Abstract
The cyanobacterium Aphanizomenon flos-aquae (AFA), from Upper-Klamath Lake, Oregon, are used to produce blue-green algal (BGA) dietary supplements. The periodic co-occurrence of hepatotoxin-producing contaminant species prompted the Oregon Health Division to establish a limit of 1 μg/g microcystin (MC) for products sold in Oregon in 1997. At the federal level, the current good manufacturing practice (CGMP) regulations for dietary supplements require manufacturers establish a specification, and test, for limits on contaminants that may adulterate finished products. Despite this, several previous international surveys reported MC in BGA supplements in excess of 1 μg/g. The objectives of this study were (1) identify a reliable, easy to use test kit for the detection of MC in dried BGA materials and (2) use this kit to assess the occurrence of MC contamination in AFA-BGA dietary supplements in the U.S. A commercial protein phosphatase inhibition assay (PPIA), based on the enzyme PP2A, was found to have acceptable relative enzyme inhibition and accuracy for the majority of MC variants tested, including those most commonly identified in commercial samples, making the kit fit for purpose. Using the PPIA kit, 51% (26 of 51) distinct AFA-BGA products had MC ≥0.25 μg/g (the detection limit of the kit), 10 products had MC concentrations between 0.5 and 1.0 μg/g, and 4 products exceeded the limit (1.1–2.8 μg/g). LC-MS/MS confirmed PPIA results ≥0.5 μg/g and determined that MC-LA and MC-LR were the main congeners present. PPIA is a reliable method for the detection of MC contamination in dried BGA dietary supplements produced in the U.S. While the majority of AFA-BGA products contained ≥0.25 μg/g MC, most were at or below 1.0 μg/g, suggesting that manufacturers have adopted this level as a specification in these products; however, variability in recommended serving sizes prevented further analysis of consumer exposure based on the concentrations of MC contamination found.
Keywords: Food safety, Food analysis
1. Introduction
Numerous dietary supplement products containing either blue-green algae (BGA) or green algae, primarily the cyanobacterial species Aphanizomenon flos-aquae, Spirulina (Arthrospira platensis), and/or the chlorophyte Chlorella (C. pyrenoidosa, C. vulgaris, or C. regularis), are currently available to U.S. consumers. Within these products, these species can occur either alone or in combination, as powders, liquids, capsules, or tablets, and either as the main ingredient or mixed with multiple additional natural ingredients. Whereas Chlorella and Spirulina raw ingredients are commonly produced through aquaculture, Aphanizomenon flos-aquae (AFA) is wild harvested, mainly from Upper-Klamath Lake (UKL), Klamath Falls, Oregon where it currently supports a $100,000,000 annual health supplement industry based on the harvested and dried material (Oregon Department of Agriculture, 2015). Carmichael et al. (2000) described the processes commonly used to harvest AFA from UKL, including quality assurance/quality control (QA/QC) programs typically used during harvesting and production to assure product quality and safety. Among these QA/QC procedures were methods used to screen for the presence of neurotoxins and hepatotoxins that can be produced by various co-occurring cyanobacterial species. As noted by Carmichael et al. (2000), cyanobacterial species belonging to the genus Microcystis and Oscillatoria are normal components in Klamath Lake, with both genera being shown to produce toxic cyclic peptides (microcystins and nodularins; herein referred to as MC) that can contaminate AFA raw materials. All MC possess a common chemical structure comprised of seven amino acids: three common d-amino acids, 2 novel nonproteinogenic d-amino acids (Mdha and Adda which both play important roles in bioactivity), and 2 variable l-amino acids, after which most of the MC variants are named (e.g. MC-LR = leucine (L), arginine (R) in the 2 variable positions) (Duy et al., 2000). Around 100 MC variants have been described thus far involving substitution of the two l-amino acids and/or methylation or demethylation at selected sites within the cyclic peptide (Duy et al., 2000, US EPA, 2015). MC is a potent hepatotoxin and liver carcinogen and is a threat to both humans and livestock worldwide, mainly through exposure to contaminated recreational and drinking waters (US EPA, 2015). Due to this potential issue of MC contamination in AFA raw materials from UKL, the State of Oregon's Health Division estimated a safe concentration of 1 μg MC per g dry weight for BGA products consumed by adults, which the Oregon Department of Agriculture adopted as a regulatory standard for products sold in Oregon in 1997 (Gilroy et al., 2000). Despite this limit, researchers in Canada (Lawrence et al., 2001), Germany (Heussner et al., 2012), and Italy (Vichi et al., 2012) reported commercially available BGA products, containing AFA from UKL, with MC at or above the 1 μg/g limit. The primary objective of this study was to evaluate the presence and concentrations of MC in AFA-containing BGA dietary supplements currently available to U.S. consumers.
Numerous methods have been employed for screening or confirmation of MC contamination in BGA-containing dietary supplement products including enzyme-linked immunosorbent assays (ELISAs) typically targeting the conserved Adda group, antibody-based surface plasmon resonance (SPR) biosensors, DNA-based polymerase chain reaction (PCR) assays screening for the presence of MC producing genes (i.e., screening for the potential for MC production), and chemical confirmatory methods such as Liquid Chromatography with UV detection (LC-UV) and Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) (Lawrence et al., 2001; Heussner et al., 2012; Vichi et al., 2012; Parker et al., 2015; Yakes et al., 2015). The mechanism of hepatotoxicity of MC to mammals involves the potent inhibition of protein phosphatases comprising PP1, PP2A, and related enzymes, which dephosphorylate serine, threonine, and histidine residues in proteins and are critical to the control of several biological processes (MacKintosh et al., 1995). This bioactivity has been exploited to develop in-vitro assays based on protein phosphatase inhibition measuring the inhibitory effects of MC on either PP1 or PP2A towards p-nitrophenyl phosphate (An and Carmichael, 1994; Heresztyn and Nicholson, 2001). These Protein Phosphatase Inhibition Assays (PPIAs) have also been used successfully to monitor BGA dietary supplements for MC contamination (Heussner et al., 2012), but to date the lack of a verified commercially available PPIA test kit has limited the standardization required for the routine use of PPIA assays as a regulatory monitoring tool. Furthermore, most of the existing rapid monitoring tools for the detection of MC, including PPIAs, were designed for the testing of drinking and source waters with calibration at either the World Health Organization (WHO) provisional guideline value of 1 μg/L MC in drinking water (WHO, 1998), or at the recreational water exposure response guidelines that have been adopted by several U.S. states [range 4–20 μg/L] (https://www.epa.gov/nutrient-policy-data/guidelines-and-recommendations) [Accessed 3 January 2018]. One such commercially available kit is the PPIA-based MicroCystest (ZEULAB S.L., Zaragoza, Spain) (Sevilla et al., 2009) which was previously evaluated for the testing of source waters through the Environmental Technology Verification (ETV) Program of the U.S. Environmental Protection Agency (EPA) (McKernan et al., 2011), and is now distributed in the U.S. as the Microcystin/Nodularin PP2A Kit (Abraxis LLC, Warminster, Pennsylvania). At present, the kit as sold is designed for testing water samples, but the manufacturer has developed a modified protocol, supplied upon request, to specifically test dried BGA materials as are found in BGA dietary supplements (Elena Dominguez, ZEULAB, personnel communication). To accomplish the primary objective of this study we first evaluated the performance of the MicroCystest for use on BGA-containing dietary supplements, and then used this test to assess the prevalence of MC contamination above the Oregon 1 μg/g limit in BGA dietary supplements currently available to U.S. consumers. For confirmation, LC-MS/MS was performed following the previously validated method of Parker et al. (2015) on any samples equal to or exceeding 0.5 μg/g MC.
