Abstract
The signaling networks regulating antimicrobial activity during urinary tract infection (UTI) are incompletely understood. Interleukin-6 (IL-6) levels increase with UTI severity, but the specific contributions of IL-6 to host immunity against bacterial uropathogens are unknown. To clarify this we tested whether IL-6 activates the Stat3 transcription factor, to drive a program of antimicrobial peptide gene expression in infected urothelium during UTI. Transurethral inoculation of uropathogenic Escherichia coli led to IL-6 secretion, urothelial Stat3 phosphorylation, and activation of antimicrobial peptide transcription, in a Toll-like receptor 4-dependent manner in a murine model of cystitis. Recombinant IL-6 elicited Stat3 phosphorylation in primary urothelial cells in vitro, and systemic IL-6 administration promoted urothelial Stat3 phosphorylation and antimicrobial peptide expression in vivo. IL-6 deficiency led to decreased urothelial Stat3 phosphorylation and antimicrobial peptide mRNA expression following UTI, a finding mirrored by conditional Stat3 deletion. Deficiency in IL-6 or Stat3 was associated with increased formation of intracellular bacterial communities, and exogenous IL-6 reversed this phenotype in IL-6 knockout mice. Moreover, chronic IL-6 depletion led to increased renal bacterial burden and severe pyelonephritis in C3H/HeOuJ mice. Thus, IL-6/Stat3 signaling drives a transcriptional program of antimicrobial gene expression in infected urothelium, with key roles in limiting epithelial invasion and ascending infection.
Keywords: urinary tract infection, antimicrobial peptide, IL-6, Stat3, urothelium, intracellular bacterial community
Introduction
Urinary tract infections (UTI) represent a modern public health crisis, particularly in light of UTI prevalence, widespread prescription of broad-spectrum antibiotics, and rising antimicrobial resistance among bacterial uropathogens.1–3 To care for patients with UTI, alternative treatments and prophylactic measures must be developed.4–6 The urothelium serves as the front line of urinary tract defense against invading microorganisms, with key roles in barrier function, pathogen detection, production of antimicrobial substances, and phagocyte recruitment.7, 8 Augmenting the innate immune properties of urothelium represents an attractive approach to UTI prevention and treatment.
The urothelium secretes and responds to chemokines and cytokines as an important component of its response to UTI.9 For example, urothelial interleukin (IL)-8 production and receptor expression serve critical roles in neutrophil recruitment.10–12 Likewise, IL-6 is rapidly secreted by urothelial cells following Escherichia coli exposure.13–15 Studies have implicated bacterial determinants, such as lipopolysaccharide (Lps) and P-fimbriae, in eliciting urothelial IL-6 production.16–18 In bladder urothelium, Lps engages the Toll-like receptor (Tlr) 4, triggering an intracellular signaling cascade that leads to IL-6 secretion.13, 15, 18
Studies suggest IL-6 secretion carries functional significance during UTI. Uropathogenic E. coli (UPEC) strains – unlike laboratory E. coli strains – suppress IL-6 secretion by urothelial cells in vitro, and this is a proposed escape mechanism for UPEC to evade the host immune response.19, 20 UPEC virulence correlates inversely with IL-6 production, such that low IL-6 elicitation confers a survival advantage for UPEC during cystitis.19, 21, 22 IL-6 expression is also indicative of clinical UTI severity: children with pyelonephritis and renal scarring have elevated urine and serum IL-6 levels compared to those with cystitis.23–26 Animal models similarly demonstrate an association between IL-6 production and UTI severity.27, 28 In a model of complicated UTI in which mice underwent urethral obstruction following transurethral UPEC inoculation, IL-6 knockout (KO) animals experienced higher mortality rates, and survivors demonstrated higher renal UPEC burden and worse renal inflammation than controls.29 The functional significance of IL-6 during uncomplicated cystitis and acute pyelonephritis, however, remains unexplored.
Moreover, the signaling pathways and transcriptional targets activated by IL-6 have not been evaluated during UTI. Following IL-6 binding to its receptor, the Signal Transducer and Activator of Transcription (Stat) 3 undergoes Jak-dependent tyrosine phosphorylation, leading to homodimerization and translocation to the nucleus, where phosphorylated (p)-Stat3 regulates transcription.30, 31 Evidence in human immunodeficiency syndromes and mouse models supports a critical role for Stat3 in defending epithelial surfaces against bacterial pathogens.32–35 Stat3 transcriptional targets encode a variety of molecules with roles in antibacterial defense, including antimicrobial peptides (AMPs).33, 35
AMPs promote immunity through bactericidal, bacteriostatic, and immunomodulatory activities.36–38 AMP deficiency increases host UTI susceptibility, but little is known regarding signaling pathways that regulate AMP production during UTI. IL-6 and Stat3 have been implicated in transcriptional regulation of multiple AMPs (including Camp, Hamp, Lcn2, RegIIIγ, and RegIIIβ) by epithelial cells outside of the urinary tract33, 35, 39–44 We hypothesized that IL-6 induction by UPEC triggers p-Stat3 dependent AMP expression during UTI, and that genetic deletion of IL-6 or Stat3 would impair the host antimicrobial response. We tested this hypothesis in mouse models of experimental UTI initiated by UPEC.
Results
Urinary IL-6 secretion, urothelial Stat3 phosphorylation, and AMP expression during UTI
We investigated the temporal relationship between urinary IL-6 secretion, bladder p-Stat3 levels, and AMP expression in a murine model of UPEC cystitis. Following transurethral UPEC inoculation, urinary IL-6 secretion peaked 2 hours post inoculation (hpi) and returned to near baseline by 16 hpi (Figure 1A). Bladder p-Stat3 levels increased within 2 hpi, peaked at 6 hpi, and returned to baseline by 72 hpi. Total Stat3 levels were unaffected (Figure 1B). P-Stat3 localized to basal and intermediate bladder urothelial cell nuclei (Figure 1C).
Figure 1. Experimental UTI triggers IL-6 secretion, urothelial p-Stat3, and AMP mRNA expression.

