little hair cell loss during the exposure.
Fig. 7.
Exposure to 104 dB SPL for 2 h has little effect on the reticular lamina in older mice. Images show whole mounts from the basal turn of the cochlea of C57BL/6J, BALB/cJ and CBA/J mouse immediately after noise exposure immunolabeled for DAPI (A,D,G), Phalloidin (B,E,H) or ZO-1 (C,F,I). ZO-1 Images are from different animals. Scale bar: 13 μm (A,G,B,E,H) and 6 μm (C,F,I).
3.6. 119 dB exposures cause reticular lamina disruption in all mice
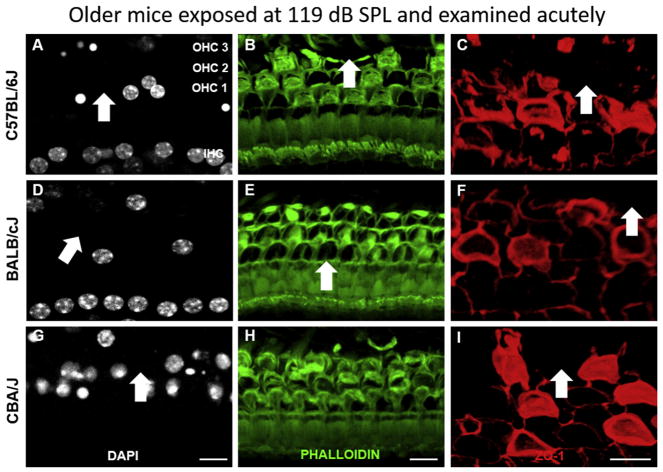
Figs. 8 and 9 show the corresponding analysis of acute surface preparations from young and older mice exposed for 2 h at 119 dB SPL. Extensive OHC loss and disruption of the reticular lamina appear, irrespective of strain or age (white arrows). Surface preparations could not discern whether disruptions of the reticular lamina at this exposure level corresponded to holes or tears, although both are likely. Such tears have already been demonstrated in mice and other animals for high level noise exposures (e.g., Hirose and Liberman, 2003).
Fig. 8.
Exposure to 119 dB SPL for 2 h promotes rapid hair cell loss and openings in the reticular lamina in young mice. Images show whole mounts from the basal turn of the cochlea of C57BL/6J, BALB/cJ and CBA/J mouse immediately after noise exposure immunolabeled for DAPI (A,D,G), Phalloidin (B,E,H) or ZO-1 (C,F,I). Arrows indicate some regions of missing/pyknotic OHCs or lamina disruption. ZO-1 Images are from different animals. Scale bar: 13 μm (A,G,B,E,H) and 6 μm (C,F,I).
Fig. 9.
Exposure to 119 dB SPL for 2 h promotes rapid hair cell loss and openings in the reticular lamina in older mice. Images show whole mounts from the basal turn of the cochlea of C57BL/6J, BALB/cJ and CBA/J mouse immediately after noise exposure immunolabeled for DAPI (A,D,G), Phalloidin (B,E,H) or ZO-1 (C,F,I). Arrows indicate some regions of missing/pyknotic OHCs or lamina disruption. ZO-1 Images are from different animals. Scale bar: 13 μm (A,G,B,E,H) and 6 μm (C,F,I).
3.7. Acute loss of ZO-1 in the medial and lateral organ of Corti coincide
We noted that when ZO-1 appears acutely disrupted medially in the hair cell region of the organ of Corti, it is also nearly absent laterally from the junctions between Hensen’s and Claudius cells. Fig. 10 shows both medial and lateral organ of Corti (separated by dashed lines) in eight example mice. The mice were chosen so that some show disrupted ZO-1 in the medial organ (119 dB, bottom) and some do not (104 dB, top). In each of these mice, the normal or abnormal appearance of ZO-1 in the hair cell region predicts the presence or near absence of ZO-1 in cell-cell junctions of the lateral organ.
