Abstract
Objectives
To evaluate the effects of Allium cepa (A. cepa) on levels of oxidants, antioxidants, and immunological markers in bronchoalveolar lavage fluids (BALF) of sensitized rats.
Materials and Methods
Oxidant/antioxidant markers and cytokines in BALF of control rats treated with saline (group C), ovalbumin-sensitized rats (group S), rats treated with 1.25 μg/mL dexamethasone and 3 doses of A. cepa extract (35, 70, and 140 mg/kg body weight [BW]/day) (S + AC) were investigated. Comparison of the results between groups was performed using analysis of variance with the Tukey-Kramer post hoc test.
Results
The oxidant markers nitrogen dioxide (NO2), nitrate (NO3–), and malondialdehyde (MDA), and immunological markers interleukin (IL)-4 and immunoglobulin E (IgE) were significantly higher, but the antioxidant markers superoxide dismutase (SOD), catalase (CAT), thiol, and interferon (IFN)-γ, and the IFN-γ/IL-4 ratio were lower in sensitized rats compared to control rats (p < 0.001 to p < 0.01). Compared to group S, the levels of the following markers were significantly lower: NO2, NO3–, and IgE in groups treated with the A. cepa extract, MDA and IL-4 levels in groups treated with 70 and 140 mg/kg BW/day of the A. cepa extract, and all these markers as well as IFN-γ in rats treated with dexamethasone (p < 0.001 to p < 0.05). However, there were significantly higher levels of SOD and CAT and an increased IFN-γ/IL-4 ratio (groups treated with 70 and 140 mg/kg BW/day of the A. cepa extract), and levels of thiol and IFN-γ (group treated with 140 mg/kg BW/day of the A. cepa extract) as well as SOD, CAT, and thiol (dexamethasone-treated group) versus group S (p < 0.00 to p < 0.05).
Conclusion
A. cepa showed antioxidant and immunomodulatory properties in sensitized rats.
Keywords: Asthma, Allium cepa, Antioxidants, Immunomodulation
Significance of the Study
• In this study, the effects of Allium cepa extract on oxidant, antioxidant, and immunological marker levels were evaluated in bronchoalveolar lavage fluid of sensitized rats. The A. cepa extract showed antioxidant and immunomodulatory effects in bronchoalveolar lavage fluid of sensitized rats, which could indicate the therapeutic potential of this extract in asthma.
Introduction
Bronchial asthma is the most common chronic disease worldwide [1] which involves the immune system [2]. Many cells are involved in its pathogenesis, such as dendritic cells (Th2), lymphocytes, eosinophils, mast cells, neutrophils, macrophages, epithelial cells, fibroblasts, and smooth muscle cells [2]. These cells release inflammatory mediators such as histamine, prostaglandin D2, leukotrienes, cytokines, chemokines, oxidative markers, and nitric oxide [2, 3]. In an immune-mediated disorder like asthma, Th2 cytokines such as IL-4, IL-5, and IL-13 initiate allergic responses by increasing the infiltration of eosinophils and enhancement of the production of IgE and Th1 cytokines (interferon [IFN]-γ), which inhibit Th2 responses [3]. Inflammatory cells such as macrophages and eosinophils generate reactive oxygen species, and hence oxidative stress is increased in patients with asthma [4]. Increased oxidative stress is associated with disease severity and may enhance the inflammatory response [4]. The main therapeutic strategies used for treatment of asthma focus on reducing airway inflammation, but there is no definite cure for reducing the airway remodeling observed in this dis ease [1].
Allium cepa belongs to the Liliaceae family, grows around the world, and has been used as a food ingredient [1]. A. cepa contains pharmacologically active constituents, including flavonoids (quercetin), organosulfur compounds (propyl thiosulfinate), and phenol components, which possess anti-allergic [5], anti-inflam matory, and antioxidant activities [6]. Graefe et al. [7] showed that the bioavailability of the active quercetin metabolites (glucuronides) in human blood is about 5 times higher than that of quercetin and only about 66% of that of the corresponding plant extract. Peak concentrations of quercetin were observed 7.0 ± 2.9 h after ingestion, and its elimination half-life was about 11 h. Also, the plant matrix influences both the rate and extent of absorption. This plant acts as a booster of the immune system [8], and its effect on cytokines reflects its immunomodulatory property [9]. Anti-asthmatic properties of the extract of A. cepa and its constituent quercetin on cytokines, inflammatory cells, and smooth muscle contraction were shown in a murine model of asthma [10]. Allergic contact dermatitis or irritation and anemia were reported for A. cepa oil or its aqueous extract [11]. However, no toxic effect was observed in mice following the oral administration of 1,000 mg/kg body weight (BW)/day of A. cepa extract [10]. Moreover, no mutagenicity or oxidative DNA damage was induced by propyl propane thiosulfinate derived from organosulfur compounds isolated from A. cepa [12, 13].
