Abstract
Proteinuria encompasses diverse etiologies including both genetic diseases and acquired forms such as diabetic and hypertensive nephropathy. The basis of proteinuria is a disturbance in size selectivity of the glomerular filtration barrier, which largely depends on the podocyte: a terminally differentiated epithelial cell type covering the outer surface of the glomerulus. Compromised podocyte structure is one of the earliest signs of glomerular injury. The phenotype of diverse animal models and podocyte cell culture firmly established the essential role of the actin cytoskeleton in maintaining functional podocyte structure. Podocyte foot processes (FPs), actin-based membrane extensions, contain two specially and molecularly distinct “hubs” that control actin dynamics: a slit diaphragm and focal adhesions. While loss of FPs, encompasses dis-assembly of slit diaphragm multiprotein complexes, as long as cells are attached to the glomerular basement membrane, focal adhesions will be the sites in which stress due to filtration flow is counteracted by forces generated by the actin network in FPs. Numerous studies within last twenty years identified actin binding and regulatory proteins as well as integrins as essential components of signaling and actin dynamics at focal adhesions in podocytes, suggesting that some of them might become novel druggable targets for proteinuric kidney diseases. Here we review evidence supporting the idea that current treatments for chronic kidney diseases (CKD) beneficially and directly target the podocyte actin cytoskeleton associated with focal adhesions and suggest that therapeutic reagents that target the focal adhesion-regulated actin cytoskeleton in FPs have potential to modernize treatments for CKD
Keywords: podocytes, actin, dynamin, Bis-T-23
The common link between proteinuria and podocyte injury is the actin cytoskeleton
The presence of high molecular weight proteins like albumin in the urine (proteinuria) is the first sign that the selectivity of the kidney filtration barrier has been compromised. As of now, proteinuria is considered one of the strongest risk factors for future loss of kidney function. Most proteinuric kidney diseases and all nephrotic diseases (proteinuria > 3.5g/day) demonstrate a change in podocyte morphology on ultrastructural level (1, 2). Podocyte structure is conventionally divided into three types of subcellular compartments: the cell body, microtubule-driven membrane extensions termed primary processes, and actin-driven membrane extensions termed FPs. FPs of neighboring cells are connected by a podocyte-unique multiprotein complex termed slit diaphragm. Podocyte injury often leads to loss of FPs, a process termed “FPs effacement” (3, 4), and molecular reorganization or loss of the slit diaphragm (5). FP effacement is the result of dis-regulation of the actin cytoskeleton, providing a direct link between proteinuria and actin cytoskeleton dynamics.
Given the connection between podocyte structure and function, concerted efforts have been made in deciphering the three-dimensional architecture of podocytes. Focused ion beam/scanning electron microscopy (FIB/SEM) identified a tortuous ridge-like prominence, which protrudes from either the basal surface of the primary processes or from the cell body (6). The FPs – previously believed to be terminal projections that only protruded from the primary processes – also branched from this ridge-like prominence. This ridge-like prominence generated a more direct link between the cell body and the FPs than originally thought. The authors suggested that the ridge-like prominences might serve as an adhesion apparatus for the direct attachment of the cell body and the primary processes to the GBM, and as a connecting apparatus of FPs to the cell body. Those novel insights suggest that in addition to the structural role of podocytes in maintaining kidney filtration, the newly identified ridge-like prominence might play an important role in regulating global organization of the actin cytoskeleton in FPs as well as cell signaling between neighboring podocytes, the glomerular basement membrane (GBM) and endothelial cells.
Podocytes have remarkable transformational abilities in response to cellular stress. While podocyte injury often leads to FPs effacement, if the podocytes are still attached to the GBM they have the ability to reform FPs (7). Because FPs effacement and re-formation are membrane-modifying processes, they are both driven by distinct re-organization of the actin cytoskeleton. During FPs effacement, the actin backbone is disassembled and the cellular protrusions lose their delicate appearance to a more ‘squattish’ form. At later stages, a dense layer of actin develops at the GBM-facing side of the cells (8). This common stress response is seen only on ultrastructural analysis using electron microscopy and can be observed in most proteinuric diseases. During FPs regeneration, the dense actin layer is dissolved and novel FPs branch from the cells (9). Re-formation of the FPs can be achieved only by induction of actin polymerization in the membrane vicinity. Both FPs effacement and reformation require funneling distinct signals into a common hub(s) that controls assembly and dis-assembly of actin (10, 11). FPs contain two physically and molecularly distinct hubs that control actin dynamics, a slit diaphragm and focal adhesions.
Do current treatments affect the actin cytoskeleton in podocytes?
Despite the absence of inflammatory cells in biopsy, many diseases with direct podocyte involvement are considered immune-cell mediated because treatment with classical immunosuppressant drugs leads to remission in a significant number of patients. This paradigm has been deemed valid for more than 40 years (12). A major conceptual shift from immune-cell mediated to podocyte-mediated diseases occurred by realizing that many inheritable forms of focal segmental glomerulosclerosis (FSGS) are caused by mutations in proteins that are important for podocyte function (reviewed in (13)). Those discoveries provided direct evidence of the essential role of podocytes in the glomerulus, and suggested that direct targeting of the pathogenic pathways within podocytes might have a beneficial effect on glomerular diseases. This original idea was subsequently expanded to argue that current treatments for glomerular diseases such as ACE (Angiotensin-Converting Enzyme) inhibitors, ARB (Angiotensin Receptor Blockers), as well as diverse immunosuppressants all exhibit their antiproteinuric effects in part by directly targeting actin cytoskeleton within the podocyte (reviewed in (13)).
One of the earliest attempts to link the effect of anti-proteinuric drugs with actin cytoskeleton dynamics in podocytes was in 2008 (14). Cyclosporin A (CsA) is an inhibitor of serine/threonine phosphatase calcineurin, known for its role in regulating T cell activation via regulating the nuclear factor of activated T cells (NFAT) signaling. It was suggested that CsA blocked calcineurin-mediated dephosphorylation of the podocyte-specific adaptor protein synaptopodin, which then protected synaptopodin from deleterious cathepsin L-mediated proteolysis in injured podocytes(15). Since synaptopodin was implicated in effecting RhoA (16) and Cdc42 (17) signaling, and since those GTPases are major regulators of the actin cytoskeleton in general (see below), these data suggested that CsA ultimately exhibited a protective effect on the actin cytoskeleton in podocytes by stabilizing the RhoA/Cdc42 signaling pathway. Moreover, CsA has a direct effect on cofilin-1 expression and CsA also regulates phosphorylation of the WAVE1, an actin nucleator and a key regulator of Arp 2/3-mediated actin polymerization(18, 19).
