Abstract
Immune cell function and metabolism are closely linked. Many studies have now clearly demonstrated that alterations in cellular metabolism influence immune cell function and that, conversely, immune cell function determines the cellular metabolic state. Less well understood, however, are the effects of systemic metabolism or whole organism nutritional status on immune cell function and metabolism. Several studies have demonstrated that undernutrition is associated with immunosuppression, which leads to both increased susceptibility to infection and protection against several types of autoimmune disease, whereas overnutrition is associated with low-grade, chronic inflammation that increases the risk of metabolic and cardiovascular disease, promotes autoreactivity, and disrupts protective immunity. Here, we review the effects of nutritional status on immunity and highlight the effects of nutrition on circulating cytokines and immune cell populations in both human studies and mouse models. As T cells are critical members of the immune system, which direct overall immune response, we will focus this review on the influence of systemic nutritional status on T cell metabolism and function. Several cytokines and hormones have been identified which mediate the effects of nutrition on T cell metabolism and function through the expression and action of key regulatory signaling proteins. Understanding how T cells are sensitive to both inadequate and overabundant nutrients may enhance our ability to target immune cell metabolism and alter immunity in both malnutrition and obesity.
Keywords: malnutrition, obesity, T cells, cellular metabolism, inflammation, leptin
Introduction
Nutritional imbalance is a major challenge for living organisms to achieve systemic homeostasis and maintain normal physiology. Mammals have developed processes to control systemic nutrient utilization and storage. For example, excess nutrients are converted and stored in adipose tissue, liver, and muscle during times when nutrients are abundant. By contrast, stored nutrients are metabolized to provide energy and building blocks to maintain vital physiological processes when nutrient availability is low.
From these processes, adipose tissue volume changes in response to under- or overnutrition. This change in adipose tissue volume, in turn, influences the secretion of hormones and cytokines from adipose tissue (adipocytokines). Many of these adipocytokines have important immune signaling functions which can influence immune cell biology and alter immune response.
Here, we will review the effects of nutritional changes on hormones and cytokines that influence immune cell function and metabolism in mouse models, and we will specifically examine how nutritionally regulated changes in immune cells alter immunity in the context of both autoimmune disease and infection response in both human and animal studies. We will also highlight some of the promising metabolic targets that may be useful in the development of novel treatments for immunity-related disorders.
Key Signaling Molecules Altered in Response to Changes in Nutritional Status
Many cytokines and hormones are changed in response to over- or undernutrition. One of the earliest reports of this is with tumor necrosis factor-alpha (TNF-α). TNF-α can be secreted from adipose tissue, and its expression is increased during obesity but decreased following weight loss (1–3). TNF-α is a well-known pro-inflammatory cytokine that is essential for the acute phase reaction. Studies from the 1990s demonstrated that TNF-α was essential for the development of insulin resistance in high-fat diet-induced obesity in mice (1) and that deletion of TNF-α protected high-fat diet-induced obese mice from developing insulin resistance (4). TNF-α can be secreted from both adipose tissue-localized macrophages, which are increased in obesity, and from adipocytes (5, 6). Following these early studies showing the importance of TNF-α in mediating metabolic disease in obesity, multiple other cytokines and hormones were also found to play a similar role.
Interleukin-6 (IL-6) is another cytokine that is secreted by many immune and non-immune cells in the adipose tissue including adipocytes, macrophages, pre-adipocytes, and T cells, in response to tissue damage (7). IL-6 has broad pleiotropic functions leading to the expansion of many types of immune cells including B cells and T cells (8, 9). IL-6 can signal through binding to the IL-6 receptor dimerized with gp130 on the surface of cells; however, IL-6 effects can also be mediated through trans-signaling, in which IL-6 binds to soluble IL-6 receptor, thereby permitting IL-6 to act on any cell that expresses gp130. In that way, IL-6 receptor trans-signaling contributes to the broad pleiotropic effect of IL-6 (10). In general, IL-6 promotes T cell survival and resistance to apoptosis (11). IL-6 also has a pro-inflammatory role promoting CD4+ T cell differentiation to the Th17 or Th1 lineages, which produce the pro-inflammatory cytokines IL-17 and interferon gamma (IFN-γ), respectively (12, 13). Increased IL-6 levels have been reported in obesity in both humans and rodents (14, 15). Pan-blocking of IL-6 signaling using an anti-IL-6 antibody (MR16-1) has been shown to ameliorate insulin resistance and reduce liver-fat accumulation in high-fat diet-fed mice (16). Blocking IL-6 trans-signaling by using gp130Fc soluble protein was found to block adipose tissue macrophage recruitment in high-fat diet-fed mice, but did not inhibit insulin resistance (17). Recently, it was reported that selective blocking of IL-6 signaling in T cells improves glucose homeostasis and ameliorates liver steatosis in high-fat diet-fed mice, but only early in the development of obesity (18).
Leptin is another well-described adipocytokine known to influence immune cells. Leptin is a hormone secreted by adipocytes in proportion to adipocyte mass and is therefore increased in obesity and decreased in malnutrition. Leptin is best known for its role in influencing systemic metabolism by signaling in the hypothalamus to suppress appetite and increase energy expenditure (19). However, leptin can also communicate energy status to other systems in the body, including the immune system (20). In that role, leptin was found to have an important developmental function in the maturation of hematopoietic cells on which the leptin receptor (LepR) is expressed (21). In addition to this developmental function, leptin deficiency has also been associated with the loss of cell-mediated immunity (22). The first reported immune function for leptin was its ability to regulate macrophage phagocytosis and pro-inflammatory cytokine production: lack of leptin or its receptor was found to reduce phagocytic activity and the production of both IL-6 and TNF-α (23). In addition, LepR is expressed on the surface of T cells (24), and deletion of LepR on T cells leads to a marked decrease in T cell number and function as well as polarization to Th1 and Th17 cell subsets, ultimately leading to immune deficiency characterized by an increased susceptibility to intracellular infections (25–27).
In addition to TNF-α, IL-6, and leptin, many other hormones and cytokines are influenced by changes in systemic metabolism and are summarized in Table 1.
Table 1.
Key immune signaling molecules that change in response to nutritional status.
| Molecule | Class | Obesity | Malnutrition | Secreted by | Immune function | Reference |
|---|---|---|---|---|---|---|
| Leptin | Adipocytokine | Increased | Decreased | Adipocytes | Pleiotropic hormone with many targets and many functions in immune cells Induces Th1 and Th17 polarization |
(28) |
| Adiponectin | Adipocytokine | Decreased | Conflicting reports—may be context-dependent | Adipocytes | Polarization of monocytes and macrophages toward M2 phenotype Suppresses NK cell, eosinophil, neutrophil, γδ T cell, and dendritic cell activation and inflammatory cytokine production |
(29) |
| Resistin | Adipocytokine | Increased | No clear correlation; more investigation needed | Adipocytes, macrophages | Stimulates the production of TNF-α and IL-12 in macrophages | (30) |
| Visfatin | Adipocytokine | Increased | No clear correlation; more investigation needed | Adipocytes | Stimulates the production of TNF-α, IL-1β, and IL-6 | (30, 31) |
| TNF-α | Cytokine | Increased | Mixed results, may depend on context | Adipocytes | Neutrophil chemotaxis | (4, 15, 32–34) |
| IL-1β | Cytokine | Increased | Mixed results, may depend on context | Non-adipocyte cells in adipose tissue | Stimulates macrophage activity | (35, 36) |
| IL-6 | Cytokine | Increased | Decreased | Adipocytes, macrophages | Recruitment of macrophages; polarization toward pro-inflammatory classically activated macrophages | (15, 37) |
| IL-8 | Cytokine | Increased | Decreased | Adipocytes, macrophages | Induces neutrophil chemotaxis | (38–40) |
| IL-10 | Cytokine | Mixed—may be context-dependent | Increased | Treg cells, iNKT cells, DCs, adipocytes, macrophages | Broad anti-inflammatory function | (41–43) |
| IL-33 | Cytokine | Decreased | Increased | DCs, macrophages, epithelial cells | Maintains adipose tissue-resident Treg cell function, promotes Th2 response, promotes alternatively activated macrophage polarization | (44, 45) |
| IL-1RA | Cytokine | Increased | Unknown | Macrophages, epithelial cells | Inhibits IL-1α and IL-1β activity | (46–48) |
| MCP-1 | Chemokine | Increased | Increased | Macrophages, adipocytes | Macrophage recruitment | (39, 49) |
| MIF | Chemokine | Increased | Decreased | Adipocytes, lymphocytes | Inhibits macrophage migration | (50) |
| MIP-1α | Chemokine | Increased | Unknown | Adipocytes | Enhances macrophage migration | (51) |
| MIP-1β | Chemokine | Increased | Unknown | Adipocytes | Enhances macrophage migration | (51) |
Immune Cells Affected by Changes in Nutritional Status
The hormone and cytokine changes seen in response to obesity and malnutrition are closely linked to changes in immune cell populations. Several types of immune cells residing in the adipose tissue are affected by changes in the above-listed cytokine and hormone levels and in turn contribute to altered cytokine production in states of under- or overnutrition. These adipose tissue-localized immune cells can be affected by changes in nutritional status through both paracrine effects (due to their proximity to adipocytes) and systemic/endocrine effects of secreted adipose factors.
