Abstract
Objective
Collaborative care for depression is effective and cost-effective in primary care settings. However, there is minimal evidence to inform the choice of on-site versus off-site models. This study examined the cost-effectiveness of on-site practice-based collaborative care (PBCC) versus off-site telemedicine-based collaborative care (TBCC) for depression in Federally Qualified Health Centers (FQHCs).
Methods
Multi-site randomized pragmatic comparative cost-effectiveness trial. 19,285 patients were screened for depression, 14.8% (n=2,863) screened positive (PHQ9 ≥10) and 364 were enrolled. Telephone interview data were collected at baseline, 6-, 12-, and 18-months. Base case analysis used Arkansas FQHC healthcare costs and secondary analysis used national cost estimates. Effectiveness measures were depression-free days and quality-adjusted life years (QALYs) derived from depression-free days, Medical Outcomes Study SF-12, and Quality of Well Being scale (QWB). Nonparametric bootstrap with replacement methods were used to generate an empirical joint distribution of incremental costs and QALYs and acceptability curves.
Results
Mean base case FQHC incremental cost-effectiveness ratio (ICER) using depression-free days was $10.78/depression-free day. Mean base case ICERs using QALYs ranged from $14,754/QALY (depression-free day QALY) to $37,261/QALY (QWB QALY). Mean secondary national ICER using depression-free days was $8.43/depression-free day and using QALYs ranged from $11,532/QALY (depression-free day QALY) to $29,234/QALY (QWB QALY).
Conclusions
These results support the cost-effectiveness of the TBCC intervention in medically underserved primary care settings. Results can inform the decision about whether to insource (make) or outsource (buy) depression care management in the FQHC setting within the current context of Patient-Centered Medical Home, value-based purchasing, and potential bundled payments for depression care.
The www.clinicaltrials.gov # for this study is NCT00439452.
INTRODUCTION
According to the 2010 Census, 19.3% of the US population resides in rural areas, which is a risk factor for poor detection and treatment of mental health disorders(1). Possible explanations for this urban/rural disparity include longer travel distances, lack of mental health specialists co-located in primary care settings, weak linkages to off-site mental health specialists, limited mental health insurance coverage, and higher levels of stigma(2).
Collaborative care for depression has been shown to be highly effective(3-6), and cost-effective (7-10) in urban settings, but is difficult to implement in federally-designated mental health professional shortage areas (85% of rural counties)(11). While collaborative care for depression can be adapted successfully for rural primary care settings using telemedicine technologies(12), it is critical to also assess the cost-effectiveness of this approach.
Federally Qualified Health Centers (FQHCs) are a critical component of the healthcare safety net and are located in medically underserved areas. In 2012, FQHCs served approximately 21 million patients and this number could double by 2015 with the passage of the Patient Protection and Affordable Care Act(13). Three-quarters of FQHC patients live in poverty, half live in rural areas, one-third are uninsured, and two-thirds are members of racial/ethnic minority groups. Mental health problems are the most commonly reported reasons for visits to FQHCs(14), yet only 5.5% of encounters are with on-site mental health specialists(15).
Requirements for Patient-Centered Medical Home (PCMH) recognition and anticipation of bundled payments for depression care are focusing FQHCs’ attention and resources on depression recognition and management. National Committee for Quality Assurance PCMH recognition requires team-based care that emphasizes care coordination. Although the Centers for Medicare & Medicaid Services (CMS) Bundled Payments for Care Improvement initiative does not currently include depression in their list of clinical condition episodes, it is anticipated that depression will be added in the future.
A common decision facing clinics striving for PCMH recognition and preparing for bundled payments is whether to insource or outsource care management services. To inform this decision, we conducted a cost-effectiveness analysis of these two alternative approaches to providing depression care management in FQHCs. The on-site insource approach, practice-based collaborative care (PBCC), focused on improving depression outcomes using local providers. The off-site outsource approach, telemedicine-based collaborative care (TBCC), focused on utilizing off-site specialists to support local primary care providers.
METHODS
Design Overview
This multi-site pragmatic randomized trial employed a comparative effectiveness design(16). Patients were randomized to either TBCC or PBCC, both of which represent potentially feasible approaches to adapting the evidence-based collaborative depression care model for routine delivery in medically underserved areas. The intervention and evaluation methods are described in detail elsewhere(12) and summarized here.
Setting and Participants
Six FQHCs were approached and five (83.3%) agreed to participate. Participating FQHCs employed between 1.3 and 9.7 PC physician FTEs, served between 5,362 and 13,050 unique PC patients, and operated one to six clinics across multiple locations. None of the participating clinic locations had an on-site MH specialist. From 2007-2009, 19,285 patients were screened for depression, 14.8% (n=2,863) screened positive (PHQ9≥10) and 364 were enrolled. We excluded patients with schizophrenia, bipolar disorder, or acute suicide ideation. Patients (stratified by clinic) were randomized to PBCC or TBCC. Blinded follow-up telephone interviews were completed for 87% (318/364) at 6 months, 79% (287/364) at 12 months and 78% (283/364) at 18 months. This study was approved by the University of Arkansas for Medical Sciences Institutional Review Board. After complete description of the study to the patients, written informed consent was obtained.
