Abstract
We aim to determine the incidence of early myocardial dysfunction after out-of-hospital cardiac arrest, risk factors associated with its development, and association with outcome. A retrospective chart review was performed among consecutive out-of-hospital cardiac arrest (OHCA) patients who underwent echocardiography within 24 h of return of spontaneous circulation at three urban teaching hospitals. Our primary outcome is early myocardial dysfunction, defined as a left ventricular ejection fraction < 40% on initial echocardiogram. We also determine risk factors associated with myocardial dysfunction using multivariate analysis, and examine its association with survival and neurologic outcome. A total of 190 patients achieved ROSC and underwent echocardiography within 24 h. Of these, 83 (44%) patients had myocardial dysfunction. A total of 37 (45%) patients with myocardial dysfunction survived to discharge, 39% with intact neurologic status. History of congestive heart failure (OR 6.21; 95% CI 2.54–15.19), male gender (OR 2.27; 95% CI 1.08–4.78), witnessed arrest (OR 4.20; 95% CI 1.78–9.93), more than three doses of epinephrine (OR 6.10; 95% CI 1.12–33.14), more than four defibrillations (OR 4.7; 95% CI 1.35–16.43), longer duration of resuscitation (OR 1.06; 95% CI 1.01–1.10), and therapeutic hypothermia (OR 3.93; 95% CI 1.32–11.75) were associated with myocardial dysfunction. Cardiopulmonary resuscitation immediately initiated by healthcare personnel was associated with lower odds of myocardial dysfunction (OR 0.40; 95% CI 0.17–0.97). There was no association between early myocardial dysfunction and mortality or neurological outcome. Nearly half of OHCA patients have myocardial dysfunction. A number of clinical factors are associated with myocardial dysfunction, and may aid providers in anticipating which patients need early diagnostic evaluation and specific treatments. Early myocardial dysfunction is not associated with neurologically intact survival.
Keywords: Cardiac arrest, Out-of-hospital, Myocardial dysfunction, Echocardiography, Neurological outcome
Introduction
Out-of-hospital cardiac arrest (OHCA) presents a considerable health burden in the United States, with an estimated incidence of 300,000 persons each year [1, 2]. Even though return of spontaneous circulation (ROSC) is achieved in 30–40% of OHCA patients, only 7% of patients receiving CPR will survive to hospital discharge, with significant geographic variability [1, 2].
Post-cardiac arrest syndrome (PCAS) is a complex constellation of multi-organ failure comprised of brain injury, systemic ischemia–reperfusion response, and post-cardiac arrest myocardial dysfunction, which contributes significantly to morbidity after cardiac arrest [2, 3]. Myocardial dysfunction is an important cause of post-resuscitation circulatory failure that may lead to early mortality after ROSC [3, 4]. It is defined as reversible global dysfunction due to stunned myocardium in the absence of coronary occlusion [5–8]. Although post-cardiac arrest myocardial dysfunction is a potentially treatable complication, the severity and duration of the myocardial dysfunction may be associated with outcome [3, 4, 7].
We aim to evaluate the incidence of post-cardiac arrest myocardial dysfunction, factors associated with its development, and its impact on outcome. Theoretically, factors that amplify ischemia–reperfusion injury should be associated with the presence and severity of post-arrest myocardial dysfunction. Therefore, we hypothesize that there would be an association between pre-hospital factors, including medical history, witnessed cardiac arrest, initial rhythm, bystander cardiopulmonary resuscitation (CPR), number of defibrillations, cumulative doses of epinephrine administrated during resuscitation, total downtime, and development of myocardial dysfunction after resuscitation.
Methods
Study design
We performed a retrospective data analysis utilizing the Penn Alliance for Therapeutic Hypothermia (PATH) database. PATH is an internet-based registry focusing on post-arrest care, which is hosted by the University of Pennsylvania.
In this study, consecutive OHCA patients treated in three urban teaching hospitals between May 2005 and December 2011 were screened for inclusion. Patients were included in this study if they were over the age of 17, suffered a non-traumatic arrest, achieved ROSC, and had a documented echocardiogram performed within 24 h of their cardiac arrest. Exclusion criteria included the initiation of extracorporeal life support or a preexisting “Do Not Resuscitate” order. Research utilizing data in PATH was approved by the University of Pennsylvania Institutional Review Board with a waiver of informed consent.
