Abstract
Objective
This study sought to evaluate the protective effect of ethanolic leaf extract of Moringa oleifera on testosterone-induced benign prostatic hyperplasia (BPH) in male Sprague-Dawley rats.
Materials and Methods
BPH was induced in rats by the administration of testosterone propionate (3 mg/kg, s.c., in olive oil) for 4 weeks. M. oleifera (50, 100, or 200 mg/kg), celecoxib (20 mg/kg), or M. oleifera (50 mg/kg) + celecoxib (20 mg/kg) were orally administered daily 15 min before testosterone. On day 29, blood was collected to measure the levels of serum testosterone and prostate-specific antigen before the animals were sacrificed. The prostates were weighed, assayed, and histologically examined.
Results
M. oleifera significantly reduced the testosterone-induced increase in prostate weight (20.16%), prostate index (65.85%), serum testosterone (72.86%), and prostate-specific antigen (48.49%). Testosterone caused a significant increase in malondialdehyde (73%) as well as a reduction in glutathione (62.5%), superoxide dismutase (50%), and catalase (64%) activities which were attenuated by M. oleifera with a peak effect obtained at 100 mg/kg. The disruption of prostate histoarchitecture by testosterone was also ameliorated by M. oleifera.
Conclusion
M. oleifera prevented testosterone-induced BPH through enhancement of antioxidant defence mechanisms, and hence could be used as an adjunct in the treatment of BPH.
Keywords: Celecoxib, Prostatic index, Antioxidant, Prostate-specific antigen, Oxidative stress
Significance of the Study
• In this study, 28-day consecutive treatment of rats with testosterone caused a significant increase in prostate weight, prostate index, serum testosterone, and prostate-specific antigen, suggestive of an ameliorative effect of Moringa oleifera leaf extract on testosterone-induced benign prostatic hyperplasia (BPH). Moreover, testosterone-induced disruption of prostate morphology as well as an increase in oxidative stress parameters were attenuated by M. oleifera. Hence, M. oleifera could be a potential phytotherapeutic agent in the treatment of BPH.
Introduction
Benign prostatic hyperplasia (BPH) is the most common urological disease in elderly men, affecting well over 42% of men in their 50s and more than 80% of octogenarians [1]. BPH is a chronic, slowly progressive disease which begins as a simple micronodular hyperplasia that evolves into macroscopic nodular enlargement [2]. It is the most common cause of lower urinary tract symptoms in men. It clinically manifests as urinary hesitancy or straining in initiating urination, causing deterioration in urinary function and quality of life. Several stimuli, including infectious agents, hormones, urinary reflux, metabolic syndrome, ageing process, and autoimmune response, have been described as triggers for the dysregulation of the prostatic immune system through different molecular pathways involving the development of inflammatory infiltrates [3]. Based on the pathophysiology, subsequent tissue damage and chronic tissue healing could result in the development of BPH nodules [2, 3].
The mainstay in the clinical management of BPH are α1-blockers and/or 5α-reductase inhibitors, but not without adverse effects. The majority of the populace in developing countries depend on herbal medicines for their health care needs [4]; hence, this could be a better alternative for the discovery of cheap and safer drugs for BPH. Moringa oleifera Lam (Moringaceae), also known as the “drumstick,” “horseradish,” or miracle tree, is a fast-growing, drought-resistant tree cultivated across the lowland dry tropics worldwide for its nutritious leaves [5]. It is an affordable and readily available source of major essential nutrients and nutraceuticals. The different parts of the M. oleifera tree, including roots, bark, leaves, flowers, fruits, and seeds, are traditionally used in various therapeutic applications, including abdominal tumours, hysteria, scurvy, paralysis, helminthic bladder, prostate problems, sores, and skin infections [6]. Dried leaves of M. oleifera are a good source of polyphenol: total flavonoid concentration in dried leaves ranges from 5.059 to 12.16 mg/g of dried extract [7]. Myricetin, quercetin, and kaempferol are the main flavonoids found in M. oleifera leaves, respectively [8]. Infusion of M. oleifera leaves in alcohol is used by traditional healers in Southwest Nigeria for the treatment of prostate and bladder problems.
