Abstract
Background
Epicardial adipose tissue is an emerging cardio metabolic risk factor. Although an association between epicardial fat thickness (EFT) and left ventricle (LV) hypertrophy in hypertensive patients is known, the relationship between abnormal LV geometric patterns and EFT has yet to be investigated. The aim of the present study was to investigate the relationship between EFT and abnormal LV geometric patterns in hypertensive patients.
Methods
Measurements were obtained from 343 patients with untreated essential hypertension (mean age 51.6 ± 5.5 years) and 52 healthy control subjects (mean age 51.8 ± 4.5 years). Four different geometric patterns (NG; normal geometry, CR; concentric remodeling, EH; eccentric hypertrophy, and CH; concentric hypertrophy) were determined according to LV mass index (LVMI) and relative wall thickness (RWt). EFT was measured using transthoracic echocardiography. High sensitive C-reactive protein (hs-CRP) and other biochemical markers were measured in all participants.
Results
The highest EFT and hs-CRP values were determined in the CH group (EFT = 8.9 ± 2.1 mm) compared with the controls (EFT = 5.7 ± 1.5 mm), followed by the NG (EFT = 5.9 ± 1.6 mm), CR (EFT = 5.9 ± 1.3 mm) and EH groups (EFT = 6.5 ± 1.6 mm) (all p < 0.05). In addition, the EFT values of the EH group were higher than the control, NG and CR groups (all p < 0.05). Multivariate linear regression analysis showed that EFT was independently associated with LV geometry (β = 0.161, p = 0.032), total cholesterol level (β = -0.129, p = 0.003), triglyceride level (β = 0.266, p < 0.001), hs-CRP level (β = 0.349, p < 0.001), and creatinine level (β = 0.108, p = 0.010).
Conclusions
EFT is independently associated with abnormal LV geometry, LV hypertrophy, creatinine level, and low grade chronic inflammation.
Keywords: C-reactive protein, Epicardial fat thickness, Hypertension, Hypertrophy
INTRODUCTION
Epicardial adipose tissue (EAT) is the large visceral fat storage located between the epicardium and pericardium and around the large coronary arteries.1 It has been reported that adipose tissue has important active endocrine and paracrine effects which contribute to the pathogenic mechanism of atherogenesis via cytokines.2 EAT volume has been shown to be independently associated with TIMI risk score in patients with non-ST elevation myocardial infarction and unstable coronary artery disease.3 EAT may therefore serve as a simple tool to predict cardiometabolic risk because of its relationships with metabolic syndrome, coronary artery disease, subclinical atherosclerosis and hypertension, and its thickness can easily and accurately be measured using echocardiography.4,5
An association between epicardial fat thickness (EFT) and hypertension has been shown in previous studies.5-11 In addition, several previous studies have reported that EFT is associated with left ventricle hypertrophy (LVH) in hypertensive patients.12,13 In a recent study by Alp et al., the authors demonstrated that EFT was a predictor of concentric hypertrophy in obese children, however they did not observe any statistically significant differences between hypertensive and normotensive patients. Despite these findings, data on the relationship between EFT and abnormal left ventricular (LV) geometric patterns such as normal geometry (NG), concentric remodeling (CR) and hypertrophic geometric patterns [eccentric hypertrophy (EH) and concentric hypertrophy (CH)] in hypertensive adults are lacking.14 Previous studies have demonstrated that abnormal LV geometric patterns are associated with a greater risk of hypertensive complications.15-17 The goal of the present study was to investigate the relationship between EFT and abnormal LV geometric patterns in hypertensive patients.
METHODS
Measurements were obtained from 343 patients with newly diagnosed essential hypertension (mean age 51.6 ± 5.5 years) and 52 control subjects (mean age 51.8 ± 4.6 years). All of the patients were consecutively enrolled from the Hypertension Outpatient Clinic admissions in Adana Numune Training and Research Hospital. After a 15-minute rest, the blood pressure readings of the participants were recorded. The participants were asked to avoid caffeinated drinks, alcohol, tobacco products, and heavy workout for 30 minutes before the measurements. Their blood pressure was measured in their unclothed right arm while the individual was sitting. A sphygmomanometer and stethoscope were used to measure systolic blood pressure (SBP) and diastolic blood pressure (DBP). Hypertensive patients were defined as those with three clinic blood pressure measurements of > 140/90 mmHg taken at 1-week intervals in the absence of any previous anti-hypertensive treatment to exclude the pharmacological effects on hemodynamics or ventricular hypertrophy and function. The patients were classified into four different geometric pattern groups (NG, CR, EH and CH) according to their LV mass index (LVMI) and relative wall thickness (RWt). The control group was completely healthy and had multiple blood pressure measurements all < 140/90 mmHg, and they were age- and gender-matched to the hypertensive patients.
