Abstract
Wound healing is the critical event for maintaining skin function and barrier. Inflammatory state in which a variety of cells are activated and accumulated is important for wound healing. Bacterial infection in cutaneous wound is a common problem and causes delay of wound healing. Our previous study demonstrated that the salmon nasal cartilage proteoglycan (PG) has an immunomodulatory effect in various mouse models of inflammatory disease. In this study, we investigated the effect of PG on healing process of Staphylococcus aureus-infected wound. PG accelerated wound closure in the initial phase of both infected and non-infected wound healing. In addition, the bacterial number in wounds of the PG-treated mice was significantly lower than that in the vehicle group. Neutrophil and macrophage infiltration was intensively observed in the PG-treated mice on day 2 after S. aureus inoculation, whereas neutrophil and macrophage influx was highly detected on day 6 in the vehicle control. Moreover, the production of TGF-β and IL-6 in the wound tissue was significantly promoted compared to the vehicle control on day 1. In contrast, the production of IL-1β and TNF-α in PG-treated mice was significantly decreased compared to the vehicle control on day 5. These data suggested that PG modulates the inflammatory state in infected wounds leading to promote wound healing.
Keywords: Microbiology, Pharmaceutical chemistry
1. Introduction
Wound healing is an important process for retaining tissue homeostasis leading to restoration of barrier function. Inflammatory response is critically involved in the initial phase of wound healing. The inflammatory response requires initial recruitment of neutrophils and macrophages for defense against invasive microbes [1, 2]. The inflammatory response is followed by migration and proliferation of dermal and epidermal cells. Extracellular matrix, then, fills the wound space and remodels the skin function [3, 4]. In the wound healing process, several cytokines play an important role in wound healing. Tumor necrosis factor (TNF)-α and interleukin (IL)-6 produced by neutrophils and macrophages induce inflammation to removal of foreign matter [5]. Transforming growth factor (TGF)-β promotes re-epithelialization [6]. Bacterial infection in cutaneous wound is a common problem and it is one of the key reasons of wound healing impairment [7, 8]. Staphylococcus aureus is one of the most common bacteria causing skin and soft tissue infections, including impetigo, folliculitis and cellulitis [9, 10]. In addition, S. aureus has high potential for tissue invasion and causes life-threatening infections such as endocarditis and sepsis [11]. Proinflammatory cytokines such as IL-1β and IL-6 responses are upregulated throughout the S. aureus infection [12].
Proteoglycan (PG) is a constituent of extracellular matrix and is widely distributed in connective tissues such as skin, bone, cartilage and vascular wall by forming a complex with collagen, fibronectin, laminin, hyaluronic acid and other glycoproteins. PG consists of core protein and one or more covalently attached glycosaminoglycan chain(s). We have previously demonstrated that PG extracted from salmon nasal cartilage has a potent effect on suppression of inflammatory responses induced by heat-killed Escherichia coli in mouse macrophages [13]. Daily oral administration of PG attenuates the severity of experimental inflammatory colitis [14], autoimmune encephalomyelitis [15] and collagen-induced arthritis [16]. In addition, PG has been shown to be involved in cellular proliferation, adhesion and effective for wound healing in vitro [17, 18, 19]. However, the healing effect of PG on infected wound is not clear.
In this study, we investigated the effect of PG on the initial healing of S. aureus-infected skin wound because anti-inflammatory response is critical for wound healing. To clarify the effect of PG, we assessed histology, recruitment of phagocytes and cytokine responses in the skin wound of PG-treated mice.
2. Materials and methods
2.1. Mice
Male BALB/c mice, 8-week-old, were purchased from Clea Japan, Tokyo, Japan, and maintained in a temperature-controlled room (22 °C) on a 12-h light-dark cycle at Institute for Animal Experimentation, Hirosaki University Graduate School of Medicine. All animal experiments were carried out in accordance with the Guidelines for Animal Experimentation of Hirosaki University. All mouse experiments were approved by the Committee on the Ethics of Animal Experimentation of Hirosaki University (Permission number: M10003).
2.2. Preparation of PG
Salmon nasal cartilage PG was purchased from Kakuhiro Co., Ltd., Aomori, Japan. Lyophilized PG powder was dissolved in sterile distilled water (DW) at concentrations ranging from 0.4–10.0 mg/mL. DW was used as control.