3.8. EP reduction versus noise duration adheres to different numerical models at 104 and 119 dB
Anatomic differences between young and older mice for 104 dB noise exposures may mean that young mice possess more fragile OHCs and/or a more mechanically fragile organ of Corti. At 119 dB SPL, the injury likely includes both holes and tears in all our mice. In our consideration of the patterns in Fig. 4, we noted that both the slope of the EP decrease with time and final EP appeared to differ between young CBA and BALB mice exposed at 104 dB versus all mice exposed at 119 dB. Although these plots are derived from many mice, if individual mice could be repeatedly sampled without artifact, we would expect them to reproduce the same trends during the exposure. One interpretation of the different slopes and endpoints might be that the different rates and degrees of EP decline reflect different rates in the formation of openings in the reticular lamina. During an ongoing noise exposure, the EP changes in a manner that reflects changes in conductance between endolymphatic and perilymphatic spaces. In a normal cochlea, the size of standing currents through hair cells is set by the electrochemical gradient for K+ ions between scala media and hair cells and the single channel conductance of the transducer channels. Acoustic over-stimulation that produces rapid hair cell death and holes in the reticular lamina or direct tears in the lamina, adds uncontrolled leakage conductance. The slope of EP decline and final EP depend on the size of the added conductance, electrochemical diffusion in scala media, the voltage gradient between the scalae, and the rate of K+ re-cycling to the stria. At least on a time scale of hours, holes and tears will be set in an open state, so the rate of electrical diffusion is dependent upon the size of the openings and the thermodynamics of K+ diffusion. These can be modeled using a Boltzmann function. To generate as many independent fits as possible, we separated strain data of Fig. 4 by gender, yielding up to 14 separate data sets that could be used to derive estimates of EPfinal. Of these, 11 produced unique Boltzmann equations, shown in Fig. 11A–K and summarized in Fig. 11L. Most fitted curves featured a clear EPinitial, a range of times over which the EP declined, and a plateau for EPfinal. It was not clear in all cases that the EP reached a single final value, or whether it would have continued to decrease for longer exposures. Nevertheless, Boltzmann fits to seven data sets for 119 dB exposures and four data sets for 104 dB exposures yielded no overlap of EPfinal.
Fig. 11.
Replot of data from Fig. 4 broken down into 11 different data sets (From a possible 14. See Methods) by strain and gender, with subsequent fit of data to Boltzmann functions. Fig. 10L summarizes derived EPinitial and EPfinal by data set. Fits obtained for 104 and 119 dB exposures did not overlap with regard to EPfinal.
3.9. For 119 dB exposures the EP largely recovers for most groups, but collapses in young B6 mice
We previously explored the recovery of the EP after a 2-hr 110 dB SPL exposure in mice >3 mos of age (Ohlemiller et al., 2011a), noting that the EP typically recovers, with the exception of ~25% of BALB mice. The sheer number of conditions tested in the present study made a thorough examination of recovery impractical. Instead we simply examined recovery for the most extreme exposure,119 dB for 2 h. The scatter plot of Fig. 12 shows recovery at 1 mo post-exposure for each strain and age group. Horizontal dashed lines depict the lowest EP recorded in each strain for ages up to 1 yr from our previous studies (Ohlemiller, 2009). For most strains and ages, most data lie above the dashed lines, supporting a trend toward recovery. One exception is young CBAs, yet the most glaring exception is the case of young B6 mice, wherein the EP invariably collapsed to ≤5 mV. No recovery was apparent even out to 3 mos in these mice, so that this loss of the EP is likely permanent. Two of 10 older B6 mice also showed a similar collapse, so that a stochastic process is suggested. These findings in B6 are strikingly at odds with the seeming resilience of the EP in both young and older B6 mice exhibited in Figs. 1–3.
Fig. 12.

Scatter plot of EPs for animals allowed to survive 1 mo after exposure to 119 dB SPL noise. Data are organized by strain and age (young versus older). Data for B6 mice also include 3 mo post-exposure survival. Horizontal dashed lines indicate the lowest EP measured in each strain for mice up to 1 yr of age from previous work (Ohlemiller, 2009).
3.10. EP collapse after 119 dB exposure in young B6 mice reflects persistent loss of tight junctions
Confocal analysis of young B6 mice exposed to 119 dB SPL and allowed to recover for 1 mo or more indicated these animals fail to recover from the acute loss of ZO-1 in both medial and lateral organ of Corti. Fig. 13 compares ZO-1 expression in a young B6 mouse examined 1 mo after a 2 h 119 dB exposure, an unexposed age-matched control, and a similarly-exposed older B6 mouse allowed to recover for 2 mos. The exposed older mouse resembles the unexposed control with respect to appearance of ZO-1. The disruption of ZO-1 labeling in the OHC region, and near absence of ZO-1 in the lateral organ of young B6 mice resembles the acute changes in ZO-1 in these mice after 119 dB exposure (Fig. 10). Among the strains and ages examined, young B6 mice may uniquely lack the ability to restore tight junctional integrity to the reticular lamina. This could account for their reliably poor EP recovery after intense noise exposure (Fig. 12). As also suggested by Fig. 12, some older B6 mice may show the same lack of recovery, but all samples obtained from older mice exposed at 119 dB (not shown) showed normal ZO-1 staining in the lateral organ.
