Abstract
Background
Gallic acid (3,4,5-trihydroxybenzoic acid) is a natural polyphenol and strong natural antioxidant found abundantly in red wine and green tea. The aim of this study was to examine the anti-inflammatory effect of a novel gallic acid-eluting stent in a porcine coronary restenosis model.
Methods
Fifteen pigs were randomized into three groups; in which a total of 30 coronary arteries (10 in each group) were implanted with gallic acid-eluting stents (GESs, n = 10), gallic acid and sirolimus-eluting stents (GSESs, n = 10), or sirolimus-eluting stents (SESs, n = 10). Histopathologic analysis was performed 28 days after stenting.
Results
There were no significant differences in injury score and fibrin score among the groups, however there were significant differences in the internal elastic lamina (4.0 ± 0.83 mm2 in GES vs. 3.0 ± 0.53 mm2 in GSES vs. 4.6 ± 1.43 mm2 in SES, p < 0.0001), lumen area (2.3 ± 0.49 mm2 in GES vs. 1.9 ± 0.67 mm2 in GSES vs. 2.9 ± 0.56 mm2 in SES, p < 0.0001), neointimal area (1.7 ± 0.63 mm2 in GES vs. 1.1 ± 0.28 mm2 in GSES vs. 1.7 ± 1.17 mm2 in SES, p < 0.05), and percent area of stenosis (42.4% ± 9.22% in GES vs. 38.2% ± 12.77% in GSES vs. 33.9% ± 15.64% in SES, p < 0.05). The inflammation score was significantly lower in the GES and GSES groups compared to that in the SES group [1.0 (range: 1.0 to 2.0) in GES vs. 1.0 (range: 1.0 to 1.0) in GSES vs. 1.5 (range: 1.0 to 3.0) in SES, p < 0.05].
Conclusions
The GES group had a greater percent area of stenosis than the SES group. Although gallic acid in the GES and GSES groups did not show a synergistic effect in suppressing neointimal hyperplasia, it resulted in greater inhibition of the inflammatory reaction in the porcine coronary restenosis model than in the SES group.
Keywords: Inflammation, Percutaneous coronary intervention, Restenosis, Stent
INTRODUCTION
Coronary artery atherosclerosis and occlusion are among the leading causes of morbidity and mortality in industrialized countries. Common treatment of coronary occlusion includes balloon angioplasty (BA) and bare-metal stent (BMS) and/or drug-eluting stent (DES) implantation. Recently, bioresorbable vascular scaffolds and polymer-free DESs have been developed and used for treatment.1-3
Sirolimus (CYPHER®, Cordis Corporation, Johnson & Johnson, Warren, NJ, USA) and paclitaxel (Taxus®, Boston Scientific, Boston, MA, USA) eluting stents were introduced as first-generation intracoronary DESs. Sirolimus, also known as rapamycin, was first isolated from streptomyces hygroscopius.4 Rapamycin is commonly used to coat coronary stents, and it is an antibiotic with potent antiproliferative, immunosuppressive, and antimigratory properties.
Taxol (generic name: paclitaxel) is isolated from the evergreen Pacific yew (Taxus brevifolia). Paclitaxel has anti-vascular smooth muscle cell (VSMC) proliferation and anti-cancer effects. Thus, the first-generation of DESs with paclitaxel were used in patients with acute myocardial infarction to prevent in-stent restenosis. This was the first plant-based substance used in a coronary stent.
Although the advent of DESs has reduced restenosis rates by 50-90% compared with BA and BMSs, DESs are associated with several limitations including late stent thrombosis (LST) and chronic inflammation at the stented lesion.5-10 Sirolimus-eluting stents (SESs) have been reported to have superior clinical outcomes to paclitaxel-eluting stent in comparative trials.11-13
Gallic acid (3,4,5-trihydroxybenzoic acid) is a phenolic acid and a phytochemical. It has been shown to have anti-inflammatory, antioxidative, antimicrobial, and anticancer effects.14 Sirolimus and gallic acid have different mechanisms of inhibiting inflammation and neointimal hyperplasia.15-17 Gallic acid induces apoptosis and suppresses VSMCs by producing the hydroxyl radical.18 Sirolimus has also been shown to suppress the proliferation of VSMCs by inhibiting cell cycle-dependent kinases and delaying the phosphorylation of retinoblastoma protein,19 suggesting that a synergistic effect may be achieved by combining sirolimus and gallic acid. Therefore, in this study, we investigated the inhibitory effect of gallic acid-eluting stents (GESs) and gallic acid and sirolimus-eluting stents (GSESs) on vascular inflammation and smooth muscle cell growth in a porcine coronary restenosis model.
METHODS
Materials
Poly-L-lactide (PLLA; 0.80-1.2 dL/g of inherent viscosity in chloroform at 0.1 w/v% at 25 °C) and poly (D, L-lactide-co-glycolide) at a ratio of 50:50 (0.45-0.60 dL/g of inherent viscosity in chloroform at 0.1 w/v% at 25 °C) was purchased from EVONIK (UK). Sirolimus was purchased from LC Laboratories (USA). Phosphate-buffered saline (PBS) and gallic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and solvents were of analytical grade and used without further purification.