2.3. Mouse model of excisional wound and PG administration
Skin wound was made on the back of mice as previously described [20]. In brief, mice were anesthetized with an intraperitoneal administration of an anesthetic mixture [0.075 mg/mL medetomidine (Zenoaq, Tokyo, Japan), 0.4 mg/mL midazolam (Sandoz, Tokyo, Japan) and 0.5 mg/mL butorphanol (Meiji Seika Pharma Co., Ltd., Tokyo, Japan)] at 100 μL/10 g body weight. Hair on back skin was removed using a mechanical shaver. After cleaning the skin with 70% ethanol, 6-mm-diameter circular full-thickness wound was made using a skin biopsy punch (disposable biopsy punch, Igarashi Ika Kogyo, Tokyo, Japan). Ten μL of PG (0.4, 2.0 and 10.0 mg/mL) or DW as the vehicle control was applied on the wound region once a day for 14 days. Pictures of the wound were taken daily to monitor wound healing. The wound area in photographs was measured using image analysis software (Photoshop CS6; Adobe Systems, San Jose, CA). The data was expressed as a percentage of wound area relative to the initial wound size.
2.4. Bacterial strain and culture condition
S. aureus strain 834, a clinical isolate [21], was used for infection of mice in this study. The bacterial cells were grown in tryptic soy broth (BD Diagnosis Systems, Sparks, MD) at 37 °C for 15 h, harvested by centrifugation and washed with phosphate-buffered saline (PBS). The washed bacterial cells were diluted with PBS to 2.5 × 109 colony-forming units (CFU)/mL adjusted by spectrophotometric measurement at 550 nm.
2.5. Inoculation of S. aureus into skin wounds
Mice were inoculated with 20 μL of 2.5 × 109 CFU/mL of S. aureus at the sites of skin wound immediately after wounding. Ten μL of PG (10 mg/mL) or DW was applied on the wound region once a day for 6 days. Skin lesion tissues were collected for further analysis at the indicated time after S. aureus inoculation.
2.6. Histological analysis
Skin lesion tissues were excised and fixed in 4% (w/v) paraformaldehyde buffer at 4 °C for overnight. Tissues were then embedded in paraffin and cut into 5-μm thick sections. Deparaffinized sections were stained with hematoxylin and eosin (H&E). To observe localization of bacterial cells, the sections were stained with crystal violet, Lugol's iodine solution and picric acid. The stained sections were observed under BZ-X700 microscope (Keyence, Tokyo, Japan) and wound length was measured.
2.7. Quantitation of viable bacterial cells in wound tissues
Wound tissues were collected to determine the number of viable bacterial cells in the infected wounds on day 2 after S. aureus inoculation. Individual tissues were homogenized in sterile Dulbecco's Modified Eagle medium (DMEM, Nissui Pharmaceutical Co., Tokyo, Japan). Each sample was plated in triplicate on tryptic soy agar plates. Plates were incubated for 16 h at 37 °C. The number of viable bacterial cells in the wound was determined by counting the colonies on the plates. The data are described as log10 number of CFU/wound.
2.8. Immunohistochemistry
Immunofluorescent analysis was conducted to determine localization of neutrophils and macrophages in the infected wound tissue. Wound tissues were excised and fixed as mentioned above. The tissues were then soaked in PBS containing 30% sucrose and frozen in optimal cutting temperature medium (Sakura Finetek Japan, Tokyo, Japan) at −80 °C. The frozen tissues were cut at 10-μm thickness and the sections were incubated with PBS containing 2% normal goat serum. The sections were stained with rat anti-mouse Ly6G IgG (diluted 1:500; Abcam, United Kingdom) or rat anti-mouse F4/80 IgG (diluted 1:200; Serotec Product, United Kingdom) at 4 °C overnight and then with Alexa 568-conjugated donkey anti-rat IgG (1:200; Invitrogen). The stained sections were observed under BZ-X700 microscope. Ly6G positive cells and F4/80 positive cells were randomly counted from eight histological sections.
2.9. Measurement of cytokines
Wound tissues were homogenized in DMEM containing complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and then were centrifuged for 10 min at 1000×g. Supernatants were used to determine TGF-β, IL-6, IL-1β and TNF-α levels using commercial ELISA kits according to the manufacturer's recommendation.
