Abstract
Antiplatelet therapy is a key component in the treatment of acute coronary syndrome (ACS). The management of ACS has evolved considerably over recent years with the development of new and more potent antiplatelet agents. Clinical trials on ACS have demonstrated that potent antiplatelet agents can more effectively reduce cardiovascular events. However, there is a tipping point between safety and efficacy, beyond which the risk of bleeding and other adverse effects can outweigh the benefits of antiplatelet therapy. Striking a balance between safety and efficacy remains a major challenge. A consensus meeting of an expert panel composed of Taiwanese experts was held to provide recommendations for the management of adverse effects in patients with ACS receiving antiplatelet therapy. The common adverse effects of antiplatelet therapy include upper gastrointestinal bleeding, ecchymosis, hematuria, epistaxis and ticagrelor-related dyspnea. In this study, a literature review of these adverse events was performed and recommendations for the management were made.
Keywords: Acute coronary syndrome, Antiplatelet therapy, Expert consensus, Guidance
INTRODUCTION
Acute coronary syndrome (ACS) occurs when coronary atherosclerotic plaques rupture or erode, triggering a cascade of events that lead to platelet aggregation and thrombin generation, both of which play pivotal roles in atherothrombosis.1 After adhering to subendothelial collagen, platelets become activated and release adenosine diphosphate (ADP) from dense granules. Binding of ADP to the G protein-coupled P2Y12 receptor on the surface of platelets activates complex signal transduction processes, resulting in the activation of glycoprotein (GP) IIb/IIIa receptors which bind to fibrinogen and form bridges between adjacent activated platelets to establish firm platelet aggregation.2 P2Y12 receptor activation is not only essential in the aggregation of platelets, it also mediates thromboxane A2 production and P-selectin expression in activated platelets.3 In addition to the release of ADP from dense granules, activated platelets also release α-granule contents including fibrinogen, von Willebrand factor, and other adhesive proteins and proinflammatory cytokines. Platelets contribute to the thrombogenic and inflammatory states in ACS.
P2Y12 inhibitors, such as ticagrelor and clopidogrel, inhibit ADP-mediated pathways to reduce the platelet release of granule contents, prevent GPIIb/IIIa receptor activation, and reduce platelet aggregation. In addition to inhibiting platelet aggregation, ticagrelor and clopidogrel have been shown to reduce lipopolysaccharide-induced platelet-monocyte aggregate formation and inflammatory cytokine production in humans, demonstrating the mechanism by which inhibition of the platelet P2Y12 receptor also reduces systemic inflammation.4 There is substantial evidence showing that inflammation is a critical factor in the pathogenesis of ACS. Levels of the inflammatory biomarker, C-reactive protein, are increased and have prognostic value in ACS.5 Laboratory observations on human coronary atherosclerotic plaques indicate that inflammation is a specific mechanism responsible for plaque fragility and subsequent rupture.6 A previous study used intravascular ultrasound to observe all three major coronary arteries in ACS patients. The study found that 79% of the patients with ACS had at least one additional plaque rupture beyond the culprit lesion, and 12.5% of the patients had one or more plaque ruptures in all three of the major coronary arteries.7 The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study was conducted to identify lesion-related factors that predict the risk of recurrent adverse cardiac events after ACS.8 The results showed that the risk of recurrent cardiac events was highest during the first 1 to 1.5 years after discharge of the index ACS event. The accumulative recurrent cardiac event rate at 3 years was 20.4%, and nearly half of these events were related to non-culprit lesions found at the index ACS admission. These non-culprit lesions that were responsible for recurrent events were frequently angiographically mild and most were thin-cap fibroatheromas. These data suggest that the use of dual antiplatelet therapy (DAPT) with potent antiplatelet agents in ACS can be used for both the stented culprit lesion and also to cover non-culprit unstable plaques.
Aspirin and clopidogrel have been the mainstay of treatment for ACS for many years. However, a substantial number of ACS patients continue to experience recurrent ischemic events during DAPT with aspirin and clopidogrel. It has been reported that the platelet inhibitory effect of clopidogrel is diminished in patients who carry a genetic variant, loss-of-function CYP2C19 allele, due to significantly lower levels of the active metabolite of clopidogrel.9 A higher rate of major adverse cardiovascular events compared with noncarriers was also observed.9 This has led to dedicated efforts to develop more potent antiplatelet agents for ACS. Currently, a new generation P2Y12 inhibitors including ticagrelor and prasugrel have replaced clopidogrel as first-line antiplatelet agents for ACS treatment. However, the risks of bleeding and other adverse effects have been reported with more potent antiplatelet agents, especially in elderly patients.10 In-hospital bleeding is also a risk factor for poor clinical outcomes for ACS patients.11 A consensus meeting of an expert panel was held in Taiwan to provide recommendations and guidance on the management of adverse effects in patients with ACS receiving these antiplatelet therapies. The common adverse effects include upper gastrointestinal (GI) bleeding, ecchymosis, hematuria, epistaxis and ticagrelor-related dyspnea. In this study, a literature review of these events during antiplatelet therapy was performed and recommendations for management were provided. Because ticlopidine, cilostazol and dipyridamole are rarely used in ACS patients, their adverse effects were not discussed in the consensus meeting.
