Abstract
Background
Petrous face meningiomas (PFM) are challenging tumors due to their proximity to the cranial nerves, brainstem and critical vasculature. The objective of this study is to present surgical outcomes and support an anatomic classification for PFM based on clinical presentation.
Methods
A retrospective chart review was performed, and 51 PFM were identified. Tumors were classified by location along the petrous face into anterior, middle and posterior. Presentation and outcomes were analyzed with logistic regression.
Results
The median follow up was 31.6 months. Tumors were WHO Grade I (n=50), with one WHO Grade II tumor. Location was anterior (22%), middle (14%), posterior (53%), and overlapping (12%). Median tumor diameter was 3.0 cm (0.8–6.2 cm). Anterior location was associated with facial pain/numbness on presentation (p<0.0001), middle location with hearing loss/vestibular dysfunction (p=0.0035), and posterior with hydrocephalus (p=0.0190), headache (p=0.0039) and vertigo (p=0.0265). Extent of resection was gross-total (63%), near-total (14%), and sub-total (25%). The observed radiographic recurrence rate was 15%. Mean progression free survival (PFS) after diagnosis was 9.1 years with 2-, 5-, and 10-year PFS rates of 91.8%, 78.6%, and 62.9%, respectively. The complication rate was 27%. Neither age, location, or approach were associated with complications.
Conclusions
PFMs present with distinct clinical syndromes based on their location along the petrous face: anterior with trigeminal symptoms, middle with auditory/vestibular symptoms, and posterior with symptoms of mass effect/hydrocephalous. Surgical resection is associated with excellent long-term survival and a low rate of recurrence that can be managed with radiotherapy.
Keywords: Petrous, Petrous Face, Meningioma, Cerebellopontine Angle, Complications, Outcomes
Introduction
Meningiomas are the most common primary intracranial tumor in adults and make up approximately one third of central nervous system neoplasms.1 They arise from the arachnoid cap cells of the meninges and are typically slow growing and rarely malignant. However, when located adjacent to eloquent brain or cranial nerves, or when they grow large enough, they can produce local mass effect and become symptomatic.2 The primary treatment is surgical resection or radiotherapy with excellent outcomes.3 Tumors in challenging locations, such as deep along the skull base and around the cranial nerves, have higher rates of subtotal resection and complications.2,4–6 Meningiomas in the cerebellopontine angle grow from the petrous face and are surgically challenging due to their proximity to the brainstem, posterior fossa vasculature and cranial nerves (CN).2,5,7 The primary approaches for surgical resection of petrous face tumors are the retrosigmoid, far-lateral and transpetrosal approaches, which are selected based on tumor location along the petrous face.8–10 Here, we present our surgical outcomes and complications using the Desgeorges and Sterkers classification11 and describe the correlation between clinical symptoms and tumor location.
Methods
Study design
We conducted a retrospective chart review of all patients who underwent resection of meningioma between 1991 to 2015 at a single institution. Patients were included in the study based on the follow inclusion criteria: 1) radiographic presence of a tumor on the petrous face based on magnetic resonance imaging; 2) underwent surgical resection for the tumor at the author’s institution; 3) had adequate surgical documentation and pre-operation tumor imaging, and 4) had a pathologically confirmed meningioma. We excluded all petroclival meningiomas, which we classified as tumors with more than 25% of the tumor extending medial to the petro-occipital fissure, or invasion superiorly into the cavernous sinus. The manuscript was drafted consistent with the STROBE statement12. The IRB at the author’s institution approved this study (IRB# 13-12587).
Variables and Tumor Classification
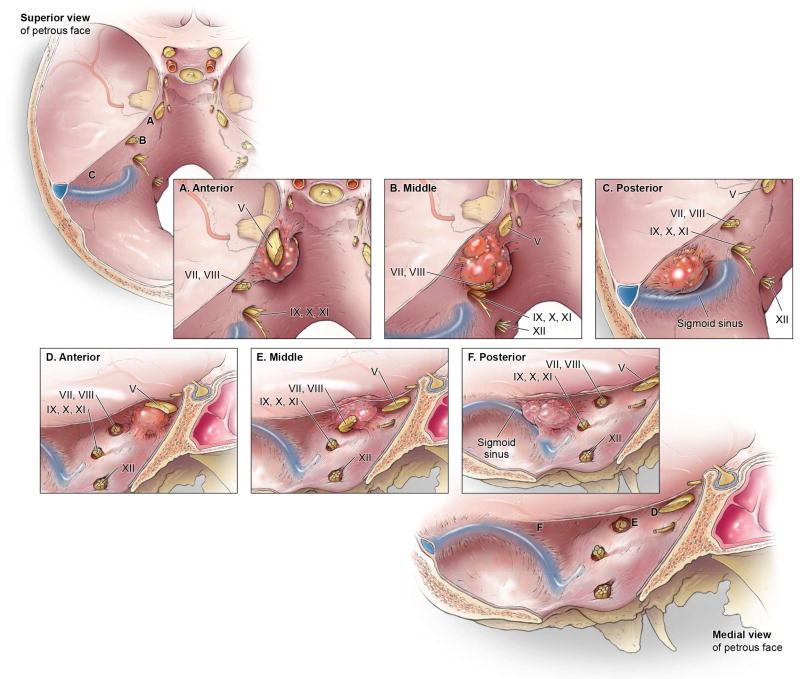
Demographic variables included age, sex, and follow-up duration. Clinical variables included presenting symptoms, pre- and post-operative neurological deficits, length of hospital stay, and surgical complications. Tumor variables included size, location and WHO Grade. The contemporary WHO grade at the time of resection was reported. Tumors were classified based on the central site of dural attachment. The modified Desgeorges and Sterkers criteria were used (Figure 1). Anterior (A) tumors included those that were located between the petrous apex and the anterior/medial lip of the IAC. Middle (M) tumors included those that were based superior to the IAC and bordered or extended into the IAC. Posterior (P) tumors included those whose dural base was between the IAC and the sigmoid sinus. Surgical variables included surgical approach, presence of pre-operative embolization, extent of resection and tumor recurrence. Extent of resection was classified radiographically as gross total, near total (>95%), and subtotal (< 95%) resection. Cases that had a radiographic gross total resection, but where the surgeon had reported that small residual tumor had been left behind in the operative report were counted as near total resections.
