Abstract
Insulin resistance is the critical condition for the development of metabolic syndromes including type II diabetes and heart disease. To investigate the active components of Angelica dahurica root which is known to increase insulin sensitivity, its methanol extract was subfractionated. The ethyl acetate (EtOAc) fraction of the Angelica dahurica root extract significantly promoted adipocyte differentiation in 3T3-L1 preadipocyte cells. Among the three compounds isolated from the EtOAc extract (bergapten (1), imperatorin (2) and phellopterin (3)), phellopterin (3) induced the highest adipocyte differentiation at 25 and 50 μg/mL. In addition, treatment with imperatorin (2) and phellopterin (3) increased the mRNA expression of peroxisome proliferator-activated receptors γ (PPARγ). In diabetic animal model induced by high-fat diets (HFD) and streptozotocin (STZ), administration of phellopterin ((3), 1 mg/kg and 2 mg/kg) significantly reduced the levels of blood glucose, triglycerides and total cholesterol. Taken together, these results indicate that phellopterin (3) enhances adipocytes differentiation in 3T3-L1 preadipocytes, phellopterin (3) significantly prevents HFD/STZ-induced type Ⅱ diabetes. The present study also provides phellopterin (3) may be a valuable therapeutic alternative for enhancing insulin sensitivity through promotion of adipocyte differentiation and by increasing mRNA expression levels of PPARγ, which is a major mediator of insulin sensitivity.
Keywords: Pharmaceutical science, Physiology
1. Introduction
Angelica dahurica (Fisch. ex Hoffm.) Benth. & Hook. f. ex Franch. & Sav. is a perennial herb belonging to the Umbelliferae family and contains various types of total coumarins [1, 2, 3, 4]. Thus, the dried roots have been used in Korean traditional medicine as a treatment for erythema, pains, sinusitis, and inflammation [1, 2].
The peroxisome proliferator-activated receptors γ (PPARγ) plays a role in lowering insulin resistance by modulating the metabolism of fatty acids [3, 4]. PPARγ regulates adipocyte differentiation and increases the number of small insulin-sensitive adipocytes, which are improtant in imparting insulin sensitivity [4]. Thus, PPARγ is one of the targets of anti-diabetic agents [5], and combining such agents with others that have different targets, can further improves insulin sensitivity [6]. PPARγ agonist activity, determined indirectly by adipocyte differentiation and triglyceride accumulation, indicates potential antidiabetic property [7]. Rosiglitazone, an agonist of PPARγ, has been widely used as an anti-diabetic drug for the treatment of the type II, but its adverse effects on the cardiovascular system, including increasing the risk of myocardial infarction, is a clinical concern [8, 9].
To search for a PPARγ mimetic with anti-diabetic activity from a natural source, we determined the adipocyte differentiation potential in 3T3-L1 cells treated with methanol extract from A. dahurica or with its subfractions. Three compounds, bergapten (1), imperatorin (2) and phellopterin (3), were obtained from the EtOAc fraction of A. dahurica. Phellopterin (3) is a naturally occurring furanocoumarin and its role as a partial benzodiazepine receptor has been described [10]. However, the anti-diabetic activity of phellopterin (3) has not been clearly identified. In this study, not only the adipocyte differentiation rate and up-regulation of the PPARγ mRNA level by phellopterin (3) treatment were determined, also the improvement in the type Ⅱ diabetic status of streptozotocin (STZ)-induced diabetic mice treated with phellopterin (3) was assessed.
2. Results and discussion
Increase in adipocyte differentiation rate indicates that cells become more sensitive to insulin [4]. To identify the effects of Angelica dahurica on adipocyte differentiation, 3T3-L1 cells were exposed to various concentrations of Angelica dahurica root extract (ADE) or its subsequent fractions. ADE was fractionated with n-hexane, dichloromethane, ethyl acetate, n-butanol, and distilled water (Fig. 1). The ADEs did not show any significant cell cytotoxicity up to 100 μg/mL (data not shown). Although both the ADE and its ethyl acetate (EtOAc) subfraction significantly promoted adipocyte differentiation in a dose-dependent manner, the EtOAc fraction was most efficient (Table 1).