The exclusion criteria were secondary hypertension, heart failure, positive history or clinical signs of ischaemic heart disease, positive stress test and myocardial perfusion scintigraphy, cerebrovascular disease, severe valvular heart disease, atrial fibrillation, usage of any drugs, severe renal insufficiency (serum creatinine: ≥ 1.5 mg/dl in men and ≥ 1.4 mg/dl in women), major non-cardiovascular diseases and known diabetes or fasting glucose level ≥ 126 mg/dl. The Local Ethics Committee assessed and approved this study, and written informed consent for participation in the study was obtained from all participants.
Blood pressure was measured using a mercury sphygmomanometer. Body mass index (BMI) was calculated as the weight divided by height squared (kg/m2). Body surface area of all subjects was calculated in m2. Blood samples were drawn in the morning after a 20-minute rest following a fasting period of 12 hours. Plasma uric acid, triglycerides, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and fasting glucose were measured using an automated analyzer (Aeroset, Abbott, Abbott Park, IL, USA) with commercial kits (Abbott). High sensitive C-reactive protein (hs-CRP) was measured using an autoanalyzer (Aeroset) with a commercial spectrophotometric kit (Scil Diagnostics GmbH, Viernheim, Germany).
Echocardiography
Standard two-dimensional and Doppler echocardiography was performed using a commercially available echocardiographic machine (Vivid 7R GE Medical Systems, Horten, Norway) with a 2.5-3.5 MHz transducer. Measurements were made during normal breathing at end expiration. LV end-systolic diameter, LV end-diastolic diameter (LVEDD), end-diastolic interventricular septal thickness (IVSth) and end-diastolic LV posterior wall thickness (PWth) were measured at end-diastole according to the established standards of the American Society of Echocardiography.18 LV ejection fraction (EF) was determined according to the biplane Simpson’s method.
Left ventricular mass (LVM) was calculated using the Devereux formula: LVM = (1.04[(LVEDD + IVSth + PWth)3 – (LVEDD)3] – 13.6).19 LVMI was calculated using the following formula: LVM/body surface area. LVH was defined according to more stringent criteria as LVMI values exceeding 125 g/m2 in men and 110 g/m2 in women.20 RWt was measured at end diastole as the ratio of (2 × LV posterior wall thickness)/LVEDD. Increased relative wall thickness (RWT) was defined as ≥ 0.45.16
LV geometric patterns
Geometric patterns were based on the upper normal limits for LVMI and RWT: (1) normal geometry (NG; normal LVMI and normal RWT); (2) concentric remodeling (CR: normal LVMI and increased RWT); (3) concentric hypertrophy (CH; increased LVMI and increased RWT); and (4) eccentric hypertrophy (EH; increased LVMI and normal RWT).3
Measurement of EFT
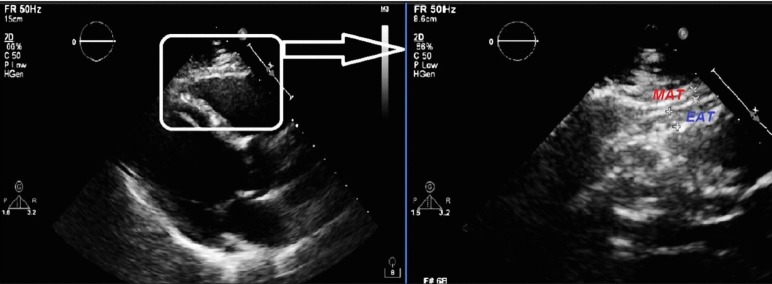
Standard parasternal long-axis and short-axis views from 2-dimensional images were used to measure EFT on the right ventricle in individuals placed in the left lateral decubitus position. Images from standard parasternal long- and short-axis views were digitally stored and reviewed by a single echocardiologist blinded to the clinical data in Adana Numune Training and Research Hospital. Echocardiographically, EFT is generally identified as the relatively echo free space between the outer wall of the myocardium and the visceral layer of pericardium, with its thickness being measured perpendicularly on the free wall of the right ventricle at end systole in three cardiac cycles21 (Figure 1). The average value of three cardiac cycles from each echocardiographic view was determined. EFT in 45 randomly selected subjects was re-measured by the same echocardiologist 1 week later. Inter-observer variability was assessed by calculating the coefficient of variation, which was < 7% for all measurements. Any discrepancy was resolved by consensus. The coefficient of intra-observer variation was 2.1%.