Data collection
Patient demographics, cardiac arrest characteristics, details regarding prehospital and emergency department care, as well as patient outcomes were obtained from the PATH registry [9, 10]. Each patient record in PATH consists of 30 data elements required for quality assurance purposes from all participating institutions, and 100 additional data elements are required for research participation. Data were collected retrospectively at each of the participating institutions and entered into PATH via a secure, password-protected internet portal by a trained healthcare provider. Before entering data, these clinicians are first trained by the PATH database manager using a structured approach including mock case entry and case review. All participants are provided with a standardized data dictionary for PATH data elements and frequent telephone contact is made with each data abstracter early in registry participation. Additionally, a formal auditing process is conducted on a selection of cases to ensure data integrity, with feedback given to site abstractors for correction. The database manager has access to all entered records from each participating hospital.
Additional chart review was performed at three PATH institutions to obtain echocardiogram results, which were not available in the PATH registry. Transthoracic or transesophageal echocardiogram data were abstracted from clinical echocardiography reports interpreted by attending cardiologists by a single physician-abstractor who was trained by the principal investigator prior to the study. If there was more than one echocardiogram performed within 24 h, the final one performed within the initial 24 h period was used for analysis. The principal investigator met regularly with the data abstractor to review abstracted charts and ensure data quality. The initial 30 charts were also reviewed by the principal investigator to ensure data quality and appropriate categorization. A kappa statistic was not calculated because agreement on left ventricular ejection fraction values was 100%. The abstractor was not blinded to the study hypothesis, but was blinded to patient outcomes.
The most readily available quantitative measure of myocardial function is the left ventricular ejection fraction (LVEF). According to 2010 Heart Failure Society of America guidelines, preserved LVEF is variably defined [11]. The American Heart Association (AHA) suggests that LVEF < 40% may indicate heart failure or cardiomyopathy while an LVEF between 40 and 55% implies damage [12]. Accordingly, myocardial dysfunction is defined as LVEF < 40% in this study, and is the variable of interest.
Outcome measures
Our primary outcome is post-cardiac arrest myocardial dysfunction. Secondary outcomes include survival to hospital discharge with favorable neurological outcome. Neurological outcome is measured using the cerebral performance category (CPC) score and dichotomized into favorable versus poor outcome. Favorable neurological outcome is defined as CPC score of 1 or 2 at hospital discharge.
Statistical analyses
Descriptive statistics are used to summarize subject characteristics including median and interquartile range (IQR) for continuous variables, and number (n) and percentage (%) for categorical variables. We use Wilcoxon rank-sum test for continuous variables in univariate comparison and Chi-square tests for categorical variables. Multivariate logistic regression is then completed to adjust for variables influencing LVEF. Regression results are reported as odds ratios (ORs) with 95% confidence intervals (CI). Statistical analysis was performed using STATA 11.0 software (STATA 11.0, Statacorp, College Station, TX, USA).
Results
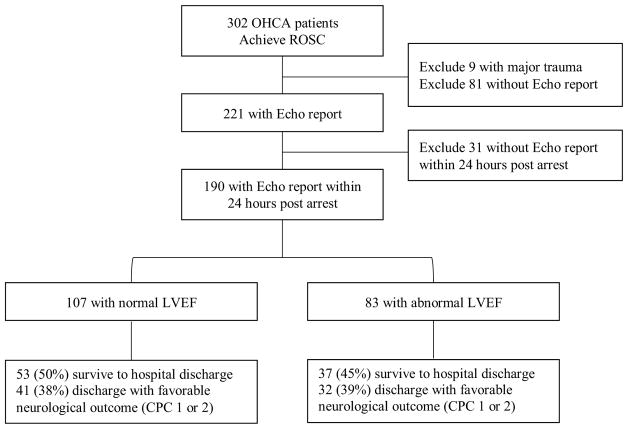
Between May 2005 and December 2011, 302 patients with OHCA successfully achieved ROSC and were transported to one of the three study hospitals. Nine patients (3%) were excluded because of major trauma as the cause of cardiac arrest (Fig.1). Median age in the cohort of patients with echocardiography performed within the study window is 60 years (IQR 48, 70) and 53% are male (Table 1). The majority of the cohort is black (56%). Most patients had a cardiac etiology for their arrests (78%) and a nonshockable rhythm (66%), and fewer than half received bystander CPR (47%) (Table 2). Echocardiography was performed in 221 patients (75%) and 190 (86%) of those patients had an echocardiogram performed within 24 after ROSC. A total of 83 patients (44%) had myocardial dysfunction within 24 h after ROSC. There is no difference in the median time from ROSC to echocardiogram when comparing patients with and without myocardial dysfunction (5.3 vs. 7.1 h, p = 0.18).