Hence, with regard to alterations in the oxidant/antioxidant balance and cytokine changes in asthma and due to its antioxidant and immunomodulatory effects, the objective of the present study was to evaluate the effects of A. cepa extract on bronchoalveolar lavage fluid (BALF) levels of oxidant and antioxidant markers, IgE, and cytokines in sensitized rats.
Materials and Methods
Plant and Extract
A. cepa (onion) was collected and peeled, and its juice was obtained using a fruit juice producer (Pars Khazar, Iran). The extract was concentrated by evaporation using a rotary evaporator at 60–70°C and then dehydrated in an oven.
Experimental Groups and Ovalbumin Sensitization
In this study, 36 male Wistar rats weighing 200–250 g were studied. The rats were kept in the animal house under standard conditions (at 22 ± 2°C at a 12-h light/dark cycle) with clean filtered air (Maximiser, Thorens Caging System Inc., Hazleton, PA, USA) at the School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. Three rats were kept in each cage, and they had free access to water and food.
The rats were sensitized intraperitoneally once a day for 3 days with 1 mg/kg ovalbumin (OVA) dissolved in 0.9% saline + 100 mg aluminum hydroxide as adjuvant. Thirty rats were then exposed to 1% OVA aerosol produced by a nebulizer (DeVilbiss Health Care Ltd., Feltham, UK) for 20 min/day with an air flow of 8 L/min on days 6, 9, 12, 15, 18, and 21. Exposure of rats to OVA was done in a 0.8-m3 chamber [14].
The 36 rats were divided into 6 groups (n = 6 in each group) as follows: (a) control rats received intraperitoneal and inhaled saline (group C); (b) sensitized but untreated rats (group S); (c) sensitized rats treated with 1.25 μg/mL dexamethasone added to the drinking water during the sensitization period (on days 1–21) (group S + D); and (d–f) sensitized groups treated with A. cepa extract at 3 doses (0.175, 0.35, or 0.7 mg/mL, respectively) added to the drinking water during the sensitization period (days 1–21), which corresponded to 35, 70, and 140 mg/kg BW/day, respectively. The doses were chosen based on the study of Oliveira et al. [10]. Each rat drank an average of 40 mL water/day. Experiments on rats were done according to the national laws and the Guidelines for the Use and Care of Laboratory Animals (National Institutes of Health, USA). The study was approved by the Ethics Committee of the Mashhad University of Medical Sciences.
BALF Preparation
All rats were sacrificed on day 22, their chests opened, and trachea and lungs were separated. The left lung was lavaged 5 times with 1 mL saline (a total of 5 mL). The BALF was centrifuged at 2,500 g at 4°C for 10 min. Supernatants were collected and stored at −80°C until analysis.
Measurement of Oxidant and Antioxidant Levels in BALF
Total stable oxidation products of NO metabolism (NO2–/NO3–) in BALF supernatant were evaluated using the Griess reagent. The Griess reagent contains sulfanilamide (SULF; Sigma, USA) and N-(1-naphthyl)ethylenediamine dihydrochloride (NEDD). The frozen BALF samples were thawed at 25°C and deproteinized using zinc sulfate solution (Sigma). The liquefied BALF was then centrifuged at 12,000 g for 10 min. Next, 300 μL of the clear supernatant were mixed with Griess reagent containing 300 μL SULF (2% w/v) in 5% HCl and 300 μL NEDD (0.1% w/v; Sigma) in water in a test tube. To reduce nitrate to nitrite, 300 μL of saturated vanadium trichloride solution (Sigma) in 1 M HCl were added and incubated for 2 h at 30°C in the dark. Then, the absorbance of samples was assessed at 540 nm against a blank containing the same concentrations of ingredients but no biological sample. Linear regression was used to determine NO concentrations using a standard curve plotted for NaNO2. The final results are expressed as micromoles [14].