A) Urine IL-6/Cr levels peak 2 hpi following transurethral UPEC inoculation. Urine was pooled from 6 mice/experimental group at each time point. Bars indicate mean ± standard deviation of triplicate IL-6/Cr values. #: p = 0.0073, Kruskal-Wallis test. B) Western blotting demonstrates a transient increase in p-Stat3 levels in UPEC infected bladders. C) Immunofluorescence microscopy identifies p-Stat3 reactive nuclei (pink staining) localized mainly within the basal and intermediate urothelial layers. Green staining demonstrates E-cadherin, and DAPI-positive nuclei are indicated in blue. The white dotted line indicates the basement membrane. Scale bars represent 16.25 microns, 60x original magnification. D) QRT-PCR identifies bladder AMP mRNA induction during experimental UTI. * p < 0.05, compared to uninfected, Mann-Whitney U test, n=4 bladders/time point. E) RegIIIβ and RegIIIγ proteins (green) localize to the urothelium during urinary tract infection. Scale bars represent 16.25 microns (RegIIIβ) and 25 microns (RegIIIγ), 60x and 40x original magnification, respectively.
Next, we quantified transcript levels of putative IL-6/Stat3 target genes encoding AMPs in bladder RNA extracts. RegIIIγ, RegIIIβ, Lcn2, and Hamp mRNA levels were significantly increased within 6 to 16 hpi, coinciding with peak bladder pStat3 levels (Figure 1D). RegIIIγ and RegIIIβ localized to infected urothelium (Figure 1E).45 Thus, experimental UTI initiates a temporal sequence of urinary IL-6 secretion, urothelial Stat3 phosphorylation with nuclear localization, and AMP transcription.
Stat3 phosphorylation and AMP production are IL-6 dependent
We tested the role of IL-6 in Stat3 phosphorylation and AMP expression by performing experimental UTI in IL-6 knockout (KO) mice. We observed significant reduction in p-Stat3 levels in IL-6 KO bladders 6 hpi compared to wild type (WT) controls, whereas total Stat3 expression was unchanged (Figure 2A). Similarly, urothelial p-Stat3 immunoreactivity was absent in IL-6 KO bladders at 6 hpi (Figure 2B). Since IL-6 is required for urothelial Stat3 phosphorylation, we predicted that IL-6 deficiency leads to reduced expression levels of putative Stat3 transcriptional targets. Accordingly, we investigated expression of Stat3 target genes encoding AMPs in uninfected and infected bladders from IL-6 KO and WT animals. Uninfected IL-6 KO and WT bladders expressed comparable AMP mRNA levels (data not shown). At 6hpi, however, IL-6 KO bladders expressed significantly lower RegIIIγ, RegIIIβ, and Hamp mRNA levels compared to WT (Figure 2C). Another putative IL-6/Stat3 transcriptional target, Lcn2, trended lower in IL-6 KO mice but did not reach statistical significance. We confirmed reduced urinary RegIIIγ protein levels in IL-6 KO mice by ELISA (Figure 2D). Thus, IL-6 is necessary for urothelial Stat3 phosphorylation and AMP transcription during UTI.
Figure 2. IL-6 is necessary for urothelial Stat3 phosphorylation and AMP mRNA production following UTI.
A) Western blot detects attenuated p-Stat3 induction 6hpi in IL-6 KO bladders compared to C57BL/6J controls. Total Stat3 is unchanged in both strains. B) Immunofluorescence demonstrates the absence of p-Stat3 reactivity in IL-6 KO urothelium 6 hpi, compared to C57BL/6J controls. Positive p-Stat3 reactivity is marked by pink nuclear staining and highlighted by arrows. Green: E-cadherin; Blue: nuclei; scale bars represent 25 microns, 40x original magnification. C) Impaired expression of RegIIIβ, RegIIIγ, and Hamp transcripts in IL-6 KO bladders 6 hpi compared to WT C57BL/6J mice; * p = 0.0379 and # p = 0.0002 Mann-Whitney U test, n=8 mice/group. D) Reduced RegIIIγ/Cr levels in IL-6 KO versus WT C57BL/6J urine 24 hpi. #p = 0.0286, Mann-Whitney U test, n = 6 mice/group.
IL-6 drives Stat3 phosphorylation and AMP expression
Next, we tested the direct effect of IL-6 on Stat3 phosphorylation in cultured urothelial cells. We identified brisk induction of p-Stat3 in primary human urothelial cells following IL-6 exposure (Figure 3A), and observed similar responses in transformed human and murine urothelial lines (Figure S1A). In contrast, we observed little or no IL-6 and p-Stat3 induction following UPEC incubation in these same cell lines (Figure S1B). Next, we investigated whether IL-6 administration is sufficient to drive Stat3 phosphorylation and AMP mRNA expression in vivo in the absence of UTI. Intraperitoneal injection of recombinant, murine IL-6 led to p-Stat3 in bladders and kidneys (Figure 3B). In addition, there was significant induction of Lcn2, RegIIIβ, and RegIIIγ AMP mRNA levels, though not to the magnitude observed during experimental UTI (Figure 3C). Thus, systemic IL-6 administration in the absence of UTI is sufficient to induce bladder p-Stat3 and AMP mRNA expression.
Figure 3. IL-6 drives urothelial Stat3 phosphorylation and AMP production in the absence of UTI.
A) Primary human urothelial cells exhibit p-Stat3 induction in response to recombinant human IL-6 treatment. B) Intraperitoneal injection of 100 ng recombinant murine IL-6 induces p-Stat3 in WT bladders and kidneys within 1 hour as compared to animals injected with PBS carrier alone. C) IL-6 treatment induces Lcn2, RegIIIβ, and RegIIIγ mRNA in the absence of UTI. Animals underwent intraperitoneal injection of IL-6 or carrier, and were euthanized 4 hours later. *p = 0.0023 and #p = 0.007, Mann-Whitney U test. Data shown represent 4 mice/condition from one of two independent experiments.