ADVERSE EFFECTS OF ANTIPLATELET THERAPY FOR ACS
Currently, DAPT with aspirin and P2Y12 inhibitors is recommended in international and local guidelines as first-line therapy for up to 1 year following ACS.12-14 In the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) study, DAPT with aspirin plus clopidogrel was compared with aspirin monotherapy in patients with ACS. Although the primary composite outcome of cardiovascular death, nonfatal myocardial infarction, or stroke was significantly reduced with DAPT, more major and minor bleeding events were also noted in the DAPT group.15 The Platelet Inhibition and Patient Outcomes (PLATO) trial compared DAPT with aspirin plus ticagrelor and aspirin plus clopidogrel in patients with ACS with or without ST-segment elevation. The results showed that ticagrelor was associated with a reduction in the primary composite outcome and stent thrombosis compared with clopidogrel. However, non-coronary artery bypass grafting-related major bleeding and dyspnea occurred more frequently in the ticagrelor group than in the clopidogrel group.16
The most common adverse effect associated with antiplatelet agents is bleeding, which commonly occurs in the GI tract, nose, urinary tract, subcutaneous/dermal tissue or at puncture or surgical sites. Compared with a placebo, aspirin itself is associated with a higher risk of bleeding which commonly occurs in the GI tract. Concerns exist regarding the risks of neutropenia and thrombocytopenia in association with ticlopidine therapy.17 Currently, ticlopidine is used only if patients are allergic to clopidogrel and other newer P2Y12 inhibitors. An association between ticagrelor and dyspnea was observed in the PLATO trial.16 More frequent incidents of dyspnea were observed in patients receiving ticagrelor compared to those taking clopidogrel, and 0.9% of the patients discontinued the drug because of dyspnea. In the ONSET/OFFSET trial, 123 aspirin-treated patients with stable coronary artery disease were randomized to receive either ticagrelor, clopidogrel, or placebo for 6 weeks following a double-blind, double-dummy design, and dyspnea was observed among 38.6%, 9.3%, and 8.3% of the patients in the ticagrelor, clopidogrel, and placebo groups, respectively.18
UPPER GI BLEEDING IN PATIENTS WITH ANTIPLATELET THERAPY
Aspirin monotherapy per se is associated with a wide spectrum of adverse GI side effects including ulcers, bleeding, and perforation. The risk of GI complaints or GI bleeding has been reported to be higher with aspirin than with clopidogrel.19 GI bleeding has also been reported to be more frequent in patients receiving DAPT than antiplatelet monotherapy.15 The PLATO study provides a valuable opportunity to directly compare the risk of GI bleeding among DAPT with ticagrelor vs. clopidogrel.16 Ticagrelor was reported to demonstrate a potent and faster onset of antiplatelet effect than clopidogrel, contributing significantly to reduced adverse cardiovascular events in ACS. Overall, the ticagrelor and clopidogrel groups did not differ significantly regarding the risk of major bleeding (11.6% and 11.2%, respectively). However, non-procedure-related bleeding was more common with ticagrelor than with clopidogrel (Table 1).20 The most common location of non-procedure-related bleeding was the GI tract. GI bleeding accounted for one-third of all bleeding events and occurred in 31.5% and 36.9% of the ticagrelor and clopidogrel groups, respectively.
Table 1. Primary location of non-procedure-related bleeding in the PLATO study.
| Ticagrelor (n = 9,235) | Clopidogrel (n = 9,186) | |
| Any spontaneous major bleeding | ||
| Patients | 235 | 180 |
| Events | 664 | 483 |
| Primary location of bleeding, numbers of events (%) | ||
| Gastrointestinal | 209 (31.5) | 178 (36.9) |
| Epistaxis | 133 (20.0) | 70 (14.5) |
| Urinary | 58 (8.7) | 54 (11.2) |
| Subcutaneous/dermal | 64 (9.6) | 44 (9.1) |
| Intracranial | 36 (5.4) | 18 (3.7) |
| Pericardial | 15 (2.3) | 15 (3.1) |
| Hemoptysis | 16 (2.4) | 11 (2.3) |
| Intraocular | 3 (0.5) | 4 (0.8) |
| Retroperitoneal | 4 (0.6) | 3 (0.6) |
| Intra-articular | 2 (0.3) | 1 (0.2) |
| Cardiac catheterization/PCI access site | 2 (0.3) | 0 (0.0) |
| Other bleeding site | 122 (18.4) | 85 (17.6) |
Adapted and modified from reference 20. PCI, percutaneous coronary intervention.