Figure 1. Anatomic sketch showing tumor location along the petrous face.
Tumor location from a superior (A-C) and medial view (D-F) of anterior (A, D), middle (B, E) and posterior (C, F) petrous face tumors. Published with permission. Copyright Kenneth X. Probst.
Bias
This is a retrospective study, and thus, is limited by selection and observer bias. All subjects treated at the author’s institution who had information regarding the variables of interest in the chart were included.
Statistical Analysis
All statistical analyses were performed in JMP (JMP®, Version 13.0. SAS Institute Inc., Cary, NC). Demographic data was assembled and analyzed in the standard fashion. For categorical data, Pearson’s chi-squared tests were reported. Kaplan-Meier analysis was used for radiographic progression free survival.
Results
Patient Demographics
Data was collected as part of a retrospective outcomes study on 2120 patients that underwent resection for meningioma at our institution from 1991–2015. We identified 51 patients that had an isolated petrous face meningioma and met the inclusion criteria for our study (Table 1). The median age of these patients was 62 years old (range 37–90) and 41 (80%) were women. The median follow-up was 31.6 months (range 1–191). Presenting symptoms included headache (43%), ataxia (27%) vertigo (37%), and hydrocephalus (25%). More than half (60%) of the subjects had at least one cranial palsy, with the majority being trigeminal or auditory symptoms.
Table 1.
Patient Demographics & Tumor Characteristics
| Patients (#) | 51 |
| Median Age (yrs, range) | 62 (37–90) |
| Median Follow Up (mo, range) | 31.6 (1–190) |
| Sex (female, %) | 41 (80) |
| Presenting symptoms (#, %) | |
| Headache | 22 (43) |
| Ataxia | 14 (27) |
| Vertigo | 19 (37) |
| Hydrocephalus | 13 (25) |
| Cranial Neuropathy | |
| Any | 31 (61) |
| CN III, IV, VI | 2 (4) |
| CN V | 15 (29) |
| CN VII | 1 (2) |
| CN VIII | 18 (35) |
| Incidental | 4 (8) |
| WHO Grade (n, %) | |
| Grade I | 50 (98) |
| Grade II | 1 (2) |
| Largest tumor diameter (cm) | |
| Median (Range) | 3.0 (0.8–6.2) |
| Location on Petrous Face (n, %) | |
| Anterior | 11 (21) |
| Middle | 7 (14) |
| Posterior | 27 (53) |
| Anterior and Middle | 1 (2) |
| Middle and Posterior | 4 (8) |
| Anterior, Middle and Posterior | 1 (2) |
| Extent of Resection (n, %) | |
| Gross-total | 32 (63) |
| Near-total | 7 (14) |
| Sub-total | 12 (24) |
| Adjuvant Radiosurgery (n, %) | 10 (24) |
| Residual tumor | 2 (4) |
| Recurrent tumor | 8 (16) |
| Recurrence Rate (n, %) | 8 (15.7) |
Abbreviations: CN, cranial nerve; yrs, years; mo, months
Tumor Characteristics and Presentation Syndromes
Tumor grade and distribution based on petrous face location are shown in Table 1. The majority of tumors were WHO Grade I (98%). There were no WHO Grade III tumors in this series. Anterior, middle and posterior location were defined as discussed in the methods and demonstrated in Figure 1, based on the modified Desgeorges and Sterkers classification. Figure 2 shows case examples of each type of tumor. Tumors that overlapped multiple regions were grouped into anterior/middle (AM), middle/posterior (MP) and anterior/middle/posterior (AMP). Roughly half of the tumors (53%) were posterior only, followed by anterior (22%) and isolated middle (14%). The mean largest tumor diameter was 3.2 cm (standard deviation 1.3 cm).