Impaired bladder AMP mRNA expression in Stat3 knockout mice
To directly test the role of Stat3 in AMP mRNA expression, we performed experimental UTI in Stat3 conditional knockout mice. To delete Stat3 throughout the urothelium, we made use of UBC-CreERT2; Stat3fl/fl mice (Stat3Δ mice), in which expression of a tamoxifen-inducible Cre recombinase is driven by the constitutive UBC promoter.46 Tamoxifen-treated Cre(−);Stat3fl/fl littermates were used as controls (Stat3flox mice). We confirmed Cre-mediated recombination following tamoxifen administration by RT-PCR (Figure 4A) based on detection of a truncated Stat3 transcript.47 Western blotting demonstrated markedly reduced p-Stat3 and total Stat3 protein levels in UPEC infected Stat3Δ bladders (Figure 4B). Loss of Stat3 led to significant reduction in Hamp, RegIIIγ, and RegIIIβ mRNA levels in Stat3Δ bladders 6 hpi (Figure 4C) and undetectable RegIIIγ protein in Stat3Δ urine 6hpi (Figure 4D). Thus, Stat3 is required for AMP mRNA expression during experimental UTI.
Figure 4. AMP expression is significantly reduced in Stat3Δ mice.
A) RT-PCR demonstrates unique detection of a truncated Stat3 mRNA in Stat3Δ bladders after tamoxifen injection. B) Reduced baseline total Stat3 protein levels as well as total Stat3 protein and pStat3 at 6 hpi in Stat3Δ and Stat3flox bladders; C) Significantly decreased Hamp, RegIIIβ, and RegIIIγ mRNA levels in Stat3Δ bladders 6 hpi versus Stat3flox controls (*p = 0.0022, Mann-Whitney U test). n=6 mice/condition from two independent experiments. D) RegIIIγ protein secretion is undetectable in Stat3Δ urine 6 hpi, compared to Stat3flox controls; n.d.= not detectable; #p = 0.03, student’s t test, n = 6 mice/group from two independent experiments.
IL-6 deficiency leads to increased bladder epithelial cell invasion and bacteriuria following experimental cystitis
To determine the consequence of IL-6 deficiency on UPEC clearance during acute cystitis, we measured bacterial burden in IL-6 KO versus WT tissues following experimental UTI. We observed significant increases in urine bacterial burden in IL-6 KO mice at 24, 48, and 72 hpi (Figure 5A). We observed a transient increase in UPEC burden recovered from IL-6 KO bladders 6 hpi that resolved by 24 hpi (Figure 5B). In contrast, IL-6 deficiency did not lead to significant alterations in renal bacterial burden, nor did we observe increased mortality in IL-6 KO as reported previously in obstructed infected mice.29
Figure 5. IL-6 deficiency leads to increased bacteriuria and IBC formation.
A) Higher magnitude of UPEC recovered from IL-6 KO urine compared to WT C57BL/6J controls. *p=0.0021; **p=0.0440; #p=0.0045, Mann-Whitney U test. B) Transient increase in bladder UPEC burden 6 hpi in IL-6 KO (#p = 0.038, Mann-Whitney U test), that was not subsequently sustained. C) Increased IBC in IL-6 KO bladders 6 and 16 hpi compared to WT C57BL/6J controls, that normalizes by 24 hpi. *p = 0.0043; **p=0.0286, Mann-Whitney U test. D) This increased IBC phenotype in IL-6 KO bladders is diminished in the setting of recombinant IL-6 protein given intravesically 1 hour before infection and again 2 hpi prior to mice being sacrificed at 6hpi. *p=0.0317, Mann-Whitney U test. E) There is a similar increase in IBC inStat3Δ bladders compared to control Stat3flox bladders. *p=0.0216, Mann-Whitney U test.
To clarify the early difference in bladder colonization between IL-6 KO and WT controls, we enumerated intracellular bacterial communities (IBC). IBC number increased significantly in IL-6 KO at 6 and 16 hpi, normalizing by 24 hpi (Figure 5C). The increase in IBC number 6 hpi was reversed by intravesical delivery of recombinant murine IL-6 (Figure 5D). We also observed increased IBC numbers in Stat3Δ mice 6 hpi (Figure 5E), consistent with a role for IL-6/Stat3 signaling in regulating intracellular UPEC in vivo.
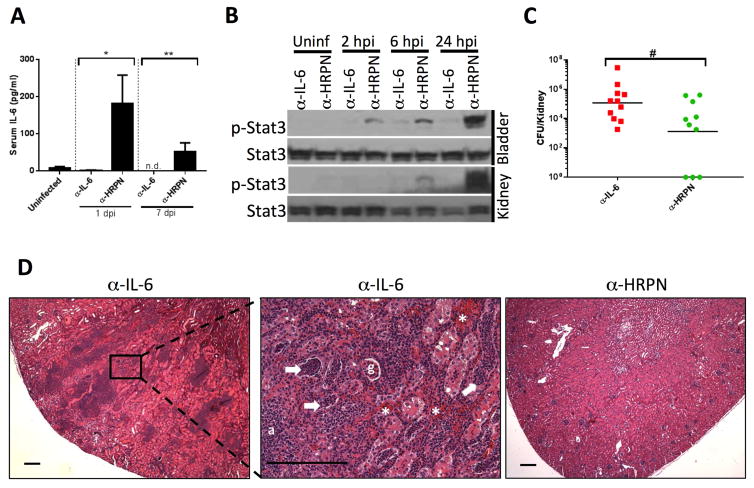
IL-6 neutralization leads to increased renal bacterial burden and inflammation
We reasoned that the genetic background of IL-6 KO mice (C57BL/6J) limits the proclivity of these animals to acute pyelonephritis. Therefore, we evaluated the impact of IL-6 neutralization on pyelonephritis susceptibility in C3H/HeOuJ mice, which develop pyelonephritis at a high frequency after transurethral inoculation of UPEC despite an intact Tlr4 locus.27, 48 Systemic delivery of an IL-6 neutralizing antibody (α–IL-6) led to reduced IL-6 levels and decreased bladder and kidney p-Stat3 in response to UPEC, compared to animals treated with isotype control antibody (α-HRPN; Figure 6A and 6B). IL-6 neutralization led to increased renal bacterial burden 7 days post infection (dpi; Figure 6C), accompanied by renal abscess formation, neutrophil casts, and interstitial hemorrhage (Figure 6D).