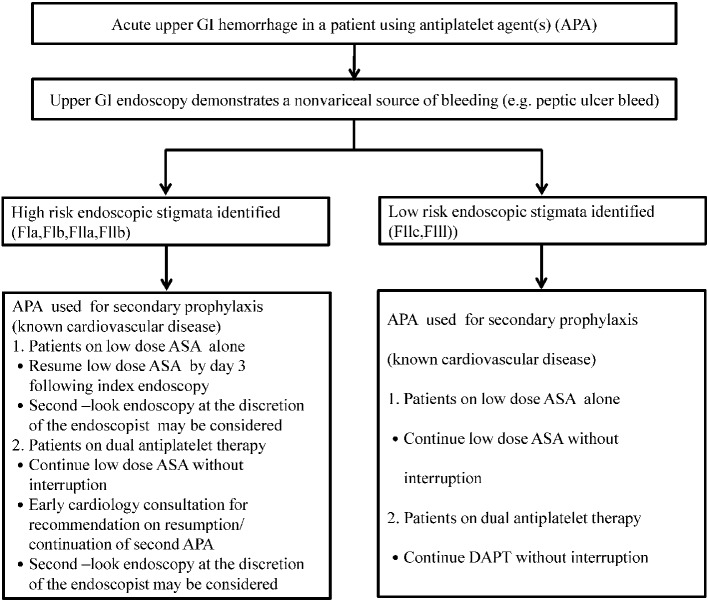
The European Society of Gastrointestinal Endoscopy (ESGE) guidelines on the management of non-variceal upper GI bleeding makes recommendations for the step-wise care of patients with acute upper GI bleeding.21 First, hemodynamic status is assessed and resuscitation initiated as needed. Second, patients are risk-stratified based on features such as hemodynamic status, comorbidities, and age. Endoscopy is typically performed within 24 hours with further management depending on the endoscopic examination results. Intravenous proton pump inhibitors (PPIs) with a bolus followed by continuous infusion are administered. Finally, in patients with clinical evidence of rebleeding following successful initial endoscopic hemostasis, the guidelines recommend repeat endoscopy with hemostasis if indicated, while surgery should be considered if the second attempt at hemostasis fails.21 In peptic ulcer bleeding, the Forrest classification has been proven to be useful in predicting the risk of rebleeding and mortality. The Forrest classification is defined as follows: FIa spurting hemorrhage, FIb oozing hemorrhage, FIIa nonbleeding visible vessel, FIIb an adherent clot, FIIc flat pigmented spot, and FIII clean base ulcer. Figure 1 shows the algorithm for the management of patients with acute upper GI bleeding who are under antiplatelet treatment. The scientific evidence of the suggestions in Figure 1 were described in the ESGE guidelines.21 Currently, the prophylactic use of PPIs is suggested in patients who need DAPT but are at risk of GI bleeding. Previous studies have suggested that pantoprazole and rabeprazole have the lowest drug-drug interactions with clopidogrel.22 No interactions between the concomitant use of PPIs and prasugrel or ticagrelor have been reported.
Figure 1.
The algorithm for the management of patients with acute upper GI bleeding who are under antiplatelet treatment. Adapted and modified from reference 21. APA, antiplatelet agent; ASA, aspirin; DAPT, dual antiplatelet therapy; GI, gastrointestinal.
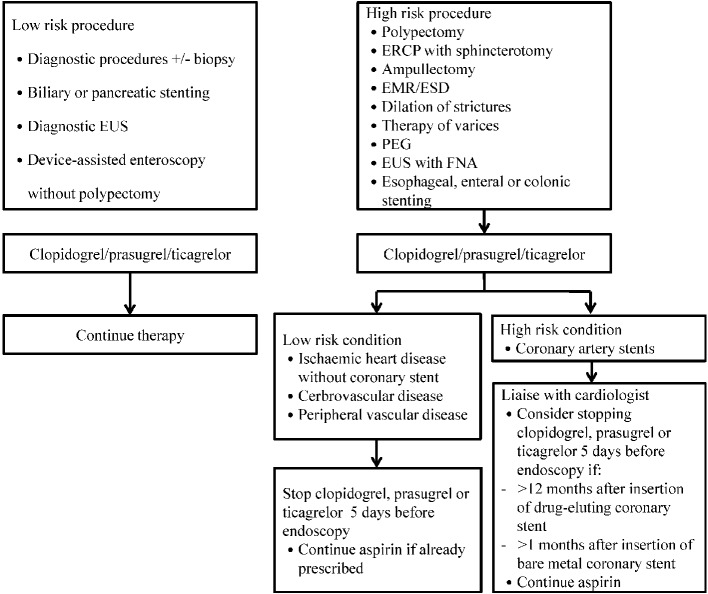
Recommendations for the management of patients on antiplatelet therapy undergoing low-risk or high-risk elective endoscopic procedures are outlined in the British Society of Gastroenterology/ESGE guidelines.23 For low-risk endoscopic procedures, continuing P2Y12 inhibitors as single or DAPT is recommended. For high-risk procedures, patients with a low risk of thrombosis are advised to discontinue P2Y12 inhibitors 5 days before the procedure, whereas patients on DAPT should continue taking aspirin. Patients at high thrombotic risk should remain on aspirin and consult a cardiologist regarding the risk/benefits of discontinuing P2Y12 inhibitors. Lastly, antiplatelet therapy, if discontinued, should be resumed 48 hours after the procedure, depending on the bleeding and thrombotic risks.23 Figure 2 shows the algorithm for the management of patients who are under antiplatelet treatment and need elective endoscopic procedures.
