Abstract
We successfully treated a 22-year-old woman with eosinophilic gastroenteritis (EGE) using a multiple food-elimination diet. The patient was diagnosed with EGE based on histopathological findings and initially treated with oral prednisolone. The symptoms immediately improved, although they recurred when prednisolone was tapered to a low dose. We then treated her with a multiple food-elimination diet, including milk, soy, eggs, wheat, nuts, seafood, and rice. During dietary treatment, we identified dairy products and eggs as causative foods of the symptoms, and we ceased prednisolone. Similar to eosinophilic esophagitis, an elimination diet may be effective for adult patients with EGE.
Introduction
Eosinophilic gastroenteritis (EGE) is an uncommon inflammatory disorder characterized by eosinophilic infiltration of the gastrointestinal (GI) tract.1,2 Although the pathogenesis remains obscure, food allergens are considered to be triggering and aggravating factors for delayed Th2-type allergic reaction in the GI tract of affected patients, similarly to eosinophilic esophagitis (EoE).3,4 An empiric 6-food elimination diet, which eliminates 6 food groups (wheat, milk, eggs, nuts, soy, seafood) and then reintroduces them one at a time, is the most frequent dietary therapy used for patients with EoE, and this method has been shown to lead to clinicohistologic remission in three-quarters of treated patients.5,6 However, the effects of dietary therapy for EGE remain to be fully elucidated.7-9
Case Report
A 22-year-old woman with steroid-dependent EGE was referred to our institution for treatment with food-elimination diet therapy. She had been diagnosed with EGE on the basis of histopathological findings showing dense eosinophil infiltration of >20/high-power field (HPF) in the lamina propria of the ileum; secondary causes of eosinophilia, such as medication or infection, were excluded by taking medical history and stool studies. The patient was initially treated with oral prednisolone at a local hospital. Her symptoms immediately improved, but they recurred when the prednisolone was tapered to a low dose.
Upon admission to our department, she was treated with oral prednisolone (20 mg). A physical examination showed tenderness in the left lower abdomen. Serum immunoglobulin E (IgE) was 2,158 IU/mL, and antigen-specific IgE antibody was positive only for shrimp. Patch and scratch tests were negative for the common ingredients tested. Abdominal computed tomography scans revealed no obvious abnormal findings. Transanal double-balloon enteroscopy showed prominent villous atrophy in the ileum, with no obvious abnormality found in other GI tracts (Figure 1). Histopathological results showed edema and fibrosis of the ileum mucosa, with a maximum eosinophil infiltration of 23/HPF in the ileum, even on continuous administration of prednisolone (Figure 2).
Figure 1.
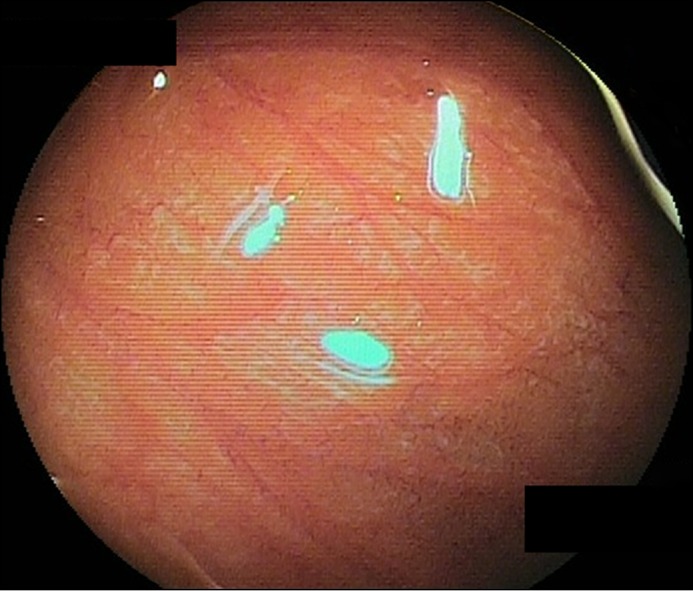
Transanal double-ballon enteroscopy showing prominent villous atrophy in the ileum.
