Abstract
Purpose
To conduct a systematic review that examined the effect of dance interventions on balance, gait and functional mobility outcomes in adults with neurological conditions other than Parkinson's disease.
Methods
A systematic search of relevant databases was conducted. Data extraction and methodological appraisal were performed by two independent authors.
Results
Nine studies were included (4 pre-post studies with no control group, 3 case reports, and 2 controlled studies) and results of the methodological quality assessment ranged from poor to good. Study groups included stroke, multiple sclerosis, spinal cord injury, and Huntington's disease. Dance interventions varied in frequency, type and duration, and only 1 study reported intensity. Study dropout rates ranged from 20–44%, and 88–100% of dance classes were attended. Only 3 studies mentioned adverse events, of which there were none. A summary of results revealed significant changes in spatiotemporal gait parameters, Berg Balance Scale scores, Timed Up and Go test and six-minute walk test that were similar to or greater than those previously reported in a review of dance for individuals with Parkinson's disease.
Conclusions
There is emerging evidence to support the use of dance as a feasible intervention for adults with neurological conditions. Further investigation of the effects of dance with randomized controlled trials using larger sample sizes and better reporting of the intervention, participant tolerance, and adverse events is warranted.
Keyword: Rehabilitation
1. Introduction
One in 3 Canadians are affected by a disease, disorder or injury of the nervous system in their lifetimes [1]. This includes neurological conditions (and prevalence per 100,000 population in Canada) such as stroke (980), multiple sclerosis (290), spinal cord injury (360) traumatic brain injury (410) and Huntington's disease (10) [2]. Neurological conditions frequently require rehabilitation, and from Canadian inpatient rehabilitation data (2005–2006) available for 5 conditions (stroke, multiple sclerosis (MS), spinal cord injury (SCI), Parkinson's disease (PD), and head injury), it is estimated they account for 31.7% of the total inpatient rehabilitation days for all conditions [3]. Walking, balance and mobility are almost invariably affected by these neurological conditions. Gait and balance impairments have a profound impact on an individual's ability to perform activities of daily living and consequently, quality of life [4, 5]. It is no surprise then, that balance and gait are important to address from the perspectives of both the patient and the therapist. For example, 25–45% of stroke rehabilitation time is spent addressing gait dysfunction [6], while improvement of gait function is a commonly stated goal by individuals undergoing neurologic rehabilitation [7, 8, 9].
Improvements in gait, mobility and balance are made with rehabilitation. For example, functional standing balance (as measured by the Berg Balance Scale) improves and gait velocity increases significantly with rehabilitation after stroke and incomplete SCI [10, 11]. Inpatient rehabilitation for individuals with MS improves wheelchair mobility and transfers [12] as well as function measured by the Functional Independence measure which includes two items related to mobility (walking and stairs) [13] Despite these gains, balance and gait remain significantly impaired compared to healthy adults. For example, velocity at discharge from stroke rehabilitation (0.84 ± 0.33 m/s) is ∼45% slower than healthy older adults of similar age [10, 14], and adults with incomplete SCI achieve Berg Balance Scale scores (29.1 ± 20.6 out of 56 points), 47% lower than scores achieved by healthy older adults (60–69 years) [11, 14]. Persistent slow walking speeds at discharge from rehabilitation are of particular concern given the associated outcomes such as risk of death, hospitalization, falls [15] and limitations in community ambulation [16]. Clearly, new therapeutic approaches and interventions for balance, mobility and gait are needed. Dance instruction as therapy is a promising intervention that may address this need.
Dance is a worldwide human activity that involves complex whole body movements through space synchronized to music [17]. Older adults who regularly dance have a more stable gait pattern, better balance and faster reaction times than older adults who do not dance [18, 19]. Dance also has the same aerobic benefits as jogging and walking programs [20]. Adults who dance recreationally describe it as an enjoyable experience and perceive a number of benefits such as emotional and social well-being and stress reduction [17]. Interest in the use of dance as a therapeutic tool to address both psychological and physical impairments is increasing with one Cochrane review reporting positive effects on quality of life and fatigue in people with cancer [21]. Furthermore, dance has been used extensively for the treatment of gait and balance dysfunction in individuals with PD and a number of systematic reviews on the topic exist [22, 23, 24]. Tango is the most frequently employed dance form in the treatment of PD [23]. However, evidence suggests that other dance forms including non-partnered dance are just as effective [23, 25]. Dance as therapy for PD has multiple benefits including improvements in balance, gait and motor impairment [23]. Furthermore, improvements made with dance as therapy may be greater than gains made with traditional group exercise classes [26] or in some circumstances, conventional physiotherapy [27]. A recent systematic review and meta-analysis also confirmed balance and gait outcomes in favour of dance interventions for PD over control groups or other interventions [23].
The aim of this study was to conduct a systematic review of the literature in order to describe how dance is being used and investigated in adults with neurological conditions that affect the central nervous system and feature motor and sensory impairments that impact gait and balance. Our objectives were to: 1) describe dance interventions used in adult neurologic populations using the FITT principle (frequency, intensity, time/duration, type); 2) describe the feasibility of dance interventions in terms of participant dropout, reporting of adverse events and adherence; and 3) investigate the evidence for the effect of dance on balance, gait, and functional mobility outcomes in adult neurologic populations.
2. Methods
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [28]. We modeled our approach on the most recent review conducted by Shanahan and colleagues [23] on the use of dance in PD. We defined a dance intervention as any regular intervention program that featured learning movement patterns synchronized to music.
2.1. Literature search
An electronic search of the literature was conducted in February 2015 by an Information Specialist. The following electronic databases were included: MEDLINE and MEDLINE in-process, PubMed (supplemental search), Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Physiotherapy Evidence Database (PEDro). Search strategies using combinations of subject headings and keywords specific to each database were developed to identify appropriate studies; for example, the search terms used in MEDLINE and MEDLINE in-process were “dancing, dance therapy”, “gait”, and “postural balance” (a full MEDLINE search strategy is included in Table 1). Updated searches using the same search strategies were conducted in July 2015, May 2016, December 2016 and August 2017. Reference lists from relevant articles were also examined to identify additional studies missed by the electronic search.
Table 1.