Preparation of gallic acid-, gallic acid and sirolimus-, and sirolimus-eluting stents (Figure 1)
Figure 1.
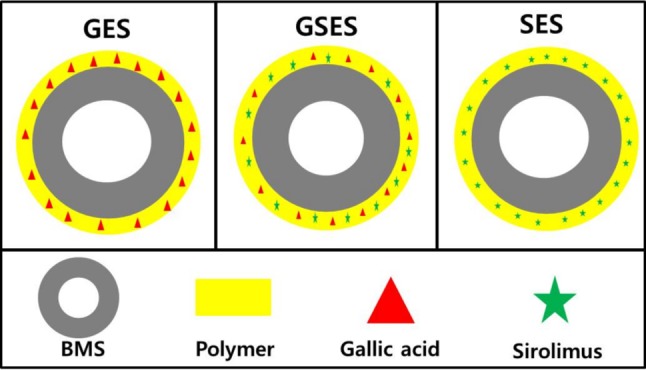
Schematic illustration of the GES, GSES, and SES. GES, gallic acid-eluting stent; GSES, gallic acid- and sirolimus-eluting stent; SES, sirolimus-eluting stent.
The BMS (Chonnam National University Hospital stent) used in the study was made by laser-cut processing of a cobalt-chromium alloy tube (L605 Co-Cr alloy) followed by electropolishing to a strut thickness of < 70 μm.20,21 An ultrasonic spray method was used to apply coatings to the prepared BMS (3 × 16 mm). The required amount of poly (L-lactide) (PLA) and gallic acid was dissolved in 5 mL of tetrahydrofuran (THF), and the drug solution was then dissolved in the polymer solution. The stents to be coated were cleaned with ethanol and distilled water and then vacuum-dried for 24 h. The sprayed liquid consisted of the polymer/drug solution dissolved in THF and diluted to 2% by weight. Coating application required a flow rate of 50 μL/min. The stents were placed on a mandrel attached to a rotating shaft, coated, and vacuum-dried for 24 h. The surface morphologies of the GESs, GSESs, and SESs were then examined.
Coated stent evaluation
We used a scratch method under a scanning electron microscopy (SEM; SNE-1500M, SEC Co., Ltd., Korea) and reflection spectrometry (RS; F40, Filmetrics, Inc., USA) using a wavelength range of 400-850 nm to measure the thickness of the coated layer on each stent. The optical index of refraction was assumed to be n = 1.50 of PLA. To determine the total amount of gallic acid on a BMS, the coated stent was sonicated in 5 mL of acetonitrile (ACN) for 1 h to dissolve the coated layer with the drug and then analyzed using an ultraviolet-visible spectrophotometer (UV; UV-1800, Shimadzu, Japan) at 241.5 nm. In vitro drug release was measured using a simple shaking method with UV.22
All stents were expanded to a 3.0-mm diameter, and then immersed in 5 mL of phosphate buffered saline (PBS) in colored vials and subjected to 100 rpm shaking at 37 °C. The stent was taken out at each designated time point, and the PBS was replaced with fresh solution at the specified times. The drugs remaining on the stents were dissolved in ACN and measured with a UV-visible spectrophotometer at 241.5 nm.
Animal preparation and stent implantation
The study animals were Yorkshire × Landrace F1 crossbred, castrated male swine with an average age of 7-9 weeks. The animal experiments for coronary stenting were conducted as described previously.23 Pigs were selected randomly in this study and the coronary artery size was highly variable. Therefore, the balloon pressure was adjusted according to the vessel size to accommodate the stent diameter. The stent was deployed by inflating the balloon (3 × 20 mm) and the resulting stent-to-coronary artery ratio was 1.3:1. The diameter of the implanted coronary stent (stent-to-artery ratio) was adjusted with reference to the 7-F guiding catheter diameter (2.31 mm). The stented pigs underwent follow-up angiography after 4 weeks. The pigs were anesthetized on the day of follow-up with zolazepam and tiletamine (2.5 mg/kg; Zoletil50®, Virbac, Caros, France), xylazine (3 mg/kg; Rompun®, Bayer AG, Leverkusen, Germany), and azaperone (6 mg/kg; Stresnil® , Janssen-Cilag, Neuss, Germany). The pigs were sacrificed with 20 mL of potassium chloride by intracoronary injection under deep anesthesia after follow-up angiography.
Study groups
The pigs were randomly divided into three groups: GES (3.0 × 16 mm, n = 10), GSES (3.0 × 16 mm, n = 10), and SES (3.0 × 16 mm, n = 10) groups. A total of 15 pigs (30 coronary arteries) were used in this study. A GES, GSES, or SES was implanted in the left anterior descending and left circumflex artery of each pig in a randomized manner.