Macrophages comprise more than 50% of adipose tissue-resident immune cells and are, therefore, the most abundant immune cells in the adipose tissue (52). During obesity, an influx of macrophages into the adipose tissue takes place in response to the secretion of monocyte chemoattractant protein-1 from the adipose tissue (53). These macrophages become polarized into pro-inflammatory, classically activated macrophages (previously termed M1 macrophages) in response to IFN-γ, which is secreted by effector T cells (Teff cells) and other immune cells in the adipose tissue (54). This increase in inflammatory macrophage population leads to an increase in TNF-α secretion in addition to other inflammatory molecules secreted by inflammatory macrophages, including IL-1β, IL-6, and IL-12. Adipose tissue macrophages in obesity contribute to the formation of the crown-like structure that forms around necrotic adipocytes, a very distinctive histological feature of the adipose tissue during obesity. In lean individuals, alternatively activated macrophages (previously termed M2 macrophages) secrete anti-inflammatory cytokines including IL-10, IL-4, and IL-1 receptor agonists which promote immune modulatory functions (55). The shift in macrophage populations during obesity plays a central role in the maintenance of inflammation and the rise of obesity-associated pathologies including insulin resistance and non-alcoholic fatty liver disease.
In addition to macrophages, other smaller populations of innate immune cells are found in the adipose tissue and change in number and function in response to obesity. Neutrophils and mast cells are found to increase and become activated in the adipose tissue during obesity (56). By contrast, eosinophil numbers decrease in adipose tissue during obesity (57), which is relevant because eosinophils secrete IL-4, a cytokine that helps maintain the alternatively activated population of macrophages within the adipose tissue (57).
Several lymphocyte populations are also found in the adipose tissue. B lymphocytes (B cells) have been found to accumulate in the adipose tissue during obesity (58). Although B cells are best known for the production of antibodies, they also express inflammatory cytokines, such as IL-2 and IL-12, which influence T cell differentiation into Th1 versus Th2 cells (59). Adipose tissue also harbors a large population of natural killer T (NKT) cells (60); these cells are known for the expression of an invariant form of the T cell receptor (TCR) that interacts with a lipid antigen presenting protein, CD1d, which is highly expressed on adipocytes (61). NKT cells secrete different types of cytokines depending on the lipid antigen presented by CD1d. During normal weight conditions, NKT cells modulate inflammation by secreting anti-inflammatory cytokines such as IL-4 and IL-10 (62). In obesity, NKT cells decrease in number, at the same time adipocytes express lower levels of CD1d. This change altogether reduces the amount of anti-inflammatory cytokines secreted by NKT cells and contributes to the complications of obesity (62).
T lymphocytes (T cells) represent the most abundant lymphocyte population and second most abundant immune cell in the adipose tissue behind macrophages (63). Both CD4+ and CD8+ T cells are found in the adipose tissue. During obesity, the proportion of adipose CD8+ T cells to CD4+ T cells increases (64). In addition, proportions of inflammatory CD4+ T cell subsets increase in obesity, whereas regulatory T cells (Treg cells) decrease (64, 65). This change in T cell populations during obesity contributes to the pro-inflammatory state of the adipose tissue: both CD8+ T cells and pro-inflammatory CD4+ Th1 cells express the pro-inflammatory cytokine IFN-γ, whereas effector CD4+ Th17 cells express the pro-inflammatory cytokine IL-17.
During normal physiological conditions, the adipose tissue represents a major depot of Treg cells in the body (66, 67). These cells represent more than 50% of CD4+ T cells in lean adipose tissue (66). Treg cells are responsible for suppressing inflammation through the secretion of anti-inflammatory cytokines such as TGF-β and IL-10 (58, 63). During obesity, the proportion of adipose tissue Treg cells decreases dramatically as adipose tissue volume increases. Due to their role in maintaining self-tolerance and in dampening excessive inflammatory response, the reduction in Treg cell number during obesity (68) induces a significant shift in immune cell populations and cytokine production toward a pro-inflammatory state (65). Paradoxically, adipose tissue Treg cells were also found to contribute to age-associated insulin resistance, and deletion of Treg cells could protect against age-associated, but not obesity-associated, insulin resistance (69).
In contrast to the effects of obesity on immune cells, malnutrition leads to a decrease in immune cell number. This has been shown particularly in the case of T cells: mice fasted 48 h had large and significantly decreased thymocyte and splenocyte counts compared to fed control mice (26, 70, 71). Both total T cell and CD4+ T cell numbers from spleens of fasted mice were decreased by 40–50% compared to fed control animals (26, 71). Other studies have shown that mice fed a protein-deficient diet had atrophic spleens and decreased T cell numbers compared to chow-fed control mice (72, 73). A similar finding was seen in human studies. Malnourished children had decreased CD4+ and CD8+ T cell numbers in whole blood compared to well-nourished children (74). Moreover, childhood malnutrition causes atrophy of primary lymphoid organs, leading to reduced T and B cell numbers and a generalized state of leukopenia (75). These reductions in immune cell number in malnutrition contribute to functional deficiencies, which will be discussed in further detail below.
Effect of Nutritional Status on Immune Cell Metabolism
Although it is clear that systemic metabolism influences immune cell function, we are only just starting to understand how changes in nutrition can influence metabolism at the cellular level. This is an important consideration, as immune cell metabolism and immune cell function are intrinsically tied. Previous studies have demonstrated a link between cellular metabolism and function for several types of immune cells (76, 77), but we will focus our discussion here on T cells. Multiple studies have now shown that changes in T cell metabolism can influence T cell differentiation and function, whereas changes in T cell function can likewise influence T cell metabolism.
The energy requirement of naïve T cells performing immune surveillance is satisfied through oxidative phosphorylation of lipids, amino acids, and glucose-derived pyruvate to ATP in the mitochondria (78). This process is highly efficient at producing ATP, but does not provide biosynthetic precursors that are necessary for proliferation or growth. Naïve T cells are arrested in the G0 stage of the cell cycle and this state of homeostatic quiescence is actively maintained (79). Without TCR stimulation, CD4+ T cells fail to undergo homeostatic proliferation, downregulate Glut1, and die from apoptosis (80, 81). Following activation, however, T cells need to rapidly grow, proliferate, and generate cytokines to direct a functional immune response. Given the growth and proliferation requirement of an activated T cell, these cells must be prepared to increase the biosynthesis of cellular products including lipids, proteins, and nucleotides which are needed for rapid cell division (78), and for these reasons, a metabolic switch is required.
Upon activation, the metabolic state of T cells resembles that of cancer cells (82). These rapidly proliferating cells increase glucose uptake, glycolysis, and reduction of pyruvate to lactate even in the presence of oxygen, a process aptly named aerobic glycolysis (83). Warburg noticed this effect in his early studies of blood leukocytes, and more recent studies have confirmed the “Warburg effect” in thymocytes and T cells (84, 85). A state of rapid ATP usage and massive biosynthetic requirement make the process of glycolysis a more efficient way for cancer cells and activated T cells to proliferate. TCA cycle intermediates can be used as precursors in biosynthetic pathways to support the growing need for lipid, protein, and nucleotide synthesis that precedes cellular division (78). Conversion of pyruvate to lactate ensures that reducing equivalents of NAD+ are restored, allowing the process of glycolysis to continue (83).
The upregulation of the glycolytic metabolic program in activated T cells is controlled by several key signaling pathways and transcription factors. Both TCR signaling and signaling through CD28 induce the activation of the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt pathway, which is partially responsible for the upregulation of a glycolytic metabolic program (80, 86). The PI3K/Akt pathway leads to the activation of mammalian target of rapamycin (mTOR) which forms two functional complexes: mammalian target of rapamycin complex 1 (mTORC1) and mammalian target of rapamycin complex 2 (mTORC2). mTOR is an important integrator of environmental signals, allowing the cell to respond to signals from the TCR, co-stimulation, cytokines, and nutrient availability (87–89). The activation of Akt and mTOR has been shown to lead to an increase in aerobic glycolysis by increasing the transcription of glycolytic genes and transcription factors including c-Myc and hypoxia-inducible factor (HIF)-1α (87, 88). In addition, constitutive Akt expression leads to an increase in surface expression of the glucose transporter Glut1 and thereby increases glucose uptake, whereas blocking Akt or PI3K decreases T cell glucose uptake. The inhibition of mTORC1 by treatment with rapamycin has been shown to prevent T cell growth and proliferation (90, 91). Knockout of the mTOR inhibitor AMPKα1 decreases the ability of T cells to respond to metabolic stress and decreases their ability to transition between anabolic and catabolic metabolisms (92). mTOR also regulates protein translation as part of mTORC1 through interaction with the translation initiation factor 4E-binding protein (4E-BP) and p70s6K. By phosphorylating 4E-BP and p70s6K, mTOR activates these two proteins to increase protein translation in the cell (88).
At the transcriptional level, c-Myc is responsible for regulating glucose and glutamine metabolism. Specifically, c-Myc has been shown to upregulate multiple glycolytic genes upon T cell activation, including Glut1, hexokinase2 (HK2), and pyruvate kinase muscle isozyme 2 (93). Glut1 is a critical glucose transporter expressed in T cells and is upregulated upon activation. Glut1 can be stored in vesicles intracellularly and transported to the cell surface to increase glucose uptake following T cell activation, and Glut1 transcription and translation are increased following T cell activation (94). In cancer cells, c-Myc has been shown to control the transition to glycolytic metabolism in hypoxic conditions along with HIF-1-α (95). c-Myc is upregulated following T cell activation and has been demonstrated by several groups to induce cell cycle progression and glycolytic metabolism (96, 97). Without this transition, T cells would not be able to exit G0 and enter the rapid expansion phase of the immune response. This has been confirmed in a T cell specific knockout of c-Myc, in which T cells fail to proliferate and were unable to upregulate glycolytic metabolism (95). c-Myc also upregulates glutaminolysis which can feed into anaplerosis, so that TCA cycle intermediates can be used for biosynthesis. For example, citrate is a TCA cycle intermediate that can be used as a precursor for lipid synthesis (95). HIF-1-α is also increased upon T cell activation and has been shown to increase the expression of glycolytic genes along with c-Myc (95, 98). HIF-1-α is also important in the differentiation of T cells, particularly toward Th17 cells.