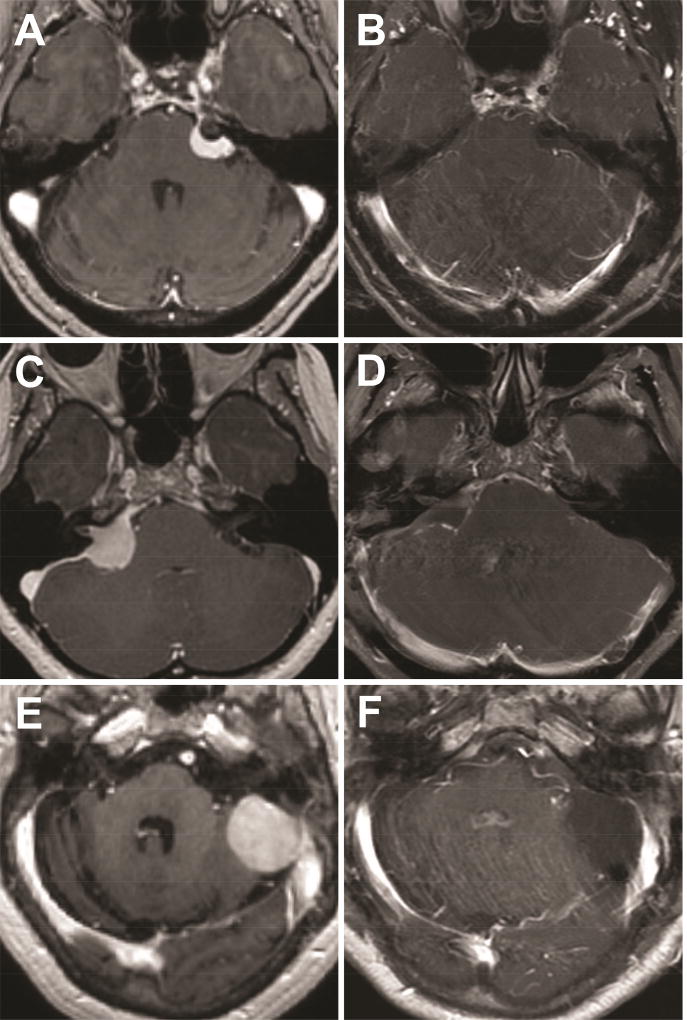
Figure 2. Case examples.
Pre-operative (A, C, E) and post-operative (B, D, F) T1-weighted post-contrast images of an anterior (A, B), middle (C, D) and posterior (E, F) petrous face meningiomas.
Tumor size and presenting symptoms varied significantly by location (Table 2). Given the smaller number of tumors overlapping multiple locations, the AM, MP, and AMP tumors were grouped together for analysis. There were no significant differences in patient age at operation or gender between locations. The largest tumor diameter was used as a surrogate for tumor size. Anterior tumors were significantly smaller than other locations, with overlapping tumors being the largest, followed by posterior and then middle.
Table 2.
Clinical Syndromes and Tumor Characteristics by Tumor Location
| Anterior | Middle | Posterior | Overlapping (AM/MP/AMP) |
p-value | |
|---|---|---|---|---|---|
| Patient Demographics | |||||
| Total (n, %) | 11 (22) | 7 (14) | 27 (53) | 6 (12) | |
| Female (n, %) | 10 (91) | 4 (57) | 23 (85) | 4 (67) | 0.2325 |
| Median Age (yrs, range) | 63 (48–74) | 51 (40–77) | 62 (37–84) | 69 (46–90) | 0.5326* |
| Tumor Demographics | |||||
| Largest Tumor Diameter (cm, range) | 1.8 (0.8–3.2) | 3 (2.6–3.5) | 3.8 (1.1–5.9) | 4.0 (2.8–6.2) | 0.0003* |
| Tumor Size ≥3 cm (n, %) | 1 (9) | 4 (57) | 17 (63) | 5 (83) | 0.0079 |
| Tumor Size < 3 cm (n, %) | 10 (91) | 3 (43) | 10 (37) | 1 (17) | |
| WHO Grade I (n, %) | 11 (100) | 7 (100) | 26 (96) | 6 (100) | 0.8238 |
| WHO Grade II (n, %) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | |
| Clinical Symptoms (n, %) | |||||
| Hydrocephalus | 0 (0) | 0 (0) | 10 (37) | 3 (50) | 0.0190 |
| Headache | 0 (0) | 2 (29) | 17 (63) | 3 (50) | 0.0039 |
| Ataxia | 0 (0) | 2 (29) | 10 (37) | 2 (33) | 0.1376 |
| Vertigo | 1 (9) | 1 (14) | 15 (55) | 2 (33) | 0.0265 |
| Cranial Neuropathy | |||||
| Any | 10 (91) | 6 (86) | 10 (37) | 5 (83) | 0.0034 |
| Extraocular (III, IV, VI) | 0 (0) | 0 (0) | 1 (4) | 1 (17) | 0.3442 |
| Trigeminal (V) | 10 (91) | 3 (43) | 2 (7) | 0 (0) | < 0.0001 |
| Facial (VII) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0.0932 |
| Auditory (VIII) | 0 (0) | 4 (57) | 9 (33) | 5 (83) | 0.0035 |
p-value for Pearson’s Chi-Square unless noted
p-value for Wilcoxon test
Each tumor location presented with statistically significant clinical syndromes (Table 2). Anterior tumors almost all presented with trigeminal symptoms, either numbness or trigeminal neuralgia (p < 0.0001). Anterior tumors never presented with hydrocephalus, headache, and ataxia. Most middle petrous face tumors presented with a cranial nerve deficit (86%, p = 0.0034), with the majority having auditory (57%) or trigeminal symptoms (43%), and only one case presenting with facial weakness. Posterior and overlapping tumors presented most commonly with symptoms attributable to mass effect—hydrocephalus, headache, ataxia, and vertigo. Posterior petrous face and large overlapping tumors also often had auditory impairment as well.