2.10. Statistical analysis
Data were expressed as means ± standard deviations, and p < 0.05 from unpaired student's t test in Figs. 1, 2, and 3 or Dunnet test analysis in Figs. 4 and 5 were used to determine the significance of the differences.
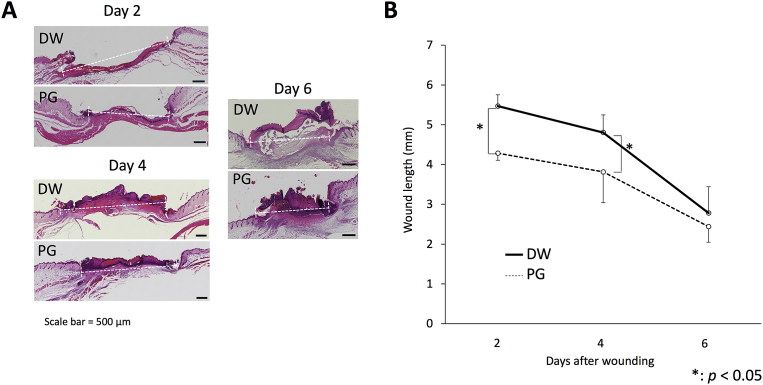
Fig. 1.
Effect of PG on S. aureus-inoculated wound length. Mice were inoculated with 20 μL of 2.5 × 109 CFU/mL of S. aureus at the sites of skin wound immediately after wounding, and then treated with 10 μL of 10 mg/mL PG daily. On days 2, 4 and 6 after infection, the skin tissues were collected and paraffin sections were prepared. After staining with H&E, the length of infected wound was measured (5 mice per group per each experiment). The pictures of histology are representative (A). The length of infected wound is expressed as the means ± SD of 2 independent experiments (B). An asterisk (p < 0.05) indicates that the value is significantly different from the DW control group.
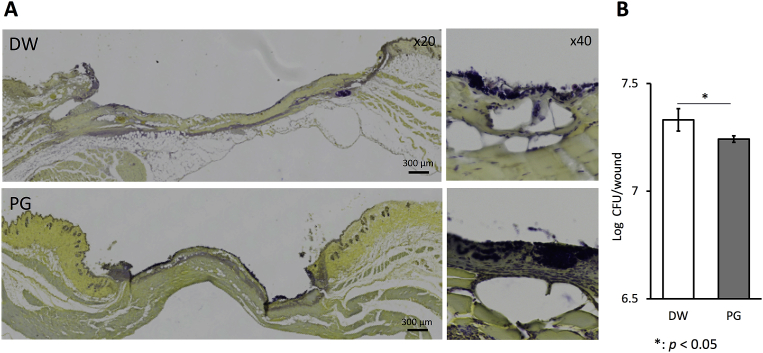
Fig. 2.
Effect of PG on S. aureus infection in wound. Mice were inoculated with 20 μL of 2.5 × 109 CFU/mL of S. aureus at the sites of skin wound immediately after wounding, and then treated with 10 μL of 10 mg/mL PG daily. On day 2, the skin tissues were collected and paraffin sections were prepared. The sections were stained with crystal violet, Lugol's iodine solution and picric acid (A). Bacterial cells (dark blue) localized on yellow background of mouse tissue. The bacterial number of homogenates of whole wounds was determined (B). The data are representative (A) and the results are expressed as the means ± SD of 2 independent experiments (5 mice per group per each experiment) (b). An asterisk (p < 0.05) indicates that the value is significantly different from the DW control group.
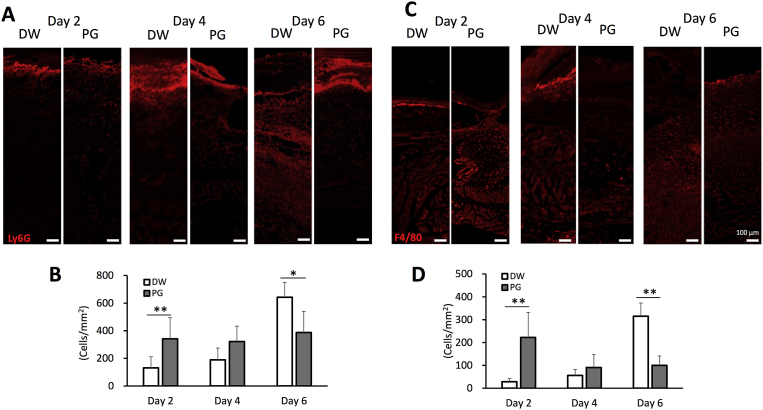
Fig. 3.