Differentiation of CD4+ T cells into functionally distinct subsets is associated with alterations in the cellular metabolic phenotype. In general, Teff cells are pro-glycolytic and depend heavily upon an increased glucose uptake, as well as glutamine metabolism, to fuel effector function, as described above. Inhibition of glycolysis prevents differentiation into these pro-inflammatory subsets (99). Regulatory T cells (Treg) and memory T cells (Tmem), however, have a decreased glucose metabolism and predominantly utilize lipid oxidation to fuel suppressive and memory function, respectively (100–102). Although Treg cells have a decreased glucose metabolism in comparison to Teff cells, glycolysis is essential for Treg cell migration into sites of inflammation (103).
The first evidence of T cell differentiation depending on metabolism came from studies using the drug rapamycin. Treatment with rapamycin prevented T cell growth and proliferation and promoted differentiation of Treg cells, rather than Teff cells (90). Further evidence came from studies using whole-body or T cell-specific knockout of mTOR, in which only Treg cells were produced under activation conditions (104). Failure to produce Teff cells in the absence of mTORC1 demonstrates that glycolytic metabolism, as driven by mTORC1, is required for Teff cell differentiation and cytokine production. Moreover, increasing the activity of AMPK by treatment with metformin increased Treg cell numbers and decreased Teff cells, further demonstrating that blocking glycolytic metabolism promotes the Treg cell lineage (100). Metformin-treated T cells also had lower Glut1 levels upon activation, demonstrating a decreased glycolytic state compared to untreated T cells in these studies (100).
Not only does the activity of mTORC1 promote Teff cell differentiation over Treg cells but differential activity of mTORC1 and mTORC2 promotes distinct Teff subsets (78, 98, 105). mTORC1 activity is upregulated in Th1 and Th17 cells, whereas Th2 cells show an increased activity of mTORC2. Treg cells also demonstrate an increased AMPK activity, which leads to inhibition of mTOR. Tmem express both TRAF6 and AMPK, which promote lipid oxidation and thereby suppress the glycolytic phenotype of activated Teff cells (102).
Leptin Promotes Glycolytic Metabolism in Activated T Cells
One well-established connection between systemic nutritional status and immune cell metabolism is through leptin. As mentioned above, leptin is secreted by adipocytes in proportion to adipocyte mass and leptin levels, thereby trending with nutritional status (106). In states of malnutrition or following fasting, circulating leptin levels are decreased, whereas in obesity, leptin levels are increased. Leptin acts directly on CD4+ T cells through the LepR to direct changes in T cell metabolism and function (26, 27, 107–110). Since T cell metabolism and function are intimately linked, any change in immune cell metabolism can lead to a change in the function of that cell, altering cellular proliferation, differentiation, and cytokine production. Leptin has been shown to promote both CD4+ T cell inflammatory cytokine production and glucose metabolism. Indeed, T cells unable to respond to leptin had impaired upregulation of glucose uptake and glycolysis following T cell activation (26). In the context of malnutrition, fasting-induced hypoleptinemia caused activated CD4+ T cells to produce less inflammatory cytokines IFN-γ and IL-2 (26, 27). That functional defect did not extend to naïve T cells or Treg cells, however, presumably because those cells do not depend on increased glycolytic metabolism to fuel immune surveillance or regulatory function. In subsequent studies, Th17 cells from fasted mice were found to be functionally deficient and metabolically less glycolytic, whereas Treg cells from fasted mice did not experience a functional or a metabolic defect (27). The metabolic status of Th17 cells derived from fasted mice was assessed by extracellular flux analysis, and these cells were found to have a decreased lactate production as well as a decreased mitochondrial respiration compared to Th17 cells from ad libitum fed mice. The functional and metabolic defects of Th17 cells were restored when fasted mice received leptin injections or when T cells isolated from fasted mice were activated in the presence of leptin in vitro. Leptin can affect many types of immune system; however, these effects of leptin on T cells were shown to be cell-intrinsic, as Th17 cells from T cell-specific LepR conditional knockout mice showed a decreased glucose uptake and glycolysis as well as a decreased glycolytic enzyme expression, whereas glucose metabolism and function of Treg cells from T cell-specific LepR conditional knockout mice were unaffected. Altogether, these studies show that leptin is a systemic hormone that communicates nutritional status to immune cells by directly increasing Teff cell glucose metabolism and thereby fueling Teff cell function.
The effect of leptin on immune cells is now well documented; however, other nutritionally regulated hormones may also mediate the communication between systemic nutritional status and immune cell function. One such candidate hormone is insulin. Insulin is secreted from pancreatic beta cells following an increase in blood glucose; however, insulin levels also become elevated in states of obesity due to insulin resistance of metabolic tissues including muscle and adipose tissue. Insulin is best known for its role in promoting glucose uptake and glycolysis in metabolic tissues through its ability to upregulate surface expression of the glucose transporter Glut4 and increase the activities of the glycolytic enzymes hexokinase and phosphofructokinase (111–116). Insulin is also able to regulate lipid and protein metabolism. Interestingly, insulin receptors are expressed on activated CD4+ T cells (117). One recent study in rats showed that inducible knockdown of insulin receptor in T cells led to a decreased T cell glucose metabolism and cytokine production (118). Further studies are needed to define the mechanism by which changes in systemic nutrition alter circulating levels of insulin and thereby influence immune cell metabolic and functional processes.
Effect of Nutritional Status on Immune-Mediated Disease
Autoimmunity
Given the role of leptin in regulating the balance between Teff and Treg cells, multiple studies have examined the effect of leptin on autoimmune diseases. One well-studied example is the autoimmune disease multiple sclerosis (MS). The autoimmunity of MS depends upon the activation of Teff, particularly Th1 and Th17 cells, which produce inflammatory cytokines that promote inflammation. Conversely, Treg cells influence immune response by suppressing inflammation caused by Teff and other inflammatory immune cells and thereby protect against autoimmunity. The balance of Teff (Th1, Th17) to Treg cells in MS is, therefore, a critical determinant of inflammation and autoimmune disease. Multiple human studies have shown that active MS is associated with a decreased Treg cell number and proliferation (119–121).
Nutritional status appears to influence MS susceptibility. Clinical studies have shown an association between adolescent obesity and an increased risk of developing MS, particularly in women (122–125). Consistent with that observation, leptin levels have been found to be increased in patients with MS in both serum and cerebrospinal fluid and are associated with increased inflammatory cytokines (119, 120, 126). In addition, LepR expression was increased on the surface of CD8+ T cells from relapsing–remitting MS patients in relapse, as compared to patients in remission or controls (127).
The role of leptin in promoting T cell inflammation has also been well described in a mouse model of MS, experimental autoimmune encephalomyelitis (EAE). Serum leptin levels have been shown to be increased in EAE and correlate with disease severity (128, 129). Early studies in EAE showed that leptin injections worsened EAE disease in female mice and increased disease susceptibility in male mice, while promoting inflammatory cytokine release (130). Moreover, leptin-deficient mice were found to be resistant to EAE, but this protection was lost when mice were treated with recombinant leptin protein (128, 131). Leptin-neutralizing antibodies likewise protected against T cell response and EAE disease in mice (107).
Since leptin levels decrease with fasting and calorie restriction, the effect of fasting on EAE disease severity has been examined by several groups. Fasting-induced hypoleptinemia resulted in a reduced EAE disease severity (27, 129, 132, 133). This fasting-induced protection against EAE was reversed, in part, when fasted mice received leptin injections (27). Moreover, T cells recovered from draining lymph nodes in fasted EAE mice showed a decreased production of the Th1 and Th17 cytokines IFN-γ and IL-17, respectively, as well as a decrease in the expression of glycolytic proteins Glut1 and HK2 (27); again, these fasting-induced changes in Teff cell cytokine production and glucose metabolism were reversed in T cells from fasted mice receiving leptin injections.
Leptin signaling has also been implicated in several other autoimmune diseases. In systemic lupus erythematosus (SLE), leptin levels have been reported to be elevated in human patients and correlate with severity in a mouse model of the disease (134, 135). Decreasing leptin signaling through genetic knockout or antibody blockade protected against disease and increased Treg cell numbers in SLE mice (134). Additional studies in SLE models demonstrated an increased Th17 response, which could be attenuated with the neutralization of leptin (110). Leptin has also been reported to increase Th17 activity in Hashimoto’s thyroiditis and collagen-induced arthritis (108, 109). This provides further evidence for the role of leptin in promoting Th17 differentiation and activation, thus promoting autoimmune pathology. Although not strictly an autoimmune disease, it is also notable that in an allogeneic skin-transplant model, leptin-deficient mice showed an increase in graft survival relative to wild-type mice (136). Altogether, leptin signaling appears to provide an important link between nutritional status and autoimmune disease through its effects on T cell metabolism and function.