Figure 1.
Measurement of epicardial fat thickness by echocardiography. EAT, epicardial adipose tissue; MAT, mediastinal adipose tissue.
Statistical analysis
All analyses were conducted using SPSS version 17.0 (SPSS for Windows 17.0, Chicago, IL). The distribution of continuous variables was assessed using the one-sample Kolmogorov Smirnov test. Comparisons of categorical variables between groups were performed using the chi-square test. Comparisons among multiple groups (controls and patients according to LV geometric pattern) were performed using one-way analysis of variance (ANOVA) with Tukey’s post hoc test for continuous variables. An overall significance level 0.05 was considered in post hoc comparisons (Table 1 and 2), and for simplicity p < 0.05 was defined as being statistically significant. Correlations between EFT and LV geometry, baseline, hemodynamic and echocardiographic parameters were assessed using the Pearson correlation test. Multiple linear regression analysis was performed to identify the independent associations of EFT among the hypertensive patients. An "abnormal LV geometric pattern" was a dummy variable with normal geometry as 0 and otherwise as 1. A two-tailed p < 0.05 was considered to be statistically significant.
Table 1. Comparison of baseline and laboratory characteristics among the groups.
| Variables | Control (n = 52) | Normal geometry (n = 99) | Concentric remodeling (n = 80) | Eccentric hypertrophy (n = 69) | Concentric hypertrophy (n = 95) | p value |
| Baseline findings | ||||||
| Age, years | 51.8 ± 4.6 | 52.4 ± 4.3 | 50.8 ± 4.9 | 52.1 ± 3.8 | 51.1 ± 7.8 | 0.286 |
| Gender, male | 26 (50%) | 48 (48.5%) | 37 (46.3%) | 29 (42.0%) | 44 (46.3%) | 0.506 |
| BMI, kg/m2 | 26.6 ± 1.1* | 27.4 ± 2.7* | 30.4 ± 4.7† | 29.0 ± 2.4 | 29.4 ± 4.2 | < 0.001 |
| SBP, mmHg | 116.2 ± 7.8‡ | 150.8 ± 17.9§ | 158.0 ± 20.3** | 153.3 ± 18.1** | 166.6 ± 17.4 | < 0.001 |
| DBP, mmHg | 71.3 ± 7.1‡ | 95.4 ± 12.6* | 102.5 ± 11.2** | 99.8 ± 14.6** | 110.0 ± 13.4 | < 0.001 |
| Heart Rate, b/m | 75.9 ± 8.6 | 78.9 ± 12.1 | 79.0 ± 8.3 | 76.3 ± 10.2 | 77.2 ± 9.6 | 0.231 |
| Smoking, n (%) | 20 (38.5%) | 29 (29.3%) | 23 (28.8%) | 29 (42.0%) | 32 (33.7%) | 0.353 |
| Laboratory | ||||||
| Glucose, mg/dl | 89.1 ± 8.2 | 88.4 ± 8.4 | 90.3 ± 9.1 | 89.5 ± 3.4 | 90.7 ± 9.6 | 0.347 |
| TC, mg/dl | 169.2 ± 23.2‡ | 179.6 ± 23.7§ | 191.3 ± 23.1 | 187.6 ± 22.9 | 193.0 ± 37.4 | < 0.001 |
| Triglyceride, mg/dl | 141.8 ± 21.8† | 151.3 ± 30.3† | 152.5 ± 27.3† | 163.8 ± 40.4** | 176.9 ± 41.7 | < 0.001 |