MEDLINE search strategy used Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid (1946-present) on December 21, 2016.
| Line | Search term (s) | Results |
|---|---|---|
| 1 | dancing/ or dance therapy/ | 2737 |
| 2 | danc*.tw. | 6032 |
| 3 | DMT.tw. | 1659 |
| 4 | ((dance or movement) adj5 therap*).tw. | 2292 |
| 5 | 1 or 2 or 3 or 4 | 10406 |
| 6 | Gait/ | 24301 |
| 7 | exp Gait Disorders, Neurologic/ | 6257 |
| 8 | Postural Balance/ | 19847 |
| 9 | gait*.tw. | 40617 |
| 10 | balanc*.tw. | 260919 |
| 11 | (equilibrium adj4 (body or postur* or musculoskeletal or disorder*)).tw. | 1121 |
| 12 | 6 or 7 or 8 or 9 or 10 or 11 | 315260 |
| 13 | 5 and 12 | 625 |
| 14 | (exp child/ or exp infant/) not ((exp child/ or exp infant/) and (exp adolescent/ or exp aged/ or exp adult/)) | 1258211 |
| 15 | 13 not 14 | 596 |
| 16 | limit 15 to English language | 558 |
2.2. Study selection
The titles of all search results were screened independently by two authors (JW, EP) to identify potentially relevant studies for further review. Abstracts of articles identified as potentially relevant were assessed to determine if they met the inclusion criteria. Inclusion criteria included studies in English that investigated: 1) an adult population with a neurological condition affecting the central nervous system associated with motor and/or sensory impairments and is known to impair balance and/or mobility; 2) the effects of a dance intervention as determined by at least one objective measure of gait, balance or functional mobility that had published psychometric properties (i.e. reliability and validity) taken before and after the intervention. Studies were excluded if: 1) the dance intervention was combined with another intervention (e.g. speech therapy); and 2) the study investigated adults with PD, as a recent review of dance for people with PD was conducted [23]. Disagreements were first resolved by consensus and in the case where consensus was not reached they were resolved by a third author (KKP). Given the overall aim of this review to describe the use of dance interventions for neurological conditions, there were no restrictions on publication type. This enabled a fulsome view of the state of the field.
2.3. Data extraction
Articles that met the inclusion criteria were reviewed independently by two authors (JW, EP) to extract details related to the outcomes of interest. Any conflicts in data extraction were resolved by a third author (KKP). Authors of included studies were contacted for disclosure of any unpublished work.
Details regarding each publication such as the year of publication, study design and demographics of the study group (i.e. disease population, age, and number of participants) were extracted. For the first study objective, characteristics of the dance interventions were extracted including the frequency, intensity, time/duration and type (FITT principles). For the second objective, indicators of feasibility including the number of participants who dropped out, the number and type of adverse events, and adherence to the intervention were extracted. Finally, for the third objective, both valid and reliable clinical performance-based measures and instrumented laboratory measures of balance, gait and functional mobility were considered acceptable. In cases where a study used more than one measure for an outcome of interest (e.g. spatiotemporal parameters of gait and Dynamic Gait Index used in the same study), the results of both measures were extracted. Thus, different measures (e.g. Functional Reach Test and Berg Balance Scale) could be extracted within a specific outcome of interest (e.g. balance) across the included studies.
2.4. Data analysis
Publication details for each of the included studies were summarized in table format. The median and first (Q1) and third quartiles (Q3) were calculated for study sample size and FITT descriptors of the dance intervention including frequency, time and duration. Changes in balance, gait, and functional mobility outcomes were calculated as the difference between the post-intervention value and the pre-intervention value. Even though some studies collected follow-up measures, only pre- and post-intervention values were considered to facilitate comparisons across studies. Finally, for studies that reported spatiotemporal gait parameters, only measures of preferred forward paced gait were extracted, again to facilitate comparison. Changes in outcomes were compared against published minimal important difference (MID) values when such values were available for the corresponding measure and patient population. A meta-analysis was not planned given the anticipated heterogeneity in populations, study designs, and outcomes. Thus, study results were categorized as either positive, negative or no significant change based on the statistical tests if reported (indicated by an asterisk) or based on the authors' conclusions about the results for case reports. This categorization of study results was summarized in a table and modelled after a similar approach employed in a systematic review of self-management in chronic obstructive pulmonary disease [29].
2.5. Risk of bias in individual studies
Included studies were evaluated for methodological quality using a tool matched to study design. For pre-post studies with no control group, the study question, participant selection, sample size, description of the intervention and outcome measures, rate of follow-up, and statistical methods were examined using the Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group [30]. For case reports, the Quality Assessment Tool for Case Series Studies [31] was used to evaluate the clarity of the research question, definition of the study population and intervention, and statistical methods. Components of the study design, participant selection, allocation to intervention and control groups, blinding, and the appropriateness of data collection tools and analysis were assessed for controlled studies using the Quality Assessment Tool of Controlled Intervention Studies [32]. Two reviewers (JW, EP) independently rated components of the scale as “Yes”, “No”, “Not applicable”, “Not recorded” or “Cannot determine” using the accompanying guidance document, which were then used to guide the overall rating for the study as “Good”, “Fair” or “Poor”. Any differences between reviewers were discussed in order to reach consensus, and in the case where consensus could not be reached, a third author resolved the conflict (KKP).