Protective Immunity
Like any other physiological system, the development of the immune system is affected by nutritional status. One salient example for this is the thymic atrophy and increase in thymocyte apoptosis observed during malnutrition early in life (137–139). This has a devastating effect on the ability of the immune system to mount a successful immune response to infection. Moreover, many epidemiological studies have shown dysfunction in both innate and adaptive immunity during malnutrition (138). This explains the increased susceptibility to many kinds of infection in the malnourished individuals, such as influenza, tuberculosis, Streptococcus pneumonia and gastrointestinal infections (140–143), and the poor response to vaccines (138).
Obesity, however, is also associated with susceptibility to a number of infections (144). One example of this is with influenza. This was first reported when studies on the H1N1 strain of influenza showed a connection between obesity and poor disease outcome (145). Indeed, obesity was found to be a risk factor for developing H1N1 infection and was associated with a longer length of stay in the intensive care unit and with higher rates of mortality (146–148). Following the discovery that obesity increased risk and mortality from H1N1 flu, obesity that was subsequently found to be is an independent risk factor for increased morbidity and mortality from all strains of influenza.
In addition to increased susceptibility to influenza, individuals with obesity are also at an increased risk from other infections: obese individuals have an increased risk of developing complications such as sepsis, pneumonia, and bacteremia following surgical procedures (149); they are more prone to Helicobacter pylori infection (150), and obese children were found to have three times greater risk of being asymptomatic carriers of Neisseria meningitides (151). In addition, obesity is associated with a lower antibody response to select vaccinations including influenza, hepatitis B, and tetanus (152).
In the case of malnutrition, the lack of protective immunity can be easily traced back to the developmental defects associated with inadequate nutrients and the lack of nutritional signals such as leptin that are critical for fueling immune cell proliferation and function. On the other hand, the susceptibility to infection and poor vaccine response associated with obesity seems unexpected when factoring in that obesity is accompanied with a low-grade inflammation and constant activation of immune cells. In the case of influenza, obesity has been found to be associated with impaired memory response (153). One possible explanation for this is that the systemic metabolic environment in obesity promotes a cellular metabolism in immune cells which supports short-lived effector cells over the generation of long-term memory cells.
Targeting Immune Metabolism for Disease Treatment
As we have documented here, many signaling cascades are affected by nutritional status and subsequently have an effect on immune cell metabolism and function. For that reason, immune cell metabolism represents an attractive target to improve response in both malnutrition and obesity. Here, we will highlight several signaling molecules and metabolic enzymes that play central roles in the metabolic reprogramming of immune cells and are critical for mounting an immune response and initiating inflammatory reaction. These metabolic molecules serve as potential targets to reverse the effects of obesity and malnutrition on immunodeficiency and inflammation, respectively.
Glut1
Activated immune cells are dependent on a glycolytic metabolism to fuel rapid ATP production and provide biosynthetic materials for growth and proliferation. For that reason, activated immune cells require a large influx of glucose to fuel glycolysis (154). This suggests the glycolysis pathway as a potential target for the control of inflammation. Multiple reports have shown that the inhibition of glycolysis by 2-deoxyglucose (2-DG) can block CD4+ T cell proliferation, inflammatory macrophage polarization, and B cell survival (155–157). Although 2-DG shows a potent anti-inflammatory activity and has shown tolerability in clinical trials for the treatment of prostate cancer, cardiac adverse reactions to 2-DG were reported (158, 159), and alternative targets for the inhibition of glucose metabolism are required.
Glucose transport represents the most upstream, rate-limiting step for glycolysis. There are approximately 13 members of the glucose transporter family expressed to various extents in different tissues. The best-described glucose transporter is Glut4, which is an insulin-sensitive glucose transporter expressed on metabolic tissues including muscle, adipose tissue, and liver. However, Glut4 is not expressed on T cells (160). Rather, T cell glucose uptake is largely dependent on the ubiquitously expressed glucose transporter Glut1. Glut1 expression is upregulated in classically activated macrophages, activated Teff cells, and B cells, all of which depend on Glut1 for an increased glucose uptake during activation (154, 160, 161). Glut1 may, therefore, provide a more appropriate target for blocking glycolysis in activated immune cells than 2-DG. Treating macrophages with the Glut1 inhibitor Fasentin was found to inhibit the production of IL-1β (162). Currently, there are several trials to synthesize more selective small molecule inhibitors for Glut1 (163–165). The bioavailable Glut1 inhibitor BAY-876 represents one of the most potent Glut1 inhibitors (IC50 = 2nM) and shows more than 100-fold selectivity against the other glucose transporters (164). Unpublished data from our laboratory showed the ability of BAY-876 to selectively inhibit glucose uptake in activated CD4+ T cells. Although there is limited information about targeting Glut1 in immune cells, the new generation of selective Glut1 inhibitors may have the potential to be used as a therapy for the control of inflammation. Caution must be taken with this class of inhibitors, though, as Glut1 is critical for glucose transport to the brain and Glut1 mutations have been described in disorders of seizures and developmental delay (166, 167).
PFKFB3
One mechanism by which glycolysis is regulated is through the metabolite fructose-2,6-bisphosphate (F26BP), which is an allosteric activator of 6-phosphofructo-1-kinase: a rate-limiting enzyme in the glycolysis pathway that phosphorylates fructose 6-phosphate (F6P) to yield fructose 1,6-bisphosphate (168). The intracellular levels of F26BP are regulated by a family of bifunctional enzymes known as 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase enzymes (PFKFB 1 through 4) which can convert F6P into F26BP and vice versa based on the phosphorylation levels of the enzyme (169). PFKFB3 expression is upregulated in activated macrophages and T cells (170, 171). In T cells, treatment with the PFKFB inhibitor 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) has been shown to block both glycolysis and activation (171). In vivo, 3PO treatment of BALB/c mice challenged with methylated BSA was found to inhibit delayed-type hypersensitivity, highlighting the viability of targeting PFKFB activity for the control of inflammation (171). Unfortunately, the number of selective PFKFB small molecule inhibitors is limited; the only other PFKFB inhibitor, in addition to 3PO, is PFK15. PFK15 is a more potent PFKFB inhibitor than 3PO and showed antiproliferative activity in Jurkat T cell leukemia cells and several solid tumor-derived cell lines, but its effect on primary immune cells has yet to be investigated (172). Similar to targeting Glut1, targeting PFKFB activity may provide an additional tool to block glycolysis; however, more potent and selective inhibitors are needed, as are additional studies to investigate the effect of targeting such enzymes on immune function.
BCAT1
Most activated immune cells are dependent on glycolysis for the rapid generation of energy, which limits the flow of pyruvate to the TCA cycle. However, TCA cycle intermediates are essential for proliferation and immune cell functions, and activated immune cells generally overcome this by metabolizing amino acids. This requires the upregulation of many genes involved in amino acid transport and catabolism.
Branched-chain aminotransferase (BCAT) is the enzyme responsible for the transamination of branched-chain amino acids (BCAAs): leucine, isoleucine, and valine. There are two isoforms of the BCAT enzymes: cytosolic BCAT1 and mitochondrial BCAT2. Transamination of BCAAs is the initial reaction toward the formation of branched-chain α-keto acids, which are decarboxylated to produce coenzyme A (CoA) derivatives (173). Leucine transamination leads to the formation of glutamate and α-ketoisocaproate. The α-ketoisocaproate molecule is subsequently metabolized to form acetoacetate and acetyl-CoA, which is then oxidized in the TCA cycle, similar to glutamate which enters the TCA cycle in the form of α-ketoglutarate.
Branched-chain aminotransferase 2 is highly expressed in many tissues including the kidneys, skeletal muscle, and tissues of the digestive system, while BCAT1 is expressed in a small number of tissues including placenta, adult brain, peripheral neurons, and a limited number of embryonic tissues (174). BCAT1 was found to be highly expressed in human monocyte-derived macrophages compared to BCAT2 (175). Blocking the activity of BCAT1 by the leucine analog ERG240 was found to inhibit the induction of cis-aconitate decarboxylase (IRG1) expression by LPS which is a crucial step for the activation for macrophages (175). ERG240 treatment was able to reduce oxygen consumption and glycolysis in human macrophages. Consistent with this phenotype, the administration of ERG240 ameliorated the severity of crescentic glomerulonephritis in rats and collagen-induced arthritis in mice (175).
F1F0-ATPase
In contrast to acutely activated T cells being dependent on glycolysis, chronically activated T cells involved in autoimmune disease are more dependent on oxidative phosphorylation for energy production (176–178). This suggests that components of the oxidative phosphorylation pathway may be targets to block the expansion of autoreactive T cells in autoimmune diseases. The ATP synthase F1F0-ATPase catalyzes the final step of energy production of the respiratory chain in the mitochondria. F1F0-ATPase was found to be the target for the 1,4-benzodiazepine (Bz-423) which is reported to induce apoptosis of pathogenic lymphoid cells in lupus mouse models (179–181). Bz-423 was also reported to induce apoptosis of alloreactive T cells in a graft-versus-host disease model (182). Based on this, F1F0-ATPase gained special attention as a viable drug target for the treatment of autoimmune diseases. Currently, there are ongoing clinical trials for the treatment of inflammatory bowel disease and ulcerative colitis using the F1F0-ATPase small molecule inhibitor LYC-30937.