| HDL, mg/dl | 40.4 ± 4.5## | 42.5 ± 5.6†† | 38.9 ± 4.9** | 40.0 ± 6.3 | 41.4 ± 7.1 | 0.001 |
| LDL, mg/dl | 100.5 ± 24.7# | 106.8 ± 23.0# | 121.9 ± 20.0 | 114.8 ± 21.1 | 116.1 ± 32.5 | < 0.001 |
| Hs-CRP, mg/dl | 0.65 ± 0.20 | 0.67 ± 0.21 | 0.73 ± 0.26 | 0.77 ± 0.23‡‡ | 0.93 ± 0.28§§ | < 0.001 |
| Creatinine, mg/dl | 0.74 ± 0.19† | 0.73 ± 0.14† | 0.88 ± 0.15** | 0.78 ± 0.14† | 0.92 ± 0.18 | < 0.001 |
| Uric acid, mg/dl | 3.8 ± 0.7‡ | 4.5 ± 0.9 | 4.4 ± 1.0 | 4.5 ± 0.8 | 4.4 ± 0.9 | < 0.001 |
BMI, body mass index; CH, concentric hypertrophy; CR, concentric remodeling; DBP, diastolic blood pressure; EH, eccentric hypertrophy; HDL, high density lipoprotein; Hs-CRP, high sensitive C reactive protein; LDL, low density lipoprotein; NG, normal geometry; SBP, systolic blood pressure; TC, total cholesterol.
* p < 0.05 vs. CR, EH and CH groups; # p < 0.05 vs. CR, CH and EH groups; † p < 0.05 vs. EH and CH groups; ‡ p < 0.05 vs. NG, CR, EH and CH groups; § p = 0.05 vs. CR and CH groups; ** p < 0.05 vs. CH group; ## p < 0.05 vs. NG group; †† p < 0.05 vs. CR and EH groups; ‡‡ p < 0.05 vs. control and NG groups; §§ p < 0.05 vs. control, NG, CR and EH groups.
Table 2. Comparison of echocardiografic findings among the groups.
| Variables | Control (n = 52) | Normal geometry (n = 99) | Concentric remodeling (n = 80) | Eccentric hypertrophy (n = 69) | Concentric hypertrophy (n = 95) | p value |
| LAD, mm | 30.7 ± 3.8* | 33.1 ± 4.3# | 34.3 ± 3.8 | 34.4 ± 2.7 | 34.2 ± 3.7 | < 0.001 |
| LVID, mm | 46.8 ± 3.6† | 46.4 ± 4.3† | 42.4 ± 3.8‡ | 49.6 ± 3.7§ | 46.0 ± 3.5 | < 0.001 |
| EF, % | 65.9 ± 3.9 | 66.1 ± 3.7 | 67.0 ± 4.6§ | 66.5 ± 5.0 | 65.1 ± 4.2 | 0.066 |
| IVSth, mm | 9.3 ± 0.7# | 9.4 ± 1.0# | 10.6 ± 1.3§ | 10.9 ± 0.6§ | 13.4 ± 1.5 | < 0.001 |
| PWth, mm | 8.7 ± 0.8# | 8.4 ± 0.8# | 10.8 ± 1.1‡ | 9.8 ± 0.9§ | 12.1 ± 1.1 | < 0.001 |
| RWth, mm | 0.37 ± 0.05** | 0.36 ± 0.05‡ | 0.51 ± 0.04‡ | 0.39 ± 0.03§ | 0.52 ± 0.06 | < 0.001 |
| LVMI, g/m2 | 88.0 ± 13.9# | 88.1 ± 10.6# | 96.7 ± 10.8‡ | 127.1 ± 9.8§ | 148.7 ± 24.0 | < 0.001 |
| E/A ratio | 1.4 ± 0.24* | 1.1 ± 0.36‡ | 0.99 ± 0.20§ | 0.97 ± 0.28§ | 0.79 ± 0.21 | < 0.001 |
| EFth, mm | 5.7 ± 1.5‡ | 5.9 ± 1.6‡ | 5.9 ± 1.3‡ | 6.5 ± 1.6§ | 8.9 ± 2.1 | < 0.001 |
E/A, early left ventricle filling velocity/active left ventricle filling velocity; EF, ejection fraction; Efth, epicardial fat thickness; IVSth, interventricular septal thickness; LAD, left atrial diameter; LVID, left ventricle internal diameter; LVMI, left ventricle mass index; PWth, posterior wall thickness; RWth, relative wall thickness.