3. Results
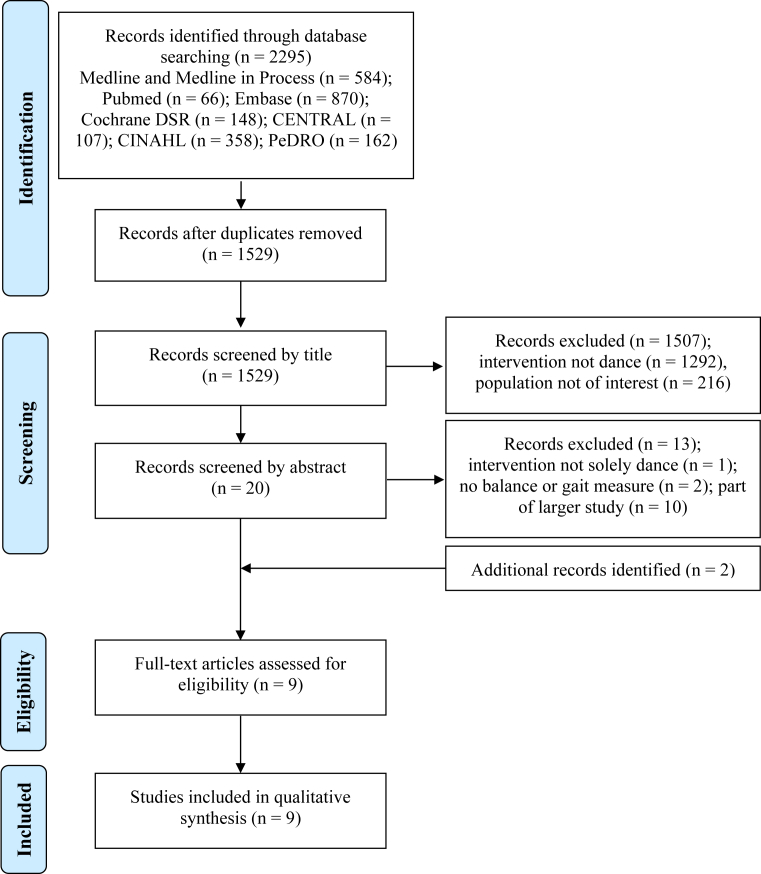
Nine articles met the inclusion criteria. A flowchart detailing the search and review process is included in Fig. 1. In one case, 3 search results (2 conference abstracts and 1 full article) were combined as they were reporting on one larger study [33]. There were 4 pre-post studies with no control group [33, 34, 35, 36], 3 case reports [37, 38, 39], and 2 controlled studies [40, 41]. The results of 5 of these studies were published as full articles in academic journals [33, 36, 37, 39, 40], 3 studies were published as abstracts in academic journals [34, 35, 41], and 1 study was a published as a graduate thesis [38]. The authors of the 3 studies published as abstracts were contacted to request further information and to determine if there was a full article submitted or in press. Only one author replied with details about the study population and provided an updated sample size; however, no further published results were available at the time [34]. Table 2 lists the included studies and summarizes the neurological patient group studied and features of the dance intervention used.
Fig. 1.
Search strategy and results based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Table 2.
Summary of study and dance intervention characteristics for included studies.
| Study | Study design | Population | Participants [n; age (standard deviation)] | Dropouts/adherence | Adverse events | Type of dance/activity | Frequency of intervention | Intensity | Time of assessment |
|---|---|---|---|---|---|---|---|---|---|
| Demers and McKinley, 2015 [33] | Pre-post study (no control group) | Stroke (sub-acute) | n = 9; mean age 63.7 (11.7) years | n = 7 | None | Jazz dance and merengue | 45 minutes, 2 classes/week for 4 weeks | Moderate intensity, measured with Borg scale | 1 week pre-intervention and 1-week post-intervention |
| Lachance et al., 2013 (abstract) [34] | Pre-post study (no control group) | Motor deficits | n = 16; age not reported | Not reported | Not reported | Dance therapy program based on Laban movement theory | 90 minutes, 1 class/week for 12 weeks | Not reported | Pre-intervention and post-intervention, and 3 months post-intervention |
| Hong et al., 2013 (abstract) [35] | Pre-post study (no control group) | Spinal cord injury | n = 15; mean age 42.9 (12.1) years | Adherence 93.3 (7.8)% classes | Not reported | Rumba, tango, and salsa | 120 minutes, 2 classes/week for 6 weeks | Not reported | Pre-intervention and post-intervention |
| Mandelbaum et al., 2015 [36] | Pre-post study (no control group) | Multiple sclerosis | n = 8; mean age 49.5 (12.7) years | n = 2, 100% classes attended by 7 participants, 1 class missed by 1 participant due to schedule conflict | None | Salsa | 60 minutes, 2 classes/week for 4 weeks + practice at home 30 minutes/week |
Not reported | Baseline, post-intervention, 3-months and 6-months post-intervention |
| Hackney et al., 2012 [37] | Case report | Stroke (chronic) | n = 1; age 73 years | None | Not reported | Tango | 90 minutes, 20 classes total over 11 weeks | Not reported | 1-week pre-intervention and 1-week post-intervention, and 4 weeks post-intervention |
| Dureska, 2007 (thesis) [38] | Case report | Stroke (chronic) | n = 1; age 48 years | Unspecified number of missed classes | Not reported | Ballet | 90 minutes, 3 classes/week for 8 weeks | Not reported | 1-week and 2-weeks pre-intervention, 4-weeks, 8-weeks, and 1-month post-intervention |
| Salgado and de Paula Vasconcelos, 2010 [39] | Case report | Multiple sclerosis | n = 1; age 45 years | None, 1 participant missed 1 class | Not reported | Free and guided dance movements | 100 minutes, 2 classes/week for 20 weeks | Not reported | Pre-intervention and post-intervention |
| Kloos et al., 2013 [40] | Cross-over, single-blinded, controlled study | Huntington's disease | n = 18; mean age 50.7 (14.7) years | n = 6, 100% of sessions completed | None | Video game exercise program (Dance Dance Revolution) | 45 minutes, 2 classes/week for 6 weeks | Not reported | Pre-intervention A, post-intervention A, and post-intervention B |
| Sapezinskiene et al., 2009 (abstract) [41] | Controlled study (nature of control not specified) | Spinal cord injury | n = 108; age not reported | Not reported | Not reported | Dance movement program | 12 weeks | Not reported | Pre-intervention and post-intervention |
3.1. Participants
The median sample size was 9 (Q1 = 1, Q3 = 16) participants. Seven of the 9 studies listed the age or mean age and standard deviation for their sample, which ranged from 42.9 (12.1) [35] to 73 years of age [37]. The patient population receiving the dance intervention in each study was as follows: stroke (3 studies) [33, 37, 38], MS (2 studies) [36, 39], SCI (2 studies) [35, 41], Huntington's disease (1 study) [40], and individuals with motor deficits (1 study) [34]. The authors of this final study were contacted for more details regarding the individuals included in their study group. They reported that the group included people with various conditions such as SCI, traumatic brain injury/acquired brain injury, MS, stroke, and fibromyalgia, where some individuals had slight cognitive impairments but no behavioural issues.