Fig. 1.
Flow diagram of patient enrollment, exclusions, and outcomes. OHCA out of hospital cardiac arrest, ROSC return of spontaneous circulation, Echo echocardiography, LVEF left ventricular ejection fraction
Table 1.
Demographic characteristics and co-morbidities
| All patients (n = 190) | LVEF < 40% (n = 83) | LVEF ≥ 40% (n = 107) | p value | |
|---|---|---|---|---|
| Gender, n (%) | 0.003 | |||
| Male | 101 (53) | 55 (65) | 46 (43) | |
| Female | 90 (47) | 29 (35) | 61 (57) | |
| Age, median (IQR), years | 60 (48, 70) | 60 (48, 70) | 60 (48, 71) | 0.811 |
| Race, n (%) | 0.22 | |||
| Black | 105 (56) | 48 (58) | 57 (54) | |
| White | 72 (38) | 31 (38) | 41 (39) | |
| Other | 11 (6) | 3 (4) | 8 (7) | |
| Preexisting conditions, n (%) | ||||
| Previous CHF | 48 (25) | 30 (36) | 18 (17) | |
| Coronary artery disease | 46 (24) | 28 (33) | 18 (17) | 0.003 |
| Previous MI | 22 (12) | 12 (14) | 10 (9) | 0.008 |
| Hypertension | 94 (49) | 35 (42) | 59 (55) | 0.288 |
| Arrhythmia | 6 (14) | 12 (14) | 14 (13) | 0.064 |
| Diabetes mellitus | 65 (34) | 24 (29) | 41 (38) | 0.81 |
| Stroke | 14 (7) | 6 (7) | 8 (7) | 0.158 |
| Renal insufficiency | 35 (18) | 14 (17) | 21 (20) | 0.93 |
LVEF left ventricular ejection fraction, CHF congestive heart failure, MI myocardial infarct, IQR interquar-tile range
Table 2.
Out-of-hospital cardiac arrest event characteristics and post-arrest care
| All patients (n = 190) | LVEF < 40% (n = 83) | LVEF ≥ 40% (n = 107) | p valuea | |
|---|---|---|---|---|
| Cardiac etiology | 0.088 | |||
| Yes | 148 (78) | 69 (84) | 79 (74) | |
| No | 41 (22) | 13 (16) | 28 (26) | |
| Witnessed event | 0.131 | |||
| Yes | 130 (68) | 62 (74) | 68 (64) | |
| No | 61 (32) | 22 (26) | 39 (36) | |
| Location | 0.266 | |||
| Health care facility | 58 (30) | 22 (26) | 36 (34) | |
| Non-healthcare facility | 133 (70) | 62 (74) | 71 (66) | |
| CPR | 0.173 | |||
| Bystander | 41 (21) | 21 (25) | 20 (19) | |
| Health Professional | 61 (32) | 21 (25) | 40 (37) | |
| No | 89 (47) | 42 (50) | 47 (44) | |
| First pulseless rhythm, n (%) | 0.079 | |||
| Shockable | 65 (34) | 34 (40) | 31 (29) | |
| Non-shockable | 126 (66) | 50 (60) | 76 (71) | |
| Number of defibrillations | 0.016 | |||
| No | 81 (45) | 28 (36) | 53 (52) | |
| 1–4 | 48 (27) | 20 (26) | 28 (28) | |
| > 4 | 50 (28) | 30 (38) | 20 (20) | |
| Epinephrine | 0.17 | |||
| No dose | 47 (25) | 16 (19) | 31 (29) | |
| 1–3 doses | 74 (39) | 31 (38) | 43 (40) | |
| > 3 doses | 68 (36) | 30 (43) | 33 (31) | |
| Duration of resuscitation, median (IQR), min | 21 (13,41) | 25 (18, 47) | 20 (10, 34) | 0.001 |