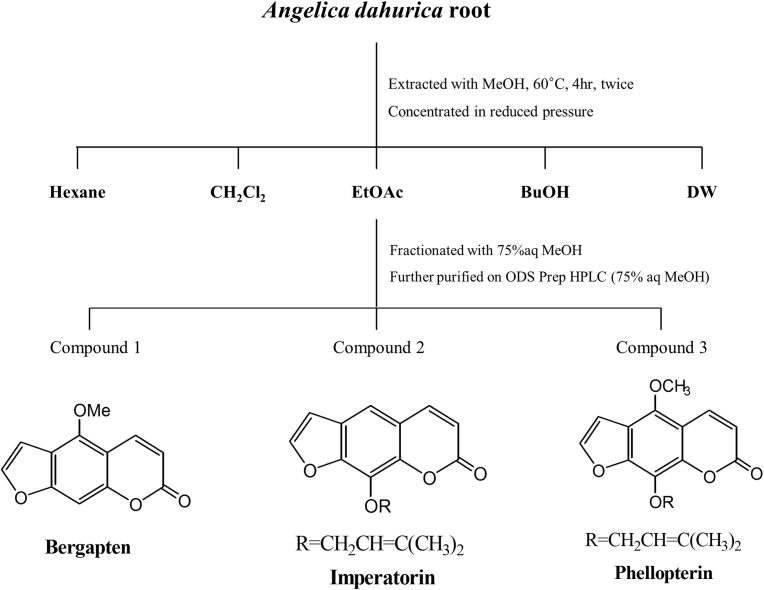
Fig. 1.
Isolation of bergapten, imperatorin, and phellopterin from Angelica dahurica root. CH2Cl2, dichloromethane; EtOAc, ethyl acetate; BuOH, n-butanol; DW, distilled water; MeOH, methanol.
Table 1.
Changes in adipocyte differentiation with treatments of Angelica dahurica extract and its fractions in 3T3-L1 cells.1
| Extract | Fraction | Sub-fractionated and purified | Adipocyte differentiation (%) |
|||
|---|---|---|---|---|---|---|
| Treatment concentration | ||||||
| 12.5 μg/mL | 25 μg/mL | 50 μg/mL | 100 μg/mL | |||
| ADE2 |
NE3 |
115.8 ± 1.43∗ |
120.4 ± 2.04* |
130.5 ± 1.29* |
||
| ADE | n-hexane | NE | 86.7 ± 5.64∗ | 94.6 ± 3.78** | 90.1 ± 2.68** | |
| CH2Cl2 | NE | 84.5 ± 5.36∗∗ | 90.4 ± 7.26** | 92.3 ± 3.93** | ||
| EtOAc | NE | 214.5 ± 4.03∗ | 228.4 ± 3.15** | 245.6 ± 3.67** | ||
| n-BuOH | NE | 72.6 ± 3.68∗ | 80.1 ± 4.97** | 80.6 ± 4.64** | ||
| H2O |
NE |
68.3 ± 4.23∗ |
70.3 ± 4.69* |
77.6 ± 5.06* |
||
| EtOAc | Bergapten (1) | 123.76 ± 9.00 | 127.92 ± 16.86 | 91.70 ± 8.35 | NE3 | |
| Imperatorin (2) | 150.89 ± 18.15∗ | 169.73 ± 51.62∗ | 189.60 ± 22.95* | NE | ||
| Phellopterin (3) |
223.43 ± 23.10∗ |
334.06 ± 64.28∗∗ |
336.30 ± 15.16** |
NE |
||
| Rosiglitazone | 239.31 ± 26.95∗ | 247.7 ± 4.65∗∗ | 250.7 ± 6.34∗∗ | 330.7 ± 5.64∗∗ | ||
∗P < 0.05; ∗∗P < 0.01 vs. untreated control.
All values (the means ± SEM) are expressed as a percentage of untreated control (n = 3).
Angelica dahurica extract with methanol.
No experiment.
Since the EtOAc fraction effectively stimulated adipocyte differentiation compared to the other fractions, we further subfractionated the EtOAc fraction with 75% aq. MeOH and then separated the individual compounds using ODS Prep HPLC (5 nm ODS, 75% aq. MeOH, Waters Spherisorb, Ireland). The structures of the three compounds isolated were identified using 1H-NMR and 13C-NMR spectroscopy.