Levels of malondialdehyde (MDA), an index of lipid peroxidation, were measured. MDA reacts with thiobarbituric acid (TBA) as a TBA-reactive substance to produce a red-colored complex with maximum absorbance at 535 nm. For MDA measurement, 2 mL of TBA/trichloroacetic acid/HCl was added to 1 mL of BALF supernatant, and the mixture was heated in a water bath for 40 min. Then, the mixture was centrifuged at 1,000 g for 10 min, and absorbance was measured at 535 nm [14].
Total thiol concentration was measured using 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) reagent which reacts with thiol moieties to produce a yellow-colored complex with maximum absorbance at 412 nm. Briefly, 1 mL Tris-ethylenediaminetetraacetic acid (Tris-EDTA) buffer (pH 8.6) was added to 50 μL serum supernatant in 1-mL cuvettes, and sample absorbance was read at 412 nm against Tris-EDTA buffer alone (A1). Then, 20 μL DTNB reagent (10 mM in methanol) was added to the mixture, and absorbance was read again after 15 min at room temperature (RT) (A2). The absorbance of the DTNB reagent alone was also read as a blank (B). Total thiol concentration (mM) was calculated using the following equation [14]:
Total thiol concentration (mM) = (A2 – A1 – B) × 1.07/0.05 × 13.6.
Superoxide dismutase (SOD) was assessed using a colorimetric assay involving the production of superoxide by pyrogallol autooxidation and the prevention of superoxide-dependent diminution of the tetrazolium dye (3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide [MTT]) to formazan by SOD [14]. One unit of SOD activity was defined as the quantity of enzyme required for 50% prevention in the MTT reduction rate.
Catalase (CAT) activity was assessed based on the rate constant, k, (dimension: s–1, k) of hydrogen peroxide decomposition. The reduction in absorbance at 240 nm per minute and the rate constant of the enzyme were determined. Activities were defined as k (rate constant) per liter [14].
Measurement of Cytokines and IgE in BALF
Levels of IL-4, IFN-γ, and IgE were measured by enzyme-linked immunosorbent assay (Abcam, Cambridge, UK). The tests were done according to the manufacturer's instructions [15]. Briefly, 100 μL of detection antibody was added to all wells (except for the blank), mixed, and incubated for 16–24 h at RT. Plates were washed 3 times, and standards or supernatants added to the respective wells in duplicate. After incubation, the plates were washed again and incubated with 200 μL of the conjugate for 60 min at RT. Plates were then washed 3 times, and 200 μL of substrate were added and incubated for 15 min at RT in the dark. The reaction was ended by the addition of 50 μL of stop solution, and the color produced was measured using an automated microplate spectrophotometer. Total cytokine concentrations were determined as picograms per milligram of gingival tissue. Results were calculated using the standard curves plotted for each assay. The assays were carried out in a blind fashion in duplicate [16]. The interassay coefficients of variation for IFN-γ, IL-4, and IgE were < 12, 5.9, and < 10%, respectively.
Statistical Analysis
Analysis of variance (ANOVA) was used for comparisons among groups with the Tukey-Kramer post hoc test. Significance was considered as p < 0.05. InStat software (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analyses. Data are presented as means ± SEM.
Results
Effect of A. cepa on Oxidant and Antioxidant Markers in BALF
The volume of water used by animals did not significantly vary among the different groups. NO2, NO3–, and MDA levels were higher in the BALF in group S than in the control group (p < 0.001 for all cases). NO2 and NO3–levels were significantly reduced in rats treated with dexamethasone and one of the 3 doses of A. cepa, and MDA level was lower in rats treated with dexamethasone plus 70 and 140 mg/kg BW/day A. cepa compared to group S (p < 0.001 for all cases; Fig. 1). The effects of the A. cepa extract on MDA and NO3– levels and the effect of 35 and 70 mg/kg BW/day A. cepa on NO2 were significantly lower compared to that of dexamethasone treatment (p < 0.001to p < 0.05; Fig. 1).
Fig. 1.