Interventions
PBCC involved two types of providers: on-site PC providers and on-site nurse depression care managers (DCM). Each clinic location employed a half-time DCM funded by the study. All DCMs received one day of training in depression care management, a care manager training manual, and access to a web-based decision support system (https://www.netdss.net/)(17). DCM encounters were conducted either face-to-face or by telephone depending on patient preference. The initial DCM encounter included: PHQ9 symptom monitoring; education/activation; barrier assessment/resolution; and establishing self-management goals (e.g., planning physical, rewarding, and social activities). Follow-up encounters, included the monitoring of: PHQ9 symptoms, medication adherence, side-effects, and engagement in planned self-management activities. PBCC DCMs received no supervision from a mental health specialist. Patients could be referred to off-site specialists (e.g., Community Mental Health Centers). Progress notes were entered into the patients’ paper medical record. Patients received the intervention for up to 12 months.
TBCC involved five types of providers: on-site PC providers and off-site DCM (RN), clinical pharmacist (PharmD), psychologist (PhD) and psychiatrist (MD). The off-site team was funded by the study and located at the University of Arkansas for Medical Sciences. All DCM encounters were conducted by telephone and followed the protocol described above. DCM progress notes were faxed to the Health Center. During weekly meetings, the DCM received psychiatric supervision and discussed new patients and patients failing treatment, and offered stepped-care treatment recommendations to PC providers via the DCM progress notes. If the patient did not respond to the initial antidepressant, the telephone pharmacist conducted a medication history and provided medication management recommendations as needed. If the patient did not respond to two trials, a psychiatry consultation via interactive video was scheduled. At any time, patients had access to cognitive behavioral therapy delivered via interactive video.
Depression Outcomes
It has been previously reported that the TBCC group experienced a significantly greater treatment response (odds ratio=7.74, 95% CI=3.94–15.20), remission (odds ratio=12.69, 95% CI=4.81–33.46), and overall group-by-time interaction effect for depression severity on the 20-item Hopkins Symptom Checklist (SCL-20) (chi-square=40.51, df=3, p<.001) with greater reductions in the TBCC group(12).
Cost-effectiveness Outcomes
Primary effectiveness outcomes for the cost-effectiveness analysis were depression-free days derived from the SCL-20(18) and QALYs calculated using a depression-free day to quality-adjusted life year (QALY) conversion(19), Medical Outcomes Study SF-12 standard gamble to QALY conversion(20), and Quality of Well-Being scale (QWB)(21). Generic QALYs from the SF-12 and QWB are reported because generic QALYs are the recommended unit of effectiveness for the base case cost-effectiveness analysis(22).
Depression-free days were calculated using the formula originally developed by Lave and colleagues(23) and adapted for the SCL-20(18). SCL-20 score of .5 or less was considered depression free, a score of 1.7 or higher was considered fully symptomatic, and scores in between were assigned a linear proportional value. Using variations of the depression-free day definition (.25 to .75 for depression-free and 1.5 to 2.0 for fully symptomatic) resulted in minimal depression-free day differences. To determine the incremental depression-free day-QALYs, we divided the 18-month depression-free day difference by 365 then multiplied by the lower (.2) and upper (.4) bound of the QALY increase associated with going from fully symptomatic to depression-free(19).
QALYs derived from the SF-12 used standard gamble preference weights(20) which transform SF-12 data into a preference-weighted index score that varies from 0 (death) to 1.0 (perfect health). Similarly, the QWB subscales represent preference-weighted scores which are subtracted from 1.0 (perfect health) to determine the QWB index score ranging from 0 (death) to 1.0 (perfect health)(24).
Intervention costs and health care costs were collected using a societal perspective (healthcare utilization and patient costs) and were adjusted to reflect year 2009 dollars. The societal perspective was recommended by US Public Health Panel on Cost-effectiveness in Health and Medicine. Fixed intervention costs included the cost of DCM education materials, DCM training, and interactive video equipment (TBCC only). There was one DCM for TBCC and six DCMs for PBCC. DCM training costs used 2009 Bureau of Labor Statistics median hourly wage for registered nurses (eight hours of training time) plus 25% fringe benefit. Equipment costs included interactive video stations, routers, and installation depreciated over the course of the study. The annual depreciation rate was 18.33% (from US Bureau of Economic Analysis for medical equipment) over four years (total duration of recruitment and intervention). Variable intervention costs included the time spent by intervention personnel delivering the intervention. Time costs for intervention personnel were estimated using 2009 Bureau of Labor Statistics hourly wage data plus 25% fringe benefit (http://www.bls.gov/oes/oes_dl.htm). DCM time was estimated using the number of encounters from chart review and an estimated 1.5 hours for initial encounter and 1.0 hours for follow-up encounters (includes time to get the patient on the phone, conduct the phone interview, and chart the encounter). Variable TBCC intervention costs also included pharmacist, psychologist, and psychiatrist time and T1 line monthly charges for tele-mental health encounters. Intervention clinician time was estimated by the number of progress notes written by each provider and the time spent in team meetings. For the base case analysis, we assumed that 40% of T1 charges were attributable to TBCC based on reports in the literature that 40% of patients seen at an university-based telepsychiatry service had a primary depression diagnosis(25). Sensitivity analyses varied T1 cost assumptions from 0 to 100%.
Healthcare costs were based on patient self-reported service utilization using the Quality Improvement for Depression (QID) collaboration service utilization instrument. Patients were asked about service utilization for physical health problems and mental health problems (“personal or emotional problems such as feeling down or anxious, or for alcohol or drug problems”).
