Abstract
Background
Chronic kidney disease (CKD) and inflammation play critical roles in atherosclerosis. There is limited evidence regarding the relationship between CKD and patients receiving second-generation drug-eluting stents for coronary artery disease.
Objective
This study aimed to investigate the effect of CKD on cardiovascular and renal events in patients undergoing percutaneous coronary intervention (PCI) with everolimus-eluting stents (EES).
Methods
We analyzed 504 consecutive patients with stable angina pectoris and significant coronary artery stenosis treated with EES. CKD was defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2 before coronary angiography. The primary outcome was the occurrence of major adverse renal and cardiovascular events (MARCE) including cardiac death, revascularization, heart failure, cerebral infarction, worsening renal function > 25% from baseline, and renal replacement therapy at 1 year.
Results
Patients were divided into the a MARCE (n = 126) and a non-MARCE (n = 378) group. The incidence of CKD was 51% in all subjects (including those on hemodialysis) and was significantly higher in the MARCE group than in the non-MARCE group (p = 0.00001). Multivariate logistic regression analysis identified that CKD was independently associated with MARCE (adjusted odds ratio 2.03, 95% confidence interval 1.21–3.39, p = 0.007). Patients were divided into four groups based on CKD and C-reactive protein (CRP) level prior to initial coronary angiography. Cox proportional hazards analysis revealed that patients with CKD and high CRP (≥0.3 mg/dL) had the worst prognosis (hazard ratio 4.371, 95% confidence interval 2.634–7.252, p = 0.00001) compared to patients without CKD and with low CRP.
Conclusion
CKD combined with CRP predicted more clinical events in patients undergoing PCI with EES.
Keywords: Chronic kidney disease, Percutaneous coronary intervention, Everolimus-eluting stent, Cardiovascular and renal event, C-reactive protein
Introduction
First-generation drug-eluting stents (DES) have led to dramatic reductions in the rate of in-stent restenosis (ISR) compared with bare metal stents [1]. However, stent thrombosis and stent fracture with first-generation DES have emerged as significant hazards [2]. Furthermore, second-generation DES reduce the incidence of ISR and stent thrombosis due to their superior designs incorporating biocompatible polymers, thinner struts, and integrity of the stent with favorable mechanical properties [3]. Everolimus-eluting stents (EES) play a major role in the second-generation DES era. These stents are more efficacious and safe compared to the first-generation DES and show good results in patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI).
Chronic kidney disease (CKD) increases the mortality and adverse event rate in patients with cardiovascular disease along with the traditional risk factors such as hypertension, diabetes mellitus, dyslipidemia, and smoking. Recent epidemiological studies and clinical trials suggest that CKD is an independent risk factor for cardiovascular disease [4, 5, 6]. In a previous study [7], we reported the impact of CKD on the severity of CAD and prognosis in the first-generation DES era. However, only a few studies have been conducted on CKD patients treated with second-generation DES in real-world practice [8]. Inflammation has an important pathogenic link between atherosclerosis and CKD [9]. C-reactive protein (CRP) is reported to reflect inflammation and to be a biomarker for ischemic heart disease [10]; however, the combined impact of CKD and CRP on CAD has not been fully elucidated.
In the present study, we examined the impact of CKD on renal and cardiovascular events in patients undergoing PCI with EES in the second-generation DES era. With a view of the underlying pathogenesis, we studied the influence of CRP and CKD on renal and cardiovascular events.
Methods
Study Population
Between October 2010 and November 2012, we registered 2,141 consecutive patients undergoing coronary angiography at Fukuyama City Hospital, Fukuyama, Japan. Among them, 798 patients underwent a PCI procedure. We analyzed 504 consecutive patients (789 lesions) with stable angina pectoris and sig nificant coronary artery stenosis treated with EES who completed clinical follow-up. The flowchart of the present study is shown in online supplementary Figure S1 (for all online suppl. material, see www. karger.com/doi/10.1159/000486971). In this study, EES included XIENCE V (Abbot Vascular, Santa Clara, CA, USA) and PROMUS (Boston Scientific, Natick, MA, USA) stents. Patients with acute coronary syndrome (unstable angina and acute myocardial infarction), cardiogenic shock, valvular heart disease, or cardiomyopathy were excluded.