* p < 0.05 vs. NG, CR, EH and CH group; # p < 0.05 vs. CR, EH and CH groups; † p < 0.05 vs. CR and EH groups; ‡ p < 0.05 vs. EH and CH groups; § p < 0.05 vs. CH group; ** p < 0.05 vs. CR and CH groups. Other abbreviations as Table 1.
RESULTS
The present study enrolled 343 hypertensive patients including 99 with NG, 80 with CR, 69 with EH and 95 with CH.
Baseline and laboratory characteristics
Table 1 shows comparisons of baseline and laboratory characteristics between groups. The highest triglyceride and hs-CRP levels were detected in the CH group compared with the control, NG, CR and EH groups (all p < 0.05). In addition, the hs-CRP level in the EH group was higher than in the control and NG groups (both p < 0.05). Moreover, there were significant differences in BMI, SBP, DBP, total cholesterol, LDL cholesterol, HDL cholesterol, creatinine and uric acid levels between the controls and patient groups (all p < 0.05).
Echocardiographic findings
Comparisons of echocardiographic findings are shown in Table 2. Left atrial diameter (LAD), LVID, IVSth, PWth, relative wall thickness (RWth), LVMI and early left ventricle filling velocity/active left ventricle filling velocity (E/A) were different among the groups (all p < 0.05). The highest EFT values were detected in the CH group compared to the control, NG, CR and EH groups (all p < 0.05). In addition, the EFT value in the EH group was higher than in the control, NG and CR groups (all p < 0.05). On the other hand, the EFT values were similar between the control, NG and CR groups (all p > 0.05). Comparisons of EFT values according to groups are shown in Figure 2.
Figure 2.
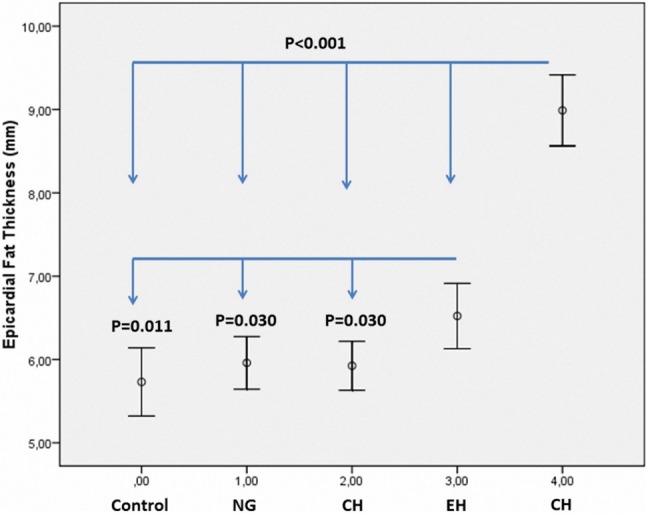
Comparison of epicardial fat thickness among the groups.
Bivariate and multivariate relationships of EFT (Table 3)
Table 3. Bivariate and multivariate relationships of epicardial fat thickness.
| Variables | Pearson correlation coefficient | p value | Standardized β regression coefficients | p value |
| TC, mg/dl | 0.138 | 0.006 | -0.129 | 0.003 |
| Triglyceride, mg/dl | 0.424 | < 0.001 | 0.266 | < 0.01 |
| Creatinin, mg/dl | 0.274 | < 0.001 | 0.108 | 0.010 |
| Hs-CRP, mg/dl | 0.541 | < 0.001 | 0.349 | < 0.01 |
| Abnormal LV geometric patterns | 0.539 | < 0.001 | 0.161 | 0.032 |
EFT was associated with total cholesterol (r = 0.138, p = 0.006), triglyceride level (r = 0.424, p < 0.001), creatinine level (r = 0.274, p < 0.001), hs-CRP level (r = 0.541, p < 0.001), and LV geometry (r = -0.539, p < 0.001) in bivariate analysis. EFT was independently associated with LV geometry (β = 0.161, p = 0.032), total cholesterol level (β = -0.129, p = 0.003), triglyceride level (β = 0.266, p < 0.001), hs-CRP level (β = 0.349, p < 0.001), and creatinine level (β = 0.108, p = 0.010) in multivariate linear regression analysis.