| Hypothermia | 0.067 | |||
| Yes | 160 (84) | 75 (89) | 85 (79) | |
| No | 31 (16) | 9 (11) | 22 (21) | |
| Duration of ROSC to echo median (IQR), h | 6.25 (3.80, 11.38) | 5.28 (3.18, 13.25) | 7.07 (4.1, 11.22) | 0.198 |
LVEF left ventricular ejection fraction, CPR cardiopulmonary resuscitation, IQR interquartile range, ROSC return of spontaneous circulation, Echo echocardiography
Comparing patients with LVEF LVEF < 40% to those with LVEF ≥ 40%
When comparing patients with and without myocardial dysfunction, there is no difference in survival or favorable neurologic outcome. Men are more likely than women to have myocardial dysfunction (65 vs. 35%, p = 0.003) (Table 1). Duration of resuscitation is significantly longer in patients with myocardial dysfunction (25 vs. 20 min, p = 0.002). Patients who have myocardial dysfunction are more likely to have a history of congestive heart failure (CHF) (63 vs. 38%, p = 0.002) or coronary artery disease (34 vs. 17%, p = 0.007). Finally, an OHCA event involving more than four defibrillations is more common in the myocardial dysfunction group (64 vs. 36%, p = 0.001).
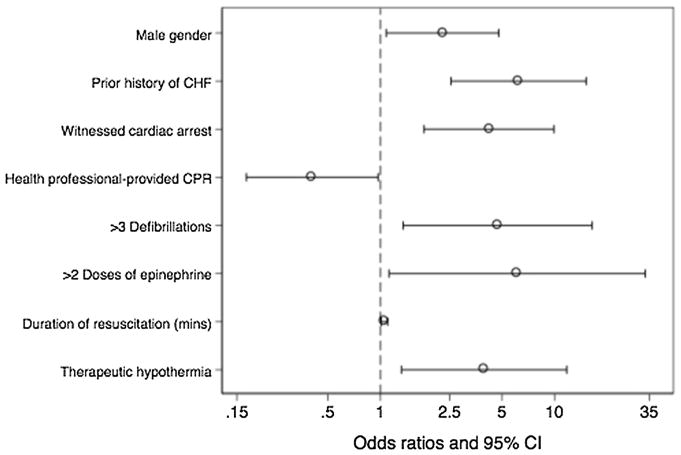
In multivariate analysis, a number of factors are associated with myocardial dysfunction, including history of CHF, male gender, witnessed arrest, more than three doses of epinephrine, more than four defibrillations, longer duration of resuscitation, and therapeutic hypothermia (Fig. 2). CPR immediately initiated by health professional personnel is associated with better myocardial function. In addition, there is a significant interaction between duration of resuscitation and more than three doses of epinephrine used during resuscitation (OR 0.95; 95% CI 0.9–1.0).
Fig. 2.
Factors significantly associated with myocardial dysfunction within 24 h post-arrest (multivariable analysis). Odds ratio > 1 indicates increased likelihood of myocardial dysfunction and odds ratio < 1 indicates decreased likelihood. CHF congestive heart failure, CPR cardiopulmonary resuscitation, mins minutes, CI confidence interval
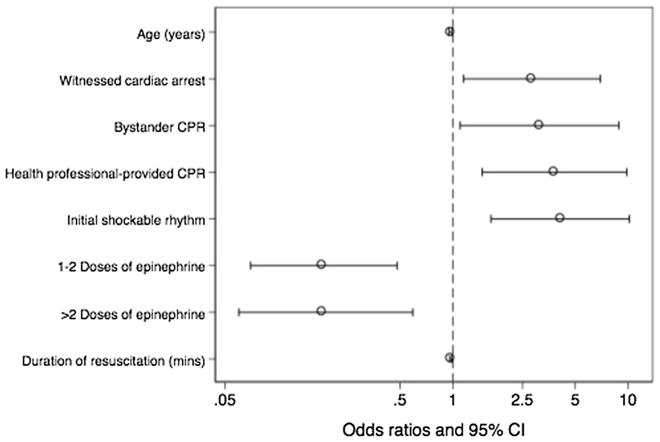
Bystander CPR, immediate CPR from a healthcare professional, witnessed arrest, and shockable rhythm are associated with favorable neurologic outcome on multivariable analysis (Fig. 3). Advanced age, administration of epinephrine during resuscitation and longer duration of resuscitation are associated with poor outcome. There is no association between early myocardial dysfunction and neurological outcome.