2. Materials and methods
2.1. Standards and reagents
Lyophilized analytical standards of microcystin-LA, LF, LR, LW, LY, RR, YR, and nodularin, all with manufacturer reported purities of ≥95%, were purchased from Enzo Life Sciences, Inc. (Framingdale, New York, USA). Stock solutions were prepared at concentrations of 10 μg/mL in methanol. Certified reference materials (CRMs) of microcystin-LR, RR, and nodularin, in methanol, as well as anatoxin-a, cylindrospermopsin, and saxitoxin were purchased from the National Research Council Canada (Ottawa, Ontario, Canada). All MC standards were stored in the dark at −20 °C. All other standards were stored according to the manufacturer's recommendations. For LC-MS/MS and PPIA analyses, Optima LC/MS Grade acetonitrile, methanol, and water were purchased from Fisher Scientific (Pittsburgh, PA, USA) and used for sample preparation and LC eluents. Trifluoroacetic acid (Chromasolv® for HPLC, >99.0%) and Tween 20 for PPIA extractions were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.2. Data analysis
All statistical analyses were performed using GraphPad Prism v5.01 (GraphPad Software Inc., La Jolla, CA, USA).
2.3. Evaluation of a commercial PPIA kit for the testing of dried BGA dietary supplements
Microcystin/Nodularin PP2A microtiter plate test kits (Product No. 520032) for the detection of total MCs in water were purchased from Abraxis LLC (Warminster, Pennsylvania, USA). A supplemental procedure for the analysis of dried algae as dietary supplements, detailed below, was provided by ZEULAB S.L. (Zaragoza, Spain). The kits were used as directed according to the manufacturer's recommendations in terms of dynamic range, storage, and stability.
2.3.1. Sample preparation and analysis by PPIA
A supplemental procedure for the analysis of dried algae as dietary supplements was provided by ZEULAB S.L. (Zaragoza, Spain): 100 mg of sample was weighed into a 15 mL disposable polypropylene centrifuge tube followed by the addition of 5 mL of 80:20 (v/v) methanol/deionized water containing 0.1% (v/v) trifluoroacetic acid and 0.1% (v/v) Tween 20. Next, samples were mixed gently for 30 minutes at room temperature, protected from light, using a roller mixer at 33 rpm (Stuart, Bibby Scientific Limited, Staffordshire, UK), centrifuged at 4,000 × g for 5 minutes, and a sub-sample of the supernatant was diluted 1:20 with deionized water. From this point on, samples were analyzed according to the kit included instructions. MC concentration (in μg/g) was calculated using the following equation: C (μg/L) × FD × V (L)/M (g), where C = MC concentration in the assay (μg/L), FD = dilution factor (20), V = volume of the extract solution (0.005 L), M = sample weight (0.1 g).
2.3.2. Relative enzyme inhibition
The relative inhibition of the PP2A enzyme used in the MicroCystest kit was measured as the response of the various commercially available MCs that could potentially occur in BGA-containing dietary supplements relative to the kit provided MC-LR standard. Standard curves were prepared in deionized water for MC-LA, LF, LR, LW, LY, RR, YR, and nodularin (from Enzo Life Sciences) at concentrations of 0.25, 0.50, 1.0, and 2.5 μg/L (the same concentrations as the kit-provided MC-LR standard). For comparison, MC-LR, RR, and nodularin CRMs were purchased from the Certified Reference Materials Program of the National Research Council, Canada, and prepared at the same concentrations. For all assays, each standard concentration was run in triplicate wells and results were averaged. Each standard from each manufacturer was analyzed in independent replicates on different days (n = 4 combined for MC-LR, RR, and nodularin; n = 2 for MC-LA, LF, LW, LY, YR). Average absorbance values (n = 3) at 405 nm were compared to the average absorbance values (n = 3) for the kit supplied MC-LR standards of the same concentration for each plate run using the following equation [(average absorbance at 405 nm for Enzo or NRC standard/average absorbance at 405 nm for kit supplied MC-LR standard) × 100] providing a % relative inhibitory response for each MC congener compared to the kit supplied MC-LR standard. Average responses and standard deviations across all concentrations were calculated to assess overall relative enzyme inhibitory activity for the various MC congeners within the working range of the kit. Paired students t-tests were performed for results from MC-LR, RR, and NOD from NRC, Canada and Enzo Life Sciences to test for significant differences in the responses between these certified and non-certified standards.
2.3.3. Accuracy
To assess the accuracy of MC measurements in BGA-containing dietary supplements, MC-LA, LF, LR, LW, LY, RR, YR, and nodularin were spiked individually into a representative powdered BGA supplement containing 100% Spirulina (species not identified by manufacturer). AFA-based BGA products were not used as a blank matrix because preliminary analysis of representative products in powdered, capsule, and tablet form did not identify any products below the limit of detection for MC contamination as determined by LC-MS/MS (Parker et al., 2015, unpublished data). Following the supplemental procedure for the analysis of dried algae as dietary supplements, as described above in the Sample Preparation section, 100 mg of Spirulina powder was weighed into a 15 mL disposable polypropylene centrifuge tube followed by the addition of 5 mL of extraction solvent. These suspensions were fortified individually with 0.50, 1.0, or 2.0 μg/g of each standard resulting in 24 fortified samples. These concentrations represented 1/2X, 1X, and 2X of the Oregon 1 μg/g limit and were all within the manufacturer stated working range of the PPIA kit (0.25–2.5 μg/g). All fortifications were performed as described above in independent tests (n = 2) on different days (n = 48 analyses of fortified samples in total). For all assays, spiked samples were analyzed in triplicate wells and results were averaged. Average percent accuracy [(measured concentration/spiked concentration) × 100] and relative standard deviations (RSDs) were calculated for each MC standard.
2.3.4. Specificity
To test the specificity of the PPIA kit for MC, the potentially co-occurring non-MC cyanobacterial toxins, anatoxin-a, cylindrospermopsin, and saxitoxin, were prepared as solutions at concentrations of 0.25, 0.50, 1.0, and 2.5 μg/L in deionized water and analyzed as described in the cross-reactivity section. In addition, CRM MC-LR from NRC was spiked into 100 mg of Spirulina powder at concentrations of 0.50, 1.0, or 2.0 μg/g in the presence of 2.5 μg/g anatoxin-a, cylindrospermopsin, or saxitoxin and analyzed as described in the accuracy section. For spikes into matrix, the specificity index for MC-LR in the presence of each potentially interfering compound was calculated by dividing the determined MC-LR concentration when spiked alone by the determined MC-LR concentration when spiked together with an abundance of the individual potentially co-occurring compounds. Students t-tests were also performed to test for differences between results for MR-LR spiked into Spirulina alone compared to spikes in the presence of an abundance of anatoxin-a, cylindrospermopsin, and saxitoxin.