Fig. 13.
Examples of ZO-1 staining in the organ of Corti of B6 mice 1 or 2 mos after 119 dB noise exposure. Exposed young mouse (B) shows loss of cell-cell tight junctions (arrows) that appear intact in age-matched unexposed control (A) and exposed older B6 mouse (C). Scale Bars: A: 12 μm, B: 17 mμ, C: 10 μm.
4. Discussion
From the largest collection of EP recordings in any animal model to date, we characterized dynamic aspects of noise-related EP reduction in three inbred mouse strains in two age groups. Our collective findings suggest that acute EP reduction from a single noise exposure can reflect lateral wall pathology, loss of reticular lamina integrity, or both. Evidence for acute breach of the lamina was confined to high exposure levels in older mice, but was also found at lower exposure levels in younger animals in a manner that depended on genetic background. Based on the different rate and extent of EP reduction at 104 versus 119 dB (Figs. 4 and 12), we anticipated that reticular lamina breach would be limited to holes at points of OHC loss at 104 dB, but would also include tears at 119 dB. Surprisingly, young CBA and BALB mice exposed at 104 showed evidence of both holes and tears, suggesting that these may both have the same proximate cause. Since young B6 mice showed little EP reduction at this noise level and little evidence of either holes or tears, it is tempting to speculate that all three events are related. That is, a genetically-based tendency in young CBA and BALB mice toward noise-related lateral wall pathology may somehow drive injury to the organ of Corti that manifests as both holes and tears. These reduce the EP even further. Our confocal observations after 2-hr exposure do not clarify whether there are exposure conditions that cause holes without tears, or whether one may promote the other. If there exist a range of exposure conditions in young CBA and BALB mice that cause only holes, they may lie between 90 and 100 dB (Figs. 1 and 2), or might be found for exposures shorter than 2 h. The coincidence of holes and tears in our young CBA and BALB material for a 104 dB exposure suggests that tears need not reflect purely mechanical trauma while holes primarily indicate lethal metabolic injury to OHCs. That these features are not seen in older CBA and BALB mice after 104 dB exposure further suggests that the organ of Corti in young mice is more vulnerable to metabolic perturbations than in older mice. Thus, the cochlear lateral wall in young mice need not be particularly fragile, but rather, the organ of Corti in young mice is more affected by metabolic events surrounding acute EP reduction. These may include disruption of the flow of nutrients from spiral ligament or strial blood vessels to the organ of Corti and alterations of K+ homeostasis (Chang et al., 2008; Forge et al., 2013; Ohlemiller, 2015). Interplay between the organ of Corti and lateral wall dysfunction may partly account for how the early vulnerable period to noise manifests among species and on different genetic backgrounds.
Based on recovery of the EP one mo after a 119 dB SPL exposure (Fig. 12), breaches of the reticular lamina are substantially repaired in most cases. In CBA mice, recovery of the EP was often incomplete, particularly in young CBAs, wherein the majority of animals suggested incomplete recovery (Fig. 12). In the present study we did not test for recovery of the EP over the mild-to-moderate exposure range, but previously reported incomplete recovery in some BALBs for 110 dB exposures. Partial EP recovery in BALBs was not clear in the present data (Fig. 12), but that may reflect actual differences between 110 versus 119 dB exposures, or simply a small sample size. The most glaring exceptions to EP recovery, however, were young B6 mice, wherein collapse of the EP reliably corresponded to the absence of ZO-1 in cell-cell junctions of the lateral organ of Corti (Fig. 13).