Effect of PG on recruitment of neutrophils and macrophages to S. aureus-inoculated wounds. Mice were inoculated with 20 μL of 2.5 × 109 CFU/mL of S. aureus at the sites of skin wound immediately after wounding, and then treated with 10 μL of 10 mg/mL PG daily. On days 2, 4 and 6 after infection, the skin tissues were collected and frozen sections were prepared. Immunofluorescent staining was performed using anti-Ly6G antibody for neutrophils (A) and anti-F4/80 antibody for macrophages (C). Ly6G+ cells (B) and F4/80+ cells (D) were randomly counted from eight histological sections. The data are representative of 2 independent experiments (4 mice per group per each experiment) (B, D). An asterisk (p < 0.05) and double asterisks (p < 0.01) indicate that the value is significantly different from the control group, respectively.
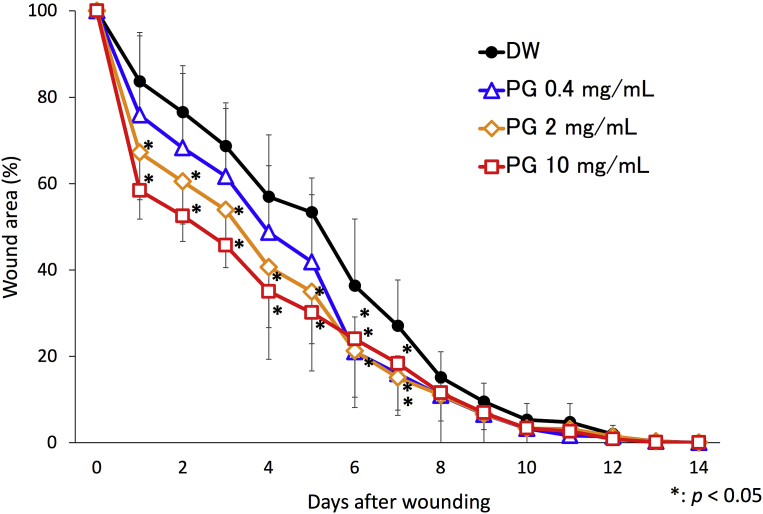
Fig. 4.
Effect of PG on skin wound healing. Skin wound (6-mm-diameter circular full-thickness) was made on back of mice using a skin biopsy punch as described in materials and methods. Ten μL of PG (0.4, 2.0 and 10.0 mg/mL) or DW as vehicle control was applied on the wound region once a day for 14 days. The data was expressed as a percentage of wound area relative to the initial wound size. The results are expressed as the means ± SD of 3 independent experiments (5 mice per group per each experiment). An asterisk (p < 0.05) indicates that the value is significantly different from the DW control group.
Fig. 5.
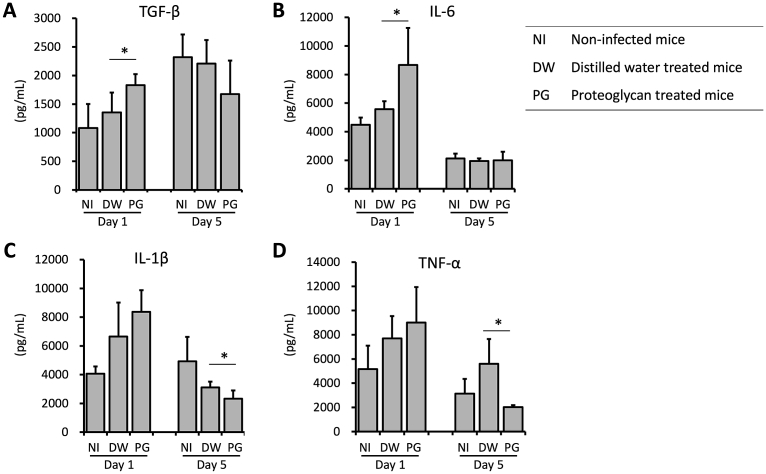
Effect of PG on cytokine production during S. aureus infection in wound. Mice were inoculated with 20 μL of 2.5 × 109 CFU/mL of S. aureus at the sites of skin wound immediately after wounding, and then treated with 10 μL of 10 mg/mL PG daily. On days 1 and 5, skin tissues were collected and homogenized. Production of TGF-β (A), IL-6 (B), IL-1β (C) and TNF-α (D) in wound tissues was determined by ELISA. The results are expressed as the means ± SD of 2 independent experiments (5 mice per group per each experiment). An asterisk (p < 0.05) indicates that the value is significantly different from the DW control group. PG group: S. aureus-infected and PG-treated, DW group: S. aureus-infected and PG-untreated control, NI group: non-infected and PG-untreated control.