Protocol
Patients were divided into two groups according to the incidence of cardiovascular events. We compared the following variables between the two groups: traditional coronary risk factors, medical histories, CKD, laboratory findings, angiographic and PCI findings, and medications. The patients were clinically followed up 1 year after discharge. The outcome measure was the incidence of subsequent major adverse renal and cardiovascular events (MARCE). Follow-up evaluation of the coronary artery (such as coronary angiography) was scheduled approximately 8 months after PCI, regardless of clinical symptoms. In addition, we measured CRP to further delineate the pathophysiology of CKD and clinical events. Subjects with active infection or cancer were excluded.
Cardiac Catheterization and PCI Procedure
Significant stenosis was defined as > 50% luminal reduction in the left main trunk and > 75% luminal reduction in the left anterior descending, left circumflex, or right coronary artery. Most patients had a positive stress test and objective evidence of ischemia as seen on stress electrocardiography, cardiac scintigraphy, and fractional flow reserve at the initial PCI and at the time of revascularization. Each coronary angiogram was analyzed using the automated edge-detection system and/or by careful visual inspection by at least two cardiologists with expertise in coronary catheter intervention.
Stent implantation procedures were performed according to standard techniques. Intravascular ultrasound (IVUS) imaging was used for nearly all PCI procedures by Japanese operators. The recommended antiplatelet regimen was aspirin (100 mg/day) and thienopyridine (clopidogrel 75 mg/day or ticlopidine 200 mg/day) for 1 year after the PCI procedure. ISR was defined as diameter stenosis > 50% within the stent.
Blood Sampling
Blood samples were obtained from fasting patients early in the morning on the day of coronary angiography. The concentration of serum lipids was measured using automated enzymatic methods. The concentration of low-density lipoprotein cholesterol was calculated using the Friedewald equation [11]. Glycated hemoglobin was expressed in units as defined by the Japan Diabetic Society [12]. Serum creatinine and CRP were measured automatically using an enzyme assay in all patients before initial coronary angiography.
Definition of Risk Factors
Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or the current use of antihypertensive agents. Diabetes mellitus was defined as the presence of any of the following: fasting plasma glucose levels ≥126 mg/dL, random plasma glucose levels ≥200 mg/dL, or a history of treatment for diabetes mellitus. Dyslipidemia was defined as the use of lipid-lowering agents or meeting one or more of the following criteria from the first fasting blood sample: low-density lipoprotein cholesterol ≥140 mg/dL, triglycerides ≥150 mg/dL, or high-density lipoprotein cholesterol < 40 mg/dL. The estimated glomerular filtration rate (eGFR) was calculated using the equation from the Modification of Diet in Renal Disease Study Group, with coefficients modified for Japanese patients [13]: eGFR (mL/min/1.73 m2) = 194 × serum creatinine–1.094 × age–0.287 (× 0.739 if female). CKD was defined as an eGFR < 60 mL/min/1.73 m2. The upper normal limit of CRP was defined as 0.3 mg/dL.
Definition of MARCE
The outcome measure was the incidence of MARCE defined as one of the following conditions: cardiac death, revascularization due to de novo lesion or target lesion restenosis, and hospitalization because of heart failure, cerebral infarction, renal replacement therapy (new dialysis, renal transplant), and worsening renal function > 25% from baseline within 1 year after discharge. A de novo lesion was defined as acute myocardial infarction, unstable angina pectoris, or new stable coronary artery lesion.