Histopathological and micro-computed tomography analysis
Histopathological evaluation of each artery was performed by an experienced cardiovascular pathologist. The specimens were embedded, and sections of ~3-5-μm thickness were obtained at ~1-mm intervals and stained with hematoxylin and eosin (H&E) and Carstairs’ stain for histological analysis. The histopathological sections were measured using a calibrated microscope, digital video imaging system, and microcomputer program (Visus 2000 Visual Image Analysis System, IMT Tech, CA, USA). Borders were manually traced for lumen area, and the area was circumscribed by the internal elastic lamina and the innermost border of the external elastic lamina (i.e., external elastic lamina area). Morphometric analysis was used to calculate the neointimal area of a given vessel as the measured internal elastic lamina area minus the lumen area. Measurements were made on five cross sections from proximal and distal ends, and three midpoints of each stented segment. Histopathological stenosis was calculated as 100 × [1 – (lesion lumen area/lesion internal elastic lamina area)].24 The harvested stent specimens were stored in formaldehyde solution. A 1.5-mL Eppendorf tube was filled with clay, and the clay was formed into a V shape to hold the stent during contrast agent staining. The stents were taken from the solution and placed vertically in the V-shaped opening in the clay. Each stent had to be fixed in the clay so that it would not move inside the tube. The contrast agent used was omnihexol. One milliliter of the contrast agent was then placed in a 5-mL syringe and injected through the opening at the center of the stent. The stent was incubated with the contrast agent overnight and subjected to micro-computed tomography (CT) imaging.25 All results were interpreted by two independent pathologists in a blinded fashion.
Classification of in-stent restenosis using angiographic patterns
Coronary angiograms were reviewed by independent pathologists who classified the lesions according to the following criteria (Table 1).26
Table 1. In-stent restenosis criteria on angiography.
| Class | Definition |
| I | Focal ISR group. Lesions are ≤ 10 mm long and positioned at the unscaffolded segment (ie, articulation or gap), stent body, proximal or distal margin, or combination of these sites (multifocal ISR). |
| II | “Diffuse intrastent” ISR. Lesions are > 10 mm long and confined to the stent(s) without extending outside the margins. |
| III | “Diffuse proliferative” ISR. Lesions are > 10 mm long and extend beyond the stent margin(s). |
| IV | ISR with “total occlusion.” Lesions have a TIMI flow grade of 0. |
ISR, in-stent restenosis.
Evaluation of arterial injury score
Arterial injury at each stent strut site was determined according to the anatomic structures penetrated by each stent strut. A numeric value was assigned as previously described by Schwartz et al.: 0 = no injury; 1 = break in the internal elastic membrane; 2 = perforation of the media; and 3 = perforation of the external elastic membrane to the adventitia.24 The average injury score for each segment was calculated by dividing the sum of the injury scores by the total number of stent struts at the examined section (Table 2).27
Table 2. Histopathological quantification of arterial injury, inflammation, and fibrin scores.
| Score | Injury |
| 0 | Internal elastic lamina intact; endothelium typically denuded; media compressed but not lacerated. |
| 1 | Internal elastic lamina lacerated; media typically compressed but not lacerated. |
| 2 | Internal elastic lacerated; media visibly lacerated; external elastic lamina intact but compressed. |
| 3 | External elastic lamina lacerated; typically large lacerations of media extending through the external elastic lamina; coil wires sometimes residing within adventitia. |
| Score | Inflammation |
| 0 | < 25% struts with < 10 inflammatory cells. |
| 1 | Up to 25% struts with > 10 inflammatory cells. |
| 2 | 25%-50% struts with > 10 inflammatory cells. |
| 3 | > 50% struts with > 10 inflammatory cells. |
| Score | Fibrin |
| 0 | There was no fibrin deposition. |
| 1 | Focal residual fibrin involving any portion of the artery and for moderate fibrin deposition adjacent to the strut involving < 25% of the circumference of the artery. |
| 2 | Moderate fibrin deposition involving > 25% of the circumference of the artery or heavy deposition of fibrin adjacent to and between stent struts involving < 25% of the circumference of the artery. |
| 3 | Heavy deposition of fibrin involving > 25% of the circumference of the artery. |
Evaluation of inflammation scores and fibrin scores
The inflammation score for each individual stent strut was graded as follows: 0 = no inflammatory cells surrounding the stent strut; 1 = light, noncircumferential lymphohistiocytic infiltration surrounding the strut; 2 = localized, noncircumferential, moderate-to-dense cellular aggregates surrounding the stent strut; and 3 = circumferential, dense lymphohistiocytic cell infiltration of the stent strut. The inflammation score for each cross section was calculated by dividing the sum of the individual inflammation scores by the total number of stent struts at the examined section (Table 2).28 Ordinal data for fibrin were collected for each stent section using a scale of 0-3 as previously reported (Table 2).29
Ethical statement
This animal study was approved by the Ethics Committee of Chonnam National University Medical School and Chonnam National University Hospital (CNU IACUC-H-2016-03), and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Statistical analysis
Statistical analysis was performed with commercially available software (SPSS Version 15, Chicago, IL, USA). Data were presented as mean ± SD. The unpaired Student’s t test was used to compare each stent group, and analysis of variance (ANOVA) was used for comparisons of the three stent groups. Ordinal measurements such as injury, fibrin, and inflammation scores were analyzed using the Kruskal-Wallis test. The Mann-Whitney test was used to compare ordinary values in each stent group. Non-parametric results were presented as median and interquartile range. p values < 0.05 were considered to be statistically significant.