Antiproliferative, antioxidant, pro-apoptotic [8], anti-quorum sensing, DNA-protective [9], anti-inflammatory, anti-hyperglycaemic, hepatoprotective, and nephroprotective [10] effects of M. oleifera have been reported. Similarly, toxicological evaluation of the aqueous leaf extract of M. oleifera showed that it is relatively safe when administered orally [11]. This study sought to evaluate the effect of ethanolic leaf extract of M. oleifera on testosterone-induced BPH in rats and to investigate possible synergy when co-administered with celecoxib (COX-2 inhibitor).
Materials and Methods
Laboratory Animals
Forty-nine male Sprague-Dawley rats weighing 200–250 g were obtained from the Laboratory Animal Centre, College of Medicine, University of Lagos, Lagos, Nigeria and were divided into 7 groups (7 in each group). The rats were allowed to acclimatize for a week prior to experiments. All the rats were housed in a well-ventilated room under a 12 h light/dark cycle and had access to feed and water ad libitum. The experimental procedures adopted were in accordance with the Health Research and Ethics Committee of the College of Medicine, University of Lagos and the US National Institute of Health Guidelines for Care and Use of Laboratory Animals in Biomedical Research (2011).
Plant Material
The M. oleifera leaves used in this study were obtained from Mushin Herbal Market, Mushin, Nigeria. Botanical identification and authentication was done by Mr. Oyebanji Oyetola (Herbarium Curator) and Prof. J.D. Olowokudejo of the Department of Botany, University of Lagos, Nigeria. A voucher specimen was deposited in the Herbarium for reference purposes (No. LUH 6516).
Drugs and Chemicals
Testosterone propionate (TESTOSTTM; Laborate Pharmaceuticals Ltd., India), celecoxib (CelebrexTM; Pfizer Pharmaceuticals Ltd., lllertissen, Germany), olive oil (Goya En Spana S.A.U, Spain), testosterone ELISA kit (Cayman, Ann Arbor, MI, USA), prostate-specific antigen (PSA) (Novatein Biosciences, Woburn, MA, USA), ethanol, thiobarbituric acid, bovine serum albumin, 5, 5′-dithiobis (2-nitrobenzoic acid) (DTNB), trichloroacetic acid, glacial acetic acid, sodium chloride, and sodium hydroxide were purchased from Sigma Aldrich (St, Louis, MO, USA)
Preparation of Plant Extract
The collected M. oleifera leaves were air dried (320 g), and then pulverized into powder. The powdered leaf was soaked in 3.75 L of ethanol (70%) with intermittent agitation for 72 h. The extract was filtered and the filtrate obtained was oven dried at 40°C, with a percentage yield of 7.5% w/w (24 g; deep brown). The choice of ethanolic extraction was based on folkloric preparation.
Quantitative Phytochemical Analysis
Preliminary quantitative analyses of phytochemical constituents of M. oleifera were done using previously reported protocols: total phenolic content [12], total flavonoid content [12], and total antioxidant capacity [13] (n = 3).
In vitro Antioxidant Assays
1,1 Diphenyl-2-Picrylhydrazyl Radical Scavenging Assay
The free radical scavenging activity of M. oleifera was assayed based on the ability of the extract to bleach stable 1,1 diphenyl-2-picrylhydrazyl (DPPH) radicals. Briefly, 500 μL of DPPH was mixed with aliquots of gallic acid or M. oleifera (0–100 mg/mL), or dimethylsulfoxide (DMSO; control). The mixture was incubated for 30 min at 37°C in the dark and absorbance was read at 517 nm [13] (n = 3). Percentage inhibition was calculated according to the equation:
Nitric Oxide Scavenging Assay
The ability of M. oleifera to inhibit nitric oxide radicals was estimated using the Griess reaction method [14]. Griess reagent is comprised of 1% sulphanilamide in 5% v/v phosphoric acid and 0.01% naphthylethylenediamine in distilled water in equal volumes. Sodium nitroprusside (5 µM) in phosphate buffer (0.025 M, pH 7.4) was added to different concentrations of M. oleifera or gallic acid (0–100 mg/mL) and incubated for 5 h at 37°C. An equal amount of methanol was taken as control. Five hours later, 500 µL of Griess reagent was added. The absorbance of chromophore formed during the digitization of nitrite with sulphanilamide and its subsequent coupling with napthylethylenediamine was read at 546 nm (n = 3).
