Abstract
Forward masking experiments at 4 kHz have demonstrated that preceding sound can elicit changes in masking patterns consistent with a change in cochlear gain. However, the acoustic environment is filled with complex sounds, often dominated by lower frequencies, and ipsilateral cochlear gain reduction at frequencies below 4 kHz is largely unstudied in the forward masking literature. In this experiment, the magnitude of ipsilateral cochlear gain reduction was explored at 1, 2, and 4 kHz using forward masking techniques in an effort to evaluate a range of frequencies in listeners with normal hearing. Gain reduction estimates were not significantly different at 2 and 4 kHz using two forward masking measurements. Although the frequency was a significant factor in the analysis, post hoc testing supported the interpretation that gain reduction estimates measured without a masker were not significantly different at 1, 2, and 4 kHz. A second experiment provided evidence that forward masking in this paradigm at 1 kHz cannot be explained by excitation alone. This study provides evidence of ipsilateral cochlear gain reduction in humans at frequencies below the 4 kHz region.
I. INTRODUCTION
Cochlear gain improves auditory sensitivity and sharpens cochlear tuning (Ruggero et al., 1997). However, it may be advantageous for cochlear gain to adjust based on the acoustic environment. Cochlear gain reduction is hypothesized to play a role in auditory learning (Perrot et al., 1999), to protect the ear from noise-induced damage (Maison and Liberman, 2000; Marshall et al., 2014), and to improve auditory perception in noisy environments (Nieder and Nieder, 1970). A physiological mechanism that may facilitate a cochlear gain reduction is the medial olivocochlear reflex (MOCR), a bilateral auditory reflex between the brainstem and the cochlea. With stimulation, the MOCR decreases the gain provided by the outer hair cells in the cochlea (Murugasu and Russell, 1996; Cooper and Guinan, 2003), decreasing basilar membrane movement (Cooper and Guinan, 2006) with an onset and offset delay of approximately 25 ms (James et al., 2005; Backus and Guinan, 2006). This reflex is believed to be frequency-specific (Cooper and Guinan, 2006), but there is evidence that broadband stimuli are stronger elicitors (Lilaonitkul and Guinan, 2009a). Both the innervation and physiology of the MOCR have been studied in mammals. Gain reduction strength was higher at basal (higher) frequencies than at apical (lower) frequencies, with a sharp decrease in gain reduction strength at the highest frequencies (Liberman et al., 1990; Maison et al., 2003). In humans, the innervation pattern inferred by immunostaining appears similar to that of other mammals (Schrott-Fischer et al., 1994; Viana et al., 2015, Fig. 2), but the physiology is not fully known and the perceptual impact of cochlear gain reduction across the frequency is not yet clear.
Otoacoustic emissions (OAEs) have been used as an objective measure to investigate the cochlear gain reduction in humans (e.g., Collet et al., 1990; Backus and Guinan, 2006, 2007; Lilaonitkul and Guinan, 2009b). When sound (an elicitor) is presented to the ear, the magnitude of the OAE response is typically lower than when it is measured without an elicitor. This decrease in magnitude with an elicitor is believed to be related to the MOCR activity (Collet et al., 1990). Experimenters have found larger changes in stimulus-frequency otoacoustic emissions (SFOAEs) at 0.5 and 1 kHz than at 4 kHz for both ipsilateral and contralateral elicitors (Lilaonitkul and Guinan, 2009a, 2012). However, this may not truly reflect that the MOCR is stronger at low frequencies than high frequencies in humans. SFOAEs can be difficult to record at high frequencies at a satisfactory signal-to-noise ratio (SNR), resulting in fewer participants for the 4 kHz measurements (Lilaonitkul and Guinan, 2009a, 2012). It is possible that small changes in the magnitude of SFOAEs at 4 kHz are due to a measurement issue rather than a true low-frequency bias.
Psychoacoustics is another approach that has been used to investigate cochlear gain reduction in humans. Initial interest in this area was related to a phenomenon called overshoot (Zwicker, 1965), otherwise known as the temporal effect (Hicks and Bacon, 1992). The temporal effect is the improved SNR for tone detection when a tone is presented at a delay from the onset of a broadband noise masker.
The temporal effect is reduced with aspirin use (McFadden and Champlin, 1990) and noise exposure (Champlin and McFadden, 1989), two conditions that lead to an elevation in hearing thresholds, but improved detection of the signal at the beginning of the masker. Aspirin ingestion decreases frequency selectivity (Carlyon and Butt, 1993; Beveridge and Carlyon, 1996; Hicks and Bacon, 1999) and OAE fine structure (Long and Tubis, 1988), which is consistent with a decrease in cochlear gain. Bacon and Takahashi (1992) found a larger temporal effect at 4 kHz than at 1 kHz, and hearing impairment decreased the temporal effect significantly by improving signal thresholds at the onset of the noise. Strickland and Krishnan (2005) found a graded decrease in the temporal effect at frequencies in the 3–6 kHz range with increasing severity of cochlear hearing loss. Again, the individuals with hearing impairment had better thresholds in the onset condition than individuals with normal hearing. This was further evidence that the temporal effect is related to cochlear processing, and is consistent with less cochlear gain in the long-delay condition. A few studies have investigated overshoot psychophysically and with SFOAEs to evaluate the role of cochlear gain in the temporal effect (Keefe et al., 2009; Walsh et al., 2010). Although one did not find a physiological temporal effect (Keefe et al., 2009), the other did (Walsh et al., 2010), again suggesting that cochlear processing may play an important role in the temporal effect.
Further research has explored ways to control the auditory stimulus preceding the signal. Instead of manipulating the delay of the signal from the onset of the masker, additional noise (a precursor) was added before the signal (Strickland, 2001). Frequency selectivity was measured using the notched-noise technique, and it was found to decrease with a precursor. With this technique, the temporal effect was smaller at 1 kHz, and it was suggested that this was due to less active cochlear processing at that frequency (Strickland, 2001). However, this finding could also be due to an interaction between two gain reduction mechanisms. Cochlear suppression, nearly instantaneous cochlear gain reduction in response to simultaneous sound (Arthur et al., 1971), and sluggish cochlear gain reduction in response to preceding sound may interact in simultaneous masking experiments (Strickland 2004, 2008).
To remove the impact of suppression on signal threshold, experimenters have used forward masking to investigate cochlear gain reduction at 4 kHz (e.g., Roverud and Strickland, 2010; Jennings and Strickland, 2012). Further evidence has been found that preceding sound (on the MOCR time course) affects signal threshold in a way that is consistent with a decrease in frequency selectivity (Jennings et al., 2009; Jennings and Strickland, 2012) and inconsistent with temporal integration, given that increasing the duration of equally effective on- and off-frequency precursors differentially affects signal threshold (Roverud and Strickland, 2014). Cochlear gain reduction has been measured at 4 kHz using the growth of masking (GOM) technique. A decrease in the gain of estimated cochlear input-output functions has been measured with the addition of a precursor (Krull and Strickland, 2008; Jennings et al., 2009; Roverud and Strickland, 2010; Jennings and Strickland, 2012; Yasin et al., 2014).
Further psychoacoustic investigation of cochlear gain reduction has been completed using elicitors in the contralateral ear. Vinay and Moore (2008) estimated frequency selectivity as a function of signal frequency by measuring psychophysical tuning curves (PTCs) with simultaneous masking. Larger decreases in frequency selectivity were observed with contralateral elicitation at higher frequencies (2 and 4 kHz) compared to lower frequencies (0.5 and 1 kHz), as is seen in physiological studies. Wicher and Moore (2014) similarly measured PTCs with simultaneous masking. They found decreased frequency selectivity that was larger at a higher frequency (2 kHz) than a lower frequency (1 kHz). Kawase et al. (2000) measured PTCs with both simultaneous and forward masking at 2 kHz, finding evidence of decreased frequency selectivity with contralateral noise. Aguilar et al. (2013) measured PTCs at near-threshold and suprathreshold levels using forward masking. Larger decreases in frequency selectivity were observed at a lower frequency (0.5 kHz) than at a higher frequency (4 kHz). This effect was more pronounced for the suprathreshold measurements. Fletcher et al. (2016) examined changes in cochlear gain with contralateral noise by measuring temporal masking curves (TMCs). The TMCs were measured at 2 kHz and results were consistent with reduced cochlear gain with contralateral sound. Contralateral suppression of OAEs was measured in some of these studies and compared to psychoacoustic findings (Kawase et al., 2000; Wicher and Moore, 2014; Fletcher et al., 2016). Results support a relationship between cochlear gain and temporal effects in some studies (Kawase et al., 2000; Wicher and Moore, 2014), but another found no relationship between the psychoacoustic and physiological results (Fletcher et al., 2016). Overall, there is evidence of cochlear gain reduction from 0.5 to 4 kHz with contralateral stimulation.
Although low-frequency gain reduction has been measured in contralateral masking experiments as described, there are no known studies that investigate gain reduction below 4 kHz using ipsilateral forward masking. Considering that the ipsilateral MOCR may be stronger than the contralateral MOCR (Lilaonitkul and Guinan, 2012), this is a large gap in our knowledge and further investigation of ipsilateral cochlear gain reduction magnitude across frequency is needed.