Confirming and extending our earlier reports for a single 110 dB SPL exposure (Ohlemiller and Gagnon, 2007; Ohlemiller et al., 2011a, 2016), EP-versus-noise level relations differ both qualitatively and quantitatively by strain and age. Striking differences appeared across a wide range of exposures, from as little as 92 dB SPL up to 110–113 dB (Figs. 1 and 2), at which level mechanical trauma may ultimately impose a common limit on the tolerance of μm. the organ of Corti. The contribution of EP reduction to acute threshold shifts therefore varies with age and genetic makeup for a wide range of exposure conditions. For exposure levels up to 110 dB, our previous anatomic assessments indicated that EP reductions in older CBA and BALB mice (>3 mos) reflect principally lateral wall pathology. The present data suggest this pathology arises at exposure levels as low as 98 dB, producing wide variation in the EP (~30–110 mV, Fig. 1B and C) and an average EP of ~70 mV in both strains.
Caveats attending this work notably include limited and purely qualitative anatomic assessment, the use of only two noise intensities (104 and 119 dB SPL) to infer broad ‘low-to-moderate’ and ‘high’ level noise exposure EP dynamics, and combining data from many mice to infer EP changes that individual mice may undergo with a continuing exposure (Fig. 4). The latter approach was taken to avoid potential artifacts associated with multiple EP recording tracks. Our inferences about tight junctions were limited to their presence or absence, and were based on the distribution of ZO-1. This protein appears required for epithelial tight junction formation, although not necessarily for small ion regulation (Rodgers et al., 2013; Tornavaca et al., 2015; Van Itallie et al., 2009). Finally, our suggestion that EP reduction in young CBA and BALB mice is dominated by opening of the lamina does not disprove any role for heightened vulnerability of the lateral wall in young animals. Indeed, Fig. 1A suggests a modest EP reduction in younger B6 mice that does not appear in older mice. We have not yet characterized the lateral wall or Reissner’s membrane after exposure in young mice. Most likely, the younger CBA and BALB mice will show changes in the lateral wall similar to their older counterparts (Ohlemiller and Gagnon, 2007; Ohlemiller et al., 2010, 2011a), making it difficult to separate potential origins of their EP reduction. In older CBA and BALB mice, injury metrics for stria vascularis, spiral ligament, and Reissner’s membrane were each correlated with EP reduction, so that it was not necessary to invoke injury to the organ of Corti to explain those results. In the present work, qualitatively different patterns of EP reduction (Figs. 1, 2 and 12) correspond to qualitative differences in the appearance of the reticular lamina (Figs. 5–7, 13), so that it is simply not necessary to invoke additional strain-dependent changes in the lateral wall to explain our results. Light microscopy evaluation of our material is ongoing.
4.1. Noise level-versus-acute EP
Two patterns emerged in noise level-versus-EP dynamics of older mice. Older CBA and BALB mice appeared generally similar, while both diverged widely from B6. EP-versus-noise intensity relations for CBA and BALB mice are suggestive of two plateaus, a partial reduction for exposure levels ranging 98–107 dB SPL, and a more extreme reduction for exposures ≥110 dB. The complete absence of EP reduction at lower exposure levels in B6 suggests that a process that reduces the EP in CBA and BALB is absent in B6. The patterns by strain are qualitatively different and cannot be superimposed by simple translation on the X-axis. Despite similar CBA and BALB noise-EP phenotypes, these strains show different inheritance patterns of the EP-reduction phenotype when crossed with B6 mice (respectively, dominant and recessive vs. B6), and only partial overlap of related QTLs (Ohlemiller et al., 2010, 2016). Although candidate genes have been identified, the underlying genes remain unknown.
In hindsight the choice of 110 dB SPL exposures in our previous work was fortuitous, since we might have missed strain differences had we used a higher noise level (Figs. 1 and 2). Yet even had we combined data across test ages, we would still have detected strain differences for any noise level between 95 and 110 dB. Irrespective of age, the acute EP in B6 mice after 110–113 dB of octave band noise showed considerable variation that might reflect a probabilistic character of tearing of the lamina at these noise levels (Fig. 1A). There appeared, moreover, little age dependence in B6 for ages up to 16 months (Fig. 3). Although we did not count hair cells, by 9–16 mos advanced hair cell loss is expected in the basal half of the B6 mouse cochlea due to the influence of Cdh23753A and Ahl3 (Johnson et al., 1997; Morita et al., 2007). Thus, as long as the ion barrier of the reticular lamina is maintained, pronounced hair cell loss neither promotes nor prevents noise-related EP reduction. A corollary might be that advanced loss of hair cells need not render the reticular lamina more vulnerable to tearing. The first argument is also supported by the fact that BALB and B6 mice share the Cdh23753A allele and show similar age-dependent hearing loss (Johnson et al., 2000; Zheng and Johnson, 2001), yet BALBs closely resemble ‘good hearing’ CBA mice in noise-EP phenotype.