3.2. Dance intervention (FITT principle)
3.2.1. Frequency
Frequency was defined as the number of classes delivered per week. The median frequency of dance classes was 2 (Q1 = 2, Q3 = 2) times per week. Single studies delivered classes 3 times per week [38] and 1 time per week [34], respectively. One study cited the total number of classes delivered (which was 20 classes over 11 weeks) [37], and 1 study did not report the frequency of classes [41].
3.2.2. Intensity
In a study for stroke [33], the intensity of the dance intervention was described as “moderate” and the authors noted intensity was measured at the individual level with the Borg Rating of Perceived Exertion scale. However, neither the individual scores nor the group mean ratings on the Borg scale were reported in the results [33]. Two other studies in stroke did not comment specifically on intensity but made related comments on progression of dance movements [37] and rest periods [38].
3.2.3. Type
Type was defined as the genre of dance for which each study provided instruction. Two studies taught a mix of genres including jazz, merengue, tango, rumba, and salsa [33, 35]. Ballet [38], salsa [36], and tango [37] were taught exclusively in single studies. One study used a dance video game for the intervention [40]. Finally, dance therapy [34], free and guided dance movement [39] and dance movement [41] were terms used in single studies.
3.2.4. Time/duration
Duration was described as both the length of the dance class and the length of the intervention period. The length of each dance class ranged from 45 [33, 40] to 120 minutes [35], and the median dance class duration was 90 (Q1 = 56.25, Q3 = 92.5) minutes. One study did not specify the duration of the dance class [41]. The length of the intervention period ranged from 4 weeks to 5 months, and the median length of dance intervention period was 8 (Q1 = 6, Q3 = 12) weeks.
3.3. Dance intervention feasibility
Six studies [33, 36, 37, 38, 39, 40] reported on participants that dropped out. The number of participants not completing a dance intervention ranged from 2 [36] to 7 participants [33], representing 20%–44% of the original number of participants in the study group. Reasons for discontinuation of the dance intervention included discharge from hospital before the program ended [33], not liking the program [33], and illness [38]. Three studies [38, 39, 40] reported on adherence to the intervention, ranging from a single class missed by 1 participant [39], and up to 100% attendance [40]. Reasons for missed dance classes included illness, vacation and scheduling conflicts. Three studies [33, 36, 40] reported on adverse events, defined as falls and events requiring medical attention, of which there were none. One study in subacute stroke reported complaints of increased fatigue though this was reported to be no worse that the fatigue expected after a typical physiotherapy session [33].
3.4. Effect of dance on outcomes of interest
The effect of dance on outcomes, calculated as post-pre intervention change scores, is reported for each study in Table 3. A summary of findings with respect to the outcomes of interest (balance, gait, and functional mobility) for this systematic review is included in Table 4. For studies that employed more than one measure for a particular outcome of interest, the results for each measure were summarized separately.
Table 3.
Changes (post- pre) in balance, gait, and mobility outcomes.
| Study | Balance |
Gait |
Functional Mobility |
|||
|---|---|---|---|---|---|---|
| Outcome Measure | Post-pre change | Outcome Measure | Post-pre change | Outcome Measure | Post - pre change | |
| Demers and McKinley, 2015 [33] | Berg Balance Scale | +15.7 (15.8)* points | -- | -- | -- | -- |
| Lachance et al., 2013 (abstract) [34] | -- | -- | -- | -- | Timed Up and Go test | −4 s* |
| Hong et al., 2013 (abstract) [35] | Not specified | -- | -- | -- | Six-minute wheeled distance | +119.4 ft* |
| Mandelbaum et al., 2015 [36] | Berg Balance Scale | +1 point | Dynamic Gait Index | +0.5* points | Timed Up and Go test | −1 s* |
| Timed 25 foot walk test | +0.2 s | |||||
| Multiple Sclerosis Walking Scale-12 | 0 points | |||||
| Hackney et al., 2012 [37] | Berg Balance Scale | +8 points | Preferred velocity | +0.2 m/s | Six-minute walk test | +30.4 m |
| Speed variability | +0.02 m/s | |||||
| Functional Reach Test | +0.04 m | Step length variability | 0 m | |||
| Left single support time | −0.02 s | Timed Up and Go test | −1 s | |||
| Right single support time | −0.04 s | |||||
| Dureska, 2007 (thesis) [38] | Berg Balance Scale | +2 points | Velocity | −0.13 m/s | Timed Up and Go test | −5 s |
| Cadence | −5.4 steps/min | |||||
| Left step time | +0.07 s | |||||
| Left step length | −0.07 m | |||||
| Right step time | +0.003 s | |||||
| Right step length | −0.04 m | |||||
| Dynamic Gait Index | +5 points | |||||
| Salgado and de Paula Vasconcelos, 2010 [39] | -- | -- | -- | -- | Minimal Record of Disability | −1 point |
| Kloos et al., 2013 [40] | Four-square step test | −0.06 (−1.72, 0.60) s | Velocity | 0.01 (−0.08,0.10) m/s | -- | -- |
| Stride length | 0.88 (−4.57, 6.330) cm | |||||
| Tinetti Mobility test | 0.35 (−0.97, 1.67) points | Swing percent | 0.85 (−0.23, 1.92) % | |||
| Double support percent | −2.54 (−4.75, −0.34)* % | |||||
| Base of support | −0.19 (−1.30, 0.92) cm | |||||
| Sapezinskiene et al., 2009 (abstract) [41] | Tinetti Mobility test | +52.5% | -- | -- | -- | -- |
(*) indicates statistically significant differences as reported by original article, or calculated by authors.
(--) indicates outcome not measured.
Table 4.
Summary of study outcomes.
| Study | Balance | Gait | Functional mobility |
|---|---|---|---|
| Demers and McKinley, 2015 [33] | +* | -- | -- |
| Lachance et al., 2013 (abstract) [34] | -- | -- | +* |
| Hong et al., 2013 (abstract) [35] | -- | -- | +* |
| Mandelbaum et al., 2015 [36] | NS | + Dynamic Gait Index* | +* |
| Hackney et al., 2012 [37] | + | + | + |
| Dureska, 2007 (thesis) [38] | + | − Spatiotemporal gait parameters + Dynamic Gait Index |
+ |
| Salgado and de Paula Vasconcelos, 2010 [39] | -- | -- | + |
| Kloos et al., 2013 [40] | NS | +* | -- |
| Sapezinskiene et al., 2009 (abstract) [41] | + | -- | -- |
+ = positive result; NC = no change; NS = no significant result; - = negative result.