Conclusion
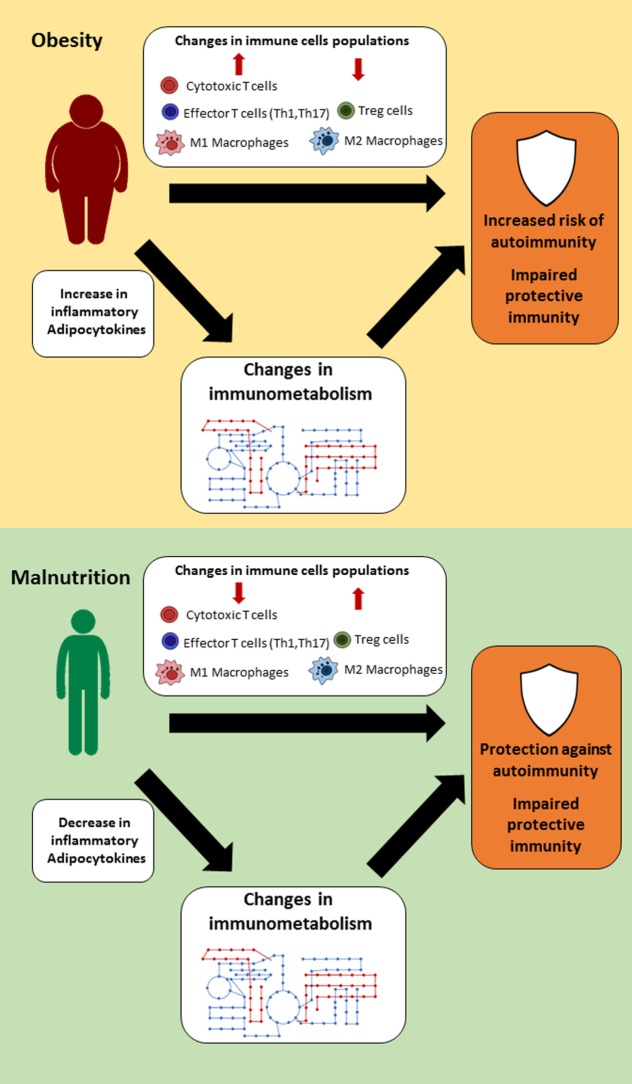
Changes in nutritional status have a wide range of effects on the body, which can influence organ size, hormone, and cytokine levels, and immune cell populations and function. This link between nutrition and immunity is mediated, in part, by a select group of adipocytokines, such as leptin, which can influence immune cell number and function through its effects on cellular metabolism (Figure 1). For that reason, leptin has been identified as a key regulator of both protective immunity and autoimmunity in the context of nutritional disorders. Other cytokines and hormones likely play a similar key role in linking nutrition and immunity. In understanding the mechanisms by which nutrition influences immunity, we can identify targets to improve or normalize immunity in cases of under- or overnutrition. Currently, there are several small molecules under investigation that show promising preclinical results and the potential for restoring immune response in both malnutrition and obesity. In summary, more studies are needed to clarify the link between nutritional status, immune metabolism, and immune function; such knowledge may pave the way for the development of novel classes of therapies that can reverse the detrimental effects of the extremes of nutritional status on immunity.
Figure 1.
Suggested links between nutritional status, immune metabolism, and immune function. In settings of extreme nutritional status (obesity or malnutrition), changes in immune cell populations, hormones, and cytokine levels lead to alternations in immune cell metabolism, which thereby influence immune function.
Author Contributions
All authors participated in the writing of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Institutes of Health (R01-DK106090 and T32-AI052077) and the National Multiple Sclerosis Society (RG-5333-A-1).
References
- 1.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (1993) 259(5091):87–91. 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest (1995) 95(5):2409–15. 10.1172/JCI117936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest (1995) 95(5):2111–9. 10.1172/JCI117899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature (1997) 389(6651):610–4. 10.1038/39335 [DOI] [PubMed] [Google Scholar]
- 5.Sewter CP, Digby JE, Blows F, Prins J, O’Rahilly S. Regulation of tumour necrosis factor-alpha release from human adipose tissue in vitro. J Endocrinol (1999) 163(1):33–8. 10.1677/joe.0.1630033 [DOI] [PubMed] [Google Scholar]
- 6.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest (2005) 115(5):1111–9. 10.1172/JCI200525102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopp A, Buechler C, Neumeier M, Weigert J, Aslanidis C, Scholmerich J, et al. Innate immunity and adipocyte function: ligand-specific activation of multiple toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) (2009) 17(4):648–56. 10.1038/oby.2008.607 [DOI] [PubMed] [Google Scholar]
- 8.Yasukawa K, Hirano T, Watanabe Y, Muratani K, Matsuda T, Nakai S, et al. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J (1987) 6(10):2939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woloski BM, Fuller GM. Identification and partial characterization of hepatocyte-stimulating factor from leukemia cell lines: comparison with interleukin 1. Proc Natl Acad Sci U S A (1985) 82(5):1443–7. 10.1073/pnas.82.5.1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci (2012) 8(9):1237–47. 10.7150/ijbs.4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med (2000) 6(5):583–8. 10.1038/75068 [DOI] [PubMed] [Google Scholar]
- 12.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A (2008) 105(47):18460–5. 10.1073/pnas.0809850105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, et al. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med (1996) 183(4):1345–55. 10.1084/jem.183.4.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia (1997) 40(11):1286–92. 10.1007/s001250050822 [DOI] [PubMed] [Google Scholar]
- 15.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab (2001) 280(5):E745–51. 10.1152/ajpendo.2001.280.5.E745 [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi K, Nishimura T, Ishiba H, Seko Y, Okajima A, Fujii H, et al. Blockade of interleukin 6 signalling ameliorates systemic insulin resistance through upregulation of glucose uptake in skeletal muscle and improves hepatic steatosis in high-fat diet fed mice. Liver Int (2015) 35(2):550–61. 10.1111/liv.12645 [DOI] [PubMed] [Google Scholar]
- 17.Kraakman MJ, Kammoun HL, Allen TL, Deswaerte V, Henstridge DC, Estevez E, et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab (2015) 21(3):403–16. 10.1016/j.cmet.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 18.Xu E, Pereira MMA, Karakasilioti I, Theurich S, Al-Maarri M, Rappl G, et al. Temporal and tissue-specific requirements for T-lymphocyte IL-6 signalling in obesity-associated inflammation and insulin resistance. Nat Commun (2017) 8:14803. 10.1038/ncomms14803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev (2002) 60(10 Pt 2):S1–14; discussion S68–84, 5–7. 10.1301/002966402320634878 [DOI] [PubMed] [Google Scholar]
- 20.Naylor C, Petri WA, Jr. Leptin regulation of immune responses. Trends Mol Med (2016) 22(2):88–98. 10.1016/j.molmed.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 21.Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A (1996) 93(25):14564–8. 10.1073/pnas.93.25.14564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandel MA, Mahmoud AA. Impairment of cell-mediated immunity in mutation diabetic mice (db/db). J Immunol (1978) 120(4):1375–7. [PubMed] [Google Scholar]
- 23.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J (1998) 12(1):57–65. 10.1096/fasebj.12.1.57 [DOI] [PubMed] [Google Scholar]
- 24.Cioffi JA, Shafer AW, Zupancic TJ, Smith-Gbur J, Mikhail A, Platika D, et al. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med (1996) 2(5):585–9. 10.1038/nm0596-585 [DOI] [PubMed] [Google Scholar]
- 25.Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med (2012) 33(1):35–45. 10.1016/j.mam.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 26.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol (2014) 192(1):136–44. 10.4049/jimmunol.1301158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerriets VA, Danzaki K, Kishton RJ, Eisner W, Nichols AG, Saucillo DC, et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol (2016) 46(8):1970–83. 10.1002/eji.201545861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Stijn CM, Kim J, Lusis AJ, Barish GD, Tangirala RK. Macrophage polarization phenotype regulates adiponectin receptor expression and adiponectin anti-inflammatory response. FASEB J (2015) 29(2):636–49. 10.1096/fj.14-253831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Y, Liu M. Adiponectin: a versatile player of innate immunity. J Mol Cell Biol (2016) 8(2):120–8. 10.1093/jmcb/mjw012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Suhaimi EA, Shehzad A. Leptin, resistin and visfatin: the missing link between endocrine metabolic disorders and immunity. Eur J Med Res (2013) 18(1):12. 10.1186/2047-783X-18-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dostálová I, Sedlácková D, Papezova H, Nedvídková J, Haluzik M. Serum visfatin levels in patients with anorexia nervosa and bulimia nervosa. Physiol Res (2009) 58:903–7. [DOI] [PubMed] [Google Scholar]
- 32.Azevedo ZMA, Luz RA, Victal SH, Kurdian B, Fonseca VM, Fitting C, et al. Increased production of tumor necrosis factor-a in whole blood cultures from children with primary malnutrition. Braz J Med Biol Res (2005) 38:171–83. 10.1590/S0100-879X2005000200005 [DOI] [PubMed] [Google Scholar]
- 33.Fock RA, Vinolo MAR, de Moura Sá Rocha V, de Sá Rocha LC, Borelli P. Protein-energy malnutrition decreases the expression of TLR-4/MD-2 and CD14 receptors in peritoneal macrophages and reduces the synthesis of TNF-α in response to lipopolysaccharide (LPS) in mice. Cytokine (2007) 40(2):105–14. 10.1016/j.cyto.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 34.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol (2016) 12(1):49–62. 10.1038/nrrheum.2015.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bing C. Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte (2015) 4(2):149–52. 10.4161/21623945.2014.979661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muñoz C, Arévalo M, López M, Schlesinger L. Impaired interleukin-1 and tumor necrosis factor production in protein-calorie malnutrition. Nutr Res (1994) 14(3):347–52. 10.1016/S0271-5317(05)80173-3 [DOI] [Google Scholar]
- 37.Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res (2009) 58(11):727. 10.1007/s00011-009-0060-4 [DOI] [PubMed] [Google Scholar]
- 38.Straczkowski M, Dzienis-Straczkowska S, Stêpieñ A, Kowalska I, Szelachowska M, Kinalska I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-α system. J Clin Endocrinol Metab (2002) 87(10):4602–6. 10.1210/jc.2002-020135 [DOI] [PubMed] [Google Scholar]
- 39.Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (2006) 30:1347. 10.1038/sj.ijo.0803259 [DOI] [PubMed] [Google Scholar]