The base case analysis used FQHC costs and the secondary analysis used national costs. Outpatient FQHC visit costs were estimated using the FQHC Prospective Payment System rates for Arkansas. Non-FQHC outpatient visit costs were estimated using Arkansas Blue Cross Blue Shield data. ER and inpatient costs were estimated using academic medical center and affiliated hospital data, including safety net providers, from the University HealthSystem Consortium (UHC) Southern Region. Medication costs approximated the discounts provided to FQHCs by the 340B Drug Pricing Program by applying the average discount for the top 10 prescribed physical health and mental health medications in this study (76% and 86%, respectively) to the lowest average wholesale Red Book of Prescription Drugs price. Patient time and mileage associated with healthcare utilization were collected from patient self-report. Patient time costs were estimated using 2009 US Census Bureau wage estimates based on age, gender, and education for employed patients and minimum wage ($7.25) for unemployed patients. Patient mileage costs were estimated using the 2009 General Services Administration reimbursement rate of $.59 per mile.
For the secondary analysis, healthcare costs were estimated from the Lifelink Health Plans Claims Database from Pharmetrics, Inc. which comprises 70 million enrollees from 80 Managed Care Organizations and is nationally representative of the commercially insured US population. Inpatient physical health per diem costs were estimated from the median allowed cost of the top 10 most frequent non-mental health ICD-9 diagnoses. Inpatient mental health per diem costs and ER costs for physical and mental health visits were estimated from their respective Clinical Classification Software (CCS) codes. Outpatient costs were estimated based on their respective CPT codes. Medication costs were estimated using the Red Book lowest average wholesale price.
Incremental cost-effectiveness ratios (ICER) are the ratio of the difference in total costs between TBCC and PBCC divided by the difference in effectiveness (depression-free days or QALYs). The base case analysis included the SF-12 QALY and outpatient, emergency room, pharmacy, patient (travel and time), intervention, and 40% of monthly T1 costs. Sensitivity analyses included 0% or 100% of the T1 costs, using alternative depression-free day to QALY conversions (.4 and .2), QWB QALYs, and adding mental health inpatient costs. Secondary analyses included cost estimates from the nationally representative Lifelink claims data.
Casemix Variables
At baseline, socio-demographic and clinical casemix factors were collected using the Depression Outcomes Module(26), Mini International Neuropsychiatric Interview(27,27), Duke Social Support and Stress Scale(28), Quality Improvement for Depression Treatment Acceptability scale(5), and the Depression Health Beliefs Inventory(29). Zip codes were used to categorize patient’s residence as rural or urban according to Rural Urban Commuting Area.
Statistical Analysis
Patients were the unit of the intent-to-treat analysis. Only patients with at least one research follow-up visit were included in the analyses. All models specified clinic as a random effect to control for intra-class correlation. Variables with missing data were imputed using multiple imputation methods. The level of missing data was .3% for four cost variables and two demographic variables and 15.7% for the SF-12 at 18 months. Due to the large number of available covariates, only those with significant differences between TBCC and PBCC at p<.20 were included in multivariate analyses. After model specification was finalized, pre-baseline costs were added as a covariate to cost models .
The depression-free day and cost outcomes were non-normally distributed so generalized linear models (GLMs) were used. The best fit for the cost data was the GLM with a gamma distribution and identity link. The depression-free day and QALY data were normally distributed so the normal distribution with identity link was used. To determine the incremental treatment effect, we used the regression coefficient for the intervention.
We used a nonparametric bootstrap with replacement method and 1000 replications to generate an empirical joint distribution of incremental costs and QALYs(30) and acceptability curves representing the probability of falling below CE ratio thresholds ranging from $0 to $100,000 per QALY(31).