(*) indicates statistically significant differences as reported by original article, or calculated by authors.
(--) indicates outcome not measured.
3.4.1. Balance
The most common balance measure was the Berg Balance Scale, which was used in 4 studies [33, 36, 37, 38]. The Tinetti Mobility Test was used in 2 studies [40, 41], and single studies used the Four-square step test [40] and the Functional Reach Test [37]. Four studies reported improvement in balance; 3 were in stroke [33, 37, 38] and the other study was in SCI [41]. All 3 studies in stroke used the Berg Balance Scale and 2 reported change values greater than the MID, which is 7 [42]. In one of these studies, statistical analysis was not performed [33]; however there was enough information available to conduct a paired t-test to determine that the pre-post change in Berg Balance Scale was significant (t = −2.97, p = 0.02). Two studies, one in MS [36], and one in Huntington's disease [40], reported no improvement in balance. Finally, 1 study in SCI reported that balance was measured but results were not reported [35]. MID values for MS, SCI, and Huntington's disease for the outcome measures used were not available.
3.4.2. Gait
Three studies measured spatiotemporal gait parameters with a pressure sensitive mat [37, 38, 40]. The Dynamic Gait Index was used in 2 studies [36, 38], and the Multiple Sclerosis Walking Scale and a timed 25-foot walk test were used by a single study [36]. Two studies, one in chronic stroke [37] and one in Huntington's disease [40] reported an improvement in spatiotemporal gait parameters. The change in velocity reported by the study in chronic stroke exceeded the MID for velocity in subacute stroke (MID = 0.16 m/s) [43]. One study in stroke reported a slower gait velocity [38], and 1 study in MS reported no improvement in gait velocity [36]. However, both of these studies reported an improvement in gait as measured by the Dynamic Gait Index [36, 38], and the decrease in velocity reported by the study in stroke did not exceed the MID [38]. There were no published MID values in MS or Huntington's disease for any of the gait parameters used.
3.4.3. Functional mobility
Four studies measured functional mobility with the Timed Up and Go test [34, 36, 37, 38], and single studies used the six-minute walk test [37], the six-minute wheeled distance [35], and the Minimal Record of Disability [39]. All 4 studies that used the Timed Up and Go test reported an improvement, which included 2 studies with stroke [37, 38], one with MS [36], and one with a study group with motor deficits [34]. Improvements were also reported in the study that used the six-minute walk test in stroke [37], and the other study that used the six-minute wheeled distance in SCI [35]. Finally, 1 study in MS reported improvement as measured by the MRD [39]. MID values for the Timed Up and Go test, six-minute walk test and six-minute wheeled distance were not available for any of the neurological populations described in these studies.
3.5. Methodological quality evaluation
No study included in this review was a randomized controlled trial. Of the 4 pre-post design studies without a control group, one had a quality rating of good, and 3 had a quality rating of fair. None of the studies had a sample size sufficiently large to provide confidence in the findings (Table 5). Of the 3 case report studies, 2 had a quality rating of good, and 1 had a quality rating of fair (Table 6). Of the 2 controlled studies, 1 had a good quality rating, and the other had a poor quality rating partly due to the fact it was an abstract and limited information was available from which the quality rating criteria could be determined (Table 7).
Table 5.
Methodological quality of pre-post studies with no control group.
| Q1 - Study question | Q2 - Eligibility criteria and study population | Q3 - Study participants representative of populations of interest | Q4 - All eligible participants enrolled | Q5 - Sample size | Q6 - Intervention clearly described | Q7 - Outcome measures clearly described, valid, and reliable | Q8 - Blinding of outcome assessors | Q9 - Follow-up rate | Q10 - Statistical analysis | Q11 - Multiple outcome measures | Q12 - Group-level interventions and individual-level outcome efforts | Quality rating | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demers and McKinley, 2015 [33] | Yes | Yes | Yes | Cannot determine | No | Yes | Yes | No | No | Not applicable | No | Not applicable | Fair |
| Lachance et al., 2013 (abstract) [34] | Yes | Cannot determine | Cannot determine | Not reported | No | No | Yes | Not reported | Not reported | Yes | No | Not applicable | Fair |
| Hong et al., 2013 (abstract) [35] | Yes | No | Cannot determine | Not reported | No | Yes | Yes | Cannot determine | Cannot determine | Yes | No | Not applicable | Fair |
| Mandelbaum et al., 2015 [36] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Not applicable | Good |
Table 6.
Methodological quality of case reports.
| Q1 - Study question | Q2 - Study population | Q3 – Consecutive cases | Q4 – Comparable subjects | Q5 – Intervention clearly described | Q6 - Outcome measures clearly described, valid, and reliable | Q7 – Length of follow-up | Q8 – Statistical analysis | Q9 – Results well-described | Quality rating | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hackney et al., 2012 [37] | Yes | Yes | Not applicable | Not applicable | Yes | Yes | Yes | Yes | Yes | Good |
| Dureska, 2007 (thesis) [38] | Yes | Yes | Not applicable | Not applicable | Yes | Yes | Yes | Yes | Yes | Good |
| Salgado and de Paula Vasconcelos, 2010 [39] | Yes | Yes | Not applicable | Not applicable | Yes | Yes | No | No | Yes | Fair |
Table 7.
Methodological quality of controlled studies.
| Q1 – Described as randomized | Q2 – Treatment allocation – adequate randomization | Q3 – Treatment allocation - concealment | Q4 – Blinding of group assignment | Q5 – Blinding of outcome assessors | Q6 – Similarity of groups at baseline | Q7 – Dropouts (overall) | Q8 – Dropouts (differential) | Q9 - Adherence | Q10 – Avoid other interventions | Q11 – Outcome measures clearly described, valid, and reliable | Q12 – Power calculation | Q13 – Prespecified outcomes | Q14 – Intention-to-treat analysis | Quality rating | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kloos et al., 2013 [40] | No | Not applicable | No | No | Yes | Cannot determine | No | No | Yes | Cannot determine | Yes | No | Yes | Not applicable | Good |
| Sapezinskiene et al., 2009 (abstract) [41] | No | Not applicable | Not reported | Not reported | Not reported | No | Not reported | Not reported | Not reported | Not reported | Cannot determine | No | Cannot determine | Not applicable | Poora |
The article was rated as “Poor” quality due to the limited information available in the published abstract. Several elements of the study may have introduced a high potential of bias, including a lack of detail regarding group assignment, a difference between groups at baseline on the primary outcome measures, and no description of the dance intervention or risk of co-intervention.