- 40.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm (2013) 2013:139239. 10.1155/2013/139239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calcaterra V, De Amici M, Klersy C, Torre C, Brizzi V, Scaglia F, et al. Adiponectin, IL-10 and metabolic syndrome in obese children and adolescents. Acta Biomed (2009) 80:117–23. [PubMed] [Google Scholar]
- 42.Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab (2003) 88(3):1055–8. 10.1210/jc.2002-021437 [DOI] [PubMed] [Google Scholar]
- 43.Mello AS, de Oliveira DC, Bizzarro B, Sá-Nunes A, Hastreiter AA, de Oliveira Beltran JS, et al. Protein malnutrition alters spleen cell proliferation and IL-2 and IL-10 production by affecting the STAT-1 and STAT-3 balance. Inflammation (2014) 37(6):2125–38. 10.1007/s10753-014-9947-5 [DOI] [PubMed] [Google Scholar]
- 44.Hasan A, Al-Ghimlas F, Warsame S, Al-Hubail A, Ahmad R, Bennakhi A, et al. IL-33 is negatively associated with the BMI and confers a protective lipid/metabolic profile in non-diabetic but not diabetic subjects. BMC Immunol (2014) 15(1):19. 10.1186/1471-2172-15-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ragusa R, Cabiati M, Guzzardi MA, D’Amico A, Giannessi D, Del Ry S, et al. Effects of obesity on IL-33/ST2 system in heart, adipose tissue and liver: study in the experimental model of Zucker rats. Exp Mol Pathol (2017) 102(2):354–9. 10.1016/j.yexmp.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 46.Jung C, Gerdes N, Fritzenwanger M, Figulla HR. Circulating levels of interleukin-1 family cytokines in overweight adolescents. Mediators Inflamm (2010) 2010:6. 10.1155/2010/958403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer J-M. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab (2002) 87(3):1184–8. 10.1210/jcem.87.3.8351 [DOI] [PubMed] [Google Scholar]
- 48.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun (2004) 323(2):630–5. 10.1016/j.bbrc.2004.08.145 [DOI] [PubMed] [Google Scholar]
- 49.Bartz S, Mody A, Hornik C, Bain J, Muehlbauer M, Kiyimba T, et al. Severe acute malnutrition in childhood: hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J Clin Endocrinol Metab (2014) 99(6):2128–37. 10.1210/jc.2013-4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hambor JE, Fleck L, Stevenson JR. Impairment of macrophage migration inhibitory factor synthesis and macrophage migration in protein-malnourished mice. Cell Immunol (1983) 81(2):306–12. 10.1016/0008-8749(83)90238-1 [DOI] [PubMed] [Google Scholar]
- 51.Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol (2008) 3(5):545–56. 10.2217/17460875.3.5.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest (2003) 112(12):1796–808. 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest (2006) 116(6):1494–505. 10.1172/JCI26498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med (2009) 15(8):914–20. 10.1038/nm.1964 [DOI] [PubMed] [Google Scholar]
- 55.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest (2007) 117(1):175–84. 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells (2014) 37(5):365–71. 10.14348/molcells.2014.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science (2011) 332(6026):243–7. 10.1126/science.1201475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature (2012) 486(7404):549–53. 10.1038/nature11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol (2008) 20(3):332–8. 10.1016/j.coi.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol (2009) 39(7):1893–901. 10.1002/eji.200939349 [DOI] [PubMed] [Google Scholar]
- 61.Huh JY, Kim JI, Park YJ, Hwang IJ, Lee YS, Sohn JH, et al. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol Cell Biol (2013) 33(2):328–39. 10.1128/MCB.00552-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity (2012) 37(3):574–87. 10.1016/j.immuni.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerriets VA, MacIver NJ. Role of T cells in malnutrition and obesity. Front Immunol (2014) 5:379. 10.3389/fimmu.2014.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med (2009) 15(8):921–9. 10.1038/nm.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med (2009) 15(8):930–9. 10.1038/nm.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, et al. Antigen-and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab (2015) 21(4):543–57. 10.1016/j.cmet.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol (2016) 34:609–33. 10.1146/annurev-immunol-032712-095948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cipolletta D, Cohen P, Spiegelman BM, Benoist C, Mathis D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARgamma effects. Proc Natl Acad Sci U S A (2015) 112(2):482–7. 10.1073/pnas.1423486112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature (2015) 528(7580):137–41. 10.1038/nature16151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest (1999) 104(8):1051–9. 10.1172/JCI6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Procaccini C, De Rosa V, Galgani M, Carbone F, Cassano S, Greco D, et al. Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J Immunol (2012) 189(6):2941–53. 10.4049/jimmunol.1200935 [DOI] [PubMed] [Google Scholar]
- 72.Pena-Cruz V, Reiss CS, McIntosh K. Sendai virus infection of mice with protein malnutrition. J Virol (1989) 63(8):3541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor AK, Cao W, Vora KP, De La Cruz J, Shieh WJ, Zaki SR, et al. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J Infect Dis (2013) 207(3):501–10. 10.1093/infdis/jis527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Najera O, Gonzalez C, Toledo G, Lopez L, Ortiz R. Flow cytometry study of lymphocyte subsets in malnourished and well-nourished children with bacterial infections. Clin Diagn Lab Immunol (2004) 11(3):577–80. 10.1128/CDLI.11.3.577-580.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savino W, Dardenne M, Velloso LA, Dayse Silva-Barbosa S. The thymus is a common target in malnutrition and infection. Br J Nutr (2007) 98(Suppl 1):S11–6. 10.1017/S0007114507832880 [DOI] [PubMed] [Google Scholar]
- 76.Saha S, Shalova IN, Biswas SK. Metabolic regulation of macrophage phenotype and function. Immunol Rev (2017) 280(1):102–11. 10.1111/imr.12603 [DOI] [PubMed] [Google Scholar]
- 77.Boothby M, Rickert RC. Metabolic regulation of the immune humoral response. Immunity (2017) 46(5):743–55. 10.1016/j.immuni.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol (2013) 31:259–83. 10.1146/annurev-immunol-032712-095956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol (2003) 24(7):380–6. 10.1016/S1471-4906(03)00141-8 [DOI] [PubMed] [Google Scholar]
- 80.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell (2000) 6(3):683–92. 10.1016/S1097-2765(00)00066-6 [DOI] [PubMed] [Google Scholar]
- 81.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol (2002) 2(8):547–56. 10.1038/nri853 [DOI] [PubMed] [Google Scholar]
- 82.Warburg O. On the origin of cancer cells. Science (1956) 123(3191):309–14. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 83.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci (2016) 41(3):211–8. 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bental M, Deutsch C. Metabolic changes in activated T cells: an NMR study of human peripheral blood lymphocytes. Magn Reson Med (1993) 29(3):317–26. 10.1002/mrm.1910290307 [DOI] [PubMed] [Google Scholar]
- 85.Greiner EF, Guppy M, Brand K. Glucose is essential for proliferation and the glycolytic enzyme-induction that provokes a transition to glycolytic energy-production. J Biol Chem (1994) 269(50):31484–90. [PubMed] [Google Scholar]
- 86.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity (2002) 16(6):769–77. 10.1016/S1074-7613(02)00323-0 [DOI] [PubMed] [Google Scholar]
- 87.Keating R, McGargill MA. mTOR regulation of lymphoid cells in immunity to pathogens. Front Immunol (2016) 7:180. 10.3389/fimmu.2016.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol (2012) 12(5):325–38. 10.1038/nri3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity (2010) 33(3):301–11. 10.1016/j.immuni.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol (2007) 7(13):1819–24. 10.1016/j.intimp.2007.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, et al. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol (2007) 178(4):2163–70. 10.4049/jimmunol.178.4.2163 [DOI] [PubMed] [Google Scholar]
- 92.MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol (2011) 187(8):4187–98. 10.4049/jimmunol.1100367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res (2009) 15(21):6479–83. 10.1158/1078-0432.CCR-09-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Widnell CC. Control of glucose transport by GLUT1: regulated secretion in an unexpected environment. Biosci Rep (1995) 15(6):427–43. 10.1007/BF01204347 [DOI] [PubMed] [Google Scholar]
- 95.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity (2011) 35(6):871–82. 10.1016/j.immuni.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindsten T, June CH, Thompson CB. Multiple mechanisms regulate c-Myc gene expression during normal T cell activation. EMBO J (1988) 7(9):2787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kelly K, Cochran BH, Stiles CD, Leder P. Cell-specific regulation of the c-Myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell (1983) 35(3 Pt 2):603–10. 10.1016/0092-8674(83)90092-2 [DOI] [PubMed] [Google Scholar]