Bergapten (1): 1H-NMR (500 MHz, CD3OD) δ8.08 (1H, d, J = 9.8 Hz, H-4), 7.72 (1H, d, J = 2.5 Hz, H-2′), 7.14 (1H, d, J = 2.5 Hz, H-3′), 7.00 (1H, s, H-8), 6.20 (1H, d, J = 9.8, H-3); 13C-NMR (125 MHz, CD3OD) δ161.33 (C-2), 158.22 (C-7), 152.20 (C-8a), 149.13 (C-5), 144.45 (C-2′), 140.02 (C-4), 114.45 (C-6), 113.58 (C-3), 106.84 (C-4a), 106.60 (C-3′), 93.01 (C-8), 59.88 (5-OCH3).
Imperatorin (2): 1H-NMR (500 MHz, CD3OD) δ7.95 (1H, d, J = 9.6 Hz, H-4), 7.85 (1H, d, J = 2.1 Hz, H-2′), 7.47 (1H, s, H-5), 6.90 (1H, d, J = 2.1 Hz, H-3′), 6.34 (1H, d, J = 9.6 Hz, H-3), 5.52 (1H, t, J = 7.0 Hz, H-2″), 4.92 (2H, d, J = 7.0Hz, H-1″), 1.69 (3H, s, H-5″), 1.65 (3H, s, H-4″); 13C-NMR (125 MHz, CD3OD) δ161.42 (C-2), 148.44 (C-7), 147.02 (C-2′), 145.34 (C-8), 143.45 (C-4), 139.53 (C-8a), 130.92 (C-3″), 126.30 (C-6), 119.49 (C-2″), 116.44 (C-4a), 113.65 (C-5), 113.42 (C-3), 106.54 (C-3′), 69.47 (C-1″), 24.52 (C-5″), 16.70 (C-4″).
Phellopterin (3): 1H-NMR (500 MHz, CD3OD) δ8.11 (1H, d, J = 9.6 Hz, H-4), 7.80 (1H, d, J = 2.3 Hz, H-2′), 7.19 (1H, d, J = 2.3 Hz, H-3′), 6.24 (1H, d, J = 9.6 Hz, H-3), 5.53 (1H, t, J = 7.0 Hz, H-2″), 4.89 (1H, d, J = 7.0 Hz, H-1″), 4.20 (3H, s, 5-OCH3), 1.70 (3H, s, H-4″), 1.64 (3H, s, H-5″); 13C-NMR (125 MHz, CD3OD) δ161.33 (C-2), 150.90 (C-7), 145.45 (C-2′), 145.45 (C-3″), 144.69 (C-8a), 144.00 (C-5), 140.05 (C-4), 139.46 (C-8), 119.55 (C-2″), 114.46 (C-6), 111.48 (C-3), 106.95 (C-4a), 105.01 (C-3′), 69.64 (C-1″), 59.91 (5-OCH3), 24.50 (C-4″), 16.61 (C-5″).
We also evaluated the differences in adipocyte differentiation when cells were treated with 12.5, 25 and 50 μg/mL concentrations of the identified compounds (Table 1). In the case of Bergapten (1), cytotoxicity was observed at a concentration of 50 μg/mL or later. And Imperatorin (2) and Phellopterin (3) were cytotoxic at a concentration of 100 μg/mL (Not showing data). For this reason, we conducted experiments at concentrations of 12.5, 25 and 50 μg/mL. Rosiglitazone strongly promoted adipocyte differentiation at a relatively low dose (12.5 μg/mL). Bergapten (1) did not induce adipocyte differentiation compared to the control. Both imperatorin (2) and phellopterin (3) significantly increased the rate of adipocyte differentiation compared to the control at 12.5, 25 and 50 μg/mL. Interestingly, treatment of phellopterin (3) increased adipocyte differentiation to higher levels than that seen in rosiglitazone-treated cells. This result suggests that phellopterin (3) has an adipocyte differentiation potential similar to rosiglitazone.
