Abstract
Background
The benign and malignant patterns of acral melanocytic nevi (AMN) and acral melanomas (AM) have been defined in a series of retrospective studies. A 3-step algorithm was developed to determine when to biopsy acral melanocytic lesions. This algorithm has only been applied to a Japanese population.
Objectives
Our study aimed to review the current management strategy of acral melanocytic lesions and to investigate the utility of the 3-step algorithm in a predominately Caucasian cohort.
Methods
A retrospective search of the pathology and image databases at Mayo Clinic was performed between the years 2006 – 2016. Only cases located on a volar surface with dermoscopic images were included. Two dermatologists reviewed all dermoscopic images and assigned a global dermoscopic pattern. Clinical and follow-up data was gathered by chart review. All lesions with known diameter and pathological diagnosis were used for the 3-step algorithm.
Results
Regular fibrillar and ridge patterns were more likely to be biopsied (p=0.01). The majority of AMN (58.1%) and AM (60%) biopsied were due to physician-deemed concerning dermoscopic patterns. 39.2% of these cases were parallel furrow, lattice-like, or regular fibrillar. When patients were asked to follow-up within a 3-6-month period, only 16.7% of the patients returned within that interval. The 3-step algorithm would have correctly identified 4/5 AM for biopsy, missing a 6mm, multi-component, invasive melanoma.
Conclusion
We found one major educational gap in the recognition of low risk lesions with high rates of biopsy of the fibrillary pattern. Recognizing low-risk dermoscopic patterns could reduce the rate of biopsy of AMN by 23.3%. We identified two major practice gaps, poor patient compliance with follow up and the potential insensitivity of the 3-step algorithm to small multi-component acral melanocytic lesions.
Introduction
Acral melanocytic nevi (AMN) and acral melanomas (AM) can be difficult to interpret dermoscopically and are associated with a worse prognosis than non-acral cutaneous melanomas (CM).1 The benign and malignant patterns of AMN and AM have been defined in a series of retrospective studies.2–11 Several clinically useful patterns have been described, including benign, malignant, and indeterminate patterns. Benign patterns include parallel furrow, lattice-like, and regular fibrillar.12 The three malignant patterns are parallel ridge, multi-component, and diffuse irregular pigmentation.2, 4, 10, 11 Indeterminate patterns include reticular, globular, homogeneous, and non-typical patterns.12
Recently, an evidence-based, 3-step algorithm was developed using clinical and dermoscopic features.12–14 In this algorithm, all parallel ridge patterns require a biopsy. Parallel furrow, lattice-like and regular fibular patterns do not require a biopsy. For any lesion not conforming to one of these four patterns, a biopsy is performed if the diameter of the lesion is greater than 7 mm. Although easy to use, the utility of the 3-step algorithm has not been confirmed in a non-Japanese population.
For indeterminate or intermediate risk acral melanocytic lesions, close clinical follow-up may be warranted instead of a biopsy. Many factors determine if clinical follow-up is appropriate, including the assessed risk of the lesion, the location of the lesion, patient preference, and patient reliability. In non-acral CM, the most important clinical features are a new or changing lesion, dermoscopic impression, and patient concerns.15, 16 Interestingly, there is little data on these clinical factors and decision making in acral locations.
Our study aimed to review the current management strategy of acral melanocytic lesions and to investigate the utility of the 3-step algorithm in a predominately Caucasian cohort. Due to the poor outcomes in AM, success was defined as 100% sensitivity to advocate for a biopsy of AM.
Methods
The institutional review board at Mayo Clinic approved this study. A retrospective search of all pathology records between 2006 and 2016 at Mayo Clinic Arizona was performed. Search terms included: acral nevus, acral melanocytic nevus, nevus, nevi, melanoma, or acral melanoma with foot, feet, plantar, toe, heel, sole, digit, hand, hands, finger, thumb, palm, or palmar. This search identified 293 cases. All pathology reports and clinical and dermoscopic photographs were reviewed to confirm the diagnosis and the location. All cases that did not have dermoscopic photographs or lesions on non-volar surfaces were excluded. 27 cases met all criteria for inclusion.