Figure 6. IL-6 neutralization in C3H/HeOuJ mice leads to severe pyelonephritis.
A) Reduction in serum IL-6 levels induced by UPEC in C3H/HeOuJ mice pretreated with α-IL-6 neutralizing antibody 24 hours before infection versus isotype control antibody (α-HRPN). *p<0.05, Kruskal-Wallis test. n=8 mice/group. B) Reduced bladder and kidney p-Stat3 induction by UPEC in C3H/HeOuJ mice treated with α-IL-6 neutralizing antibody versus isotype control (α-HRPN), at specified times after inoculation. C) Increased renal bacterial burden in mice treated with α-IL-6 neutralizing antibody versus α-HRPN 7 days post UPEC infection. #p=0.036, Mann-Whitney U test. D) H&E staining of a representative kidney following α-IL-6 neutralizing antibody treatment (left) demonstrating extensive cortical inflammation 7 dpi. The middle micrograph shows higher power magnification of the area depicted in the inset with areas of neutrophilic infiltrate, neutrophil casts in tubules (white arrows), hemorrhage (white asterisk), and abscess (a) formation. An unaffected glomerulus (g) is visible. An example of a representative kidney following α-HRPN treatment is shown for comparison (right) which demonstrates normal renal architecture. Scale bars represent 200 microns, 4x and 20x original magnification.
Stat3 phosphorylation and AMP production during UTI requires intact Tlr4 signaling
Given the requirement for Tlr4 signaling in IL-6 secretion during cystitis,13, 16, 18 we hypothesized that Stat3 phosphorylation and AMP mRNA expression display Tlr4 dependence. In order to evaluate this, we compared IL-6 production, p-Stat3, and AMP mRNA levels in infected C3H/HeOuJ (Lps-sensitive) and C3H/HeJ (Lps-resistant) mice during experimental UTI. UPEC infection did not elicit urinary or systemic IL-6 secretion in C3H/HeJ animals (Figure 7A). In addition, p-Stat3 levels were attenuated in infected C3H/HeJ bladders and kidneys compared to C3H/HeOuJ controls (Figure 7B), whereas total Stat3 protein levels were unchanged. While AMP mRNA levels did not differ at baseline between the strains in the absence of UPEC infection (data not shown), putative Stat3 transcriptional target AMPs RegIIIγ, RegIIIβ, Lcn2, Hamp, and Camp were reduced in C3H/HeJ bladders 24 hpi (Figure 7C). Thus, Tlr4 signaling regulates Stat3 phosphorylation and mRNA expression of AMPs during UTI.
Figure 7. IL-6 production, Stat3 phosphorylation, and AMP production are Tlr4-dependent.
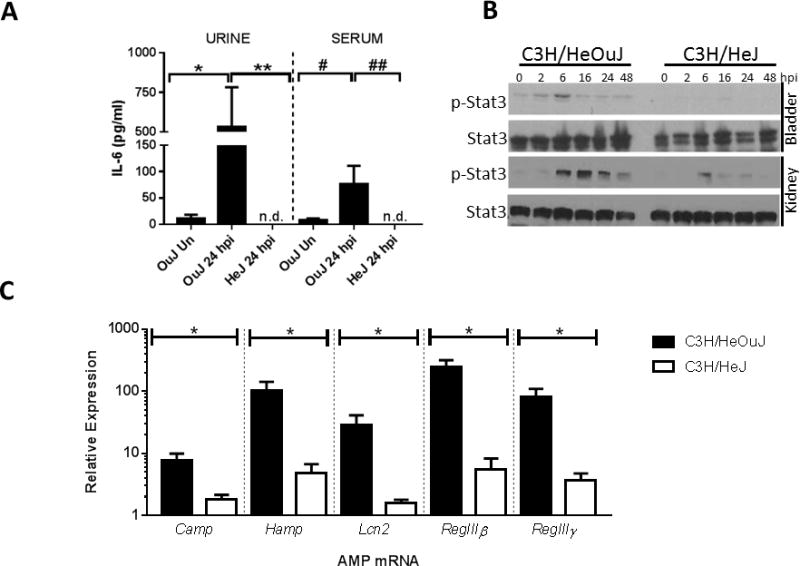
A) UPEC inoculation in LPS-responsive C3H/HeOuJ mice results in urine IL-6 secretion and accumulation of serum IL-6 24 hpi compared to uninfected (Un) controls. Urine and serum IL-6 was not detected (n.d.) in LPS resistant C3H/HeJ mice at the same time point (*p = 0.0046; **p=0.0015, #p=0.034, ##p=0.001, one-way ANOVA, n ≥ 6 mice/group [urine] or 4 mice/group [serum]). B) Western blotting indicates UPEC induced p-Stat3 expression in C3H/HeOuJ bladders and kidneys to a greater extent than that of C3H/HeJ animals. C) Reduced bladder mRNA levels of AMPs in C3H/HeJ bladders compared to C3H/HeOuJ mice 24 hpi (*p<0.01; n=5 mice/group).
Discussion
While IL-6 secretion has been associated with clinical and experimental UTI for decades,16, 27, 28 the functional significance and mechanistic action of IL-6 during acute cystitis have remained elusive. Our data support a model in which UPEC signals through Tlr4 to elicit IL-6 production, which triggers urothelial Stat3 phosphorylation and AMP expression. Of note, we identify a novel role for IL-6/Stat3 signaling in urothelial susceptibility to IBC formation, demonstrating that deletion of IL-6 or Stat3 leads to increased number of IBCs. In addition, neutralization of IL-6 leads to increased renal bacterial burden and severe inflammation. These findings have implications for our understanding of UTI pathogenesis and the roles of IL-6/Stat3 signaling in the host innate immune response to UTI.