2.4. Occurrence of MC contamination in BGA dietary supplements
To assess the occurrence of MC contamination in BGA dietary supplements available to U.S. consumers, an internet search was performed using the following search engines: Yahoo.com, Google.com/shopping, and Amazon.com using the keyword Aphanizomenon flos-aquae. From these searches, 70 products were identified. Only dry products (powders, capsules, and tablets) were targeted for purchase; fresh or frozen liquid products were excluded due to sample handling challenges explained in the discussion section. From the initial list of 70 products, 51 unique dried supplements were successfully obtained. All products were purchased between February 9 and May 6, 2016. The majority of these products (47) were marketed for human use while a limited number (4) were marketed for use with animals (2 dog, 1 cat, 1 horse). After an initial test for MC contamination using the PPIA kit, an attempt was made to re-acquire any products with detectable contamination (>0.25 μg/g) from a second source in hopes of obtaining a different product lot to test lot-to-lot variability. This effort produced an additional 9 AFA-containing BGA products of which 3 were from different product lots. An additional 2 of these 9 products were a different lot but also a different product form than the one originally purchased (e.g., powder vs. tablet from the same manufacturer). Of the 60 total AFA-containing products purchased, 35 were in the form of capsules, 16 as powders, and 9 as tablets. Fifty one of the sixty products tested contained AFA as the only algal material, 45 of which contained AFA as the only active ingredient, leaving 9 products that also contained the BGA/green algal species Spirulina and/or Chlorella. Thirteen of the products tested, containing either AFA alone or AFA in combination with Spirulina and/or Chlorella, also contained additional natural ingredients such as vitamins, enzymes, amino acids, probiotics, fiber, or additional botanical extracts. As negative controls, 7 additional BGA/green algal products containing Spirulina (4) or Chlorella (3) were purchased from websites that also offered AFA-containing products. These products represented a mixture of powdered (3), tablet (2), and capsule (2) forms. Full internet searches were not performed for Spirulina or Chlorella products because previous studies found that products containing only these ingredients were less likely to contain MC contamination (Lawrence et al., 2001; Heussner et al., 2012; Vichi et al., 2012; Parker et al., 2015; Yakes et al., 2015). Altogether, 67 algal dietary supplement products were tested for MC contamination using the Microcystin/Nodularin PP2A microtiter plate test kit following the supplemental instructions for dried algal dietary supplements as described above. For products in tablet or capsule form, 6 units were combined and homogenized for analysis. Tablets were ground using a mortar and pestle. For all products, samples were analyzed in duplicate wells, following the manufacturer's recommendations, and results were averaged. Any products found to be above the working range of the kit (>2.5 μg/g) (n = 1) were re-extracted and run as serial dilutions. Any products found to be ≥0.5 μg/g MC (1/2 the Oregon State limit) (n = 20) were tested further by LC-MS/MS to confirm PPIA findings and determine the individual MC composition.
2.5. Confirmation of MC contamination in BGA dietary supplements by LC-MS/MS
LC-MS/MS analyses were performed according to a validated method for quantitation of MC in BGA supplements (Parker et al., 2015). In brief, a SPEX Sample Prep 2010 Geno/Grinder (SPEX, Metuchen, NJ, USA) was used to homogenize each supplement sample (12 capsules, 12 tablets, or 6 g powder) in 50 mL Nalgene high speed centrifuge tubes (Fisher Scientific, Pittsburgh, PA, USA); for tablets, a 3/8 in. stainless steel grinding ball was added to each tube to simultaneously grind and homogenize the sample. To achieve complete homogenization of all products, an additional cycle at 1500 rpm was added (1400 rpm × 1 min × 5 cycles and 1500 rpm × 1 min × 2 cycles). For each BGA product, 100 mg of homogenized sample was extracted in 1 mL of 80:20 methanol:water. It was not possible to test the same extract used in the PPIA analysis by LC-MS/MS due to the presence of Tween 20 in the recommended PPIA extraction solvent, which would interfere with the LC-MS/MS analysis. Each sample was mixed at room temperature using a vortex mixer for 15 min at 1,400 rpm and an end-over-end rotating mixer for 15 min at 40 rpm. The samples were centrifuged at 14,000 × g for 10 min at 20 °C, and the supernatant was transferred to a 1.5 mL micro-centrifuge tube. A 100 μL aliquot of each sample extract was then filtered using a graphite spin column (Pierce Biotechnology, Rockford, IL, USA) as described by Parker et al. (2015). Each product extract was prepared in parallel with a pre-extraction fortified sample containing 1.0 μg/g MC-LA, LF, LR, LW, LY, RR, YR, and nodularin (all from Enzo Life Sciences). Samples were prepared in duplicate and analyzed on consecutive days. Using these fortified samples, a one-point correction factor was calculated for each MC congener to correct for matrix effects and sample preparation losses. Endogenous MC concentrations were determined using matrix-corrected solvent calibration curves (using MC-LA, LF, LR, LW, LY, RR, YR, and nodularin from Enzo Life Sciences), fitted by least squares linear regression. Neat calibration solutions were prepared daily in 80:20 methanol:water at concentrations ranging from 0.02 to 3.0 μg/g. LC-MS/MS analyses were performed using an Acquity UPLC with a 150 mm × 1 mm i.d. 1.7 μm (130 °A) C18 BEH analytical column coupled to a 5500 QTrap mass spectrometer (SCIEX, Framingham, MA, USA). Gradient conditions and MS parameters were set as described previously (Parker et al., 2015). For quantitative data analysis, Skyline v 2.6 (MacLean et al., 2010) was utilized.
3. Results
3.1. Evaluation of a commercial PPIA kit for the testing of dried BGA dietary supplements
3.1.1. Relative enzyme inhibition
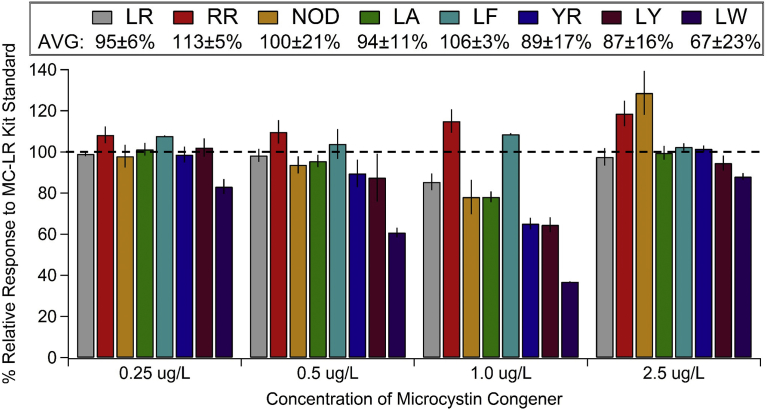
In replicate experiments, MC-LR (n = 2 for 2 suppliers, n = 4 total*), RR (n = 2 for 2 suppliers, n = 4 total*), nodularin (n = 2 for 2 suppliers, n = 4 total*), LA (n = 2), LF (n = 2), YR (n = 2), LY (n = 2), and LW (n = 2) showed relative PP2A enzyme inhibition, within the manufacturer stated working range of 0.25–2.5 μg/L, of 95 ± 6% for MC-LR, 113 ± 5% for MC-RR, 100 ± 21% for NOD, 94 ± 11% for MC-LA, 106 ± 3% for MC-LF, 89 ± 17% for MC-YR, 87 ± 16% for MC-LY, and 67 ± 23% for MC-LW compared to the kit provided MC-LR standard (Fig. 1). *For MC-LR, RR, and nodularin, results for the non-certified standards from Enzo Life Sciences were not significantly different (P > 0.05 for all) compared to the certified reference standards from NRC Canada, therefore results for the toxins for both suppliers were combined. MC-LW had a higher relative inhibition of the PP2A enzyme compared to the other MC congeners at all concentrations tested, ranging from 36 to 83% relative conversion of the chromogenic substrate (Fig. 1). [Note: this assay is based on the inhibition of the PP2A enzyme to hydrolyze a chromogenic substrate; therefore, higher relative inhibition (i.e. greater inhibitory capacity towards the PP2A enzyme) for a particular MC variant relative to the kit supplied MC-LR standard would result in less conversion of the chromogenic substrate and an overestimation of toxin present. Likewise, lower relative inhibition of a particular MC variant compared to the kit supplied MC-LR standard would result in greater conversion of the chromogenic substrate and an underestimation of toxin present]. All of the MC congeners tested, with the exception of MC-LW, performed within 85–130% at 0.25, 0.50, and 2.5 μg/L. At 1.0 μg/L, the external standards for MC-LR, LA, YR, LY, and nodularin all performed within 65–85% compared to the kit provided MC-LR standard, while MC-RR and LF performed at 108–112%.