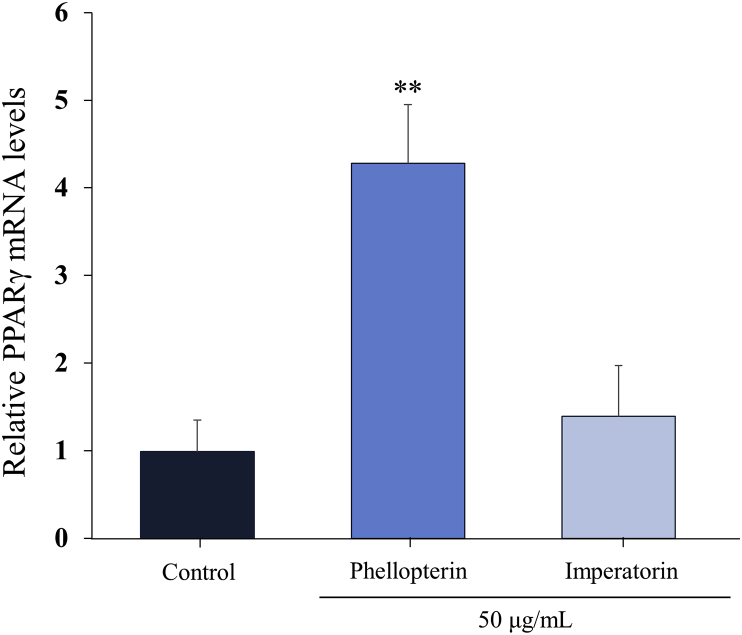
Adipocyte differentiation can be prompted by the action of PPARγ [4]. PPARγ isoforms have been identified as key regulators of glucose absorption, lipid metabolism, proliferation, and cellular differentiation [11]. PPARγ agonist thiazolidinedione have been widely used as insulin sensitizers for treatment of type II diabetes mellitus for more than 20 years [12, 13]. It shown a significant improvement of tissue (muscle and adipocytes) sensitivity to the effects of insulin, which is used in the treatment of type II diabetes mellitus [11]. Thus, we tested the effect of phellopterin (3) on changes in the PPARγ mRNA level using quantitative RT-PCR. Treatment with phellopterin ((3), 50 μg/mL) significantly augmented the mRNA expression level of PPARγ compared to control (4.30 ± 0.67 vs. 1.00 ± 0.35), whereas treatment with imperatorin (2) did not (1.40 ± 0.59). Based on these results, it is speculated that the adipocyte differentiating potential of phellopterin (3) may be, at least in part, induced by its augmentation of the mRNA expression of PPARγ in preadipocytes (Fig. 2).
Fig. 2.
Changes in mRNA expression level of PPARγ by phellopterin and imperatorin. The mRNA expression level of PPARγ on 3T3-L1 cells were determined by qRT-PCR method. ∗∗P < 0.01 vs. control.
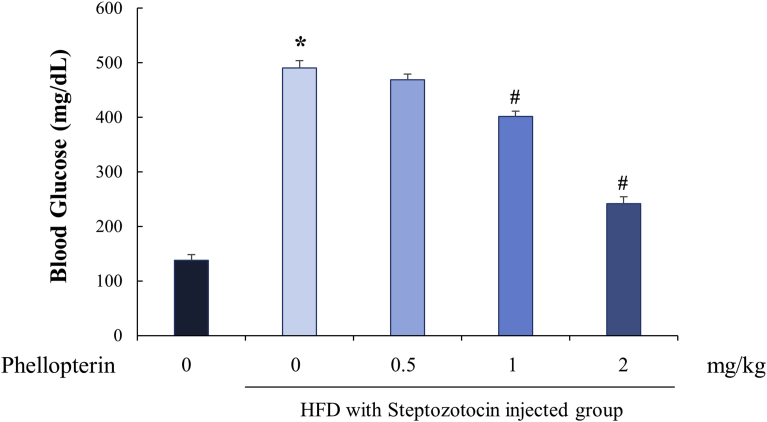
Next, we tested whether these actions of phellopterin (3) could improve insulin resistance and attenuate blood glucose levels, in the diabetic status of HFD/STZ-induced type Ⅱ diabetic mice. Because, the animal model by feeding with high-fat diet following STZ that would closely mimic the metabolism characteristics of human type II diabetes mellitus [14]. As shown in Table 2 and Fig. 3, HFD/STZ mice showed impairments in the regulation of their blood glucose and in their lipid metabolisms, which resulted in highly increased glucose, triglyceride, and total-cholesterol levels. However, through the administration of phellopterin ((3), 1 and 2 mg/kg) improved the levels of blood glucose, triglyceride and total-cholesterol in HFD/STZ mice, and this role of phellopterin appears to be concentration-dependent. This result indicates that administration of phellopterin (3) could be helpful in reducing blood glucose levels and improving lipid profiles, which are beneficial in the treatment and prevention of diabetes.
Table 2.