Statistical Analysis
Continuous variables were compared using the unpaired Student t test or one-way analysis of variance. These data are presented as means ± standard deviations. Categorical variables were compared using either the χ2 or the Fisher exact test and are expressed as frequencies with percentages. Multivariate logistic regression analysis was used to detect associations between MARCE and various risk factors including CKD, traditional coronary risk factors (age, male sex, diabetes mellitus, hypertension, dyslipidemia, smoking), medications, brain natriuretic peptide (BNP), and CRP. MARCE event time was defined as the time between discharge from hospital after the procedure and the occurrence of the first MARCE. Cumulative MARCE-free survival rates were estimated using the Kaplan-Meier method and represented patients who did not experience MARCE over the 1-year follow-up period. Survival rates were compared among groups using the log-rank test. The association between MARCE and CKD and CRP was assessed using the Cox proportional hazards model. Group differences associated with a p value < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS 20 for Windows (SPSS, Chicago, IL, USA).
Results
Factors Influencing Cardiovascular and Renal Events
Patient characteristics, laboratory data, and angiographic findings are summarized in Table 1. In the MARCE group, the prevalence of CKD, diabetes mellitus, beta-blocker therapy, and raised low-density lipoprotein, BNP, and CRP was significantly higher, and the prevalence of angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) therapy was significantly lower compared to the non-MARCE group. The angiographic and PCI findings did not differ between patients with versus without MARCE.
Table 1.
Patient characteristics, laboratory data, and angiographic findings
| Total (n = 504) | Non-MARCE (n = 378) | MARCE (n = 126) | p value | |
|---|---|---|---|---|
| Age, years | 72±9 | 72±9 | 72±8 | 0.68 |
| Male sex | 74% | 74% | 76% | 0.59 |
| Body mass index | 23.6±3.2 | 23.9±3.1 | 22.9±3.5 | 0.24 |
| Smoking | 43% | 44% | 42% | 0.76 |
| Medical history | ||||
| Previous myocardial infarction | 34% | 32% | 42% | 0.31 |
| Previous PCI | 53% | 51% | 61% | 0.33 |
| Previous coronary bypass | 5.2% | 5.6% | 3.6% | 0.67 |
| Previous pacemaker | 1.5% | 1.9% | 0% | 0.47 |
| Hypertension | 79% | 80% | 76% | 0.32 |
| Dyslipidemia | 77% | 77% | 78% | 0.89 |
| Diabetes mellitus | 52% | 48% | 63% | 0.005 |
| Chronic kidney disease | 51% | 45% | 68% | 0.00001 |
| Serum creatinine, mg/dL | 1.39±1.68 | 1.19±1.29 | 1.98±2.41 | 0.00001 |
| eGFR, mL/min/1.73 m2 | 56.7±22.0 | 59.4±20.3 | 48.5±24.8 | 0.00001 |
| eGFR ≥60 | 49% | 54% | 31% | |
| 30 ≤ eGFR < 60 | 39% | 40% | 39% | |
| eGFR <30 | 12% | 6% | 30% | |
| Hemodialysis | 7.7% | 4.8% | 16.7% | |
| LDL-C, mg/dL | 106±35 | 103±33 | 114±37 | 0.002 |
| HDL-C, mg/dL | 46±12 | 46±12 | 44±13 | 0.05 |