Fig. 1.
Relative enzyme activity, as represented by the percent relative response, for external microcystin and nodularin standards (in water) for inhibition of the PP2A enzyme to hydrolyze the chromogenic substrate in the Microcystin/Nodularin PP2A microtiter plate test kit (Product No. 520032, Abraxis LLC, Warminster, Pennsylvania) compared to the kit supplied MC-LR standard. Histograms for MC-LA, LF, YR, LY, and LW represent average responses for two independent tests run on different days (n = 2), with bars representing range. Histograms for MC-LR, RR, and nodularin (NOD), represent standards from two different suppliers, each run twice on different days. Results for these tests (n = 4) were averaged, with bars representing ±1 standard deviation. The average and standard deviation for the percent relative response across all concentrations tested was calculated for each MC congener and is presented in the legend.
3.1.2. Accuracy
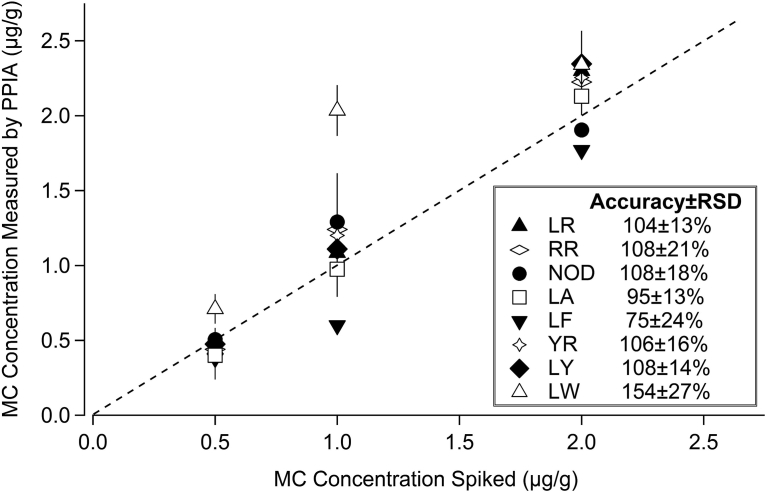
In replicate experiments, average accuracies and RSDs for MC-LR, RR, NOD, LA, LF, YR, LY, and LW spiked into a powdered Spirulina BGA dietary supplement at concentrations of 0.50, 1.0, and 2.0 μg/g were 104 ± 13% for MC-LR, 108 ± 21% for MC-RR, 108 ± 18% for NOD, 95 ± 13% for MC-LA, 75 ± 24% for MC-LF, 106 ± 16% for MC-YR, 108 ± 14% for MC-LY, and 154 ± 27% for MC-LW (Fig. 2). The largest deviations from expected results were for MC-LW (overestimated the MC concentration by 2 fold at 1.0 μg/g) and MC-LF (underestimated the MC concentration by 2 fold at 1.0 μg/g) (Fig. 2).
Fig. 2.
Accuracy, as represented by the spiked MC concentration versus the measured MC concentration, for external microcystin and nodularin standards spiked into a powdered Spirulina BGA supplement for the Microcystin/Nodularin PP2A microtiter plate test kit (Product No. 520032, Abraxis LLC, Warminster, Pennsylvania). Symbols represent average response for two independent tests run on different days (n = 2), with bars representing range. Average percent accuracy and RSDs for the 3 spiked concentrations are presented in the legend.
3.1.3. Specificity
The potentially co-occurring, non-MC cyanobacterial toxins anatoxin-a, saxitoxin, and cylindrospermopsin showed no detectable reaction in the Microcystin/Nodularin PP2A Microtiter Plate Test Kit at concentrations of 0.25–2.5 μg/L (Table 1). The specificity index for MC-LR spiked into a powdered Spirulina BGA dietary supplement at concentrations of 0.50, 1.0, and 2.0 μg/g ranged from 95 ± 4% to 98 ± 5% in the presence of 2.5 μg/g anatoxin-a, saxitoxin, or cylindrospermopsin (Table 1). Further, the results for MC-LR spiked into Spirulina at concentrations of 0.50, 1.0, and 2.0 μg/g did not differ significantly compared to MC-LR spikes of the same concentrations in the presence of 2.5 μg/g anatoxin-a, saxitoxin, or cylindrospermopsin (P > 0.05 for all).
Table 1.
Specificity of the Microcystin/Nodularin PP2A microtiter plate test kit (Product No. 520032, Abraxis LLC, Warminster, Pennsylvania) tested in the presence of potentially co-occurring cyanobacterial toxins saxitoxin (STX), anatoxin (ATX), and cylindrospermopsin (CYN). Kit tested both at varying concentrations of potential interfering compounds alone in water, and spiked into a representative BGA supplement matrix (Spirulina) at a concentration of 2.5 μg/g with MC-LR spiked at 1/2X, 1X, or 2X the 1 μg/g MC limit. The average (±1 SD) specificity index [(MC-LR alone/MC-LR in presence of potential interfering compound) × 100] was calculated for each potentially interfering compound.
| Standard | Concentration (μg/L) | Result (μg/L) | Specificity Index |
|---|---|---|---|
| STX (in H2O) | 0.25, 0.50, 1.0, 2.5 | ND | – |
| ATX (in H2O) | 0.25, 0.50, 1.0, 2.5 | ND | – |
| CYN (in H2O) |
0.25, 0.50, 1.0, 2.5 |
ND |
– |
|
Concentration (μg/g) |
Result (μg/g) |
||
| MC-LR (in Spirulina) | 0.50, 1.0, 2.0 | 0.47, 1.15, 2.37 | – |
| STX + MC-LR (in Spirulina) | 2.5 + 0.50, 1.0, 2.0 | 0.48, 1.27, 2.43 | 95 ± 4% |
| ATX + MC-LR (in Spirulina) | 2.5 + 0.50, 1.0, 2.0 | 0.46, 1.25, 2.39 | 98 ± 5% |
| CYN + MC-LR (in Spirulina) | 2.5 + 0.50, 1.0, 2.0 | 0.49, 1.21, 2.40 | 96 ± 2% |
ND: Not detected; <0.25 μg/L.