A second search of our image database of biopsied and non-biopsied lesions, collected from mid-2010 through 2016, was performed using anatomically limiting search terms: foot, feet, plantar, toe, heel, sole, digit, hand, hands, finger, thumb, palm, or palmar. A total of 5859 images were identified, and the clinical and dermoscopic images and the medical records were reviewed. All nevi and melanomas with clinical and dermoscopic images were recorded and duplicates from the pathology search were removed. All cases on non-volar surfaces were excluded. This search identified an additional 21 cases that were biopsied and 73 cases that were followed clinically.
Demographic data was collected from chart reviews, including age at diagnosis, gender, history of non-melanoma skin cancer, and history of melanoma. During the chart reviews, data on who initiated the request for a biopsy (defined as the physician-initiated when the physician documented clinical concern for the lesion being biopsied, patient-initiated when the patient presented with a lesion they were concerned of, patient directed without physician recommendation when patient concern was documented as the reason for biopsy and the physician’s examination documented the benign clinical appearance) and the reason for biopsy (new lesion, changing lesion, symptomatic, physician-deemed concerning dermoscopic pattern, patient concern) was collected. For individuals followed clinically, the recommended follow-up times and actual follow-up times were recorded. All lesions with known diameter and pathological diagnosis were used for the 3-step algorithm.
Two experienced dermatologists (DLS and ARM), blinded to the outcomes, reviewed all 121 dermoscopic images using the definitions in Table 1. Each dermoscopic image was independently assigned a global dermoscopic pattern that best represented the lesion by each reviewer (Fig. 1). All global patterns were then re-reviewed and a consensus was made on any discrepant cases.
Table 1.
Definition of dermoscopy patterns and demographics
| Low-risk Dermoscopic Patterns†
| ||||||
| Parallel Furrow | Linear pigmentation most prominent in the furrows. | |||||
| Lattice-like | Linear pigmentation following the furrows and linear bands of pigment crossing over the ridge to the next furrow. | |||||
| Regular Fibrillar | Thin, even, pigmentation tangential to furrows and ridges. | |||||
|
| ||||||
| Indeterminate Dermoscopic Patterns‡
| ||||||
| Reticular | Regular reticulated pigment network. | |||||
| Globular | Regular globules not associated with a parallel furrow pattern. | |||||
| Homogeneous | Regular, diffuse pigmentation. | |||||
| Non-typical | Not fitting with a classic benign or malignant pattern. | |||||
|
| ||||||
| High-risk Dermoscopic Patterns§
| ||||||
| Parallel Ridge | Linear pigmentation more prominent on the ridges of skin markings. | |||||
| Multi-component | Several dermoscopic patterns irregularly distributed throughout the lesion. (e.g: diffuse pigmentation, irregular dots and globules, irregular pigment network, atypical streaks, and multiple colors. Note that regions of benign patterns may be seen in the same lesion) | |||||
|
| ||||||
| Demographics
| ||||||
| Age | Gender | History of Skin Cancer | ||||
|
| ||||||
| Mean | SD | Median | Males | 45 (37.2%) | Non-melanoma | 15 (12.4%) |
| 51.7 | 16.2 | 52.0 | Females | 76 (62.8%) | Melanoma | 6 (5.0%) |
Figure 1.
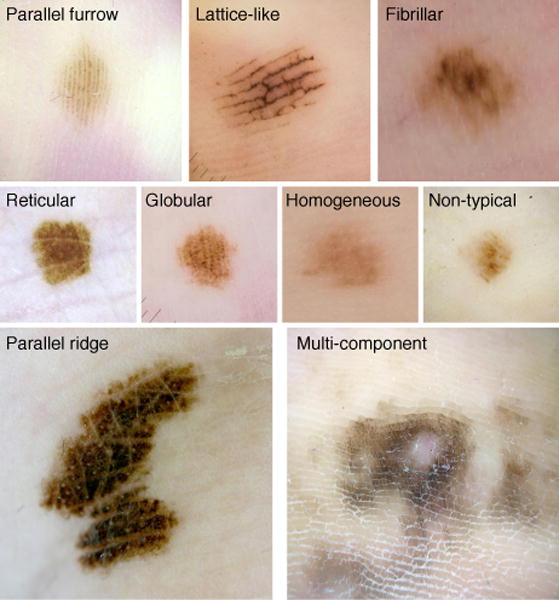
Dermoscopic patterns – Parallel furrow, Lattice-like, and Regular Fibrillar are low risk patterns, Parallel Ridge and Multi-component are high-risk patterns.