RESULTS
In general, study patients were middle-aged, low income, Caucasian females with moderate depression who were unemployed and uninsured (Table 1). The only statistically significant differences between the intervention groups was a higher level of perceived barriers to depression treatment in the TBCC group (4.0±2.1) compared with the PBCC group (3.4±2.0, p=.01).
Table 1.
Baseline Group Socio-Demographic and Clinical Characteristicsa
| Characteristics | All N=332 |
Telemedicine Based Collaborative Care N=163 |
Practice Based Collaborative Care N=169 |
P value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Age (mean±SD) | 47.9±12.4 | 48.3±12.2 | 47.6±12.6 | .60 | |||
| Male | 62 | 18.7 | 30 | 18.4 | 32 | 18.9 | .90 |
| Race/Ethnicity | .88 | ||||||
| Caucasian | 237 | 71.4 | 118 | 72.4 | 119 | 70.4 | |
| African American | 69 | 20.8 | 33 | 20.3 | 36 | 21.3 | |
| Native American | 17 | 5.1 | 7 | 4.3 | 10 | 5.9 | |
| Other | 9 | 2.7 | 5 | 3.1 | 4 | 2.4 | |
| Income | .67 | ||||||
| < $10,000 | 95 | 29.6 | 52 | 32.5 | 43 | 26.7 | |
| $10,000 - $15,000 | 76 | 23.7 | 38 | 23.7 | 38 | 23.6 | |
| $15,000 - $20,000 | 51 | 15.9 | 28 | 17.5 | 23 | 14.3 | |
| $20,000 - $30,000 | 56 | 17.5 | 24 | 15.0 | 32 | 19.9 | |
| $30,000 - $40,000 | 23 | 7.2 | 9 | 5.6 | 14 | 8.7 | |
| $40,000 - $50,000 | 12 | 3.7 | 6 | 3.8 | 6 | 3.7 | |
| >$50,000 | 8 | 2.5 | 3 | 1.9 | 5 | 3.1 | |
| Married | 151 | 45.5 | 74 | 45.4 | 77 | 45.6 | .98 |
| High school graduate | 245 | 74.0 | 117 | 72.2 | 128 | 75.7 | .47 |
| Employed | 122 | 36.9 | 52 | 31.9 | 70 | 41.7 | .07 |
| Insurance | .29 | ||||||
| Public insurance | 100 | 30.1 | 57 | 35.0 | 43 | 25.4 | |
| Private insurance | 50 | 15.1 | 24 | 14.7 | 26 | 15.4 | |
| Both insurance | 12 | 3.6 | 5 | 3.1 | 7 | 4.1 | |
| Uninsured | 170 | 51.2 | 77 | 47.2 | 93 | 55.0 | |
| Rural | 229 | 70.0 | 110 | 67.5 | 119 | 70.4 | .56 |
| Social support | .4±.2 | .4±.2 | .4±.2 | .77 | |||
| Perceived barriers | 3.7±2.0 | 4.0±2.1 | 3.4±2.0 | .01 | |||
| Perceived need | 3.0±1.5 | 3.1±1.4 | 2.9±1.5 | .15 | |||
| Perceived treatment effectiveness | 1.3±.7 | 1.4±.7 | 1.3±.7 | .42 | |||
| SCL-20 (depression severity score) | 1.9±.8 | 1.9±.8 | 1.9±.7 | .79 | |||
| SF-12 PCS (physical component score) | 36.7±13.4 | 35.8±13.2 | 37.7±13.5 | .20 | |||
| SF-12 MCS (mental component score) | 31.3±11.3 | 32.4±11.4 | 30.2±11.2 | .08 | |||
| QWB (Quality of Well-Being) | .4±.1 | .4±.1 | .4±.1 | .43 | |||
| Chronic physical illness count | 4.6±2.6 | 4.8±2.5 | 4.4±2.7 | .21 | |||
| Family history of depression | 191 | 58.4 | 102 | 63.8 | 89 | 53.3 | .06 |
| Age depression onset <18 | 129 | 40.4 | 61 | 39.4 | 68 | 41.5 | .70 |
| Prior depression episodes count | 4.2±1.6 | 4.2±1.6 | 4.2±1.6 | .87 | |||
| Prior depression treatment | 251 | 75.6 | 120 | 73.6 | 131 | 77.5 | .41 |
| Current depression Treatment | 160 | 48.2 | 76 | 46.6 | 84 | 49.7 | .57 |
| Antidepressants acceptable | 276 | 85.2 | 136 | 85.0 | 140 | 85.4 | .93 |
| Counseling acceptability | 248 | 76.5 | 125 | 78.1 | 123 | 75. | .51 |
| Current major depressive disorder | 276 | 83.1 | 130 | 79.8 | 146 | 86.4 | .11 |
| Current dysthymia | 10 | 3.0 | 6 | 3.7 | 4 | 2.4 | .54 |
| Current panic disorder | 28 | 8.4 | 13 | 8.0 | 15 | 8.9 | .77 |
| Current generalized anxiety disorder | 211 | 63.6 | 107 | 65.6 | 104 | 61.5 | .44 |
| Current post-traumatic stress disorder | 54 | 16.3 | 29 | 17.8 | 25 | 14.8 | .46 |
| Current at-risk drinking | 14 | 4.2 | 8 | 4.9 | 6 | 3.6 | .54 |
Some numbers do not add up to total number of patients because of missing data and some percentages do not add up to 100 because of rounding. Values expressed as mean (standard deviation) unless otherwise indicated
Abbreviations: SCL-20, Symptom Checklist-20 item; SF-12, Short Form 12-item
Although there were no statistically significant group differences in terms of health care costs, the overall TBCC cost was significantly greater than the overall PBCC cost because of the higher TBCC fixed and variable intervention cost (Table 2). The unadjusted average incremental intervention cost (fixed+variable) was $1,132. For the base case analysis, the adjusted TBCC total cost was significantly greater than PBCC (β=1,146, 95%CI=396-1,897, p=.003). The adjusted incremental cost ranged from $794 (95%CI=56-1533, p=.03) for 0% T1 monthly charge to $1,663 (95%CI=884-2,442, p<.001) for 100% T1 monthly charge.
Table 2.