3.2. Occurrence of MC contamination in BGA dietary supplements
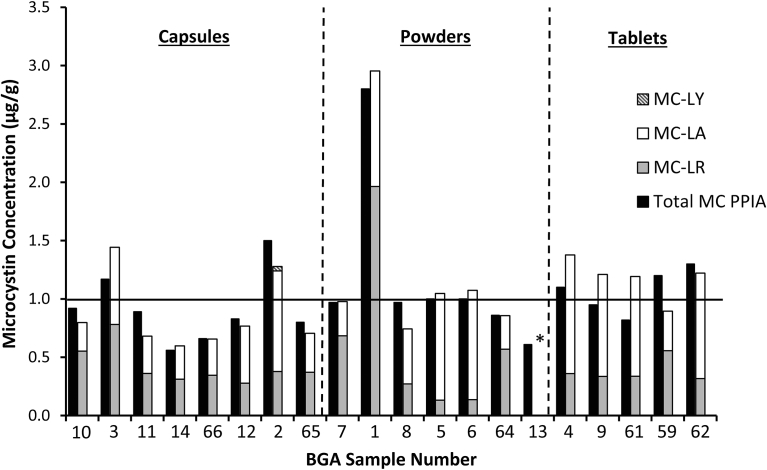
Using the Microcystin/Nodularin PP2A microtiter plate test kit, 51% (26 out of 51) of BGA dietary supplements containing AFA were found to have MC contamination above the detection limit of the kit (0.25 μg/g), 10 products contained MC concentrations between 0.5 and 1.0 μg/g (1/2X-1X the Oregon State limit), while 4 products were found to be in excess of the 1.0 μg/g Oregon State limit (1.1–2.8 μg/g) (Table 2). No trends were found between MC contamination and product form (powder, capsule, or tablet) (Table 2, Fig. 3). None of the non-AFA BGA dietary supplements containing Spirulina and/or Chlorella (n = 7) contained detectable concentrations of MC using the PPIA kit (Table 2). With the exception of 1 product (product 13), supplementary LC-MS/MS confirmation of all products found to be in excess of 0.5 μg/g MC (n = 20) confirmed the findings of the PPIA kit (Table 2, Fig. 3). Excluding product 13 as a false positive (explained further in discussion), analysis of results between PPIA and LC-MS/MS for all products >0.5 μg/g (n = 19) found the two methods to be highly correlated (Pearson's r correlation coefficient = 0.93; P < 0.0001), and not significantly different (Paired t test, P = 0.82).
Table 2.
Occurrence of microcystin (MC) contamination in Aphanizonemon flos-aquae containing blue-green algal dietary supplements as determined by protein phosphatase inhibition assay (PPIA) and liquid chromatography tandem mass spectrometry (LC-MS/MS). LC-MS/MS values represent the average for duplicate analyses (n = 2) analyzed on separate days.
| Sample Number | Active Ingredient | Form | Recommended Consumption (g/day) | PPIA (μg/g MC) | LC-MS/MS (μg/g MC) | BGA Source |
|---|---|---|---|---|---|---|
| 1 | AFA | Powder | 1 | 2.8 | 3.0 | Klamath |
| 2 | AFA | Capsule | 1.6-as needed | 1.5 | 1.3 | Klamath |
| 3 | AFA | Capsule | 1.2 | 1.2 | 1.4 | NA |
| 4 | AFA | Tablet | 1 | 1.1 | 1.4 | Klamath |
| 5 | AFA | Powder | 0.8 (1/2 for children<12) | 1.0 | 1.1 | USA |
| 6 | AFA | Powder | 1.6 (1/2 for children<12) | 1.0 | 1.1 | USA |
| 7 | AFA | Powder | 5.5–11 | 0.97 | 0.98 | Klamath |
| 8 | AFA | Powder | 1 | 0.97 | 0.74 | Klamath |
| 9 | AFA | Tablet | 2 | 0.95 | 1.2 | Klamath |
| 10 | AFA | Capsule | 1.5–3 | 0.92 | 0.80 | Klamath |
| 11 | AFA | Capsule | 0.8-as needed | 0.89 | 0.68 | Klamath |
| 12 | AFA-Mixed | Capsule | 1.1-as needed | 0.83 | 0.77 | NA |
| 13 | AFA/S/C-Mixed | Powder | 9.8 | 0.61e,f | NDe | Klamath |
| 14 | AFA | Capsule | 1–3 | 0.56 | 0.60 | Klamath |
| 15 | AFA | Powder | 1 | 0.43 | – | Klamath |
| 16 | AFA-Mixed | Capsule | 2 | 0.43 | – | NA |
| 17 | AFA | Capsule | 1.2 | 0.42 | – | Klamath |
| 18 | AFA-Mixed | Capsule | 2.2–6.6 | 0.41 | – | USA |
| 19 | AFA | Capsule | 1–3 | 0.38 | – | Klamath |
| 20 | AFA | Capsule | 1–3 | 0.36 | – | Klamath |
| 21 | AFA | Capsule | 1–3 | 0.35 | – | Klamath |
| 22 | AFA | Capsule | 1–3 | 0.34 | – | Klamath |
| 23 | AFA/S/C-Mixed | Powder | 8 | 0.30 | – | USA |
| 24a | AFA | Powder | 1.3–2.6d -as needed | 0.29 | – | Klamath |
| 25b | AFA | Powder | 0.3–1d -as needed | 0.27 | – | Klamath |
| 26 | AFA/S/C-Mixed | Powder | 8 | 0.26 | – | USA |
| 27 | AFA | Capsule | 0.5 | <0.25 | – | NA |
| 28 | AFA/S | Tablet | 0.5 | <0.25 | – | Klamath |
| 29 | AFA | Capsule | 1.5 | <0.25 | – | Klamath |
| 30 | AFA/S/C | Capsule | 3.7 | <0.25 | – | Klamath |
| 31 | AFA | Capsule | 1.5–3 | <0.25 | – | Klamath |
| 32 | AFA | Capsule | 1.5 | <0.25 | – | Klamath |
| 33 | AFA | Capsule | 1.5–3 | <0.25 | – | Klamath |
| 34 | AFA | Tablet | 0.5–1 | <0.25 | – | Klamath |
| 35 | AFA | Capsule | 1–2 | <0.25 | – | Klamath |
| 36 | AFA | Capsule | 1–3 | <0.25 | – | Klamath |
| 37 | AFA | Capsule | 1 | <0.25 | – | USA |
| 38 | AFA | Capsule | 1–3 | <0.25 | – | Klamath |
| 39 | AFA | Capsule | 1–3 | <0.25 | – | Klamath |
| 40 | AFA | Capsule | 1–3 | <0.25 | – | Klamath |
| 41 | AFA | Capsule | 1–3 | <0.25 | – | Klamath |
| 42 | AFA | Capsule | 1–3 | <0.25 | – | Klamath |
| 43 | AFA | Capsule | 1–3 | <0.25 | – | Klamath |
| 44 | AFA | Capsule | 0.6 | <0.25 | – | USA |
| 45 | AFA/C-Mixed | Tablet | 9.1 | <0.25 | – | NA |
| 46 | AFA/S/C-Mixed | Powder | 8 | <0.25 | – | USA |
| 47 | AFA/S/C-Mixed | Powder | 15 | <0.25 | – | NA |
| 48 | AFA-Mixed | Capsule | 0.6 | <0.25 | – | NA |
| 49g | AFA/S/C-Mixed | Capsule | 0.8 -as needed | <0.25 | 0.12 | Klamath |
| 50c,g | AFA-Mixed | Powder | 7–40d | <0.25 | 0.20 | USA |
| 51a,g | AFA-Mixed | Tablet | 0.75–6d | <0.25 | 0.26 | USA |
| 52g | C | Capsule | 3.69 | <0.25 | ND | NA |
| 53 | C | Capsule | 2 | <0.25 | – | Japan |
| 54 | C | Powder | 3 | <0.25 | – | Japan |
| 55 | S | Tablet | 3 | <0.25 | – | USA |
| 56 | S | Tablet | 2 | <0.25 | – | USA |
| 57 | S | Powder | 13 | <0.25 | – | USA |
| 58 | S | Powder | 1 | <0.25 | – | NA |
AFA – Aphanizomenon flos-aquae, S – Spirulina, C – Chlorella.