In this study, we examined cochlear gain reduction using forward masking paradigms at 1, 2, and 4 kHz. Techniques previously used at 4 kHz were adapted to examine the magnitude of cochlear gain reduction across frequency. We hypothesized that estimates of cochlear gain reduction across these three frequencies would not be significantly different, given the innervation and physiology of the MOCR measured in mammals (Liberman et al., 1990; Maison et al., 2003). However, the current OAE evidence in humans suggests that ipsilateral cochlear gain reduction may be stronger at lower frequencies than at higher frequencies (Lilaonitkul and Guinan, 2009a, 2012) and the temporal effect in humans, thought to be related to cochlear gain reduction, is stronger at high frequencies (Bacon and Takahashi, 1992; Strickland, 2001). This experiment was intended to explore another method to estimate ipsilateral cochlear gain reduction in humans and to aid in reconciling the differences between current human estimates and known mammalian physiology.
II. EXPERIMENT 1: PSYCHOACOUSTIC ESTIMATES OF COCHLEAR GAIN REDUCTION AT 1, 2, AND 4 KHZ
A. Methods
A psychoacoustic approach was taken to measure cochlear gain reduction at 1, 2, and 4 kHz using a forward masking technique.
1. Participants
Six participants completed this experiment (three males and three females). The age of participants ranged from 19 to 22 yr with a median age of 19 yr. Audiometric thresholds were within the normal range from 0.25 to 8 kHz [15 dB hearing level (HL) or less]. Distortion product OAEs were measured with a Bio-logic system (Natus Medical Incorporated, Pleasanton, CA) and were present from 1.5 to 10 kHz [minimum criteria of −6 dB sound pressure level (SPL) distortion product, 6 dB SNR, 10 of 12 frequencies present, absent responses not consecutive in frequency]. Immittance measurements were completed with a Tympstar (Grason-Stadler Inc., Eden Prairie, MN) and tympanograms were normal (Type A), consistent with normal middle ear function. Ipsilateral and contralateral acoustic reflex thresholds for broadband noise were above 60 dB SPL for all participants. Acoustic reflex thresholds were of interest because activation of the middle ear muscle reflex attenuates the signal reaching the inner ear and could be confounded with cochlear gain reduction. This experimental protocol was approved by the Institutional Review Board at Purdue University and all participants provided informed consent.
2. Stimuli: GOM
GOM functions are a psychoacoustic measurement to estimate the input-output function of the cochlea at the signal frequency place (Oxenham and Plack, 1997; Plack and Oxenham, 1998). These functions were measured for participants in this experiment at 2 and 4 kHz to estimate the cochlear input-output function at full gain. These functions were used to find a masker level that elevates signal threshold by approximately 5 dB. A 5-dB shift in threshold was desired so that a masker level on the lower leg of the GOM function could be selected for the gain reduction estimate measure. This region of the cochlear input-output function was of interest because it is the region of the function that would be affected by a change in cochlear gain; gain reduction shifts this portion of the function to the right (Krull and Strickland, 2008; Roverud and Strickland, 2010).
To measure a GOM function at 2 kHz, a 20-ms, 1.2-kHz masker (including 5-ms cos2 ramps at onset and offset) was presented before an 8-ms signal (including 4-ms cos2 ramps at onset and offset) to measure signal threshold for masker levels of 30 and 60–85 dB SPL (in 5-dB steps). To measure a GOM function at 4 kHz, a 20-ms, 2.4-kHz masker (including 5-ms cos2 ramps at onset and offset) was presented before a 6-ms signal (including 3-ms cos2 ramps at onset and offset) or 8-ms signal (including 4-ms cos2 ramps at onset and offset) to measure signal threshold for masker levels of 30 and 60–85 dB SPL (in 5 dB steps).
The 8-ms signal durations in this experiment are longer than the 6-ms signals previously used for this method (e.g., Jennings et al., 2009; Roverud and Strickland, 2010). Longer signal durations were used in an effort to accurately test lower frequencies. A longer signal has narrower frequency spread. This is an important consideration when testing low frequencies, where cochlear filters are narrower. The 8-ms signals used in this experiment are approximately 170 Hz wide at the 3-dB down points. Increasing the duration further would reduce frequency spread further, improving the accuracy of the assumption that participants are listening through a single auditory filter to detect the signal. However, this would be at the cost of having a signal that is now too long to assume that it is not affected by cochlear gain reduction when measuring GOM functions without a precursor. The 8-ms duration was chosen considering this trade-off.
3. Stimuli: Gain reduction estimate
Gain reduction was estimated using forward masking techniques that rely on the timing of cochlear gain reduction by the MOCR. At 2 and 4 kHz, the masker level that shifted the signal threshold by approximately 5 dB (the level with a shift closest to 5 dB) was identified from the GOM data. The masker was too short to elicit the MOCR during the signal presentation (James et al., 2005; Backus and Guinan, 2006). Signal threshold in this reference condition was then compared to a condition where the MOCR should be elicited during the signal presentation due to the presence of preceding sound, a precursor. The difference in signal threshold with and without a precursor was interpreted as an estimate of cochlear gain reduction (Krull and Strickland, 2008; Roverud and Strickland, 2010), and will be referred to as the “masker present” gain reduction estimate. This interpretation is based on previous experimental outcomes showing that forward masking by a precursor is more consistent with gain reduction than with temporal integration. This has been shown by the magnitude (Jennings et al., 2009; Roverud and Strickland, 2014; Yasin et al., 2014), time course (Roverud and Strickland, 2010, 2014), and decrease in frequency selectivity (Jennings et al., 2009; Jennings and Strickland, 2012) with a precursor.
A 0.25–8-kHz, 50-ms, pink noise precursor (including 5-ms cos2 ramps at onset and offset) was presented before the masker and signal to elicit cochlear gain reduction. Pink noise has equal energy in each octave, with a frequency roll-off of −3 dB per octave. This precursor differs from the tonal precursors used in previous experiments (e.g., Roverud and Strickland, 2010). This stimulus was chosen in an effort to make a fair comparison of the magnitude of gain reduction across frequency, since it will stimulate auditory filters across the frequency spectrum with approximately equal energy. In addition, broadband stimuli have been shown to be more effective elicitors of cochlear gain reduction (Maison et al., 2000; Lilaonitkul and Guinan, 2009a). Pink noise precursors were presented at 60 dB SPL. This level was chosen so that the level would be high enough to elicit the MOCR (Backus and Guinan, 2006) but not high enough to elicit the middle ear muscle reflex, based on the measured acoustic reflex thresholds for broadband noise above 60 dB SPL for all participants. The 50-ms duration was also chosen to produce the maximum shift in signal threshold (Roverud and Strickland, 2014).
At 1, 2, and 4 kHz, an alternative “masker absent” measure of cochlear gain reduction was made. The primary masking contribution of the masker in the masker present measurement was assumed to be excitation rather than gain reduction, given its proximity in time to the signal. Therefore, it was hypothesized that the gain reduction estimate would be the same with or without the masker present. Quiet threshold for the signal was compared to signal threshold with a precursor and 20-ms gap (masker removed). This was the only gain reduction measure completed at 1 kHz because an off-frequency masker cannot be assumed to be processed linearly at the signal place, as at 2 and 4 kHz (Cooper and Rhode, 1997). If the masker is not linear at the signal place, it could be affected by gain reduction and provide differential amounts of masking with and without a precursor present, making the magnitude of gain reduction difficult to elucidate.
4. Procedure
Testing was completed in a double-walled sound-attenuating booth. Stimuli were generated in custom matlab (2012a, MathWorks, Natick, MA) software (Bidelman et al., 2015) with a Lynx II XLR sound card (Lynx Studio Technology, Inc., Costa Mesa, CA). The stimuli then passed through a headphone buffer (TDT HB6, Tucker-Davis Technologies, Alachua, FL) and were presented to the participant via insert earphones (ER-2, Etymotic Research, Inc., Elk Grove Village, IL). The insert earphones had a flat frequency response from 0.25 to 8 kHz at the eardrum. All stimuli in this experiment were presented monaurally to the right ear in an effort to control for possible ear differences in the strength of the MOCR. High pass noise was included to limit off-frequency listening (Nelson et al., 2001) for all trials excluding quiet threshold measurements. The high pass noise began 50 ms before the first stimulus and ended 50 ms after the signal. The high pass noise frequency extended from 1.2 times the signal frequency to 10 kHz, and the spectrum level was 50 dB below the level of the signal.
Participants completed a three-interval forced choice task where only one interval included the signal. A graphical user interface indicated visually which interval was currently playing, and there were 500 ms of silence between intervals. Participants were instructed to identify the interval containing the signal by clicking a button or pressing a key. Visual feedback was given to indicate if a response was correct or incorrect. Signal level was adjusted using a two-down, one-up tracking rule to estimate a threshold of 70.7% correct on the psychometric function (Levitt, 1971).
Three one-hour sessions of training were completed at the beginning of the experiment and were not included in the final experimental data. One participant (P6) required an additional session of training to perform consistently on the forward masking task. Participants were welcome to take breaks as needed during each session.