The identification of B7-1 (CD80), a costimulatory receptor present on antigen presenting cells required to induce T cell activation also on podocyte membrane, provided the opportunity to test the idea that immunomodulators directly target podocytes in humans. Experiments using diverse animal models and podocyte tissue culture suggested that de novo expression of B7-1 in podocytes initiated their injury by deactivating essential α3β1 integrin (20, 21). Abatacept (CTLA4-Ig), a fusion protein that blocks T cell activation by binding to B7-1 and B7-2 (CD86) on antigen presenting cells with high affinity is a drug licensed for the treatment of rheumatoid arthritis. After original promising study (21), the responsiveness of FSGS patients to abatacept, or a newer version of the drug named belatacept, have been discouraging (22). In fact, while abatacept did effectively reduce the level of activated CD4+ T cells, its administration did not preserve kidney function in the streptozocininduced model of DN in mice (23), further questioning the direct effect of abatacept on podocytes.
In contrast to the negative results with immunomodulators that act by blocking T cell activation, the B-cell targeting drug rituximab exhibited moderate success in some patients with nephrotic syndrome (24, 25). Rituximab is a chimeric monoclonal antibody against the protein CD20, which is primarily found on the surface of immune system B cells where it acts by destroying them. Fornoni and colleagues suggested that in CKD patients, rituximab operates in a B-cell independent manner by directly targeting podocytes (26). Specifically, the authors hypothesized that rituximab binds sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b), a lipid-modulating phospodiesterease. Studies using podocytes in culture suggested that rituximab partially prevented SMPDL3b down-regulation, which was associated with recurrent FSGS. Since levels of SMPDL3b expression affected the actin cytoskeleton in podocytes, the study suggested that rituximab modulates podocyte actin cytoskeleton in an SMPDL3b-dependent manner.
The above-mentioned therapeutics were developed to specifically target the immune system. At present, it is hard to conclude with certainty whether the antiproteinuric effects observed in a subset of patients and/or animal models are due to their effect on podocytes, immunomodulation, or both. In contrast to immunomodulators, Renin Angiotensin Aldosteron System (RAAS) blockers are the only generally accepted and most widespread supportive anti-proteinuric therapy that has a proven influence on kidney function (27). While their antiproteinuric effect is clearly due to alterations in the blood flow in the glomerulus due to change in blood pressure, it is not surprising that a number of studies also examined the ability of RAAS to alter the actin cytoskeleton in podocytes. Both angiotensin II receptor type 1 and type 2 (AT1 and AT2) are expressed in podocytes, and their expression is elevated in proteinuric diseases (28). Durvasula and coworkers showed that RAAS inhibition can lead to reduced cell tonus and decreased apoptosis in an AT1 receptor-mediated fashion in cultured podocytes (29). This observation functionally supports the older observations that Angiotensin II infusion leads to blood pressure independent proteinuria, changes in chloride conductance of podocytes, and cytoskeletal rearrangements (30, 31). More recent data from Nijenhuis and coworkers suggested a direct negative effect of Angiotensin II on podocytes via the NFAT mediated upregulation of TRPC6 expression, indicating direct prevention of this deleterious cellular effect via ACE-inhibition (32). As for immunomodulating drugs, while these data suggest that RAAS-blockers play a role in altering podocyte structure, given their effect on blood pressure, it is impossible to indisputably attribute their anti-proteinuric effect to directly targeting podocytes.
Role of focal adhesions in podocytes
Realization that the actin cytoskeleton plays an essential role in maintaining podocyte unique structure lead to a wide spread effort by the scientific community in deciphering both podocyte-specific and canonical signaling pathways essential for regulating its actin cytoskeleton (for comprehensive review see (33)). As mentioned above, FPs contain two specially and molecularly distinct “hubs” that control actin dynamics: a slit diaphragm and focal adhesions.
Since glomerular filtration flow represents the highest extravascular fluid flow in the body, the attachment of podocytes to GBM is challenged by both tensile and shear stress forces. Wilhelm Kriz and Kevin Lemley recently suggested a distinct role of slit diaphragm in balancing the lateral components of the shear stresses on opposing FPs (34). Since this unique podocyte structure is either altered or lost in most pathological situations, it seems reasonable to suggest that slit diaphragm specific proteins might not be the best druggable targets for CKD. In contrast, number of studies suggested that focal adhesion proteins have potential to be druggable in CKD.
The mechanical forces endured at the sites of FAs are believed to result from the retrograde flow of actin in the membrane vicinity, the remodeling of dendritic actin networks into liner filaments array of the stress fibers in the vicinity of adhesions, and the bundling of actin by mysosin II, which stimulates proximal actin assembly (Figure 1). Thus, bidirectional force transmission between the internal cytoskeletal network and the exterior gets directed through the adhesion. A mature focal adhesion contains hundreds of proteins that can be grouped based on their contribution to four basic processes: receptor/matrix binding proteins, linkage to actin cytoskeleton, intracellular signal transduction, and actin polymerization (a general information on FAs can be found at https://www.mechanobio.info/topics/mechanosignaling/cell-matrix-adhesion/focal-adhesion/; or reviewed in (35).
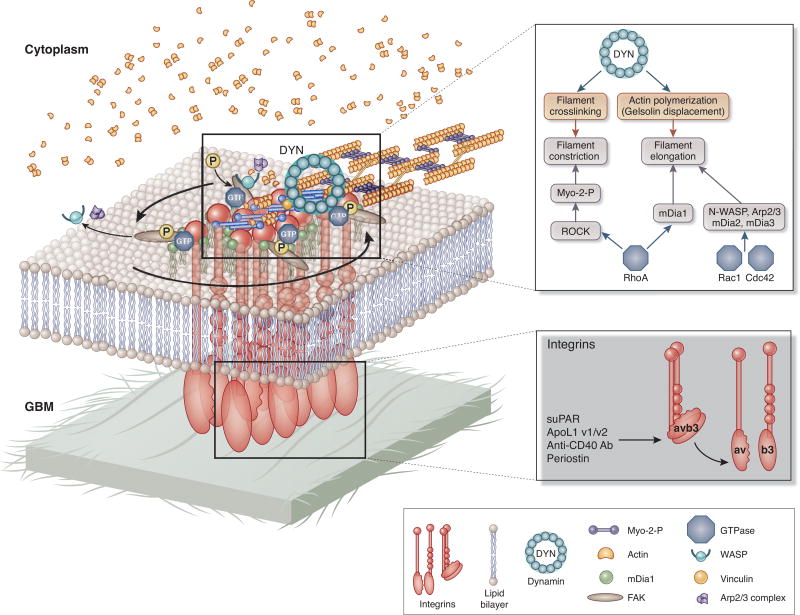
Figure 1.