DISCUSSION
To the best of our knowledge, this is the first study to investigate the relationship between EFT and abnormal LV geometric patterns in newly diagnosed hypertensive patients. The highest EFT values were detected in the patients with CH. EFT was independently associated with LV geometry as well as chronic low-grade inflammation as indicated by hs-CRP level.
Previous studies have shown a relationship between EFT and hypertension.5,11 Gastaldelli et al. reported an association between increased blood pressure and ectopic fat accumulation. In their study, both visceral fat tissue and EAT were independently associated with mean blood pressure.6 In addition, a recent report reported an association between early hypertension and increased EFT.7 Moreover, Iacobellis et al. demonstrated that EFT was correlated with DBP,8 and Sengül et al. confirmed the relationship between EFT and hypertension.9 Furthermore, Sengül et al. reported that non-dipper hypertensive patients had higher EFT compared with controls and dipper hypertensive patients.9 A similar finding was demonstrated by Ertas et al.10 In addition, a link between EFT and arterial stiffness has been reported in hypertensive patients.11 Moreover, in the present study, the patients with hypertrophic geometric patterns such as CH and EH had higher EFT values compared to those in the NG, CR and control groups.
The higher EFT values in the patients with hypertrophic geometric patterns in the present study is not surprising. Previous studies have reported a close association between increased EFT and increased ventricular myocardial mass.12,22 Previous studies have also reported that EFT was related to not only the presence of hypertension but also LVH.12 On the other hand, increased EFT has been associated with LVH regardless of the presence of hypertension.12 Several mechanisms may play a role in the relationship between EFT and LVH. EFT may be thought to have a direct influence on the myocardium.23 Previous studies have shown that EFT secretes several proinflammatory mediators, proatherogenic cytokines and growth factors.24,25 In addition, cell culture models have shown that myocardial hypertrophy can be induced by interleukin-6, transforming growth factor-β and macrophage chemotactic factor-1.26,27 Hypertrophied adipocytes in EFT release free fatty acids, which may directly diffuse into the myocardium, together with myocardial uptake of plasma free fatty acids, exacerbating myocardial steatosis, and lipotoxicity.28 Intracardiac lipotoxicity and extracardiac adiposity can lead to increased heart weight and mechanical pumping effort, which can cause LVH, LV diastolic dysfunction, cardiac failure, and increased arrhythmogenicity.28 Manzella et al. demonstrated that increased plasma fatty acid levels may stimulate cardiac autonomic nervous system activity through an increase in plasma catecholamine concentrations,29 which then induce LVH.30 Recent studies of EFT have shown that EFT reflects arterial stiffness,11 and increased arterial stiffness plays an important role in the pathogenesis of LVH. Therefore, increased EFT may explain the relationship between increased aortic stiffness and LVH in hypertensive patients. Taken together, these findings may explain the higher EFT values in hypertrophic geometric patterns.
Previous studies have reported that abnormal LV geometric patterns are associated with a greater risk of hypertensive complications.15-17 In addition, Katlandur et al. demonstrated that EFT was significantly correlated with the severity and prevalence of coronary artery disease in positive exercise test patients.31 In particular, hypertensive patients with CH have been reported to have the highest mortality and cardiovascular event rates.15 In the present study, the highest EFT values were recorded in the CH group. Similarly, the highest hs-CRP and LVMI values were also observed in the CH group. Therefore, increased EFT values in patients with CH may contribute to a poor prognosis in hypertensive patients. Patients with EH or CR have intermediate risk rates, and patients with normal LV geometry have been reported to have the lowest cardiovascular risk.15-17 The EFT values in the patients with EH, which incorporates increased LVMI and normal RWth, were higher than those in the NG and CR groups. In conclusion, the increase in EFT is synchronized with LVH in hypertensive patients. Ozturk et al. demonstrated that EFT is associated with microalbuminuria in patients with essential hypertension.32 Moreover, Aydın et al. reported that hemodialysis patients were independently associated with high EFT.33 The results of the present study suggest that measurements of EFT may be a useful tool for monitoring the follow-up of hypertensive patients with target organ damage such as LVH.