Figure 2.
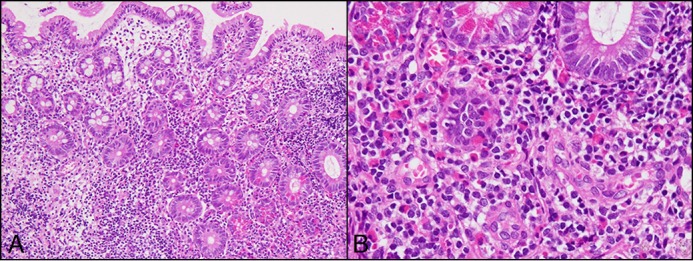
Hematoxylin & eosin (H&E) stain showing edema and fibrosis of the ileum mucosa with a maximum eosinophil infiltration of 23/high-power field in the ileum, even on continuous administration of prednisolone. (A) 10×. (B) 40×.
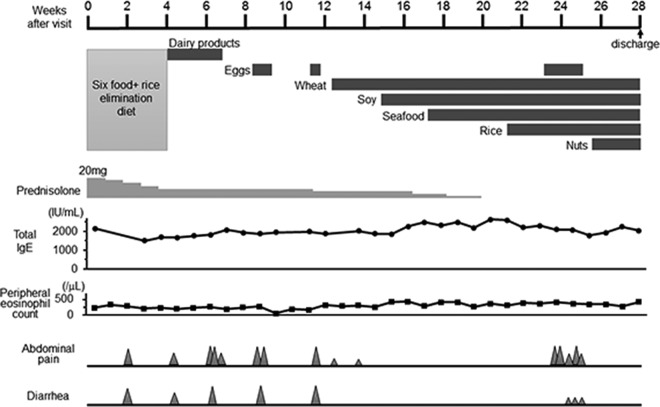
The patient suspected rice as the causative food. Therefore, we designed and started a multiple food-elimination diet, including the 6 major food groups (wheat, milk, eggs, nuts, soy, seafood) and rice (Figure 3). Four weeks later, her abdominal symptoms had improved, and prednisolone was gradually reduced to 10 mg. We reintroduced dairy products first, and 1 week later the patient reported frequent severe abdominal pain with clammy sweating, which required an anticholinergic injection. Therefore, dairy products were judged to be causative and stopped. After improvement of symptoms, we reintroduced eggs. She reported severe abdominal pain and diarrhea twice when exercising after eating eggs. The possibility of exercise-induced anaphylaxis was excluded with a basophil activation test and blood tryptase value, so eggs were also considered to be possibly causative and stopped. We subsequently reintroduced wheat, soybeans, seafood, rice and nuts, in that order, with a 2-week interval between each, along with a gradual decrease in steroid administration. There were no severe abdominal symptoms noted after reintroduction of these foods. Once again, eggs were reintroduced with a prohibition on exercise after meals; however, the patient was frequently affected by abdominal pain and diarrhea. Finally, we determined that dairy products and eggs were the causative foods for her symptoms, and prednisolone was ceased during the dietary treatment.
Figure 3.
Clinical course after initial visit to our department. During dietary treatment, we identified dairy products and eggs as causative foods of the symptoms, and prednisolone was ceased.
Endoscopy showed improvement of villous atrophy in the ileum (Figure 4). Histopathology showed eosinophilic infiltration decreased to 2–3/HPF (Figure 5). We also monitored total IgE level and peripheral eosinophil count each week during treatment, but we found no relationship between those values and the symptoms. The patient has continued the elimination diet as an outpatient, and she no longer is affected by severe abdominal symptoms. At the time of writing, she was in good condition without prednisolone administration.
Figure 4.