4. Conclusions
This systematic review revealed emerging evidence for the use of dance in adults with neurological conditions other than PD. The results suggest that dance may be feasible for adults with neurological conditions that can affect gait and balance such as stroke, MS, SCI, and Huntington's disease with no adverse events reported in the three studies that included reporting in their protocol. It is important to note that the majority of studies reviewed did not describe adverse event reporting and a lack of reporting in these studies does not imply that no adverse events occurred.
The type of dance varied across studies as did the frequency and duration of classes. These findings contrast with a systematic review of dance in PD which included studies focused primarily on tango [23]. However, previous work that compared partnered and non-partnered forms of dance reported similar gains in balance (measured with the Berg Balance Scale) and gait (measured as velocity and cadence) in people with PD [25]. The present review also suggests that gains in gait, balance and functional mobility can be made with different forms of dance across different neurological conditions. The current review revealed that intensity of the program was rarely reported when describing the dance intervention. Finally, dropout rates when reported, were slightly higher compared to previous work in the PD population for which multiple studies have reported a dropout rate of 0% [23, 26]. Future work should include greater detail with respect to tracking of adverse events, reporting the intensity of the intervention and participant tolerance as well as examine barriers to participation and adherence to dance interventions for individuals with neurological conditions other than PD.
The present review found pre-post changes in balance, gait and functional mobility outcomes associated with dance in individuals with a variety of neurological conditions that were either similar to or greater than those found in individuals with PD as reported in the systematic review by Shanahan and coauthors [23]. However, it should be emphasized that none of the studies were randomized controlled trials and the quality of 5 of the 9 studies reviewed were rated poor or fair and therefore definitive conclusions about the effects of dance cannot be made at this time. However, at this early stage of a developing rehabilitation field, a summary of results is still informative to direct future investigations of dance as an intervention. Four of the 6 studies that measured balance reported improvement post-intervention but only two of those studies were rated good quality. All 6 studies that measured functional mobility reported improvement with dance and 3 of those were rated good quality. The findings for the effects of dance on spatiotemporal parameters of gait differed between studies; for example, velocity declined in some studies and did not change in others, whereas double support time was improved in another study. The effects of dance on gait as measured by a clinical scale were more consistent; both studies that used the Dynamic Gait Index were rated as good quality and both reported improvement. Finally, some of the changes reported on gait velocity and Berg Balance Scale scores for individuals with stroke exceeded published MID cut-offs suggesting that some changes made with dance are likely to have an impact on participants' function. Given the indication of positive changes with dance in some studies with better quality ratings, further investigation of the effects of dance on balance, gait and functional mobility of people with neurological conditions other than PD is warranted.
The methodological quality ratings of the majority of included studies in the present review (which were a mix of case studies, pre-post controlled and non-controlled studies) were fair or poor. In the interest of supporting the development of the field it is worthwhile to report on common limitations so that they can be addressed in future work. The most common issue was an insufficient sample size to provide confidence in the findings. Recruitment is always a challenge for intervention research [44]. Large sample sizes for dance interventions are likely further complicated by practicalities such as room size, availability of a dance instructor with the appropriate expertise and experience, availability of staff to provide assistance and for safety, and limitations to class size such that each dance class participant can receive personalized attention from the instructor. Future investigations into the use of dance may overcome these challenges with multi-centre, controlled trials and/or providing multiple dance programs with different students throughout the study period. Such studies would necessitate standardization of the dance intervention across the multiple sites and/or groups through clear and detailed description with respect to the FITT principle as well as clear reporting guidelines with respect to adverse events, participant tolerance and adherence.
The current review has several limitations. First, the search was restricted to the English language, and therefore relevant studies published in other languages may have been missed. Second, there is a risk for bias since none of the included studies were randomized controlled trials, and several included studies lacked a control group. Finally, there was significant heterogeneity across multiple domains including study design, patient population (featuring neurological conditions with different etiology and pathophysiology), dance program (i.e. type, frequency, duration) and outcome measures employed. We were unable to perform meta-analysis given the heterogeneity in designs and outcomes. As this field develops and larger, controlled studies become available, reviews that synthesize the evidence focussing on each of the conditions included in the present review would be of value.
In conclusion, the use of dance for neurological conditions other than PD has received very little attention. Like PD, many adult neurological conditions involve damage to central nervous system structures and feature motor impairments resulting in gait, balance and mobility dysfunction (admittedly due to different underlying pathologies) that could be improved with a dance program. Thus, it is surprising that the interest in dance for PD has not translated to other neurological conditions that could benefit. The results of the current review suggest that dance may hold promise as an intervention to improve gait, balance and mobility in a variety of neurological conditions including stroke, MS, SCI, and Huntington's disease. Though not the focus of this review, certain forms of dance may elicit cardiorespiratory responses in people with neurological conditions [45] and thus may afford aerobic fitness [46] benefits in addition to gains in gait and balance. These potential benefits also warrant further investigation. Within each neurological condition there is likely a subgroup of patients that would benefit from dance. The characteristics of these subgroups remain to be determined but likely include the capacity to walk/move and maintain balance with minimal assistance and follow multi-step instructions, as these were common abilities outlined in the inclusion and exclusion criteria of many of the studies in this review [36, 40]. Future work should focus on randomized controlled studies of clearly described dance interventions with detailed reporting of participant tolerance and adverse events and larger sample sizes appropriately calculated to answer the given clinical question.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
D Brooks is a Tier 2 Canada Research Chair and KK Patterson was supported by a Focus on Stroke personnel award from the Heart and Stroke Foundation of Canada and the Canadian Stroke Network.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Jessica Babineau, Information Specialist at Toronto Rehab-University Health Network, for her expertise and assistance with the literature search.