Average Unadjusted Cost per Patient by Category Baseline to 18 months (2009 dollars)
| Characteristics | Practice Based Collaborative Care (N=169) |
Telemedicine Based Collaborative Care (N=163) |
Difference | P value | |
|---|---|---|---|---|---|
| Intervention Fixed Cost | 13.19 | 389.51 | 376.32 | <.001 | |
| Education | 2.31 | .4 | −1.91 | ||
| Training | 10.88 | 4.75 | −6.13 | ||
| Equipment | 0 | 384.36 | 384.36 | ||
| Intervention Variable Cost | 78.44 | 834.46 | 756.02 | <.001 | |
| Care manager | 78.44 | 338.7 | 260.26 | ||
| Psychologist | 0 | 119.63 | 119.63 | ||
| Pharmacist | 0 | 56.76 | 56.76 | ||
| T1 line (40%) | 0 | 222.33 | 222.33 | ||
| Psychiatrist | 0 | 97.04 | 97.04 | ||
| Outpatient Cost | 6559.42 | 7178.72 | 619.3 | .29 | |
| PH emergency | 2701.73 | 2805.35 | 103.62 | ||
| MH emergency | 233.44 | 491.60 | 258.16 | ||
| PH primary care | 958.31 | 1066.91 | 108.60 | ||
| MH primary care | 779.97 | 709.22 | −70.75 | ||
| Psychiatrist | 320.14 | 526.63 | 206.49 | ||
| PH specialist | 718.79 | 683.27 | −35.52 | ||
| PH medication | 712.61 | 749.29 | 36.68 | ||
| MH medication | 16.02 | 14.95 | −1.07 | ||
| Antidepressant medication | 118.41 | 131.50 | 13.09 | ||
| MH Inpatient Cost | 45.36 | 188.13 | 142.77 | .19 | |
| Patient Cost | 354.90 | 340.40 | −14.50 | .69 | |
| PH gas | 125.71 | 117.29 | −8.42 | ||
| PH travel + waiting | 127.69 | 127.93 | .24 | ||
| MH gas | 46.07 | 42.03 | −4.04 | ||
| MH travel + waiting | 55.43 | 53.15 | −2.28 | ||
| Total Cost | |||||
| 0% T1 line charge | 7005.94 | 8136.39 | 1130.44 | .054 | |
| 40% T1 line* | 7005.94 | 8512.46 | 1506.52 | .01 | |
| 100% T1 line | 7005.94 | 9076.57 | 2070.63 | .0007 | |
| 40% T1 line + MH inpatient cost | 7051.30 | 8700.59 | 1649.29 | .0069 | |
base case cost analysis
Abbreviations: PH, physical health; MH, mental health
The adjusted incremental effectiveness on depression-free days was significant (β=109.6, 95%CI=79.7-139.5, p<.001), and the incremental effectiveness for depression-free day QALYs was significant using both the .2 and .4 QALY difference between fully symptomatic and depression-free (β=.04, 95%CI=.029-.051 and β=.078, 95%CI=.057-.1, respectively, both p-values<.001). The adjusted incremental generic QALY effectiveness was also significant (SF-12 QALY: β=.043, 95%CI=.015-.071, p=.003 and QWB QALY: β=.038, 95%CI=.009-.067, p=.01).
The bootstrapped mean ICER using FQHC cost and depression-free days was $10.75/depression-free day. The mean range in the sensitivity analyses was $7.49 (0% T1 charge) to $15.49 (100% T1 charge). The mean ICER using FQHC cost and SF-12 QALY was $33,217/QALY (Table 3). The sensitivity analyses for the QALY estimates ranged from $14,714/QALY (depression-free day QALY.4) to $35,762/QALY (QWB QALY). The T1 charge sensitivity analyses using FQHC cost and SF-12 QALY ranged from $22,548/QALY (0% T1 charges) to $48,789/QALY (100% T1 charges). Adding inpatient mental health costs to the SF-12 QALY base case resulted in an ICER of $36,033/QALY. Figures 1 and 2 depict the base case bivariate incremental cost and QALY scatter plot and acceptability curve, respectively.
Table 3.
Bootstrapped Adjusted Incremental Outpatient Cost per QALY Results (2009 dollars)1
| Mean Incremental Cost per QALY Ratio | Interquartile Range | |
|---|---|---|
| FQHC Perspective2 | ||
| SF-12 QALY* | 33,217 | 18,744 – 39,298 |
| QWB QALY | 35,762 | 20,336 – 44,299 |
| Depression-free day .2 QALY | 29,428 | 21,588 – 36,740 |
| Depression-free day .4 QALY | 14,714 | 10,794 – 18,370 |
| National Perspective3 | ||
| SF-12 QALY* | 25,728 | 14,684 – 30,045 |
| QWB QALY | 28,017 | 16,044 – 34,418 |
| Depression-free day .2 QALY | 23,158 | 16,418 – 29,326 |
| Depression-free day .4 QALY | 11,579 | 8,209 – 14,663 |
Abbreviations: QALY, quality adjusted life year; FQHC, federally qualified health system; SF-12, Medical Outcomes Study Short Form 12-item; QWB, Quality of Well Being scale; Depression-free day .2 QALY, depression free day to QALY conversion using .2 to estimate change from fully symptomatic depression to depression free; Depression-free day .4 QALY, depression free day to QALY conversion using .4 to estimate change from fully symptomatic depression to depression free.
The final model for depression-free days included: intervention, barriers to treatment, perceived need for treatment, SF-12 physical health component score, SF-12 mental health component score, employment, family history of depression, SCL-20. The QALY and cost models were similar except the SCL-20 was replaced by the baseline QALY measure or baseline cost measure, respectively.
Cost estimates are based on Arkansas FQHC prospective payment system rates for CHC visits, Arkansas Blue Cross Blue Shield data for non-CHC outpatient visits, University HealthSystem Consortium (UHC) Southern Region per diem rates for ER and inpatient visits, estimated 340B Drug Pricing Program costs for medication, 40% T1 lines monthly charges.
Cost estimates are based on Lifelink Health Plans Claims Database for outpatient, ER, and inpatient visits, lowest average wholesale price from Red Book for medication, 40% T1 lines monthly charges
designates base case analysis
Figure 1.