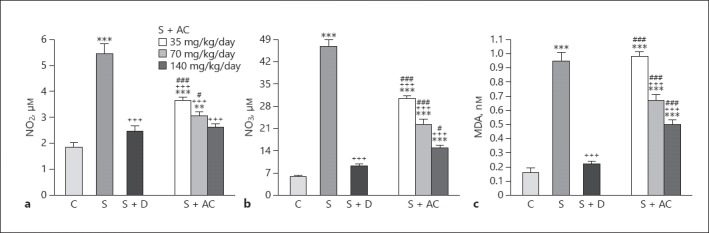
Levels of NO2 (a), NO3 (b), and malondialdehyde (MDA) (c) in the bronchoalveolar lavage fluid of control rats (C), sensitized rats (S), sensitized rats treated with dexamethasone (S + D) and Allium cepa (35, 70, and 140 mg/kg BW/day) (S + AC). ** p < 0.01, *** p < 0.001, vs. group C; +++ p < 0.001 vs. group S; # p < 0.05, ### p < 0.001, vs. group S + D.
The effects of 140 mg/kg BW/day A. cepa on all oxidant biomarkers were significantly more pronounced than that of 35 mg/kg BW/day A. cepa (p < 0.001 to p < 0.05; Table 1). In addition, the effect of the 70 mg/kg BW/day A. cepa dose on MDA and NO3– was significantly more marked than its lowest dose but less marked than the highest dose (p < 0.001 to p < 0.05; Table 1). Levels of SOD, thiol, and CAT were significantly decreased in sensitized rats compared to the control group (p ≤ 0.001 for all cases; Fig. 2). Levels of CAT and SOD in rats treated with dexamethasone and 70 and 140 mg/kg BW/day A. cepa and the level of thiol in the groups treated with dexamethasone and 140 mg/kg BW/day A. cepa were significantly increased compared to the sensitized group (p < 0.001 to p < 0.05; Fig. 2).
Table 1.
Comparison of oxidant, antioxidant, cytokine, and IgE levels
| Parameters | S + AC 35 | S + AC 70 | S + AC 140 |
|---|---|---|---|
| MDA, nM | 0.98±0.02 | 0.66±0.04*** | 0.49±0.03***, + |
| NO2, µM | 3.66±0.11 | 3.05±0.15 | 2.62+0.11* |
| NO3, µM | 30.46±0.89 | 22.21±1.85** | 15.08±0.81***, + |
| CAT, U/mL | 0.03±0.00 | 0.05±0.00*** | 0.06±0.00***, + |
| SOD, U/mL | 0.01±0.00 | 0.03±0.00*** | 0.04±0.00** |
| Thiol, µM | 0.02±0.00 | 0.04±0.00 | 0.06±0.00***, + |
| IL-4, pg/mL | 6.85±0.12 | 4.47±0.02*** | 3.75±0.06***, ++ |
| INF-γ, pg/mL | 2.48±0.03 | 2.80±0.01*** | 3.06±0.02***, ++ |
| INF-γ/IL-4 | 0.36±0.00 | 0.62±0.00*** | 0.81±0.01***, +++ |
| IgE, ng/mL | 32.64±1.42 | 24.19±0.65** | 16.67±0.55***, ++ |
Means ± SEM. S + AC 35/70/140, sensitized animals treated with 35/70/140 mg/kg BW/day Allium cepa extract. See text for further abbreviations.
p < 0.05
p < 0.01
p < 0.001, vs. S + AC 35
p < 0.05
p < 0.01
p < 0.001, vs. S + AC 70 (ANOVA).
Fig. 2.
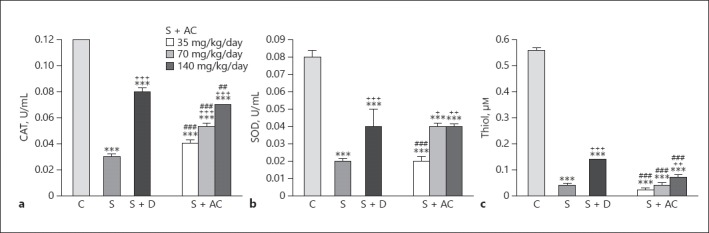
Levels of catalase (CAT) (a), superoxide dismutase (SOD) (b), and thiol (c) in the bronchoalveolar lavage fluid of control rats (C), sensitized rats (S), sensitized rats treated with dexamethasone (S + D) and Allium cepa (35, 70, and 140 mg/kg BW/day) (S + AC). *** p < 0.001 vs. group C; + p < 0.05, ++ p < 0.01, +++ p < 0.001, vs. group S; ## p < 0.01, ### p < 0.001, vs. group S + D.