Schematic view of signaling pathways that lead to actin organization at focal adhesions in podocytes. Integrins such as a3b1 bind to components of glomerular basal membrane. avb3 integrin can be activated by binding to its ligands such as suPAR, ApoL1, periostin or anti-CD40 antibody. a3b1 integrin activation leads to clustering of a3b1 integrins and initiation of actin polymerization by the action of Arp2/3 complex, cortactin and WASP-family proteins. mDia1 (formin family member) elongates actin filaments into columnar structure from the branched actin network that was previously induced by N-WASP, the Arp2/3 complex and cortactin. Actin polymerization is regulated by the action of GTPases such as Rac 1, Rho A and Cdc42. Actin filaments are crosslinked by α-actinin, and myosin II incorporates into the α-actinin-crosslinked actin filament bundles. Large GTPase dynamin can induce actin polymerization and crosslinking of actin filaments by directly binding actin filaments.
(adapted with permission from Albiges-Rizo et al. J Cell Sci. 2009 Sep 1;122(Pt 17):3037-49. doi: 10.1242/jcs.052704.0
αvβ3 integrin and its ligands in podocytes
When comes to podocytes, α3β1 Integrin is essential for maintenance of glomerular structural integrity (36). In contrast, a growing number of studies suggest that one of the “hallmarks” of podocyte injury is the activation of constitutively inactive αvβ3 integrin (37)(Figure 1), which initiated the search for its activating ligands.
Soluble urokinase activating receptor (suPAR) has been implicated as one of the ligands for αvβ3 integrin activation in podocytes (37). While suPAR per se does not seem to be highly pathogenic in wild type animals, co-administration of suPAR and human auto-antibodies against CD40 isolated from the patients with recurrent FSGS resulted in proteinuria, foot process effacement, and histological evidence of glomerular sclerosis (38, 39). No kidney injury was noted in mice deficient in CD40 or in wild-type mice that received blocking antibody to CD40. CD40 is a costimulatory protein found on antigen presenting cells and is required for their activation. Circulating anti-CD40 antibody was identified by screening about 9000 antigens in pretransplant sera for their ability to predict posttransplant FSGS recurrence with high accuracy (40).
Expending the type of mechanism for αvβ3 integrin-induced podocyte injury, recent studies identified a link between Apolipoprotein L1(ApoL1) and suPAR-driven αvβ3 integrin activation, suggesting formation of a triple complex between suPAR, ApoL1 and αvβ3 integrin (41, 42). In addition, suPAR-induced αvβ3 integrin activation has been recently linked to increase in activity of TRPC6 (Ca2+-permeable cationic channel), channel localized to the slit diaphragm (41, 42). This study established a link between αvβ3 integrin signaling at the FAs and activity of slit diaphragm channel. Importantly, identical effect was observed with serum of FSGS patient but only if the patient was in relapse state of diseases (41, 42). Furthermore, a study identified a periostin as jet anther αvβ3 integrin ligand (43). Periostin is a secreted extracellular matrix protein found in many cancers, where by binding αvβ3 or αvβ5 integrins on cancer cells leads to activation of number of downstream signaling pathways implicated in cell invasion, metastasis and the epithelial-mesenchymal transition (43). By using a combination of bioinformatics, reporter assay, and chromatin immunoprecipitation analyses, the authors found that NFκB and other proinflammatory transcription factors induce de novo expression of periostin in vitro, and that periostin and β3 integrin co-localize in renal biopsies from patients with ANCA vasculitis, inflammatory glomerulopathy, and in glomeruli of nephrotoxic serum (NTS)-induced glomerular nephropathy (GN) in mice. Mice lacking expression of periostin displayed preserved renal function and structure during NTS-induce GN, and administration of periostin antisense oligonucleotides in wild-type animals with GN reversed already established proteinuria, diminished tissue inflammation, and improved renal structure. Together, these studies identify several potential therapeutic strategies for treating CKD, such as inhibition/downregulation of αvβ3 integrin, periostin, suPAR, and removal of auto-CD40 antibodies. A monoclonal antibody that blocks αvβ3 integrin ligand occupancy inhibited the progression of albuminuria in diabetic rats, development of nephropathy in diabetic pigs and is currently tested in patients with CKD (44, 45). Data from just completed human trials (ClinicalTrials.gov Identifier: NCT02251067) are expected to provide information with regard to feasibility of targeting αvβ3 integrin signaling in diabetic nephropathy patients.
GTPases at focal adhesions
The cytoplasmic tail of active integrin binds several intracellular anchor proteins including talin, paxillin, vinculin and α-actinin (Figure 1). These anchor proteins bind directly to actin or to other anchor proteins such as cortactin and WASP, thereby linking the integrin to actin filaments. Since anchor proteins lack intrinsic enzymatic activity, no attempts have been made to asses their druggability in podocytes. Importantly, polymerization of the cortical actin cytoskeleton in the membrane vicinity precedes formation of focal adhesions. Connection between focal adhesions and actin polymerization is formed through direct interactions of vinculin with components of the actin polymerization module such as Arp2/3 complex, cortactin, gelsolin and N-WASP. Furthermore, DRF/mDia1 elongates actin filaments into columnar structures from the branched actin network. Actin filaments are subsequently crosslinked by myosin II and α-actinin. Actomyosin contractility reinforces adhesion and is required for their maturation.
Canonical small GTPases of a RhoA family are well known regulators of actin polymerization. Numerous studies that focused on the role of RhoA, Rac1 and Cdc42 signaling in podocytes, together showed that podocyte health requires a well controlled cross-talk and balance among different Rho-GTPases (reviewed in (46)). For example, podocyte-specific loss of Cdc42 leads to congenital nephropathy, whereas loss of RhoA and Rac1 did not cause a discernible renal phenotype for up to 3 months of age (47). In contrast, expression of dominant negative RhoA in podocytes induced reversible FP effacement and proteinuria (48), as did doxycycline-inducible and podocyte-specific expression of constitutively active RhoA in mice (48, 49). While the reason for the apparent contradiction in the phenotypes regarding loss of RhoA signaling in podocytes is not clear, data suggests that the basal activity of RhoA might be important for the integrity of podocytes. Similarly, while loss of Rac1 has not phenotype (47), Rac1 activation has been linked to podocyte FP effacement and proteinuria (50). Loss of function mutations in ARHGAP2, a gene encoding GTPase activating protein for Rac1, were found to be associated with familial FSGS, and Rac1 activation in podocytes as a consequence of the mutation was suggested to be responsible for the phenotype (50). Similarly, increase in Rac1 activation was also linked to increase in nuclear translocation and transcriptional activity of mineralocorticoid receptors, which was in turn linked to proteinuric kidney diseases (51). Specifically, mice lacking Rho GDP-dissociation inhibitor-α (Arhgdia−/− mice) developed progressive renal disease with massive albuminuria and glomerular lesions with focal and segmental sclerosis, along with intratubular casts and luminal dilation(52). Since Rho GDP dissociation inhibitors (RhoGDI) bind the GDP-bound inactive Rho GTPases and thus prevent them from being converted to the active GTP-bound form, researchers searched for the GTPase that was activated in Arhgdia−/− mice and found it to be Rac1. Indeed, administration of Rac-specific inhibitor, NSC23766(53), for 6 weeks significantly reduced proteinuria and ameliorated kidney damage. Finally, researchers identified increase in transcriptional activity of mineralocorticoid receptor as downsteam effectors of activated Rac1. This study suggested for the first time that Rac1 inhibitors have potential to become novel therapeutic targets in CKD.