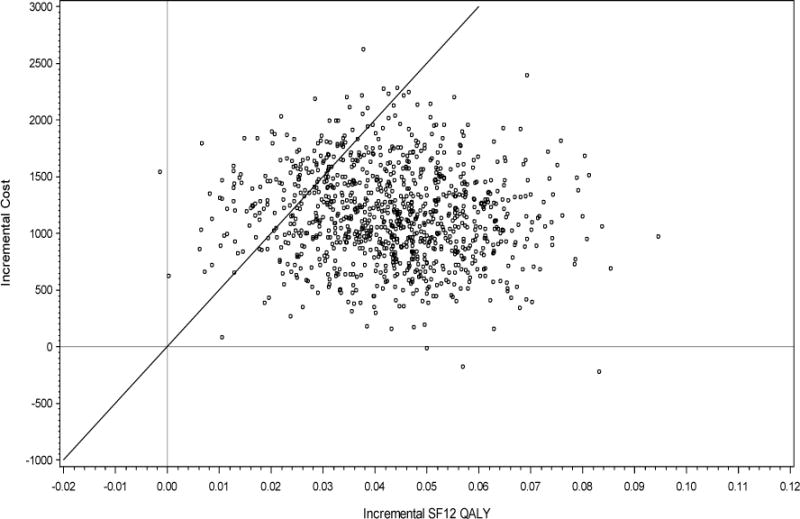
Scatter Plot Base Case Analysis using 1,000 Bootstrap Samples with Replacement*
*The proportion of bootstrapped samples below (to the right) of the $50,000 per QALY threshold line (through the origin) is 85.6%
Figure 2.
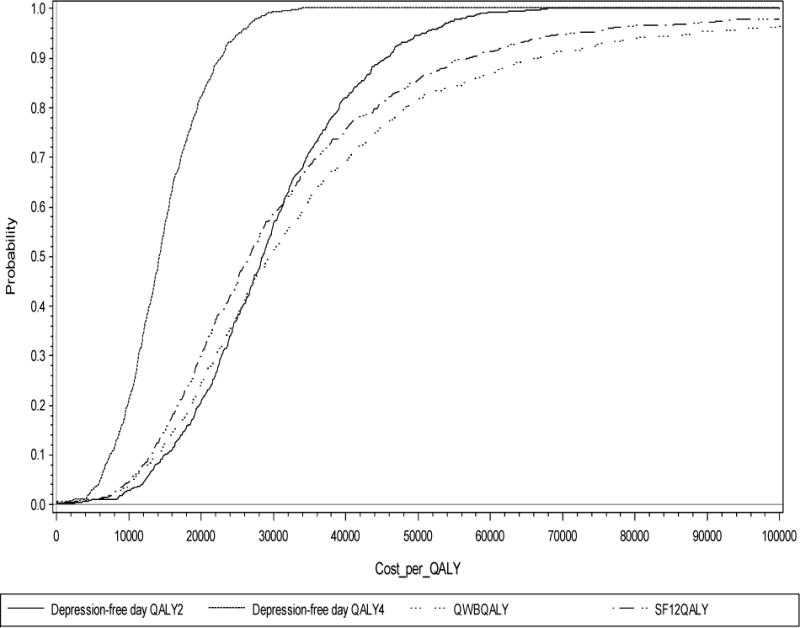
Cost per QALY Acceptability Curves
Abbreviations: Depression-free day QALY2 stands for depression-free day to QALY conversion using .2 as the improvement in QALY associated with improving from fully symptomatic depression to depression-free; depression-free day QALY4 stands for depression-free day to QALY conversion using .4 as the improvement in QALY associated with improving from fully symptomatic depression to depression-free; QWBQALY stands for QALY measure using Quality of Well-Being scale; SF12QALY stands for QALY measure using Medical Outcomes Study SF-12 to QALY conversion with standard gamble preference weights
The bootstrapped mean ICER using national cost and depression-free days was $8.46/depression-free day. The mean ICER using national cost and SF-12 QALY was $25,728/QALY. The sensitivity analyses for the QALY estimates ranges from $11,579/QALY (depression-free day QALY.4) to $28,016/QALY (QWB QALY). Adding inpatient mental health costs to the national cost SF-12 QALY analysis resulted in $28,126/QALY.
DISCUSSION
For primary care clinics lacking on-site mental health resources, there are increasing calls for collaborative care models where off-site specialists support primary care providers using telemedicine technologies(32). To our knowledge, this is the first cost-effectiveness analysis to compare the value of outsourced TBCC to insourced PBCC. The base case adjusted incremental cost of the TBCC intervention was $1,163 which is consistent with the incremental cost reported for other depression collaborative care interventions ($389 to $1,772 per capita adjusted to 2009 dollars)(7,10,18,19,33). Televideo equipment and T1 line charges accounted for 50% of the per capita TBCC direct cost. However, results clearly demonstrate that TBCC is both more effective and more cost-effective compared to PBCC. The incremental cost per depression-free day was $10.78/depression-free day, which is less than depressed patients report they are willing to pay ($14.40 per additional depression-free day adjusted to 2009 dollars)(34). Compared to usual care, the ICER for depression-free day estimated from other collaborative depression care studies ranged from $3.64/depression-free day to $85.54/depression-free day (2009 dollars)(19,35).
The mean ICER QALY results were all below the commonly used threshold of $50,000/QALY for intervention adoption. The CE ratios using depression-free day QALY.4 (which is the most commonly reported QALY measure for depression collaborative care interventions) were less than $20,000/QALY which is considered the threshold for recommending immediate adoption(22). Compared to usual care, mean ICER depression-free day QALY estimates from the other depression collaborative care studies referenced above ranged from $3,325 to $99,335/QALY adjusted to 2009 dollars.
The TBCC intervention is a cost-effective model for delivering accessible and high quality depression care to settings lacking on-site mental health resources. Thus, TBCC presents a viable option for the make or buy depression care management decision facing organizations striving for PCMH recognition. Telemedicine capability in primary care clinics is increasing within (http://aims.uw.edu) and outside (http://www.accesspsych.com) university research programs. Based on estimates from previous collaborative care interventions, approximately one DCM is needed for every 10,000 primary care patients and TBCC could feasibly cover more than one site(36). Adaptations of TBCC to enhance value and sustainability could be tested within specific settings and will be required within the changing healthcare environment(37).