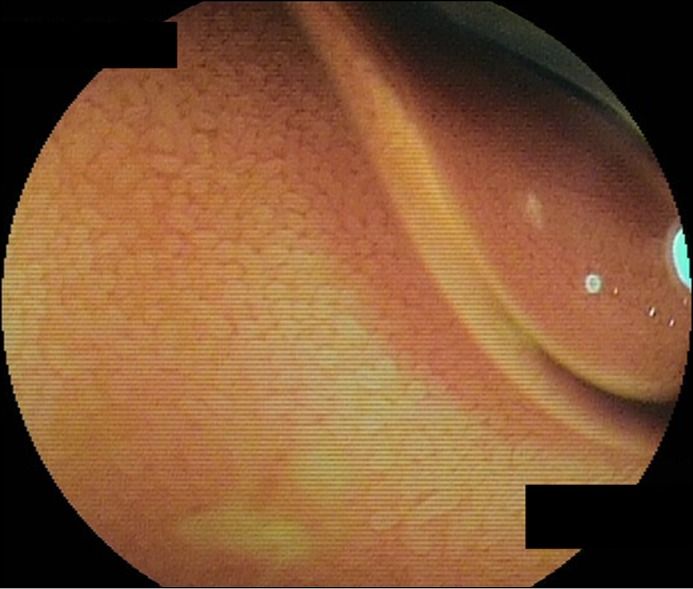
Endoscopy after 4 months of dietary treatment showing improvement of villous atrophy in lesion of ileum.
Figure 5.
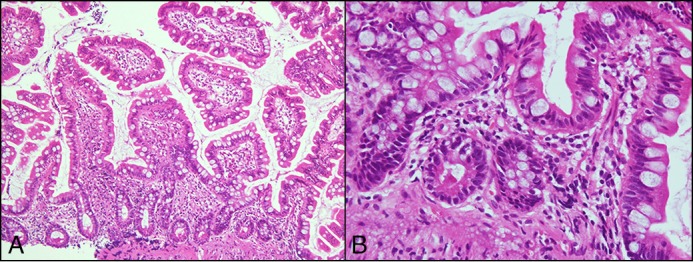
H&E stain during dietary treatment showing improvement of villous atrophy in the ileum and eosinophil infiltration decreased to 2–3/high power field. (A) 10×. (B) 40×.
Discussion
Consensus is lacking regarding optimal treatment for patients with EGE.10,11 Although corticosteroid therapy remains the mainstay to induce remission, EGE often relapses during steroid tapering or after corticosteroid withdrawal. In addition, long-term use of corticosteroids is not desirable because of serious adverse effects, including adrenocortical suppression, hyperglycemia, and cushingoid state.
Non-IgE-dependent allergic inflammation caused by a food allergen is considered to be important for the pathogenesis of EGE, so the use of elemental or elimination diets has been recommended.9 However, it is difficult to identify causative foods because an individual’s response to an elimination diet does not always correlate with laboratory and skin food allergy tests, such as allergen-specific IgE tests or patch and scratch tests. Indeed, patients with EGE are often found to be sensitized to multiple food allergens in examinations of serum allergen-specific IgE, although the clinical relevance of allergen-specific IgE is not clear.12
An empiric food-elimination diet, preferentially devoid of 6 common food allergens, is most frequently used as dietary therapy for patients with EoE because of the many disadvantages of elemental diets and the low diagnostic accuracy of skin allergy testing to identify trigger foods.6,13 A recent systematic review demonstrated that a 6-food elimination diet was effective for 72.1% of examined cases (95% CI 65.8–78.1%), with no significant differences in remission following dietary intervention between adults and children.6 As food are reintroduced sequentially, 1–2 foods are usually identified as being responsible for EoE in 65–85% of EoE patients, with milk most frequently associated with symptoms in both adults and children.14 Recently, multiple food-elimination diet therapy has also been used for pediatric patients with EGE.15,16 Therefore, we designed a multiple food-elimination diet, including 6 major foods, for adult patients with EGE.