Data collection was then conducted for the four frequency and signal duration conditions. All participants began data collection with the 6-ms, 4-kHz signal. Data collection continued with the 8-ms signals at 1, 2, and 4 kHz in a counterbalanced order. A one-hour session of training was completed for each 8-ms signal condition, and this training was again not included in the final experimental data.
For each of the the 2- and 4-kHz signals, data collection consisted of four one-hour test sessions. In the first three sessions, two runs were collected to measure quiet threshold of the signal and each fixed masker level in the GOM function. Runs with a standard deviation above 5 dB were discarded and replaced with an additional run, so that there were six total runs (to be averaged) collected in the three sessions. An additional session was completed to collect data with the precursor. Three runs of training were discarded, followed by six runs of data collection to measure signal threshold with a precursor and with a precursor and masker.
For the 1-kHz signals, data collection consisted of one one-hour test session. Six runs of signal threshold alone and six runs of signal threshold in the masker absent condition were completed.
Any levels that were reported as too loud by the participants or that led to repeated signal levels presented at the limits of the equipment were discontinued. This occurred at 85 dB SPL masker levels (the highest level used to measure GOM functions) for P1 at 2 kHz and P6 at 2 and 4 kHz.
B. Results
1. GOM
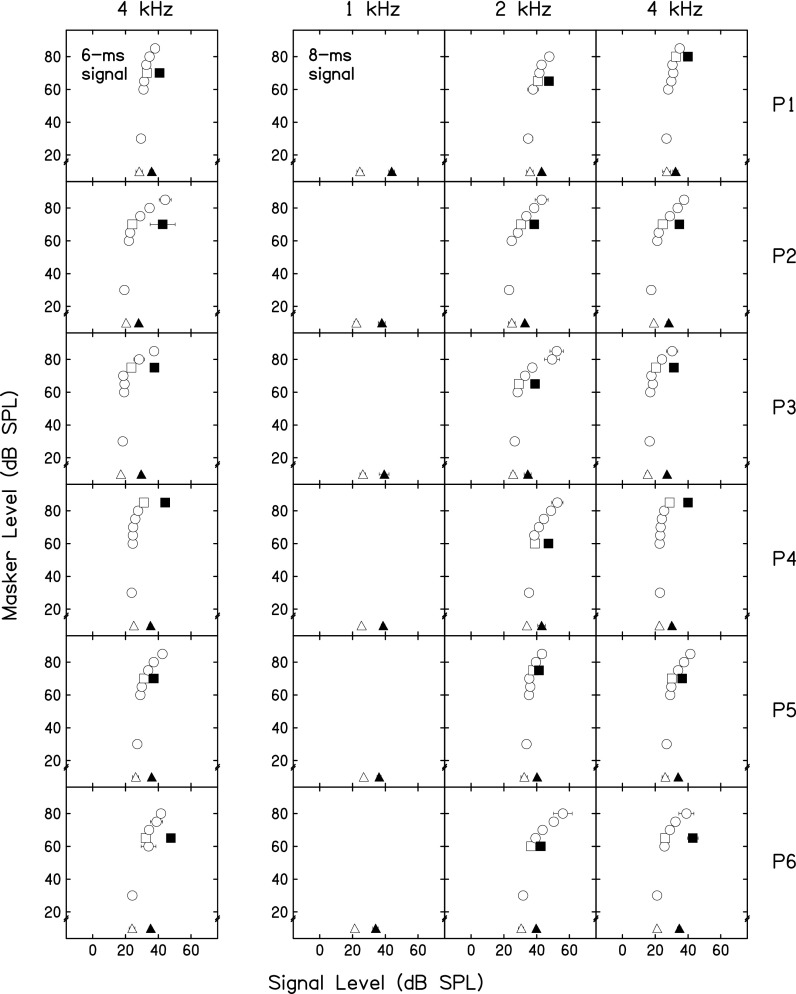
GOM functions were measured at 4 kHz with the 6-ms signal duration that has been used in previous studies (Jennings et al., 2009; Roverud and Strickland, 2010, 2014). Individual functions are shown as the open circles and open squares in the first column of Fig. 1. Functions measured in this study are qualitatively similar to those measured previously, with variability across listeners (Roverud and Strickland, 2010).
FIG. 1.
GOM and gain reduction estimates across frequency: Individual data. Open circles and squares depict GOM of a 2- or 4-kHz tone when masked by a 1.2- or 2.4-kHz tone, respectively. Error bars represent one standard deviation. Masker duration was 20 ms. Quiet threshold (signal threshold without a masker) is represented by the open triangles. Threshold for the same tone preceded by a 50-ms pink noise precursor and a 20-ms silent gap is represented by the filled triangles. Threshold for the tone preceded by both a 50-ms pink noise precursor and a masker is represented by the filled squares. The difference in signal thresholds between the square symbols is the masker present gain reduction estimate. The difference in signal thresholds between the triangle symbols is the masker absent gain reduction estimate.
GOM functions were also measured with 8-ms signals. These functions at 2 and 4 kHz are shown as open circles and open squares in the final two columns of Fig. 1.
At 4 kHz, the two signal durations yielded similar GOM functions. However, the 6-ms signal GOM functions are sometimes able to capture more of the compressive part of the input-output function, especially for P2 and P3. The GOM functions in Fig. 1 are first dominated by internal noise at low masker levels (e.g., Roverud and Strickland, 2010), and the linear lower leg of the input-output function is captured at higher masker levels. At both durations, longer or more intense maskers would be required to capture enough of the compressive region of the function to define its slope (Oxenham and Plack, 1997). The goal of this study was to examine gain reduction using a technique that does not require an estimate of compression. Because of this, no attempt was made to ensure that compression estimates would be acquired. Another psychophysical technique has revealed that compression may also be reduced with cochlear gain reduction (Yasin et al., 2014), so compression can be informative, but was not necessary to obtain a gain reduction estimate with this approach.
GOM across frequency can be examined in the final two columns of Fig. 1. Maskers are generally more effective at 2 kHz, leading to higher signal thresholds overall. However, compression is still not captured in the 2 kHz functions. This may be because there is more compression at higher frequencies (Cooper and Rhode, 1997).
This experiment demonstrates that GOM functions can be measured at 2 and 4 kHz with a longer signal duration than used previously in this laboratory (Jennings et al., 2009; Roverud and Strickland, 2010, 2014). This comes at the possible cost of an inability to estimate compression for these participants, but compression estimates can also be difficult to acquire with shorter signal durations.
2. Gain reduction estimates
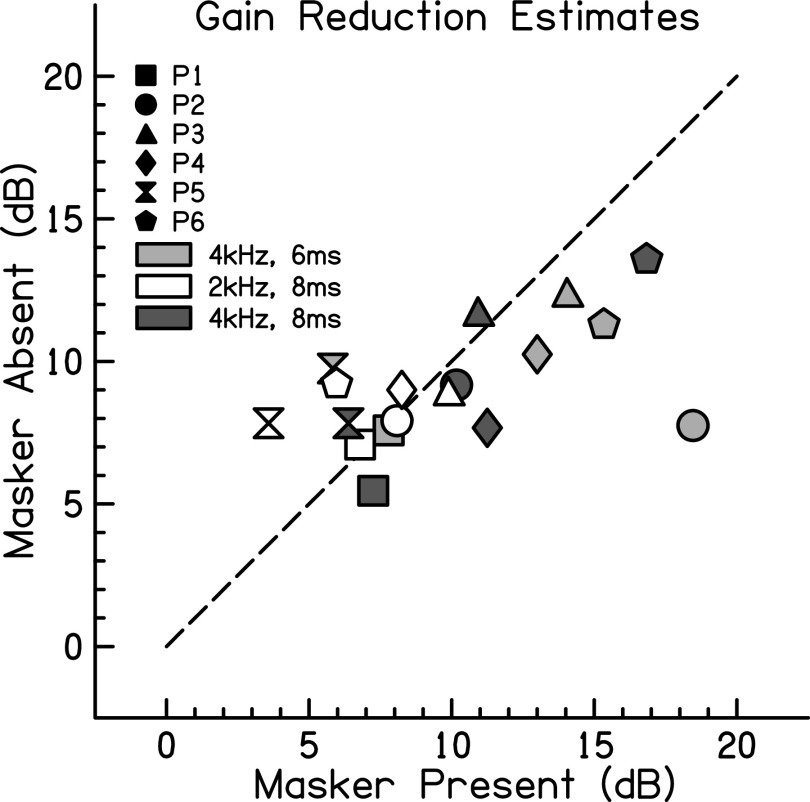
Two methods were used to examine cochlear gain reduction in this experiment, a masker present approach at 2 and 4 kHz and a masker absent approach at 1, 2, and 4 kHz. The masker present gain reduction estimate is shown for each individual in Fig. 1 as the difference between the open and filled squares. The masker absent gain reduction estimate is shown for each individual in Fig. 1 as the difference between the open and filled triangles. These results are further compared in Fig. 2, where each symbol represents data from an individual participant and each shade represents gain reduction estimates at a given signal frequency and duration. Symbols that fall close to the dotted line are cases where the two conditions give similar estimates of gain reduction. The further from the line, the more divergence there is between the two estimates. The difference in threshold across methods is always under 5 dB, with the exception of P2 in the 4-kHz, 6-ms signal condition. The signal threshold was notably variable for P2 in this condition when the precursor and masker were present (see the filled square symbol in the first column of Fig. 1). The divergence in estimates for this participant in this condition may also occur due to a practice effect, since this condition was always completed first, but other participants do not show this type of pattern. Group mean gain reduction estimates and standard deviations are shown in Table I.