Limitations of this study include the following. Electronic health record systems were not in place at the FQHCs during this study which limits generalizability. However, electronic health records would likely improve communication between the TBCC intervention team and FQHC providers. The demographic characteristics of FQHC patients (typically poor, rural, uninsured, and/or minority) differ from private sector patients which limits generalizability to the private sector.
In conclusion, this pragmatic comparative cost-effectiveness study provides evidence to support the cost-effectiveness of TBCC in medically underserved areas. These results can inform the on-site versus off-site depression care management decision facing FQHCs and other healthcare delivery systems working towards PCMH recognition, utilizing value-based purchasing, and preparing for bundled depression care payments.
Acknowledgments
The respective funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We would like to gratefully acknowledge the patients and staff at the Boston Mountain Rural Health Center, Inc., Community Clinic Northwest Arkansas, Corning Area Healthcare Inc., East Arkansas Family Health Center, Inc., Jefferson Comprehensive Care Systems, Inc., as well as staff at the Community Health Centers for Arkansas Inc. We acknowledge the important contributions of project staff including Amanda Davis, Loretta Ducker, Debbie Hodges, Choi Lai, Michael McCarther, Camille Mack, Jennifer Stephens and Vera Tate. We also acknowledge Brad Martin and Anand Shewale for providing access to LifeLink data.
This research was supported by grants from the National Institute of Mental Health (R01 MH076908, MH076908-04S1) and National Institutes of Health (UL1TR000039, KL2TR000063) and National Institute of General Medical Science (P30 GM110702).
Footnotes
Disclosure: The authors report no direct or indirect conflicts of interest relative to the subject of this manuscript.
Previous presentation: Poster presented at the Academy Health Annual Research Meeting, San Diego, CA, June 8-10, 2014.
Contributor Information
Jeffrey M. Pyne, University of Arkansas for Medical Sciences - Department of Psychiatry, 4224 Shuffield Dr, Little Rock, AR 72205, Central Arkansas Veterans Healthcare System - Psychiatry, North Little Rock, Arkansas
John C. Fortney, University of Arkansas for Medical Sciences - Department of Psychiatry, 4224 Shuffield Dr, Little Rock, AR 72205
Sip Mouden, Community Health Centers of Arkansas, Inc., North Little Rock, Arkansas.
Liya Lu, University of Arkansas for Medical Sciences - Department of Psychiatry, 4224 Shuffield Dr, Little Rock, AR 72205.
Teresa J Hudson, University of Arkansas for Medical Sciences - Department of Psychiatry, 4224 Shuffield Dr, Little Rock, AR 72205.
Dinesh Mittal, Central Arkansas Veterans Healthcare System - Psychiatry, North Little Rock, Arkansas.
Reference List
- 1.Wang PS, Berglund P, Olfson M, et al. Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:603–613. doi: 10.1001/archpsyc.62.6.603. [DOI] [PubMed] [Google Scholar]
- 2.Fortney J, Rost K, Zhang M, Warren J. The Impact of Geographic Accessibility on the Intensity and Quality of Depression Treatment. Medical Care. 1999;37(9):884–893. doi: 10.1097/00005650-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Katon W, Robinson P, Von Korff M, et al. A multifaceted intervention to improve treatment of depression in primary care. Archives of General Psychiatry. 1996;53(10):924–932. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 4.Simon GE, VonKorff M, Rutter C, Wagner E. Randomised trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. British Medical Journal. 2000;320(7234):550–554. doi: 10.1136/bmj.320.7234.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells KB, Sherbourne C, Schoenbaum M, et al. Impact of disseminating quality improvement programs for depression in managed primary care: A randomized controlled trial. Journal of the American Medical Association. 2000;283(2):212–220. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- 6.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. Journal of the American Medical Association. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 7.Schoenbaum M, Unutzer J, Sherbourne C, et al. Cost-effectiveness of practice-initiated quality improvement for depression: Results of a randomized controlled trial. Journal of the American Medical Association. 2001;286(11):1325–1330. doi: 10.1001/jama.286.11.1325. [DOI] [PubMed] [Google Scholar]
- 8.Simon GE, Ludman EJ, Rutter C. Incremental benefit and cost of telephone care management and telephone psychotherapy for depression in primary care. Archives of General Psychiatry. 2009;66(10):1081–1089. doi: 10.1001/archgenpsychiatry.2009.123. [DOI] [PubMed] [Google Scholar]
- 9.Gensichen J, Petersen JJ, Von KM, et al. Cost-effectiveness of depression case management in small practices. British Journal of Psychiatry. 2013;202:441–446. doi: 10.1192/bjp.bp.112.118257. [DOI] [PubMed] [Google Scholar]
- 10.Pyne JM, Rost KM, Zhang M, et al. The cost-effectiveness of a primary care depression intervention. Journal of General Internal Medicine. 2003;18(6):432–441. doi: 10.1046/j.1525-1497.2003.20611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.New Freedom Commission on Mental Health. Achieving the promise: Transforming mental health care in America. Final report. Rockville, MD: 2003. SMA-03-3832. [Google Scholar]