One of the major downstream effectors of RhoA is a Rho-associated protein kinase (ROCK). ROCK induces formation of stress fibers and focal adhesions by phosphorylating myosin light chain, which induces actin binding by myosin II and thus increase in contractility. Number of reports using the non-selective ROCK inhibitors Y27632 or fasudil in the setting of renal injury and proteinuria reported amelioration of proteinuria and/or kidney injury (reviewed in (54, 55)). Furthermore, administration of Y27632 was also effective in reducing proteinuria and preventing nephrin loss in puromycin aminonucleoside nephrosis (56). There are two ROCK isoforms, ROCK1 and ROCK2. Mice deficient in ROCK1 were protected from the development of diabetic nephropathy, while podocyte-specific expression of constitutively active ROCK1 exacerbated the disease (57). The hypothesis that ROCK inhibitors ameliorate podocyte damage by specifically targeting actin cytoskeleton is hard to reconcile with the role of ROCK in maintaining actomyosin contractility in the cells. Crosslinking of actin filaments by myosin is a prerequisite for formation of stable focal adhesions, and accordingly treatment of cultured podocytes with Y27632 results in dramatic loss of focal adhesions (58). It is impossible to explain why would loss of focal adhesion in already injured podocytes ameliorate proteinuria, jet a wealth of evidence suggests that inhibiting ROCK activity is beneficial for injured glomerulus. The explanation might lie in the fact that fasudil acts as a vasodilator, thus arguing that observed beneficial effects of fasudil were via changes in intrarenal hemodynamics (59), and not necessary alterations of the actin cytoskeleton in podocytes. Recently, a novel podocyte enriched focal adhesion molecule was discovered, Erythrocyte Membrane Protein Band 4.1 Like 5 (EPB41L5). which orchestrates focal adhesion formation at the leading edge of the cells (60).
Given the essential role that the actin cytoskeleton plays in podocytes, we expect that number of novel regulatory pathways will be identified in the near future. It is worth keeping in mind that there is a clear distinction between proteins that are essential for actin dynamics and those that are druggable targets. One of the essential lessons learned by our past studies is that identification of specific, druggable target(s) requires development of target-specific regents.
Role of dynamin as a regulator of actin cytoskeleton at focal adhesions
Currently, no drug has been developed that systemically and beneficially directly alters the actin cytoskeleton. While small molecules that disrupt the actin cytoskeleton are standardly used as research tools (cytochalasin D (formation of short actin filaments), latranculin A (actin depolymerization), and jasplakinolide (stabilization of actin filaments)) and have been tested as cytotoxic reagents in anti-cancer therapy in rodents (61, 62), significant side effects render them nonviable candidates for human drugs. It seemed as if beneficially and systemically targeting actin cytoskeleton dynamics might not be feasible; however a possible solution came from an unusual place. The GTPase dynamin was originally isolated in 1989 as a microtubule-associated protein (MAP) (63–65). Despite its origin, the role of dynamin in regulating microtubule dynamics has not been extensively studied, though mutations have been linked to microtubule instability in Charcot-Marie-Tooth Disease and inherited peripheral neuropathy (66). Dynamin is best known for its essential role in clathrin-mediated endocytosis, where its ability to oligomerize into higher order structures such as rings has been implicated in budding vesicles from the plasma membrane (Reviewed in (67) and Figure 2). In contrast to small canonical GTPases such as RhoA, Rac 1 and Cdc42, dynamin is a large, multidomain GTPase (Figure 2). In its native state, dynamin is a homo-dimer that possesses the intrinsic propensity to oligomerize into higher order structures. Dynamin self-assembly can be promoted by lipids, actin filaments, microtubules, and SH3-domain containing proteins (reviewed in (68) and Figure 2). One of the key differences between dynamin and small canonical GTPases is that dynamin oligomerization regulates its GTPase cycle. Internal domain within dynamin named GED (GTPase effector domain) acts as a GTPase activating protein (GAP), and given low affinity for both GTP and GDP, as of now there are no known dynamin-specific GDIs or GEFs (GTPase exchange factors).
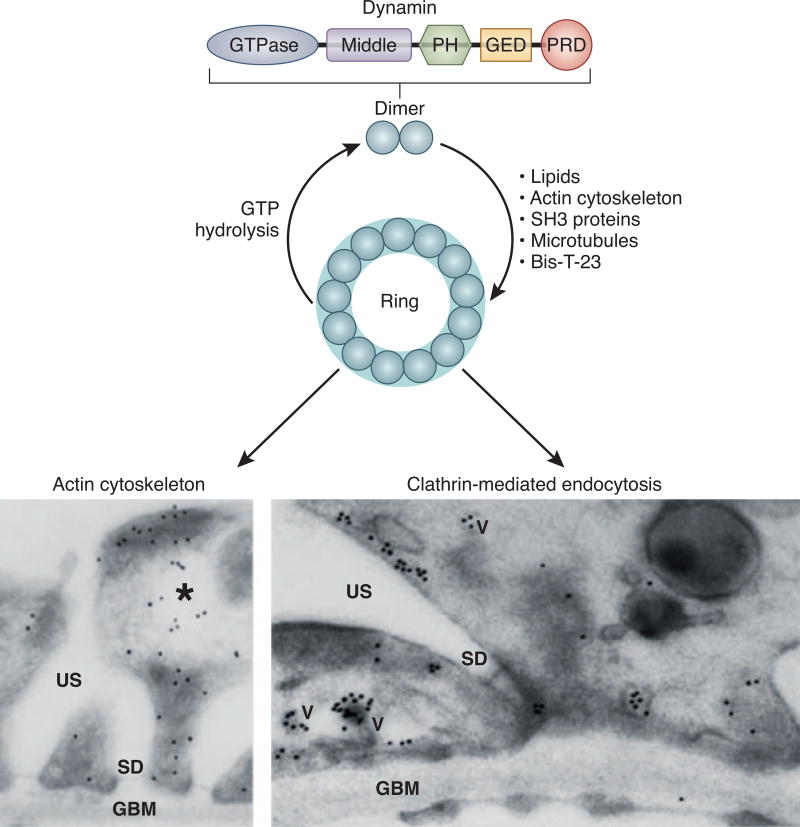
Figure 2.
Schematic overview of dynamin protein domains and its ability to oligomerize into higher order structures such as rings which can be induced by lipids, actin filaments, SH3-domain containing proteins and Bis-T23. Expression of dynamin can be clearly observed in podocyte foot processes. Here it was found using immunogold labellings as associated with the actin cytoskeleton and on clathrin-coated pits (reprinted with permission from Sever et al., J Clin Invest. 2007;117(8):2095–2104) (TEM magnification 30000× left and 45000× right).