Figure 2.
The algorithm for the management of patients who are under antiplatelet treatment and need elective endoscopic procedures. Adapted and modified from reference 23. EMR, endoscopic mucosal resection; ERCP, endoscopic retrograde cholangiopancreatography; ESD, endoscopic submucosal dissection; EUS, endoscopic ultrasound; FNA, fine needle aspiration; PEG, percutaneous endoscopic gastrostomy.
In conclusion, upper GI bleeding is a common adverse effect of DAPT. Prior to receiving DAPT, patients at high risk of GI bleeding can take oral PPIs to reduce the risk of bleeding. In the event of GI complications, a high dose of PPIs should be used and referral to a gastroenterologist should be made. When discontinuing DAPT is necessary in severe GI bleeding, the treatment should be resumed as early as possible following endoscopic procedures.
ECCHYMOSIS IN PATIENTS WITH ANTIPLATELET THERAPY
The development of purpura has been reported in patients taking antiplatelet agents. When skin lesions occur, diascopy (glass test) is widely performed for the initial diagnosis. The test is performed by applying pressure with a finger or glass slide and observing color changes on the skin. This test is used to determine whether a lesion is vascular (inflammatory or congenital), nonvascular (nevus), or hemorrhagic (purpura). Purpura does not blanch on pressure. Purpura may be palpable or nonpalpable, with the latter presenting as flat/macular lesions that exhibit two types of morphologies, namely, petechiae (lesions < 3 mm) and ecchymoses (lesions > 5 mm). As a general rule, the development of nonpalpable purpura indicates a problem in the platelet system, blood vessel, or skin tissue. Nonpalpable purpura in patients with ACS is likely attributable to antiplatelet or anticoagulant therapy, but it is crucial to consider all other clinically plausible causes of this condition before attributing it to the drug itself. For example, dengue fever, which is prevalent in Taiwan, may be a possible cause. Elderly patients undergoing prolonged steroid therapy which inhibits the regeneration of dermal connective tissues resulting in bleeding vessels should also be considered.24 Actinic purpura or senile purpura is also a nonpalpable purpura commonly seen in the elderly. It is characterized by ecchymoses on sun-exposed areas of the skin that usually last for 1-3 weeks and disappear spontaneously while other new lesions continue to develop.
Severe cases of purpura associated with antiplatelet or anticoagulant therapy are characterized by purpura in non-exposed parts of the body and the mucous membrane that rapidly lead to skin necrosis and signs of inflammation. Ticagrelor, a new agent now preferred to clopidogrel as treatment for ACS, has not yet been associated with severe skin reactions such as Stevens-Johnson syndrome or skin necrosis, whereas warfarin has been reported to induce skin necrosis in patients under warfarin treatment with or without concomitant heparin.25 In patients with purpura, a risk assessment of major bleeding should be done to rule out its association with infection, inflammation, or other clinical factors. The severity of drug-induced purpura should be assessed based on clinical judgment and the patients’ condition to determine subsequent measures including a transient dose reduction or discontinuation of the drug. In general, education aimed at facilitating the continuation of DAPT for as long as possible is necessary. Skin lesions have a great impact on the patient’s perceptions and concerns regarding the safety of the medication, and may interfere with the continuous use of DAPT. When skin lesions occur in DAPT-treated patients, it is advisable to consult a dermatologist immediately and provide all necessary information to ensure patient adherence for the entire course of 12-month DAPT to prevent recurrent cardiovascular events.