RESULTS
Surface coating and drug-releasing evaluation of the GES, GSES, and SES
Roughness and defects of the polymer-coated surface on the stent have been reported to potentially affect stent thrombosis and drug release.30,31 Our SEM findings (Figure 2) showed that the coated surface was uniform and smooth, and neither bridging nor webbing was observed in any of the stents. The coating thickness using RS was 5-8 μm. The in vitro elution of gallic acid and sirolimus from the coated stents is shown in Figure 3. Gallic acid and sirolimus were released for more than 25 days.
Figure 2.
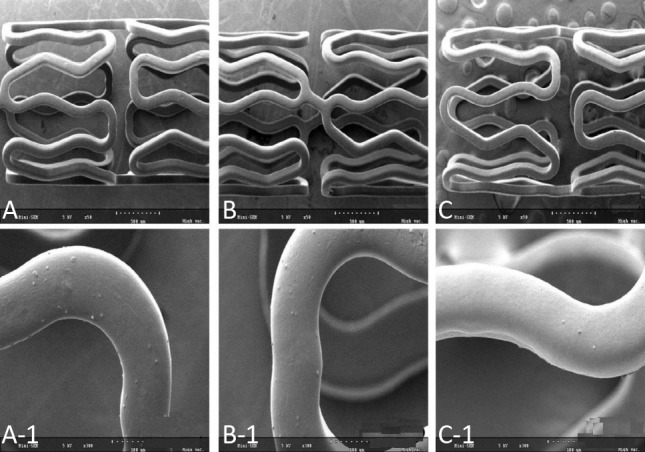
Scanning electron microscopy images (magnitude, ×50 and ×300) of the GES (A and A-1), GSES (B, B-1, and B-2), and SES (C, C-1, and C-2). GES, gallic acid-eluting stent; GSES, gallic acid and sirolimus-eluting stent; SES, sirolimus-eluting stent.
Figure 3.
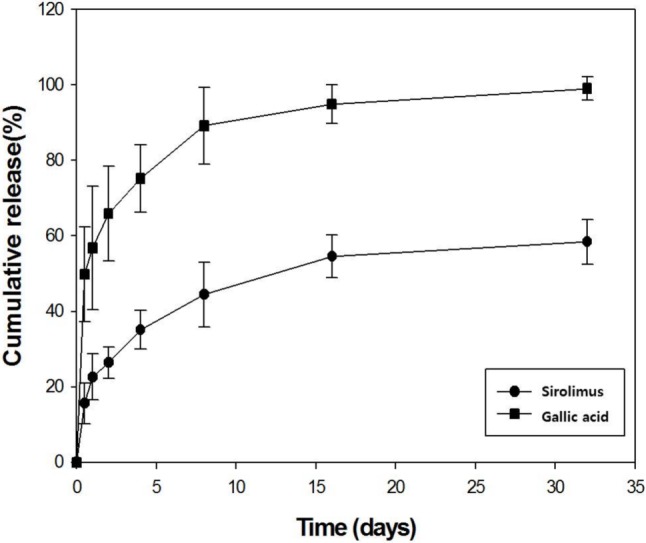
In vitro release kinetics of gallic acid (square dot) and sirolimus (round dot) on a stent over time.
Stent implantation in pigs
A total of 30 stents (10 GESs, 10 GSESs, and 10 SESs) were placed in the left anterior descending and left circumflex arteries of 15 pigs. The overall mortality rate was 0% in this study. There were no significant differences in stent-to-artery ratio among the three stent groups.
Follow-up coronary angiographic findings
All stented coronary arteries showed in-stent restenosis pattern II on follow-up coronary angiographic analysis.
Histopathological findings in the three groups (Table 3)
Table 3. Coronary artery morphometric measurements in 30 stented vessels.
| GES (n = 10, A) | GSES (n = 10, B) | SES (n = 10, C) | p value | ||
| Among | Between | ||||
| Injury score | 1.0 (1.0-1.0) | 1.0 (1.0-1.0) | 1.0 (1.0-2.0) | NS | A vs. B: p = NS |
| B vs. C: p = NS | |||||
| C vs. A: p = NS | |||||
| IEL (mm2) | 4.0 ± 0.83 | 3.0 ± 0.53 | 4.6 ± 1.43 | < 0.0001 | A vs. B: p < 0.0001 |
| B vs. C: p < 0.0001 | |||||
| C vs. A: p = NS | |||||
| Lumen area (mm2) | 2.3 ± 0.49 | 1.9 ± 0.67 | 2.9 ± 0.56 | < 0.0001 | A vs. B: p = NS |
| B vs. C: p < 0.0001 | |||||
| C vs. A: p < 0.01 | |||||
| Neointima area (mm2) | 1.7 ± 0.63 | 1.1 ± 0.28 | 1.7 ± 1.17 | < 0.05 | A vs. B: p < 0.001 |
| B vs. C: p < 0.05 | |||||
| C vs. A: p = NS | |||||
| % area stenosis (%) | 42.4 ± 9.22 | 38.2 ± 12.77 | 33.9 ± 15.64 | < 0.05 | A vs. B: p < 0.05 |
| B vs. C: p = NS | |||||
| C vs. A: p < 0.05 | |||||
| Fibrin score | 1.0 (1.0-2.0) | 2.0 (1.0-2.0) | 2.0 (0.0-3.0) | NS | A vs. B: p = NS |
| B vs. C: p = NS | |||||
| C vs. A: p = NS | |||||
| Inflammation score | 1.0 (1.0-2.0) | 1.0 (1.0-1.0) | 1.5 (1.0-3.0) | < 0.05 | A vs. B: p = NS |
| B vs. C: p < 0.05 | |||||
| C vs. A: p < 0.05 |
Injury, fibrin and inflammation scores are expressed as median (interquartile range).