- 98.Park BV, Pan F. Metabolic regulation of T cell differentiation and function. Mol Immunol (2015) 68(2 Pt C):497–506. 10.1016/j.molimm.2015.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palmer CS, Ostrowski M, Balderson B, Christian N, Crowe SM. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol (2015) 6:1. 10.3389/fimmu.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol (2011) 186(6):3299–303. 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Michalek RD, Rathmell JC. The metabolic life and times of a T-cell. Immunol Rev (2010) 236(1):190–202. 10.1111/j.1600-065X.2010.00911.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature (2009) 460(7251):103–7. 10.1038/nature08097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kishore M, Cheung KCP, Fu H, Bonacina F, Wang G, Coe D, et al. Regulatory T cell migration is dependent on glucokinase-mediated glycolysis. Immunity (2017) 47(5):875–89.e10. 10.1016/j.immuni.2017.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity (2009) 30(6):832–44. 10.1016/j.immuni.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol (2011) 12(4):295–303. 10.1038/ni.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med (1995) 1(11):1155–61. 10.1038/nm1195-1155 [DOI] [PubMed] [Google Scholar]
- 107.De Rosa V, Procaccini C, La Cava A, Chieffi P, Nicoletti GF, Fontana S, et al. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J Clin Invest (2006) 116(2):447–55. 10.1172/JCI26523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang S, Baidoo SE, Liu Y, Zhu C, Tian J, Ma J, et al. T cell-derived leptin contributes to increased frequency of T helper type 17 cells in female patients with Hashimoto’s thyroiditis. Clin Exp Immunol (2013) 171(1):63–8. 10.1111/j.1365-2249.2012.04670.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deng J, Liu Y, Yang M, Wang S, Zhang M, Wang X, et al. Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis Rheum (2012) 64(11):3564–73. 10.1002/art.34637 [DOI] [PubMed] [Google Scholar]
- 110.Yu Y, Liu Y, Shi FD, Zou H, Matarese G, La Cava A. Cutting edge: leptin-induced RORgammat expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J Immunol (2013) 190(7):3054–8. 10.4049/jimmunol.1203275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shepherd PR, Kahn BB. Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N Engl J Med (1999) 341(4):248–57. 10.1056/NEJM199907223410406 [DOI] [PubMed] [Google Scholar]
- 112.Mandarino LJ, Printz RL, Cusi KA, Kinchington P, O’Doherty RM, Osawa H, et al. Regulation of hexokinase II and glycogen synthase mRNA, protein, and activity in human muscle. Am J Physiol (1995) 269(4 Pt 1):E701–8. [DOI] [PubMed] [Google Scholar]
- 113.Dimitriadis G, Parry-Billings M, Bevan S, Leighton B, Krause U, Piva T, et al. The effects of insulin on transport and metabolism of glucose in skeletal muscle from hyperthyroid and hypothyroid rats. Eur J Clin Invest (1997) 27(6):475–83. 10.1046/j.1365-2362.1997.1380688.x [DOI] [PubMed] [Google Scholar]
- 114.Dimitriadis G, Parry-Billings M, Bevan S, Dunger D, Piva T, Krause U, et al. Effects of insulin-like growth factor I on the rates of glucose transport and utilization in rat skeletal muscle in vitro. Biochem J (1992) 285(Pt 1):269–74. 10.1042/bj2850269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract (2011) 93(Suppl 1):S52–9. 10.1016/S0168-8227(11)70014-6 [DOI] [PubMed] [Google Scholar]
- 116.Dimitriadis GD, Leighton B, Vlachonikolis IG, Parry-Billings M, Challiss RA, West D, et al. Effects of hyperthyroidism on the sensitivity of glycolysis and glycogen synthesis to insulin in the soleus muscle of the rat. Biochem J (1988) 253(1):87–92. 10.1042/bj2530087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Helderman JH, Reynolds TC, Strom TB. The insulin receptor as a universal marker of activated lymphocytes. Eur J Immunol (1978) 8(8):589–95. 10.1002/eji.1830080810 [DOI] [PubMed] [Google Scholar]
- 118.Fischer HJ, Sie C, Schumann E, Witte AK, Dressel R, van den Brandt J, et al. The insulin receptor plays a critical role in T cell function and adaptive immunity. J Immunol (2017) 198(5):1910–20. 10.4049/jimmunol.1601011 [DOI] [PubMed] [Google Scholar]
- 119.Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, et al. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc Natl Acad Sci U S A (2005) 102(14):5150–5. 10.1073/pnas.0408995102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kraszula L, Jasinska A, Eusebio M, Kuna P, Glabinski A, Pietruczuk M. Evaluation of the relationship between leptin, resistin, adiponectin and natural regulatory T cells in relapsing-remitting multiple sclerosis. Neurol Neurochir Pol (2012) 46(1):22–8. 10.5114/ninp.2012.27211 [DOI] [PubMed] [Google Scholar]
- 121.Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, et al. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med (2014) 20(1):69–74. 10.1038/nm.3411 [DOI] [PubMed] [Google Scholar]
- 122.Hedstrom AK, Lima Bomfim I, Barcellos L, Gianfrancesco M, Schaefer C, Kockum I, et al. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology (2014) 82(10):865–72. 10.1212/WNL.0000000000000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kavak KS, Teter BE, Hagemeier J, Zakalik K, Weinstock-Guttman B, New York State Multiple Sclerosis Consortium . Higher weight in adolescence and young adulthood is associated with an earlier age at multiple sclerosis onset. Mult Scler (2015) 21(7):858–65. 10.1177/1352458514555787 [DOI] [PubMed] [Google Scholar]
- 124.Gianfrancesco MA, Acuna B, Shen L, Briggs FB, Quach H, Bellesis KH, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obes Res Clin Pract (2014) 8(5):e435–47. 10.1016/j.orcp.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology (2013) 80(6):548–52. 10.1212/WNL.0b013e31828154f3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Emamgholipour S, Eshaghi SM, Hossein-nezhad A, Mirzaei K, Maghbooli Z, Sahraian MA. Adipocytokine profile, cytokine levels and foxp3 expression in multiple sclerosis: a possible link to susceptibility and clinical course of disease. PLoS One (2013) 8(10):e76555. 10.1371/journal.pone.0076555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frisullo G, Mirabella M, Angelucci F, Caggiula M, Morosetti R, Sancricca C, et al. The effect of disease activity on leptin, leptin receptor and suppressor of cytokine signalling-3 expression in relapsing-remitting multiple sclerosis. J Neuroimmunol (2007) 192(1–2):174–83. 10.1016/j.jneuroim.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 128.Matarese G, Di Giacomo A, Sanna V, Lord GM, Howard JK, Di Tuoro A, et al. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol (2001) 166(10):5909–16. 10.4049/jimmunol.166.10.5909 [DOI] [PubMed] [Google Scholar]
- 129.Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest (2003) 111(2):241–50. 10.1172/JCI200316721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Matarese G, Sanna V, Di Giacomo A, Lord GM, Howard JK, Bloom SR, et al. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur J Immunol (2001) 31(5):1324–32. [DOI] [PubMed] [Google Scholar]
- 131.Galgani M, Procaccini C, De Rosa V, Carbone F, Chieffi P, La Cava A, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol (2010) 185(12):7474–9. 10.4049/jimmunol.1001674 [DOI] [PubMed] [Google Scholar]
- 132.Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol (2008) 84(4):940–8. 10.1189/jlb.0208133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kafami L, Raza M, Razavi A, Mirshafiey A, Movahedian M, Khorramizadeh MR. Intermittent feeding attenuates clinical course of experimental autoimmune encephalomyelitis in C57BL/6 mice. Avicenna J Med Biotechnol (2010) 2(1):47–52. [PMC free article] [PubMed] [Google Scholar]
- 134.Lourenco EV, Liu A, Matarese G, La Cava A. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc Natl Acad Sci U S A (2016) 113(38):10637–42. 10.1073/pnas.1607101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang X, Qiao Y, Yang L, Song S, Han Y, Tian Y, et al. Leptin levels in patients with systemic lupus erythematosus inversely correlate with regulatory T cell frequency. Lupus (2017) 26(13):1401–6. 10.1177/0961203317703497 [DOI] [PubMed] [Google Scholar]
- 136.Moraes-Vieira PM, Bassi EJ, Larocca RA, Castoldi A, Burghos M, Lepique AP, et al. Leptin deficiency modulates allograft survival by favoring a Th2 and a regulatory immune profile. [corrected]. Am J Transplant (2013) 13(1):36–44. 10.1111/j.1600-6143.2012.04283.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prentice AM. The thymus: a barometer of malnutrition. Br J Nutr (1999) 81(5):345–7. [PubMed] [Google Scholar]
- 138.Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition—a systematic review. PLoS One (2014) 9(8):e105017. 10.1371/journal.pone.0105017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ortiz R, Cortes L, Cortes E, Medina H. Malnutrition alters the rates of apoptosis in splenocytes and thymocyte subpopulations of rats. Clin Exp Immunol (2009) 155(1):96–106. 10.1111/j.1365-2249.2008.03796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis (2004) 8(3):286–98. [PubMed] [Google Scholar]