HEMATURIA IN PATIENTS WITH ANTIPLATELET THERAPY
Blood in the urine (hematuria) can originate from any site along the urinary tract and it may be a sign of underlying disease of the urinary system. Patients on DAPT with signs of hematuria need further investigation and should not be attributed to the medication only. Macroscopic (or gross) hematuria is prevalent in older patients and warrants a thorough diagnostic evaluation to determine its cause.26 Infection, stones, and, in older patients, urinary tract malignancies are potential causes of macroscopic hematuria. The common causes of microscopic hematuria include glomerulonephropathy (16%; of which IgA nephropathy is the most common cause) and cancer (5%; of which bladder or prostate cancer are the most common).27 In contrast to gross hematuria, microscopic hematuria is often deemed to be an "insignificant" disease requiring no treatment. It is, however, strongly recommended to assess the risk factors of patients with microscopic hematuria (Table 2) for significant underlying diseases, because such assessments will often determine the cause of the hematuria.28
Table 2. Risk factors for significant disease in patients with microscopic hematuria.
| Smoking history |
| Occupational exposure to chemicals or dyes (benzenes or aromatic amines) |
| History of gross hematuria |
| Age > 40 yr |
| Previous urologic history |
| History of irritative voiding symptoms |
| History of urinary tract infection |
| Analgesic abuse |
| History of pelvic irradiation |
Adapted from reference 28.
Hematuria can be measured quantitatively by a simple dipstick test, but initial findings of microscopic hematuria using the dipstick method should be confirmed by microscopic evaluation. Patients showing the presence of significant proteinuria, red cell casts, or a predominance of dysmorphic red blood cells in the urine should receive an evaluation for renal parenchymal disease or be referred to a nephrologist.29 The time at which blood in the urine appears during urination also provides information of the potential problem. For example, blood in the initial flow of the urine is indicative of a problem in the lower urinary tract, whereas blood appearing later in the release of urine is suggestive of a problem in the upper urinary tract, and a bladder problem is indicated if blood is observed during the entire process of urination. Hematuria is rarely attributable to antiplatelet therapy only. Appropriate evaluation of patients with gross or microscopic hematuria should be performed to identify the actual cause. Urologists can be consulted to formulate a regimen suitable for such patients. More importantly, ACS patients are encouraged to continue their antiplatelet therapy if hematuria is not severe. The development of persistent hematuria in patients with DAPT represents a high probability of urinary tract disease. At this point, a urologist should be consulted to identify the cause of hematuria and initiate appropriate treatment. ACS patients are considered a high-risk group that should refrain from discontinuing DAPT as far as possible to prevent recurrent ischemic events. Hematuria is typically resolved following proper treatment. Physicians should explain the side effects to patients, encourage them to stay focused on adherence, and stress the fact that favorable clinical outcomes can be achieved with at least 12 months of DAPT.
EPISTAXIS IN PATIENTS WITH ANTIPLATELET THERAPY
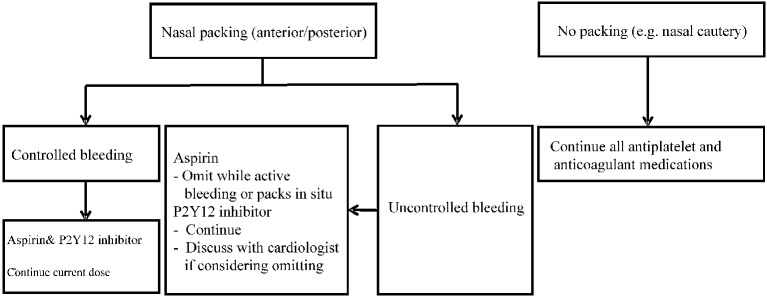
No identifiable cause has been reported in 85% of patients with epistaxis, and this type of epistaxis is labeled as "idiopathic".30 Epistaxis can be caused by both systemic and local factors. Local factors include inflammatory, infective, traumatic, anatomical, or chemical causes. Climatic changes, neoplasms, and foreign bodies should also be considered. Systemic factors of epistaxis are hematological diseases that cause coagulopathy, cardiovascular diseases, liver diseases, renal diseases, and antiplatelet or anticoagulant drugs. In the PLATO study, there was a trend that ticagrelor increased the risk of epistaxis compared to clopidogrel (RR = 1.49; 95% CI: 0.67-3.32).31 A prospective study assessed the prevalence of epistaxis in 119 patients taking antiplatelet or anticoagulant agents, in which epistaxis stopped spontaneously in 8% of the patients, 19% were controlled with cautery, 68% needed anterior or posterior packing, and only 4% required a surgical intervention to control bleeding.32 Epistaxis can be caused or exacerbated by anticoagulant and antiplatelet therapy, and in the majority of the cases, it stops spontaneously or can be controlled with three simple steps: sit up straight, lean a little bit forward, and pinch the nostrils closed. According to suggestions from the UK for epistaxis patients on antiplatelet or anticoagulant therapy (Figure 3),33 medication should be discontinued temporarily only when nasal packing is necessary. Since P2Y12 inhibitors, as opposed to aspirin, are relatively more important in the management of high risk patients, it should be continued unless cardiologists also agree to stop the drug. If no nasal packing is necessary, all drugs can be continued. Ninety percent of cases of epistaxis are resolved by supportive care, nasal packing or cautery, with very few cases requiring surgical ligation to control bleeding. Collaboration between cardiologists and otolaryngologists is necessary when taking care of these patients. In summary, epistaxis can be attributed to a number of factors. Patients on antiplatelet therapy who present with a nosebleed should consult their doctor immediately for proper treatment to prevent recurrent events. Nosebleeds are considered to be a mild side effect that can be controlled with adequate interventions. Patients are strongly recommended to continue their DAPT for at least 12 months to maximize the benefits of antiplatelet therapy.