Surgical Outcomes
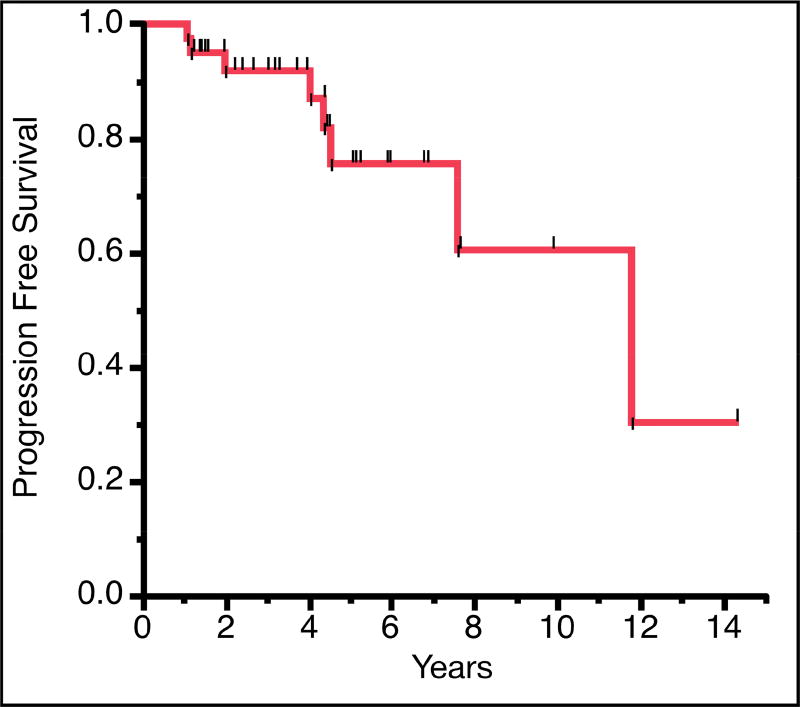
Overall, gross total resection was achieved in 32 (63%) of patients, with an additional 7 (14%) achieving near total resection (Table 1). Subtotal resection was achieved in the remaining 12 patients (24%), typically due to tumor involvement/adherence to critical neurovascular structures. The median length of hospitalization was 5.0 days (range 2–28 days). Ten (20%) of the patients received adjuvant radiosurgery with Gamma Knife or CyberKnife. No patients received radiotherapy prior to surgical resection. Two patients received CyberKnife stereotactic radiotherapy after subtotal resection, one was performed before disease progression and the other was performed after radiographic evidence of disease progression. Eight patients received Gamma Knife radiosurgery after they had tumor progression postoperatively. The radiographic observed recurrence rate was 16%. Mean follow up was 3.7 years (SD, 3.5 years). No patients died during the follow up period. A Kaplan-Meier analysis was performed for progression free survival (Figure 3). Mean radiographic progression free survival was 9.1 years with 2-, 5-, and 10-year progression-free survival rates of 91.8%, 78.6%, and 62.9%, respectively. There was no significant difference in survival based on tumor location. Post-operatively, 70% of patients reported overall improvement of their symptoms, while 30% did not have improvement in their pre-existing neurological deficits. The pre-operative symptoms that improved most frequently were gait (100%), headache (75%) and trigeminal neuralgia (63% with complete pain relief).
Figure 3. Kaplan-Meier curve of radiographic progression free survival in years.
We tested whether location on the petrous face influenced the surgical approach or clinical outcome (Table 3). Posterior and middle-posterior were the only tumors that underwent pre-operative embolization (n=7, and =1, respectively). The median hospital stay did not vary significantly based on tumor location. Three approaches were used for resection of all petrous face tumors: 1) retrosigmoid, 2) translabyrinthine, and 3) far-lateral. The retrosigmoid approach was used for all anterior petrous face tumors (n=11). Middle petrous face tumors were approached with either the retrosigmoid (n=5, 71%) or far-lateral (n=2, 29%) approach. Posterior petrous face tumors were approached with all approaches: retrosigmoid (n=18, 67%), translabyrinthine (n=1, 3%), and far-lateral (n=8, 30%). The overlapping tumors included AM (n=1), MP (n=4), and AMP (n=1) and were grouped together for statistical analysis (n=6). A translabyrinthine approach was used for the AM tumor, a retrosigmoid approach was used for the four MP tumors and a far-lateral approach was used for the one AMP tumor. The extent of resection for each tumor location did not vary significantly, but posterior tumors were the most likely to have a gross total resection (n=21, 78%; Table 3). Adjuvant radiotherapy (for sub-total resection or recurrence) did not vary significantly between the cohorts (Table 3). We tested whether tumor location (p= 0.66), tumor size (p=0.86), Simpson grade of surgery (p=0.24), extent of resection (p=0.29) or adjuvant radiotherapy (p=0.10) were associated with symptom improvement, but none of them were significant. The recurrence rate was not significantly different across tumor locations (Table 3).