References
- 1.Clarke M., Emery H., Kneebone R., Nicholas D.B. A basic annual income for the neuro-developmentally disabled in Canada. Options Polit. 2012:56–61. [Google Scholar]
- 2.Public Health Agency of Canada . Mapping Connections: an understanding of neurological conditions in Canada. In: Canada PHAo, editor. Chatper 3 Scope (Prevalence and Incidence) Government of Canada; Ottawa: 2014. [Google Scholar]
- 3.Canadian Institute for Health Information . 2007. The Burden of Neurological Diseases, Disorders and Injuries in Canada. [DOI] [PubMed] [Google Scholar]
- 4.Bethoux F., Calmels P., Gautheron V. Changes in the quality of life of hemiplegic stroke patients with time: a preliminary report. Am. J. Phys. Med. Rehabil. 1999;78(1):19–23. doi: 10.1097/00002060-199901000-00006. PubMed PMID: 9923424. [DOI] [PubMed] [Google Scholar]
- 5.Pound P., Gompertz P., Ebrahim S. A patient-centred study of the consequences of stroke. Clin. Rehabil. 1998;12(4):338–347. doi: 10.1191/026921598677661555. PubMed PMID: 9744669. [DOI] [PubMed] [Google Scholar]
- 6.Latham N.K., Jette D.U., Slavin M., Richards L.G., Procino A., Smout R.J. Physical therapy during stroke rehabilitation for people with different walking abilities. Arch. Phys. Med. Rehabil. 2005;86(12 Suppl. 2):S41–S50. doi: 10.1016/j.apmr.2005.08.128. PubMed PMID: 16373139. [DOI] [PubMed] [Google Scholar]
- 7.Bohannon R.W., Andrews A.W., Smith M.B. Rehabilitation goals of patients with hemiplegia. Int. J. Rehabil. Res. 1988;11:181–183. [Google Scholar]
- 8.Ditunno P.L., Patrick M., Stineman M., Ditunno J.F. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord. 2008;46(7):500–506. doi: 10.1038/sj.sc.3102172. PubMed PMID: 18209742. [DOI] [PubMed] [Google Scholar]
- 9.Bloom L.F., Lapierre N.M., Wilson K.G., Curran D., DeForge D.A., Blackmer J. Concordance in goal setting between patients with multiple sclerosis and their rehabilitation team. Am. J. Phys. Med. Rehabil. 2006;85(10):807–813. doi: 10.1097/01.phm.0000237871.91829.30. [DOI] [PubMed] [Google Scholar]
- 10.Patterson K.K., Mansfield A., Biasin L., Brunton K., Inness E.L., McIlroy W.E. Longitudinal changes in post-stroke spatiotemporal gait asymmetry over inpatient rehabilitation. Neurorehabil. Neural Repair. 2015;29(2):153–162. doi: 10.1177/1545968314533614. Epub 2014/05/16. [DOI] [PubMed] [Google Scholar]
- 11.Harkema S.J., Schmidt-Read M., Lorenz D.J., Edgerton V.R., Behrman A.L. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch. Phys. Med. Rehabil. 2012;93(9):1508–1517. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Freeman J.A., Langdon D.W., Hobart J.C., Thompson A.J. The impact of inpatient rehabilitation on progressive multiple sclerosis. Ann. Neurol. 1997;42(2):236–244. doi: 10.1002/ana.410420216. PubMed PMID: 9266735. [DOI] [PubMed] [Google Scholar]
- 13.Khan F., Turner-Stokes L., Stevermuer T., Simmonds F. Multiple sclerosis rehabilitation outcomes: analysis of a national casemix data set from Australia. Multiple Scler. J. 2009;15(7):869–875. doi: 10.1177/1352458509105230. [DOI] [PubMed] [Google Scholar]
- 14.Steffen T.M., Hacker T.A., Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, Berg balance scale, Timed Up & Go test, and gait speeds. Phys. Ther. 2002;82(2):128–137. doi: 10.1093/ptj/82.2.128. PubMed PMID: 11856064. [DOI] [PubMed] [Google Scholar]
- 15.Middleton A., Fritz S.L., Lusardi M. Walking speed: the functional vital sign. J. Aging Phys. Activ. 2015;23(2):314–322. doi: 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid A., Duncan P.W., Studenski S., Lai S.M., Richards L., Perera S. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38(7):2096–2100. doi: 10.1161/STROKEAHA.106.475921. PubMed PMID: 17510461. [DOI] [PubMed] [Google Scholar]
- 17.Quiroga Murcia C., Kreutz G., Clift S., Bongard S. Shall we dance? An exploration of the perceived benefits of dancing on well-being. Arts Health. 2010;2(2):149–163. [Google Scholar]
- 18.Verghese J. Cognitive and mobility profile of older social dancers. J. Am. Geriatr. Soc. 2006;54(8):1241–1244. doi: 10.1111/j.1532-5415.2006.00808.x. PubMed PMID: 16913992; PubMed Central PMCID: PMC1550765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J.G., Ishikawa-Takata K., Yamazaki H., Morita T., Ohta T. Postural stability and physical performance in social dancers. Gait Posture. 2008;27(4):697–701. doi: 10.1016/j.gaitpost.2007.09.004. PubMed PMID: 17981468. [DOI] [PubMed] [Google Scholar]
- 20.Garber C.E., McKinney J.S., Carleton R.A. Is aerobic dance an effective alternative to walk-jog exercise training? J. Sports Med. Phys. Fit. 1992;32(2):136–141. [PubMed] [Google Scholar]
- 21.Bradt J., Goodill S.W., Dileo C. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst. Rev. 2011;(10) doi: 10.1002/14651858.CD007103.pub2. PubMed PMID: 21975762. [DOI] [PubMed] [Google Scholar]
- 22.Mandelbaum R., Lo A.C. Examining dance as an intervention in Parkinson's disease: a systematic review. Am. J. Dance Ther. 2014;36(2):160–175. [Google Scholar]
- 23.Shanahan J., Morris M.E., Bhriain O.N., Saunders J., Clifford A.M. Dance for people with Parkinson disease: what is the evidence telling us? Arch. Phys. Med. Rehabil. 2015;96(1):141–153. doi: 10.1016/j.apmr.2014.08.017. PubMed PMID: 25223491. [DOI] [PubMed] [Google Scholar]
- 24.Sharp K., Hewitt J. Dance as an intervention for people with Parkinson's disease: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2014;47:445–456. doi: 10.1016/j.neubiorev.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Hackney M.E., Earhart G.M. Effects of dance on gait and balance in Parkinson's disease: a comparison of partnered and nonpartnered dance movement. Neurorehabil. Neural Repair. 2010;24(4):384–392. doi: 10.1177/1545968309353329. PubMed PMID: 20008820; PubMed Central PMCID: PMC2900796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackney M.E., Kantorovich S., Levin R., Earhart G.M. Effects of tango on functional mobility in Parkinson's disease: a preliminary study. J. Neurol. Phys. Ther. 2007;31(4):173–179. doi: 10.1097/NPT.0b013e31815ce78b. PubMed PMID: 18172414. [DOI] [PubMed] [Google Scholar]
- 27.Volpe D., Signorini M., Marchetto A., Lynch T., Morris M.E. A comparison of Irish set dancing and exercises for people with Parkinson's disease: a phase II feasibility study. BMC Geriatr. 2013;13(1):54. doi: 10.1186/1471-2318-13-54. PubMed PMID: 23731986; PubMed Central PMCID: PMC3685562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 29.Harrison S.L., Janaudis-Ferreira T., Brooks D., Desveaux L., Goldstein R.S. Self-management following an acute exacerbation of COPD: a systematic review. Chest. 2015;147(3):646–661. doi: 10.1378/chest.14-1658. PubMed PMID: 25340578. [DOI] [PubMed] [Google Scholar]
- 30.National Heart Lung and Blood Institute. Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group 2016 [cited 2016 5/1/2016]. Available from: http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after.