Effect of phellopterin treatment on the changes of triglyceride and total-cholesterol level in high-fat diet/streptozotocin (HFD/STZ)-induced diabetic mice (n = 6).
| Normal diet group | HFD with STZ injected group |
||||
|---|---|---|---|---|---|
| Treatment concentration of phellopterin (mg/kg) | |||||
| 0 | 0.5 | 1 | 2 | ||
| Body weight (g) | 32.7 ± 1.13 | 37.9 ± 1.63∗ | 37.3 ± 2.13 | 33.1 ± 1.67# | 31.1 ± 2.27# |
| Food intake (g/day) | 4.11 ± 0.61 | 5.34 ± 0.56∗ | 5.24 ± 0.64 | 5.15 ± 0.81 | 5.05 ± 0.81 |
| Total-cholesterol (mg/dL) | 520.4 ± 6.21 | 1154.8 ± 97.64∗ | 997.5 ± 70.68 | 864.4 ± 53.14# | 630.4 ± 46.78# |
| Triglyceride (mg/dL) | 170.6 ± 25.64 | 541.6 ± 26.87∗ | 481.6 ± 17.45# | 446.7 ± 18.65# | 346.7 ± 20.17# |
∗P < 0.05 vs. normal diet group.
#P < 0.05 vs. HFD with STZ injected group.
Fig. 3.
Effect of phellopterin treatment on the changes of blood glucose level in high-fat diet/streptozotocin (HFD/STZ)-induced diabetic mice (n = 6). ∗P < 0.05 vs. normal diet group and #P < 0.05 vs. HFD with STZ injected group.
3. Conclusion
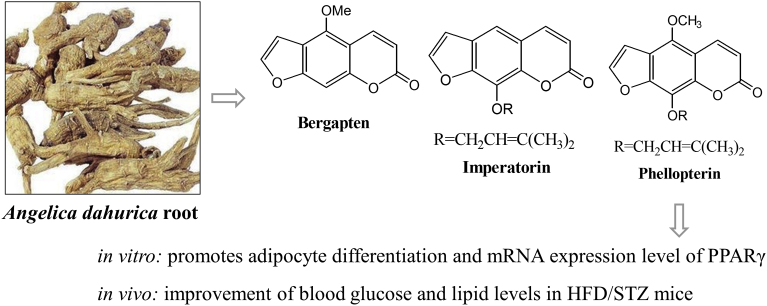
Phellopterin (3), a naturally occurring furanocoumarin isolated from A. dahurica promoted adipocyte differentiation and augmented the mRNA expression level of PPARγ. PPARγ has been identified as a therapeutic target for the prevention and treatment of both insulin resistance and metabolic syndrome. The results of present study revealed that A. dahurica and its major component, phellopterin, efficiently induced adipocyte differentiation, suggesting that it functions as a PPARγ agonist. These anti-diabetic actions resulted in the improvement of blood glucose and lipid levels in HFD/STZ mice. Therefore, the insulin-like differentiation-inducing activity of phellopterin (3) may be valuable as an interventional strategy for diabetic patients, and should be investigated further as a starting compound for the development of anti-diabetic drugs and PPARγ mimetics (see Fig. 4).
Fig. 4.
Summary of the present study. Phellopterin, isolated from Angelica dahurica root, promotes adipocyte differentiation and mRNA expression level of PPARγ. Also improvement of blood glucose and lipid levels in HFD/STZ mice. Thus, phellopterin has the potential to be developed as PPARγ mimetics in the future.
4. Materials and methods
4.1. Materials
3-Isobutyl-1-methylxanthine, insulin, and dexamethasone were purchased from Sigma (St. Louis, MO, USA). Unless indicated otherwise, chemicals were obtained from Sigma. Dulbecco's modified Eagle's medium (DMEM) along with bovine calf serum (BCS), fetal bovine serum (FBS) and antibiotics were obtained from GIBCO/BRL Life Technologies, Inc. (NY, USA). AdipoRed Assay Reagent, a product of Lonza Walkersville Inc. (Walkersville, MD, USA), was used to measure adipocyte differentiation.
4.2. Preparation of Angelica dahurica extracts
Angelica dahurica plants were collected from a farm located at Bongwha, Gyeongsangbuk-do, and proliferated at the experiment farm of the Agricultural Research Station, Dongguk University, located at Goyang, Gyeonggi-do. Samples were collected in August 2014. Dr. Se Chan Kang identified the specimens and the voucher specimen of Angelica dahurica (NMR-KR-14-00159) was deposited at the herbarium of Department of Oriental Medicine Biotechnology, Kyung Hee University. The crude extract was obtained by extracting 1 kg of dried plant twice with methanol for 4 hours at 60 °C. The extracts were concentrated for 16 hours at reduced pressure and 40 °C using a rotary evaporator, and then stored at −20 °C until use. The yield of the extracts was about 19%.