GES, gallic acid-eluting stent; GSES, gallic acid and sirolimus-eluting stent; IEL, internal elastic lamina; NS, not significant; SES, sirolimus-eluting stent.
There were no significant differences in injury score [1.0 (range: 1.0 to 1.0) in GES vs. 1.0 (range: 1.0 to 1.0) in GSES vs. 1.0 (range: 1.0 to 2.0) in SES, p = NS] or fibrin score [1.0 (range: 1.0 to 2.0) in GES vs. 2.0 (range: 1.0 to 2.0) in GSES vs. 2.0 (range: 0.0 to 3.0) in SES, p = NS] among the three groups. There were significant differences in internal elastic lamina (4.0 ± 0.83 mm2 in GES vs. 3.0 ± 0.53 mm2 in GSES vs. 4.6 ± 1.43 mm2 in SES, p < 0.0001), lumen area (2.3 ± 0.49 mm2 in GES vs. 1.9 ± 0.67 mm2 in GSES vs. 2.9 ± 0.56 mm2 in SES, p < 0.0001), neointimal area (1.7 ± 0.63 mm2 in GES vs. 1.1 ± 0.28 mm2 in GSES vs. 1.7 ± 1.17 mm2 in SES, p < 0.05), and percent area of stenosis (42.4 ± 9.22% in GES vs. 38.2 ± 12.77% in GSES vs. 33.9 ± 15.64% in SES, p < 0.05) among the three groups. GESs achieved a greater percent area of stenosis than SESs.
The inflammation score [1.0 (range: 1.0 to 2.0) in GES vs. 1.0 (range: 1.0 to 1.0) in GSES vs. 1.5 (range: 1.0 to 3.0) in SES, p < 0.05] was significantly lower in the GES and GSES groups compared to that in the SES group (Figure 4 and 5). Injury, fibrin, and inflammation scores were expressed as median (interquartile range).
Figure 4.
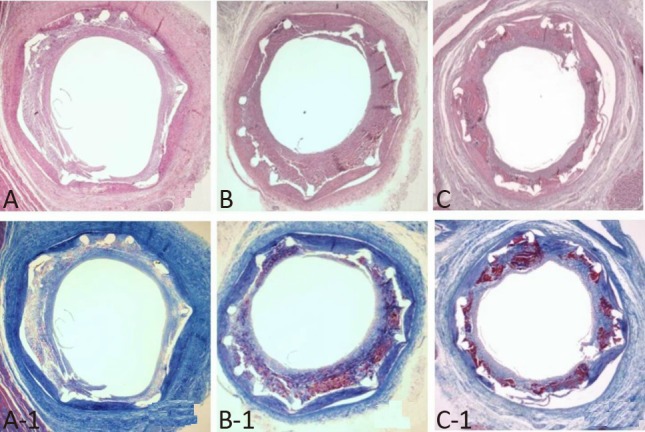
Representative images of hematoxylin and eosin staining at 4 weeks after stenting. Specimens of implanted GES (A, ×20), GSES (B, ×20), and SES (C, ×20). Carstairs’ fibrin stain (magnitude, ×20) of fibrin infiltration in implanted GES (A-1, ×20), GSES (B-1, ×20), and SES (C-1, ×20). GES, gallic acid-eluting stent; GSES, gallic acid and sirolimus-eluting stent; SES, sirolimus-eluting stent.
Figure 5.
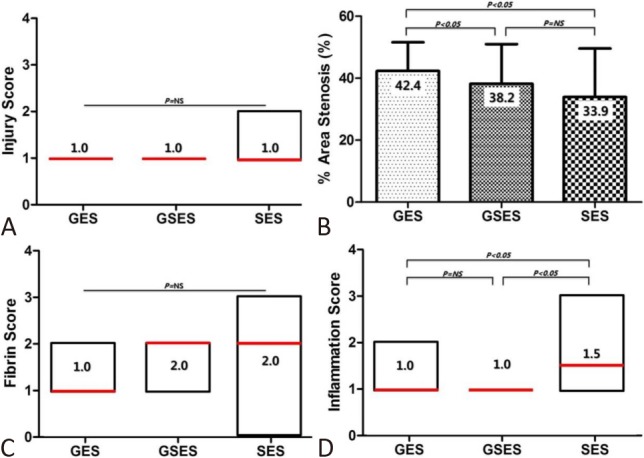
Injury score (A), percent area of stenosis (B), fibrin score (C), and inflammation score (D) of GES, GSES, and SES. A, C, and D are expressed as the median (interquartile range). GES, gallic acid-eluting stent; GSES, gallic acid and sirolimus-eluting stent; SES, sirolimus-eluting stent.