Reducing Power Assay
The ferric ion-reducing power of M. oleifera was determined according to the method of Oyaizu [15]. Briefly, 1 mL of M. oleifera (0–100 mg/mL) was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide (1%). The reaction mixture was incubated for 20 min at 50°C. Then, 2.5 mL of trichloroacetic acid (10%) was added and centrifuged for 10 min. An aliquot of 2.5 mL was mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 (0.1%). The absorbance of all solutions was measured at 700 nm and expressed as milligrams of gallic acid equivalent (GAE) per gram of powder.
Acute Toxicity Test
Acute toxicity was tested in 10 female albino mice (18–25 g) according to the OECD (Organization for Economic Co-Operation and Development) (OECD, TG420, 2001) procedure for acute toxicity. Three doses were assayed: maximum-tolerated (5,000 mg/kg, p.o.; n = 5), medium (500 mg/kg, p.o.; n = 2), and low-dose (50 mg/kg, p.o.; n = 2) levels M. oleifera, or vehicle control (10 mL/kg, p.o.; n = 1) (which did not cause any unacceptable sign of toxicity or death). The mice were closely observed for signs of toxicity during the first 30 min and hourly for 3 h, then during the first 24 h, and then daily for 14 days for delayed toxicity or mortality.
Treatment Regimens
After an acclimatization period of 7 days, 49 male Sprague-Dawley rats were randomly divided into 7 groups (n = 7) and treated for 28 consecutive days as follows: group 1: vehicle (10 mL/kg, p.o., normal), group 2: vehicle (10 mL/kg, p.o.), groups 3–5: M. oleifera (50, 100, or 200 mg/kg, p.o., respectively), group 6: celecoxib (20 mg/kg, p.o.), and group 7: M. oleifera (50 mg/kg) + celecoxib (20 mg/kg). Fifteen minutes after drug or vehicle administration, testosterone propionate (3 mg/kg, s.c.) in olive oil was given to the animals in groups 2–7. Body weights were recorded before and weekly until the end of the study.
Body and Prostate Weights
On the 29th day, the rats were anaesthetized with chloral hydrate (300 mg/kg, i.p.) and blood was collected via retro-orbital puncture. Following blood collection, the rats were euthanized and prostate tissues harvested. The prostate index was calculated as the ratio of the prostate weight to the total body weight.
Measurement of Serum Testosterone and PSA Levels
The serum levels of testosterone and PSA were assayed using ELISA (Cayman, USA and Novatein Biosciences, Woburn, MA, USA, respectively) following the manufacturer's instructions.
Histological Studies
The prostate tissues were rapidly dissected out and weighed. Sections of the ventral lobes were fixed in 10% neutral buffered formalin and embedded in paraffin for histological examinations.
Assessment of Oxidative Stress Markers
The prostate was washed with cold phosphate buffer, blotted, and weighed. A 10% (w/v) prostate in 0.03 M sodium phosphate buffer (pH 7.4) was homogenized with Ultra-Turrax T25 (USA) homogenizer at 9,500 rpm. The homogenate was stored at −20°C until use.
Estimation of Malondialdehyde Level
Malondialdehyde (MDA), an index of lipid peroxidation, was determined using the method of Buege and Aust [16]. Briefly, 500 μL of tissue homogenate in phosphate buffer (pH 7.4) were added to reaction mixture containing 300 µL of 30% trichloroacetic acid, 150 µL of 5N HCl, and 300 µL of 2% (w/v) 2-thiobarbituric acid. The mixture was heated for 15 min at 90°C and centrifuged at 12,000 g for 10 min. A pink colour supernatant was obtained, which was measured spectrophotometrically at 532 nm.
Estimation of Reduced Glutathione
Glutathione (GSH) content of the prostate as non-protein sulphydryls was estimated according to the method described by Sedlak and Lindsay [17]. GSH was determined by its reaction with DTNB (Ellman's reagent) to yield a yellow chromophore which was measured spectrophotometrically. In brief, 500 μL of prostate homogenate were added to 500 μL of 10% trichloroacetic acid and centrifuged (Remi cold centrifuge) at 2,000 g for 10 min at 4°C. An aliquot of 33 μL of supernatant was added to the reaction mixture containing 66 μL of 100 mM of DTNB and 900 μL of 0.1 M phosphate buffer (pH 7.4). The absorbance was read at 412 nm.
Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity was determined using the method of Winterbourn et al. [18]. An aliquot of 200 μL of supernatant was added to the reaction mixture containing 500 μL of 100 mM TRIS/HCl (pH 7.8), 200 μL of 75 mM nitro-blue-tetrazolium, 200 μL of 2 μM riboflavin, and 200 μL of 6 mM EDTA. The absorbance was read at 560 nm. One unit of SOD is defined as the quantity required to inhibit the rate of nitro-blue-tetrazolium reduction by 50%. The enzyme activity is expressed as units/mg protein.