4.3. Animals and treatment with the isolated compounds
Male ICR strain mice were purchased from Korea Laboratory Animal Co. (Daejeon, Korea) and were allowed to acclimatize for 2 weeks prior to the experiments. The animals were maintained under standard laboratory conditions: temperature of 21 ± 2 °C, relative humidity of 50 ± 5% and a normal photoperiod (12 h dark/light). The experimental procedures and animal care protocols were approved by the Animal Care and Use Committee of Kyung Hee University, and conformed to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH Publication No. 85-23). After having been fed a high-fat diet (HFD; rodent diet in which 60% of the energy comes from fat, Research Diet, USA) for 4 weeks, the mice were administered streptozotocin (STZ, Sigma, 100 mg/kg) dissolved in 0.1 M citrate buffer (pH 4.5) by peritoneal injection to induce type Ⅱ diabetes.
Diabetic status was determined two weeks after receiving STZ using a glucometer (Glucotrend, Roche, Germany). Mice with blood glucose concentrations less than 250 mg/dL were excluded from subsequent experiments. Intragastric delivery of saline or phellopterin ((3), 0–2 mg/kg) was carefully performed for 4 weeks. An appropriate dosing volume of 0.5 mL of saline or phellopterin (3) was determined after weighing the animals daily. At the end of the experiment, blood samples were collected from the posterior vena cava of each animal under ether anesthesia. After obtaining the serum by centrifugation (2000 x g, 15 min), glucose, triglyceride and total-cholesterol levels were analyzed using a HEMAVET950 analyzer (Drew, USA).
4.4. Cell culture and adipocyte differentiation
Mouse 3T3-L1 preadipocytes (American Type Culture Collection (Manassas, VA, USA)) were incubated in DMEM containing 10% BCS along with 100 U/mL penicillin and 100 mg/mL streptomycin under 5% CO2, at 37 °C. Cells in the confluent state were cultured in DMEM supplemented with 0.5 mM 3-isobutyl-1-methylxanthine, 10 μg/mL insulin, 0.25 μM dexamethasone and 10% FBS for 2 days, and then transferred into DMEM containing 10% FBS only.
The 3T3-L1 preadipocytes were treated with various concentrations of the isolated compounds for 9 days, and were compared to an untreated control and positive control. The cells were then immobilized with phosphate buffered saline (PBS) containing 3% formaldehyde for 1 hour at room temperature, and washed three times with PBS. In order to analyze the rate of adipocyte differentiation, the immobilized cells were treated using AdipoRed Assay Reagent for 10 minutes at room temperature and the degree of fluorescence was measured (485 nm excitation and 572 nm emission).
4.5. Quantitative RT-PCR analysis
Total RNA was extracted from 3T3-L1 adipocytes, after induced differentiation for 8 days, using PureLink RNA Mini Kit (Ambion, USA). One microgram of the total RNA was reversely transcribed with oligo (dT) primers, using the enzyme and buffer supplied in the PrimeScriptⅡ 1st strand cDNA Synthesis kit (Takara, Japan). Quantitative RT-PCR (qRT-PCR) reactions were performed on a MX3005P (Stratagene, USA). The primers used in the experiments were as follows: PPARγ (forward primer: 5′-CAAGAATACCAAAGTGCGATCAA-3′, reverse primer: 5′-GAGCTGGGTCTTTTCAGAATAATAAG-3′) and β-actin (forward primer: 5′-GAGACCTTCAACACCCC-3′, reverse primer: 5′-GTGGTGGTGAAGCTGTAGCC-3′) [15].
For qRT-PCR, SYBR Premix Ex Taq Ⅱ (Takara, Japan) was used. The thermal cycling profile consisted of a pre-incubation step at 95 °C for 10 min, followed by 40 cycles of 95 °C (15 s) and 60 °C (60 s). Relative quantitative evaluations of PPARγ and β-actin levels were performed by the comparative CT (cycle threshold) method [16].
4.6. Statistical analysis
All results are presented as means ± the standard error of the mean. Statistical significance of group differences as determined with one-way analysis of variance, and individual differences between the means of groups were analyzed using a modified t-test with Bonferroni correction; ∗P < 0.05 and ∗∗P < 0.01 were considered significant.