In addition to its role in endocytosis, dynamin is also a well-known regulator of the actin cytoskeleton (69). Dynamin co-localizes with actin at multiple cellular structures, and its GTPase activity is essential for multiple actin-driven processes such as forming growth cones, the cortical actin cytoskeleton, podosomes, formation of filopodia and F-actin comets generated by Listeria (reviewed in (70)). Consistent with its dual role in the cell, dynamin was co-localized with clathrin-coated pits and actin filaments in podocyte foot processes using electron microscopy (Figure 2). Podocyte-specific loss of dynamin results in massive proteinuria, foot processes effacement, and early death due to kidney function loss (71), establishing dynamin’s essential role in the maintenance of podocyte structure and function.
The molecular mechanism of diverse dynamin-regulated actin-driven processes is attributed to its ability to bind several actin binding and regulatory proteins. Via its C-terminal PRD, dynamin interacts with SH3-domain containing proteins such as profilin II (a major regulator of the actin based cytoskeleton) (72), syndapin/pacsin/FAP52 (73–75), Src (76) (a non-receptor tyrosine kinase essential for actin-mediated cell adhesion and motility) (77), cortactin, and Nck (reviewed in (78)). Several interactions, such as dynamin-cortactin interactions, are implicated in regulating Arp2/3 complex-driven actin polymerization. Unexpectedly, it has been shown by us that dynamin directly binds actin filaments, distinguishing itself again from other canonical regulatory GTPases that regulate the actin cytoskeleton by binding multiple downstream effectors. Direct dynamin-actin interactions can induce de novo actin polymerization in vitro, in cells, and in the whole organism (11, 58, 79). The ability to induce actin polymerization is driven by dynamin rings, which then (by an unidentified mechanism) displace the capping protein gelsolin from the barbed ends, allowing rapid actin polymerization (79). Based on current knowledge, dynamin oligomerization promotes actin polymerization in a gelsolin-dependent manner and therefore at distinct cellular sites such as the formation of lamellipodia (78), actin-dependent endocytosis in yeast (80), fusion pore expansion in neuroendocrine chromaffin cells (81), and focal adhesions (58). We have shown that ability to promote focal adhesion maturation was autonomous from RhoA (58). The ability to regulate the actin cytoskeleton via direct interactions has been shown to belong to another member of the dynamin family, Drp1, and is linked to its role in regulating mitochondrial morphogenesis (82).
Podocytes expressing a dynamin mutant with an increased propensity to form rings resulted in significantly longer foot processes ((11) and Figure 3), providing direct proof for the physiological role of dynamin-induced actin polymerization and foot processes formation. The use of a small molecule that promotes dynamin oligomerization into rings, Bis-T-23, provided additional proof (79). The original study showed that daily administration of Bis-T-23 for up to 10 days ameliorated proteinuria, restored foot processes, and removed early signs of glomerular injury (such as collagen IV depositions) in diverse rodent models of proteinuric kidney diseases (Lipopolysaccharide (LPS) and Puromycin aminonucleoside (PAN)-induced proteinuria, strepotozotocin (STZ) induced diabetic nephropathy, and mice lacking the PKCε signaling cascade (11)). Bis-T-23’s ability to restore foot processes in such diverse models further emphasizes that the commonality in podocyte injury is dis-regulation of the actin cytoskeleton in foot processes.
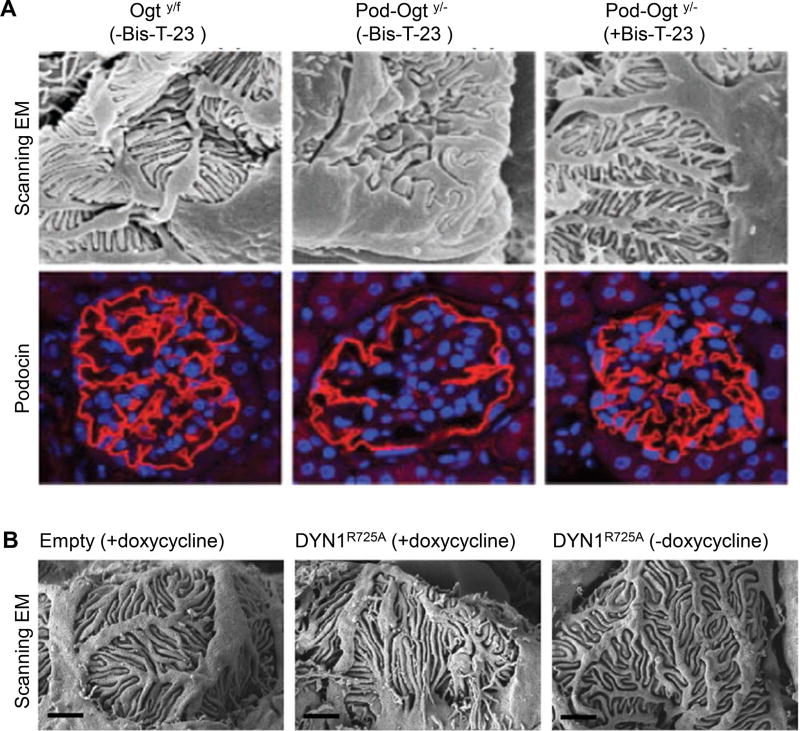
Figure 3.
Dynamin activity orchestrates foot process length and functionality. A) Scanning electron microscopy (SEM) revealed that daily administration of Bis-T-23 for 3 weeks restored foot process effacement and podocin staining in mice where podocytes lacking O-linked b-N-acetylgucosamine (O-GlcNAc) transferase (Ogt) (Podo-Ogty/− mice)(adapted with permission from Ono et al., Nephrol Dial Transplant 2017 gfw463. doi: 10.1093/ndt/gfw463) (SEM magnification 8000× and IHC magnification 400×). B) SEM after doxycycline induced overexpression of the R725A mutation in DNM1 in podocytes which is situated in the GED and renders dynamin prone to oligomerize leads to extended foot processes after doxycycline feeding (adapted with permission from Schiffer et al., Nat Med. 2015 Jun; 21(6): 601–609) (Scale bar 1µm).
Furthermore, a recent study showed that podocytes lacking O-linked b-N-acetylgucosamine (O-GlcNAc) transferase (Ogt) (Podo-Ogty/− mice) developed albuminuria at 8 weeks, increasing progressively until 32 weeks (83). The animals exhibited glomerular sclerosis, proteinuria-related tubulointerstitial lesions, and effaced foot processes with decreased podocin expression. Since O-GlcNAc modification is a post-translational modification of intracellular proteins, the loss of Ogt is expected to affect multiple signaling pathways in podocytes, including those that regulate actin cytoskeleton dynamics. Daily administration of Bis-T-23 for 3 weeks prevented albuminuria and podocyte damage (Figure 3), further supporting the ability of dynamin-driven actin polymerization to counteract multiple mechanisms that lead to dis-regulation of the actin cytoskeleton in podocytes.