Determination of Catalase Activity
Catalase activity was determined according to the method of Higashi and Peters [19]. The reaction mixture contained 250 μL of 0.01 M phosphate buffer (pH 7.0), 25 μL of tissue homogenate, and 100 μL of 2M H2O2. The reaction was stopped by the addition of 500 μL of dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in a1: 3 ratio). The absorbance was read at 620 nm and expressed as micromoles of H2O2 consumed/min/mg protein at 25°C.
Protein Estimation
Protein estimation was carried out using the method of Lowry et al. [20] and bovine serum albumin (1 mg/mL) was used as standard.
Statistical Analysis
All the data are expressed as mean ± SEM (n = 6). All statistical analyses were performed using the GraphPad Prism, version 6.01. Multiple comparisons were performed using one-way ANOVA followed by the Tukey post hoc multiple comparisons test.
Results
Preliminary Quantitative Phytochemical Analysis
Results of the preliminary quantitative analyses of phytochemical constituents of M. oleifera were as follows: total phenolic content 22.45 ± 0.12 mg/100 g GAE, total flavonoid content 13.61 ± 0.08 mg/100 g GAE, and total antioxidant capacity 26.96 ± 0.08 mg/100g GAE.
In vitro Antioxidant Activity
M. oleifera showed significant free radical scavenging activity, with an inhibitory concentration (IC50) of 60.87 µg/mL (y = 0.55•x + 16.52) while ascorbic acid had an IC50 of 41.41 µg/mL (y = 0.58•x + 26.07) on DPPH free radicals. Similarly, the nitric oxide free radical scavenging effect of M. oleifera was similar to that of ascorbic acid with an IC50 of 49.72 (y = 0.49•x + 25.53) and 40.74 µg/mL (y = 0.55•x + 27.40), respectively. The reducing capabilities of M. oleifera and ascorbic acid were 94.00 µg/mL (y = 0.004•x + 0.03) and 64.47 µg/mL (y = 0.006•x + 0.02), respectively.
Acute Toxicity Test
Acute oral administration of M. oleifera up to 5,000 mg/kg did not induce mortality but the observed toxic behavioural changes include increased respiratory rate (tachypnoea), grooming, rearing, and restlessness.
Effect on Prostate Weight and Prostate Index
Subchronic injection of testosterone for 28 consecutive days produced no significant change in body weight but significantly increased prostate weight (0.11 ± 0.04 in normal controls to 1.23 ± 0.15 in the vehicle-testosterone-treated group) and prostate index (0.29 ± 0.02 in vehicle-control-treated animals to 0.80 ± 0.07 in the vehicle-testosterone-treated group), indicating prostate hyperplasia. However, pretreatment of rats with M. oleifera prevented an increase in prostate weight and prostate index with a peak effect observed at M. oleifera 100 mg/kg (65.85% inhibition) (Table 1). In contrast, oral administration of celecoxib and combined treatment reduced the prostate index, though not significantly.
Table 1.
Effect of Moringa oleifera on body weight and prostate index
| Treatment | Prostate weight, g | Prostate index |
|---|---|---|
| Vehicle | 0.41±0.09 | 0.29±0.05 |
| Testosterone | 1.23±0.27*** | 0.80±0.19*** |
| M. oleifera 50 mg/kg | 0.52±0.16γ | 0.32±0.09γ |
| M. oleifera 100 mg/kg | 0.42±0.12γ | 0.26±0.05γ |
| M. oleifera 200 mg/kg | 0.92±0.28α | 0.54±0.17α |
| Celecoxib 20 mg/kg | 1.31±0.29 | 0.73±0.19 |
| M. oleifera 50 mg/kg + celecoxib 20 mg/kg | 1.18±0.32 | 0.69±0.12 |
Values are expressed as mean ± SD (n = 7).
p < 0.001 versus vehicle;
p < 0.05;
p < 0.001 versus vehicle-testosterone-treated animals. One-Way ANOVA followed by Tukey post hoc multiple comparison tests.