Table 3.
Clinical Outcomes by Location
| Anterior | Middle | Posterior | Overlapping (AM/MP/AMP) |
p-value | |
|---|---|---|---|---|---|
| Hospital Course | |||||
| Total (n) | 11 | 7 | 27 | 6 | |
| Embolization (n, %) | 0 (0) | 0 (0) | 7 (26) | 1 (17%) | 0.1390 |
| Median Stay (days, range) | 4 (3–16) | 6 (3–28) | 5 (2–22) | 3 | 0.5377* |
| Surgical Approach (n, %) | |||||
| Retrosigmoid | 11 (100) | 5 (71) | 18 (67) | 4 (66) | 0.2495 |
| Translabyrinthine | 0 (0) | 0 (0) | 1 (3) | 1 (17) | |
| Far-Lateral | 0 (0) | 2 (29) | 8 (30) | 1 (17) | |
| Extent of Resection (n, %) | |||||
| Gross-Total | 7 (64) | 2 (29) | 21 (78) | 2 (33) | 0.0732 |
| Near-Total | 1 (9) | 1 (14) | 4 (15) | 1 (17) | |
| Sub-Total | 3 (27) | 4 (57) | 2 (7) | 3 (50) | |
| Clinical Course (n, %) | |||||
| Radiosurgery | 1 (9) | 2 (29) | 6 (22) | 1 (17) | 0.6126 |
| Recurred | 1 (9) | 1 (14) | 6 (22) | 0 (0) | 0.8327 |
p-value for Pearson’s Chi-Square unless noted
p-value for Wilcoxon test for continuous variable
Complications
There were 22 complications in 14 patients (27%). The complications are detailed in Table 4. Of the 22 complications, nine required a return to the operating room, whether for shunt placement, CSF leak repair, or wound washout for infection. Post-operative hydrocephalus only occurred in patients with posterior petrous face tumors. Five patients had new unanticipated cranial nerve deficits that persisted at greater than six-month follow up. Other complications included a patient with cerebellar edema, but no clear venous infarction, who required take back to the operating room for sub-occipital decompression. Another patient had an anomalous vertebral artery that was injured during the exposure and repaired primarily. His surgery was aborted after the artery was repaired. He was rescheduled and subsequently underwent an uncomplicated resection of his posterior petrous meningioma. There were several medical complications including a DVT and two urinary tract infections. In addition, there was a case of meningitis presenting 17-day post-operative. The lab results and clinical presentation were consistent with a bacterial meningitis; however, no organism grew from the CSF, which may have been complicated by empiric antibiotic therapy initiated at an outside hospital prior to transfer to our institution. The patient recovered after a four-week course of intravenous antibiotics that was recommended by the infectious disease service.
Table 4.
Patient Complications
| n (%) | |
|---|---|
| Total Complications (n) | 22 |
| # Patients With Any Complication | 14 (27) |
| Complications Requiring Surgery | 9 (18) |
| 30-Day Readmissions | 7 (14) |
| Complication Details | |
| Hydrocephalus | 2 (4) |
| CSF Leak | 3 (6) |
| Wound Infection | 3 (6) |
| New Cranial Nerve Deficit | |
| Any | 5 (10) |
| V (numbness) | 1 (2) |
| VII (facial weakness) | 2 (4) |
| VIII (hearing loss) | 2 (4) |
| Other | |
| Cerebellar Edema Requiring Decompression | 1 (2) |
| Vertebral Artery Injury | 1 (2) |
| Subarachnoid Hemorrhage Requiring Shunt | 1 (2) |
| Cerebellar Hematoma Managed Medically | 1 (2) |
| Meningitis | 1 (2) |
| Pseudomeningocele | 1 (2) |
| DVT | 1 (2) |
| UTI | 2 (4) |
Abbreviations: CSF, cerebral spinal fluid; DVT, deep vein thrombosis; and UTI, urinary tract infection.
We performed univariate Pearson chi-square analysis to determine if any clinical risk factors or tumor characteristics predicted the occurrence of complications. None of the variables tested were significant predictors of any complication, which included sex (p=0.3213), WHO grade (p=0.5344), tumor location on the petrous face (p=0.7491), max tumor diameter > 3 cm (p=0.3181), adjuvant radiotherapy (p=0.6016), extent of resection (p=0.4625), and recurrence (p=0.9724). The same variables were evaluated individually for the following outcome variables: hydrocephalus, infection, new postoperative cranial nerve deficit, and 30-day readmission. None were significantly predictive of these adverse outcomes. Given the lack of significance on univariate testing and limited sample size, multivariate analysis was not performed.
Discussion
Key Results
In this series, we report our surgical outcomes and complications with petrous face meningiomas and demonstrate a significant relationship between tumor location, as defined by the modified Desgeorges and Sterkers classification, and clinical presentation. This classification system can be used as a starting point to select a surgical approach. These results are a useful reference for counseling patients on the morbidity associated with resection of these tumors and risk of recurrence.