FIG. 2.
Individual comparison of two gain reduction estimates. Symbols near the dotted line represent conditions where the gain reduction estimates with and without a masker present were similar for individual participants. Each participant is represented by a different symbol. The shading of symbols denotes the frequency and duration of the signal as described in the key.
TABLE I.
Group average gain reduction estimates (in dB). The standard deviation at each frequency and condition is shown in parentheses.
| Frequency | Masker present | Masker absent |
|---|---|---|
| 4-kHz, 6-ms signal | 12.41 (4.75) | 9.84 (1.92) |
| 1-kHz, 8-ms signal | 13.90 (3.34) | |
| 2-kHz, 8-ms signal | 7.09 (2.18) | 8.33 (0.85) |
| 4-kHz, 8-ms signal | 10.47 (3.70) | 9.24 (2.96) |
To test if changing the duration of the signal from 6 to 8 ms changed the gain reduction estimate, a two-way repeated measures analysis of variance (ANOVA) was completed with gain reduction estimate as the dependent variable and factors of duration (6- or 8-ms signal) and condition (masker present or absent) at 4 kHz. There were no significant effects of duration, F(1,5) = 2.98, p = 0.145, or condition, F(1,5) = 2.43, p = 0.180, on the gain reduction estimate. The interaction between frequency and condition also was not significant, F(1,5) = 0.55, p = 0.493. It was concluded that the change from a signal duration of 6 to 8 ms did not change the gain reduction estimates measured at 4 kHz, supporting the assumption that an 8-ms signal duration could be used to estimate cochlear gain reduction at other frequencies.
A two-way repeated measures ANOVA was completed with gain reduction estimate as the dependent variable and factors of frequency (2 and 4 kHz) and condition (masker present or absent) for the 8-ms signal duration data. There were no significant effects of frequency, F(1,5) = 3.45, p = 0.122, or condition, F(1,5) = 0.00, p = 0.987, on the gain reduction estimate. The interaction between frequency and condition also was not significant, F(1,5) = 4.52, p = 0.087.
A one-way repeated measures ANOVA was also completed to test for significant differences between the three frequencies of the masker absent condition. The mean gain reduction estimates differed in a statistically significant manner across frequency, F(2,10) = 6.59, p = 0.015. However, post hoc tests using a Bonferroni correction revealed that the gain reduction estimate at 1 kHz was borderline significantly different from the gain reduction estimate at 2 kHz (p = 0.049) and not significantly different from the gain reduction estimate at 4 kHz (p = 0.255). The gain reduction estimates at 2 and 4 kHz were not significantly different from each other (p = 1.000).
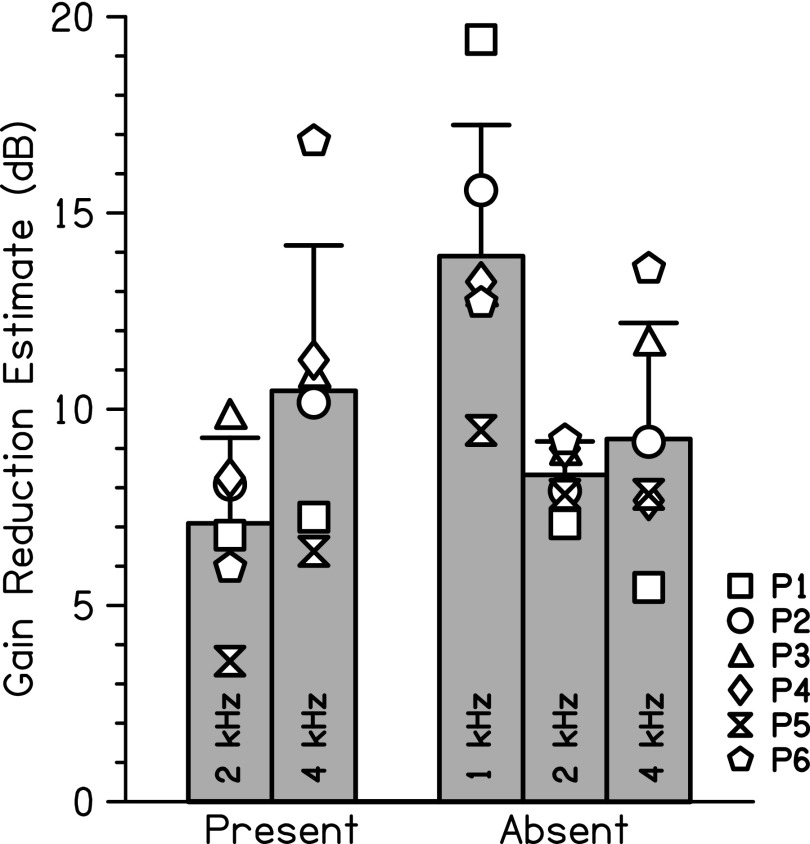
The overall interpretation of the two-way and one-way ANOVA results is that there are no significant differences across frequency and condition, given the borderline nature of the significant difference between the 1- and 2-kHz data in the masker absent condition, the lack of statistical difference between the 1- and 4-kHz data in the masker absent condition, and the lack of statistical difference between the 2- and 4-kHz data. The average gain reduction estimates for each condition and frequency are shown by the bars in Fig. 3, with individual gain reduction estimates plotted as open symbols.
FIG. 3.
Group comparison of two gain reduction estimates measured with 8-ms signals. Each bar represents averaged data for each condition (masker present or absent) and signal frequency (1, 2, or 4 kHz). Standard deviations are displayed by error bars.
III. EXPERIMENT 2: EXPLORATION OF THE FORWARD MASKING MECHANISM BY A PRECURSOR AT 1 KHZ IN THIS TEMPORAL PARADIGM
A control experiment was completed to examine whether the forward masking at 1 kHz can be attributed to excitation, rather than gain reduction, given the longer duration of ringing in a lower-frequency auditory filter. This experiment was motivated by previous experiments that have shown gain reduction effects on signal threshold for off-frequency maskers and not on-frequency maskers (e.g., Kawase et al., 2000). Because an off-frequency masker is processed linearly at the signal place, it is not affected by cochlear gain reduction by an elicitor and may be a more effective masker than a comparable on-frequency masker (Kawase et al., 2000, Fig. 6).
A. Methods
1. Participants
Three participants completed this experiment (two males and one female). Ages ranged from 21 to 30 yr, with a median age of 21 yr. Audiometric thresholds were within the normal range as required in experiment 1. P7 and P9 had present distortion product OAEs from 1.5 to 10 kHz. P8 had present transient evoked OAEs measured using a SmartTrOAE system (Intelligent Hearing Systems, Miami, FL). Tympanograms were normal for all participants. Broadband noise acoustic reflex thresholds for two of the participants (P7 and P8) were above 60 dB SPL, and the third participant (P9) had broadband noise acoustic reflex thresholds above 55 dB SPL. The same equipment was used for these audiometric tests as in experiment 1 unless otherwise specified. This protocol was also approved by the Purdue University Institutional Review Board, and the participants gave informed consent.
2. Stimuli
Forward masking was measured in this experiment with a pink noise precursor and 1-kHz signal. In experiment 1, an off-frequency masker was omitted to estimate cochlear gain reduction at 1 kHz because it cannot be assumed to be linear at that frequency (Cooper and Rhode, 1997). It was assumed that since the shift in threshold at 2 and 4 kHz was not significantly different with and without a masker, this approach could be used at 1 kHz. However, the duration of the ringing in the auditory filter increases as frequency decreases, likely changing the time course of excitatory masking. This makes it possible that the shift in signal threshold with a precursor is driven by excitation, rather than gain reduction, in this masking paradigm. This experiment was designed to test that hypothesis. Although it cannot be assumed that an off-frequency masker for a 1-kHz signal is processed linearly at the signal place, it may be processed more linearly (provided less gain) than an on-frequency masker. There is a known transition from the base to the apex where the gain applied to off-frequency sounds increases in animal models (Cooper and Rhode, 1997, Fig. 2). If there is a difference in the gain of off- and on-frequency maskers at the 1-kHz place, gain reduction masking would lead to the prediction that a precursor would cause a differential reduction in gain for on- and off-frequency maskers matched in effectiveness (on- and off-frequency maskers that produce the same signal threshold). If the precursor does not change cochlear gain, the shift in signal threshold should be the same, regardless of the masker frequency. In that case, the forward masking of the precursor would be driven by excitatory masking.
Stimuli consisted of a precursor, on-frequency masker, off-frequency masker, and signal. The precursor was a 0.25–10-kHz, 50-ms, 60-dB SPL pink noise with 5-ms cos2 ramping (similar to that used in experiment 1, but with a wider bandwidth). The on-frequency masker was a 1-kHz, 20-ms tone with 5-ms cos2 ramping. The off-frequency masker was a 0.6-kHz, 20-ms tone with 5-ms cos2 ramping. The signal was a 1-kHz, 8-ms tone with 4-ms cos2 ramping (as used in experiment 1).