The effects of all 3 doses of A. cepa extract on CAT and thiol and the effect of the 35 and 70 mg/kg BW/day doses on SOD were significantly lower than those of dexamethasone alone (p < 0.001 to p < 0.05; Fig. 1). The effects of the 140 mg/kg BW/day dose on all antioxidant biomarkers and the effect of its 70 mg/kg BW/day dose on CAT and SOD were significantly higher than those of the 35 mg/kg BW/day dose (p < 0.001 to p < 0.05; Table 1). The effects of the 140 mg/kg BW/day dose on CAT and thiol were also significantly higher than the 70 mg/kg BW/day dose (p < 0.001 to p < 0.05; Table 1).
Effect of A. cepa on Immunological Markers in BALF
Levels of IL-4 and IgE were higher, but levels of IFN-γ and the IFN-γ/IL-4 ratio were lower in sensitized than control rats (p < 0.001 for all cases; Fig. 3, 4). Levels of IgE in sensitized groups treated with all A. cepa doses, and levels of IL-4 in groups treated with the 70 and 140 mg/kg BW/day doses were significantly lower than in untreated rats (p < 0.001 for both cases; Fig. 3). In addition, the level of IFN-γ and the IFN-γ/IL-4 ratio were higher in groups treated with 70 and 140 mg/kg BW/day doses of the extract (p < 0.001 for both cases; Fig. 4). However, dexamethasone treatment caused significant reductions in IgE, IL-4, and IFN-γ levels but did not affect the IFN-γ/IL-4 ratio (p < 0.05 for IFN-γ and p < 0.001 for IgE and IL-4; Fig. 3, 4).
Fig. 3.
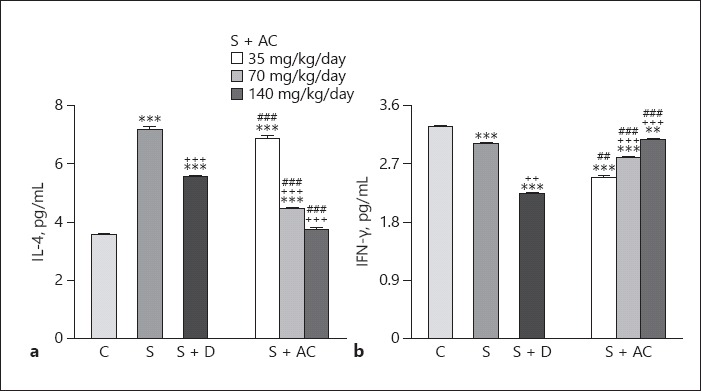
Levels of interleukin (IL)-4 (a) and interferon (IFN)-γ (b) in the bronchoalveolar lavage fluid of control rats (C), sensitized rats (S), sensitized rats treated with dexamethasone (S + D) and Allium cepa (35, 70, or 140 mg/kg BW/day) (S + AC). ** p < 0.01, *** p < 0.001, vs. group C; ++ p < 0.01, +++ p < 0.001, vs. group S; ## p < 0.01, ### p < 0.001, vs. group S + D.
Fig. 4.
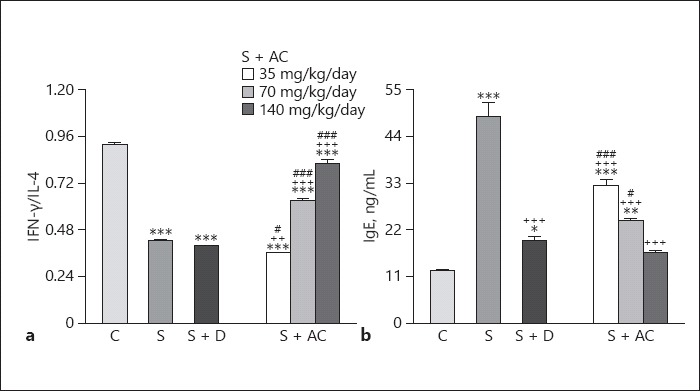
The interferon (IFN)-γ/interleukin (IL)-4 ratio (a) and IgE levels (b) in the bronchoalveolar lavage fluid of control rats (C), sensitized rats (S), sensitized rats treated with dexamethasone (S + D) and Allium cepa (35, 70, and 140 mg/kg/day) (S + AC). * p < 0.05, ** p < 0.01, *** p < 0.001, vs. group C; ++ p < 0.01, +++ p < 0.001, vs. group S; # p < 0.05, ### p < 0.001, vs. group S + D.