Increased EAT and epicardial adipokines augment the inflammatory process in the myocardium and coronary arteries by secreting various paracrine and vasocrine mediators.34 Previous studies have reported an association between EFT and inflammation in patients with hypertension and metabolic syndrome.35,36 Increased EFT has also been more strongly associated with a systemic inflammatory response than general risk factors and body fat composition.37 In addition, Alpaydın et al. reported that rheumatoid arthritis disease activity as reflected by disease activity score-28 (DAS-28) had an impact on left ventricular diastolic function and EFT in patients with rheumatoid arthritis with no traditional cardiovascular risk factors.38 Consistent with previous studies, we also found that EFT was associated with chronic low-grade inflammation as indicated by the high hs-CRP level.
A relationship between EFT and blood pressure has been reported in previous studies.6,8 Kim et al. demonstrated that enlargement of visceral fat tissue was associated with increased blood pressure which was predominantly due to activated renin-angiotensin system.39 In the present study, both SBP and DBP were associated with EFT. However, these relationships were not observed in multivariate linear regression analysis. Several studies have reported significant associations between cardiac fat and dyslipidemia.40 Moreover, the present study identified a relationship between EFT and triglyceride and total cholesterol levels. Finally, the present study demonstrates an independent relationship between EFT and creatinine level as well as LV geometry, suggesting that echocardiographic EFT measurements could be an easy-to-measure tool for the early detection of subclinical target organ damage.31,32
Study limitations
In the present study, EFT measurements obtained by echocardiography were used for comparisons. Although EFT and its volume are better visualized on high-speed computerized tomography and cardiac magnetic resonance imaging, it is not practical to use these methods for routine assessments. Echocardiographic EFT measurements are noninvasive, cheaper, do not require radiation exposure, and are easily available at the bedside. Moreover, its applicability and reliability has been validated in numerous previous studies.
CONCLUSIONS
The patients with hypertrophic geometric patterns such as EH and CH had higher EFT values. Moreover, EFT was associated with both LVH and also LV geometry, chronic low-grade inflammation and creatinine level. Measurements of EFT may be a useful and non-invasive tool for monitoring the follow-up of hypertensive patients with end organ damage such as LVH and nephropathy.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Br J Pharmacol. 2012;165:659–669. doi: 10.1111/j.1476-5381.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau DC, Dhillon B, Yan H, et al. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:2031–2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 3.Ozcan F, Turak O, Canpolat U, et al. Association of epicardial fat thickness with TIMI risk score in NSTEMI/USAP patients. Herz. 2014;39:755–760. doi: 10.1007/s00059-013-3914-z. [DOI] [PubMed] [Google Scholar]
- 4.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–1319. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Teijeira-Fernandez E, Eiras S, Grigorian-Shamagian L, et al. Epicardial adipose tissue expression of adiponectin is lower in patients with hypertension. J Hum Hypertens. 2008;22:856–863. doi: 10.1038/jhh.2008.75. [DOI] [PubMed] [Google Scholar]
- 6.Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: what is the link? Nutr Metab Cardiovasc Dis. 2010;20:481–490. doi: 10.1016/j.numecd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Sironi AM, Pingitore A, Ghione S, et al. Early hypertension is associated with reduced regional cardiac function, insulin resistance, epicardial, and visceral fat. Hypertension. 2008;51:282–288. doi: 10.1161/HYPERTENSIONAHA.107.098640. [DOI] [PubMed] [Google Scholar]
- 8.Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 9.Sengul C, Cevik C, Ozveren O, et al. Epicardial fat thickness is associated with non-dipper blood pressure pattern in patients with essential hypertension. Clin Exp Hypertens. 2012;34:165–170. doi: 10.3109/10641963.2011.577488. [DOI] [PubMed] [Google Scholar]
- 10.Ertas F, Kaya H, Acet H, et al. Increased echocardiographic epicardial fat thickness is related to impaired diurnal blood pressure profiles. Blood Press. 2012;21:202–208. doi: 10.3109/08037051.2011.649538. [DOI] [PubMed] [Google Scholar]
- 11.Natale F, Tedesco MA, Mocerino R, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr. 2009;10:549–555. doi: 10.1093/ejechocard/jep002. [DOI] [PubMed] [Google Scholar]