Statistical analysis was performed using frequencies and proportions for categorical variables, and mean, standard deviation, median, and range for continuous variables. A comparison between groups was conducted using the Wilcoxon rank sum test for continuous variables, and the Pearson Chi-squared test for categorical variables. The significance level was at 0.05. The analysis was performed using SAS version 9.4 (SAS Institute Inc.; Cary, NC).
Results
A total 121 acral melanocytic lesions were identified; 73 were clinically followed and 48 were biopsied. A total of 45 biopsied lesions were located on the soles and 3 were located on the palms. For the clinically followed lesions, 64 were located on the soles and 9 on the palms.
There were 5 patients with AM (0 male & 5 female), 43 with AMN (18 male & 25 female), and 73 with clinically followed lesions (27 male & 46 female). The ethnicities were Caucasian (70.2%), African American (17.4%), Asian (5.0%), Hispanic (3.3%) Native American (0.8%), Pacific Islander (0.8%), and unknown (2.5%). A total of 4.1% of patients had a history of melanoma, 11.6% had a history of non-melanoma skin cancer, and 0.8% had a history of both. There were no significant differences in ethnicity or gender between biopsied and clinically followed lesions or the diagnosis of AMN and AM. Individuals with a history of skin cancer were more likely to be biopsied (p=0.02). Patients who were followed clinically were significantly younger than those who underwent biopsy (49.1 yrs (SD=15.6) vs 55.6yrs (SD=16.6), p=0.04).
The dermoscopic patterns that were more likely to be biopsied than followed clinically were the regular fibrillar and parallel ridge patterns. 4 fibrillar pattern lesions were followed and 10 biopsied (p=0.01). No parallel ridge pattern lesions were followed and 5 were biopsied (p=0.01). In contrast, the parallel furrow pattern nevi were followed 21 times and biopsied 5 times (p=0.02). The remaining benign and malignant patterns had no significant difference between the rate of biopsy and clinical follow-up (Table 2).
Table 2.
Dermoscopy Pattern for Clinically Followed versus Biopsied Lesions
| Pattern | Followed (n=73) | Biopsied (n=48) | P-value |
|---|---|---|---|
| Parallel Furrow | 21 (28.8%) | 5 (10.4%) | 0.02 |
| Lattice-like | 14 (19.2%) | 6 (12.5%) | 0.33 |
| Regular Fibrillar | 4 (5.5%) | 10 (20.8%) | 0.01 |
| Reticular | 3 (4.1%) | 1 (2.1%) | 0.54 |
| Globular | 5 (6.8%) | 3 (6.3%) | 0.90 |
| Homogeneous | 4 (5.5%) | 3 (6.3%) | 0.86 |
| Non-typical | 19 (26.0%) | 12 (25.0%) | 0.90 |
| Parallel Ridge | 0 (0%) | 5 (10.4%) | 0.01 |
| Multi-component | 3 (4.1%) | 3 (6.3%) | 0.60 |
|
| |||
| Diameter of Biopsied Benign Nevi versus Biopsied Melanoma
| |||
| Mean Size (mm) | Range (mm) | P-value | |
|
| |||
| Melanoma (n=5) | 12.0 (SD=15.7) | 4.0-40.0 | 0.25 |
| Benign Nevi (n=43) | 5.2 (SD=2.8) | 1.5-15.0 | |
Of biopsied lesions, the parallel ridge pattern (p=0.01) and multicomponent (p=0.03) were more likely to be malignant patterns. All other patterns were not significantly associated with benign or malignant pathology. The malignant lesions were non-significantly larger (12.0 mm, SD=15.7, p=0.25) than the benign lesions (5.2 mm, SD=2.8) (Table 2).