Effect on Serum Level of Testosterone and PSA
Subcutaneous injection of testosterone significantly (p < 0.001) increased serum testosterone from 11.97 ± 1.78 in controls to 26.68 ± 4.90. The increase in serum testosterone was reduced by pretreatment of rats with M. oleifera with a peak effect observed at 100 mg/kg (72.86% inhibition) (Fig. 1a). Similarly, the COX-2 inhibitor celecoxib significantly reduced testosterone levels by 38.34%. Conversely, co-administration of celecoxib and M. oleifera failed to produce a significant reduction in testosterone levels. In another experiment, administration of testosterone for 28 consecutive days increased PSA by 45.45% compared with vehicle-control-treated animals. Pretreatment of rats with M. oleifera reduced PSA levels 2-fold (48.49%) compared with the vehicle-testosterone-treated group. Similarly, celecoxib administration reduced PSA levels 2.25-fold, while co-administration of celecoxib and M. oleifera markedly reduced PSA levels 2.30-fold (56.06%) (Fig. 1b).
Fig. 1.
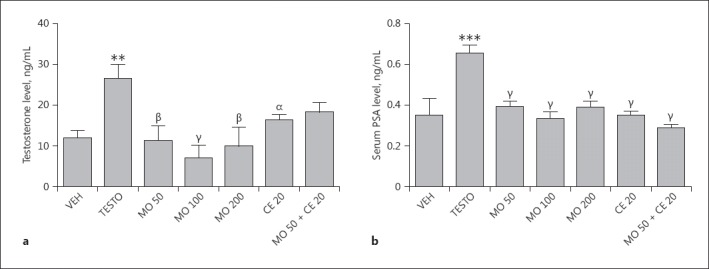
a, b Effects of M. oleifera and/or celecoxib on serum testosterone (a) or prostate-specific antigen (PSA) (b) level in rats. Values are expressed as mean ± SD (n = 6). ** p < 0.01; *** p < 0.001, versus vehicle-control-treated animals; α p < 0.05; β p < 0.01; γ p < 0.001, versus vehicle-testosterone-treated animals. One-way ANOVA followed by Tukey post hoc multiple comparison tests. VEH, vehicle-control; TESTO, vehicle-testosterone; MO, M. oleifera (50, 100, 200 mg/kg); CE, celecoxib (20 mg/kg).
Effect on in vivo Antioxidant Systems
Subcutaneous injection of testosterone significantly increased MDA levels 3-fold when compared with vehicle-control-treated animals. However, M. oleifera (50, 100, or 200 mg/kg) significantly (p < 0.001) attenuated MDA levels (F[4, 24] = 34.35, p < 0.0001) compared with vehicle-testosterone-treated animals. Similarly, pretreatment of rats with celecoxib significantly reduced levels of MDA. Interestingly, the combination of M. oleifera (50 mg/kg) + celecoxib (20 mg/kg) significantly reduced MDA level 3-fold (Fig. 2a). Subchronic injection of testosterone significantly reduced prostate GSH levels 2-fold in comparison with the vehicle-treated group. However, pretreatment of rats with M. oleifera (50, 100, or 200 mg/kg) enhanced the level of prostate GSH. Similarly, celecoxib alone and co-treatment with M. oleifera increased the level of GSH (Fig. 2b). Subchronic administration of testosterone reduced SOD activity. However, pretreatment of rats with M. oleifera increased SOD activity 2-fold with a peak effect observed at 100 mg/kg. Although pretreatment of rats with celecoxib alone produced no significant change in SOD activity, co-administration of celecoxib with M. oleifera significantly increased SOD activity (Fig. 2c). Catalase activity was also reduced by testosterone administration but pretreatment of rats with M. oleifera (50, 100, or 200 mg/kg) increased catalase activity (Fig. 2d).
Fig. 2.