- 12.Iacobellis G, Ribaudo MC, Zappaterreno A, et al. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;94:1084–1087. doi: 10.1016/j.amjcard.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 13.Nelson MR, Mookadam F, Thota V, et al. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011;24:339–345. doi: 10.1016/j.echo.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Alp H, Karaarslan S, Eklioğlu BS, et al. The effect of hypertension and obesity on left ventricular geometry and cardiac functions in children and adolescents. J Hypertens. 2014;32:1283–1292. doi: 10.1097/HJH.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 15.Koren MJ, Devereux RB, Casale PN, et al. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 16.Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 17.Krauser DG, Devereux RB. Ventricular hypertrophy and hypertension: prognostic elements and implications for management. Herz. 2006;31:305–316. doi: 10.1007/s00059-006-2819-5. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Devereux RB, Reichek N. Echocardiographic determination 14 of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 20.Guidelines Committee. European Society of Hypertension–European Society of Cardiology guidelines for management of arterial hypertension. J Hypertens. 2003;21:1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Iacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 22.Corradi D, Maestri R, Callegari S, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004;13:313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 2011;34:371–379. doi: 10.2337/dc11-s250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 26.Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci USA. 1995;92:4862–4866. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melendez GC, McLarty JL, Levick SP, et al. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225–231. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease. Pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 29.Manzella D, Barbieri M, Rizzo MR, et al. Role of free fatty acids on cardiac autonomic nervous system in noninsulin-dependent diabetic patients: effects of metabolic control. J Clin Endocrinol Metab. 2001;86:2769–2774. doi: 10.1210/jcem.86.6.7553. [DOI] [PubMed] [Google Scholar]
- 30.Kimura K, Ieda M, Kanazawa H, et al. Cardiac sympathetic rejuvenation: a link between nerve function and cardiac hypertrophy. Circ Res. 2007;100:1755–1764. doi: 10.1161/01.RES.0000269828.62250.ab. [DOI] [PubMed] [Google Scholar]
- 31.Katlandur H, Ulucan Ş, Özdil H, et al. Evaluation of echocardiographic epicardial fat thickness as a sign of cardiovascular risk in positive exercise test patients. Acta Cardiol Sin. 2016;32:684–689. doi: 10.6515/ACS20160110A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozturk MT, Ebinç FA, Okyay GU, et al. Epicardial adiposity is associated with microalbuminuria in patients with essential hypertension. Acta Cardiol Sin. 2017;33:74–80. doi: 10.6515/ACS20160418A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aydın E, Altın C, Sakallıoğlu O, et al. Epicardial adipose tissue thickness and carotid intima-Media thickness in hemodialysis patients. Acta Cardiol Sin. 2017;33:266–272. doi: 10.6515/ACS20161023A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–457. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turak O, Özcan F, Canpolat U, et al. Increased echocardiographic epicardial fat thickness and high-sensitivity CRP level indicate diastolic dysfunction in patients with newly diagnosed essential hypertension. Blood Press Monit. 2013;18:259–264. doi: 10.1097/MBP.0b013e3283651d19. [DOI] [PubMed] [Google Scholar]
- 36.Tok D, Kadife I, Turak O, et al. Increased epicardial fat thickness is associated with low grade systemic inflammation in metabolic syndrome. Turk Kardiyol Dern Ars. 2012;40:690–695. doi: 10.5543/tkda.2012.60207. [DOI] [PubMed] [Google Scholar]
- 37.Lai YH, Yun CH, Yang FS, et al. Epicardial adipose tissue relating to anthropometrics, metabolic derangements and fatty liver disease independently contributes to serum high-sensitivity C-reactive protein beyond body fat composition:a study validated with computed tomography. J Am Soc Echocardiogr. 2012;25:234–241. doi: 10.1016/j.echo.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Alpaydın S, Buyukterzi Z, Akkurt HE, et al. Impaired left ventricular diastolic functions and thickened epicardial adipose tissue in rheumatoid arthritis patients is correlated with DAS-28 score. Acta Cardiol Sin. 2017;33:182–187. doi: 10.6515/ACS20160608B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BJ, Kim BS, Kang JH. Echocardiographic epicardial fat thickness is associated with arterial stiffness. Int J Cardiol. 2013;167:2234–2238. doi: 10.1016/j.ijcard.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Whayne TF., Jr. Epicardial fat thickness in heart failure and other clinical conditions. Angiology. 2013;64:169–172. doi: 10.1177/0003319712450310. [DOI] [PubMed] [Google Scholar]