| LDL-C/HDL-C ratio | 2.4±1.0 | 2.4±0.9 | 2.6±1.0 | 0.11 |
| Glycated hemoglobin, % | 6.1±1.3 | 6.2±1.3 | 6.3±1.4 | 0.23 |
| Brain natriuretic peptide, pg/dL | 204±367 | 168±299 | 310±503 | 0.0007 |
| Left ventricular ejection fraction, % | 60.7±11.9 | 61.6±11.8 | 57.1±12.1 | 0.08 |
| C-reactive protein, mg/dL | 0.4±0.86 | 0.29±0.58 | 0.77±1.33 | 0.00001 |
| Medications | ||||
| Aspirin | 99.6% | 99.4% | 100% | 0.41 |
| ACEI/ARB | 57% | 60% | 47% | 0.01 |
| Statin | 80% | 81% | 76% | 0.27 |
| Beta-blocker | 50% | 46% | 63% | 0.001 |
| Anti-diabetes drug | 41% | 39% | 46% | 0.14 |
| Main physiological method for ischemia detection | ||||
| Stress echocardiogram | 43.0% | 43.9% | 39.3% | 0.66 |
| Stress cardiac scintigram | 25.2% | 22.4% | 35.7% | 0.15 |
| Fractional flow reserve | 9.6% | 10.3% | 7.1% | 0.61 |
| Lesion and PCI findings | ||||
| Lesion length, mm | 17.1±9.4 | 17.3±9.0 | 16.4±9.6 | 0.39 |
| Postprocedural MLD, mm | 2.36±0.62 | 2.41±0.63 | 2.27±0.66 | 0.07 |
| AHA A/B1/B2/C | 6/22/60/12% | 4/23/60/13% | 11/17/63/9% | 0.48 |
| LAD/LCX/RCA/LMT | 45/13/37/5% | 44/14/38/4% | 49/13/32/6% | 0.87 |
| Chronic total occlusion | 6% | 6% | 7% | 0.76 |
| Bifurcation technique | 13% | 13% | 14% | 0.86 |
| IVUS use | 99.3% | 99.1% | 100% | 0.61 |
Data given as mean ± standard deviation or percentage. ACEI, angiotensin-converting enzyme inhibitor; AHA, American Heart Association; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex coronary artery; LDL-C, low-density lipoprotein cholesterol; LMT, left main trunk; MARCE, major adverse renal and cardiovascular events; MLD, minimal lumen diameter; PCI, percutaneous coronary intervention; RCA, right coronary artery.
Renal Insufficiency and Cardiovascular Events
MARCE was identified in 25.0% of patients (n = 126) (online suppl. Table S1). Death, revascularization, hospitalization because of heart failure, cerebral infarction, renal replace ment therapy (new dialysis, renal transplant), and worsening renal function > 25% from baseline occurred in 10, 80, 11, 9, 0, and 16 patients, respectively. Among patients undergoing revascularization, de novo lesions and ISR were present in 53 and 27 patients, respectively.
The risk factors associated with MARCE were analyzed by multivariate logistic analysis. As shown in Table 2, CKD (adjusted odds ratio [OR] 2.03, 95% confidence interval [CI] 1.21–3.39, p = 0.007), ACEI/ARB therapy (adjusted OR 0.58, 95% CI 0.36–0.95, p = 0.03), and CRP (≥0.3 mg/dL) (adjusted OR 2.36, 95% CI 1.40–3.98, p = 0.001) were independently associated with MARCE. CKD, non-ACEI/ARB therapy, and CRP predicted an increased risk of MARCE. Kaplan-Meier curves that illustrate the percentage of MARCE-free patients over time during the first year after treatment are shown in Figure 1a. Patients with CKD had a significantly higher prevalence of cumulative MARCE compared to patients without CKD (p < 0.01).
Table 2.