4.2. Holes versus tears in the reticular lamina and the influence of age
For all strains and ages, acutely measured EPs were similarly low after exposures ≥110 dB SPL (Figs. 1 and 2). Temporal dynamics at 119 dB (Fig. 4) also indicated similar EP reduction over time, irrespective of strain or age. In chinchillas, guinea pigs and mice, high level exposures and impact exposures are associated with tears in the lamina (Fredelius, 1988; Fredelius et al., 1990; Henderson et al., 1994; Spongr et al., 1998; Thorne et al., 1984; Zheng and Hu, 2012). While few studies have included EP measures, those that have indicate EP acute reduction, ostensibly caused by added leakage conductance across the damaged reticular lamina. Most relevant to the current study is work by Hirose and colleagues (Hirose and Liberman, 2003; Wang et al., 2002), wherein exposing CBA/CaJ mice to 116 dB octave band noise was associated with EP reductions similar to those we find, along with visual evidence of tears in the lamina. For the same type of noise used in those studies, our data lower the critical noise level for the strains we examined down to 110–113 dB SPL.
In young CBA and BALB mice, hair cell death and openings in the reticular lamina may accumulate quickly for even modest exposure levels. Although our corresponding confocal images were obtained at 104 dB SPL (Fig. 6), rapid hair cell loss and gaps in the lamina may begin at levels as low as 95 dB, where the EP abruptly drops with increasing noise level (Figs. 1 and 2). Extant accounts of rapid hair cell death after exposure to noise (Oesterle, 2013) and ototoxins (Anttonen et al., 2012, 2014; Astbury and Read, 1982; Forge, 1985; Leonova and Raphael, 1997; McDowell et al., 1989; Raphael and Altschuler, 1991; Taylor et al., 2008) in guinea pig, rat, and mouse favor a general principle where scar formation coincides with hair cell elimination from the reticular lamina, and holes are prevented. Prior to the current study, exceptions have been limited to knockout mouse models (Cohen-Salmon et al., 2002; Jin et al., 2016) and focal hair cell loss in chinchillas for relatively low-level noise exposures reported by Bohne and colleagues (Harding and Bohne, 2004). The present findings indicate that acute reticular lamina breach could be a common feature of young adult cochlea, which had not been closely examined within this framework.
Young adult animals are especially vulnerable to cochlear noise injury, including greater loss of OHCs for a given degree of NIPTS (Ohlemiller et al., 2000, 2011b; Pujol, 1992; Saunders and Chen, 1982). In mice, the early vulnerable period peaks at 1–2 mos of age and ends at ~4 mos (Henry, 1982, 1983). The cellular and molecular bases of this period are not known, although it is possible that OHCs are more metabolically or mechanically fragile. Some clues may emerge from acute EP differences between young B6 mice versus young CBA and BALB mice. If, as we suggest, stability of the EP and lateral wall function in young B6 mice helps to protect the organ of Corti, one might predict that young B6 mice would be less vulnerable to noise than similarly aged CBA/J and BALB mice, at least for exposures levels not exceeding 110 dB SPL. This is what has been found (Ohlemiller et al., 2000, 2011b). Thus, our EP findings parallel the relative noise vulnerabilities of young B6, CBA, and BALB mice, but not for older mice of the same strains.
4.3. A mathematical signature of reticular lamina breach?
Noise duration-versus-EP dynamics (Figs. 4 and 11) support three distinct injury states as a function of exposure duration: An initial ‘normal’ EP (EPinitial), a transition phase, and a final EP (EPfinal). The final EP value was lower for 119 dB exposures than 104 dB exposures and all mice appeared to respond similarly. By contrast, the EP decline at 104 dB was markedly different by mouse strain and age. Young CBA and BALB mice showed progressive EP reduction from the start of the exposure, while their older counterparts showed more modest reduction to a plateau, starting around 3.75 min. This difference in rate and extent of EP reduction may reflect lateral wall versus organ of Corti injury contributions. At 119 dB, both holes and tears likely accumulate more quickly than at 104 dB. We cannot, of course, rule out that greater damage to the stria vascularis also contributed to the final EP value at 119 dB. Boltzmann fits to the present data did not clearly identify differential time courses associated with the accumulation of lateral wall pathology or holes versus tears in the reticular lamina. Follow-up studies quantifying hair cell, strial cell, and tight junction loss are needed.