Mixed – Additional non-BGA active ingredients.
“–” With the exception of negative controls, only samples ≥0.50 μg/g by PPIA were confirmed by LC-MS/MS.
ND – Not Detected (below limit of detection for each congener).
NA – Information Not Available.
Marketed for dogs.
Marketed for cats.
Marketed for horses.
Recommended dosage varies depending on weight of animal, entire range shown.
Average of replicate measurements.
False positive.
LC-MS/MS negative controls (as defined by PPIA).
Fig. 3.
Distribution of individual microcystin (MC) congeners in Aphanizonemon flos-aquae containing blue-green algal dietary supplements that tested ≥0.5 μg/g total MC by PPIA as determined by LC-MS/MS and comparison with total MC concentration as measured by PPIA. Horizontal line indicates 1 μg/g MC limit. *PPIA false positive as MC were not detected by LC-MS/MS for sample 13. Sample 13 was tested in duplicate by both methods.
Although an attempt was made to assess lot-to-lot variability in MC contamination among AFA-containing BGA products, as has been reported previously (Vichi et al., 2012), only 5 of the 9 products with detectable MC concentrations by PPIA successfully obtained from a second supplier were from a second product lot and two of these turned out to also be in a different product form (i.e., powder versus tablet) (Table 3). It is not known if products from the same manufacturer, but in different product forms with different lot numbers, use the same lot of raw material; therefore, an insufficient number of products were obtained to make any statistically supported statements about lot to lot variability in this study. If it is assumed that products 1 and 59 (powder verses tablet) and products 10 and 64 (capsule verses powder) were in fact manufactured using different lots of raw AFA, then there appears to be greater variability between product lots compared to within the same product lot (Table 3). Additional sampling will be required to determine if this observed trend holds.
Table 3.
Inter-lot and intra-lot variability in microcystin (MC) contamination in Aphanizonemon flos-aquae containing blue-green algal dietary supplements as determined by protein phosphatase inhibition assay (PPIA) and liquid chromatography tandem mass spectrometry (LC-MS/MS). LC-MS/MS values represent the average for duplicate analyses (n = 2) run on separate days.
| Original Purchase |
Second Purchase |
Different Lot | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Number | Forma | PPIA (μg/g MC) | LC-MS/MS (μg/g MC) | Sample Number | Forma | PPIA (μg/g MC) | LC-MS/MS (μg/g MC) | |
| 1 | Powder | 2.8 | 3.0 | 59 | Tablet | 1.2 | 0.90 | Yesb |
| 3 | Capsule | 1.2 | 1.4 | 60 | Capsule | 0.26 | – | Yes |
| 4 | Tablet | 1.1 | 1.4 | 61 | Tablet | 0.82 | 1.2 | Yes |
| 9 | Tablet | 0.95 | 1.2 | 62 | Tablet | 1.3 | 1.2 | Yes |
| 10 | Capsule | 0.92 | 0.80 | 63 | Powder | 0.29 | – | Yesb |
| 7 | Powder | 0.97 | 1.0 | 64 | Powder | 0.86 | 0.86 | No |
| 11 | Capsule | 0.89 | 0.68 | 65 | Capsule | 0.80 | 0.71 | No |
| 14 | Capsule | 0.56 | 0.60 | 66 | Capsule | 0.64 | 0.66 | No |
| 19 | Capsule | 0.38 | – | 67 | Capsule | 0.42 | – | No |
“–” Only samples ≥0.50 μg/g by PPIA were confirmed by LC-MS/MS.
All of these products contained AFA as the only active ingredient.
Note that these products, from the same manufacturer, were different lots but also in different product forms.
All products contaminated with microcystins were found to contain MC-LA and MC-LR in varying ratios (Fig. 3, Table 4). A third congener, MC-LY, which was reported in one BGA product tested in a previous study (Parker et al., 2015), was also detected at 0.04 μg/g (at the LC-MS/MS method limit of quantitation) in one of the products tested here (Fig. 3, Table 4). MC-LF, LW, RR, YR, and nodularin were not detected in any of the supplements examined. Four products that were below the detection limit of the PPIA kit were also tested as PPIA negative control samples by LC-MS/MS. Two AFA-mixed products (50 and 51) and one AFA/Spirulina/Chlorella-mixed product (49) contained measurable concentrations of MC, but ≤0.26 μg/g. The other PPIA negative sample, a Chlorella-based product (product 52), did not contain MC above the LC-MS/MS method limit of detection for all congeners.
Table 4.
Concentration of individual microcystin (MC) congeners in Aphanizonemon flos-aquae containing blue-green algal dietary supplements that tested ≥0.5 μg/g total MC by PPIA as determined by LC-MS/MS and comparison with total MC concentration as measured by PPIA. LC-MS/MS values represent the average for duplicate analyses (n = 2) analyzed on separate days. Sample 13 was a false positive by PPIA as MC were not detected by LC-MS/MS. Sample 13 was tested in duplicate by both methods.
| Sample Number | MC-LR (μg/g) | MC-LA (μg/g) | MC-LY (μg/g) | Total MC by LC-MS/MS | Total MC by PPIA |
|---|---|---|---|---|---|
| 1 | 2.0 | 0.99 | ND | 3.0 | 2.8 |
| 2 | 0.38 | 0.86 | 0.04 | 1.3 | 1.5 |
| 62 | 0.32 | 0.90 | ND | 1.2 | 1.3 |
| 3 | 0.78 | 0.66 | ND | 1.4 | 1.2 |
| 59 | 0.56 | 0.34 | ND | 0.90 | 1.2 |
| 4 | 0.36 | 1.0 | ND | 1.4 | 1.1 |
| 5 | 0.13 | 0.92 | ND | 1.1 | 1.0 |
| 6 | 0.14 | 0.94 | ND | 1.1 | 1.0 |
| 7 | 0.68 | 0.30 | ND | 0.98 | 0.97 |
| 8 | 0.27 | 0.47 | ND | 0.74 | 0.97 |
| 9 | 0.34 | 0.87 | ND | 1.2 | 0.95 |
| 10 | 0.55 | 0.25 | ND | 0.80 | 0.92 |
| 11 | 0.36 | 0.32 | ND | 0.68 | 0.89 |
| 64 | 0.57 | 0.29 | ND | 0.86 | 0.86 |
| 12 | 0.28 | 0.49 | ND | 0.77 | 0.83 |
| 61 | 0.34 | 0.86 | ND | 1.2 | 0.82 |
| 65 | 0.37 | 0.34 | ND | 0.71 | 0.80 |
| 13 | ND | ND | ND | ND | 0.61a |
| 66 | 0.35 | 0.31 | ND | 0.66 | 0.66 |
| 14 | 0.31 | 0.29 | ND | 0.60 | 0.56 |
ND = Not Detected.