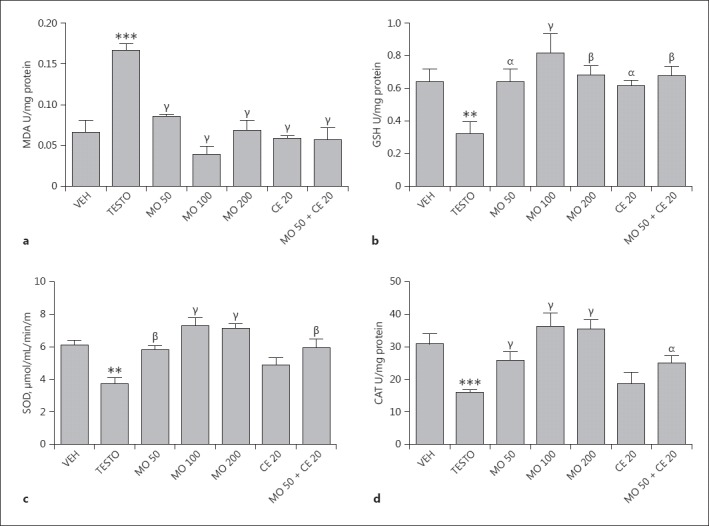
a–d Effects of M. oleifera and/or celecoxib on prostate levels of malondialdehyde (MDA) (a), glutathione (GSH) (b), superoxide dismutase (SOD) activity (c), and catalase (CAT) activity (d) in testosterone-induced BPH rats. Values are expressed as mean ± SD (n = 6). ** p < 0.01; *** p < 0.001, versus vehicle-control-treated animals; α p < 0.05; β p < 0.01; γ p < 0.001, versus vehicle-testosterone-treated animals. One-way ANOVA followed by Tukey post hoc multiple comparison tests. VEH, vehicle-control; TESTO, vehicle-testosterone; MO, M. oleifera (50, 100, 200 mg/kg); CE, celecoxib (20 mg/kg).
Histological Examination
Figure 3a shows the normal histological architecture of the prostate gland in the vehicle-treated group. However, the vehicle-testosterone-treated group showed disruption of the architecture of the prostate gland, evidenced in the increase in intraluminal secretion, thickened epithelial cells, and involution of the epithelial cells into the lumen (Fig. 3b). Figure 3c, d, e shows the effect of M. oleifera (50, 100, and 200 mg/kg, respectively): involution of the epithelial cells into the lumen which result in the narrowing of the lumen, an increase in intraluminal secretions, and thickened epithelial cells (Fig. 3c), and preservation of the integrity of prostate cells when compared with the vehicle-testosterone-treated group (Fig. 3d, e). Celecoxib (an anti-inflammatory drug) preserves the integrity of the prostate, as evidenced in the absence of intraluminal secretions and normal lumen of the acini (Fig. 3f). Co-administration of celecoxib and M. oleifera improved the cytoarchitecture of the prostate gland, as shown by the reduced intraluminal secretions in the glandular acini and increased epithelial cell occlusions into the lumen of the glandular acini (Fig. 3g).
Fig. 3.
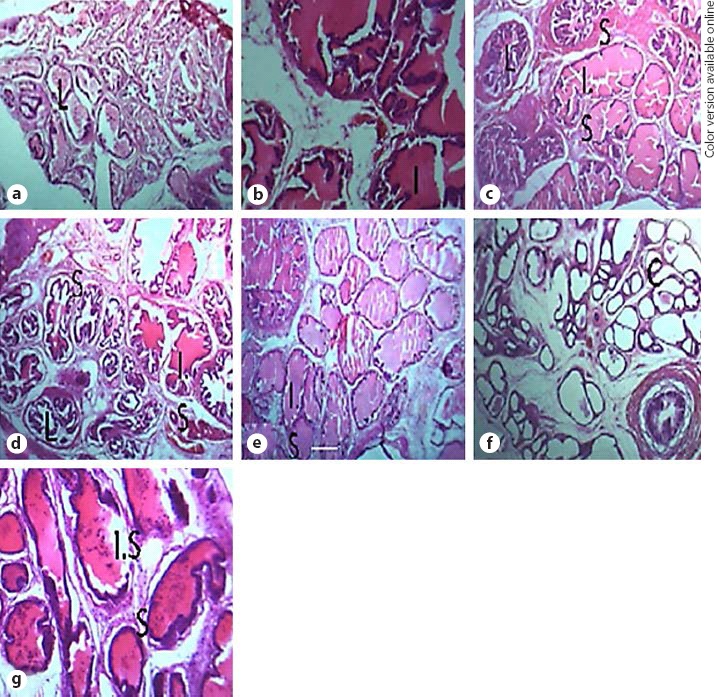
a–g Photomicrographs of prostate samples of vehicle-control normal group (a), vehicle-testosterone-treated group (b), M. oleifera 50 mg/kg (c), M. oleifera 100 mg/kg (d), M. oleifera 200 mg/kg (e), celecoxib 20 mg/kg (f), and M. oleifera 50 mg/kg + celecoxib 20 mg/kg (g). ×40. L, lumen; S, stromal compartment; I.S., intraluminal secretions; C, prostatic concretions.