3. Procedure
This experiment was completed with the same equipment and user interface described in experiment 1, again with stimuli presented to the right ear. Maskers were always presented immediately before the signal in this forward masking paradigm, and there was always a 20-ms gap in time between the precursor and signal. Two sets of data collection were completed in 1–1.5 hour sessions. The first set of conditions were completed to familiarize the participant with the stimuli and to determine the appropriate masker levels needed. Data from this set were excluded from the results.
In the first set of conditions, four runs were completed to measure quiet threshold for the signal. These thresholds were averaged and the signal was then fixed at 5 dB above threshold [dB sensation level (SL)] for the next measurement. To find the on- and off-frequency masker levels that raise signal threshold by 5 dB, the signal level was fixed at 5 dB SL and the masker level was adaptively varied. Two runs were completed at each masker frequency. Based on these thresholds a masker level was chosen. To verify that it raised signal threshold by 5 dB, the masker was then fixed and signal threshold was varied for two runs. Finally, to familiarize the participants with the precursor, signal threshold was measured with a precursor and masker present. Two runs were completed for each masker frequency.
Next, experimental data collection began. Quiet threshold was measured for the signal alone and the signal in the presence of the precursor (with a 20-ms gap between the precursor and signal) to estimate cochlear gain reduction without a masker, as in experiment 1. Signal threshold was then measured with an off-frequency masker, and subsequently, with an on-frequency masker. The masker levels were chosen in the first session to provide approximately equal masking of the signal. Finally, a precursor was added before each masker in time to examine if the precursor produces a differential shift in threshold with two equally effective maskers. Four runs were completed for each condition in this set.
B. Results
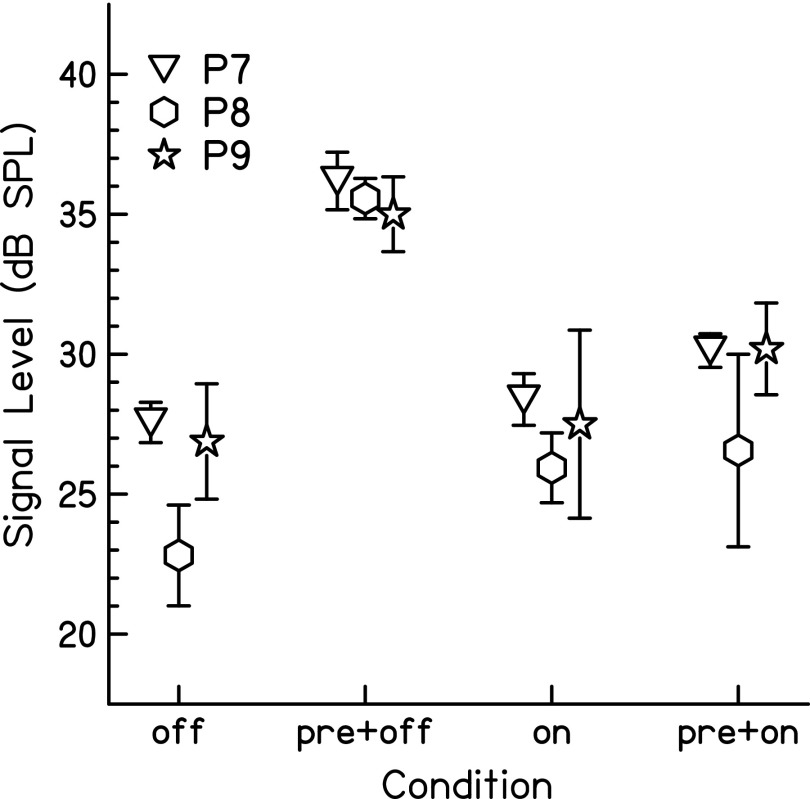
The individual results of this experiment are shown in Fig. 4. The on- and off-frequency maskers were chosen to match signal threshold at 5 dB SL for each individual. For all three participants, there is a larger shift in threshold with the addition of the precursor to the off-frequency masker condition than the on-frequency masker condition.
FIG. 4.
Individual signal threshold averages and standard deviations for the conditions with maskers measured in experiment 2. Masker conditions included a 0.6-kHz, off-frequency masker (off), a 1-kHz, on-frequency masker (on), and the same maskers with a pink noise precursor preceding the masker in time (pre+off, pre+on). Signal threshold was significantly higher in the precursor condition with an off-frequency masker than in the precursor condition with an on-frequency masker.
One-tailed, paired t-tests with a Holm-Bonferroni correction were measured to test if the signal threshold in the off-frequency precursor condition was significantly higher than the on-frequency precursor condition for each individual. Signal thresholds of P7 in the off-frequency precursor condition [mean (M) = 36.19, standard deviation (SD) = 1.03] were significantly higher than signal thresholds in the on-frequency precursor condition (M = 30.13, SD = 0.60), t(3) = 18.44, p < 0.001. Signal thresholds of P8 in the off-frequency precursor condition (M = 35.56, SD = 0.72) were significantly higher than signal thresholds in the on-frequency precursor condition (M = 26.56, SD = 3.44), t(3) = 4.71, p = 0.018. Signal thresholds of P9 in the off-frequency precursor condition (M = 35.00, SD = 1.34) were significantly higher than signal thresholds in the on-frequency precursor condition (M = 30.19, SD = 1.64), t(3) = 3.49, p = 0.020.
A one-way repeated measures ANOVA was completed with change in average signal threshold with the addition of a precursor as the dependent variable and masker frequency (0.6 and 1 kHz) as factors. The shift in threshold when a precursor was added to the 0.6 kHz masker condition was not significantly higher than the shift in threshold when a precursor was added to the 1 kHz masker condition, F(1,2) = 16.11, p = 0.057, but showed a clear trend in that direction with a small sample size. These group data can be visualized by the bars in Fig. 5, with individual data plotted as open symbols. The greater shift in threshold with the off-frequency masker is consistent with forward masking due to cochlear gain reduction. If the shift in threshold was driven by excitatory masking, a similar shift in threshold would be observed between the two masker frequencies when the same precursor was added, since the maskers were chosen to mask the signal equally.
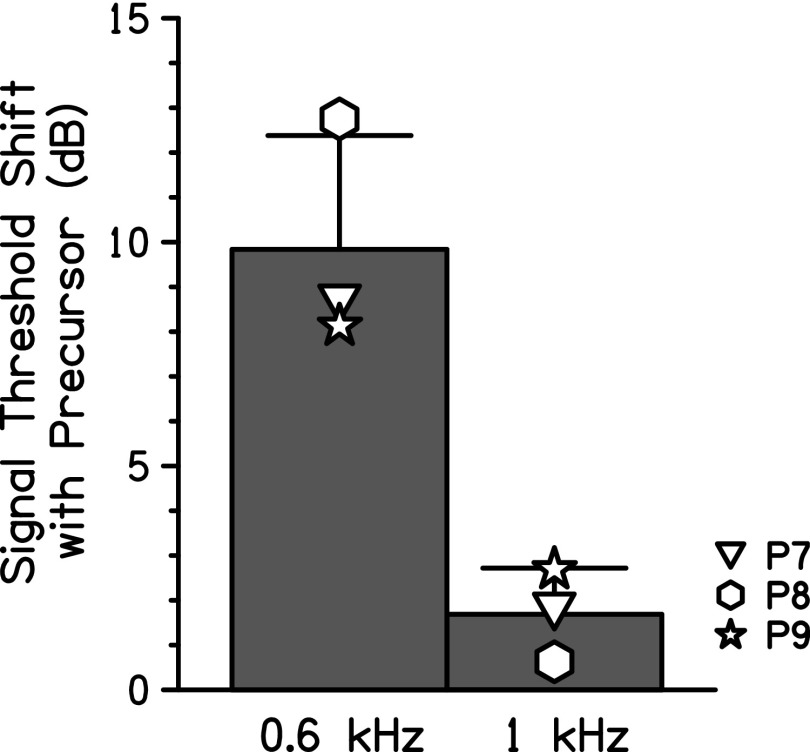
FIG. 5.
Group average difference between the 1-kHz signal thresholds measured when the signal was preceded in time by a masker alone and when the signal was preceded by a precursor and masker. The bar labeled “0.6 kHz” shows the average shift in threshold when a precursor is added to the off-frequency (0.6 kHz) masker condition. The bar labeled “1 kHz” shows the average shift in threshold when a precursor is added to the on-frequency (1 kHz) masker condition. The error bars represent one standard deviation.
Gain reduction estimates were measured for the participants in this study by omitting the masker. The gain reduction estimates for P7 (6.69 dB), P8 (6.06 dB), and P9 (7.38 dB) were compared to the shift in threshold with an off-frequency masker (the “0.6-kHz” bar in Fig. 5). This one-way repeated measures ANOVA had threshold shift with a precursor as the dependent variable and factors of condition (off-frequency masker present or absent). Signal thresholds with and without an off-frequency masker were not significantly different, F(1,2) = 2.97, p = 0.227.