Risk factors associated with MARCE
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age | 1.01 | 0.98–1.03 | 0.68 | - | ||
| Male sex | 1.14 | 0.71–1.82 | 0.60 | - | ||
| Hypertension | 0.78 | 0.48–1.27 | 0.32 | - | ||
| Dyslipidemia | 1.03 | 0.64–1.68 | 0.89 | - | ||
| Diabetes mellitus | 1.81 | 1.19–0.75 | 0.005 | 1.35 | 0.83–2.22 | 0.23 |
| Smoking | 0.93 | 0.59–1.47 | 0.75 | - | ||
| Chronic kidney disease | 2.51 | 1.64–3.84 | 0.00002 | 2.03 | 1.21–3.39 | 0.007 |
| ACEI/ARB | 0.58 | 0.39–0.89 | 0.01 | 0.58 | 0.36–0.95 | 0.03 |
| Statin | 0.76 | 0.46–1.24 | 0.27 | - | ||
| Beta-blocker | 1.99 | 1.31–3.05 | 0.001 | 1.82 | 1.11–2.99 | 0.02 |
| Anti-diabetes drug | 1.37 | 0.90–2.07 | 0.14 | - | ||
| LDL-C >106 mg/dL | 1.50 | 0.99–2.26 | 0.06 | - | ||
| BNP >204 pg/dL | 1.98 | 1.19–3.29 | 0.008 | 1.34 | 0.76–2.33 | 0.31 |
| CRP ≥0.3 mg/dL | 2.54 | 1.66–3.88 | 0.00002 | 2.36 | 1.40–3.98 | 0.001 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; CI, confidence interval; CRP, C-reactive protein; LDL-C, low-density lipoprotein cholesterol; MARCE, major adverse renal and cardiovascular events; OR, odds ratio.
Fig. 1.
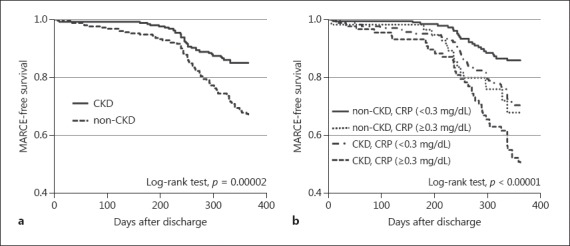
Kaplan-Meier curves for patients with and without CKD (a) and a combination of CKD and CRP (b) regarding MARCE. CKD, chronic kidney disease; CRP, C-reactive protein; MARCE, major adverse renal and cardiovascular events.
Further analysis with CRP level was performed to evaluate the pathophysiology underlying the relationship between renal insufficiency and cardiovascular events. We divided all patients into four groups: non-CKD and low CRP (< 0.3 mg/dL), CKD and low CRP, non-CKD and high CRP (≥0.3 mg/dL), and CKD and high CRP. The hazard ratios for MARCE were 2.263 (95% CI 1.379–3.714, p = 0.001) in the CKD and low CRP group and 4.371 (95% CI 2.634–7.252, p = 0.00001) in the CKD and high CRP group compared to the non-CKD and low CRP group (Table 3). The Kaplan-Meier curves and log-rank analysis showed the same result (Fig. 1b).
Table 3.
Impact of the combination of CKD and CRP in the prediction of MARCE
| HR | 95% CI | p value | |
|---|---|---|---|
| Non-CKD, CRP <0.3 mg/dL Non-CKD, CRP ≥0.3 mg/dL |
1 2.566 |
1.378–4.776 | 0.003 |
| CKD, CRP <0.3 mg/dL | 2.263 | 1.379–4.714 | 0.001 |
| CKD, CRP ≥0.3 mg/dL | 4.371 | 2.634–7.252 | 0.00001 |
CI, confidence interval; CKD, chronic kidney disease; CRP, C-reactive protein; HR, hazard ratio; MARCE, major adverse renal and cardiovascular events.
De novo lesions were identified in 10.5% of patients as the most frequent clinical event. The incidence of CKD and beta-blocker therapy as well as BNP values was significantly higher among patients with de novo lesions than among those without (online suppl. Table S2). As shown in online supplementary Table S3, multivariate logistic regression analysis indicated that CKD was independently associated with de novo lesions (adjusted OR 2.30, 95% CI 1.20–4.39, p = 0.01). Furthermore, the ratio of ISR using EES was 3.8% with IVUS guidance. This rate was not different in patients with versus without CKD. Stent thrombosis was not identified in this 1-year follow-up study, which included some patients (7.7% of the study population) with hemodialysis.
Discussion
The main findings of the present study are as follows: (1) CKD was independently associated with cumulative MARCE, especially de novo lesions, in patients with stable angina pectoris using EES, and (2) in patients with CKD, risk was stratified according to the CRP level prior to coronary angiography.