4.4. Failure of tight junction restoration after intense noise exposure in B6 mice
An especially surprising finding, particularly in light of the resilience of B6 mice emphasized above, was the complete failure of EP recovery in young B6 mice exposed to 119 dB SPL, then allowed to recover for up to 3 mos (Figs. 12 and 13). Confocal analysis indicated near absence of ZO-1 in cell-cell junctions of the lateral organ of Corti of these mice. Assuming the distribution of ZO-1 is an indication of tight junction composition and function (Rodgers et al., 2013; Tornavaca et al., 2015; Van Itallie et al., 2009), young B6 mice exhibit wholesale failure to restore an ion-tight barrier as part of reticular lamina repair. This fits with no other aspect of our findings, and seems to reflect cellular processes not assessed by acute examination. Notably, several published genetic impairments of tight junctions apparently do not promote EP reduction (Bahloul et al., 2009; Ben-Yosef et al., 2003; Kitajiri et al., 2014; Morozko et al., 2014; Nakano et al., 2009; Nayak et al., 2013). Young B6 mice exposed to supercritical noise levels may re-form tight junctions that are severely dysfunctional, leading to greater paracellular ion leakage and permanent EP collapse. We know of no other report of persistent failure to re-establish tight junctions in the reticular lamina after insults, so that this phenomenon merits further study.
Acknowledgments
Funding agencies
P30 DC04665 (R.A. Chole), R01 DC006283 (M.E. Warchol), R03 DC015320 (T. Kaur) and Washington University School of Medicine, Department of Otolaryngology.
Thanks to the WUSM Dept. of Otolaryngology for the opportunity to carry out this work. This account is dedicated to the memory and scientific contributions of Patricia M. Gagnon.
References
- Ahmad M, Bohne BA, Harding GW. An in vivo tracer study of noise-induced damage to the reticular lamina. Hear Res. 2003;175:82–100. doi: 10.1016/s0378-5955(02)00713-x. [DOI] [PubMed] [Google Scholar]
- Anttonen T, Kirjavainen A, Belevich I, Laos M, Richardson WD, Jokitalo E, Brakebusch C, Pirvola U. Cdc42-dependent structural development of auditory supporting cells is required for wound healing at adulthood. Sci Rep. 2012:2. doi: 10.1038/srep00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttonen T, Belevich I, Kirjavainin A, Laos M, Brakebusch c, Joritalo E, Pirvola U. How to bury the dead: elimination of apoptotic hair cells from the hearing organ of the mouse. J Assoc Res Otolaryngol. 2014;15:975–992. doi: 10.1007/s10162-014-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astbury PJ, Read NG. Kanamycin induced ototoxicity in the laboratory rat. Arch Toxicol. 1982;50:267–278. doi: 10.1007/BF00310859. [DOI] [PubMed] [Google Scholar]
- Bahloul A, Simmler MC, Michel V, Leibovici M, Perfettini I, Roux I, Weil D, Nouaille S, Zuo J, Zadro C, Licastro D, Gasparini P, Avan P, Hardelin JP, Petit C. Vezatin, an integral membrane protein of adherens junctions, is required for the sound resilience of cochlear cells. EMBO Mol Med. 2009;1:125–138. doi: 10.1002/emmm.200900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beluzzi O, Sacchi O, Wanke E. Identification of delayed potassium and calcium currents in the rat sympathetic neurone under voltage clamp. J Physiol Pharmacol. 1985;358:109–129. doi: 10.1113/jphysiol.1985.sp015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- Chang Q, Tang W, Ahmad S, Zhou B, Lin x. Gap junction mediated intercellular metabolite transfer in the cochlea is compromised in connexin 30 null mice. PloS One. 2008;3:e4088. doi: 10.1371/journal.pone.0004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junctional network causes hearing impairment and cell death. Curr Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Ouanounou G, Rouzaire-Dubois B. The Boltzmann equation in molecular biology. Prog Biophys Mol Biol. 2009;99:87–93. doi: 10.1016/j.pbiomolbio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Ehrenstein G, Lecar H, Nossal R. The nature of the negative resistance in bimolecular lipid membranes containing excitability-inducing material. J Gen Physiol. 1970;55:119–133. doi: 10.1085/jgp.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A. Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res. 1985;19:171–182. doi: 10.1016/0378-5955(85)90121-2. [DOI] [PubMed] [Google Scholar]