Fig. 3.
Factors significantly associated favorable neurological outcome after out of hospital cardiac arrest (multivariable analysis). Odds ratio > 1 indicates increased likelihood of myocardial dysfunction and odds ratio < 1 indicates decreased likelihood. Favorable neurological outcome is defined as Cerebral Performance Category score of 1 or 2. CHF congestive heart failure, CPR cardiopulmonary resuscitation, mins minutes, CI confidence interval
Discussion
We perform a cohort study of 190 patients after out-of-hospital cardiac arrest to examine predictors of post-arrest myocardial dysfunction and its association with outcome. We find associations between the following variables and myocardial dysfunction: male gender, history of CHF, witnessed arrest, longer duration of resuscitation, more than three doses of epinephrine, more than four defibrillations, and therapeutic hypothermia. CPR immediately initiated by a health professional is associated with a better LVEF. Mortality and neurological outcome are not significantly different when comparing patients with and without myocardial dysfunction.
Classically, post-cardiac arrest myocardial dysfunction is a transient global dysfunction that starts within minutes of arrest, becomes clinically apparent around 7 h after ROSC, and potentially recovers fully within 48–72 h [4, 5, 7, 8, 13, 14]. Our results confirm findings from previous studies that initial post-arrest myocardial dysfunction is not associated with neurologic outcome [4, 14].
Few studies have looked at clinical factors associated with post-arrest myocardial dysfunction. A previous study assessing hemodynamics in OHCA survivors reports that the amount of epinephrine used during CPR is associated with post-arrest instability, but not neurologic outcome [4]. Another study reports high doses of epinephrine as an independent predictor of post-resuscitation myocardial dysfunction, but demonstrates no association with neurologic outcome [14]. We find that a high epinephrine dose during CPR, as well as the duration of resuscitation, is associated with post-arrest myocardial dysfunction, and that there is an interaction between these two variables. Catecholamine excess has been described as a cause of myocardial dysfunction in other disease states, such as stress-induced cardiomyopathy and subarachnoid hemorrhage [15, 16]. Because epinephrine dose and duration of resuscitation are tightly associated, it is difficult to determine if this is truly a pharmacologic effect, or simply related to prolonged ischemia and amplified reperfusion after ROSC.
We also find that repeated defibrillation is associated with myocardial dysfunction. The adverse effect of repeated defibrillation on myocardial function has been reported in laboratory studies [7, 8, 17–19]. The mechanism is not well understood, but may be related to increased intra-myocardial temperature causing myocardial damage, potentiated excitation–contraction uncoupling, dose-dependent release of free radicals, and waveform-specific effects on mitochondrial function [20]. This finding, along with high doses of epinephrine and longer duration of resuscitation, suggests that individuals who develop post-cardiac arrest myocardial dysfunction may have had more difficult, intervention-intensive resuscitative efforts prior to ROSC. It is also possible that both epinephrine dose and repeated defibrillation attempts are simply markers for prolonged resuscitation, which has itself been associated with post-arrest myocardial dysfunction.
In this study, we also compare predictors of neurological outcomes to those of post-cardiac arrest myocardial dysfunction. We find that factors traditionally associated with better neurological outcome may not reliably predict preserved myocardial function. Witnessed cardiac arrest, as an independent predictor of favorable neurological outcome in multiple studies, is associated with myocardial dysfunction in our study [21]. Prior investigators demonstrate that patients with a cardiac etiology for their arrest are more likely to be witnessed, and have a higher prevalence of pre-existing cardiovascular disease, including congestive heart failure, previous myocardial infarction, angina pectoris, and hypertension [22]. In our study, there is a trend toward an increased prevalence of cardiac etiology among patients with witnessed arrest when compared with patients whose arrest was not witnessed (81 vs. 72%), but this did not reach statistical significance. It is possible that because more patients with witnessed arrest have a primary cardiac etiology or pre-existing cardiovascular disease, they are more likely to develop post-arrest myocardial dysfunction.