Interpretation
We identified significant differences in patient presentation based on tumor location (Summarized in Table 5). Anterior tumors, which are located anterior to the internal auditory canal, exert mass effect on the brainstem and trigeminal ganglion. Nearly all (91%) anterior petrous face meningiomas presented with symptoms attributable to involvement of the fifth cranial nerve. As has been reported before, cranial nerve compression from meningioma can result in a spectrum of facial numbness to trigeminal neuralgia2,13,14. Anterior tumors were significantly smaller than all other groups, which could reflect them becoming symptomatic earlier in their growth. Middle petrous face tumors (along with the combined middle and posterior petrous location) were the most likely to present with hearing loss and vestibular symptoms. This is consistent with prior reports that found normal hearing in only 20% of patients with tumors in these regions15. Although the facial nerve also travels in this anatomic region, it was relatively spared in all tumor groups, with only one patient presenting with facial weakness. This is likely due to the relatively earlier detection of vestibular symptoms (hearing loss, vertigo and tinnitus) because of their impact on activities of daily living16,17. Similar to what is observed with acoustic neuromas, the facial nerve typically requires more compression before facial motor deficits are detected. This is consistent with other reports that found hearing/vestibular dysfunction in 28% of patients, but facial weakness in only 8% of patients18. Lastly, the posterior petrous face tumors presented primarily with symptoms secondary to mass effect and/or hydrocephalus rather than focal deficits, consistent with others findings19,20. They were significantly associated with headaches, ataxia, vertigo and pre-operative hydrocephalus.
Table 5.
Summary of Presentations and Approach by Location
| Anterior | Middle | Posterior | |
|---|---|---|---|
|
| |||
| Size | Small or Medium | Medium | Small, Medium, or Large |
|
| |||
| Symptoms | Trigeminal | Audiovestibular | Small – Audiovestibular |
| Large – Hydrocephalus, Headache, Vertigo | |||
|
| |||
| Approach | Suprameatal Retrosigmoid | Retrosigmoid | Small – Retrosigmoid |
| Large – Modified far lateral | |||
Historically, there has been some ambiguity regarding the classification of petrous face meningiomas. Cushing and Eisenhart grouped them together with all posterior fossa meningiomas and called them “Meningiomas of the cerebellar chamber”.21 Many modern authors group petrous face meningiomas in with petroclival, lateral-inferior tentorial, anterior-lateral foramen magnum, and jugular foramen tumors, and label them all cerebellopontine angle meningiomas.2,14,22,23 Desgeorges and Sterkers proposed a more precise anatomical classification dividing petrous face tumors into anterior, middle, and posterior zones based on their relationship to the IAC.11 Anterior (A) tumors are those arising between the trigeminal impression and the anterior border of the IAC. Middle (M), or “median” tumors, are those arising between the anterior lip of the IAC and the labyrinth. Posterior (P) tumors were those extending from the labyrinth posteriorly to the sigmoid sinus. Schaller et al. proposed a simplified classification system by dividing tumors simply into pre- and retro-meatal, and highlighted their differences in clinical presentation, with premeatal tumors being smaller and more likely to present with auditory and facial nerve deficits, while retromeatal tumors presented with larger size and cerebellar signs.24 Peyre et al. published their results excluding petroclival meningiomas and using a similar A/M/P classification, except defined M tumors as those that invaded the auditory canal. The correlation of our symptomatic data with the anatomical classification support the use of the modified Desgeorges and Sterkers criteria (A/M/P).
While 27% of patients had a complication, we did not find any significant risk factors for complication, recurrence, or extent of resection. This may be secondary to sample size, or could be due to the fact that the cerebellopontine angle is inherently a high-risk zone in which to operate, regardless of where you are working within it. This is particularly challenging when removing adherent tumors that have compressed the brainstem and nerves for years. These findings highlights the utility of neuromonitoring in guiding the surgeon and protecting the sensitive cranial nerves13,15,25. It is also notable that only patients with posterior petrous face tumors had post-operative hydrocephalus. While this finding did not meet statistical significance, it does support the finding by Burkhardt et al. that larger tumor resections were more likely to be complicated by post-operation hydrocephalus26. These authors also identified a correlation between pre-operative embolization and new hydrocephalous; notably only posterior petrous meningiomas were embolized. In our study embolization and tumor size were not significantly associated with hydrocephalus, but we may simply lack the power to replicate this finding.