- Forge A, Jagger DJ, Kelly JJ, Taylor RR. Connexin30-mediated intercellular communication plays an essential role in epithelial repair in the cochlea. J Cell Sci. 2013;126:1703–1712. doi: 10.1242/jcs.125476. [DOI] [PubMed] [Google Scholar]
- Fredelius L. Time sequence of degeneration pattern of the organ of Corti after acoustic overstimulation. Acta Otolaryngol. 1988;106:373–385. doi: 10.3109/00016488809122260. [DOI] [PubMed] [Google Scholar]
- Fredelius L, Bagger-Sjoback D, Johansson B, Wersall J. Time-related changes in the Guinea pig cochlea after acoustic overstimulation. Ann Otol Rhinol Laryngol. 1990;99:369–378. doi: 10.1177/000348949009900510. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA. Noise-induced hair-cell loss and total exposure energy: analysis of a large data set. J Acoust Soc Am. 2004;115:2207–2220. doi: 10.1121/1.1689961. [DOI] [PubMed] [Google Scholar]
- Henderson D, Spongr V, Subramaniam M, Campo P. Anatomical effects of impact noise. Hear Res. 1994;76:101–117. doi: 10.1016/0378-5955(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Henry KR. Age-related changes in sensitivity of the postpubertal ear to acoustic trauma. Hear Res. 1982;8:285–294. doi: 10.1016/0378-5955(82)90020-x. [DOI] [PubMed] [Google Scholar]
- Henry KR. Lifelong susceptibility to acoustic trauma: changing patterns of cochlear damage over the life span of the mouse. Audiology. 1983;22:372–383. doi: 10.3109/00206098309072797. [DOI] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol. 2005;489:180–194. doi: 10.1002/cne.20619. [DOI] [PubMed] [Google Scholar]
- Jin Y, Ren N, Li S, Fu X, Sun X, Men Y, Xu Z, Zhang J, Xie Y, Xia M, Gao J. Deletion of Brg1 causes abnormal hair cell planer polarity, hair cell anchorage, and scar formation in mouse cochlea. Sci Rep. 2016;6:27124. doi: 10.1038/srep27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least 10 inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Kitajiri S-I, Katsuno T, Sasaki H, Ito J, Furuse M, Tsukita S. Deafness in occludin-deficient mice with dislocation of tricellulin and progressive apoptosis of the hair cells. Biol Open. 2014 doi: 10.1242/bio.20147799. BIO20147799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonova EV, Raphael Y. Organization of cell junctions and cytoskeleton in the reticular lamina in normal and ototoxically damaged organ of Corti. Hear Res. 1997;113:14–28. doi: 10.1016/s0378-5955(97)00130-5. [DOI] [PubMed] [Google Scholar]
- McDowell B, Davies S, Forge A. The effect of gentamicin-induced hair cell loss on the tight junctions of the reticular lamina. Hear Res. 1989;40:221–232. doi: 10.1016/0378-5955(89)90163-9. [DOI] [PubMed] [Google Scholar]
- Morita Y, Hirokawa S, Kikkawa Y, Nomura T, Yonekawa H, Shiroishi T, Takahashi S, Kominami R. Fine mapping of Ahl3 affecting both age-related and noise-induced hearing loss. Biochem Biophys Res Commun. 2007;355:117–121. doi: 10.1016/j.bbrc.2007.01.115. [DOI] [PubMed] [Google Scholar]
- Morozko EL, Nishio A, Ingham NJ, Chandra R, Fitzgerald T, Martelletti E, Borck G, Wilson E, Riordan GP, Wangemann P, Forge A, Steel KP, Liddle RA, Friedman TB, Belyantseva IA. ILDR1 null mice, a model of human deafness DFNB42, show structural aberrations of tricellular tight junctions and degeneration of auditory hair cells. Hum Mol Genet. 2014;24(3):609–624. doi: 10.1093/hmg/ddu474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Kim SH, Kim HM, Sanneman JD, Zhang Y, Smith RJH, Marcus DC, Wangemann P, Nessler RA, Banfi B. A claudin-9-based ion permeability barrier is essential for hearing. PLoS Genet. 2009;5:e1000610. doi: 10.1371/journal.pgen.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak G, Lee SI, Yousaf R, Edelmann SE, Trincot C, Van Itallie CM, Sinha GP, Rafeeq M, Jones SM, Belyantseva IA, Anderson JM, Forge A, Frolenkov G, Riazuuddin S. Tricellulin deficiency affects tight junction architecture and cochlear hair cells. J Clin Invest. 