- 31.National Heart Lung and Blood Institute. Quality assessment tool for case series studies 2014 [11/28/2016].
- 32.National Heart Lung and Blood Institute. Quality assessment of controlled intervention studies 2014 [11/28/2016].
- 33.Demers M., McKinley P. Feasibility of delivering a dance intervention for subacute stroke in a rehabilitation hospital setting. Int. J. Environ. Res. Publ. Health. 2015;12(3):3120–3132. doi: 10.3390/ijerph120303120. PubMed PMID: 25785497; PubMed Central PMCID: PMC4377955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lachance B., Poncet F., Goulet C.P., Durand T., Messier F., McKinley P. Effect of a dance therapy workshop on social participation and integration of adults with motor deficits: an exploratory study. Annals Phys. Rehabil. Med. 2013;56:e162–e163. PubMed PMID: 71224770. [Google Scholar]
- 35.Hong M., Earhart G.M., Kiratli B.J. Outcomes of short-term participation in wheelchair dancing for individuals with spinal cord injuries: a pilot study. J. Spinal Cord Med. 2013;36(5):524–567. PubMed PMID: 71232220. [Google Scholar]
- 36.Mandelbaum R., Triche E.W., Fasoli S.E., Lo A.C. A pilot study: examining the effects and tolerability of structured dance intervention for individuals with multiple sclerosis. Disabil. Rehabil. 2016;38(3):218–222. doi: 10.3109/09638288.2015.1035457. PubMed PMID: 25875049. [DOI] [PubMed] [Google Scholar]
- 37.Hackney M.E., Hall C.D., Echt K.V., Wolf S.L. Application of adapted tango as therapeutic intervention for patients with chronic stroke. J. Geriatr. Phys. Ther. 2012;35(4):206–217. doi: 10.1519/JPT.0b013e31823ae6ea. [DOI] [PubMed] [Google Scholar]
- 38.Dureska M.D. University of South Carolina; Columbia, SC: 2007. Beginning Ballet as an Intervention for Gait, Balance, and Mobility for an Individual with Post-stroke Hemiparesis: a Case Report. [Google Scholar]
- 39.Salgado R., de Paula Vasconcelos L.A. The use of dance in the rehabilitation of a patient with multiple sclerosis. Am. J. Dance Ther. 2010;32(1):53–63. [Google Scholar]
- 40.Kloos A.D., Fritz N.E., Kostyk S.K., Young G.S., Kegelmeyer D.A. Video game play (Dance Dance Revolution) as a potential exercise therapy in Huntington's disease: a controlled clinical trial. Clin. Rehabil. 2013;27(11):972–982. doi: 10.1177/0269215513487235. PubMed PMID: 23787940. [DOI] [PubMed] [Google Scholar]
- 41.Sapezinskiene L., Soraka A., Svediene L. Dance movement impact on independence and balance of people with spinal cord injuries during rehabilitation. Int. J. Rehabil. Res. 2009;32(S100-S) PubMed PMID: 2010426661. [Google Scholar]
- 42.Godi M., Franchignoni F., Caligari M., Giordano A., Turcato A.M., Nardone A. Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys. Ther. 2013;93(2):158–167. doi: 10.2522/ptj.20120171. PubMed PMID: 23023812. [DOI] [PubMed] [Google Scholar]
- 43.Tilson J.K., Sullivan K.J., Cen S.Y., Rose D.K., Koradia C.H., Azen S.P. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys. Ther. 2010;90(2):196–208. doi: 10.2522/ptj.20090079. PubMed PMID: 20022995; PubMed Central PMCID: PMC2816032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanton S., Morris D.M., Prettyman M.G., McCulloch K., Redmond S., Light K.E. Lessons learned in participant recruitment and retention: the EXCITE trial. Phys. Ther. 2006;86(11):1520–1533. doi: 10.2522/ptj.20060091. PubMed PMID: 17079752. [DOI] [PubMed] [Google Scholar]
- 45.Terada K., Satonaka A., Terada Y., Suzuki N. Cardiorespiratory responses during wheelchair dance in bedridden individuals with severe athetospastic cerebral palsy. Gazz. Med. Ital. 2016;176(6):241–247. [Google Scholar]
- 46.Terada K., Satonaka A., Terada Y., Suzuki N. Training effects of wheelchair dance on aerobic fitness in bedridden individuals with severe athetospastic cerebral palsy rated to GMFCS level V. Eur. J. Phys. Rehabil. Med. 2017;53(5):744–750. doi: 10.23736/S1973-9087.17.04486-0. [DOI] [PubMed] [Google Scholar]