Discussion
In the present study, treatment of rats with testosterone propionate for 28 days increased prostate weight and prostate index as well as levels of serum testosterone and PSA, indicating BPH [1]. In addition, testosterone increased levels of prostate MDA and reduced GSH, SOD, and catalase activity, suggestive of oxidative stress. The pretreatment of rats with M. oleifera ameliorated the effect of testosterone as shown by the reduction of prostate weight, prostate index, serum testosterone, and PSA. Interestingly, M. oleifera enhanced antioxidant defence mechanisms. Histological examination also showed that oral administration of the leaf extract of M. oleifera attenuated testosterone-induced prostatic hyperplasia. The role of inflammation in BPH is well known [2]. In this study, celecoxib (COX-2 inhibitor) alone or in combination with M. oleifera ameliorated testosterone-induced BPH, justifying the roles of inflammation [21].
Several studies have alluded to the fact that an increase in prostate index is indicative of the development of BPH [22, 23, 24]. Pretreatment of rats with M. oleifera leaf extract caused a significant reduction in the prostate index with a peak effect observed at 100 mg/kg. It is of interest to note that these results were consistent with histological examination of the prostate tissues.
Subchronic administration of testosterone increased serum levels of testosterone and PSA, confirming the induction of BPH. Androgens play a permissive role in BPH pathogenesis; thus, in clinical practice, inhibitors of 5α-reductase (which converts testosterone to the more potent androgen dihydrotestosterone) have proven effective in the management of BPH, confirming an essential role for androgens in the pathophysiology of BPH [25]. The increase in serum testosterone and PSA induced by testosterone was attenuated by pretreatment with M. oleifera. Moreover, animals treated with celecoxib alone and co-treatment with M. oleifera (50 mg/kg) had significant attenuation of serum testosterone and PSA, corroborating the findings of previous studies [26, 27].
In the present study, testosterone caused a significant increase in MDA and a deficit in GSH, SOD, and catalase, suggestive of oxidative stress. Oxidative stress is considered to be one of the mechanisms that trigger the chain of reactions involved in the development and progression of BPH [28]. Interestingly, the pretreatment of rats with M. oleifera resulted in attenuation of testosterone-induced lipid peroxidation, GSH, SOD, and catalase deficit. Moreover, co-administration of celecoxib 20 mg/kg and M. oleifera significantly enhanced antioxidant defence mechanisms of the prostate [9, 11, 13].
A wide variety of polyphenols and phenolic acids as well as flavonoids, glucosinolates, and possibly alkaloids are believed to be responsible for the observed effects [29]. Our in vitro antioxidant assays showed the potentials of M. oleifera in scavenging of DPPH and nitric oxide radicals as well as its reductive capability. These findings corroborated the reports of Leone et al. [29]; of the various plant parts of M. oleifera, the leaves possessed the highest content of total phenolics, β-carotene, and lycopene. Leaves also showed maximum antioxidant potential in the nitric oxide scavenging assay and deoxyribose degradation assay [30].
Conclusion
Findings from this study showed that the ethanolic leaf extract of M. oleifera prevented testosterone-induced BPH possibly through enhancement of antioxidant defence mechanisms or inhibition of inflammatory mediators. Thus, M. oleifera could be a potential phytotherapeutic agent in the management of BPH in men.