Since dynamin is best known for its role in endocytosis, it seems surprising that administration of Bis-T-23 does not affect endocytosis (58, 79). The reason for this is that dynamin role in endocytosis is dependent on its ability to oligomerize around a membrane. Since this process exhibits very fast kinetics by itself, Bis-T-23 cannot alter it. In another words, something that goes with maximum kinetics, such as dynamin oligomerization on lipids, cannot be made faster by Bis-T-23. The same rational can explain why Bis-T-23 does not cause toxic effects in the animals (11, 83). Data suggest that as long as the dynamin oligomerization cycle is intact in the cell, and that applies for both, membrane and actin induced oligomerization, the dynamin oligomerization cycle proceeds with such fast kinetics that they cannot be altered by Bis-T-23. Therefore, Bis-T-23 will exhibit its effect only when and where dynamin oligomerization cycle becomes a rate-limiting step. Together, these data provide compelling experimental proof of the potential to induce actin polymerization in podocytes by inducing dynamin oligomerization, which suggests jet another path towards developing novel supportive therapeutics to treat diverse glomerular diseases.
Is there a need for personalized strategies for the treatment of nephrotic syndrome?
One major challenge in the development of much needed novel therapeutics for CKD is that most of our treatment regimens are based on studies with comparably low patient numbers with low statistical power, a good example being the FSGS-CT study (84). The largest NIH funded study on FSGS tried to compare the efficacy of cyclosporine to mycophenolate mofetil in combination with dexamethasone pulses. Due to a lack of recruitment and logistical difficulties with 53 centers recruiting only 1–2 patients, the study has a center bias and is underpowered (84). Moreover, patients with family history or a high Body Mass Index (BMI) were not completely excluded. This readily demonstrates the difficulty in designing a randomized controlled trial for the treatment of nephrotic syndrome. Additionally, the lack of unified definitions of “steroid resistance” or “partial/full remission” between centers makes guidelines questionable. Current treatment regimens result in trial and error since most centers start with steroids and switch to calcineurine inhibitors before “experimental” regimens. We expose our patients daily to what is considered the most effective approach but accept that treatment has the likelihood of being inefficient with a high risk of strong, and potentially life-threatening, adverse effects. Currently, we strongly believe that the difference in treatment responses clearly indicates the necessity to pursue a more detailed characterization of podocyte phenotypes in different kidney diseases. Additionally, we believe that “FSGS” should no longer be considered a diagnosis: current histopathology only describes one common phenotype of podocyte stress, which is not a “true” diagnosis. While we clearly and desperately need novel options for our vulnerable patients, the current opinion is strongly in favor of establishing genetic, epigenetic, and signaling signatures as a path towards personalized and/or precision medicine. While this is an important trend towards identification of diverse mechanistic treatment options we believe the more efficient route is, developing novel therapeutics that directly target the actin cytoskeleton that can be applied more general as supportive therapy options that enhance tissue regeneration.
Identification of the actin cytoskeleton as the common most downstream effector in highly diverse models of glomerular injury and the “proof of the concepts” that the actin cytoskeleton can be targeted specifically in podocytes both suggest a global approach to treat CKD: targeting the actin cytoskeleton. Instead of trailing in the wake of cancer therapies, nephrology is given the chance to become a leader in developing novel therapeutic approaches for its patients.
Acknowledgments
This work was supported by DFG-grant (SCHI587/6-1) and BMBF-grant 01GM1518A to M.S, and by the National Institutes of Health (R01 DK093773, DK087985, and DK101350) to S.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
M.S. has nothing to disclose. S.S. is co-founders of the biotech TRISAQ and hold patents and stock in the space of proteinuric kidney disease. She is also an inventor on pending and issued patents related to anti-proteinuric therapies. She stands to gain royalties from present and future commercialization.
References
- 1.Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338(17):1202–11. doi: 10.1056/NEJM199804233381707. [DOI] [PubMed] [Google Scholar]
- 2.Bergon E, Granados R, Fernandez-Segoviano P, Miravalles E, Bergon M. Classification of renal proteinuria: a simple algorithm. Clin Chem Lab Med. 2002;40(11):1143–50. doi: 10.1515/CCLM.2002.201. [DOI] [PubMed] [Google Scholar]
- 3.Kriz W, Kretzler M, Nagata M, Provoost AP, Shirato I, Uiker S, et al. A frequent pathway to glomerulosclerosis: deterioration of tuft architecture-podocyte damage-segmental sclerosis. Kidney Blood Press Res. 1996;19(5):245–53. doi: 10.1159/000174083. [DOI] [PubMed] [Google Scholar]
- 4.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. The podocyte's response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2013;304(4):F333–47. doi: 10.1152/ajprenal.00478.2012. [DOI] [PubMed] [Google Scholar]
- 5.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24(4):333–5. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 6.Ichimura K, Miyazaki N, Sadayama S, Murata K, Koike M, Nakamura K, et al. Three-dimensional architecture of podocytes revealed by block-face scanning electron microscopy. Sci Rep. 2015;5:8993. doi: 10.1038/srep08993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal Change Disease. Clin J Am Soc Nephrol. 2017;12(2):332–45. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirato I, Sakai T, Kimura K, Tomino Y, Kriz W. Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. Am J Pathol. 1996;148(4):1283–96. [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian B, Sun H, Yan P, Charoonratana VT, Higgs HN, Wang F, et al. Mice with mutant Inf2 show impaired podocyte and slit diaphragm integrity in response to protamine-induced kidney injury. Kidney Int. 2016;90(2):363–72. doi: 10.1016/j.kint.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashworth S, Teng B, Kaufeld J, Miller E, Tossidou I, Englert C, et al. Cofilin-1 inactivation leads to proteinuria--studies in zebrafish, mice and humans. PLoS One. 2010;5(9):e12626. doi: 10.1371/journal.pone.0012626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiffer M, Teng B, Gu C, Shchedrina VA, Kasaikina M, Pham VA, et al. Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models. Nat Med. 2015;21(6):601–9. doi: 10.1038/nm.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2(7880):556–60. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 13.Muller-Deile J, Schiffer M. Podocyte directed therapy of nephrotic syndrome-can we bring the inside out? Pediatr Nephrol. 2016;31(3):393–405. doi: 10.1007/s00467-015-3116-4. [DOI] [PubMed] [Google Scholar]