3. Results
3.1. PG administration accelerated initial healing of skin wound
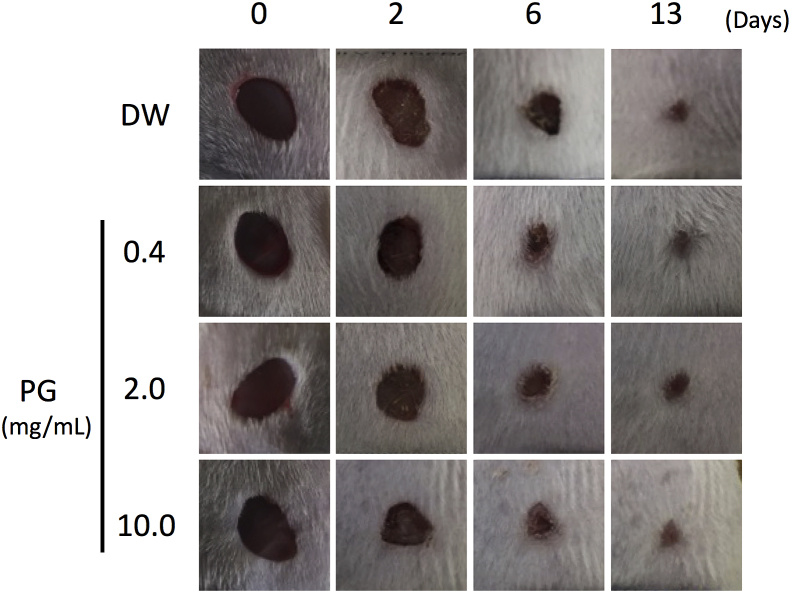
To investigate the effect of PG on wound healing, digital images of the wounds were captured at a fixed distance and angle for planimetric measurement. PG significantly accelerated wound closure compared with the vehicle control in the initial phase until day 7 after wounding (Fig. 4). The effect of PG on the initial wound closure was shown in a dose dependent manner (Fig. 4). Although wound contraction of PG-treated mice was occurred earlier than that of vehicle control, the period of time for complete repair was comparable (Fig. 6).
Fig. 6.
Effect of PG on skin wound healing. Representative digital images of non-infected wounds were captured over 13 days during wound-healing period.
3.2. PG administration induced accumulation of neutrophils and macrophages in the early healing process of skin wound
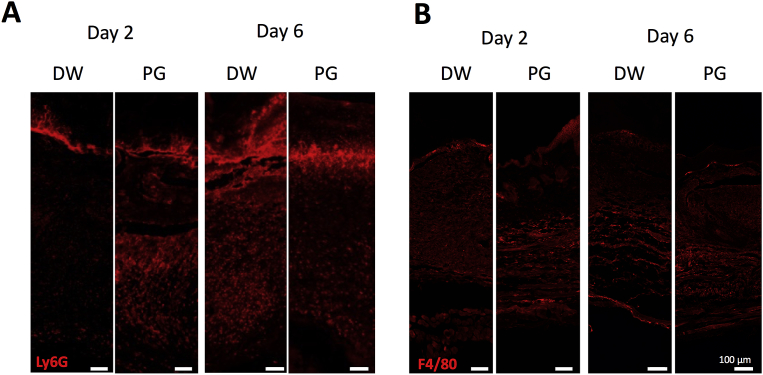
To determine accumulation of immune cells in wound area, we carried out immunofluorescent staining using anti-Ly6G antibody or anti-F4/80 antibody for neutrophils and macrophages, respectively. Neutrophil infiltration was intensively observed in the PG-treated mice on day 2 after wounding (Fig. 7A). In contrast, neutrophil influx was highly detected on day 6 in the vehicle control (Fig. 7A). There was no difference in macrophage infiltration between the PG-treated mice and the control animals (Fig. 7B).