We also demonstrate that the use of therapeutic hypothermia (TH) is associated with myocardial dysfunction. The association between TH and myocardial function is complex, and should be interpreted cautiously. Therapeutic hypothermia as part of a standardized treatment for comatose survivors of cardiac arrest is recommended by the AHA due to its beneficial effect on both survival and neurological outcomes, but its effect on post-resuscitation myocardial function remains unclear [23]. One study documents moderately reduced myocardial function among OHCA patients at the time of admission that increases upon induction of TH [24]. Animal models show that moderate TH preserves myocardial function, stabilizes hemodynamics, and decreases myocardial damage; others have not demonstrated such effects [25–30]. A sub-analysis of a recent, large randomized trial demonstrates a decrease in cardiac index in patients treated with TH at 33 °C compared with 36 °C that is entirely attributable to heart rate; myocardial systolic function is not different between the groups [31]. In our study, one possible explanation for the association between TH and myocardial function is that patients who receive TH are different from those who do not. A total of 11 patients did not receive TH because they regained sufficient neurologic function shortly after ROSC; these patients will generally fare better than comatose patients with regard to neurologic outcome, survival, and, likely, myocardial function. In fact, only three of these patients had documented myocardial dysfunction.
Post-cardiac arrest myocardial dysfunction is a common component of PCAS, which can lead to early death [4, 32, 33]. In a recent large randomized trial, 46% of patients who died did so before thorough neurologic prognostication could occur because of a cardiac or hemodynamic cause [34]. These findings reinforce the need for early, aggressive hemodynamic evaluation and support. In a small pilot study, our group shows that early goal-directed hemodynamic optimization combined with TH is feasible and is associated with improved outcomes when compared to historical controls [35]. Other authors have suggested that every patient admitted to the ICU after ROSC should be screened for post-cardiac arrest myocardial dysfunction because of its significant treatment implications, and because it can be easily identified with appropriate monitoring tools [36]. Echo-cardiography is a safe, widely available, and relatively inexpensive clinical tool, which can provide both anatomical and functional information. The AHA guideline for post-cardiac arrest care suggests performing echocardiographic evaluation within 24 h to help guide therapeutic decisions [23]. It is possible that patients with risk factors for post-arrest myocardial dysfunction, as identified in this manuscript, might benefit from even more aggressive, earlier screening and early treatment [36–38]
Limitations
This study has several limitations. Because this is a retrospective cohort registry study, the quality of data mainly relies on the accuracy of existing documentation, which may be incomplete or incorrect. We define myocardial dysfunction according to left ventricular function, so it is possible that patient with other forms of myocardial dysfunction, namely right ventricular dysfunction, were missed. Another factor is the limited availability of documentation of pre-arrest myocardial function in these individuals, which precludes the ability to account for baseline myocardial dysfunction. It is possible that some patients may have already had a low LVEF before their arrest. One study finds that LVEF decreases approximately 25% among in-hospital cardiac arrest survivors within 72 h regardless of their LVEF before cardiac arrest [37]. Importantly, we adjusted for a history of CHF in the multivariate analysis to control for a potential difference in baseline function. We also were unable to obtain follow up echocardiograms on all patients, so we could not demonstrate whether post-cardiac arrest myocardial dysfunction is transient or sustained. All patients in this study were treated at tertiary centers with comprehensive post-arrest care. Finally, selection bias may exist because patients who have echocardiographic evaluation after resuscitation may be significantly different from those who do not.
Conclusions
In survivors of out-of-hospital cardiac arrest, myocardial dysfunction is common in the early post-resuscitation phase. In our study, early myocardial dysfunction is not associated with survival or neurologic outcome. Systematic screening for post-arrest myocardial dysfunction should be considered to improve knowledge of this potentially dangerous sequela, and provide evidence to direct future management of these patients.
Acknowledgments
Funding sources/disclosures NJ receives Grant support from the National Institutes of Health (U01HL123008-02). During part of the study period, the PATH database was supported by an unrestricted educational grant from Gaymar/Stryker.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no competing interests.
Statement of human and animal rights This study was approved by the Institutional Review Board at the University of Pennsylvania.