Tlr4-dependent IL-6 secretion links UPEC infection to Stat3 phosphorylation and AMP expression
In this study, we found that Stat3 phosphorylation and AMP mRNA induction during cystitis depend on intact Tlr4 signaling. We were unable to elicit robust Stat3 phosphorylation in urothelial cells treated with live UPEC or Lps alone (Figure S1A and data not shown), however, arguing that Stat3 phosphorylation does not arise as a direct consequence of Tlr4 signaling. Rather, we demonstrate that IL-6 serves a critical role in triggering urothelial Stat3 phosphorylation and AMP mRNA expression during cystitis. Previous work has established that secretion of IL-6 by bladder urothelial cells is Tlr4-dependent, since inhibition of Lps or Tlr4 signaling results in impaired IL-6 production following E. Coli treatment.18 While UPEC strains are capable of suppressing IL-6 production by urothelial cell lines in vitro,19, 20 our in vivo data are consistent with a model in which bacterial endotoxin signals through Tlr4 to activate IL-6 secretion, leading to Stat3 phosphorylation and AMP transcription. In this manner, IL-6 secretion may serve to amplify the host response to UPEC by triggering antimicrobial defense mechanisms in surrounding urothelial cells.
IL-6/Stat3 regulates IBC number with implications for UPEC virulence
The importance of IL-6/Stat3 signaling is highlighted by our finding of increased IBC numbers in IL-6 KO and Stat3 KO bladders, along with the demonstration that intravesical IL-6 administration reduces IBC number in IL-6 KO bladders. IL-6 elicitation by UPEC treated urothelial cells has been previously shown to vary inversely with virulence, suggesting that UPEC fitness in the urinary tract may be achieved in part by blunting the initial burst of IL-6 production.21 Our findings support this concept, as a reduction in IL-6 secretion should limit Stat3 phosphorylation by urothelial cells – favoring UPEC invasion and increased IBC formation, as observed in IL-6 KO and Stat3 KO mice. These findings parallel the observations of Schilling and coworkers, who demonstrated that Tlr4-hyporesponsive C3H/HeJ mice had large foci of intracellular bacteria in the absence of a normal inflammatory response.18 Together, these findings are consistent with a model in which Tlr4/IL-6/Stat3-dependent signaling limits IBC formation in the urothelium. To our knowledge, this pathway encompasses the only known host genes implicated in controlling IBC number.
Impact of the IBC bottleneck on ascending infection and chronic UTI
IBC formation represents a significant population bottleneck in the UPEC life cycle during cystitis, with only 0.01-0.001% of the initial UPEC inoculum contributing to IBC formation.49 Thus, mechanisms that facilitate increased IBC formation are associated with higher UPEC virulence, leading to UTI chronicity and upper tract infection.49 Indeed, Schwartz and coworkers demonstrated that mice that developed persistent bacteriuria and chronic cystitis had increased bacterial diversity reflecting increased IBC formation during acute infection compared to those mice able to clear infection two weeks after inoculation.49 We hypothesize that increased IBC numbers - arising from host deficiency in IL-6 or Stat3 or via UPEC suppression of IL-6 production - effectively widen the population bottleneck, increasing UPEC diversity and heightening host susceptibility to chronic UTI and ascending infection.50
Functional significance of AMP production during UTI
We identify AMP production as one antibacterial mechanism impacted by IL-6 and Stat3 deficiency. In particular, this study identified three AMPs as transcriptional targets of IL-6/Stat3 signaling during UTI: Hepcidin, RegIIIβ, and RegIIIγ. Hepcidin, encoded by the Hamp gene, exhibits bacteriostatic activity toward E. coli, and its deficiency leads to increased renal UPEC burden, but the consequence on IBC formation was not evaluated in this study.51 RegIIIβ exhibits bactericidal activity against Gram-negative bacteria and is implicated in clearance of salmonellosis in vivo,52 but its function has not yet been evaluated during UTI. In contrast, RegIIIγ antimicrobial activity is restricted to Gram-positive organisms, and its deficiency did not impact UPEC burden, though IBC number was not evaluated. Further studies are required to determine if higher IBC numbers in infected IL-6 and Stat3 KO mice arise as a consequence of reduced AMP production. While it is conceivable that a collective deficiency in AMP production favors increased IBC formation, it is equally plausible that other downstream targets of IL-6/Stat3 signaling account for this phenotype.
Conclusions
We have implicated IL-6/Stat3 signaling in urothelial AMP production and inhibition of IBC formation during UTI. Based on our observations, we speculate that genetic or acquired deficiencies in IL-6/Stat3 signaling may lead to increased susceptibility to recurrent UTI and pyelonephritis due to alterations in IBC formation and AMP production. In this regard, it is noteworthy that genitourinary infections are a recognized side effect of tocilizumab, a humanized monoclonal antibody directed toward IL-6.53 Conversely, strategies that trigger IL-6/Stat3 signaling may promote AMP production, limit IBC formation, and hasten UTI resolution.
Methods
Mice and Genotyping
Experiments were conducted with the approval of our Institutional Laboratory Animal Care and Use Committee (Welfare Assurance Number A3544-01), Protocol AR13-00057. All mice were purchased from Jackson Laboratories (Bar Harbor, ME). Except for the C3H/HeOuJ and C3H/HeJ strains, all animals were on a pure C57BL/6J background. Animals were PCR genotyped according to the company website. To confirm Cre/LoxP recombination in Stat3Δ mice, we performed RT-PCR to detect the truncated Stat3 mRNA in which exons 18–20 are deleted, using primers 5′-TGGGCATCAATCCTGTGGTAT-3′ and 5′-GCTGCTGCTTGGTGTATGGCTCTA-3′, which generate a 514 bp product (flox) or 226 bp product (Δ).