Figure 3.
The algorithm for the management of patients with epistaxis who are under antiplatelet treatment. Adapted and modified from reference 33.
DYSPNEA IN PATIENTS WITH ANTIPLATELET THERAPY
Dyspnea is a side effect of ticagrelor. It is important to note that there may be a propensity to attribute dyspnea to ticagrelor when in reality it is due to other clinical problems, such as pulmonary congestion in patients with new-onset heart failure after myocardial infarction, or it may simply be the patient’s subjective perception.34 For proper management of patients complaining of dyspnea when taking ticagrelor, all clinically plausible causes of dyspnea must be considered before attributing it to the medication itself. Episodes of dyspnea tend to occur early (1 to 50 days) after the initiation of ticagrelor treatment. This side effect is mostly mild or moderate in intensity, and led to treatment discontinuation in only about 0.9% of cases in the PLATO study. The PLATO study also found that episodes of dyspnea were observed in 14.5% of ticagrelor-treated patients and 8.7% of clopidogrel-treated patients, while severe cases of dyspnea were found in 0.4% and 0.3% of patients receiving ticagrelor and clopidogrel, respectively.35 There was no loss of treatment effect in the ticagrelor-treated patients who experienced dyspnea as a side effect because the mortality rate was still lower among the ticagrelor-treated patients with dyspnea compared with clopidogrel-treated patients with dyspnea. These results imply that the occurrence of dyspnea does not influence the efficacy of ticagrelor, even in patients with asthma, congestive heart failure, or chronic obstructive pulmonary disease.35 In the ONSET/OFFSET trial, cardiac and pulmonary function tests were evaluated in patients with stable coronary artery disease treated with ticagrelor, clopidogrel, or a placebo. The results showed no significant changes in the left ventricular ejection fraction and pulmonary function test of all treatment groups.18 Ticagrelor-induced dyspnea has been attributed to increased plasma levels of adenosine because ticagrelor increases adenosine concentrations by inhibiting cellular reuptake of adenosines.36 Pulmonary vagal C fibers are stimulated by increased adenosine levels through the A1R receptor and A2AR receptor, which mediate the sensation of dyspnea. On the other hand, adenosine exerts beneficial biological effects on the cardiovascular system because it has a negative chronotropic effect, reduces ischemia/reperfusion injury, and increases coronary vasodilation.36
Regarding management of dyspnea, the patients’ comorbidities critically influence the prognosis of ticagrelor-treated patients with dyspnea. ACS patients with new-onset dyspnea should be assessed for comorbidities first with medical tests such as chest X-ray, echocardiography, pulmonary function test, or tests for N-terminal pro B-type natriuretic peptide.34,35 Subsequently, clinical judgment can be made based on the results of these medical tests. Patients should be closely monitored to identify the actual cause of dyspnea and determine whether it is attributable to the medication itself. Ticagrelor-induced dyspnea is generally mild in intensity, exerting no effect on the patient’s daily activities, and can be resolved quickly. Doctors should therefore encourage patients to follow their medical advice and emphasize the importance of continuing their medication to achieve maximum efficacy. For patients with persistent, severe dyspnea rendering the patients unable to tolerate the discomfort, alternative regimens should be considered to replace ticagrelor. Figure 4 shows the flow chart of management of dyspnea during antiplatelet treatment.
Figure 4.