Fig. 7.
Effect of PG on recruitment of neutrophils and macrophages to non-infected wounds. On days 2 and 6 after infection, the skin tissues were collected and frozen sections were prepared. Immunofluorescent staining was performed using anti-Ly6G antibody for neutrophils (A) and anti-F4/80 antibody for macrophages (B). The data are representative of 2 independent experiments (3 mice per group per each experiment).
3.3. PG administration accelerated initial wound healing of S. aureus-infected skin
To determine whether PG promotes the initial healing of infected wound, skin wound was infected with S. aureus and then treated with 10 μL of 10 mg/mL PG once a day. On days 2, 4 and 6 after infection, the skin tissues were collected and the length of infected wound was measured. The length of PG-treated wound was significantly shorter than that of the vehicle control on days 2 and 4 after infection (Fig. 1A, B).
3.4. PG administration reduced bacterial number on day 2 after infection
To determine the effect of PG on bacterial invasion into wound tissue, we observed the localization of S. aureus and enumerated the bacterial number in the wound tissues. Most of all bacterial cells localized on the surface of crust in the PG-treated mice and the vehicle control (Fig. 2A). The bacterial number in PG-treated mice was significantly lower than that in the vehicle group (Fig. 2B).
3.5. PG administration induced accumulation of neutrophils and macrophages in the early healing process of infected wound
To determine accumulation of immune cells in wound area, we carried out immunofluorescent staining using anti-Ly6G antibody or anti-F4/80 antibody for neutrophils and macrophages. Neutrophil infiltration was intensively observed in the PG-treated mice on days 2 and 4 after S. aureus inoculation (Fig. 3A). Neutrophil number of the PG-treated mice was 160% increase compared with the control group (Fig. 3B). In contrast, neutrophil influx was highly detected on day 6 in the vehicle control (Fig. 3A). The number of neutrophils in PG-administered mice was 40% decrease compared with the vehicle control (Fig. 3B). The similar pattern of macrophage infiltration was also found. Macrophage infiltration was intensively observed on day 2 after S. aureus inoculation in the PG-treated mice (Fig. 3C). The number of macrophages in the PG-treated mice was 670% increase compared with the control group (Fig. 3D). On day 4, nearly the same macrophage infiltration was found in both PG-treated and control mice (Fig. 3C, D). On day 6, the number of macrophages in the vesicle control was significantly higher than that in the PG-treated mice (Fig. 3D). The macrophage influx in the PG-treated mice was 70% decrease compared with that in vehicle control (Fig. 3D).
3.6. Cytokine production was promoted in the early phase and suppressed in middle phase of wound healing by PG
To determine whether PG affects cytokine production, we measured TGF-β, IL-6, IL-1β and TNF-α levels in the wound tissues. In the PG-treated mice, the production of TGF-β (35%) and IL-6 (56%) in the wound tissues was significantly promoted compared with the vehicle control on day 1 (Fig. 5A, B). However, the production of IL-1β (25%) and TNF-α (64%) in the PG-treated mice was significantly decreased compared with the vehicle control on day 5 (Fig. 5C, D).
4. Discussion
It has been reported that closing and moist environment promotes wound healing and reduces the pain [22]. In this study, PG administration accelerated initial healing of non-infected skin wound in a dose-dependent manner (Fig. 4). Similarly, PG administration accelerated initial healing of skin wound infected with S. aureus (Fig. 1). In addition, PG administration reduced bacterial number in the early phase of healing (Fig. 2). PG shows no antibiotic activity against S. aureus (unpublished data). Therefore, the reduction of bacterial number is possibly mediated by host immune response. Neutrophil and macrophage influx is an early inflammatory response of wound healing. This event is essential for the clearance of bacteria and cellular debris in infected wound [23]. Bacterial clearance by neutrophils is a key event requiring for wound healing [24]. Alternatively, wound healing is delayed by continuous influx of neutrophils [25]. PG administration enhanced the accumulation of neutrophils and macrophages in the early healing process of infected wound, whereas fewer neutrophils were observed in the wound tissue in the middle phase (Fig. 3). The comparable results were observed in non-infected wound (Fig. 7). These results suggest that PG administration enhanced the accumulation of neutrophils and macrophages only in the early phase.