- 14.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14(9):931–8. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaddanapudi S, Altintas MM, Kistler AD, Fernandez I, Moller CC, Wei C, et al. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J Clin Invest. 2011;121(10):3965–80. doi: 10.1172/JCI58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8(5):485–91. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 17.Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Young Choi H, Hyung Chang J, et al. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171(2):415–27. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Ding F, Wang S, Li B, Ding J. Cyclosporine A protects podocytes by regulating WAVE1 phosphorylation. Sci Rep. 2015;5:17694. doi: 10.1038/srep17694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Zhang X, Li X, Wang X, Wang S, Ding J. Cyclosporine A protects podocytes via stabilization of cofilin-1 expression in the unphosphorylated state. Exp Biol Med (Maywood) 2014;239(8):922–36. doi: 10.1177/1535370214530365. [DOI] [PubMed] [Google Scholar]
- 20.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113(10):1390–7. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369(25):2416–23. doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garin EH, Reiser J, Cara-Fuentes G, Wei C, Matar D, Wang H, et al. Case series: CTLA4-IgG1 therapy in minimal change disease and focal segmental glomerulosclerosis. Pediatr Nephrol. 2015;30(3):469–77. doi: 10.1007/s00467-014-2957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norlin J, Nielsen Fink L, Helding Kvist P, Douglas Galsgaard E, Coppieters K. Abatacept Treatment Does Not Preserve Renal Function in the Streptozocin-Induced Model of Diabetic Nephropathy. PLoS One. 2016;11(4):e0152315. doi: 10.1371/journal.pone.0152315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buscher AK, Beck BB, Melk A, Hoefele J, Kranz B, Bamborschke D, et al. Rapid Response to Cyclosporin A and Favorable Renal Outcome in Nongenetic Versus Genetic Steroid-Resistant Nephrotic Syndrome. Clin J Am Soc Nephrol. 2016;11(2):245–53. doi: 10.2215/CJN.07370715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravani P, Bonanni A, Ghiggeri GM. Randomised controlled trial comparing ofatumumab to rituximab in children with steroid-dependent and calcineurin inhibitor-dependent idiopathic nephrotic syndrome: study protocol. BMJ Open. 2017;7(3):e013319. doi: 10.1136/bmjopen-2016-013319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3(85):85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toto RD. Proteinuria reduction: mandatory consideration or option when selecting an antihypertensive agent? Curr Hypertens Rep. 2005;7(5):374–8. doi: 10.1007/s11906-005-0074-4. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Han GD, Miyauchi N, Hashimoto T, Nakatsue T, Fujioka Y, et al. Angiotensin II type 1 and type 2 receptors play opposite roles in regulating the barrier function of kidney glomerular capillary wall. Am J Pathol. 2007;170(6):1841–53. doi: 10.2353/ajpath.2007.060484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65(1):30–9. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 30.Gloy J, Henger A, Fischer KG, Nitschke R, Mundel P, Bleich M, et al. Angiotensin II depolarizes podocytes in the intact glomerulus of the Rat. J Clin Invest. 1997;99(11):2772–81. doi: 10.1172/JCI119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma R, Lovell HB, Wiegmann TB, Savin VJ. Vasoactive substances induce cytoskeletal changes in cultured rat glomerular epithelial cells. J Am Soc Nephrol. 1992;3(5):1131–8. doi: 10.1681/ASN.V351131. [DOI] [PubMed] [Google Scholar]
- 32.Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, et al. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol. 2011;179(4):1719–32. doi: 10.1016/j.ajpath.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perico L, Conti S, Benigni A, Remuzzi G. Podocyte-actin dynamics in health and disease. Nat Rev Nephrol. 2016;12(11):692–710. doi: 10.1038/nrneph.2016.127. [DOI] [PubMed] [Google Scholar]
- 34.Kriz W, Lemley KV. Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int. 2017;91(6):1283–6. doi: 10.1016/j.kint.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 35.Reiser J, Sever S, Faul C. Signal transduction in podocytes--spotlight on receptor tyrosine kinases. Nat Rev Nephrol. 2014;10(2):104–15. doi: 10.1038/nrneph.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterk LM, de Melker AA, Kramer D, Kuikman I, Chand A, Claessen N, et al. Glomerular extracellular matrix components and integrins. Cell Adhes Commun. 1998;5(3):177–92. doi: 10.3109/15419069809040290. [DOI] [PubMed] [Google Scholar]
- 37.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17(8):952–60. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei C, Sigdel TK, Sarwal MM, Reiser J. Circulating CD40 autoantibody and suPAR synergy drives glomerular injury. Ann Transl Med. 2015;3(19):300. doi: 10.3978/j.issn.2305-5839.2015.11.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spinale JM, Mariani LH, Kapoor S, Zhang J, Weyant R, Song PX, et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int. 2015;87(3):564–74. doi: 10.1038/ki.2014.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delville M, Sigdel TK, Wei C, Li J, Hsieh SC, Fornoni A, et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med. 2014;6(256):256ra136. doi: 10.1126/scitranslmed.3008538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim EY, Roshanravan H, Dryer SE. Changes in podocyte TRPC channels evoked by plasma and sera from patients with recurrent FSGS and by putative glomerular permeability factors. Biochim Biophys Acta. 2017;1863(9):2342–54. doi: 10.1016/j.bbadis.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayek SS, Koh KH, Grams ME, Wei C, Ko YA, Li J, et al. A tripartite complex of suPAR, APOL1 risk variants and alphavbeta3 integrin on podocytes mediates chronic kidney disease. Nat Med. 2017;23(8):945–53. doi: 10.1038/nm.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakoura N, Kavvadas P, Kormann R, Dussaule JC, Chadjichristos CE, Chatziantoniou C. NFkappaB-Induced Periostin Activates Integrin-beta3 Signaling to Promote Renal Injury in GN. J Am Soc Nephrol. 2017;28(5):1475–90. doi: 10.1681/ASN.2016070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maile LA, Busby WH, Gollahon KA, Flowers W, Garbacik N, Garbacik S, et al. Blocking ligand occupancy of the alphaVbeta3 integrin inhibits the development of nephropathy in diabetic pigs. Endocrinology. 2014;155(12):4665–75. doi: 10.1210/en.2014-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maile LA, Gollahon K, Wai C, Dunbar P, Busby W, Clemmons D. Blocking alphaVbeta3 integrin ligand occupancy inhibits the progression of albuminuria in diabetic rats. J Diabetes Res. 2014;2014:421827. doi: 10.1155/2014/421827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kistler AD, Altintas MM, Reiser J. Podocyte GTPases regulate kidney filter dynamics. Kidney Int. 2012;81(11):1053–5. doi: 10.1038/ki.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott RP, Hawley SP, Ruston J, Du J, Brakebusch C, Jones N, et al. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol. 2012;23(7):1149–54. doi: 10.1681/ASN.2011121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Ellis MJ, Gomez JA, Eisner W, Fennell W, Howell DN, et al. Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int. 2012;81(11):1075–85. doi: 10.1038/ki.2011.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22(9):1621–30. doi: 10.1681/ASN.2010111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akilesh S, Suleiman H, Yu H, Stander MC, Lavin P, Gbadegesin R, et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest. 2011;121(10):4127–37. doi: 10.1172/JCI46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14(12):1370–6. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 52.Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18(39):5373–80. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 53.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101(20):7618–23. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng Y, LoGrasso PV, Defert O, Li R. Rho Kinase (ROCK) Inhibitors and Their Therapeutic Potential. J Med Chem. 2016;59(6):2269–300. doi: 10.1021/acs.jmedchem.5b00683. [DOI] [PubMed] [Google Scholar]