The effects of the 35 mg/kg BW/day A. cepa dose on IL-4 and the IFN-γ/IL-4 ratio as well as the effects of the 35 and 70 mg/kg BW/day A. cepa doses on IgE were significantly lower than those of dexamethasone (p < 0.01 for the IFN-γ/IL-4 ratio and p < 0.001 for the other cases; Fig. 3, 4). However, the effect of the 70 and 140 mg/kg BW/day A. cepa doses on IL-4 and IFN-γ levels as well as the IFN-γ/IL-4 ratio and the effect of the 140 mg/kg BW/day A. cepa dose on IgE level were significantly more marked than the effect of dexamethasone (p < 0.001 for all cases; Fig. 3, 4). The effects of the 70 and 140 mg/kg BW/day A. cepa doses on all immunological parameters were significantly more pronounced than the 35 mg/kg BW/day dose (p < 0.001 to p < 0.01; Table 1). The effects of the 140 mg/kg BW/day dose on immunological parameters were also significantly higher than the 70 mg/kg BW/day dose (p < 0.001 to p < 0.01; Table 1).
Discussion
In this study, the level of the oxidant markers NO2, NO3–, and MDA were lower but antioxidant markers CAT, SOD, and thiol were higher in the BALF of the sensitized rats than the control rats. Levels of IL-4 were also higher but IFN-γ and the IFN-γ/IL-4 ratio were lower in the BALF of sensitized than control rats. Previous studies in sensitized rats using similar methods of sensitization to the method used in this study also showed changes in oxidant and antioxidant levels as well as changes in IL-4 and IFN-γ levels, and the IFN-γ/IL-4 ratio [15, 17, 18], which support the findings of the present study.
Studies have reported the role of oxidative stress in asthmatic airway inflammation [19], reflected by increased levels of oxidants in a rat model of asthma and in asthmatic patients [20], and increased plasma levels of MDA in the BALF of asthmatic patients [21].
Marked antioxidant properties of red and yellow A. cepa skin have been shown to be due to the presence of flavonoids and organosulfur compounds [22], and the antioxidant effect of A. cepa was also documented in A. cepa-enriched bread [23]. The results of the present study suggest the potential therapeutic effect of A. cepa extract on asthma due to its antioxidant effect.
The A. cepa extract also reduced IgE and IL-4 levels but increased the IFN-γ/IL-4 ratio in the BALF of sensitized rats, indicating that A. cepa has immunomodulatory effects.
The suppressive effects of the plant and its constituents, such as quercetin, on IL-6, TNF-α, and IL-1β, and expression of COX-2, iNOS, NF-κB, and MAPKs have been reported [24]. The inhibitory effect of quercetin on the production of IL-4, a Th2 cytokine, and its stimulatory effect on the production of IFN-γ, a Th1 cytokine [25], were also shown in asthmatic mice, which support the findings of the present study and indicate that the plant is able to contribute to the treatment of asthma by increasing the Th1/Th2 balance and decreasing the levels of IgE.
The effects of A. cepa on oxidant and antioxidant markers, cytokines, and IgE in sensitized mice were also supported by the study of Oliveira et al. [10]. In a murine model of Blomia tropicalis-induced asthma, Oliveira et al. [10] showed the suppressive effect of A. cepa L. extract and quercetin on inflammatory activities such as eosinophil infiltration in the lung, eosinophil peroxidase, the cytokines IL-4 and IL-5, as well as smooth muscle contraction. The effect of the extract on eosinophil infiltration and eosinophil peroxidase was greater than that of quercetin. The effect of the extract and quercetin on cytokines was similar, but the effect of quercetin on tracheal smooth muscles relaxation was greater than that of the extract. They studied the effect of A. cepa extract (100 and 1,000 mg/kg/day for 6 days) and quercetin in rats sensitized with B. tropicalis, but in the present study the A. cepa extract was tested on OVA-sensitized rats for 21 days. Various pharmacological effects, including antioxidant and anti-allergic effects, immune system stimulation, improved Th1/Th2 balance, and reduction of pro inflammatory cytokine levels have been reported for onion-derived quercetin supporting its possible contribution to the therapeutic effects of A. cepa in bronchial asthma [26].