Physicians directed the biopsy of 37/43 (70.2%) AMN and 3/5 (60%) AM. Patients directed the biopsy with physician agreement and recommendation in 1/43 (2.3%) AMN and 1/5 (20%) AM. Patients directed the biopsy without physician recommendation in 1/43 (2.3%) AMN and 0/5 AM. It was unclear who directed the biopsy in 4/43 (9.3%) AMN and 1/5 (20%) AM. A total of 25/43 (58.1%) AMN and 3/5 (60%) AM were biopsied due to physician-deemed concerning dermoscopic patterns. Changing lesions and new lesions were the reasons for biopsy in 9/43 (20.9%) and 7/43 (16.3%) AMN and 0/5 and 1/5 (20%) AM respectively. Difficult to access locations were not reasons for biopsy for any lesion. Patient concerns without physician agreement were the reasons for biopsy in 1/43 (2.3%) AMN. 1/43 (2.3%) AMN and 1/5 (20%) AM were biopsied for unknown reasons.
When physician-deemed concerning dermoscopic patterns were the reason for biopsy, upon review of the dermoscopy images, 39.2% of cases were either parallel furrow, lattice-like, or regular fibrillar. 42.9% of the cases were indeterminate patterns including homogeneous, reticular, globular, or non-typical patterns. 17.9% of the cases were parallel ridge or multi-component.
For non-biopsied acral lesions, physician recommendations were as follows: 64.4% for no close follow-up (defined as no follow-up besides an annual skin exam), 32.9% for close follow-up within 3-6 month, and 2.7% did not have specific recommendations. The mean recommended follow-up interval was 4.4 months. When close follow-up was recommended, 16.7% of the patients followed up within the recommended time interval, 33.3% of patients followed up outside of the recommended time interval (mean=19.4 months), and 50.0% did not follow-up.
All biopsied volar acral lesions with a known lesion diameter, 40 AMN and 5 AM, were reviewed using the 3-step algorithm (Fig. 2). The 3-step algorithm correctly identified 4/5 (80%) AMs for biopsy. A multi-component pattern (atypical network, atypical lattice, and atypical globules), 0.5 mm Breslow thickness, 6 mm in diameter lesion was missed (Fig. 3). The sensitivity of the 3-step algorithm was 80.0%, specificity 87.8%, PPV of 44.4% and the NPV 97.2% (see Supplemental).
Figure 2.
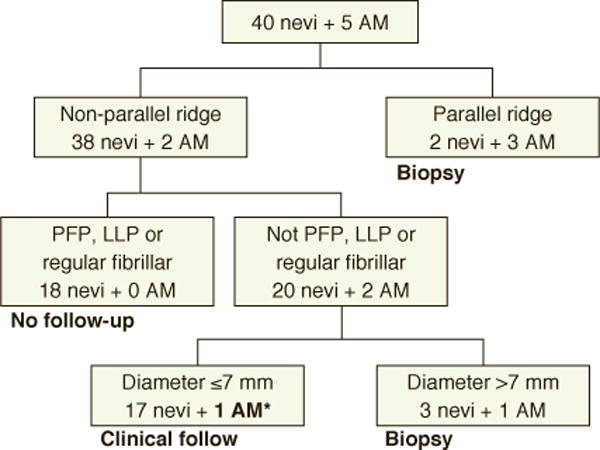
3-Step Algorithm –*6mm, multicomponent acral melanoma; Parallel furrow pattern (PFP), lattice-like pattern (LLP), acral melanoma (AM)
Figure 3.
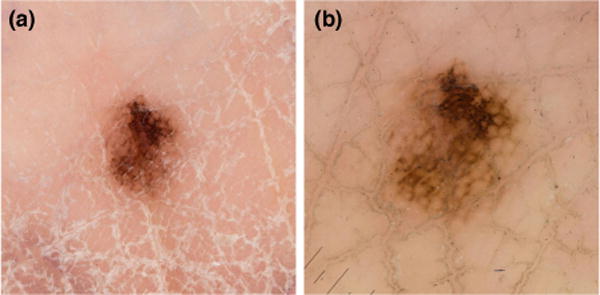
Potentially missed acral melanoma by 3-step algorithm- A 6mm, 0.5mm Breslow thickness, multi-component pattern. a) clinical photograph b) contact dermoscopy
Discussion
Acral melanocytic lesions remain a diagnostic challenge. Our study examined the clinical and dermoscopic features of biopsied and followed AMN and AM to review our current management strategy. We found one major educational gap and two major practice gaps including: the recognition of low risk benign lesions, poor patient compliance with follow up, and the potential insensitivity of the 3-step algorithm to small multi-component acral melanocytic lesions. Physicians often do not recognize regular fibrillar as a benign pattern. Of nearly 40% of the lesions biopsied due to physician-deemed concerning dermoscopic pattern, the patterns were low-risk and did not require biopsy, regardless of size. Overall, patients have poor follow-up to re-examine acral lesions within a 3-6-month time frame. Only 16.7% of patient followed up on time and 50% did not follow up. Lastly, we confirmed the utility of the 3-step algorithm in a predominately Caucasian population, albeit missing on 6mm AM with a multi-component pattern. The addition of the multi-component pattern as high-risk, requiring a biopsy, to the 3-step algorithm will ameliorate this potential pitfall.