Management Strategy
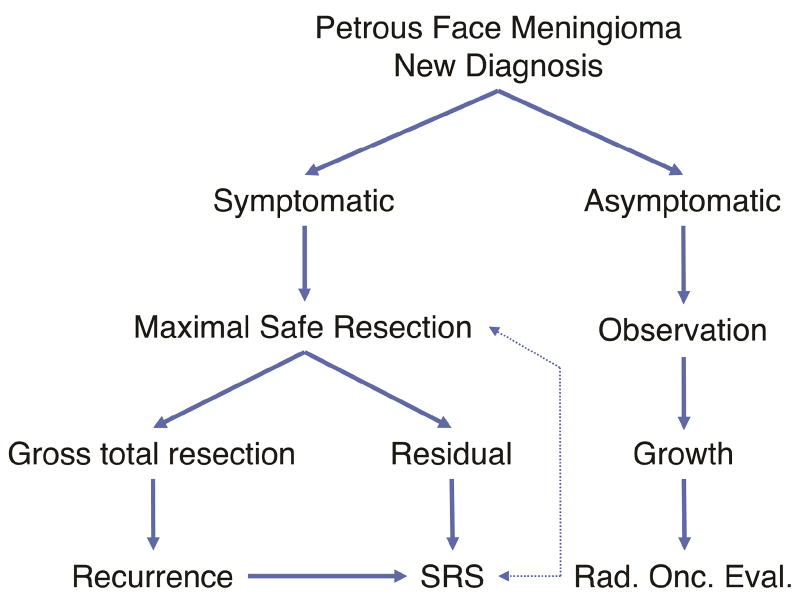
Management of petrous face meningiomas should be a joint decision made with each patient and a collaborative team of skull base neurosurgeons and radiation oncologists. Our standard algorithm for treatment is shown in Figure 4. The most important branch is whether the patient is symptomatic or asymptomatic. For asymptomatic patients, we typically observe them, and if the tumor grows, we will perform radiosurgery, or radiotherapy if the tumor is large. For symptomatic patients, we perform maximal safe resection. Patients who have residual are presented at our tumor board and can elect for observation or radiosurgery to the residual.
Figure 4. Management strategy for petrous face meningiomas.
Flow chart showing suggested management of a newly diagnosed petrous face meningioma and at recurrence.
The cornerstone of management for symptomatic patients is maximal safe resection. All of the anterior petrous face tumors and a majority (71%) of middle petrous face tumors were resected with a retrosigmoid approach. Posterior tumors had a more diverse set of operations, with retrosigmoid, translabyrinthine, and far-lateral each being used (Table 3) based on the individual tumor’s features and the patient’s symptoms. There was no difference in extent of resection between tumor locations. This is likely due to the fact that the primary limiting factor in extent of resection is adherence of the tumor to critical structures. Any residual or recurrent tumors were managed successfully with radiosurgery.
Limitations
This study is a retrospective study and is limited by recall bias. We could only evaluate patients that had adequate documentation of pre- and post-operative examinations and available imaging. Finally, the outcomes were assessed based on review of notes in the electronic medical record, which are limited by provider documentation and access to information that was recorded prior to implementation of the electronic medical record.
Generalizability
These results are those of specialized surgeons with a high-volume skull-base practice, and thus, may not be generalizable to all surgeons or institutions. Nevertheless, the principles and techniques should be generalizable to other experienced neurosurgeons and are widely applicable in that setting.
Conclusions
Petrous face meningiomas present with distinct clinical syndromes that are significantly associated with anterior, middle and posterior anatomical classification. Anterior tumors present with symptoms attributable to the trigeminal ganglion, middle petrous face tumors frequently present with symptoms in the vestibulocochlear nerve and posterior tumors present with symptoms secondary to hydrocephalous and mass effect. Resection of these tumors is associated with a significant rate of complications, but a low rate of recurrence that can be managed with radiotherapy.
Highlights.
Petrous face meningiomas can be classified based on their location along the petrous face into anterior, middle and posterior.
A petrous face meningioma’s location along the petrous face is significantly correlated with symptom presentation and tumor size.
Careful classification of a tumor along the petrous face can help guide selection of surgical approach.
Acknowledgments
The authors would like to thank Kenneth X. Probst for the artwork used Figure 1. This work was supported by the Linda Wolfe Memorial Meningioma Research Fund and the National Cancer Institute of the National Institutes of Health (1F32CA213944-01 to S.T.M.).
Abbreviations
- PFM
Petrous Face Meningioma
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work has not been previously published or presented at the time of submission.