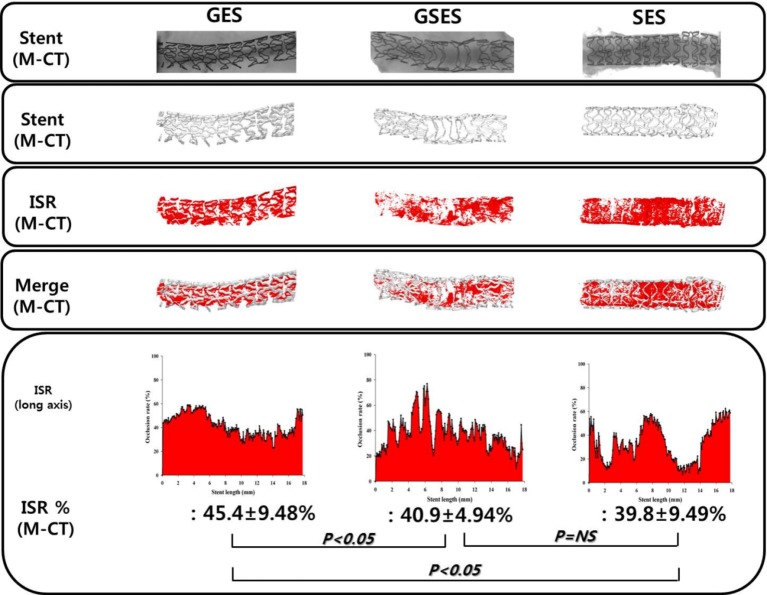
Micro-CT analysis
Percent area of stenosis of the stented arteries detected using micro-CT was significantly lower in the GSES and SES groups than in the GES group (45.4 ± 9.48% in GES vs. 40.9 ± 4.94% in GSES vs. 39.8 ± 9.49% in SES, p < 0.05) (Figure 6).
Figure 6.
Micro-CT analysis and representative images of in-stent restenosis of GES, GSES, and SES. GES, gallic acid-eluting stent; GSES, gallic acid and sirolimus-eluting stent; SES, sirolimus-eluting stent.
DISCUSSION
The present study was conducted to investigate the anti-inflammatory and anti-smooth muscle proliferative effects of gallic acid-eluting stents with/without sirolimus compared with sirolimus-eluting stents in a porcine coronary restenosis model. The results showed that gallic acid had a mild suppressive effect on vascular inflammation in the stented arteries. In addition, the injury score results showed that adequate pressure was applied according to the variable artery size.
The unpaired Student’s t test revealed no statistically significant difference in percent area stenosis between the SES and GSES groups (Figures 4 and 5). Conversely, the sirolimus-containing groups (SESs and GSESs) demonstrated significantly decreased neointimal hyperplasia compared to the GESs without sirolimus. Although the inflammation score was significantly lower in the two groups with gallic acid (GESs and GSESs), it was too weak to inhibit neointimal proliferation.
Macrocyclic immunosuppressive drugs such as sirolimus (rapamycin) bind to immunophilins to exert immunosuppressive effects. Sirolimus blocks the G1 to S cell cycle by interacting with mammalian target of rapamycin (mTOR) protein. This mechanism of action has been associated with inhibition of VSMC migration and proliferation after stenting.32
Previous small and large animal studies reported that sirolimus could inhibit mammalian VSMC proliferation, and that its systemic administration could significantly reduce neointimal hyperplasia in a porcine coronary angioplasty model.19,33,34 Thus, first generation DESs used sirolimus to prevent the proliferation of VSMCs after percutaneous coronary interventions. Coronary SESs have also been shown to significantly reduce target lesion revascularization compared with BMSs in long-term follow-up in patients with acute myocardial infarction.35
There have been several attempts to use natural products such as phytochemicals (phytoncides), paclitaxel, and artemisinin derivatives to coat coronary stents to prevent the side effects of other synthetically coated drugs and polymers.31,36-43 The success of natural product-eluting stents has demonstrated the possibility of using natural products in coronary stents. Based on these previous reports, a new candidate for natural products was found.
Gallic acid (trihydroxybenzoic acid) is a phenolic acid and phytochemical which is found in sumac, witch hazel, oak bark, tea leaves, grapes, blackberries, gallnuts, and many plants. It has antioxidant, antimicrobial, and anti-inflammatory effects.14
In a previous study in which gallic acid was coated on a metal plate to evaluate the inhibitory effect on human umbilical artery smooth muscle cell adhesion and proliferation, the coated surface showed remarkable inhibitory activity.44 In the present study, gallic acid exhibited insufficient suppression of neointimal proliferation, although the anti-inflammatory effect of gallic acid was noted. The results of this study suggest the feasibility of using natural substances to coat coronary stents. In our future research, stents using other natural polymers such as dextran or without polymers are under development.45
CONCLUSIONS
Gallic acid added to coronary stents (GESs, GSESs) showed very mild anti-inflammatory effects at 1 month compared with SESs in a porcine coronary restenosis model. Although gallic acid itself did not show a sufficient effect on neointimal hyperplasia, the application of natural products to coronary stents has shown potential for use in other surface-coated medical devices.
Acknowledgments
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2017R1C1B 1001956).