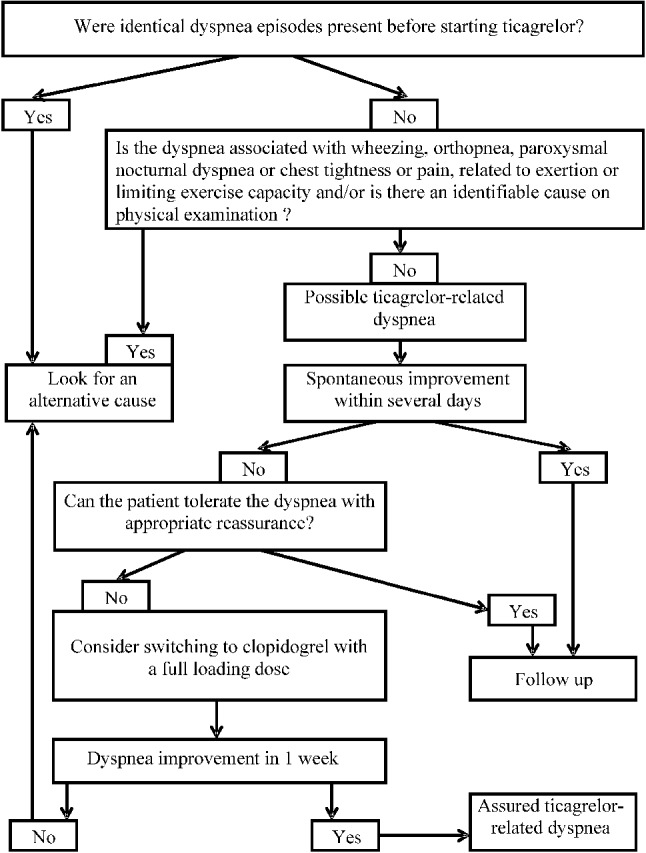
The algorithm for the management of patients with dyspnea who are under ticagrelor treatment. Adapted and modified from reference 3434.
CONCLUSIONS
Despite its prominent efficacy in lowering the risk of recurrent ischemic events, antiplatelet therapy is inevitably associated with adverse effects that cannot be overlooked. This article described the common adverse effects of antiplatelet therapy and made recommendations for managing these events. The overall management recommendations are summarized in Table 3.
Table 3. Management recommendations of adverse effects of antiplatelet therapy.
| Adverse effects | Recommendations |
| Upper GI bleeding | • Prior to receiving DAPT, patients at high risk of GI bleeding can orally administer PPI to reduce the risk of bleeding. |
| • In the event of GI bleeding, a high dose of PPI should be used and refer the patient to a gastroenterologist. | |
| • For severe GI bleeding, temporary discontinuation of antiplatelet agent can be considered. | |
| • When discontinuing DAPT is necessary, the treatment should be resumed as early as possible following endoscopic procedures. | |
| Ecchymosis | • Diascopy (glass test) is used to determine whether the skin lesions are hemorrhagic. |
| • Ecchymosis is a subcutaneous spot of bleeding arising as a result of old age, exposure to sunlight, bleeding disorder, or medication. | |
| • Ecchymosis is considered a mild side effect that usually can be resolved with supportive care. | |
| Hematuria | • Development of hematuria in patients with DAPT still represents a high probability of urinary tract disease. |
| • Urologist should be consulted, the cause of hematuria identified, and the appropriate treatment administered. | |
| • Hematuria is typically resolved following proper treatment. | |
| Epistaxis | • Nosebleed is considered a mild side effect that can be controlled with adequate intervention. |
| • Patients are strongly recommended to continue their DAPT for at least 12 months to maximize the benefits of antiplatelet therapy. | |
| Dyspnea | • Medical conditions should be assessed before attributing dyspnea to ticagrelor. |
| • Ticagrelor-induced dyspnea is generally mild in intensity, exerting no effect on the patient’s activities of daily life and can be resolved quickly. | |
| • Ticagrelor does not influence the patient’s cardiac and pulmonary function. | |
| • If persistent, severe dyspnea occurs, rendering patients unable to tolerate the discomfort, alternative regimen should be considered to replace ticagrelor. |
DAPT, dual antiplatelet therapy; GI, gastrointestinal; PPI, proton-pump inhibitor.
Acknowledgments
The adverse effect management consensus meeting was held by AstraZeneca Taiwan. This work was supported by AstraZeneca Taiwan. The authors would like to thank Mr. Kevin Hung, AstraZeneca Taiwan for his administrative support. The funders had no role in the publication of this study.