For our case, an antigen-specific IgE antibody was identified only for shrimp, and there was no relationship between total IgE level and symptoms. With the use of this food-elimination diet, we identified dairy products and eggs as causative foods, and the patient’s symptoms disappeared with a diet free of those foods. These findings support the pathogenic role of non-IgE-mediated hypersensitivity in EGE.
Disclosures
Author contributions: E. Okimoto, M. Okada, H. Mikami, H. Sonoyama, N. Oshima, and N. Ishimura wrote and edited the manuscript. A. Araki, N. Ishikawa, and R. Maruyama evaluated histological findings. J. Hirai supported nutritional treatment. S. Ishihara and Y. Kinoshita edited the manuscript. N. Ishimura is the article guarantor.
Financial disclosure: This project was supported by Health and Labor Sciences Research Grants, Research on Intra-ctable Diseases, from the Ministry of Health, Labour, and Welfare in Japan.
Informed consent was obtained for this case report.
References
- 1.Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: A clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31(1):54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004;113(1):11–28. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita Y, Furuta K, Ishimura N, Ishihara S. Elevated plasma cytokines in Japanese patients with eosinophilic esophagitis and gastroenteritis. Digestion. 2012;86(3):238–43. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell JM, Collins MH, Stucke EM, et al. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J Allergy Clin Immunol. 2014;134(5):1114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucendo AJ, Molina-Infante J, Arias A, et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5(3):335–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias A, González-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: A systematic review and meta-analysis. Gastroenterology. 2014;146(7):1639–48. [DOI] [PubMed] [Google Scholar]
- 7.Pineton de Chambrun G, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9(1):950–56. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita Y, Furuta K, Ishimaura N, et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol. 2013;48(3):333–39. [DOI] [PubMed] [Google Scholar]
- 9.Lucendo AJ, Serrano-Montalbán B, Arias A, Redondo O, Tenias JM. Efficacy of dietary treatment for inducing disease remission in eosinophilic gastroenteritis. J Pediatr Gastroenterol Nutr. 2015;61(1):56–64. [DOI] [PubMed] [Google Scholar]
- 10.Abou Rached A, El Hajj W. Eosinophilic gastroenteritis: Approach to diagnosis and management. World J Gastrointest Pharmacol Ther. 2016;7(4):513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinoshita Y, Ishimura N, Oshima N, Mikami H, Okimoto E, Jiao DJ, Ishihara S. Recent progress in the research of eosinophilic esophagitis and gastroenteritis. Digestion. 2016;93(1):7–12. [DOI] [PubMed] [Google Scholar]
- 12.Ishimura N, Furuta K, Sato S, Ishihara S, Kinoshita Y. Limited role of allergy testing in patients with eosinophilic gastrointestinal disorders. J Gastroenterol Hepatol. 2013;28(8):1306–13. [DOI] [PubMed] [Google Scholar]
- 13.Groetch M, Venter C, Skypala I, et al. Dietary therapy and nutrition management of eosinophilic esophagitis: A Work Group Report of the American Academy of Allergy, Asthma, and Immunology. J Allergy Clin Immunol Pract. 2017;5(2):312–24. [DOI] [PubMed] [Google Scholar]
- 14.González-Cervera J, AJ L. Eosinophilic esophagitis: An evidence-based approach to therapy. J Investig Allergol Clin Immunol. 2016;26(1):8–18. [PubMed] [Google Scholar]
- 15.Yamada Y, Kato M, Isoda Y, Nishi A, Jinbo Y, Hayashi Y. Eosinophilic gastroenteritis treated with a multiple-food elimination diet. Allergol Int. 2014;63(Suppl 1):53–56. [DOI] [PubMed] [Google Scholar]
- 16.Ko HM, Morotti RA, Yershov O, Chehade M. Eosinophilic gastritis in children: Clinicopathological correlation, disease course, and response to therapy. Am J Gastroenterol. 2014;109(8):1277–85. [DOI] [PubMed] [Google Scholar]