Parallel furrow, lattice-like, and regular fibrillar patterns are considered low-risk in the 3-step algorithm, and within our study none of these patterns were malignant. We found that parallel furrow is less likely to be biopsied (p=0.02), while regular fibrillar and parallel ridge were more likely to be biopsied (p=0.01, p=0.01). This suggests that parallel furrow is an easily recognized benign pattern, and parallel ridge is an easily recognized malignant pattern. Our results demonstrate that there is an incomplete understanding and interpretation of the regular fibrillar pattern, which is actually a secondary pattern that results from shear displacement of the stratum corneum.3 Better education is needed on how to examine fibrillar pattern acral melanocytic lesions. The best technique is to utilize a tangential, oblique dermoscopic angle when visualizing acral melanocytic lesions.3 This determines the origin of the pigment as furrow or ridge, noting that the latter is a malignant pattern.
The clinical factors that led to biopsy or clinical follow-up were examined. Individuals with a history of skin cancer were more likely to be biopsied (p=0.02) and younger individuals were more likely to be followed clinically (49.1 vs 55.6 yrs, p=0.04). Additionally, all individuals with AM were female. The current study is underpowered to establish age, sex, and history of skin cancer as additional diagnostic classifiers for the analysis of acral melanocytic lesions. Future studies should be undertaken to determine which clinical classifiers are independent predictors of malignancy. The reasons for biopsy included physician-deemed concerning dermoscopic features in 58.1% and 60% of AMN and AM respectively, a changing lesion in 20.9% and 0% of AMN and AM respectively, and a new lesion in 16.3% for AMN and 20% for AM respectively. Previous studies have investigated the reasons for biopsies of non-acral cutaneous nevi and CM, including dermoscopic impressions, new lesions, and changing lesions.15, 16 In those studies, the strongest predictor of biopsy was “physician concern,” including atypical gross or dermoscopic appearances.
Our study found that physician-deemed concerning dermoscopic patterns were the strongest predictors of biopsy of AMN and AM. Reinterpretations of the physician-deemed concerning patterns by 2 trained dermoscopists found that 39.2% were benign parallel furrow, lattice-like, or regular fibrillar patterns. This suggests the need for additional dermoscopy training for both residents and physicians. A recent survey of 139 dermatology chief residents found that 94% use dermoscopy; however, only 48% trained with a pigmented lesions specialist.17 In our series, better training in acral-pigmented lesions may have decreased the biopsy rate of AMN by 23.3% (see Supplemental).
In our review of clinically followed acral melanocytic lesions, only 16.7% of the patients followed up within the recommended time interval, 33% followed up outside of the time interval, and 50% never returned. This finding was unexpected and may be practice changing. Rates of follow-up for non-acral melanocytic lesions, in Europe and Australia, show an inverse relationship between compliance and follow-up interval with compliance rates of 84%, 46-63%, and 30% for 3-month, 6-month, and 12-month follow-up respectively.18–20 Due to poor clinical follow-up overall and the inverse relationship between follow-up interval and compliance, we caution any clinical follow-up of non-benign pattern acral melanocytic lesions. For reliable patients, we recommend clinical follow-up intervals of less than 3 months with indeterminate dermoscopic patterns and biopsy for high-risk acral dermoscopic patterns.