IV. DISCUSSION
Cochlear gain reduction was estimated with two psychoacoustic forward masking techniques. The first technique examined the change in a masked signal threshold with the addition of a precursor. The second omitted the masker, assuming that its only contribution was excitatory masking and that its omission would give a similar gain reduction estimate. No significant differences were found between the gain reduction estimates at 2 and 4 kHz, and the two techniques did not result in significantly different gain reduction estimates overall. The masker absent condition was used to explore gain reduction at 1 kHz, and post hoc testing supported the interpretation that it was not significantly different from the estimates at 2 and 4 kHz. As frequencies become more apical, filters become narrower (e.g., Glasberg and Moore, 1990) and this leads to a longer duration of ringing on the basilar membrane, which could lengthen the time course of excitatory masking. Because of this, another experiment was performed to explore the source of the masking of the 1-kHz signal by the precursor. On- and off-frequency maskers were matched in effectiveness, meaning that levels were chosen to raise the signal threshold approximately equally. When a precursor is added to the paradigm and presented before the masker, there is a greater increase in signal threshold with an off-frequency masker than an on-frequency masker. This pattern is consistent with the precursor providing masking driven by cochlear gain reduction and inconsistent with masking driven by excitation.
A. Comparison to physiological studies
The gain reduction estimated in this study can be compared to estimates made in other experiments. Individual cochlear gain reduction estimates in this study ranged from 6 to 19 dB at 1 kHz, 4 to 10 dB at 2 kHz, and 5 to 17 dB at 4 kHz for a moderate level (60 dB SPL), ipsilateral elicitor.
Medial olivocochlear innervation in other mammals is strongest at frequencies on the high end of their audible range (Liberman et al., 1990; Maison et al., 2003). Based on the aforementioned physiological measurements of both innervation density and neural threshold shift with MOCR stimulation, it was expected that cochlear gain reduction estimates would be similar across the three frequencies tested in this experiment. Frequency was a significant factor in the analysis, but post hoc testing did not result in a clear distinction of how gain reduction compares across the three frequencies tested. In the OAE literature, suppression of SFOAEs has been found to be strong at 1 kHz and much weaker at 4 kHz (Lilaonitkul and Guinan, 2012). The results of this study suggest that cochlear gain reduction in humans at 4 kHz is not weak, and SFOAEs may be limited in their ability to capture changes in SFOAE magnitude at higher frequencies.
These results can also be compared to physiological estimates made in the basal turn of the cochlea of animal models with electrical stimulation of the MOCR at the fourth ventricle. Gain reduction estimates measured in the present study are consistent with those made by Russell and Murugasu (1997) and Dolan et al. (1997).
B. Comparison to psychoacoustic studies
1. Forward masking
Other forward masking experiments show similar gain reduction estimates for ipsilateral stimuli at 4 kHz. Krull and Strickland (2008) measured 10–20 dB of gain reduction across participants with a 60-dB SPL, 40-ms, 4-kHz tonal precursor presented before a 40-ms off-frequency masker and 6-ms signal. They calculated the gain change by fitting the GOM functions measured with and without a precursor, but a similar change in gain could be obtained by simply examining the shift in the lower leg of the function at one masker level. Jennings et al. (2009) found 8–27 dB of gain reduction across participants using a 50- or 60-dB SPL, 160-ms, 4-kHz tonal precursor presented before a 20-ms off-frequency masker and 6-ms signal, using a similar model fitting to that of Krull and Strickland (2008). Again, similar changes in gain could be estimated from the data by examining the shift in threshold with the addition of a precursor at a masker level on the lower leg of the GOM function. Roverud and Strickland (2010) measured 9–20 dB of gain reduction across participants, using a “two-point gain change” technique that was the inspiration for the technique used in the present study. The precursor in this experiment was a 100-ms, 40-dB SPL, 4-kHz pure tone, followed by a 20-ms off-frequency masker and 6-ms signal.
Yasin et al. (2014) used the fixed-duration masking curve method to estimate cochlear gain reduction. They measured signal threshold for a 25-ms masker-signal complex with a 500-ms narrowband noise precursor centered on 4 kHz. The precursor was presented before the masker at delays of 0-, 50-, 100-, and 200-ms. The masker was either on- or off-frequency, and a comparison was made between on- and off-frequency masker data to estimate cochlear gain. For a 60-dB SPL precursor, maximum gain reduction was calculated as 21 dB on average (Yasin et al., 2014, Fig. 6) for the participants.
2. Simultaneous masking
In simultaneous masking experiments with sound presented ipsilaterally, the temporal effect can be interpreted as a reflection of a reduction in active cochlear processing (Strickland, 2001), and thus can give an estimate of gain reduction magnitude. The temporal effect must be measured at a range of signal levels to estimate the change in the cochlear input-output function in the two temporal conditions. Strickland (2001) found little effect at 1 kHz and approximately 15–20 dB of gain reduction on average at 4 kHz (Strickland, 2001, Figs. 10 and 11). Strickland (2004) measured a temporal effect at 1 kHz in one participant with other participants showing no temporal effect at this frequency. At 4 kHz, gain reduction was estimated as approximately 20 dB when the temporal effect data were fitted with input-output functions (Strickland, 2004, Fig. 5).
Overall, simultaneous masking experiments show a robust temporal effect at 4 kHz and a smaller or absent temporal effect at 1 kHz. This is in contrast to the results of this experiment. It is possible that suppression, the nearly instantaneous reduction in cochlear gain when two sounds are presented simultaneously, played a role in reducing the temporal effect seen at 1 kHz, although there is some evidence that adaptation of suppression does not occur in overshoot (Fletcher et al., 2015).
C. Ipsilateral and contralateral gain reduction
The results of this study provide evidence that frequencies below 4 kHz are subject to ipsilateral gain reduction, despite the fact that the temporal effect is small at low frequencies. Exploration of contralateral cochlear gain reduction has revealed behavioral evidence of cochlear gain reduction at 0.5, 2, and 4 kHz. Since the addition of preceding sound to the contralateral ear has no physical interaction with sound in the ipsilateral ear, behavioral measures of contralateral gain reduction have been more easily interpreted as such. Cochlear gain reduction elicited in the contralateral ear has been primarily explored by examining changes in cochlear tuning with contralateral sound.
Vinay and Moore (2008) measured PTCs with and without contralateral sound. They found that contralateral sound decreased the sharpness of tuning at 2 and 4 kHz and increased the sharpness of tuning at 0.5 and 1 kHz. This difference was attributed to the difference in active cochlear processing in the base and apex of the cochlea. Wicher and Moore (2014) also examined PTCs at 1 and 2 kHz. They used narrowband and broadband contralateral sound to elicit gain reduction and found that broadband sounds broadened the tuning at 2 kHz. No effects on the tuning curves could be measured at 1 kHz or with a narrowband sound at 2 kHz. Aguilar et al. (2013) used a forward masking approach to examine changes in tuning with contralateral broadband noise at 0.5 and 4 kHz. They found broadened tuning at 0.5 kHz and no change in tuning at 4 kHz with contralateral stimulation. Kawase et al. (2000) used both forward and simultaneous masking to measure PTCs at 2 kHz with and without contralateral broadband noise. They found larger effects of broadened tuning with the forward masking paradigm, when the signal was delayed from the offset of the masker.
This body of research shows that broadened tuning occurs at 0.5, 2, and 4 kHz with contralateral sound, consistent with cochlear gain reduction. These studies found little to no shift in on-frequency masker threshold with contralateral sound, and thus the magnitude of contralateral gain reduction can be estimated in these studies by examining the shift in the off-frequency masked threshold with an elicitor. Gain reduction was approximately 5 dB at 0.5 kHz (Vinay and Moore, 2008; Aguilar et al., 2013), at 2 kHz (Kawase et al., 2000; Vinay and Moore, 2008; Wicher and Moore, 2014), and at 4 kHz (Vinay and Moore, 2008; Aguilar et al., 2013). Overall gain reduction estimates were higher when measured ipsilaterally in the present study; approximately 15 dB of gain reduction was observed at 1 kHz and approximately 10 dB of gain reduction was observed at 2 and 4 kHz.
The relationship between ipsilateral and contralateral gain reduction magnitude is still debated, but anatomical data in cat suggest that the ipsilateral reflex may be twice as strong as the contralateral reflex (Warr, 1992). In addition, research using OAEs has found that narrowband elicitation of gain reduction is stronger in the ipsilateral reflex, but wideband elicitation is similar for the ipsilateral and contralateral reflexes (Lilaonitkul and Guinan, 2009a). A recent study investigated contralateral gain reduction more directly, finding approximately 1 dB effects at 2 kHz (Fletcher et al., 2016). Another investigation, using compound action potentials in humans, found approximately 2 dB of attenuation with contralateral broadband noise (Lichtenhan et al., 2016). Further research is needed to examine how the ipsilateral and contralateral MOCR function as a system.
D. Two forward masking techniques proposed to measure gain reduction
In this study, two forward masking techniques were used to estimate cochlear gain reduction. The masker present technique was based on previous work at 4 kHz (Krull and Strickland, 2008; Jennings et al., 2009; Roverud and Strickland, 2010) and extended to examine cochlear gain reduction at 2 kHz. The masker absent technique was based on the observation that a similar shift in threshold occurs when the masker is omitted at 4 kHz (Roverud and Strickland, 2010).