PG administration significantly augmented IL-6 and TGF-β production in the wound tissue in the early phase of healing (Fig. 5A, B). IL-6 has the mitogenic and proliferative effects on keratinocytes and is a neutrophil chemoattractant [26, 27]. TGF-β has been shown to play a critical role in inflammation, angiogenesis, re-epithelialization, and connective tissue regeneration [28]. In the middle phase of healing, PG administration reduced proinflammatory cytokine production such as TNF-α and IL-1β (Fig. 5C, D). TNF-α and IL-1β are important proinflammatory cytokines that raise inflammation for bacterial clearance and healing of infected wound, while prolonged inflammation by these cytokines causes the development of chronic wounds [29]. Our results suggested that PG administration accelerates the inflammatory response in the initial step of healing in the infected wounds leading to promote infected wound healing. This finding implied that early resolution of wounds may contribute to faster healing. The mechanism of enhancement of inflammatory response by PG in the initial step of wound healing is still unclear. TNF-stimulated gene 6 has been shown to be an important receptor involving in glycosaminoglycan-induced cell-mediated inflammation [30]. Moreover, the binding of salmon nasal PG to CD44 on mouse fibroblasts is the primary mechanism for the effect on in vitro wound closure [19]. Therefore, these receptors may be involved in inflammatory response and wound healing promoted by PG administration. We have reported that PG has an anti-inflammatory effect on mouse models of various inflammatory diseases. The anti-inflammatory effect of PG depends on induction of Foxp3+ regulatory T cells [14, 15]. Therefore, regulatory T cells might be induced in infected wounds in the middle phase and contribute to suppression of inflammatory response. Regarding high molecular weight of PG, a component(s) of PG which is responsible for wound healing action remains to be clarified. Finally, this finding implied that PG may therefore be a useful substance in the drug delivery of wound healing active agent.
In conclusion, our present results demonstrated that PG has a prophylactic effect by modulation of inflammatory responses in infected wounds. These results suggest the existence of novel interaction of extracellular matrix components in inflammatory responses during bacterial infections on wound healing.
Declarations
Author contribution statement
Shouhei Hirose: Performed the experiments; Wrote the paper.
Kouji Narita: Performed the experiments.
Krisana Asano: Analyzed and interpreted the data.
Akio Nakane: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was partly supported by Regional Innovation Strategy Support Program, MEXT (AN).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Krisana Asano, Email: krisana@hirosaki-u.ac.jp.
Akio Nakane, Email: a27k03n0@hirosaki-u.ac.jp.
References
- 1.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;4(276):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 2.Singer A.J., Clark R.A.F. Cutaneous wound healing. N. Engl. J. Med. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Hackam D.J., Ford H.R. Cellular, biochemical, and clinical aspects of wound healing. Surg. Infect. (Larchmt) 2002;3(Suppl. 1):S23–S35. doi: 10.1089/sur.2002.3.s1-23. [DOI] [PubMed] [Google Scholar]
- 4.Harding K.G., Moore K., Phillips T.J. Wound chronicity and fibroblast senescence–implications for treatment. Int. Wound J. 2005;2(4):364–368. doi: 10.1111/j.1742-4801.2005.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrientos S., Stojadinovic O., Golinko M.S. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 6.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 7.Madsen S.M., Westh H., Danielsen L. Bacterial colonization and healing of venous leg ulcers. APMIS. 1996;104(12):895–899. doi: 10.1111/j.1699-0463.1996.tb04955.x. [DOI] [PubMed] [Google Scholar]
- 8.Grimble S.A., Magee T.R., Galland R.B. Methicillin resistant Staphylococcus aureus in patients undergoing major amputation. Eur. J. Vasc. Endovasc. Surg. 2001;22(3):215–218. doi: 10.1053/ejvs.2001.1436. [DOI] [PubMed] [Google Scholar]