The 3-step algorithm correctly identified 4/5 volar AM (Fig. 2). However, a 6 mm, multi-component (atypical network, atypical lattice, and atypical globules), invasive AM was missed. This lesion was located at the junction of the heel and the arch on the volar foot. The combination of a “low” and “high” mechanical stress area may account for the atypical appearance.21 We advocate for the inclusion of the multi-component pattern as a high-risk dermoscopic pattern that should be biopsied regardless of lesion size. Multiple studies have found a positive correlation between size and malignancy in melanocytic lesions with non-benign volar dermoscopic patterns.10, 12, 22 The 7-mm cutoff should be used when deciding to biopsy indeterminate lesions. Previous reports have demonstrated that parallel ridge, multi-component, and irregular diffuse pigmentation are all malignant patterns on the volar surface.2, 4, 11 Based on our findings, the multi-component and the parallel ridge patterns should be biopsied regardless of lesion diameter.
Our study is limited in its retrospective design. Dermoscopic and clinical photos were only obtained on clinically concerning or biopsied lesions, which led to selection bias. Our sample size was small, limiting our study’s analysis of the 3-step algorithm. However, despite the small number of volar AM, the 3-step algorithm missed 1/5 AM. Future studies are warranted to examine the 3-step algorithm with the inclusion of the multi-component pattern in a larger sample population as well as to examine age, sex, and history of skin cancer as additional risk classifiers.
Conclusions
Acral melanocytic lesions can be difficult to interpret. The regular fibrillar pattern had the highest biopsy rate of benign lesions. Recognizing that parallel furrow, lattice-like and regular fibrillar are low-risk dermoscopic patterns could reduce the rate of biopsy of AMN by 23.3%. Patients have poor compliance with clinical follow-up of acral melanocytic lesions. Therefore, caution should be used when following any non-benign pattern acral melanocytic lesion. Clinical follow-up of less than 3 months for indeterminate dermoscopic patterns and biopsy of all high-risk dermoscopic patterns may be appropriate. The 3-step algorithm is useful to decide when to biopsy acral melanocytic lesions. To improve the sensitivity, the multi-component pattern should be added as a size independent high-risk lesion to the 3-step algorithm.
Supplementary Material
Supplemental Table: Calculations for sensitivity, specificity, PPV, NPV, and biopsy reduction percentage.
Acknowledgments
We would like to thank National Institutes of Health: National Heart, Lung, and Blood Institute# T35HL007479 grant for the support of CMC’s time.
NIH NHLBI# T35HL007479, Short-Term Training: Students in Health Professional Schools supported C.M. Costello’s time. There was no additional funding source.
Footnotes
None of the authors have any conflicts of interest to disclose.
References
- 1.Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145(4):427–34. doi: 10.1001/archdermatol.2008.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altamura D, Altobelli E, Micantonio T, Piccolo D, Fargnoli MC, Peris K. Dermoscopic patterns of acral melanocytic nevi and melanomas in a white population in central Italy. Archives of dermatology. 2006;142(9):1123–8. doi: 10.1001/archderm.142.9.1123. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki A, Saida T, Koga H, Oguchi S, Suzuki T, Tsuchida T. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53(2):230–6. doi: 10.1016/j.jaad.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 4.Saida T, Miyazaki A, Oguchi S, Ishihara Y, Yamazaki Y, Murase S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140(10):1233–8. doi: 10.1001/archderm.140.10.1233. [DOI] [PubMed] [Google Scholar]
- 5.Saida T, Oguchi S, Miyazaki A. Dermoscopy for acral pigmented skin lesions. Clin Dermatol. 2002;20(3):279–85. doi: 10.1016/s0738-081x(02)00219-5. [DOI] [PubMed] [Google Scholar]
- 6.Saida T, Oguchi S, Ishihara Y. In vivo observation of magnified features of pigmented lesions on volar skin using video macroscope. Usefulness of epiluminescence techniques in clinical diagnosis. Arch Dermatol. 1995;131(3):298–304. [PubMed] [Google Scholar]