REFERENCES
- 1.Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136:1155–1166. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas MR, Storey RF. Effect of P2Y12 inhibitors on inflammation and immunity. Thromb Haemost. 2015;114:490–497. doi: 10.1160/TH14-12-1068. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: a review of the evidence. J Am Coll Cardiol. 2005;45:1157–1164. doi: 10.1016/j.jacc.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Thomas MR, Outteridge SN, Ajjan RA, et al. Platelet P2Y12 inhibitors reduce systemic inflammation and its prothrombotic effects in an experimental human model. Arterioscler Thromb Vasc Biol. 2015;35:2562–2570. doi: 10.1161/ATVBAHA.115.306528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 6.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 7.Rioufol G, Finet G, André-Fouët X, et al. Multiple ruptures of atherosclerotic plaques in acute coronary syndrome. Endocoronary ultrasonography study of three arteries. Arch Mal Coeur Vaiss. 2002;95:157–165. [PubMed] [Google Scholar]
- 8.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 9.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 10.Wei CC, Lee SH. Predictors of mortality in elderly patients with non-ST elevation acute coronary syndrome - data from Taiwan Acute Coronary Syndrome Full Spectrum Registry. Acta Cardiol Sin. 2017;33:377–383. doi: 10.6515/ACS20170126A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu CY, Lin TH, Lai WT. The management and prognostic factors of acute coronary syndrome: evidence from the Taiwan Acute Coronary Syndrome Full Spectrum Registry. Acta Cardiol Sin. 2017;33:329–338. doi: 10.6515/ACS20161205A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 14.Li YH, Yeh HI, Tsai CT, et al. 2012 guidelines of the Taiwan Society of Cardiology for the management of ST-segment elevation myocardial infarction. Acta Cardiol Sin. 2012;28:63–89. doi: 10.6515/ACS.202007_36(4).20200619A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 16.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 17.Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998;339:1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 18.Storey RF, Bliden KP, Patil SB, et al. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J Am Coll Cardiol. 2010;56:185–193. doi: 10.1016/j.jacc.2010.01.062. [DOI] [PubMed] [Google Scholar]
- 19.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 20.Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2011;32:2933–2944. doi: 10.1093/eurheartj/ehr422. [DOI] [PubMed] [Google Scholar]
- 21.Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1–46. doi: 10.1055/s-0034-1393172. [DOI] [PubMed] [Google Scholar]
- 22.Agewall S, Cattaneo M, Collet JP, et al. Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur Heart J. 2013;34:1708–1713, 1713a-13b. doi: 10.1093/eurheartj/eht042. [DOI] [PubMed] [Google Scholar]
- 23.Veitch AM, Vanbiervliet G, Gershlick AH, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48:385–402. doi: 10.1055/s-0042-102652. [DOI] [PubMed] [Google Scholar]
- 24.Asboe-Hansen G. Influence of corticosteroids on connective tissue. Dermatologica. 1976;152(Suppl 1):127–132. doi: 10.1159/000257873. [DOI] [PubMed] [Google Scholar]
- 25.Kakagia DD, Papanas N, Karadimas E, Polychronidis A. Warfarin-induced skin necrosis. Ann Dermatol. 2014;26:96–98. doi: 10.5021/ad.2014.26.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritchie CD, Bevan EA, Collier SJ. Importance of occult haematuria found at screening. Br Med J (Clin Res Ed) 1986;292:681–683. doi: 10.1136/bmj.292.6521.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology. 2001;57:599–603. doi: 10.1016/s0090-4295(01)00919-0. [DOI] [PubMed] [Google Scholar]
- 28.Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology. 2001;57:604–610. doi: 10.1016/s0090-4295(01)00920-7. [DOI] [PubMed] [Google Scholar]
- 29.Grossfeld GD, Wolf JS, Jr., Litwan MS, et al. Asymptomatic microscopic hematuria in adults: summary of the AUA best practice policy recommendations. Am Fam Physician. 2001;63:1145–1154. [PubMed] [Google Scholar]
- 30.Pallin DJ, Chng YM, McKay MP, et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46:77–81. doi: 10.1016/j.annemergmed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 31.DiNicolantonio JJ, D'Ascenzo F, Tomek A, et al. Clopidogrel is safer than ticagrelor in regard to bleeds: a closer look at the PLATO trial. Int J Cardiol. 2013;168:1739–1744. doi: 10.1016/j.ijcard.2013.06.135. [DOI] [PubMed] [Google Scholar]
- 32.Smith J, Siddiq S, Dyer C, et al. Epistaxis in patients taking oral anticoagulant and antiplatelet medication: prospective cohort study. J Laryngol Otol. 2011;125:38–42. doi: 10.1017/S0022215110001921. [DOI] [PubMed] [Google Scholar]
- 33.Biggs TC, Baruah P, Mainwaring J, et al. Treatment algorithm for oral anticoagulant and antiplatelet therapy in epistaxis patients. J Laryngol Otol. 2013;127:483–488. doi: 10.1017/S0022215113000492. [DOI] [PubMed] [Google Scholar]
- 34.Parodi G, Storey RF. Dyspnoea management in acute coronary syndrome patients treated with ticagrelor. Eur Heart J Acute Cardiovasc Care. 2015;4:555–560. doi: 10.1177/2048872614554108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Storey RF, Becker RC, Harrington RA, et al. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J. 2011;32:2945–2953. doi: 10.1093/eurheartj/ehr231. [DOI] [PubMed] [Google Scholar]
- 36.Cattaneo M, Schulz R, Nylander S. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol. 2014;63:2503–2509. doi: 10.1016/j.jacc.2014.03.031. [DOI] [PubMed] [Google Scholar]