It may not seem surprising that similar estimates of gain reduction are measured with and without a masker present. However, there is some evidence that gain reduction may reduce the spontaneous rate of auditory nerve fibers (Guinan and Gifford, 1988). This could cause a smaller change in signal threshold without a masker, since both the response to the signal and the background activity would be reduced by cochlear gain reduction. Fletcher et al. (2016) measured cochlear gain reduction using the TMC method, and concluded that their contralateral elicitor did not have a large effect on quiet threshold but did affect masked thresholds. In this experiment, it is shown that for this paradigm that is not the case; a similar shift in signal threshold is seen with a masker present or absent.
An attempt was made to examine an even lower frequency, 1 kHz. The masker present condition was not measured at this frequency initially because it is too low in frequency to safely assume that an off-frequency masker is processed linearly at the signal place (Cooper and Rhode, 1997). This is important because if the masker is not processed linearly, the gain reduction provided by the precursor could affect the gain of the masker at the signal frequency place, changing the amount of excitatory masking it provides to the signal.
In the second experiment, higher signal thresholds were measured when the precursor was presented before an off-frequency masker than when the precursor was presented before an on-frequency masker that produced approximately the same signal threshold without a precursor. This suggests that the off-frequency masker at this signal place is provided less gain than an on-frequency masker. If the gain applied to a 0.6 kHz sound at the 1 kHz place was the same as an on-frequency masker, a similar shift in threshold would be seen when the precursor was added to each condition.
It is possible that the assumptions made about the time course of cochlear gain reduction and excitatory masking change with frequency. Future experiments could explore this possibility by examining the change in signal threshold as on-frequency precursors are increased in duration, as done in Roverud and Strickland (2014) at 4 kHz. If assumptions are not violated, this masker absent technique could be used to explore gain reduction at even lower frequencies, since it does not have the constraint of requiring a linear off-frequency masker. In addition, the results of the second experiment suggest that an off-frequency masker at 1 kHz is noticeably more linear than an on-frequency masker in humans.
V. CONCLUSIONS
-
(1)
Psychoacoustic methods previously used to measure cochlear gain reduction at 4 kHz can be applied to lower frequencies, as long as assumptions are not violated with a change in frequency.
-
(2)
The masker present and masker absent measurements were not significantly different at 1, 2, and 4 kHz.
-
(3)
Ipsilateral gain reduction estimates were similar at 1, 2, and 4 kHz when measured in a forward masking paradigm. The mechanism of forward masking in this temporal paradigm and with this population is more consistent with cochlear gain reduction by the precursor than additivity of masking between the precursor and masker.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (National Institute on Deafness and Other Communication Disorders, NIDCD) Grant Nos. T32-DC000030, T32-DC000046, and R01-DC008327. This research was also supported by the Purdue Research Foundation.
References
- 1. Aguilar, E. , Eustaquio-Martin, A. , and Lopez-Poveda, E. A. (2013). “Contralateral efferent reflex effects on threshold and suprathreshold psychoacoustical tuning curves at low and high frequencies,” J. Assoc. Res. Otolaryngol. 14, 341–357. 10.1007/s10162-013-0373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arthur, R. M. , Pfeiffer, R. R. , and Suga, N. (1971). “Properties of ‘two-tone inhibition’ in primary auditory neurones,” J. Physiol. 212, 593–609. 10.1113/jphysiol.1971.sp009344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Backus, B. C. , and Guinan, J. J. (2006). “Time-course of the human medial olivocochlear reflex,” J. Acoust. Soc. Am. 119, 2889–2904. 10.1121/1.2169918 [DOI] [PubMed] [Google Scholar]
- 4. Backus, B. C. , and Guinan, J. J. (2007). “Measurement of the distribution of medial olivocochlear acoustic reflex strengths across normal-hearing individuals via otoacoustic emissions,” J. Assoc. Res. Otolaryngol. 8, 484–496. 10.1007/s10162-007-0100-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bacon, S. P. , and Takahashi, G. A. (1992). “Overshoot in normal-hearing and hearing-impaired subjects,” J. Acoust. Soc. Am. 91, 2865–2871. 10.1121/1.402967 [DOI] [PubMed] [Google Scholar]
- 6. Beveridge, H. A. , and Carlyon, R. P. (1996). “Effects of aspirin on human psychophysical tuning curves in forward and simultaneous masking,” Hear. Res. 99, 110–118. 10.1016/S0378-5955(96)00091-3 [DOI] [PubMed] [Google Scholar]
- 7. Bidelman, G. M. , Jennings, S. G. , and Strickland, E. A. (2015). “PsyAcoustX: A flexible MATLAB® package for psychoacoustics research,” Front. Psychol. 6, 1–11. 10.3389/fpsyg.2015.01498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carlyon, R. P. , and Butt, M. (1993). “Effects of aspirin on human auditory filters,” Hear. Res. 66, 233–244. 10.1016/0378-5955(93)90143-O [DOI] [PubMed] [Google Scholar]
- 9. Champlin, C. A. , and McFadden, D. (1989). “Reductions in overshoot following intense sound exposures,” J. Acoust. Soc. Am. 85, 2005–2011. 10.1121/1.397853 [DOI] [PubMed] [Google Scholar]
- 10. Collet, L. , Kemp, D. T. , Veuillet, E. , Duclaux, R. , Moulin, A. , and Morgon, A. (1990). “Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects,” Hear. Res. 43, 251–262. 10.1016/0378-5955(90)90232-E [DOI] [PubMed] [Google Scholar]
- 11. Cooper, N. P. , and Guinan, J. J. (2003). “Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity,” J. Physiol. 548, 307–312. 10.1113/jphysiol.2003.039081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooper, N. P. , and Guinan, J. J. (2006). “Efferent-mediated control of basilar membrane motion,” J. Physiol. 576, 49–54. 10.1113/jphysiol.2006.114991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper, N. P. , and Rhode, W. S. (1997). “Mechanical responses to two-tone distortion products in the apical and basal turns of the mammalian cochlea,” J. Neurophysiol. 78, 261–270. 10.1152/jn.1997.78.1.261 [DOI] [PubMed] [Google Scholar]
- 14. Dolan, D. F. , Guo, M. H. , and Nuttall, A. L. (1997). “Frequency-dependent enhancement of basilar membrane velocity during olivocochlear bundle stimulation,” J. Acoust. Soc. Am. 102, 3587–3596. 10.1121/1.421008 [DOI] [PubMed] [Google Scholar]
- 15. Fletcher, M. , de Boer, J. , and Krumbholz, K. (2015). “Is off-frequency overshoot caused by adaptation of suppression?,” J. Assoc. Res. Otolaryngol. 16, 241–253. 10.1007/s10162-014-0498-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fletcher, M. D. , Krumbholz, K. , and de Boer, J. (2016). “Effect of contralateral medial olivocochlear feedback on perceptual estimates of cochlear gain and compression,” J. Assoc. Res. Otolaryngol. 17, 559–575. 10.1007/s10162-016-0574-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glasberg, B. R. , and Moore, B. C. J. (1990). “Derivation of auditory filter shapes from notched-noise data,” Hear. Res. 47, 103–138. 10.1016/0378-5955(90)90170-T [DOI] [PubMed] [Google Scholar]
- 18. Guinan, J. J. , and Gifford, M. L. (1988). “Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. II. Spontaneous rate,” Hear. Res. 33, 115–128. 10.1016/0378-5955(88)90024-X [DOI] [PubMed] [Google Scholar]
- 19. Hicks, M. L. , and Bacon, S. P. (1992). “Factors influencing temporal effects with notched-noise maskers,” Hear. Res. 64, 123–132. 10.1016/0378-5955(92)90174-L [DOI] [PubMed] [Google Scholar]
- 20. Hicks, M. L. , and Bacon, S. P. (1999). “Effects of aspirin on psychophysical measures of frequency selectivity, two-tone suppression, and growth of masking,” J. Acoust. Soc. Am. 106, 1436–1451. 10.1121/1.427146 [DOI] [PubMed] [Google Scholar]
- 21. James, A. L. , Harrison, R. V. , Pienkowski, M. , Dajani, H. R. , and Mount, R. J. (2005). “Dynamics of real time DPOAE contralateral suppression in chinchillas and humans,” Int. J. Audiol. 44, 118–129. 10.1080/14992020400029996 [DOI] [PubMed] [Google Scholar]
- 22. Jennings, S. G. , and Strickland, E. A. (2012). “Evaluating the effects of olivocochlear feedback on psychophysical measures of frequency selectivity,” J. Acoust. Soc. Am. 132, 2483–2496. 10.1121/1.4742723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jennings, S. G. , Strickland, E. A. , and Heinz, M. G. (2009). “Precursor effects on behavioral estimates of frequency selectivity and gain in forward masking,” J. Acoust. Soc. Am. 125, 2172–2181. 10.1121/1.3081383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawase, T. , Ogura, M. , Hidaka, H. , Sasaki, N. , Suzuki, Y. , and Takasaka, T. (2000). “Effects of contralateral noise on measurement of the psychophysical tuning curve,” Hear. Res. 142, 63–70. 10.1016/S0378-5955(00)00010-1 [DOI] [PubMed] [Google Scholar]