- 141.Verhagen LM, Gomez-Castellano K, Snelders E, Rivera-Olivero I, Pocaterra L, Melchers WJ, et al. Respiratory infections in Enepa Amerindians are related to malnutrition and Streptococcus pneumoniae carriage. J Infect (2013) 67(4):273–81. 10.1016/j.jinf.2013.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ritz BW, Aktan I, Nogusa S, Gardner EM. Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J Nutr (2008) 138(11):2269–75. 10.3945/jn.108.093633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Petri WA, Jr, Mondal D, Peterson KM, Duggal P, Haque R. Association of malnutrition with amebiasis. Nutr Rev (2009) 67(Suppl 2):S207–15. 10.1111/j.1753-4887.2009.00242.x [DOI] [PubMed] [Google Scholar]
- 144.Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) (2013) 37(3):333–40. 10.1038/ijo.2012.62 [DOI] [PubMed] [Google Scholar]
- 145.Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med (2011) 8(7):e1001053. 10.1371/journal.pmed.1001053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis (2011) 52(3):301–12. 10.1093/cid/ciq152 [DOI] [PubMed] [Google Scholar]
- 147.Diaz E, Rodriguez A, Martin-Loeches I, Lorente L, Del Mar Martin M, Pozo JC, et al. Impact of obesity in patients infected with 2009 influenza A(H1N1). Chest (2011) 139(2):382–6. 10.1378/chest.10-1160 [DOI] [PubMed] [Google Scholar]
- 148.Riquelme R, Jimenez P, Videla AJ, Lopez H, Chalmers J, Singanayagam A, et al. Predicting mortality in hospitalized patients with 2009 H1N1 influenza pneumonia. Int J Tuberc Lung Dis (2011) 15(4):542–6. 10.5588/ijtld.10.0539 [DOI] [PubMed] [Google Scholar]
- 149.Choban PS, Flancbaum L. The impact of obesity on surgical outcomes: a review. J Am Coll Surg (1997) 185(6):593–603. 10.1016/S1072-7515(97)00109-9 [DOI] [PubMed] [Google Scholar]
- 150.Arslan E, Atilgan H, Yavasoglu I. The prevalence of Helicobacter pylori in obese subjects. Eur J Intern Med (2009) 20(7):695–7. 10.1016/j.ejim.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 151.Uberos J, Molina-Carballo A, Fernandez-Puentes V, Rodriguez-Belmonte R, Munoz-Hoyos A. Overweight and obesity as risk factors for the asymptomatic carrier state of Neisseria meningitidis among a paediatric population. Eur J Clin Microbiol Infect Dis (2010) 29(3):333–4. 10.1007/s10096-009-0849-7 [DOI] [PubMed] [Google Scholar]
- 152.Hemalatha Rajkumar PB. The impact of obesity on immune response to infection and vaccine: an insight into plausible mechanisms. Endocrinol Metab Synd (2013) 2(2):113. 10.4172/2161-1017.1000113 [DOI] [Google Scholar]
- 153.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol (2010) 184(6):3127–33. 10.4049/jimmunol.0903220 [DOI] [PubMed] [Google Scholar]
- 154.Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem (2014) 289(11):7884–96. 10.1074/jbc.M113.522037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Blair D, Dufort FJ, Chiles TC. Protein kinase Cbeta is critical for the metabolic switch to glycolysis following B-cell antigen receptor engagement. Biochem J (2012) 448(1):165–9. 10.1042/BJ20121225 [DOI] [PubMed] [Google Scholar]
- 156.Cao Y, Rathmell JC, Macintyre AN. Metabolic reprogramming towards aerobic glycolysis correlates with greater proliferative ability and resistance to metabolic inhibition in CD8 versus CD4 T cells. PLoS One (2014) 9(8):e104104. 10.1371/journal.pone.0104104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao Q, Chu Z, Zhu L, Yang T, Wang P, Liu F, et al. 2-Deoxy-d-glucose treatment decreases anti-inflammatory M2 macrophage polarization in mice with tumor and allergic airway inflammation. Front Immunol (2017) 8:637. 10.3389/fimmu.2017.00637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol (2013) 71(2):523–30. 10.1007/s00280-012-2045-1 [DOI] [PubMed] [Google Scholar]
- 159.Stein M, Lin H, Jeyamohan C, Dvorzhinski D, Gounder M, Bray K, et al. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate (2010) 70(13):1388–94. 10.1002/pros.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab (2014) 20(1):61–72. 10.1016/j.cmet.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol (2014) 192(8):3626–36. 10.4049/jimmunol.1302062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dror E, Dalmas E, Meier DT, Wueest S, Thevenet J, Thienel C, et al. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol (2017) 18(3):283–92. 10.1038/ni.3659 [DOI] [PubMed] [Google Scholar]
- 163.Ung PM, Song W, Cheng L, Zhao X, Hu H, Chen L, et al. Inhibitor discovery for the human GLUT1 from homology modeling and virtual screening. ACS Chem Biol (2016) 11(7):1908–16. 10.1021/acschembio.6b00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Siebeneicher H, Cleve A, Rehwinkel H, Neuhaus R, Heisler I, Muller T, et al. Identification and optimization of the first highly selective GLUT1 inhibitor BAY-876. ChemMedChem (2016) 11(20):2261–71. 10.1002/cmdc.201600276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther (2012) 11(8):1672–82. 10.1158/1535-7163.MCT-12-0131 [DOI] [PubMed] [Google Scholar]
- 166.De Vivo DC, Trifiletti RR, Jacobson RI, Ronen GM, Behmand RA, Harik SI. Defective glucose transport across the blood–brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N Engl J Med (1991) 325(10):703–9. 10.1056/NEJM199109053251006 [DOI] [PubMed] [Google Scholar]
- 167.Brockmann K, Wang D, Korenke CG, von Moers A, Ho YY, Pascual JM, et al. Autosomal dominant glut-1 deficiency syndrome and familial epilepsy. Ann Neurol (2001) 50(4):476–85. 10.1002/ana.1222 [DOI] [PubMed] [Google Scholar]
- 168.Van Schaftingen E, Jett MF, Hue L, Hers HG. Control of liver 6-phosphofructokinase by fructose 2,6-bisphosphate and other effectors. Proc Natl Acad Sci U S A (1981) 78(6):3483–6. 10.1073/pnas.78.6.3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol (2009) 86(3):174–9. 10.1016/j.yexmp.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 170.Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol (2010) 185(1):605–14. 10.4049/jimmunol.0901698 [DOI] [PubMed] [Google Scholar]
- 171.Telang S, Clem BF, Klarer AC, Clem AL, Trent JO, Bucala R, et al. Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation. J Transl Med (2012) 10:95. 10.1186/1479-5876-10-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Clem BF, O’Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA, II, et al. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther (2013) 12(8):1461–70. 10.1158/1535-7163.MCT-13-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tom A, Nair KS. Assessment of branched-chain amino acid status and potential for biomarkers. J Nutr (2006) 136(1 Suppl):324S–30S. 10.1093/jn/136.1.324S [DOI] [PubMed] [Google Scholar]
- 174.Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab (2004) 286(1):E64–76. 10.1152/ajpendo.00276.2003 [DOI] [PubMed] [Google Scholar]
- 175.Papathanassiu AE, Ko JH, Imprialou M, Bagnati M, Srivastava PK, Vu HA, et al. BCAT1 controls metabolic reprogramming in activated human macrophages and is associated with inflammatory diseases. Nat Commun (2017) 8:16040. 10.1038/ncomms16040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Benke PJ, Drisko J, Ahmad P. Increased oxidative metabolism in phytohemagglutinin-stimulated lymphocytes from patients with systemic lupus erythematosus is associated with serum SSA antibody. Biochem Med Metab Biol (1991) 45(1):28–40. 10.1016/0885-4505(91)90005-6 [DOI] [PubMed] [Google Scholar]
- 177.Kuhnke A, Burmester GR, Krauss S, Buttgereit F. Bioenergetics of immune cells to assess rheumatic disease activity and efficacy of glucocorticoid treatment. Ann Rheum Dis (2003) 62(2):133–9. 10.1136/ard.62.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wahl DR, Petersen B, Warner R, Richardson BC, Glick GD, Opipari AW. Characterization of the metabolic phenotype of chronically activated lymphocytes. Lupus (2010) 19(13):1492–501. 10.1177/0961203310373109 [DOI] [PubMed] [Google Scholar]
- 179.Johnson KM, Chen X, Boitano A, Swenson L, Opipari AW, Jr, Glick GD. Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem Biol (2005) 12(4):485–96. 10.1016/j.chembiol.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 180.Bednarski JJ, Warner RE, Rao T, Leonetti F, Yung R, Richardson BC, et al. Attenuation of autoimmune disease in Fas-deficient mice by treatment with a cytotoxic benzodiazepine. Arthritis Rheum (2003) 48(3):757–66. 10.1002/art.10968 [DOI] [PubMed] [Google Scholar]
- 181.Blatt NB, Bednarski JJ, Warner RE, Leonetti F, Johnson KM, Boitano A, et al. Benzodiazepine-induced superoxide signals B cell apoptosis: mechanistic insight and potential therapeutic utility. J Clin Invest (2002) 110(8):1123–32. 10.1172/JCI0216029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gatza E, Wahl DR, Opipari AW, Sundberg TB, Reddy P, Liu C, et al. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci Transl Med (2011) 3(67):67ra8. 10.1126/scitranslmed.3001975 [DOI] [PMC free article] [PubMed] [Google Scholar]