Dexamethasone decreased the levels of oxidant markers, and IgE and IL-4 levels, but improved antioxidant levels. However, dexamethasone did not increase IFN-γ levels or the IFN-γ/IL-4 ratio. The effects of the plant on the levels of oxidant and antioxidant markers NO3–, MDA, CAT, and thiol were less marked than those of dexamethasone. However, the effects of the extract on IL-4, IFN-γ, and the IFN-γ/IL-4 ratio were more marked, but the effect on IgE was comparable to that of dexamethasone. Therefore, A. cepa showed less oxidant and antioxidant activity than dexamethasone but demonstrated more specific immunomodulatory effects by reducing levels of the Th2 cytokine IL-4 and increasing levels of the Th1 cytokine IFN-γ and the IFN-γ/IL-4 ratio resulting in enhanced Th1/Th2 bias. However, the effect of the plant extract on other cytokines such as IL-8 and tumor necrosis factor should be evaluated in further studies [27]. It should also be noted that different results may also be related to the type of species chosen. In fact, it was shown that components and activities of onion are different on the basis of cultivars [28].
The role of thiol, an early marker of oxidative stress in asthma, in the reduction of SOD [29] and CAT activity in asthmatic patients was stressed previously [30], which was in agreement with the changes observed in sensitized animals in this study. In addition, similar to the findings of the present study, upregulation of the release of Th2 cytokines (mainly IL-4, IL-5, and IL-13) and downregulation of the release of IFN-γ were also noticed in asthmatic patients [30]. Therefore, the results of the present and previous studies [7] on antioxidant and anti-inflammatory properties of A. cepa suggest that the plant could be of therapeutic value in asthma, which should be confirmed in further studies.
Conclusion
A. cepa extract significantly lowered the levels of oxidant markers, IgE, and IL-4 but increased the levels of antioxidant markers, IFN-γ, and the IFN-γ/IL-4 ratio in sensitized rats. Therefore, the plant extract could have potential in the treatment of asthma through antioxidant and immunomodulatory mechanisms.
Acknowledgments
This work was financially supported by a grant from the Research Council of the Mashhad University of Medical Sciences (Code: 910898), Mashhad, Iran.
References
- 1.Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Bloemen K, Verstraelen S, Van Den Heuvel R, et al. The allergic cascade: review of the most important molecules in the asthmatic lung. Immunol Lett. 2007;113:6–18. doi: 10.1016/j.imlet.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Garcia G, Godot V, Humbert M. New chemokine targets for asthma therapy. Curr Allergy Asthma Rep. 2005;5:155–160. doi: 10.1007/s11882-005-0090-0. [DOI] [PubMed] [Google Scholar]
- 4.Nakagome K, Nagata M. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx. 2011;38:555–563. doi: 10.1016/j.anl.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Roldan E, Sanchez-Moreno C, de Ancos B, et al. Characterisation of onion (Allium cepa L.) by-products as food ingredients with antioxidant and antibrowning properties. Food Chem. 2008;108:907–916. doi: 10.1016/j.foodchem.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 6.Lee BK, Jung Y-S. Allium cepa extract and quercetin protect neuronal cells from oxidative stress via PKC-ε inactivation/ERK1/2 activation. Oxid Med Cell Longev. 2016;2016:2495624. doi: 10.1155/2016/2495624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graefe EU, Wittig J, Mueller S, et al. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J Clin Pharmacol. 2001;41:492–499. doi: 10.1177/00912700122010366. [DOI] [PubMed] [Google Scholar]
- 8.El-Aasr M, Fujiwara Y, Takeya M, et al. Onionin A from Allium cepa inhibits macrophage activation. J Nat Prod. 2010;73:1306–1308. doi: 10.1021/np100105u. [DOI] [PubMed] [Google Scholar]
- 9.Ueda H, Takeuchi A, Wako T. Activation of immune responses in mice by an oral administration of bunching onion (Allium fistulosum) mucus. Biosci Biotechnol Biochem. 2013;77:1809–1813. doi: 10.1271/bbb.130084. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira TT, Campos KM, Cerqueira-Lima AT, et al. Potential therapeutic effect of Allium cepa L. and quercetin in a murine model of Blomia tropicalis induced asthma. Daru. 2015;23:18. doi: 10.1186/s40199-015-0098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javed MS, Khan MI, Randhawa MA, et al. Garlic (Allium sativum L.) as antimicrobial and antioxidant agents in beef sausages. Pak J Food Sci. 2011;21:22–32. [Google Scholar]