Declarations
Author contribution statement
Hyo Sang Han: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hyelin Jeon: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Se Chan Kang: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was financially supported by the Kyung Hee University in 2016 under grant (KHU-20160597).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kang S.W., Kim C.Y., Song D.G., Pan C.H., Cha K.H., Lee D.Y., Um B.H. Rapid identification of ruranocoumarins in Angelica dahurica using the Online LC-MMR-MS and their nitric oxide inhibitory activity in RAW264.87 cells. Phytochem. Anal. 2010;21:322–327. doi: 10.1002/pca.1202. [DOI] [PubMed] [Google Scholar]
- 2.Sarker S.D., Nahar L. Natural medicine: the genus Angelica. Curr. Med. Chem. 2004;11:1479–1500. doi: 10.2174/0929867043365189. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia V., Viswanathan P. Insulin resistance and PPAR insulin sensitizers. Curr. Opin. Investig. Drugs. 2006;7:891–897. PMID: 17086933. [PubMed] [Google Scholar]
- 4.Lehrke M., Lazar M.A. The many faces of PPAR gamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee R., Davies P.J., Crombie D.L., Bischoff E.D., Cesario R.M., Jow L., Hamann L.G., Boehm M.F., Mondon C.E., Nadzan A.M., Paterniti J.R., Heyman R.A. Sensitization of diabetic and obese mice to insulin by retinoid X receptor aonists. Nature. 1997;386 doi: 10.1038/386407a0. 407–140. [DOI] [PubMed] [Google Scholar]
- 6.Kahn S.E., Haffner S.M., Heise M.A., Herman W.H., Holman R.R., Jones N.P., Kravitz B.G., Lachin J.M., O'Neill M.C., Zinman B., Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 7.Larsen T.M., Toubro S., Astrup A. PPAR gamma agonists in the treatment of type Ⅱ diabetes: is increased fatness commensurate with long-term efficacy? Int. J. Obes. Relat. Metab. Disord. 2003;27:147–161. doi: 10.1038/sj.ijo.802223. [DOI] [PubMed] [Google Scholar]
- 8.Erdmann E., Charbonnel B., Wilcox R. Thiazolidinediones and cardiovascular risk - a question of balance. Curr. Cardiol. Rev. 2009;5:155–165. doi: 10.2174/157340309788970333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 10.Dekermendjian K., Ai J., Nielsen M., Sterner O., Shan R., Wittt M.-R. Characterization of the furanocoumarin phellopterin as a rat brain benzodiazepine receptor partial. Neurosci. Lett. 1996;211:151–154. doi: 10.1016/s0304-3940(96)13183-9. [DOI] [PubMed] [Google Scholar]
- 11.Bermúdez V., Finol F., Parra N., Parra M., Pérez A., Peñaranda L., Vílchez D., Rojas J., Arráiz N., Velasco M. PPAR-gamma agonists and their role in type 2 diabetes mellitus management. Am. J. Ther. 2010;17:274–283. doi: 10.1097/MJT.0b013e3181c08081. [DOI] [PubMed] [Google Scholar]
- 12.Koh Y.J., Park B.H., Park J.H., Han J., Lee I.K., Park J.W., Koh G.Y. Activation of PPAR gamma induces profound multilocularization of adipocytes in adult mouse white adipose tissues. Exp. Mol. Med. 2009;41:880–895. doi: 10.3858/emm.2009.41.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell E., Kuhn P., Xu W. Nuclear receptor coractors in PPARgamma-mediated adipogenesis and adipocyte energy metabolism. PPAR Res. 2007;2007:53843. doi: 10.1155/2007/53843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo H.J., Kwak J.H., Kang S.C. Anti-diabetic properties of Daphniphyllum macropodum fruit and its active compound. Biosci. Biotechnol. Biochem. 2014;78:1392–1401. doi: 10.1080/09168451.2014.923289. [DOI] [PubMed] [Google Scholar]
- 15.Huang C., Zhang Y., Gong Z., Sheng X., Li Z., Zhang W., Qin Y. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARgamma pathway. Biochem. Biophys. Res. Commun. 2006;348:571–578. doi: 10.1016/j.bbrc.2006.07.095. [DOI] [PubMed] [Google Scholar]
- 16.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]