Experimental UTI
One week prior to experimental UTI, Stat3Δ and Stat3flox mice received three 100 μl intraperitoneal injections of tamoxifen (Sigma, 20 mg/kg in corn oil); each injection was spaced 48 hours apart. Experimental UTI was induced in female mice age 6–8 weeks with UPEC strain CFT073, isolated from the blood and urine of a patient with pyelonephritis.54 CFT073 was inoculated from a glycerol stock and grown statically in LB medium for 16 hours at 37°C. The inoculum was 108 colony forming units (CFU) in 50 μl phosphate buffered saline (PBS). After infection, tissue was isolated and bacterial burden assessed.55, 56 We followed a published protocol for IBC enumeration.49
IL-6 Administration and Neutralization
For systemic administration in the absence of UTI, 100 ng murine IL-6 (Peprotech, Rocky Hill, NJ) or PBS/0.1% bovine serum albumin carrier was injected intraperitoneally in 100 μl total volume. For local administration during UTI, 100 ng IL-6 or carrier was injected intravesically in 50 μl one hour before UPEC inoculation and repeated 2 hours after inoculation. For neutralization, female C3H/HeOuJ mice received an intraperitoneal injection of 1 mg anti-mouse IL-6 (BioXCell, clone MP5-20F3, in 200 μl) or rat IgG1 isotype control (BioXCell, clone HRPN) 24 hours prior to UPEC infection. The injection was repeated 1, 3, and 6 dpi.
ELISA
Commercial ELISAs detected IL-6 (R&D Systems, Minneapolis, MN) and RegIIIγ (Cloud-Clone Corporation, Katy, TX). Urine IL-6 and RegIIIγ levels were normalized to urine creatinine (Cr) in a colorimetric assay (Oxford Biomedical Research, Rochester Hills, MI).
RNA Extraction and QRT-PCR
RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA). Quantitative (q)RT-PCR was performed using gene-specific primer pairs (Table 1) and SybrGreen.45, 57 Relative expression changes were calculated by the 2^-ddCT method, normalizing to a pool of uninfected, wild-type, bladder or kidney cDNA.58
Table 1.
QRT-PCR Primers
| Gene | Forward | Reverse |
|---|---|---|
| Camp | 5′-TTCAAGGAACAGGGGGTGG-3′ | 5′-AGGCTCGTTACAGCTGATGTC-3′ |
| Defb14 | 5′-CATTCCTACCAAAAACCCTC-3′ | 5′-CTTCTACTTCTTCTTTCGGC-3′ |
| Gapdh | 5′-CTGGAGAAACCTGCCAAGTA-3′ | 5′-TGTTGCTGTAGCCGTATTCA-3′ |
| Hamp | 5′-GAGCAGCACCACCTATCTCC-3′ | 5′-TTGGTATCGCAATGTCTGCC-3′ |
| Lcn2 | 5′-ATATGCACAGGTATCCTCAG-3′ | 5′-GAAACGTTCCTTCAGTTCAG-3′ |
| RegIII β | 5′-GTCAAGAGCTTCTGGATTTC-3′ | 5′-GCTTTTTCTAGGTGAGTCTTC-3′ |
| RegIIIγ | 5′-GACAAGATGCTTCCCCGTAT-3′ | 5′-GGCATCTTTCTTGGCAACTT-3′ |
Western Blotting and Immunostaining
Protein extraction, western blotting, and immunostaining were performed as described. Primary antibodies were purchased from Cell Signaling (Beverly, MA) except for rabbit α-RegIIIγ (Dr. Lora Hooper, University of Texas at Southwestern) and sheep α-RegIIIβ (R&D systems). Primary antibodies were detected with AlexaFluor 488- or AlexaFluor 594-conjugated secondary antibodies raised in donkey (Jackson Immunoresearch, West Grove, PA). Negative controls sections were incubated with irrelevant species-specific antibody or secondary antibody alone.
Primary Urothelial Cells and Cell Lines
Human primary bladder epithelial cells (ATCC, Manassas, VA) were grown in serum-free, prostate epithelial cell basal medium with supplements. Human 5637 cells were purchased from ATCC and cultured in RPMI with L-glutamine and 10% fetal bovine serum. MBT-2 and MB-49 cells (Dr. Timothy Ratliff, Purdue University) were cultured in DMEM with 10% fetal bovine serum, 4.5 g/L glucose, 4 mM L-glutamine, and 1 mM sodium pyruvate. Cells were serum-starved 24 hours before experiments. Recombinant IL-6 (R&D Systems) was added to a final concentration of 25 ng/ml. For UPEC stimulation, overnight static cultures of CFT073 were diluted based on OD600 to multiplicity of infection of 20.
Statistical Analysis
Mann-Whitney U test compared bacterial burden, IL-6 and RegIIIγ protein levels, and relative mRNA expression levels (Graph Pad, La Jolla, CA). For comparisons involving more than two groups, the Kruskal-Wallis test was used. p values of < 0.05 were considered significant.
Supplementary Material
A) Cells were stimulated with IL-6 or UPEC for the indicated times, and protein expression was measured by western blotting. IL-6 led to brisk Stat3 phosphorylation, but UPEC did not induce significant p-Stat3 levels. B) IL-6 levels were measured by ELISA at the indicated times following UPEC exposure. Each data point indicates the mean ± standard deviation of triplicate IL-6 values. UPEC treatment resulted in negligible increase from baseline IL-6 secretion. Time “0” indicates baseline IL-6 secretion by each cell line in the absence of UPEC.
Acknowledgments
Sources of support: NIDDK 5K08DK102594 (B.B.), Urology Care Foundation Research Scholar Award Program and the American Urological Association North Central Section Research Scholar Fund I (C.C.), and a grant from the Society for Pediatric Urology (C.C.)
We thank Dr. Lora Hooper (UT Southwestern) for providing α-RegIIIγ antisera. We thank Dr. Timothy Ratliff (Purdue University) for provision of MB-49 and MBT-2 urothelial cells. This work was supported by NIDDK 5K08DK102594 (B.B.), the Urology Care Foundation Research Scholar Award and the American Urological Association North Central Section Research Scholar Fund I (C.C.), and a grant from the Society for Pediatric Urology (C.C.). We thank Dr. John David Spencer (Nationwide Children’s Hospital) and Dr. Annelie Brauner (Karolinska Institute, Stockholm, Sweden) for critical review of the manuscript.