- 12.Mellado-García P, Maisanaba S, Puerto M, et al. In vitro toxicological assessment of an organosulfur compound from Allium extract: cytotoxicity, mutagenicity and genotoxicity studies. Food Chem Toxicol. 2017;99:231–240. doi: 10.1016/j.fct.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Mansour HA, Mahfouz H, Maher N. Anti-mutagenic potential of algal extracts on chromosomal aberrations in Allium cepa L. Acta Biol Hung. 2017;68:137–149. doi: 10.1556/018.68.2017.2.2. [DOI] [PubMed] [Google Scholar]
- 14.Shakeri F, Soukhtanloo M, Boskabady MH. The effect of hydro-ethanolic extract of Curcuma longa rhizome and curcumin on total and differential WBC and serum oxidant, antioxidant biomarkers in rat model of asthma. Iran J Basic Med Sci. 2017;20:155–165. doi: 10.22038/ijbms.2017.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boskabady MH, Mehrjardi SS, Rezaee A, et al. The impact of Zataria multiflora Boiss extract on in vitro and in vivo Th1/Th2 cytokine (IFN-γ/IL-4) balance. J Ethnopharmacol. 2013;150:1024–1031. doi: 10.1016/j.jep.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Napimoga MH, Clemente-Napimoga JT, Macedo CG, et al. Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J Nat Prod. 2013;76:2316–2321. doi: 10.1021/np400691n. [DOI] [PubMed] [Google Scholar]
- 17.Boskabady MH, Keyhanmanesh R, Khameneh S, et al. Potential immunomodulation effect of the extract of Nigella sativa on ovalbumin sensitized guinea pigs. J Zhejiang Univ Sci B. 2011;12:201–209. doi: 10.1631/jzus.B1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrami G, Boskabady MH, Jalali S, et al. The effect of the extract of Crocus sativus on tracheal responsiveness and plasma levels of IL-4, IFN-gamma, total NO and nitrite in ovalbumin sensitized guinea-pigs. J Ethnopharmacol. 2013;147:530–535. doi: 10.1016/j.jep.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Sackesen C, Ercan H, Dizdar E, et al. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J Allergy Clin Immunol. 2008;122:78–85. doi: 10.1016/j.jaci.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 21.Ozaras R, Tahan V, Turkmen S, et al. Changes in malondialdehyde levels in bronchoalveolar fluid and serum by the treatment of asthma with inhaled steroid and β2-agonist. Respirology. 2000;5:289–292. doi: 10.1046/j.1440-1843.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 22.Albishi T, John JA, Al-Khalifa AS, et al. Antioxidative phenolic constituents of skins of onion varieties and their activities. J Funct Foods. 2013;5:1191–1203. [Google Scholar]
- 23.Gawlik-Dziki U, Swieca M, Dziki D, et al. Quality and antioxidant properties of breads enriched with dry onion (Allium cepa L.) skin. Food Chem. 2013;138:1621–1628. doi: 10.1016/j.foodchem.2012.09.151. [DOI] [PubMed] [Google Scholar]
- 24.Ahn NK, Kang BK, Kim KB, et al. Anti-inflammatory effect of ethanol extract from onion (Allium cepa L.) peel on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells and mice ears. J Korean Soc Food Sci Nutr. 2015;44:1612–1620. [Google Scholar]
- 25.Park HJ, Lee CM, Jung ID, et al. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int Immunopharmacol. 2009;9:261–267. doi: 10.1016/j.intimp.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Mlcek J, Jurikova T, Skrovankova S, et al. Quercetin and its anti-allergic immune response. Molecules. 2016;21:623. doi: 10.3390/molecules21050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempuraj D, Madhappan B, Christodoulou S, et al. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol. 2005;145:934–944. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma K, Assefa AD, Kim S, et al. Evaluation of total phenolics, flavonoids and antioxidant activity of 18 Korean onion cultivars: a comparative study. J Sci Food Agric. 2014;94:1521–1529. doi: 10.1002/jsfa.6450. [DOI] [PubMed] [Google Scholar]
- 29.Zinellu A, Fois AG, Sotgia S, et al. Plasma protein thiols: an early marker of oxidative stress in asthma and chronic obstructive pulmonary disease. Eur J Clin Invest. 2016;46:181–188. doi: 10.1111/eci.12582. [DOI] [PubMed] [Google Scholar]
- 30.Yang LL, Huang MS, Huang CC, et al. The association between adult asthma and superoxide dismutase and catalase gene activity. Int Arch Allergy Immunol. 2011;156:373–380. doi: 10.1159/000324448. [DOI] [PubMed] [Google Scholar]