- 12.Fortney JC, Pyne JM, Mouden SB, et al. Practice-based versus telemedicine-based collaborative care for depression in rural federally qualified health centers: A pragmatic randomized comparative effectiveness trial. American Journal of Psychiatry. 2013;170(4):414–425. doi: 10.1176/appi.ajp.2012.12050696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lardiere MR, Jones E, Perez M. National Association of Community Health Centers (NACHC) 2010 Assessment of Behavioral Health Services Provided in Federally Qualified Health Centers. Health Resources and Services Administration U30CS16089; 2011. [Google Scholar]
- 14.Druss BG, Bornemann T, Fry-Johnson YW, et al. Trends in Mental Health and Substance Abuse Services at the Nation’s Community Health Centers: 1998-2003. American Journal of Public Health. 2006;96:1779–1784. doi: 10.2105/AJPH.2005.076943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Association of Community Health Centers. US Health Center fact sheet. Bethesda, MD: 2007. [Google Scholar]
- 16.Dobscha SK, Corson K, Hickam DH, et al. Depression decision support in primary care: A cluster randomized trial. Annals of Internal Medicine. 2006;145(7):477–487. doi: 10.7326/0003-4819-145-7-200610030-00005. [DOI] [PubMed] [Google Scholar]
- 17.Fortney JC, Pyne JM, Steven CA, et al. A web-based clinical decision support system for depression care management. American Journal of Managed Care. 2010;16(11):849–854. [PMC free article] [PubMed] [Google Scholar]
- 18.Simon GE, Katon WJ, VonKorff M, et al. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. American Journal of Psychiatry. 2001;158:1638–1644. doi: 10.1176/appi.ajp.158.10.1638. [DOI] [PubMed] [Google Scholar]
- 19.Katon WJ, Schoenbaum M, Fan MY, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Archives of General Psychiatry. 2005;62:1313–1320. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- 20.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Medical Care. 2004;42(9):851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan RM, Bush JW, Berry CC. Health status: Types of validity and the index of well-being. Health Services Research. 1976;11(4):478–507. [PMC free article] [PubMed] [Google Scholar]
- 22.Gold M. Cost-Effectiveness in Health and Medicine: Report of the Panel on Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 23.Lave JR, Frank RG, Schulberg HC, Kamlet MS. Cost-effectiveness of treatments for major depression in primary care practice. Archives of General Psychiatry. 1998;55(7):645–651. doi: 10.1001/archpsyc.55.7.645. [DOI] [PubMed] [Google Scholar]
- 24.Pyne JM, Patterson TL, Kaplan RM, et al. Assessment of the quality of life of patients with major depression. Psychiatric Services. 1997;48(2):224–230. doi: 10.1176/ps.48.2.224. [DOI] [PubMed] [Google Scholar]
- 25.Hilty DM, Yellowlees PM, Nesbitt TS. Evolution of telepsychiatry to rural sites: changes over time in types of referral and in primary care providers’ knowledge, skills and satisfaction. General Hospital Psychiatry. 2006;28:367–373. doi: 10.1016/j.genhosppsych.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Smith GR, Jr, Burnam A, Burns BJ, et al. Depression Outcomes Module (DOM) In: American Psychiatric Association, editor. Handbook of Psychiatric Measures. Washington, DC: 2000. [Google Scholar]
- 27.Lecrubier Y, Sheehan DV, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry. 1997;12(5):224–231. [Google Scholar]
- 28.Parkerson GR, Jr, Michener JL, Wu LR, et al. Associations among family support, family stress, and personal functional health status. Journal of Clinical Epidemiology. 1989;42(3):217–229. doi: 10.1016/0895-4356(89)90058-9. [DOI] [PubMed] [Google Scholar]
- 29.Edlund MJ, Fortney JC, Reaves CM, Pyne JM, Mittal D. Belief about depression and depression treatment among depressed veterans. Medical Care. 2008;46(6):581–589. doi: 10.1097/MLR.0b013e3181648e46. [DOI] [PubMed] [Google Scholar]
- 30.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: A non-parametric approach to confidence interval estimation. Health Economics. 1997;6(4):327–340. doi: 10.1002/(sici)1099-1050(199707)6:4<327::aid-hec282>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 31.Hunink MGM, Bult JR, De Vries J, Weinstein MC. Uncertainty in decision models analyzing cost-effectiveness: The joint distribution of incremental costs and effectiveness evaluated with a nonparametric bootstrap method. Medical Decision Making. 1998;18(3):337–346. doi: 10.1177/0272989X9801800312. [DOI] [PubMed] [Google Scholar]
- 32.Simon GE, Ludman EJ. Should mental health interventions be locally grown or factory-farmed? American Journal of Psychiatry. 2013;170:362–365. doi: 10.1176/appi.ajp.2013.13010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyne JM, Fortney JC, Tripathi S, et al. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Archives of General Psychiatry. 2010;67(8):812–821. doi: 10.1001/archgenpsychiatry.2010.82. [DOI] [PubMed] [Google Scholar]
- 34.Unutzer J, Katon WJ, Russo J, et al. Willingness to pay for depression treatment in primary care. Psychiatric Services. 2003;54:340–345. doi: 10.1176/ps.54.3.340. [DOI] [PubMed] [Google Scholar]
- 35.Simon GE, Manning WG, Katzelnick DJ, et al. Cost-effectiveness of systematic depression treatment for high utilizers of general medical care. Archives of General Psychiatry. 2001;58(2):181–187. doi: 10.1001/archpsyc.58.2.181. [DOI] [PubMed] [Google Scholar]
- 36.Liu CF, Fortney J, Vivell S, et al. Time allocation and caseload capacity in telephone depression care management. American Journal of Managed Care. 2007;13:652–660. [PubMed] [Google Scholar]
- 37.Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implementation Science. 2013;8:117. doi: 10.1186/1748-5908-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]