References
- 1.De Nunzio C, Presicce F, Tubaro A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat Rev Urol. 2016;13:613–626. doi: 10.1038/nrurol.2016.168. [DOI] [PubMed] [Google Scholar]
- 2.De Nunzio C, Albisinni S, Gacci M, et al. The role of inflammation in the progression of benign prostatic hyperplasia. Curr Bladder Dysfunct Rep. 2013;8:142–149. [Google Scholar]
- 3.Krušlin B, Tomas D, Džombeta T, et al. Inflammation in prostatic hyperplasia and carcinoma-basic scientific approach. Front Oncol. 2017;7:77. doi: 10.3389/fonc.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong HL, Liao CH, Kuo HC. Long-term combination therapy with α-blockers and 5α-reductase inhibitors in benign prostatic hyperplasia: patient adherence and causes of withdrawal from medication. Int Neurourol J. 2016;20:356–362. doi: 10.5213/inj.1632526.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brilhante RSN, Sales JA, Pereira VS, et al. Research advances on the multiple uses of Moringa oleifera: a sustainable alternative for socially neglected population. Asian Pac J Trop Med. 2017;10:621–630. doi: 10.1016/j.apjtm.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Farooq F, Rai M, Tiwari A, et al. Medicinal properties of Moringa oleifera: an overview of promising healer. J Med Plants Res. 2012;6:4368–4374. [Google Scholar]
- 7.Sreelatha S, Jeyachitra A, Padma PR. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol. 2011;49:1270–1275. doi: 10.1016/j.fct.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Coppin JP, Xu Y, Chen H, et al. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J Funct Foods. 2013;5:1892–1899. [Google Scholar]
- 9.Singh BN, Singh BR, Singh RL, et al. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem Toxicol. 2009;47:1109–1116. doi: 10.1016/j.fct.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Omodanisi EI, Aboua YG, Oguntibeju OO. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa Oleifera in diabetes-induced nephrotoxic male Wistar rats. Molecules. 2017;22:E439. doi: 10.3390/molecules22040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awodele O, Oreagba IA, Odoma S, et al. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). J Ethnopharmacol. 2012;39:330–336. doi: 10.1016/j.jep.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Spiridon L, Bodirlau R, Teaca CA. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Cent Eur J Biol. 2011;6:388–396. [Google Scholar]
- 13.Ishola IO, Akinyede AA, Robert AK, et al. Hepatoprotective and antioxidant activities of Hepacare®, a herbal formulation against carbon tetrachloride-induced liver injury. Drug Res (Stuttg) 2015;65:30–39. doi: 10.1055/s-0034-1371829. [DOI] [PubMed] [Google Scholar]
- 14.d'Ischia M, Novellino L. Nitric oxide-induced oxidation of alpha-tocopherol. Bioorg Med Chem. 1996;4:1747–1753. doi: 10.1016/0968-0896(96)00191-5. [DOI] [PubMed] [Google Scholar]
- 15.Oyaizu M. Studies on products of browning reactions: anti-oxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. [Google Scholar]
- 16.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 17.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 18.Winterbourn CC, Benatti U, De Flora A. Contributions of superoxide, hydrogen peroxide, and transition metal ions to auto-oxidation of the favism-inducing pyrimidine aglycone, divicine, and its reactions with haemoglobin. Biochem Pharmacol. 1986;35:2009–2015. doi: 10.1016/0006-2952(86)90734-3. [DOI] [PubMed] [Google Scholar]
- 19.Higashi T, Peters T., Jr Studies on rat liver catalase. I. Combined immunochemical and enzymatic determination of catalase in liver cell fractions. J Biol Chem. 1963;238:3945–3951. [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Kirschenbaum A, Klausner AP, Lee R, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56:671–676. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 22.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 23.Cam K. BPH: how useful is a visual prostate symptom score for patients? Nat Rev Urol. 2011;8:536–537. doi: 10.1038/nrurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 24.Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8:29–41. doi: 10.1038/nrurol.2010.207. [DOI] [PubMed] [Google Scholar]
- 25.Jhang JF, Jiang YH, Kuo HC. Adding Cyclooxygenase-2 inhibitor to alpha blocker for patients with benign prostate hyperplasia and elevated serum prostate specific antigen could not improve prostate biopsy detection rate but improve lower urinary tract symptoms. Int J Clin Pract. 2013;67:1327–1333. doi: 10.1111/ijcp.12220. [DOI] [PubMed] [Google Scholar]
- 26.Udensi UK, Tchounwou PB. Oxidative stress in prostate hyperplasia and carcinogenesis. J Exp Clin Cancer Res. 2016;35:139. doi: 10.1186/s13046-016-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minciullo PL, Inferrera A, Navarra M, et al. Oxidative stress in benign prostatic hyperplasia: a systematic review. Urol Int. 2015;94:249–254. doi: 10.1159/000366210. [DOI] [PubMed] [Google Scholar]
- 28.Aryal M, Pandeya A, Bas BK, et al. Oxidative stress in patients with benign prostate hyperplasia. JNMA J Nepal Med Assoc. 2007;46:103–106. [PubMed] [Google Scholar]
- 29.Leone A, Spada A, Battezzati A, et al. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Int J Mol Sci. 2015;16:12791–12835. doi: 10.3390/ijms160612791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma AR, Vijayakumar M, Mathela CS, et al. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47:2196–2201. doi: 10.1016/j.fct.2009.06.005. [DOI] [PubMed] [Google Scholar]