References
- 1.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selesnick DSH, Nguyen DTD, Gutin DPH, Lavyne DMH. Posterior petrous face meningiomas. Otolaryngol Neck Surg. 2001;124(4):408–413. doi: 10.1067/mhn.2001.113663. [DOI] [PubMed] [Google Scholar]
- 3.Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. doi: 10.3171/2014.7.JNS131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Alkemade H, de Leau M, Dieleman EMT, et al. Impaired survival and long-term neurological problems in benign meningioma. Neuro Oncol. 2012;14(5):658–666. doi: 10.1093/neuonc/nos013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu F, Karampelas I, Megerian Ca, Selman WR, Bambakidis NC. Petroclival meningiomas: an update on surgical approaches, decision making, and treatment results. Neurosurg Focus. 2013;35(6):1–10. doi: 10.3171/2013.9.FOCUS13319. [DOI] [PubMed] [Google Scholar]
- 6.Diluna ML, Bulsara KR. Surgery for petroclival meningiomas: A comprehensive review of outcomes in the skull base surgery era. Skull Base. 2010;20(5):337–342. doi: 10.1055/s-0030-1253581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bambakidis NC, Gonzalez LF, Amin-Hanjani S, et al. Combined skull base approaches to the posterior fossa. Technical note. Neurosurg Focus. 2005;19(2):E8. doi: 10.3171/foc.2005.19.2.9. [DOI] [PubMed] [Google Scholar]
- 8.Nickele CM, Akture E, Gubbels SP, Başkaya MK. A Stepwise Illustration of the Translabyrinthine Approach to a Large Cystic Vestibular Schwannoma. Neurosurg Focus. 2012;33(3):E11. doi: 10.3171/2012.7.FOCUS12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cıkla Ulaş, Kujoth Gregory C, Başkaya Mustafa K. A stepwise illustration of the retrosigmoid approach for resection of a cerebellopontine meningioma. Neurosurg Focus. 2014;36(V1Supplement):1. doi: 10.3171/2014.V1.FOCUS13445. [DOI] [PubMed] [Google Scholar]
- 10.Moscovici S, Umansky F, Spektor S. “Lazy” far-lateral approach to the anterior foramen magnum and lower clivus. Neurosurg Focus. 2015;38(4):E14. doi: 10.3171/2015.2.FOCUS14784. [DOI] [PubMed] [Google Scholar]
- 11.Desgeorges M, Sterkers O. Anatomo-radiological classification of meningioma of the posterior skull base. Neurochirurgie. 1994;40(5):273–295. [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal V, Babu R, Grier J, et al. Cerebellopontine angle meningiomas: postoperative outcomes in a modern cohort. Neurosurg Focus. 2013;35(6):E10. doi: 10.3171/2013.10.FOCUS13367. [DOI] [PubMed] [Google Scholar]
- 14.Shulev Y, Trashin A, Gordienko K. Secondary Trigeminal Neuralgia in Cerebellopontine Angle Tumors. Skull Base. 2011;21(5):287–294. doi: 10.1055/s-0031-1284218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baguley DM, Beynon GJ, Grey PL, Hardy DG, Moffat DA. Audio-vestibular findings in meningioma of the cerebello-pontine angle: a retrospective review. J Laryngol Otol. 1997;111(11):1022–1026. doi: 10.1017/s0022215100139258. [DOI] [PubMed] [Google Scholar]
- 16.Roser F, Nakamura M, Dormiani M, Matthies C, Vorkapic P, Samii M. Meningiomas of the cerebellopontine angle with extension into the internal auditory canal. J Neurosurg. 2005;102(1):17–23. doi: 10.3171/jns.2005.102.1.0017. [DOI] [PubMed] [Google Scholar]
- 17.Sanders RD, Gillig PM. Cranial Nerve VIII: Hearing and Vestibular Functions. Psychiatry (Edgmont) 2010;7(3):17–22. [PMC free article] [PubMed] [Google Scholar]
- 18.Sheehan JP, Starke RM, Kano H, et al. Gamma Knife radiosurgery for posterior fossa meningiomas: a multicenter study. J Neurosurg. 2015;122(6):1479–1489. doi: 10.3171/2014.10.JNS14139. [DOI] [PubMed] [Google Scholar]
- 19.Velho V, Agarwal V, Mally R, Palande DA. Posterior fossa meningioma “our experience” in 64 cases. Asian J Neurosurg. 2012;7(3):116–124. doi: 10.4103/1793-5482.103710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwamb R, Dalpiaz A, Miao Y, Gonka J, Khan SA. Clinical manifestations of hydrocephalus: A review. Neurol Clin Neurosci. 2014;2(6):173–177. [Google Scholar]
- 21.Cushing H, Eisenhardt L. Meningiomas: Their Classification, Regional Behaviour, Life History, and Surgical Ends Results. Springfield, IL: Charles C. Thomas; 1938. [Google Scholar]
- 22.Mehdorn HM, Buhl RM. Petrous Meningiomas I: An Overview. In: Lee JH, editor. Meningiomas. London: Springer London; 2009. pp. 433–441. [Google Scholar]
- 23.Baroncini M, Thines L, Reyns N, Schapira S, Vincent C, Lejeune J-P. Retrosigmoid approach for meningiomas of the cerebellopontine angle: results of surgery and place of additional treatments. Acta Neurochir (Wien) 2011;153(10):1931–40. doi: 10.1007/s00701-011-1090-6. [DOI] [PubMed] [Google Scholar]
- 24.Schaller B, Merlo A, Gratzl O, Probst R. Premeatal and retromeatal cerebellopontine angle meningioma. Two distinct clinical entities. Acta Neurochir (Wien) 1999;141(5):465–471. doi: 10.1007/s007010050326. [DOI] [PubMed] [Google Scholar]
- 25.D’Amico RS, Banu MA, Petridis P, et al. Efficacy and outcomes of facial nerve–sparing treatment approach to cerebellopontine angle meningiomas. J Neurosurg. 2017 Feb;:1–11. doi: 10.3171/2016.10.JNS161982. [DOI] [PubMed] [Google Scholar]
- 26.Burkhardt Jan-Karl, Zinn Pascal O, Graenicher Muriel, et al. Predicting postoperative hydrocephalus in 227 patients with skull base meningioma. Neurosurg Focus. 2011;30(5):E9. doi: 10.3171/2011.3.FOCUS117. [DOI] [PubMed] [Google Scholar]