CONFLICTS OF INTEREST
The authors and CG Bio. Co. Ltd have no conflicts of interest relevant to this article to report. CG Bio. Co. Ltd only served to coat the coronary stent. There was no funding offered.
REFERENCES
- 1.Liang HW, Kao HL, Lin YH, et al. Everolimus-eluting bioresorbable vascular Scaffold in real world practice - a single center experience. Acta Cardiol Sin. 2017;33:250–257. doi: 10.6515/ACS20160901A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee WC, Wu CJ, Chen CJ, et al. Thirty-day and one-year clinical outcomes of bioresorbable vascular Scaffold implantation: a single-center experience. Acta Cardiol Sin. 2017;33:614–623. doi: 10.6515/ACS20170714A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J, Zhang Q, Chen L, et al. Three-year clinical outcomes of a polymer-free paclitaxel-eluting microporous stent in real-world practice: final results of the safety and efficacy registry of the Yinyi stent (SERY-I). Acta Cardiol Sin. 2017;33:28–33. doi: 10.6515/ACS20160131B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 5.Cutlip DE, Chauhan MS, Baim DS, et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol. 2002;40:2082–2089. doi: 10.1016/s0735-1097(02)02597-4. [DOI] [PubMed] [Google Scholar]
- 6.Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 7.Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 8.Shuchman M. Trading restenosis for thrombosis? New questions about drug-eluting stents. N Engl J Med. 2006;355:1949–1952. doi: 10.1056/NEJMp068234. [DOI] [PubMed] [Google Scholar]
- 9.Tsimikas S. Drug-eluting stents and late adverse clinical outcomes lessons learned, lessons awaited. J Am Coll Cardiol. 2006;47:2112–2115. doi: 10.1016/j.jacc.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Sung SH, Chen TC, Cheng HM, et al. Comparison of clinical outcomes in patients undergoing coronary intervention with drug-eluting stents or bare-metal stents: a nationwide population study. Acta Cardiol Sin. 2017;33:10–19. doi: 10.6515/ACS20160608A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morice MC, Colombo A, Meier B, et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA. 2006;295:895–904. doi: 10.1001/jama.295.8.895. [DOI] [PubMed] [Google Scholar]
- 12.Mehilli J, Dibra A, Kastrati A, et al. Randomized trial of paclitaxel- and sirolimus-eluting stents in small coronary vessels. Eur Heart J. 2006;27:260–266. doi: 10.1093/eurheartj/ehi721. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Kim HS, Lee SW, et al. Prospective randomized comparison of sirolimus- versus paclitaxel-eluting stents for the treatment of acute ST-elevation myocardial infarction: pROSIT trial. Catheter Cardiovasc Interv. 2008;72:25–32. doi: 10.1002/ccd.21510. [DOI] [PubMed] [Google Scholar]
- 14.Choubey S, Varughese LR, Kumar V, Beniwal V. Medicinal importance of gallic acid and its ester derivatives: a patent review. Pharm Pat Anal. 2015;4:305–315. doi: 10.4155/ppa.15.14. [DOI] [PubMed] [Google Scholar]
- 15.Martin KA, Merenick BL, Ding M, et al. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem. 2007;282:36112–36120. doi: 10.1074/jbc.M703914200. [DOI] [PubMed] [Google Scholar]
- 16.Marks AR. Rapamycin: signaling in vascular smooth muscle. Transplant Proc. 2003;35:231S–233S. doi: 10.1016/s0041-1345(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 17.Ou TT, Lin MC, Wu CH, et al. Gallic acid attenuates oleic acid-induced proliferation of vascular smooth muscle cell through regulation of AMPK-eNOS-FAS signaling. Curr Med Chem. 2013;20:3944–3953. doi: 10.2174/09298673113209990175. [DOI] [PubMed] [Google Scholar]
- 18.Qiu X, Takemura G, Koshiji M, et al. Gallic acid induces vascular smooth muscle cell death via hydroxyl radical production. Heart Vessels. 2000;15:90–99. doi: 10.1007/s003800070038. [DOI] [PubMed] [Google Scholar]
- 19.Poon M, Marx SO, Gallo R, et al. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98:2277–2283. doi: 10.1172/JCI119038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae IH, Lim KS, Park JK, et al. Mechanical behavior and in vivo properties of newly designed bare metal stent for enhanced flexibility. J Ind Eng Chem. 2015;21:1295–1300. [Google Scholar]
- 21.Lim KS, Bae IH, Kim JH, et al. Mechanical and histopathological comparison between commercialized and newly designed coronary bare metal stents in a porcine coronary restenosis model. Chonnam Med J. 2013;49:7–13. doi: 10.4068/cmj.2013.49.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma X, Oyamada S, Gao F, et al. Paclitaxel/sirolimus combination coated drug-eluting stent: in vitro and in vivo drug release studies. J Pharm Biomed Anal. 2011;54:807–811. doi: 10.1016/j.jpba.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim KS, Park JK, Jeong MH, et al. Biodegradable polymer-based sirolimus coating stent in a porcine coronary restenosis model. Clin Exp Thromb Hemost. 2014;1:59–65. [Google Scholar]