2013;123:4036–4049. doi: 10.1172/JCI69031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC. Changes in the adult vertebrate auditory sensory epithelium after trauma. Hear Res. 2013;297:91–98. doi: 10.1016/j.heares.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Heidbreder AF. Vulnerability to noise-induced hearing loss in ‘middle-aged’ and young adult mice: a dose-response approach in CBA, C57BL, and BALB inbred strains. Hear Res. 2000;149:239–247. doi: 10.1016/s0378-5955(00)00191-x. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Lett JM, Gagnon PM. Cellular correlates of age-related endocochlear potential reduction in a mouse model. Hear Res. 2006;220:10–26. doi: 10.1016/j.heares.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Genetic dependence of cochlear cells and structures injured by noise. Hear Res. 2007;224:34–50. doi: 10.1016/j.heares.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–83. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Rosen AD, Gagnon PM. A major effect QTL on chromosome 18 for noise injury to the mouse cochlear lateral wall. Hear Res. 2010;260:47–53. doi: 10.1016/j.heares.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Rosen AR, Rellinger EA, Montgomery SC, Gagnon PM. Different cellular and genetic basis of noise-related endocochlear potential reduction in CBA/J and BALB/cJ mice. J Assoc Res Otolaryngol. 2011a;12:45–58. doi: 10.1007/s10162-010-0238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Rybak Rice ME, Rellinger EA, Ortmann AJ. Divergence of noise vulnerability in cochleae of young CBA/J and CBA/CaJ mice. Hear Res. 2011b;272:13–20. doi: 10.1016/j.heares.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Chapter 3: a question of balance: free radicals in inner ear homeostasis. In: Miller J, Le Prell CG, editors. Free Radicals in ENT Medicine. Springer; New York: 2015. pp. 21–55. [Google Scholar]
- Ohlemiller KK, Kiener AL, Gagnon PM. QTL mapping of endocochlear potential differences between C57BL/6J and BALB/cJ mice. J Assoc Res Otolaryngol. 2016;17:173–194. doi: 10.1007/s10162-016-0558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol R. Sensitive developmental period and acoustic trauma: facts and hypotheses. In: Dancer AL, editor. Noise-induced Hearing Loss. Mosby; St. Louis: 1992. pp. 196–203. [Google Scholar]
- Raphael Y, Altschuler RA. Scar formation after drug-induced cochlear insult. Hear Res. 1991;51:173–183. doi: 10.1016/0378-5955(91)90034-7. [DOI] [PubMed] [Google Scholar]
- Rodgers LS, Beam MT, Anderson JM, Fanning AS. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J Cell Sci. 2013;126:1565–1575. doi: 10.1242/jcs.113399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JC, Chen CS. Sensitive periods of susceptibility to auditory trauma in mammals. Environ Health Perspect. 1982;44:63–66. doi: 10.1289/ehp.824463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spongr VP, Henderson D, McFadden SL. Confocal microscopic analysis of the chinchilla organ of Corti following exposure to high-level impact noise. Scand Audiol Suppl. 1998;48:15–26. [PubMed] [Google Scholar]
- Taylor RR, Nevill G, Forge A. Rapid hair cell loss: a mouse model for cochlear lesions. J Assoc Res Otolaryngol. 2008;9:44–64. doi: 10.1007/s10162-007-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PR, Gavin JB, Herdson PB. A quantitative study of the sequence of topographical changes in the organ of Corti following acoustic trauma. Acta Otolaryngol. 1984;97:69–81. doi: 10.3109/00016488409130966. [DOI] [PubMed] [Google Scholar]
- Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol. 2015;208:821–838. doi: 10.1083/jcb.201404140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576(1):11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Hu BH. Cell-cell junctions: a target of acoustic overstimulation in the sensory epithelium of the cochlea. BMC Neurosci. 2012;13:71. doi: 10.1186/1471-2202-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR. Hearing loss associated with the modifier of deaf waddler (mdfw) locus corresponds with age-related hearing loss in 12 inbred strains of mice. Hear Res. 2001;154:45–53. doi: 10.1016/s0378-5955(01)00215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]