PPIA false positive.
4. Discussion
Upper-Klamath Lake in southern Oregon, USA is the primary source for the cyanobacteria Aphanizomenon flos-aquae (AFA) used in the production of AFA blue-green algal (BGA) dietary supplements in the U.S. Depending on the time of year, other algal species known to occur in the UKL system include the diatom Fragilaria, and the BGA species Anabaena flos-aquae, Coelosphaerium, and Microcystis aeruginosa (Carmichael et al., 2000). M. aeruginosa is a known producer of the cyclic peptide hepatotoxins microcystins (MC), which can contaminate the target AFA harvested from UKL. In 1996, an extensive bloom of M. aeruginosa prompted the Oregon Health Division to issue a health advisory for water contact due to elevated concentrations of MC in UKL waters (Gilroy et al., 2000). Potential concern for MC contamination in Oregon-produced AFA BGA dietary supplements prompted a survey of commercial products by the Oregon Health Division which resulted in the finding of 72% (63 of 87) products containing >1 μg/g contamination with concentrations of total MCs in select products as high as 18 μg/g (Gilroy et al., 2000). Using the World Health Organization provisional guideline value of 1 μg/L MC in drinking water (WHO, 2003), the Oregon Health Division estimated a safe limit of 1 μg/g MC contamination in BGA dietary supplements consumed by adults and the Oregon Department of Agriculture adopted this limit as a regulatory standard for products sold in Oregon in 1997 (Gilroy et al., 2000). Due to a clerical error, the Oregon State limit for MC contamination in AFA BGA products was unintentionally deleted from the Oregon Administrative Rule and was proposed to be re-adopted in 2015 (Brown, 2015). To date, this proposed re-adoption has yet to be finalized. The U.S. Food and Drug Administration (FDA) has not established a regulatory limit for MC in these products, but the current good manufacturing practice (CGMP) regulations for dietary supplements do require manufacturers establish a specification, and test, for limits on those types of contamination that may adulterate, or lead to adulteration of, the finished dietary supplement (21 CFR 111.70(e): https://www.ecfr.gov/cgi-bin/text-idx?SID=7426007f2d43dcf286c3478222f7847e&mc=true&node=pt21.2.111&rgn=div5) [Accessed 3 January 2018]. Since the initial survey by Gilroy et al. (2000) that led to the establishment of the original Oregon State limit, follow-up studies in Canada, Germany, and Italy all found products in excess of 1 μg/g MC (Lawrence et al., 2001; Heussner et al., 2012; Vichi et al., 2012). Earlier studies such as Lawrence et al. (2001) found concentrations in select products as high as 35 μg/g MC, while later studies such as Heussner et al. (2012) and Vichi et al. (2012) found lower maximum concentrations in the range of 5–6 μg/g MC, with many products around the 1 μg/g MC limit.
In the present study, 51% (26 of 51) of AFA-containing BGA dietary supplement products purchased in the U.S. contained detectable concentrations (>0.25 μg/g) of MC contamination as determined using a commercial protein phosphatase inhibition assay (PPIA). Among the products that were positive for MC contamination, 46% (12 of 26) were close to or over the 1 μg/g MC limit, with 11 products close to the limit (1.0 ± 0.18; n = 11, range 0.83–1.5), and only 1 product significantly exceeding this limit (2.8 μg/g MC). This, along with the previous observation that maximum MC concentrations dropped in more recent surveys such as Heussner et al. (2012) and Vichi et al. (2012) compared to earlier studies such as Lawrence et al. (2001) and Gilroy et al. (2000), with many products in the more recent surveys containing MC in the immediate range of 1 μg/g, suggests that most manufacturers have established a specification and are testing for MC contamination at the previous Oregon State limit.
When the Oregon Health Division performed their risk assessment to establish a total daily intake (TDI) of 2.4 μg/day MC (for a 60 kg adult), which was used by the Oregon Department of Agriculture to establish the 1 μg/g MC limit in BGA dietary supplements, a daily intake of 2 g BGA/day was assumed (Gilroy et al., 2000). Among the BGA products tested in this study, the manufacturer recommended daily consumption ranged from 0.5 to 11 g/day for products containing AFA only, and up to 15 g/day for products containing additional BGA species and/or additional active ingredients, excluding products marketed to pets. Furthermore, four of these products (products 2, 11, 12, and 49 from Table 2) contained starting daily consumption recommendations followed by statements such as “Gradually increase based on individual needs”, “Build to desired amount”, or “More may be safely taken as needed”, with no upper limit recommended. Three of these four products (products 2, 11, and 12 from Table 2) contained MC contamination in the range of the previous 1 μg/g Oregon State limit that was based on a daily consumption of 2 g/day (1.1 ± 0.37 μg/g MC as measured by PPIA, 0.92 ± 0.34 μg/g MC as measured LC-MS/MS, n = 3). Lastly, while most of the products tested here were marketed specifically for adult use, two products (products 5 and 6 from Table 2) with MC contamination at 1 μg/g (1.0 μg/g MC as measured by PPIA, 1.1 μg/g MC as measured by LC-MS/MS, for both) had recommended dosages for children under 12 of half the recommended adult dose.
Although not a primary focus of this study, several AFA-containing BGA products were found and purchased that were specifically marketed for pets (2 dog, 1 cat, and 1 horse). Two of these products had detectable concentrations of MC contamination but close to the detection limit of the PPIA kit (product 24 at 0.29 μg/g MC and product 25 at 0.27 μg/g MC from Table 2). Both were from the same manufacturer, but one was marketed for dogs and one for cats. Both products had recommended starting daily consumption rates that depended on the weight of the animal, but both also had the additional statement on suggested use of “One or more times daily as desired”. Bautista et al. (2015) documented a case of apparent hepatic toxicity in a dog after dietary supplementation with an AFA-containing BGA product that was found to contain 1.1 μg/g MC, with resolution of symptoms upon treatment and discontinuation of product use.
The commercial protein phosphatase inhibition assay (PPIA) evaluated here is a reliable option for the detection of MC contamination in dried BGA containing dietary supplements in powered, capsule, and tablet form, with acceptable relative enzyme inhibition, accuracy, and specificity for the majority of the most prevalent MC congeners. Even the less common congeners MC-LW and LF were detected in spiked samples within a factor of 2 at the 1 μg/g MC limit. Furthermore, using a recently validated LC-MS/MS method, all PPIA results were confirmed for samples ≥0.5 μg/g MC, with the exception of one false positive sample. The single sample (product 13 from Table 2) that was positive for MC contamination in duplicate PPIA tests at an average concentration of 0.61 μg/g MC, but was negative in replicate LC-MS/MS analyses, was a mixed product both in terms of BGA species and additional active ingredients. Only 100 mg of the 9.8 g serving size was AFA meaning that if the AFA was the source of the protein phosphatase inhibition it would have needed to contain nearly 60 μg/g MC. With 55 additional active ingredients in this product, including vitamins, probiotics, enzymes, and botanical extracts from numerous additional plant species, it is more likely that another component contained inhibitory activity towards the PP2A enzyme causing the false positive result. Additional work will be required to confirm this and determine the source of the PP2A inhibition in this product.