Informed consent For this type of study, informed consent is not required.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichol G, Rumsfeld J, Eigel B, et al. Essential features of designating out-of-hospital cardiac arrest as a reportable event: a scientific statement from the American Heart Association Emergency Cardiovascular Care Committee; Council on Cardiopulmonary, Perioperative, and Critical Care; Council on Cardiovascular Nursing; Council on Clinical Cardiology; and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2008;117(17):2299–2308. doi: 10.1161/CIRCULATIONAHA.107.189472. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. Resuscitation. 2008;79(3):350–379. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40(12):2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Bailen M, Aguayo de Hoyos E, Ruiz-Navarro S, et al. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66(2):175–181. doi: 10.1016/j.resuscitation.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104(24):2981–2989. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- 7.Kern KB, Hilwig RW, Rhee KH, et al. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28(1):232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 8.Kern KB. Postresuscitation myocardial dysfunction. Cardiol Clin. 2002;20(1):89–101. doi: 10.1016/s0733-8651(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 9.Leary M, Grossestreuer AV, Iannacone S, et al. Pyrexia and neurologic outcomes after therapeutic hypothermia for cardiac arrest. Resuscitation. 2013;84(8):1056–1061. doi: 10.1016/j.resuscitation.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Grossestreuer AV, Abella BS, Leary M, Perman SM, Fuchs BD, Kolansky DM, Beylin ME, Gaieski DF. Time to awakening and neurologic outcome in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation. 2013;84(12):1741–1746. doi: 10.1016/j.resuscitation.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 comprehensive heart failure practice guideline. J Cardiac Fail. 2010;16(6):e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 12.American Heart Association. [Accessed 30 Apr 2014];Ejection Fraction Heart Failure Measurement. 2014 https://www.heart.org/HEARTORG/Conditions/HeartFailure/SymptomsDiagnosisofHeartFailure/Ejection-Fraction-Heart-Failure-Measurement_UCM_306339_Article.jsp Updated July 9, 2014.
- 13.Deantonio HJ, Kaul S, Lerman BB. Reversible myocardial depression in survivors of cardiac arrest. Pacing Clin Electrophysiol PACE. 1990;13(8):982–985. doi: 10.1111/j.1540-8159.1990.tb02144.x. [DOI] [PubMed] [Google Scholar]
- 14.Chang WT, Ma MH, Chien KL, et al. Postresuscitation myocardial dysfunction: correlated factors and prognostic implications. Intensive Care Med. 2007;33(1):88–95. doi: 10.1007/s00134-006-0442-9. [DOI] [PubMed] [Google Scholar]
- 15.Tak Boland TA, Lee VH, Bleck TP. Stress-induced cardiomyopathy. Crit Care Med. 2015;43(3):686–693. doi: 10.1097/CCM.0000000000000851. [DOI] [PubMed] [Google Scholar]
- 16.Wybraniec MT, Mizia-Stec K, Krzych Ł. Neurocardiogenic injury in subarachnoid hemorrhage: a wide spectrum of catecholamine-mediated brain–heart interactions. Cardiol J. 2014;21(3):220–228. doi: 10.5603/CJ.a2014.0019. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi H, Weil M, Tang W, et al. Myocardial dysfunction after electrical defibrillation. Resuscitation. 2002;54(3):289–296. doi: 10.1016/s0300-9572(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 18.Caterine MR, Spencer KT, Pagan-Carlo LA, et al. Direct current shocks to the heart generate free radicals: an electron paramagnetic resonance study. J Am Coll Cardiol. 1996;28(6):1598–1609. doi: 10.1016/s0735-1097(96)00333-6. [DOI] [PubMed] [Google Scholar]
- 19.Zaugg CE, Ziegler A, Lee RJ, et al. Postresuscitation stunning: postfibrillatory myocardial dysfunction caused by reduced myofilament Ca2+ responsiveness after ventricular fibrillation-induced myocyte Ca2+ overload. J Cardiovasc Electrophysiol. 2002;13(10):1017–1024. doi: 10.1046/j.1540-8167.2002.01017.x. [DOI] [PubMed] [Google Scholar]