In the present study, the risk ratio of MARCE in patients with CKD was twofold higher than that in patients without CKD. These results suggest that CKD is an independent risk factor for cardiovascular events and that CKD adversely affects the outcomes of patients treated with EES for stable angina pectoris. The incidence of CKD in this study was 51%, which was higher than the 46% incidence cited in our previous report conducted in the first-generation DES era [7]; however, this difference was not statistically significant. The prevalence of CKD in this study was higher than that reported in previous studies on CKD. We believe that our results will contribute to the real-world clinical management of CKD patients. There was a trend towards a lower prevalence of hypertension in the MARCE group, and the reduced use of ACEI/ARB therapy might lead to cardiovascular events. The same pattern was found in the analysis of de novo lesions. Some studies revealed that ARB therapy resulted in regression of plaque volume [14]. Thus, sufficient ACEI/ARB therapy may prevent the development of de novo lesions as the most frequent cardiovascular event.
The mechanisms that underlie the association between renal insufficiency and CAD have not been fully elucidated. Previous studies showed that renal insufficiency is associated with low-grade inflammation, oxidative stress, activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system, and bone mineral disorder [9, 15]. As such, CKD modification of these factors could also contribute to the pathogenesis of atherosclerosis. In our study, renal insufficiency was associated with a higher CRP value. This results in pro gressive endothelial dysfunction, atherosclerosis, and more cardiovascular events. A prior study showed that CRP was related to the presence of CAD and clinical outcomes [16]. In addition, invasive coronary imaging studies have reported the vulnerability of target and non-target vessel plaque in patients with CKD [17, 18]. These results stress the clinical implication of the relation between CKD and CRP and the importance of optimizing the medical therapy for CKD patients [19]. Taken together, our findings suggest that the combination of CKD and CRP may have clinical value in the secondary prevention of CAD.
EES provided good results in this study. The ISR rate was 3.8% despite the prevalence of CKD. This is a better outcome compared to first-generation DES [2]. Moreover, there was no patient with stent thrombosis. Lee et al. [20] reported the prognosis of CKD patients after second-generation DES PCI in acute coronary syndrome and stable angina pectoris, and despite a lower proportion of patients with complex lesions compared to the current study, the ISR rate at 1 year was similar to that reported herein, even though frequent IVUS monitoring was not performed. Our study showed that EES with IVUS guidance had good efficacy and safety for the treatment of stable coronary artery lesions in patients with CKD. It revealed that CKD is an independent risk factor in patients with CAD in the latest DES era. A prospective interventional trial to evaluate whether imaging devices detect new coronary artery lesions in patients with CKD, as well as studies to evaluate outcomes following intensive treatments for CKD, are warranted.
The present study was subject to the following limitations. First, we performed a single-center, retrospective, observational study without a long follow-up period. This may have affected the impact of renal function on outcomes. Second, some patients with CKD were categorized based on a single eGFR measurement. This eGFR value was derived from a single serum creatinine level measured on the day of coronary angiography. This creatinine value may have been influenced by medication or acute clinical status. Third, we did not collect data concerning the course of renal function in these patients, either before or after angiography. There is a common tendency to refrain from coronary angiography, which can decrease eGFR. As such, we could not address the influence of these factors on outcomes. Fourth, we included some patients with end-stage renal disease who required dialysis. The data for these patients may have affected the relationships between risk factors and health outcomes. Fifth, there were no data on the prevalence of proteinuria among our CKD patients.
Conclusion
CKD is independently associated with cardiovascular and renal events in patients undergoing PCI with EES. In patients with CKD, the risk of cardiovascular and renal events increased if the baseline CRP level prior to the initial coronary angiography was higher than the upper normal limit.
Statement of Ethics
Informed consent for study participation was obtained from each patient in accordance with the Helsinki declaration. The study was approved by the Institutional Ethics Committee.