- 9.McCaig L.F., McDonald L.C., Mandal S. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg. Infect. Dis. 2006;12(11):1715–1723. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran G.J., Krishnadasan A., Gorwitz R.J. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006;355(7):666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 11.Lowy F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 12.Megyeri K., Mandi Y., Degre M., Rosztoczy I. Induction of cytokine production by different staphylococcal strains. Cytokine. 2002;19:206–212. doi: 10.1006/cyto.2002.0876. [DOI] [PubMed] [Google Scholar]
- 13.Sashinami H., Takagaki K., Nakane A. Salmon cartilage proteoglycan modulates cytokine responses to Escherichia coli in mouse macrophages. Biochem. Biophys. Res. Commun. 2006;351(4):1005–1010. doi: 10.1016/j.bbrc.2006.10.146. [DOI] [PubMed] [Google Scholar]
- 14.Mitsui T., Sashinami H., Sato F. Salmon cartilage proteoglycan suppresses mouse experimental colitis through induction of Foxp3+ regulatory T cells. Biochem. Biophys. Res. Commun. 2010;402(2):209–215. doi: 10.1016/j.bbrc.2010.09.123. [DOI] [PubMed] [Google Scholar]
- 15.Sashinami H., Asano K., Yoshimura S., Mori F., Wakabayashi K., Nakane A. Salmon proteoglycan suppresses progression of mouse experimental autoimmune encephalomyelitis via regulation of Th17 and Foxp3+ regulatory T cells. Life Sci. 2012;91(25–26):1263–1269. doi: 10.1016/j.lfs.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura S., Asano K., Nakane A. Attenuation of collagen-induced arthritis in mice by salmon proteoglycan. BioMed Res. Int. 2014;2014:406453. doi: 10.1155/2014/406453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danen E.H., Yamada K.M. Fibronectin, integrins, and growth control. J. Cell. Physiol. 2001;189(1):1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- 18.Sano M., Shang Y., Nakane A. Salmon nasal cartilage proteoglycan enhances growth of normal human dermal fibroblast through Erk1/2 phosphorylation. Biosci. Biotechnol. Biochem. 2017;81(7):1379–1385. doi: 10.1080/09168451.2017.1318695. [DOI] [PubMed] [Google Scholar]
- 19.Ito G., Kobayashi T., Takeda Y. Proteoglycan from salmon nasal cartridge promotes in vitro wound healing of fibroblast monolayers via the CD44 receptor. Biochem. Biophys. Res. Commun. 2015;456(3):792–798. doi: 10.1016/j.bbrc.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 20.Galiano R.D., Michaels J.V., Dobryansky M. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2014;12(4):485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakane A., Okamoto M., Asano M. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect. Immun. 1995;63(4):1165–1172. doi: 10.1128/iai.63.4.1165-1172.1995. PMID: 7890367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field C.K., Kerstein M.D. Overview of wound healing in a moist environment. Am. J. Surg. 1994;167:S2–S6. doi: 10.1016/0002-9610(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim M.-H., Liu W., Borjesson D.L. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J. Investig. Dermatol. 2008;128(7):1812–1820. doi: 10.1038/sj.jid.5701223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller L.S., O’Connell R.M., Gutierrez M.A. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24(1):79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Jun J.-I., Kim K.-H., Lau L.F. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat. Commun. 2015;6:7386. doi: 10.1038/ncomms8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallucci R.M., Sloan D.K., Heck J.M. Interleukin 6 indirectly induces keratinocyte migration. J. Investig. Dermatol. 2004;122(3):764–772. doi: 10.1111/j.0022-202X.2004.22323.x. [DOI] [PubMed] [Google Scholar]
- 27.Sato M., Sawamura D., Ina S. In vivo introduction of the interleukin 6 gene into human keratinocytes: induction of epidermal proliferation by the fully spliced form of interleukin 6, but not by the alternatively spliced form. Arch. Dermatol. Res. 1999;291(7–8):400–404. doi: 10.1007/s004030050429. [DOI] [PubMed] [Google Scholar]
- 28.Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez-Fernandez A., Inada M., Balbin M. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) FASEB J. 2007;21(10):2580. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris E.N., Weigel P.H. Functional aspects of the hyaluronan and chondroitin sulfate receptors. In: Vasta G.R., Ahmed H., editors. Animal Lectins: a Functional View. CRC Press; Boca Raton: 2009. pp. 171–192. [Google Scholar]