The survey of BGA dietary supplement products available in the U.S. also revealed that some manufacturers are now offering various BGA, and other, algal species also in liquid form. These products were not included in this study as it was unclear how to test them using the PPIA kit. Options include diluting 100 μL of liquid product directly into the extraction solvent, filtering the product and testing 100 μg of wet material, or lyophilizing the product and testing 100 μg of dried material. In the future, a decision will need to be made on whether these products containing AFA should be tested for possible MC contamination based on a wet weight (either whole liquid or suspended algae) or dry weight basis before appropriate modifications can be made to the PPIA, or other, testing procedure.
5. Conclusions
While the majority of AFA-containing BGA dietary supplement products tested here did contain detectable concentrations of MC contamination (>0.25 μg MC/g as determined by PPIA), most of these products were at or below 1 μg/g as determined by PPIA and LC-MS/MS suggesting that most manufacturers have established a specification and are testing for MC contamination at the previous Oregon State limit. However, it should be noted that this limit was developed based on a daily consumption estimate of 2 g/day, and variability on recommended serving sizes by the various BGA dietary supplement manufacturers observed in this study (0.5–15 g/day with several products providing no upper recommended limit) prevented additional analysis of consumer exposure based on the concentrations of MC contamination found. Further, the previous Oregon State limit was estimated based on an average adult weighing 60 kg, and it is not known if this value can be extrapolated to children and animals, for whom some of the manufacturers of AFA-containing BGA dietary supplements are currently marketing these products.
Declarations
Author contribution statement
David Marsan, Stephen Conrad, Whitney Stutts: Performed the experiments; Analyzed and interpreted the data.
Christine Parker: Performed the experiments.
Jonathan Deeds: Conceived and designed the experiments; Wrote the paper.
Funding statement
David Marsan was supported by the Ratcliffe Environmental Entrepreneurs Fellowship (REEF) Program and the Living Marine Resources Cooperative Science Center (LMRCSC).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to acknowledge the FDA CFSAN Office of Dietary Supplement Programs (ODSP) for performing the initial internet search for AFA-containing BGA products and critical review of the manuscript, and Betsy Yakes (FDA CFSAN Office of Regulatory Science) for assistance with data analysis.
References
- An J., Carmichael W.W. Use of a colorimetric protein phosphatase inhibition assay and enzyme linked immunoassay for the study of microcystins and nodularins. Toxicon. 1994;32:1495–1507. doi: 10.1016/0041-0101(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Bautista A.C., Moore C.E., Lin Y., Cline M.G., Benitah N. Hepatopathy following consumption of a commercially available blue-green algal dietary supplement in a dog. BMC Vet. Res. 2015;11:136. doi: 10.1186/s12917-015-0453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. Rule caption: sets acceptable microcystin level and analytical laboratory procedure for Blue-Green Algae. Oregon Bull. 2015;54(1):8. http://sos.oregon.gov/archives/Documents/oar/2015-oregon-bulletin-january.pdf Available: [Google Scholar]
- Carmichael W.W., Drapeau C., Anderson D.M. Harvesting of Aphanizonemon flos-aquae Ralfs ex Born. & Flash. Var. flos-aquae (Cyanobacteria) from Klamath Lake for human dietary use. J Appl. Phycol. 2000;12:585–595. [Google Scholar]
- Duy T.N., Lam P.K.S., Shaw G.R., Connell D.W. Toxicology and risk assessment of freshwater cyanobacterial (blue-green algal) toxins in water. Rev. Environ. Contam. Toxicol. 2000;163:113–186. doi: 10.1007/978-1-4757-6429-1_3. [DOI] [PubMed] [Google Scholar]
- Gilroy D.J., Kauffman K.W., Hall R.A., Huang X., Chu F.S. Assessing potential health risks from microcystin toxins in blue-green algae dietary supplements. Environ. Health Perspect. 2000;108:435–439. doi: 10.1289/ehp.00108435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresztyn T., Nicholson B.C. Determination of cyanobacterial hepatotoxins directly in water using a protein phosphatase inhibition assay. Water Res. 2001;35:3049–3056. doi: 10.1016/s0043-1354(01)00018-5. [DOI] [PubMed] [Google Scholar]
- Heussner A.H., Mazija L., Fastner J., Dietrich D.R. Toxin content and cytotoxicity of algal dietary supplements. Toxicol. Appl. Pharmacol. 2012;265:263–271. doi: 10.1016/j.taap.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Lawrence J.F., Niedzwiadek B., Menard C., Lau B.P.Y., Lewis D., Kuper-Goodman T. Comparison of liquid chromatography/mass spectrometry, ELISA, and phosphatase assay for the determination of microcystins in blue-green algae products. J. AOAC Int. 2001;84:1035–1044. [PubMed] [Google Scholar]
- MacKintosh R.W., Dalby K.N., Campbell D.G., Cohen P.T.W., Cohen P., MacKintosh C. The cyanobacterial toxin microcystin binds covalently in cysteine-273 on protein phosphatase 1. FEBS Lett. 1995;371:236–240. doi: 10.1016/0014-5793(95)00888-g. [DOI] [PubMed] [Google Scholar]
- MacLean B., Tomazela D.M., Shulman N., Chambers M., Finney G.L., Frewen B., Kern R., Tabb D.L., Liebler D.C., MacCoss M.J. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan J., James R., Gregg A., Dindal A. U.S. Environmental Protection Agency; Washington, DC: 2011. Environmental Technology Verification Report and Statements for Zeu Inmunotec, S.L., Microcystin Test Kit: MicroCystest.https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=235712 EPA/600/R-11/064, Available: (Accessed 14 March 2018) [Google Scholar]
- Oregon Department of Agriculture . 2015. Klamath Headwaters Agricultural Water Quality Management Area Plan.http://www.oregon.gov/ODA/shared/Documents/Publications/NaturalResources/KlamathAWQMAreaPlan.pdf Available: [Google Scholar]
- Parker C.H., Stutts W.L., DeGrasse S.L. Development and validation of a liquid chromatography-tandem mass spectrometry method for the quantitation of microcystins in blue-green algal dietary supplements. J. Agric. Food Chem. 2015;63:10303–10312. doi: 10.1021/acs.jafc.5b04292. [DOI] [PubMed] [Google Scholar]
- Sevilla E., Smienk H., Razquin P., Mata L., Peleato L. Optimization of intracellular microcystin-LR extraction for its analysis by protein phosphatase inhibition assay. Water Sci. Tech. 2009;60(7):1903–1909. doi: 10.2166/wst.2009.527. [DOI] [PubMed] [Google Scholar]
- US EPA (US Environmental Protection Agency) Office of Water, Health and Ecological Criteria Division; Washington DC, USA: 2015. Drinking Water Health Advisory for the Cyanobacterial Microcystin Toxins. EPA Document Number: 820R15100. [Google Scholar]
- Vichi S., Lavorini P., Funari E., Scardala S., Testai E. Contamination by Microcystis and microcystins of blue-green algae food supplements (BGAS) on the Italian market and possible risk for the exposed population. Food Chem. Toxicol. 2012;50:4493–4499. doi: 10.1016/j.fct.2012.09.029. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) second ed. World Health Organization; Geneva: 1998. Guidelines for Drinking-water Quality. (Addendum to Volume 2. Health Criteria and Other Supporting Information). [Google Scholar]
- Yakes B.J., Handy S.M., Kanyuck K.M., DeGrasse S.L. Improved screening of microcystin genes and toxins in blue-green algal dietary supplements with PCR and a surface plasmon resonance biosensor. Harmful Algae. 2015;47:9–16. [Google Scholar]