- 7.Madankumar R, Gumaste PV, Martires K, Schaffer PR, Choudhary S, Falto-Aizpurua L, et al. Acral melanocytic lesions in the United States: Prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74(4):724–30.e1. doi: 10.1016/j.jaad.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Barquet V, Dufrechou L, Nicoletti S, Acosta MA, Magliano J, Martinez M, et al. Dermoscopic patterns of 158 acral melanocytic nevi in a Latin American population. Actas dermo-sifiliograficas. 2013;104(7):586–92. doi: 10.1016/j.adengl.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Elwan NM, Eltatawy RA, Elfar NN, Elsakka OM. Dermoscopic features of acral pigmented lesions in Egyptian patients: a descriptive study. International journal of dermatology. 2016;55(2):187–92. doi: 10.1111/ijd.12882. [DOI] [PubMed] [Google Scholar]
- 10.Braun RP, Thomas L, Dusza SW, Gaide O, Menzies S, Dalle S, et al. Dermoscopy of acral melanoma: a multicenter study on behalf of the international dermoscopy society. Dermatology (Basel, Switzerland) 2013;227(4):373–80. doi: 10.1159/000356178. [DOI] [PubMed] [Google Scholar]
- 11.Phan A, Dalle S, Touzet S, Ronger-Savle S, Balme B, Thomas L. Dermoscopic features of acral lentiginous melanoma in a large series of 110 cases in a white population. Br J Dermatol. 2010;162(4):765–71. doi: 10.1111/j.1365-2133.2009.09594.x. [DOI] [PubMed] [Google Scholar]
- 12.Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. The Journal of dermatology. 2011;38(1):25–34. doi: 10.1111/j.1346-8138.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 13.Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Archives of dermatology. 2011;147(6):741–3. doi: 10.1001/archdermatol.2011.136. [DOI] [PubMed] [Google Scholar]
- 14.Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Archives of dermatology. 2007;143(11):1423–6. doi: 10.1001/archderm.143.11.1423. [DOI] [PubMed] [Google Scholar]
- 15.Puig S, Argenziano G, Zalaudek I, Ferrara G, Palou J, Massi D, et al. Melanomas that failed dermoscopic detection: a combined clinicodermoscopic approach for not missing melanoma. Dermatol Surg. 2007;33(10):1262–73. doi: 10.1111/j.1524-4725.2007.33264.x. [DOI] [PubMed] [Google Scholar]
- 16.Soares TF, Laman SD, Yiannias JA, Connolly SM, Lim KK, Wu Q, et al. Factors leading to the biopsy of 1547 pigmented lesions at Mayo Clinic, Scottsdale, Arizona, in 2005. International journal of dermatology. 2009;48(10):1053–6. doi: 10.1111/j.1365-4632.2009.04137.x. [DOI] [PubMed] [Google Scholar]
- 17.Wu TP, Newlove T, Smith L, Vuong CH, Stein JA, Polsky D. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68(6):1000–5. doi: 10.1016/j.jaad.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Schiffner R, Schiffner-Rohe J, Landthaler M, Stolz W. Long-term dermoscopic follow-up of melanocytic naevi: clinical outcome and patient compliance. Br J Dermatol. 2003;149(1):79–86. doi: 10.1046/j.1365-2133.2003.05409.x. [DOI] [PubMed] [Google Scholar]
- 19.Argenziano G, Mordente I, Ferrara G, Sgambato A, Annese P, Zalaudek I. Dermoscopic monitoring of melanocytic skin lesions: clinical outcome and patient compliance vary according to follow-up protocols. Br J Dermatol. 2008;159(2):331–6. doi: 10.1111/j.1365-2133.2008.08649.x. [DOI] [PubMed] [Google Scholar]
- 20.Menzies SW, Gutenev A, Avramidis M, Batrac A, McCarthy WH. Short-term digital surface microscopic monitoring of atypical or changing melanocytic lesions. Archives of dermatology. 2001;137(12):1583–9. doi: 10.1001/archderm.137.12.1583. [DOI] [PubMed] [Google Scholar]
- 21.Costello CM, Pittelkow MR, Mangold AR. Acral Melanoma and Mechanical Stress on the Plantar Surface of the Foot. The New England journal of medicine. 2017;377(4):395–6. doi: 10.1056/NEJMc1706162. [DOI] [PubMed] [Google Scholar]
- 22.Rubegni P, Cevenini G, Nami N, Argenziano G, Saida T, Burroni M, et al. A simple scoring system for the diagnosis of palmo-plantar pigmented skin lesions by digital dermoscopy analysis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2013;27(3):e312–9. doi: 10.1111/j.1468-3083.2012.04651.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table: Calculations for sensitivity, specificity, PPV, NPV, and biopsy reduction percentage.