- 20.Chalkias A, Xanthos T. Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart Fail Rev. 2012;17(1):117–128. doi: 10.1007/s10741-011-9255-1. [DOI] [PubMed] [Google Scholar]
- 21.Vukmir RB Sodium Bicarbonate Study Group. Witnessed arrest, but not delayed bystander cardiopulmonary resuscitation improves prehospital cardiac arrest survival. Emerg Med J. 2004;21(3):370–373. doi: 10.1136/emj.2003.008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engdahl J, Bang A, Karlson BW, et al. Characteristics and outcome among patients suffering from out of hospital cardiac arrest of non-cardiac aetiology. Resuscitation. 2003;57(1):33–41. doi: 10.1016/s0300-9572(02)00433-1. [DOI] [PubMed] [Google Scholar]
- 23.Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;3(132):S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mottillo S, Sharma K, Eisenberg MJ. Therapeutic hypothermia in acute myocardial infarction: a systematic review. Can J Cardiol. 2011;27(5):555–561. doi: 10.1016/j.cjca.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Zobel C, Adler C, Kranz A, et al. Mild therapeutic hypothermia in cardiogenic shock syndrome. Crit Care Med. 2012;40(6):1715–1723. doi: 10.1097/CCM.0b013e318246b820. [DOI] [PubMed] [Google Scholar]
- 26.Hsu CY, Huang CH, Chang WT, et al. Cardioprotective effect of therapeutic hypothermia for postresuscitation myocardial dysfunction. Shock (Augusta, Ga. ) 2009;32(2):210–216. doi: 10.1097/SHK.0b013e318196ee99. [DOI] [PubMed] [Google Scholar]
- 27.Chenoune M, Lidouren F, Adam C, et al. Ultrafast and whole-body cooling with total liquid ventilation induces favorable neurological and cardiac outcomes after cardiac arrest in rabbits. Circulation. 2011;124(8):901–911. doi: 10.1161/CIRCULATIONAHA.111.039388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotberg M, van der Pals J, Olivecrona GK, et al. Mild hypothermia reduces acute mortality and improves hemodynamic outcome in a cardiogenic shock pig model. Resuscitation. 2010;81(9):1190–1196. doi: 10.1016/j.resuscitation.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 29.Meybohm P, Gruenewald M, Albrecht M, et al. Hypothermia and postconditioning after cardiopulmonary resuscitation reduce cardiac dysfunction by modulating inflammation, apoptosis and remodeling. PLoS One. 2009;4(10):e7588. doi: 10.1371/journal.pone.0007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeng M, Mortensen UM, Kristensen J, Kristiansen SB, Andersen HR. Hypothermia during reperfusion does not reduce myocardial infarct size in pigs. Basic Res Cardiol. 2006;101(1):61–68. doi: 10.1007/s00395-005-0550-7. [DOI] [PubMed] [Google Scholar]
- 31.Bro-Jeppesen J, Hassager C, Wanscher M, et al. Targeted temperature management at 33 vs 36 °C and impact on systemic vascular resistance and myocardial function after out-of-hospital cardiac arrest: a sub-study of the target temperature management trial. Circ Cardiovasc Interv. 2014;7(5):663–672. doi: 10.1161/CIRCINTERVENTIONS.114.001556. [DOI] [PubMed] [Google Scholar]
- 32.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 33.El-Menyar AA. Postresuscitation myocardial stunning and its outcome: new approaches. Crit Pathw Cardiol. 2004;3(4):209–215. doi: 10.1097/01.hpc.0000147142.44327.df. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 vs 36 °C after cardiac arrest. N Engl J Med. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 35.Gaieski DF, Band RA, Abella BS, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–424. doi: 10.1016/j.resuscitation.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Bougouin W, Cariou A. Management of postcardiac arrest myocardial dysfunction. Curr Opin Crit Care. 2013;19(3):195–201. doi: 10.1097/MCC.0b013e3283607740. [DOI] [PubMed] [Google Scholar]
- 37.Hovdenes J, Laake JH, Aaberge L, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand. 2007;51(2):137–142. doi: 10.1111/j.1399-6576.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez MM, Berg RA, Nadkarni VM, Vianna CB, Kern KB, Timerman S, Ramires JA. Left ventricular systolic function and outcome after in-hospital cardiac arrest. Circulation. 2008;117(14):1864–1872. doi: 10.1161/CIRCULATIONAHA.107.740167. [DOI] [PubMed] [Google Scholar]