Footnotes
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Copp HL, Shapiro DJ, Hersh AL. National ambulatory antibiotic prescribing patterns for pediatric urinary tract infection, 1998–2007. Pediatrics. 2011;127:1027–1033. doi: 10.1542/peds.2010-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman AL. Urologic diseases in North America Project: trends in resource utilization for urinary tract infections in children. J Urol. 2005;173:949–954. doi: 10.1097/01.ju.0000152092.03931.9a. [DOI] [PubMed] [Google Scholar]
- 3.Spencer JD, Schwaderer A, McHugh K, et al. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol. 2010;25:2469–2475. doi: 10.1007/s00467-010-1625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLellan LK, Hunstad DA. Urinary Tract Infection: Pathogenesis and Outlook. Trends Mol Med. 2016;22:946–957. doi: 10.1016/j.molmed.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barber AE, Norton JP, Spivak AM, et al. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719–724. doi: 10.1093/cid/cit284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godaly G, Ambite I, Puthia M, et al. Urinary Tract Infection Molecular Mechanisms and Clinical Translation. Pathogens. 2016:5. doi: 10.3390/pathogens5010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spencer JD, Schwaderer AL, Becknell B, et al. The innate immune response during urinary tract infection and pyelonephritis. Pediatr Nephrol. 2014;29:1139–1149. doi: 10.1007/s00467-013-2513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luthje P, Brauner H, Ramos NL, et al. Estrogen supports urothelial defense mechanisms. Sci Transl Med. 2013;5:190ra180. doi: 10.1126/scitranslmed.3005574. [DOI] [PubMed] [Google Scholar]
- 9.Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr Opin Microbiol. 1999;2:99–105. doi: 10.1016/s1369-5274(99)80017-4. [DOI] [PubMed] [Google Scholar]
- 10.Agace WW, Hedges SR, Ceska M, et al. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest. 1993;92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godaly G, Hang L, Frendeus B, et al. Transepithelial neutrophil migration is CXCR1 dependent in vitro and is defective in IL-8 receptor knockout mice. J Immunol. 2000;165:5287–5294. doi: 10.4049/jimmunol.165.9.5287. [DOI] [PubMed] [Google Scholar]
- 12.Hang L, Frendeus B, Godaly G, et al. Interleukin-8 receptor knockout mice have subepithelial neutrophil entrapment and renal scarring following acute pyelonephritis. J Infect Dis. 2000;182:1738–1748. doi: 10.1086/317599. [DOI] [PubMed] [Google Scholar]
- 13.Schilling JD, Martin SM, Hunstad DA, et al. CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect Immun. 2003;71:1470–1480. doi: 10.1128/IAI.71.3.1470-1480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedges S, Agace W, Svensson M, et al. Uroepithelial cells are part of a mucosal cytokine network. Infect Immun. 1994;62:2315–2321. doi: 10.1128/iai.62.6.2315-2321.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuelsson P, Hang L, Wullt B, et al. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect Immun. 2004;72:3179–3186. doi: 10.1128/IAI.72.6.3179-3186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Man P, van Kooten C, Aarden L, et al. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect Immun. 1989;57:3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wullt B, Bergsten G, Connell H, et al. P-fimbriae trigger mucosal responses to Escherichia coli in the human urinary tract. Cell Microbiol. 2001;3:255–264. doi: 10.1046/j.1462-5822.2001.00111.x. [DOI] [PubMed] [Google Scholar]
- 18.Schilling JD, Mulvey MA, Vincent CD, et al. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166:1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- 19.Hunstad DA, Justice SS, Hung CS, et al. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect Immun. 2005;73:3999–4006. doi: 10.1128/IAI.73.7.3999-4006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilbert DW, Pascal KE, Libby EK, et al. Uropathogenic Escherichia coli dominantly suppress the innate immune response of bladder epithelial cells by a lipopolysaccharide- and Toll-like receptor 4-independent pathway. Microbes Infect. 2008;10:114–121. doi: 10.1016/j.micinf.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Storm DW, Patel AS, Horvath DJ, Jr, et al. Relationship among bacterial virulence, bladder dysfunction, vesicoureteral reflux and patterns of urinary tract infection in children. J Urol. 2012;188:236–241. doi: 10.1016/j.juro.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Billips BK, Schaeffer AJ, Klumpp DJ. Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder. Infect Immun. 2008;76:3891–3900. doi: 10.1128/IAI.00069-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tramma D, Hatzistylianou M, Gerasimou G, et al. Interleukin-6 and interleukin-8 levels in the urine of children with renal scarring. Pediatr Nephrol. 2012;27:1525–1530. doi: 10.1007/s00467-012-2156-2. [DOI] [PubMed] [Google Scholar]
- 24.Sheu JN, Chen MC, Chen SM, et al. Relationship between serum and urine interleukin-6 elevations and renal scarring in children with acute pyelonephritis. Scand J Urol Nephrol. 2009;43:133–137. doi: 10.1080/00365590802478742. [DOI] [PubMed] [Google Scholar]
- 25.Sheu JN, Chen MC, Lue KH, et al. Serum and urine levels of interleukin-6 and interleukin-8 in children with acute pyelonephritis. Cytokine. 2006;36:276–282. doi: 10.1016/j.cyto.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez LM, Robles B, Marugan JM, et al. Urinary interleukin-6 is useful in distinguishing between upper and lower urinary tract infections. Pediatr Nephrol. 2008;23:429–433. doi: 10.1007/s00467-007-0670-4. [DOI] [PubMed] [Google Scholar]