Disclosure Statement
None of the authors have any conflicts of interest to declare.
Supplementary Material
Supplementary data
References
- 1.Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 2.Kimura T, Morimoto T, Nakagawa Y, et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation. 2012;125:584–591. doi: 10.1161/CIRCULATIONAHA.111.046599. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Midei M, Newman W, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299:1903–1913. doi: 10.1001/jama.299.16.1903. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 6.Papachristidis A, Lim WY, Voukalis C, et al. Determinants of mortality in patients with chronic kidney disease undergoing percutaneous coronary intervention. Cardiorenal Med. 2016;6:169–179. doi: 10.1159/000442897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dan K, Miyoshi T, Ueeda M, et al. Impact of chronic kidney disease on left main coronary artery disease and prognosis in Japanese patients. Circ J. 2012;76:2266–2272. doi: 10.1253/circj.cj-11-1455. [DOI] [PubMed] [Google Scholar]
- 8.Wańha W, Kawecki D, Roleder T, et al. Long-term percutaneous coronary intervention outcomes of patients with chronic kidney disease in the era of second-generation drug-eluting stents. Cardiorenal Med. 2017;7:85–95. doi: 10.1159/000452745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Bottiglieri T, McCullough PA. The central role of endothelial dysfunction in cardiorenal syndrome. Cardiorenal Med. 2017;7:104–117. doi: 10.1159/000452283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zebrack JS, Muhlestein JB, Horne BD, et al. C-reactive protein and angiographic coronary artery disease: independent and additive predictors of risk in subjects with angina. J Am Coll Cardiol. 2002;39:632–637. doi: 10.1016/s0735-1097(01)01804-6. [DOI] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Geistanger A, Arends S, Berding C, et al. Statistical methods for monitoring the relationship between the IFCC reference measurement procedure for hemoglobin A1c and the designated comparison methods in the United States, Japan, and Sweden. Clin Chem. 2008;54:1379–1385. doi: 10.1373/clinchem.2008.103556. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 14.Hirohata A, Yamamoto K, Miyoshi T, et al. Impact of olmesartan on progression of coronary atherosclerosis: a serial volumetric intravascular ultrasound analysis from the OLIVUS (impact of olmesartan on progression of coronary atherosclerosis: evaluation by intravascular ultrasound) trial. J Am Coll Cardiol. 2010;55:976–982. doi: 10.1016/j.jacc.2009.09.062. [DOI] [PubMed] [Google Scholar]
- 15.McCullough PA. Why is chronic kidney disease the “spoiler” for cardiovascular outcomes? J Am Coll Cardiol. 2003;41:725–728. doi: 10.1016/s0735-1097(02)02955-8. [DOI] [PubMed] [Google Scholar]
- 16.Bruke AP, Tracy RP, Kolodgie F, et al. C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105:2019–2023. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 17.Miyagi M, Ishii H, Murakami R, et al. Impact of renal function on coronary plaque composition. Nephrol Dial Transplant. 2010;25:175–181. doi: 10.1093/ndt/gfp423. [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Yonetsu T, Jia H, et al. Nonculprit coronary plaque characteristics of chronic kidney disease. Circ Cardiovasc Imaging. 2013;6:448–456. doi: 10.1161/CIRCIMAGING.112.000165. [DOI] [PubMed] [Google Scholar]
- 19.Rozenbaum Z, Benchetrit S, Minha S, et al. The effect of admission renal function on the treatment and outcome of patients with acute coronary syndrome. Cardiorenal Med. 2017;7:169–178. doi: 10.1159/000455239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JM, Kang J, Lee E, et al. Chronic kidney disease in the second-generation drug-eluting stent era: pooled analysis of the Korean Multicenter Drug-Eluting Stent Registry. JACC Cardiovasc Interv. 2016;9:2097–2109. doi: 10.1016/j.jcin.2016.06.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