- 55.Wakino S, Kanda T, Hayashi K. Rho/Rho kinase as a potential target for the treatment of renal disease. Drug News Perspect. 2005;18(10):639–43. doi: 10.1358/dnp.2005.18.10.959578. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Ellis MJ, Fields TA, Howell DN, Spurney RF. Beneficial effects of the Rho kinase inhibitor Y27632 in murine puromycin aminonucleoside nephrosis. Kidney Blood Press Res. 2008;31(2):111–21. doi: 10.1159/000121531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15(2):186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu C, Lee HW, Garborcauskas G, Reiser J, Gupta V, Sever S. Dynamin Autonomously Regulates Podocyte Focal Adhesion Maturation. J Am Soc Nephrol. 2017;28(2):446–51. doi: 10.1681/ASN.2016010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Versteilen AM, Blaauw N, Di Maggio F, Groeneveld AB, Sipkema P, Musters RJ, et al. rho-Kinase inhibition reduces early microvascular leukocyte accumulation in the rat kidney following ischemia-reperfusion injury: roles of nitric oxide and blood flow. Nephron Exp Nephrol. 2011;118(4):e79–86. doi: 10.1159/000322605. [DOI] [PubMed] [Google Scholar]
- 60.Schell C, Rogg M, Suhm M, Helmstadter M, Sellung D, Yasuda-Yamahara M, et al. The FERM protein EPB41L5 regulates actomyosin contractility and focal adhesion formation to maintain the kidney filtration barrier. Proc Natl Acad Sci U S A. 2017;114(23):E4621–E30. doi: 10.1073/pnas.1617004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang FY, Mei WL, Li YN, Tan GH, Dai HF, Guo JL, et al. The antitumour activities induced by pegylated liposomal cytochalasin D in murine models. Eur J Cancer. 2012;48(14):2260–9. doi: 10.1016/j.ejca.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 62.Konishi H, Kikuchi S, Ochiai T, Ikoma H, Kubota T, Ichikawa D, et al. Latrunculin a has a strong anticancer effect in a peritoneal dissemination model of human gastric cancer in mice. Anticancer Res. 2009;29(6):2091–7. [PubMed] [Google Scholar]
- 63.Shpetner HS, Vallee RB. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989;59(3):421–32. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- 64.Scaife R, Margolis RL. Biochemical and immunochemical analysis of rat brain dynamin interaction with microtubules and organelles in vivo and in vitro. J Cell Biol. 1990;111(6 Pt 2):3023–33. doi: 10.1083/jcb.111.6.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maeda K, Nakata T, Noda Y, Sato-Yoshitake R, Hirokawa N. Interaction of dynamin with microtubules: its structure and GTPase activity investigated by using highly purified dynamin. Mol Biol Cell. 1992;3(10):1181–94. doi: 10.1091/mbc.3.10.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanabe K, Takei K. Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. J Cell Biol. 2009;185(6):939–48. doi: 10.1083/jcb.200803153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antonny B, Burd C, De Camilli P, Chen E, Daumke O, Faelber K, et al. Membrane fission by dynamin: what we know and what we need to know. EMBO J. 2016;35(21):2270–84. doi: 10.15252/embj.201694613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sever S, Chang J, Gu C. Dynamin rings: not just for fission. Traffic. 2013;14(12):1194–9. doi: 10.1111/tra.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu C, Yaddanapudi S, Weins A, Osborn T, Reiser J, Pollak M, et al. Direct dynamin-actin interactions regulate the actin cytoskeleton. EMBO J. 2010;29(21):3593–606. doi: 10.1038/emboj.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol. 2012;13(2):75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soda K, Balkin DM, Ferguson SM, Paradise S, Milosevic I, Giovedi S, et al. Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest. 2012;122(12):4401–11. doi: 10.1172/JCI65289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Witke W, Podtelejnikov AV, Di Nardo A, Sutherland JD, Gurniak CB, Dotti C, et al. In mouse brain profilin I and profilin II associate with regulators of the endocytic pathway and actin assembly. EMBO J. 1998;17(4):967–76. doi: 10.1093/emboj/17.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merilainen J, Lehto VP, Wasenius VM. FAP52, a novel, SH3 domain-containing focal adhesion protein. J Biol Chem. 1997;272(37):23278–84. doi: 10.1074/jbc.272.37.23278. [DOI] [PubMed] [Google Scholar]
- 74.Qualmann B, Roos J, DiGregorio PJ, Kelly RB. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol Biol Cell. 1999;10(2):501–13. doi: 10.1091/mbc.10.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ritter B, Modregger J, Paulsson M, Plomann M. PACSIN 2, a novel member of the PACSIN family of cytoplasmic adapter proteins. FEBS Lett. 1999;454(3):356–62. doi: 10.1016/s0014-5793(99)00830-3. [DOI] [PubMed] [Google Scholar]
- 76.Gout I, Dhand R, Hiles ID, Fry MJ, Panayotou G, Das P, et al. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993;75(1):25–36. [PubMed] [Google Scholar]
- 77.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 78.Schafer DA. Regulating actin dynamics at membranes: a focus on dynamin. Traffic. 2004;5(7):463–9. doi: 10.1111/j.1600-0854.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 79.Gu C, Chang J, Shchedrina VA, Pham VA, Hartwig JH, Suphamungmee W, et al. Regulation of dynamin oligomerization in cells: the role of dynamin-actin interactions and its GTPase activity. Traffic. 2014;15(8):819–38. doi: 10.1111/tra.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palmer SE, Smaczynska-de R, II, Marklew CJ, Allwood EG, Mishra R, Johnson S, et al. A dynamin-actin interaction is required for vesicle scission during endocytosis in yeast. Curr Biol. 2015;25(7):868–78. doi: 10.1016/j.cub.2015.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gonzalez-Jamett AM, Momboisse F, Guerra MJ, Ory S, Baez-Matus X, Barraza N, et al. Dynamin-2 regulates fusion pore expansion and quantal release through a mechanism that involves actin dynamics in neuroendocrine chromaffin cells. PLoS One. 2013;8(8):e70638. doi: 10.1371/journal.pone.0070638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ji WK, Hatch AL, Merrill RA, Strack S, Higgs HN. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife. 2015;4:e11553. doi: 10.7554/eLife.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ono S, Kume S, Yasuda-Yamahara M, Yamahara K, Takeda N, Chin-Kanasaki M, et al. O-linked beta-N-acetylglucosamine modification of proteins is essential for foot process maturation and survival in podocytes. Nephrol Dial Transplant. 2017 doi: 10.1093/ndt/gfw463. [DOI] [PubMed] [Google Scholar]
- 84.Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80(8):868–78. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]