- 27.Hannan TJ, Mysorekar IU, Hung CS, et al. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz DJ, Conover MS, Hannan TJ, et al. Uropathogenic Escherichia coli superinfection enhances the severity of mouse bladder infection. PLoS Pathog. 2015;11:e1004599. doi: 10.1371/journal.ppat.1004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalil A, Tullus K, Bartfai T, et al. Renal cytokine responses in acute Escherichia coli pyelonephritis in IL-6-deficient mice. Clin Exp Immunol. 2000;122:200–206. doi: 10.1046/j.1365-2249.2000.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin- 6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 32.Backert I, Koralov SB, Wirtz S, et al. STAT3 activation in Th17 and Th22 cells controls IL-22-mediated epithelial host defense during infectious colitis. J Immunol. 2014;193:3779–3791. doi: 10.4049/jimmunol.1303076. [DOI] [PubMed] [Google Scholar]
- 33.Choi SM, McAleer JP, Zheng M, et al. Innate Stat3-mediated induction of the antimicrobial protein Reg3gamma is required for host defense against MRSA pneumonia. J Exp Med. 2013;210:551–561. doi: 10.1084/jem.20120260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steward-Tharp SM, Laurence A, Kanno Y, et al. A mouse model of HIES reveals pro- and anti-inflammatory functions of STAT3. Blood. 2014;123:2978–2987. doi: 10.1182/blood-2013-09-523167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wittkopf N, Pickert G, Billmeier U, et al. Activation of intestinal epithelial Stat3 orchestrates tissue defense during gastrointestinal infection. PLoS One. 2015;10:e0118401. doi: 10.1371/journal.pone.0118401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali AS, Townes CL, Hall J, et al. Maintaining a sterile urinary tract: the role of antimicrobial peptides. J Urol. 2009;182:21–28. doi: 10.1016/j.juro.2009.02.124. [DOI] [PubMed] [Google Scholar]
- 37.Becknell B, Schwaderer A, Hains DS, et al. Amplifying renal immunity: the role of antimicrobial peptides in pyelonephritis. Nat Rev Nephrol. 2015;11:642–655. doi: 10.1038/nrneph.2015.105. [DOI] [PubMed] [Google Scholar]
- 38.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol. 2007;18:2810–2816. doi: 10.1681/ASN.2007050611. [DOI] [PubMed] [Google Scholar]
- 39.Ratsimandresy RA, Indramohan M, Dorfleutner A, et al. The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway. Cell Mol Immunol. 2017;14:127–142. doi: 10.1038/cmi.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu MJ, Feng D, Wu H, et al. Liver is the major source of elevated serum lipocalin-2 levels after bacterial infection or partial hepatectomy: a critical role for IL-6/STAT3. Hepatology. 2015;61:692–702. doi: 10.1002/hep.27447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietrangelo A, Dierssen U, Valli L, et al. STAT3 is required for IL-6-gp130- dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 42.Verga Falzacappa MV, Vujic Spasic M, Kessler R, et al. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 43.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miraglia E, Nylen F, Johansson K, et al. Entinostat up-regulates the CAMP gene encoding LL-37 via activation of STAT3 and HIF-1alpha transcription factors. Sci Rep. 2016;6:33274. doi: 10.1038/srep33274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spencer JD, Jackson AR, Li B, et al. Expression and Significance of the HIP/PAP and RegIIIgamma Antimicrobial Peptides during Mammalian Urinary Tract Infection. PLoS One. 2015;10:e0144024. doi: 10.1371/journal.pone.0144024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruzankina Y, Pinzon-Guzman C, Asare A, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moh A, Iwamoto Y, Chai GX, et al. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest. 2007;87:1018–1028. doi: 10.1038/labinvest.3700630. [DOI] [PubMed] [Google Scholar]
- 48.Hopkins W, Gendron-Fitzpatrick A, McCarthy DO, et al. Lipopolysaccharide-responder and nonresponder C3H mouse strains are equally susceptible to an induced Escherichia coli urinary tract infection. Infect Immun. 1996;64:1369–1372. doi: 10.1128/iai.64.4.1369-1372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz DJ, Chen SL, Hultgren SJ, et al. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun. 2011;79:4250–4259. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walters MS, Lane MC, Vigil PD, et al. Kinetics of uropathogenic Escherichia coli metapopulation movement during urinary tract infection. MBio. 2012:3. doi: 10.1128/mBio.00303-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Houamel D, Ducrot N, Lefebvre T, et al. Hepcidin as a Major Component of Renal Antibacterial Defenses against Uropathogenic Escherichia coli. J Am Soc Nephrol. 2016;27:835–846. doi: 10.1681/ASN.2014101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Ampting MT, Loonen LM, Schonewille AJ, et al. Intestinally secreted C-type lectin Reg3b attenuates salmonellosis but not listeriosis in mice. Infect Immun. 2012;80:1115–1120. doi: 10.1128/IAI.06165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yun H, Xie F, Delzell E, et al. Comparative Risk of Hospitalized Infection Associated With Biologic Agents in Rheumatoid Arthritis Patients Enrolled in Medicare. Arthritis Rheumatol. 2016;68:56–66. doi: 10.1002/art.39399. [DOI] [PubMed] [Google Scholar]
- 54.Mobley HL, Green DM, Trifillis AL, et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Becknell B, Eichler TE, Beceiro S, et al. Ribonucleases 6 and 7 have antimicrobial function in the human and murine urinary tract. Kidney Int. 2015;87:151–161. doi: 10.1038/ki.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li B, Haridas B, Jackson AR, et al. Inflammation drives renal scarring in experimental pyelonephritis. Am J Physiol Renal Physiol. 2017;312:F43–F53. doi: 10.1152/ajprenal.00471.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Cells were stimulated with IL-6 or UPEC for the indicated times, and protein expression was measured by western blotting. IL-6 led to brisk Stat3 phosphorylation, but UPEC did not induce significant p-Stat3 levels. B) IL-6 levels were measured by ELISA at the indicated times following UPEC exposure. Each data point indicates the mean ± standard deviation of triplicate IL-6 values. UPEC treatment resulted in negligible increase from baseline IL-6 secretion. Time “0” indicates baseline IL-6 secretion by each cell line in the absence of UPEC.