- 24.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992;19:267–274. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- 25.Che HL, Bae IH, Lim KS, et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials. 2012;33:8548–8556. doi: 10.1016/j.biomaterials.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 26.Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–1878. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 27.Yeh JS, Oh SJ, Hsueh CM. Frequency of vascular inflammation and impact on neointimal proliferation of drug eluting stents in porcine coronary arteries. Acta Cardiol Sin. 2016;32:570–577. doi: 10.6515/ACS20151013G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz RS, Edelman E, Virmani R, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008;1:143–153. doi: 10.1161/CIRCINTERVENTIONS.108.789974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki T, Kopia G, Hayashi S, et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001;104:1188–1193. doi: 10.1161/hc3601.093987. [DOI] [PubMed] [Google Scholar]
- 30.Lim KS, Park JK, Jeong MH, et al. Comparison of sirolimus loaded PLGA-PEG co-polymer coronary stent and bare metal stent in a porcine coronary restenosis model. Macromol Res. 2014;22:639–646. [Google Scholar]
- 31.Kang SN, Kim SE, Choi J, et al. Comparison of phytoncide with sirolimus as a novel drug candidate for drug-eluting stent. Biomaterials. 2015;44:1–10. doi: 10.1016/j.biomaterials.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Mohacsi PJ, Tuller D, Hulliger B, Wijngaard PL. Different inhibitory effects of immunosuppressive drugs on human and rat aortic smooth muscle and endothelial cell proliferation stimulated by platelet-derived growth factor or endothelial cell growth factor. J Heart Lung Transplant. 1997;16:484–492. [PubMed] [Google Scholar]
- 33.Gregory CR, Huang X, Pratt RE, et al. Treatment with rapamycin and mycophenolic acid reduces arterial intimal thickening produced by mechanical injury and allows endothelial replacement. Transplantation. 1995;59:655–661. doi: 10.1097/00007890-199503150-00002. [DOI] [PubMed] [Google Scholar]
- 34.Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- 35.Di Lorenzo E, Sauro R, Varricchio A, et al. Long-term outcome of drug-eluting stents compared with bare metal stents in ST-segment elevation myocardial infarction: results of the paclitaxel- or sirolimus-eluting stent versus bare metal stent in Primary Angioplasty (PASEO) Randomized Trial. Circulation. 2009;120:964–972. doi: 10.1161/CIRCULATIONAHA.108.830372. [DOI] [PubMed] [Google Scholar]
- 36.Cao Q, Jiang Y, Shi J, et al. Artemisinin inhibits the proliferation, migration, and inflammatory reaction induced by tumor necrosis factor-alpha in vascular smooth muscle cells through nuclear factor kappa B pathway. J Surg Res. 2015;194:667–678. doi: 10.1016/j.jss.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Lee KP, Park ES, Kim DE, et al. Artemisinin attenuates platelet-derived growth factor BB-induced migration of vascular smooth muscle cells. Nutr Res Pract. 2014;8:521–525. doi: 10.4162/nrp.2014.8.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang D, Yuan W, Lv C, et al. Dihydroartemisinin supresses inflammation and fibrosis in bleomycine-induced pulmonary fibrosis in rats. Int J Clin Exp Pathol. 2015;8:1270–1281. [PMC free article] [PubMed] [Google Scholar]
- 39.Yu WY, Kan WJ, Yu PX, et al. Anti-inflammatory effect and mechanism of artemisinin and dihydroartemisinin. Zhongguo Zhong Yao Za Zhi. 2012;37:2618–2621. [PubMed] [Google Scholar]
- 40.Jang S, Jeong MH, Lim KS, et al. Effect of stents coated with artemisinin or dihydroartemisinin in a porcine coronary restenosis model. Korean Circ J. 2017;47:115–122. doi: 10.4070/kcj.2016.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone GW, Lansky AJ, Pocock SJ, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360:1946–1959. doi: 10.1056/NEJMoa0810116. [DOI] [PubMed] [Google Scholar]
- 42.De Luca G, Dirksen MT, Kelbaek H, et al. Paclitaxel-eluting versus bare metal stents in primary PCI: a pooled patient-level meta-analysis of randomized trials. J Thromb Thrombolysis. 2015;39:101–112. doi: 10.1007/s11239-014-1091-4. [DOI] [PubMed] [Google Scholar]
- 43.Fujimori H, Hisama M, Shibayama H, Iwaki M. Protecting effect of phytoncide solution, on normal human dermal fibroblasts against reactive oxygen species. J Oleo Sci. 2009;58:429–436. doi: 10.5650/jos.58.429. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Xiong K, Qi P, et al. Gallic acid tailoring surface functionalities of plasma-polymerized allylamine-coated 316L SS to selectively direct vascular endothelial and smooth muscle cell fate for enhanced endothelialization. ACS Appl Mater Interfaces. 2014;6:2647–2656. doi: 10.1021/am405124z. [DOI] [PubMed] [Google Scholar]
- 45.Lee SY, Bae IH, Park DS, et al. Comparison of dextran-based sirolimus-eluting stents and PLA-based sirolimus-eluting stents in vitro and in vivo. J Biomed Mater Res A. 2017;105:301–310. doi: 10.1002/jbm.a.35898. [DOI] [PubMed] [Google Scholar]