- 25. Keefe, D. H. , Schairer, K. S. , Ellison, J. C. , Fitzpatrick, D. F. , and Jesteadt, W. (2009). “Use of stimulus-frequency otoacoustic emissions to investigate efferent and cochlear contributions to temporal overshoot,” J. Acoust. Soc. Am. 125, 1595–1604. 10.1121/1.3068443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krull, V. , and Strickland, E. A. (2008). “The effect of a precursor on growth of forward masking,” J. Acoust. Soc. Am. 123, 4352–4357. 10.1121/1.2912440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- 28. Liberman, M. C. , Dodds, L. W. , and Pierce, S. (1990). “Afferent and efferent innervation of the cat cochlea: Quantitative analysis with light and electron microscopy,” J. Comp. Neurol. 301, 443–460. 10.1002/cne.903010309 [DOI] [PubMed] [Google Scholar]
- 29. Lichtenhan, J. T. , Wilson, U. S. , Hancock, K. E. , and Guinan, J. J. (2016). “Medial olivocochlear efferent reflex inhibition of human cochlear nerve responses,” Hear. Res. 333, 216–224. 10.1016/j.heares.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lilaonitkul, W. , and Guinan, J. J. (2009a). “Human medial olivocochlear reflex: Effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths,” J. Assoc. Res. Otolaryngol. 10, 459–470. 10.1007/s10162-009-0163-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lilaonitkul, W. , and Guinan, J. J. (2009b). “Reflex control of the human inner ear: A half-octave offset in medial efferent feedback that is consistent with an efferent role in the control of masking,” J. Neurophysiol. 101, 1394–1406. 10.1152/jn.90925.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lilaonitkul, W. , and Guinan, J. J. (2012). “Frequency tuning of medial-olivocochlear-efferent acoustic reflexes in humans as functions of probe frequency,” J. Neurophysiol. 107, 1598–1611. 10.1152/jn.00549.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Long, G. R. , and Tubis, A. (1988). “Modification of spontaneous and evoked otoacoustic emissions and associated psychoacoustic microstructure by aspirin consumption,” J. Acoust. Soc. Am. 84, 1343–1353. 10.1121/1.396633 [DOI] [PubMed] [Google Scholar]
- 34. Maison, S. , Adams, J. C. , and Liberman, M. C. (2003). “Olivocochlear innervation in the mouse: Immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization,” J. Comp. Neurol. 455, 406–416. 10.1002/cne.10490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maison, S. , and Liberman, M. C. (2000). “Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength,” J. Neurosci. 20, 4701–4707. 10.1523/JNEUROSCI.20-12-04701.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maison, S. , Micheyl, C. , Andeol, G. , Gallego, S. , and Collet, L. (2000). “Activation of medial olivocochlear efferent system in humans: Influence of stimulus bandwidth,” Hear. Res. 140, 111–125. 10.1016/S0378-5955(99)00196-3 [DOI] [PubMed] [Google Scholar]
- 37. Marshall, L. , Miller, J. A. L. , Guinan, J. J. , Shera, C. A. , Reed, C. M. , Perez, Z. D. , Delhorne, L. A. , and Boege, P. (2014). “Otoacoustic-emission-based medial-olivocochlear reflex assays for humans,” J. Acoust. Soc. Am. 136, 2697–2713. 10.1121/1.4896745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McFadden, D. , and Champlin, C. A. (1990). “Reductions in overshoot during aspirin use,” J. Acoust. Soc. Am. 87, 2634–2642. 10.1121/1.399056 [DOI] [PubMed] [Google Scholar]
- 39. Murugasu, E. , and Russell, I. J. (1996). “The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea,” J. Neurosci. 16, 325–332. 10.1523/JNEUROSCI.16-01-00325.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nelson, D. A. , Schroder, A. C. , and Wojtczak, M. (2001). “A new procedure for measuring peripheral compression in normal-hearing and hearing-impaired listeners,” J. Acoust. Soc. Am. 110, 2045–2064. 10.1121/1.1404439 [DOI] [PubMed] [Google Scholar]
- 41. Nieder, P. , and Nieder, I. (1970). “Stimulation of efferent olivocochlear bundle causes release from low level masking,” Nature 227, 184–185. 10.1038/227184a0 [DOI] [PubMed] [Google Scholar]
- 42. Oxenham, A. J. , and Plack, C. J. (1997). “A behavioral measure of basilar-membrane nonlinearity in listeners with normal and impaired hearing,” J. Acoust. Soc. Am. 101, 3666–3675. 10.1121/1.418327 [DOI] [PubMed] [Google Scholar]
- 43. Perrot, X. , Micheyl, C. , Khalfa, S. , and Collet, L. (1999). “Stronger bilateral efferent influences on cochlear biomechanical activity in musicians than in non-musicians,” Neurosci. Lett. 262, 167–170. 10.1016/S0304-3940(99)00044-0 [DOI] [PubMed] [Google Scholar]
- 44. Plack, C. J. , and Oxenham, A. J. (1998). “Basilar-membrane nonlinearity and the growth of forward masking,” J. Acoust. Soc. Am. 103, 1598–1608. 10.1121/1.421294 [DOI] [PubMed] [Google Scholar]
- 45. Roverud, E. , and Strickland, E. A. (2010). “The time course of cochlear gain reduction measured using a more efficient psychophysical technique,” J. Acoust. Soc. Am. 128, 1203–1214. 10.1121/1.3473695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roverud, E. , and Strickland, E. A. (2014). “Accounting for nonmonotonic precursor duration effects with gain reduction in the temporal window model,” J. Acoust. Soc. Am. 135, 1321–1334. 10.1121/1.4864783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ruggero, M. A. , Rich, N. C. , Recio, A. , Narayan, S. S. , and Robles, L. (1997). “Basilar-membrane responses to tones at the base of the chinchilla cochlea,” J. Acoust. Soc. Am. 101, 2151–2163. 10.1121/1.418265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Russell, I. J. , and Murugasu, E. (1997). “Medial efferent inhibition suppresses basilar membrane responses to near characteristic frequency tones of moderate to high intensities,” J. Acoust. Soc. Am. 102, 1734–1738. 10.1121/1.420083 [DOI] [PubMed] [Google Scholar]
- 49. Schrott-Fischer, A. , Egg, G. , Kong, W.-J. , Renard, N. , and Eybalin, M. (1994). “Immunocytochemical detection of choline acetyltransferase in the human organ of Corti,” Hear. Res. 78, 149–157. 10.1016/0378-5955(94)90020-5 [DOI] [PubMed] [Google Scholar]
- 50. Strickland, E. A. (2001). “The relationship between frequency selectivity and overshoot,” J. Acoust. Soc. Am. 109, 2062–2073. 10.1121/1.1357811 [DOI] [PubMed] [Google Scholar]
- 51. Strickland, E. A. (2004). “The temporal effect with notched-noise maskers: Analysis in terms of input-output functions,” J. Acoust. Soc. Am. 115, 2234–2245. 10.1121/1.1691036 [DOI] [PubMed] [Google Scholar]
- 52. Strickland, E. A. (2008). “The relationship between precursor level and the temporal effect,” J. Acoust. Soc. Am. 123, 946–954. 10.1121/1.2821977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Strickland, E. A. , and Krishnan, L. A. (2005). “The temporal effect in listeners with mild to moderate cochlear hearing impairment,” J. Acoust. Soc. Am. 118, 3211–3217. 10.1121/1.2074787 [DOI] [PubMed] [Google Scholar]
- 54. Viana, L. M. , O'Malley, J. T. , Burgess, B. J. , Jones, D. D. , Oliveira, C. A. , Santos, F. , Merchant, S. N. , Liberman, L. D. , and Liberman, M. C. (2015). “Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue,” Hear. Res. 327, 78–88. 10.1016/j.heares.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vinay, and Moore, B. C. J. (2008). “Effects of activation of the efferent system on psychophysical tuning curves as a function of signal frequency,” Hear. Res. 240, 93–101. 10.1016/j.heares.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 56. Walsh, K. P. , Pasanen, E. G. , and McFadden, D. (2010). “Overshoot measured physiologically and psychophysically in the same human ears,” Hear. Res. 268, 22–37. 10.1016/j.heares.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Warr, W. B. (1992). “Organization of olivocochlear efferent systems in mammals,” in , edited by Webster D. B., Popper A. N., and Fay R. R. ( Springer, New York), Chap. 7, pp. 410–448. [Google Scholar]
- 58. Wicher, A. , and Moore, B. C. J. (2014). “Effect of broadband and narrowband contralateral noise on psychophysical tuning curves and otoacoustic emissions,” J. Acoust. Soc. Am. 135, 2931–2941. 10.1121/1.4871358 [DOI] [PubMed] [Google Scholar]
- 59. Yasin, I. , Drga, V. , and Plack, C. J. (2014). “Effect of human auditory efferent feedback on cochlear gain and compression,” J. Neurosci. 34, 15319–15326. 10.1523/JNEUROSCI.1043-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zwicker, E. (1965). “Temporal effects in simultaneous masking by white-noise bursts,” J. Acoust. Soc. Am. 37, 653–663. 10.1121/1.1909389 [DOI] [PubMed] [Google Scholar]